Abstract
Objectives
The objective of this study was to identify characteristics associated with an increased risk of anxiety and mood disorder prior to 25 years of age, in children who sustained a traumatic brain injury (TBI) prior to age 10.
Methods
This population-based study identified 562 TBI cases from a 1976–1982 birth cohort in Olmsted County, Minnesota. TBI cases were manually confirmed and classified by injury severity. Separate Cox proportional hazards regression models were fit to estimate the association of TBI and secondary non-TBI related characteristics with the risk of a subsequent clinically determined anxiety or mood disorder. Multivariable-adjusted population attributable risk (PAR) estimates were calculated for TBI characteristics.
Results
Older age at initial TBI and extracranial injury at time of initial TBI were significantly associated with an increased risk of anxiety (adjusted HR [95% CI]: 1.33 [1.16, 1.52] per 1-year increase and 2.41 [1.26, 4.59]), respectively. Older age at initial TBI was significantly associated with an increased risk of a mood disorder (adjusted HR 1.17 [1.08–1.27]).
Conclusion
In individuals sustaining a TBI prior to age 10, age at injury greater than 5 years old was the largest contributor to development of a mood or anxiety disorder.
Keywords: Brain Injuries, Traumatic, Psychiatry and Psychology, brain diseases, Depressive Disorders, Anxiety Disorders, Bipolar Disorder
Introduction
Pediatric traumatic brain injury (TBI) is associated with more than 60,000 hospitalizations, 600,000 Emergency Department visits, and 7000 deaths annually in the United States, thus representing an important public health concern.(1, 2) In addition to chronic cognitive and physical impairment,(3–5) associations have been observed between pediatric TBI and the development of psychiatric sequelae, including mood and anxiety disorders.(6–10) Mood and anxiety disorders in the general pediatric population can persist into adulthood and are increasing in prevalence and associated disability.(11, 12) The prevalence of mood disorders in particular increase with age between age 10 and age 18, while the prevalence of anxiety disorders remains stable across age groups.(11) Psychiatric symptoms associated with pediatric TBI have been found to place a child at risk for additional negative health outcomes, including cognitive impairment, chronic pain, and sleep disorders.(13–16) Although mood and anxiety disorders have been described after pediatric TBI, the actual incidence of psychiatric disorders in children with TBI is uncertain, with estimates ranging as wide as 10% - 100%.(7)
Identifying the factors that place a child at increased risk of psychiatric sequalae after a TBI can assist in the development of both screening and treatment interventions. Several risk factors have been identified that contribute to the risk of developing a mood or anxiety disorder up to several years after pediatric TBI. These have included TBI related characteristics such as intraparenchymal brain lesions and older age at injury,(10, 17–19) as well as secondary factors such as a family history of a psychiatric disorder, low socioeconomic status, and prior psychosocial stressors.(18, 20, 21)
Although associations have been observed between pediatric TBI and short-term development of mood and anxiety disorders, there is limited understanding of the risk factors for developing these disorders into young adulthood.(22) Evidence is particularly limited for individuals who sustain TBI at less than 10 years, which accounts for a high proportion of all childhood TBI, especially for those sustaining TBI between ages 0–4.(2, 6) In particular, the Centers for Disease control report that children ages 0–4 have the highest rate of TBI-related ED visits (2194 per 100,000), greater than two times the rate of those in the next highest age group (887 per 100,000) in those aged 15–24.(2)
Research on anxiety disorders after childhood TBI is especially sparse, limited by small sample sizes and short follow up, thus the incidence of anxiety disorders after childhood TBI is not known, particularly in the long term.(21) The extent to which the TBI related characteristics in comparison to secondary non-TBI characteristics – such as demographic, socioeconomic, comorbid conditions, and other psychosocial factors – contributes to the risk of clinically determined psychiatric disorder by adulthood after early childhood TBI remains unclear.(6, 7) Understanding these associations over the long term is important because there is evidence that the neurobehavioral effects of pediatric TBI may not be apparent early after injury and the initial effects of early brain injury may worsen over time as social and cognitive demands increase.(23, 24)
A population-based birth cohort has the potential to provide unique insights into childhood and adolescent mental health outcomes.(25) Longitudinal studies allow for the opportunity to assess whether psychiatric changes after pediatric TBI resolve over time or continue to persist.(10) This study utilized a longitudinal birth cohort to identify the risk factors associated with a long-term clinically determined mood and anxiety disorder in children who sustained a TBI prior to age 10. We further determined the attributable risk of TBI related characteristics – including age at injury, TBI severity, hospitalization for TBI, skull fracture, and extracranial injury – to the risk of developing a mood and anxiety disorder by age 25, after adjusting for known risk factors for development of mood and anxiety disorders in the general population.
Materials and Methods
Study Population
This study was approved by the Olmsted Medical Center and Mayo Clinic Institutional Review Boards. The population for this cohort study was assembled using birth certificate data available for Olmsted County, Minnesota, obtained from the Minnesota Department of Health and using the resources of the Rochester Epidemiology Project (REP). The REP is a medical record linkage system that provides longitudinal data from all outpatient and inpatient medical professionals in the community for all individuals who resided in Olmsted County, Minnesota, from 1966 to the present.(26, 27) Diagnoses assigned at each visit are coded and maintained in continuously updated files that are automatically indexed for retrieval as part of the REP infrastructure.(27)
This study utilized a birth cohort of 8,548 individuals born from January 1, 1976 through December 31, 1982 to mothers who resided in the townships comprising Minnesota Independent School District 535 in Olmsted County, Minnesota at the time of the child’s delivery.(28, 29) The birth cohort was initially developed to study the incidence of learning disabilities (LD) and Attention Deficit Hyperactivity Disorder (ADHD) and was therefore restricted to the subset of 5718 children who were still in the community after 5 years of age.(28, 30) Individuals with severe intellectual disability (n=19) or who declined research authorization in accordance with Minnesota law (n=181) were subsequently excluded, yielding a cohort of 5,518 individuals.
Identification of TBI Cases
The methods for identifying TBI cases and categorizing each case by injury severity have been described in detail elsewhere.(31, 32) The REP records of the 5,518 cohort members were reviewed for any Hospital Adaptation of the International Classification of Diseases, Eighth Revision (H-ICDA) or International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code suggestive of TBI (Supplement 1). Medical records that contained a code suggestive of TBI prior to age 10 were screened for the following: 1) loss of consciousness, nausea, vomiting, or headache association with the injury, 2) hospital admission secondary to the injury, 3) hospitalization for rehabilitation, or 4) Computed Tomography or X-ray performed within 2 weeks of the injury. Individuals that screened positive were then fully abstracted by manual record review for case confirmation, demographics, maternal education level, medical history, and mechanism of injury. TBI was defined as a traumatically induced injury that contributed to physiological disruption of brain function, and confirmed events were those that noted documentation of concussion with loss of consciousness, traumatic amnesia, neurological signs of brain injury such as seizures, evidence of intracranial injury, skull fracture, and/or postconcussive symptoms.
The Mayo Classification System for TBI Severity was then used to categorize TBI into the following categories: Definite (consistent with moderate-severe TBI), Probable (consistent with mild TBI), or Possible (consistent with concussive TBI) (Supplement 2). This classification system is valid in all age groups and has been shown to classify TBI events more accurately than single indicator systems (e.g., length of posttraumatic amnesia, initial Glasgow Coma Score, length of loss of consciousness).(31, 33) The Mayo Classification System stratifies each TBI case by injury severity based on the strength of available information in the medical record and is of particular value particularly in retrospective studies where data can be limited by variably missing indicators of injury severity.(31) The following characteristics at the time in the initial TBI were recorded: age at injury, TBI severity category, hospitalization for TBI, skull fracture at time of TBI, mechanism of injury, and extracranial injury at time of TBI. The date of the case’s TBI diagnosis was defined as the “index date.”
Patient characteristics
Demographic and socioeconomic characteristics obtained from the child’s birth certificate included sex and race of the child, maternal and paternal age, as well as maternal and paternal education. In addition, socioeconomic status at birth was measured by the HOUSES index, which is an individual measure of socioeconomic status derived from four items of real property data (estimated building value of unit, square footage, number of bedrooms, number of bathrooms) from the County Assessor’s office.(34) The HOUSES index has been previously validated as a marker of socioeconomic status.(34–36) The HOUSES Index was determined using data from 1985 real property data (the oldest historical data available) for the address where the child lived at birth, but only for addresses with a street address (e.g., PO Box address could not be matched to HOUSES). Each property item corresponding to individual’s address was standardized into a z-score and were aggregated into an overall z-score for the four items such that a higher HOUSES score indicated higher SES. This standardized z-score of HOUSES index was then converted to quartiles within the county population, not within the study cohort. Therefore, the HOUSES quartiles of this cohort are not equally distributed among Q1 though Q4.
Birth characteristics included gestational age, birth weight, and size for gestational age. Size for gestational age during this era was defined using the 10th and 90th percentiles previously reported for each gestational age of 30,772 deliveries during 1962 to 1969 in Cleveland, Ohio of single live births as reported by Brenner et al.(37) Gestational age was confirmed by manual review of each medical record and was classified into 7 categories: extremely preterm (23–27 weeks), very preterm (28–31 weeks), moderate or late preterm (32–36 weeks), early term (37–38 weeks), full term (39–40) weeks, late term (41 weeks), and Postterm (42–43 weeks).(38)
Family history characteristics included maternal depression prior to initial TBI. Maternal depression was classified by manual record review using the same criteria of identifying mood and anxiety disorders of each child with TBI (described below).
Lastly, time dependent covariates included recurrent TBI prior to age 10, along with diagnosis of ADHD and history of LD. The identification of LD among the birth cohort members has been previously described.(28, 29, 39) Through a contractual agreement with the school district, the results of academic achievement tests and cognitive assessments administered to members of the birth cohort during their years in the local school district were accessed. LD in mathematics, reading, or written language was determined based on whether each individual met criteria using either of two regression-based formulas or one non-regression-based discrepancy formula using cognitive and achievement scores.(29, 40)
Identification of Mood and Anxiety Disorders
The methods for identifying and classifying mood and anxiety disorders were based on methodology from a prior birth cohort study.(41) Depression, anxiety, bipolar disorder, suicidal ideation, and self-injurious behavior HICDA and ICD-9 diagnosis codes that were assigned by a REP-affiliated practitioner up until the individual’s 25th birthday were electronically obtained for all TBI cases. A cut off of 25 years at time of diagnosis was chosen as this age generally corresponds to a more inclusive definition of adolescence, spanning between 10 and 24 years of age.(42) Diagnosis codes were classified into either a depressive, anxiety, or bipolar diagnosis (Supplemental 3). Cases were classified by the study statistician (A.L.W.) as having a depressive, anxiety, or bipolar diagnosis if an individual had an associated diagnosis on 2 or more visit dates greater than 30 days apart. Individuals could be classified as having more than 1 comorbid clinical diagnosis.
Based on criteria determined by Kirsch et al.,(41) a subset of these medical records were then manually reviewed (D.E.) if any of the following criteria were met: 1) depression, anxiety, or bipolar disorder diagnostic code first assigned before 5 years of age; 2) only 2 visit dates greater than 30 days apart from the same diagnostic category; 3) less than 4 visit dates with bipolar – related codes; 4) classification for depression or anxiety met but individual had a single visit date for another diagnostic category (i.e., depression, anxiety, or bipolar); 5) did not meet classification criteria for either diagnostic category but had at least one suicide related code; or 6) depression/anxiety related codes only included 2 adjustment disorder codes. Of these medical records, the reviewer (D.E.) then classified an individual as having a depressive, anxiety, or bipolar related diagnosis if any of the following criteria were met prior to age 25: 1) diagnosis of a depressive, anxiety, or bipolar disorder confirmed by a psychiatrist/psychologist; 2) pharmacotherapy prescribed for associated psychiatric disorder; or 3) evidence of patient participating in counseling services/psychotherapy for the associated disorder.(43)
We assessed the reliability of medical record abstraction of depressive, anxiety, and bipolar diagnoses by having a Pediatric Neuropsychologist (D.M.M.) review the complete medical record for a random sample of 30 patients. This abstractor did not have access to the results of the initial abstraction. The agreement on presence or absence of a depressive, anxiety, or bipolar related disorder was 87% (26/30; 13 both agreed on present and 13 both agreed on absent), with a kappa value of 73% (95% CI: 49 – 98), indicating adequate agreement.
Statistical Analysis
Data management and statistical analyses were performed using SAS version 9.4 software and RStudio version 1.3.1093.1. Because of the small number of individuals diagnosed with bipolar disorder, depression and bipolar disorder diagnoses were combined into a single category of “mood disorder”. Separate parallel analyses were performed for each psychiatric disorder of interest: a) anxiety and b) mood disorder. For each analysis, duration of follow-up was calculated from the index date (i.e. date of first TBI) until either diagnosis of the psychiatric disorder or last clinical visit date prior to their 25th birthday, whichever came first. Individuals diagnosed with the psychiatric disorder prior to their initial TBI were excluded from the analysis. Univariate Cox proportional hazards regression models were fit to evaluate the association of patient characteristics with the risk of the psychiatric disorder and the strength of the association was summarized using the hazard ratio (HR) and corresponding 95% confidence interval derived from each model. For each continuously scaled characteristic (maternal/paternal age at birth, gestational age, birth weight, and age at TBI), a penalized smoothing spline was used to model a potentially nonlinear relationship with the risk of the psychiatric disorder, before proceeding to assuming a linear relationship. Patient characteristics that were not static at the time of the TBI (e.g. diagnosis of ADHD, LD, and repeat TBI prior to age 10) were evaluated as time-dependent covariates.
Five separate multivariate Cox models were fit to evaluate the association of each TBI characteristic (age at injury, injury severity, hospitalization, skull fracture, extracranial injury) with the risk of the psychiatric disorder after adjusting for the following set of potential confounders that were determined a priori from the literature: sex, size for gestational age, maternal education, and history of maternal depression at the time of the individual’s birth.(44–46) All calculated p-values were two-sided and p-values less than 0.05 were considered statistically significant.
A multivariable-adjusted population attributable risk (PAR) estimate was calculated separately for each of the binary TBI characteristics using methods proposed by Chen, Lin, and Zeng(47) for cohort studies with censored event times implemented in the paf package in R using a Cox model, adjusted for the same four forementioned non-TBI covariates.(48) PAR is the proportion of disease in a population that could be prevented by elimination of an exposure or risk factor.(49) For age at initial TBI, age was dichotomized as ≥5 vs. <5 years, based on stratification for the youngest age group most commonly used by the Centers for Disease Control (0–4).(50) Reported values of PAR correspond to 10 years after the TBI.
Results
Demographics and Presentation Characteristics
Out of 5,518 individuals in the cohort, TBI prior to 10 years of age was identified in 566 individuals. Of these 566 TBI cases, 1 had a TBI-related death at 5 years of age and 3 did not have research authorization at the time of the current study and were excluded, resulting in 562 TBI cases. There were 238 females (42.3%) and 324 males (57.7%) in the cohort, and 546 (97.2%) were white Demographic and socioeconomic characteristics of the TBI cases are summarized in the first column of Table 1. Of the 562 cases of TBI identified, 8 had Definite TBI (1.4%), 140 had Probable TBI (24.9%), and 414 had Possible TBI (73.7%). The mean age of TBI for all severities was 4.7 years (SD 2.8). Skull fracture occurred in 49 (8.7%) individuals, 63 (11.2%) individuals were hospitalized for their injury, and extracranial injury of any sort occurred in 144 (25.6%) individuals. The most common cause of injury was falls, occurring in 344/562 individuals (data not shown). There were 80 individuals (14.2 %) whose mechanism of injury was getting hit by an object, though there were no cases found of abuse / non-accidental trauma found.
Table 1.
Non-TBI characteristics evaluated univariately for an association with anxiety or mood disorder among 562 individuals with TBI prior to 10 years of age
| Characteristic |
Overall distribution† |
Outcome=Anxiety | Outcome =Mood disorder | ||||
|---|---|---|---|---|---|---|---|
| No. with the outcome | Unadjusted HR (95% CI) |
No. with the outcome | Unadjusted HR (95% CI) |
||||
| Demographic | |||||||
| Sex | |||||||
| Female | 238 (42.3) | 33 | 2.74 (1.46, 5.12) | 73 | 2.61 (1.75, 3.90) | ||
| Male | 324 (57.7) | 14 | Referent | 36 | Referent | ||
| Child race | |||||||
| White | 546 (97.2) | 46 | 1.64 (0.23, 11.89) | 106 | 1.28 (0.41, 4.04) | ||
| Non-white | 16 (2.8) | 1 | Referent | 3 | Referent | ||
| Socioeconomic at birth | |||||||
| Maternal age (years) | 26.2 (4.7) | 47 | 1.28 (0.94, 1.73) ‡ | 109 | 1.01 (0.83, 1.24) ‡ | ||
| Paternal age (years) | 28.6 (5.3) | 47 | 1.27 (0.98, 1.64) ‡ | 109 | 1.07 (0.89, 1.28) ‡ | ||
| Maternal education | |||||||
| Less than high school | 45 (8.0%) | 7 | 4.66 (1.63, 13.32) | 9 | 1.36 (0.64, 2.87) | ||
| High school graduate | 169 (30.1%) | 7 | Referent | 29 | Referent | ||
| Some college | 180 (32.0%) | 18 | 2.58 (1.08, 6.18) | 38 | 1.32 (0.81, 2.14) | ||
| College graduate | 121 (21.5%) | 10 | 2.14 (0.81, 5.62) | 18 | 0.89 (0.50, 1.61) | ||
| Not documented | 47 (8.4%) | 5 | -- | 15 | -- | ||
| Paternal education | |||||||
| Less than high school | 30 (5.3%) | 1 | 0.47 (0.06, 3.69) | 7 | 1.05 (0.46, 2.39) | ||
| High school graduate | 150 (26.7%) | 9 | Referent | 29 | Referent | ||
| Some college | 123 (21.9%) | 12 | 1.84 (0.77, 4.37) | 24 | 1.09 (0.63, 1.87) | ||
| College graduate | 184 (32.7%) | 17 | 1.74 (0.78, 3.91) | 27 | 0.80 (0.48, 1.36) | ||
| Not documented | 75 (13.3%) | 8 | -- | 22 | -- | ||
| HOUSES Index | |||||||
| 1 (lowest SES) | 90 (16.0%) | 11 | 2.31 (0.80, 6.66) | 22 | 1.54 (0.81, 2.94) | ||
| 2 | 72 (12.8%) | 10 | 2.84 (0.97, 8.32) | 12 | 1.05 (0.50, 2.22) | ||
| 3 | 103 (18.3%) | 7 | 1.45 (0.46, 4.57) | 22 | 1.49 (0.78, 2.84) | ||
| 4 (highest SES) | 101 (18.0%) | 5 | Referent | 16 | Referent | ||
| Unable to determine | 196 (34.9%) | 14 | -- | 37 | -- | ||
| Family history | |||||||
| Maternal depression prior to initial TBI | |||||||
| Yes | 50 (8.9%) | 7 | 2.36 (1.05, 5.26) | 10 | 1.31 (0.68, 2.52) | ||
| No | 512 (91.1%) | 40 | Referent | 99 | Referent | ||
| Birth | |||||||
| Gestational age | |||||||
| Preterm (≤36 weeks) ^ | 28 (5.0%) | 4 | 2.52 (0.83, 7.66) | 8 | 1.39 (0.66, 2.94) | ||
| Early term (37–38 weeks) | 79 (14.1%) | 7 | 2.21 (0.89, 5.49) | 15 | 1.29 (0.72, 2.30) | ||
| Full term (39–40 weeks) | 271 (48.2%) | 14 | Referent | 49 | Referent | ||
| Late term (41 weeks) | 90 (16.0%) | 16 | 3.94 (1.92, 8.07) | 20 | 1.34 (0.80, 2.26) | ||
| Postterm (42–45 weeks) | 79 (14.1%) | 5 | 1.14 (0.41, 3.18) | 16 | 1.06 (0.60, 1.87) | ||
| Not documented | 15 (2.7%) | 1 | -- | 1 | -- | ||
| Birth weight (grams) | 3487 (530) | 47 | 1.15 (0.88,1.51)‡ | 109 | 1.08 (0.90, 1.29)‡ | ||
| Size for gestational age | |||||||
| Small | 16 (2.8%) | 3 | 3.13 (0.96, 10.18) | 4 | 1.78 (0.65, 4.85) | ||
| Average | 414 (73.7%) | 37 | Referent | 83 | Referent | ||
| Large | 117 (20.8%) | 6 | 0.58 (0.25, 1.39) | 21 | 0.96 (0.60, 1.56) | ||
| Unable to determine | 15 (2.7%) | 1 | -- | 1 | -- | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; SD, standard deviation. The HR (95% CI) are bolded if the 95% CI does not contain 1, indicating statistical significance at the 0.05 level.
Results presented as frequency and percentage (of 562 patients) for categorical variables and mean (SD) for continuous variables.
Hazard ratio per a 1-year increase in the child’s age, per a 5-year increase in the age of the parent, and per 500-gram decrease in birth weight, respectively.
Of the 28 individuals who were born preterm, 2 were very preterm (28–31 weeks) and 26 were moderate or later preterm (32–36 weeks).
Anxiety and Mood Disorders
Among the 562 TBI cases, just one individual was diagnosed with anxiety or mood disorders prior to the index date - a male diagnosed with depression at age 7 who experienced a TBI at 9 years of age. A total of 115 TBI cases received either an anxiety (n=47) or mood disorder (n=109) diagnosis between the initial TBI and their 25th birthday; the median age at first diagnosis was 18.1 years (interquartile range (IQR), 16.3–21.7). Among the remaining patients without a subsequent diagnosis of an anxiety or mood disorder, the median age at their last visit to REP-affiliated practitioner prior to their 25th birthday was 23.1 (IQR, 20.4–25.5) years (data not shown).
Univariate Analysis
The panels in Figure 1 depict the relationship between continuously scaled characteristics and the risk of subsequent anxiety. For maternal age, birth weight, and age at initial TBI it is reasonable to assume a linear relationship with the risk of anxiety, whereas for gestational age there is a potential non-linear relationship and therefore this variable was subsequently assessed using gestational age categories. Similar patterns were observed when these characteristics were evaluated for an association with the risk of a subsequent mood disorder (data not shown). Tables 1 and 2 summarize the unadjusted hazard ratios from the of the univariate analyses evaluating non-TBI characteristics of interest (Table 1) and TBI characteristics (Table 2) for an association with either a subsequent anxiety or mood disorder. Female sex, maternal education level, gestational age, maternal depression prior to initial TBI, older age at first childhood TBI, and having a Definite TBI diagnosis (vs. Possible TBI) were all significantly associated with an increased risk of anxiety disorder. Female sex and older age at first TBI were also significantly associated with an increased risk of mood disorder. Recurrent TBI prior to age 10, ADHD diagnosis, and LD diagnosis, each evaluated as time-dependent covariates, were not identified as significantly associated with an increased risk of anxiety or mood disorder (data not shown).
Figure 1.
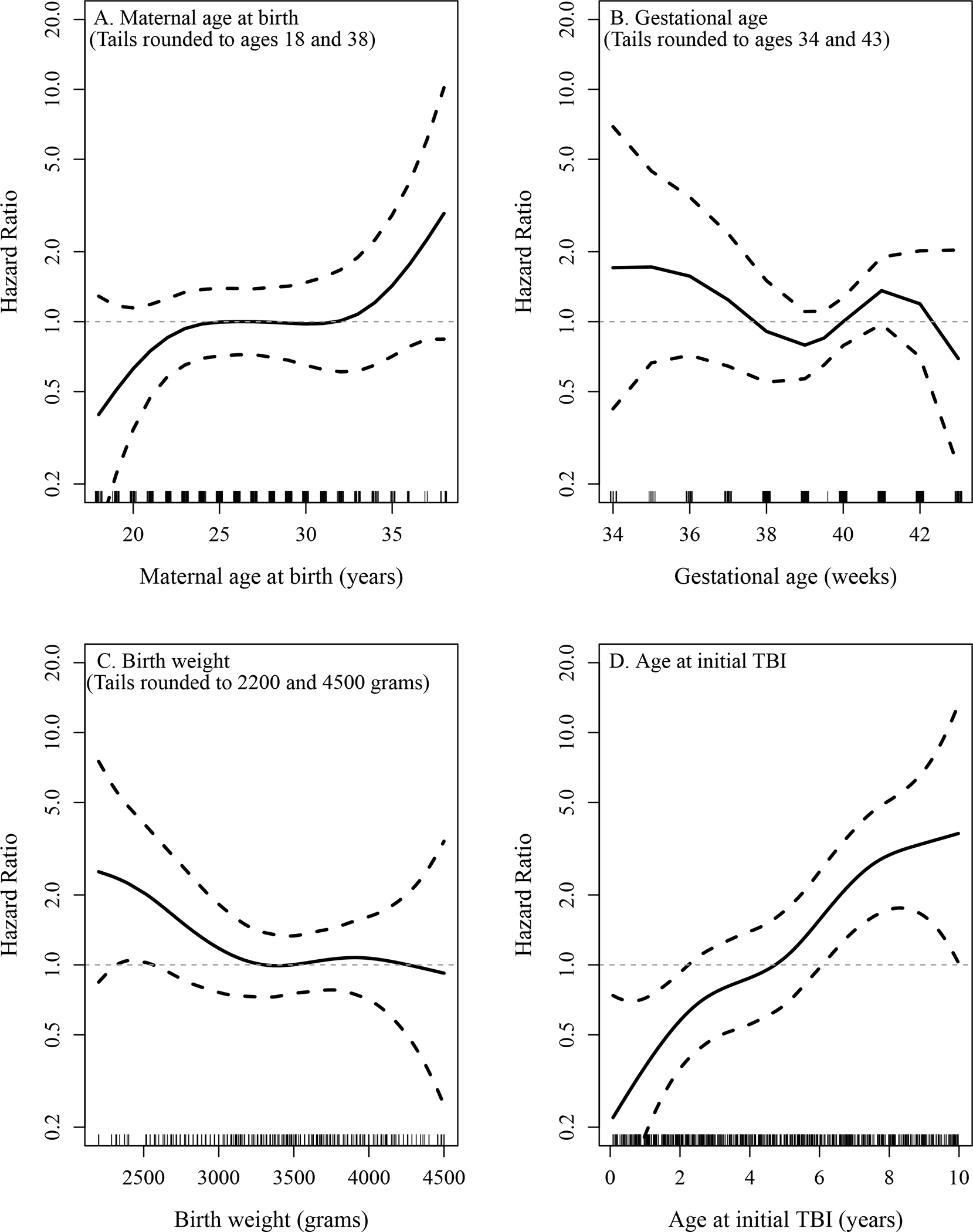
Graphical depiction of the association of maternal age at birth (A), gestational age (B), birth weight (C), and age at initial TBI (D), respectively, with the risk of anxiety as estimated by modelling each covariate using a smoothing spline in a separate Cox model. The dashed lines denote 95% confidence bands for the hazard ratio (HR). For illustrative purposes the values in the tails of the distribution of each covariate have been rounded prior to fitting the Cox models. Each graph was generated such that the reference point with a HR of 1.0 was set at the mean of each covariate.
Table 2.
TBI characteristics evaluated for an association with anxiety or mood disorder among 562 individuals with TBI prior to 10 years of age
| Characteristic at time of initial TBI | Overall distribution† | Outcome=Anxiety | Outcome =Mood disorder | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. with the outcome | Unadjusted HR (95% CI) |
Adjusted HR (95% CI)‡ | No. with the outcome | Unadjusted HR (95% CI) | Adjusted HR (95% CI)‡ | ||||
| Age (years) | 4.7 (2.8) | 47 | 1.32 (1.15, 1.52) § | 1.33 (1.16, 1.52) § | 109 | 1.14 (1.06, 1.24) § | 1.17 (1.08, 1.27) § | ||
| TBI severity | |||||||||
| Definite | 8 (1.4%) | 2 | 4.82 (1.14, 20.30) | 4.89 (1.08, 22.12) | 3 | 2.94 (0.92, 9.33) | 2.95 (0.91, 9.59) | ||
| Probable | 140 (24.9%) | 14 | 1.22 (0.65, 2.29) | 1.24 (0.65, 2.37) | 27 | 0.92 (0.59, 1.42) | 0.95 (0.61, 1.48) | ||
| Possible | 414 (73.7%) | 31 | Referent | Referent | 79 | Referent | Referent | ||
| Hospitalized | |||||||||
| Yes | 63 (11.2%) | 7 | 1.33 (0.60, 2.97) | 1.76 (0.76, 4.12) | 14 | 1.12 (0.64, 1.96) | 1.31 (0.74, 2.32) | ||
| No | 499 (88.8%) | 40 | Referent | Referent | 95 | Referent | Referent | ||
| Skull fracture | |||||||||
| Yes | 49 (8.7%) | 8 | 1.71 (0.80, 3.68) | 1.92 (0.86, 4.24) | 10 | 0.85 (0.44, 1.64) | 0.93 (0.48, 1.80) | ||
| No | 513 (91.3%) | 39 | Referent | Referent | 99 | Referent | Referent | ||
| Extracranial injury | |||||||||
| Yes | 144 (25.6%) | 16 | 1.62 (0.88, 2.96) | 2.41 (1.26, 4.59) | 26 | 0.92 (0.59, 1.42) | 1.05 (0.66, 1.65) | ||
| No | 418 (74.4%) | 31 | Referent | Referent | 83 | Referent | Referent | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; SD, standard deviation. The HR (95% CI) are bolded if the 95% CI does not contain 1, indicating statistical significance at the 0.05 level.
Results presented as frequency and percentage (of 562 patients) for categorical variables and mean (SD) for continuous variables.
Adjusted for variables significant in Table 2:Each of the 5 TBI characteristics was evaluated in a separate multivariable Cox proportional hazards regression model adjusted for the following covariates: sex, size for gestational age, maternal education, and history of maternal depression at the time of the individual’s birth.
Hazard ratio per a 1-year increase in the child’s age at initial TBI
Multivariable Analysis
The 5 TBI characteristics – age at injury, injury severity, hospitalization, extracranial injury, and skull fracture at time of TBI – were each evaluated separately for associated risk of subsequent psychiatric disorders; this was done after adjusting for characteristics that are known to be associated with depression and anxiety disorders in the general pediatric and adolescent population that were found to be significant in our univariate analysis.(44–46) Upon adjusting for non-TBI characteristics, both older age at initial TBI (HR 1.33 [95% CI 1.16–1.52]), having a Definite TBI (vs. Possible TBI: HR 4.89 [95% CI 1.08–22.12]) and extracranial injury (HR 2.41 [95% CI 1.26, 4.59]) at time of initial TBI were significantly associated with an increased risk of anxiety when evaluated separately (Table 2). Only older age at initial TBI (HR 1.17 [95 CI 1.08, 1.27]) was significantly associated with an increased risk of mood disorders after adjusting for potential covariates (Table 2).
Among the 5 TBI characteristics, the multivariable-adjusted population attributable risk (PAR) of subsequent anxiety was highest for older age at initial TBI (54.5% using an age cutoff of 5), followed by 21.1% for extracranial injury, 6.7% for definite vs. possible TBI, 6.8% for skull fracture, and 6.1% for hospitalization. Likewise, the multivariable-adjusted PAR of subsequent mood disorder was highest for older age at initial TBI (35.8% using an age cutoff of 5).
Discussion
In this longitudinal birth cohort study, older age at TBI, Definite TBI, female sex, maternal education either less than high school or some college, late term pregnancy, as well as maternal depression were univariately associated with development of anxiety disorder by age 25. After adjusting for several characteristics known to be associated with anxiety and mood disorders in the general pediatric population, older age at injury remained significantly associated with an anxiety disorder. Additionally, extracranial injury at time of initial TBI was significantly associated with an increased risk of subsequent anxiety, as was Definite TBI. Only older age at the time of the initial TBI was associated with development of a mood disorder. The multivariable-adjusted PAR for both anxiety and mood disorder was highest for older age at TBI, indicating that among TBI related factors, age at injury attributed to the greatest risk of an anxiety or mood disorder.
The age at which pediatric TBI occurs has important implications as there may be a relative vulnerability to TBI at different ages depending on the stage of brain development.(3, 24) In this study, the multivariable-adjusted population attributable risk (PAR) for subsequent anxiety and mood disorder was highest for age at initial TBI between ages 5–10 (54.5%) when compared to ages 0–4. Because the greatest incidence of childhood TBI occurs from ages 0–4,(2) it is reassuring that individuals who sustained a TBI at this age were at a relatively lower risk of developing an anxiety or mood disorder by early adulthood. Our results are consistent with previous studies that have found older age at injury to be an important predictor of psychiatric outcome. In a nationwide Swedish cohort assessing a wide range of psychological long term outcomes, older age at injury was a risk factor for psychiatric inpatient hospitalization for individuals with TBI compared to siblings without TBI, with the least significant association found for individuals injured between ages 0–4.(51) Max et al. showed that age at injury was a significant predictor of both depressive and anxiety disorders 6 months after TBI, where the mean age at injury for children with a new anxiety disorder was 8.4 years and 11.9 years for development of a depressive disorder.(18, 52)
A diffuse biomechanical injury has the potential to alter developmental outcomes as the injury and subsequent recovery occurs while the brain is developing.(53) While there is greater evidence describing mechanisms of cognitive outcomes after different ages of TBI,(54) the mechanisms that may contribute to psychiatric disorders during various stages of development remains to be elucidated, particularly in the long term.(24) There is evidence that TBI occurring between ages 7–9 may be associated with worse cognitive outcomes, suggesting that this time period may represent a critical period of brain development.(55) In addition, there is research showing that the neural mechanisms between cognitive and emotional outcomes are highly intertwined.(56) Studies have shown anatomical correlates including left inferior frontal and right frontal white matter lesions correlating with depression in children,(18) though age related factors associated with neuro-anatomical correlates are unknown. It is also possible that chronic behavioral and psychological conditions may develop relating to cognitive difficulties and subsequent changes in poor academic and job performance, thus resulting in behavioral/psychiatric concerns.(24) Overall, further research is needed to understand the neuroanatomic and neurophysiological correlates of psychiatric outcomes after childhood TBI during various stages of development. Given that the sample in this study was predominantly individuals who sustained mild TBI (Mayo Classification Possible and Probable TBI), it is less likely that solely TBI related pathophysiological changes attributed the findings of a differential outcome between younger and relatively older age groups. Rather, it is more likely that other factors independent of the injury – including a variety of personal, family, and psychosocial variables - contributed to outcomes found in this sample. While we accounted for several birth, family, and socioeconomic factors, there may be other factors that we did not account for that contributed to this association.
While increasing injury severity has been more consistently found to be a risk factor for worse cognitive impairment after pediatric TBI,(5) TBI severity has not consistently been found to increase risk for a long term clinically diagnosed mood or anxiety disorder.(6) A recent scoping review concluded that depression is largely a secondary outcome after pediatric TBI, rather than directly relating to severity of injury.(6) Consistent with prior analyses of population based samples with TBI, injuries in this study were predominated by Possible or Probable TBI,(33, 57) with only a few individuals with Definite TBI (n = 8). While Definite TBI compared to Possible TBI was significantly associated with an anxiety disorder, (adjusted HR 4.89 [95% CI 1.08–22.12]), the importance of this finding should be interpreted with caution due to the small number of Definite TBI cases in the sample. The birth cohort sample included TBI sustained prior to the widespread implementation of computed tomography for TBI. Though 63 (11.2%) of individuals were hospitalized and 49 (8.7%) of individuals in the sample sustained a skull fracture, classification of Definite TBI cases may have been limited by a lack of characterization of intracranial hemorrhage. While several longitudinal studies have identified intracranial lesions in the frontal lobes to be associated with a mood and anxiety disorders up to several years after pediatric TBI,(17, 52) further research is needed to understand neuroimaging correlates to the development of these disorders into adulthood.
Among other TBI related variables we studied, extracranial injury was significantly associated with later development of an anxiety disorder. The impact of extracranial injuries such as orthopedic trauma concomitant with TBI may be associated with immobility, hospitalization, and delayed return to functional independence, which can result in increased psychiatric sequelae.(7) In this study, extracranial injury was not quantified by a validated scoring system but rather abstracted as a dichotomous variable. Thus, conclusions about the impact of non-head injury are limited regarding the differential impact of the severity and specific type of extracranial injury. Previous literature has shown that Mean Abbreviated Injury Scale scores for non-head injuries did not significantly differ between children who developed or did not develop a new psychiatric disorder up to a year after TBI in one study,(58) and that behavioral problems at 12 months after TBI was not associated with extracranial injuries at the time of mTBI.(59)
Taken as a whole, these results suggest that age at time of TBI may be an important characteristic to consider when assessing for a long-term risk of a mood or anxiety disorder after a pediatric TBI occurring before age 10. In assessing for this risk, clinicians should consider screening children who sustain TBI for known risk factors for development of mood and anxiety disorders in the general population, such as female sex, decreased maternal education, low fetal weight percentile, and family history of depression. While our study did show that extracranial injury and Definite TBI were significantly associated with an anxiety disorder, extracranial injury severity and type was not quantified, and the sample of Definite TBI was very small (n=8). Future studies may consider quantifying extracranial injury, further understanding the impact of intracranial lesions and the risk in those with severe TBI, and better understand the differential impact of pediatric TBI after age 10 to understand the impact of injury severity and relative older age at injury on the long-term risk of mood and anxiety disorders. Ultimately, future research may consider a prediction tool that identifies children at highest risk for adverse psychiatric outcomes, with prospective studies then assessing whether early intervention targeting modifiable risk factors and targeted treatment strategies for individuals at highest risk decreases the incidence of psychiatric sequelae after childhood TBI.
There were several strengths to this study. We used a longitudinal birth cohort which allowed for abstraction of characteristics in multiple domains including birth related characteristics, family history of depression defined by medical review, an individual measure of socioeconomic status (HOUSES index),(34) as well as research defined and confirmed ADHD and LD diagnoses. In addition, TBI cases were manually confirmed and classified by injury severity using a validated classification system,(31) and outcomes were defined based on prior studies and with use of manual record review with adequate reliability.(41) The longitudinal design overall allowed for long-term associations to be made, which has otherwise been scantily reported in the literature.(6, 7)
There were several limitations as well. While we accounted for risk factors in several domains, there may be unmeasured confounders/mediating variables that were unaccounted for, such as the impact of early life stress, family environment, or specific adverse childhood events which are known risk factors for mood and anxiety disorders.(60, 61) Though an individual measure of socioeconomic status was used, 35% of study subjects did not have HOUSES index, largely due to incomplete addresses at birth (e.g., PO Box addresses). The HOUSES index can be investigated in future studies where SES at specific periods (e.g., at birth, at time of TBI, at the time of diagnosis of depression or anxiety), cumulative SES, or change of SES affect development of depression or anxiety.(62) Although we did find female sex to be a risk factor for both a mood and anxiety disorder in our univariate analysis and adjusted for sex in our multivariate model, we did not assess for the relative risk of sex specifically in our PAR analysis. Future research should focus on the relative impact of sex on the incidence of new onset mood and anxiety disorders after pediatric TBI. There were very few cases of bipolar disorder identified in this cohort, and depressive disorders were groups with bipolar disorders in a broad category of mood disorders. While this methodology has been used in a previous study,(41) further research is needed to understand risk factors specifically for bipolar disorder after childhood TBI. Finally, while age, sex, and ethnic characteristics of Olmsted County residents are similar to those of the state of Minnesota and the Upper Midwest, the understanding of the risk in other urban as well as ethnically or socioeconomically diverse populations in the United States was limited in this study.(27, 63, 64)
Supplementary Material
Acknowledgments
This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic.
Abbreviations
- ADHD
Attention Deficit Hyperactivity Disorder
- aHR
adjusted hazard ratio
- H-ICDA
Hospital Adaptation of the International Classification of Diseases, Eighth Revision
- ICD-9
International Classification of Diseases, Ninth Revision
- IQR
interquartile range
- LD
learning disabilities
- PAR
Population attributable risk
- REP
Rochester Epidemiology Project
- TBI
traumatic brain injury
Footnotes
Conflicts of Interest and Financial Disclosure
Dr. Esterov received training or funding through the Center for Clinical and Translational Science (funded through the Small Grants Program) and assistance as part of a postdoctoral master’s degree in Clinical and Translational Research (assistance with design, methods).
This publication was supported by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Contributor Information
Dmitry Esterov, Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, Minnesota.
Julie Witkowski, Mayo Clinic School of Graduate Medical Education, Mayo Clinic College of Medicine and Science, Rochester, Minnesota; Department of Physical Medicine and Rehabilitation, Northwestern Medicine, Wheaton, Illinois.
Dana M. McCall, Mayo Clinic School of Graduate Medical Education, Mayo Clinic College of Medicine and Science, Rochester, Minnesota; Gundersen Health System, La Crosse, Wisconsin.
Chung-Il Wi, Department of Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, Minnesota.
Amy L. Weaver, Division of Clinical Trials and Biostatistics, Mayo Clinic, Rochester, Minnesota.
Allen W. Brown, Department of Physical Medicine and Rehabilitation, Mayo Clinic, Rochester, Minnesota.
References
- 1.Dewan MC, Mummareddy N, Wellons JC, 3rd, Bonfield CM. Epidemiology of Global Pediatric Traumatic Brain Injury: Qualitative Review. World Neurosurg. 2016;91:497–509.e1. Epub 2016/03/29. doi: 10.1016/j.wneu.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. (2018). Report to Congress: The Management of Traumatic Brain Injury in Children, National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. Atlanta, GA. [Google Scholar]
- 3.Babikian T, Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology. 2009;23(3):283–96. Epub 2009/05/06. doi: 10.1037/a0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andruszkow H, Deniz E, Urner J, Probst C, Grun O, Lohse R, Frink M, Krettek C, Zeckey C, Hildebrand F. Physical and psychological long-term outcome after traumatic brain injury in children and adult patients. Health Qual Life Outcomes 12, 26 (2014). doi: 10.1186/1477-7525-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumane S, Câmara-Costa H, Francillette L, Araujo M, Toure H, Brugel D, Laurent-Vannier A, Ewing-Cobbs L, Meyer P, Dellatolas G, Watier L, Chevignard M. Functional outcome after severe childhood traumatic brain injury: Results of the TGE prospective longitudinal study. Ann Phys Rehabil Med. 2021;64(1):101375. doi: 10.1016/j.rehab.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Laliberte Durish C, Pereverseff RS, Yeates KO. Depression and Depressive Symptoms in Pediatric Traumatic Brain Injury: A Scoping Review. J Head Trauma Rehabil. 2018;33(3):E18–e30. Epub 2017/09/20. doi: 10.1097/htr.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery CA, Barlow KM, Brooks BL, Max JE, Villavicencio-Requis A, Gnanakumar V, Robertson HL, Schneider K, Yeates KO. A Systematic Review of Psychiatric, Psychological, and Behavioural Outcomes following Mild Traumatic Brain Injury in Children and Adolescents. The Canadian Journal of Psychiatry. 2016;61(5):259–269. doi: 10.1177/0706743716643741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Max JE, Wilde EA, Bigler ED, MacLeod M, Vasquez AC, Schmidt AT, Chapman SB, Hotz G, Yang TT, Levin HS. Psychiatric disorders after pediatric traumatic brain injury: a prospective, longitudinal, controlled study. J Neuropsychiatry Clin Neurosci. 2012;24(4):427–36. Epub 2012/12/12. doi: 10.1176/appi.neuropsych.12060149. [DOI] [PubMed] [Google Scholar]
- 9.Albicini M, McKinlay A. Anxiety Disorders in Adults With Childhood Traumatic Brain Injury: Evidence of Difficulties More Than 10 Years Postinjury. J Head Trauma Rehabil. 2018;33(3):191–9. doi: 10.1097/HTR.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 10.Luis CA, Mittenberg W. Mood and Anxiety Disorders Following Pediatric Traumatic Brain Injury: A Prospective Study. J Clin Exp Neuropsychol. 2002;24(3):270–9. doi: 10.1076/jcen.24.3.270.982. [DOI] [PubMed] [Google Scholar]
- 11.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–9. Epub 2010/09/22. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baranne ML, Falissard B. Global burden of mental disorders among children aged 5–14 years. Child Adolesc Psychiatry Ment Health. 2018;12(1):19. doi: 10.1186/s13034-018-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tham SW, Fales J, Palermo TM. Subjective and objective assessment of sleep in adolescents with mild traumatic brain injury. J Neurotrauma. 2015;32(11):847–52. Epub 2015/02/25. doi: 10.1089/neu.2014.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Max JE. Neuropsychiatry of pediatric traumatic brain injury. Psychiatr Clin North Am. 2014;37(1):125–40. Epub 2014/01/14. doi: 10.1016/j.psc.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor SS, Zatzick DF, Wang J, Temkin N, Koepsell TD, Jaffe KM, Durbin D, Vavilala MS, Dorsch A, Rivara FP. Association between posttraumatic stress, depression, and functional impairments in adolescents 24 months after traumatic brain injury. J Trauma Stress. 2012;25(3):264–71. Epub 2012/06/26. doi: 10.1002/jts.21704. [DOI] [PubMed] [Google Scholar]
- 16.Tham SW, Palermo TM, Wang J, Jaffe KM, Temkin N, Durbin D, Rivara FP. Persistent pain in adolescents following traumatic brain injury. J Pain. 2013;14(10):1242–9. Epub 2013/08/02. doi: 10.1016/j.jpain.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Max JE, Friedman K, Wilde EA, Bigler ED, Hanten G, Schachar RJ, Saunders AE, Dennis M, Ewing-Cobbs L, Chapman SB, et al. Psychiatric disorders in children and adolescents 24 months after mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2015;27(2):112–20. Epub 2015/04/30. doi: 10.1176/appi.neuropsych.13080190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Max JE, Keatley E, Wilde EA, Bigler ED, Schachar RJ, Saunders AE, Ewing-Cobbs L, Chapman SB, Dennis M, Yang TT, et al. Depression in children and adolescents in the first 6 months after traumatic brain injury. Int J Dev Neurosci. 2012;30(3):239–45. Epub 2011/12/17. doi: 10.1016/j.ijdevneu.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasa RA, Gerring JP, Grados M, Slomine B, Christensen JR, Rising W, Denckla MB, Riddle MA. Anxiety After Severe Pediatric Closed Head Injury. J Am Acad Child Adolesc Psychiatry. 2002;41(2):148–56. doi: 10.1097/00004583-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Grados MA, Vasa RA, Riddle MA, Slomine BS, Salorio C, Christensen J, Gerring J. New onset obsessive-compulsive symptoms in children and adolescents with severe traumatic brain injury. Depression and Anxiety. 2008;25(5):398–407. doi: 10.1002/da.20398. [DOI] [PubMed] [Google Scholar]
- 21.Albicini M, McKinlay A. A systematic review of anxiety disorders following mild, moderate and severe TBI in children and adolescents. In: Durbano F, editor. A fresh look at anxiety disorders. IntechOpen; 2015; p. 199–24. [Google Scholar]
- 22.Lumba-Brown A, Yeates KO, Sarmiento K, Breiding MJ, Haegerich TM, Gioia GA, Turner M, Benzel EC, Suskauer SJ, Giza CC, et al. Diagnosis and Management of Mild Traumatic Brain Injury in Children: A Systematic Review. JAMA Pediatr. 2018;172(11):e182847–e. doi: 10.1001/jamapediatrics.2018.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinlay A, Grace R, Horwood J, Fergusson D, MacFarlane M. Adolescent psychiatric symptoms following preschool childhood mild traumatic brain injury: evidence from a birth cohort. J Head Trauma Rehabil. 2009;24(3):221–7. Epub 2009/05/23. doi: 10.1097/HTR.0b013e3181a40590. [DOI] [PubMed] [Google Scholar]
- 24.Babikian T, Merkley T, Savage RC, Giza CC, Levin H. Chronic Aspects of Pediatric Traumatic Brain Injury: Review of the Literature. J Neurotrauma. 2015;32(23):1849–60. Epub 2015/09/29. doi: 10.1089/neu.2015.3971. [DOI] [PubMed] [Google Scholar]
- 25.Katusic SK, Colligan RC, Myers SM, Voigt RG, Yoshimasu K, Stoeckel RE, Weaver AL. What can large population-based birth cohort study ask about past, present and future of children with disorders of development, learning and behaviour? J Epidemiol Community Health. 2017;71(4):410–6. Epub 2017/02/09. doi: 10.1136/jech-2016-208482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 27.Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clinic Proceedings. 2012;87(12):1202–13. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth cohort, 1976–1982, Rochester, Minn. Mayo Clin Proc. 2001;76(11):1081–92. Epub 2001/11/13. doi: 10.4065/76.11.1081. [DOI] [PubMed] [Google Scholar]
- 29.Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. Math learning disorder: incidence in a population-based birth cohort, 1976–82, Rochester, Minn. Ambul Pediatr. 2005;5(5):281–9. Epub 2005/09/20. doi: 10.1367/a04-209r.1. [DOI] [PubMed] [Google Scholar]
- 30.Barbaresi WJ, Katusic SK, Colligan RC, Pankratz VS, Weaver AL, Weber KJ, Mrazek DA, Jacobsen SJ. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatr Adolesc Med. 2002;156(3):217–24. Epub 2002/03/07. doi: 10.1001/archpedi.156.3.217. [DOI] [PubMed] [Google Scholar]
- 31.Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, Perkins PK. The Mayo classification system for traumatic brain injury severity. Journal of Neurotrauma. 2007;24(9):1417–24. doi: 10.1089/neu.2006.0245. [DOI] [PubMed] [Google Scholar]
- 32.Brown AW, Leibson CL, Mandrekar J, Ransom JE, Malec JF. Long-Term Survival After Traumatic Brain Injury: A Population-Based Analysis Controlled for Nonhead Trauma. The Journal of Head Trauma Rehabilitation. 2014;29(1):E1–E8. doi: 10.1097/HTR.0b013e318280d3e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J, Malec JF. Incidence of traumatic brain injury across the full disease spectrum: a population-based medical record review study. Epidemiology. 2011;22(6):836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juhn YJ, Beebe TJ, Finnie DM, Sloan J, Wheeler PH, Yawn B, Williams AR. Development and Initial Testing of a New Socioeconomic Status Measure Based on Housing Data. J Urban Health. 2011;88(5):933–44. doi: 10.1007/s11524-011-9572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi PY, Ryu E, Hathcock MA, Olson JE, Bielinski SJ, Cerhan JR, Rand-Weaver J, Juhn YJ. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health. 2016;70(3):286. doi: 10.1136/jech-2015-205925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butterfield MC, Williams AR, Beebe T, Finnie D, Liu H, Liesinger J, Sloan J, Wheeler PH, Yawn B, Juhn YJ. A two-county comparison of the HOUSES index on predicting self-rated health. J Epidemiol Community Health. 2011;65(3):254. doi: 10.1136/jech.2008.084723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenner WE, Edelman DA, Hendricks CH. A standard of fetal growth for the United States of America. Am J Obstet Gynecol. 1976;126(5):555–64. Epub 1976/11/01. doi: 10.1016/0002-9378(76)90748-1. [DOI] [PubMed] [Google Scholar]
- 38.ACOG Committee Opinion No 579: Definition of term pregnancy. Obstet Gynecol. 2013;122(5):1139–40. Epub 2013/10/24. doi: 10.1097/01.AOG.0000437385.88715.4a. [DOI] [PubMed] [Google Scholar]
- 39.Katusic SK, Colligan RC, Weaver AL, Barbaresi WJ. The forgotten learning disability: epidemiology of written-language disorder in a population-based birth cohort (1976–1982), Rochester, Minnesota. Pediatrics. 2009;123(5):1306–13. Epub 2009/05/01. doi: 10.1542/peds.2008-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katusic SK, Barbaresi WJ, Colligan RC, Weaver AL, Leibson CL, Jacobsen SJ. Case definition in epidemiologic studies of AD/HD. Ann Epidemiol. 2005;15(6):430–7. Epub 2005/06/22. doi: 10.1016/j.annepidem.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Kirsch AC, Huebner ARS, Mehta SQ, Howie FR, Weaver AL, Myers SM, Voigt RG, Katusic SK. Association of Comorbid Mood and Anxiety Disorders With Autism Spectrum Disorder. JAMA Pediatr. 2020;174(1):63–70. doi: 10.1001/jamapediatrics.2019.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223–8. Epub 2018/09/01. doi: 10.1016/s2352-4642(18)30022-1. [DOI] [PubMed] [Google Scholar]
- 43.Handbook of DSM-5 Disorders in Children and Adolescents. Switzerland: Springer International; 2017. [Google Scholar]
- 44.Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, Miller E. Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry. 2017;7(5):e1139–e. doi: 10.1038/tp.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Lieshout RJ, Boyle MH, Favotto L, Krzeczkowski JE, Savoy C, Saigal S, Schmidt LA. Impact of extremely low-birth-weight status on risk and resilience for depression and anxiety in adulthood. J Child Psychol Psychiatry. 2018;59(5):596–603. Epub 2017/10/04. doi: 10.1111/jcpp.12826. [DOI] [PubMed] [Google Scholar]
- 46.Elovainio M, Pulkki-Råback L, Hakulinen C, Ferrie JE, Jokela M, Hintsanen M, Raitakari OT, Keltikangas-Järvinen L. Childhood and adolescence risk factors and development of depressive symptoms: the 32-year prospective Young Finns follow-up study. J Epidemiol Community Health. 2015;69(11):1109. doi: 10.1136/jech-2014-205352. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Lin DY, Zeng D. Attributable fraction functions for censored event times. Biometrika. 2010;97(3):713–26. doi: 10.1093/biomet/asq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L pAfffcsd, R package version 1.0. 2014. http://cran.r-project.org/web/packages/paf/index.html. Accessed 8 July 2021.
- 49.Zapata-Diomedi B, Barendregt JJ, Veerman JL. Population attributable fraction: names, types and issues with incorrect interpretation of relative risks. Br J Sports Med. 2018;52(4):212–3. doi: 10.1136/bjsports-2015-095531. [DOI] [PubMed] [Google Scholar]
- 50.Peterson AB, Xu L, Daugherty J Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014. Centers for Disease Control and Prevention, US Department of Health and Human Services. 2019. [Google Scholar]
- 51.Sariaslan A, Sharp DJ, D’Onofrio BM, Larsson H, Fazel S. Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes. PLOS Med. 2016;13(8):e1002103. doi: 10.1371/journal.pmed.1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Max JE, Keatley E, Wilde EA, Bigler ED, Levin HS, Schachar RJ, Saunders A, Ewing-Cobbs L, Chapman SB, Dennis M, Yang TT. Anxiety disorders in children and adolescents in the first six months after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2011;23(1):29–39. Epub 2011/02/10. doi: 10.1176/appi.neuropsych.23.1.2910.1176/jnp.23.1.jnp29. [DOI] [PubMed] [Google Scholar]
- 53.Giza CC, Kolb B, Harris NG, Asarnow RF, Prins ML. Hitting a moving target: Basic mechanisms of recovery from acquired developmental brain injury. Dev Neurorehabil. 2009;12(5):255–68. Epub 2009/12/04. doi: 10.3109/17518420903087558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor HG, Alden J. Age-related differences in outcomes following childhood brain insults: an introduction and overview. J Int Neuropsychol Soc. 1997;3(6):555–67. Epub 1998/02/04. [PubMed] [Google Scholar]
- 55.Crowe LM, Catroppa C, Babl FE, Rosenfeld JV, Anderson V. Timing of traumatic brain injury in childhood and intellectual outcome. J Pediatr Psychol. 2012;37(7):745–54. Epub 2012/06/07. doi: 10.1093/jpepsy/jss070. [DOI] [PubMed] [Google Scholar]
- 56.Bell MA, Wolfe CD. Emotion and cognition: an intricately bound developmental process. Child Dev. 2004;75(2):366–70. Epub 2004/04/02. doi: 10.1111/j.1467-8624.2004.00679.x. [DOI] [PubMed] [Google Scholar]
- 57.Esterov D, Bellamkonda E, Mandrekar J, Ransom JE, Brown AW. Cause of Death after Traumatic Brain Injury: A Population-Based Health Record Review Analysis Referenced for Nonhead Trauma. Neuroepidemiology. 2021:1–8. Epub 2021/04/12. doi: 10.1159/000514807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Max JE, Pardo D, Hanten G, Schachar RJ, Saunders AE, Ewing-Cobbs L, Chapman SB, Dennis M, Wilde EA, Bigler ED, et al. Psychiatric Disorders in Children and Adolescents Six-to-Twelve Months After Mild Traumatic Brain Injury. J Nuropsychiatry Clin Neurosci. 2013;25(4):272–82. doi: 10.1176/appi.neuropsych.12040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor HG, Orchinik LJ, Minich N, Dietrich A, Nuss K, Wright M, Bangert B, Rusin J, Yeates KO. Symptoms of Persistent Behavior Problems in Children With Mild Traumatic Brain Injury. J Head Trauma Rehabil. 2015;30(5):302–10. Epub 2015/01/30. doi: 10.1097/htr.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilman SE, Kawachi I, Fitzmaurice GM, Buka SL. Socio-economic status, family disruption and residential stability in childhood: relation to onset, recurrence and remission of major depression. Psychol Med. 2003;33(8):1341–55. Epub 2003/10/30. doi: 10.1017/S0033291703008377. [DOI] [PubMed] [Google Scholar]
- 61.Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders. 2004;82(2):217–25. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 62.Chen E, Martin AD, Matthews KA. Trajectories of socioeconomic status across children’s lifetime predict health. Pediatrics. 2007;120(2):e297–303. Epub 2007/07/04. doi: 10.1542/peds.2006-3098. [DOI] [PubMed] [Google Scholar]
- 63.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–60. Epub 2012/02/07. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epi. 2012;41(6):1614–24. Epub 2012/11/20. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


