Abstract
Bovine tuberculosis (BTB) has become an economically important disease in dairy herds found in and around Addis Ababa City and is emerging in regional cities like Gondar, Hawassa and Mekelle because of the establishment of dairy farms in the milk sheds of these cities. A cross-sectional study to estimate the prevalence of BTB and identify associated risk factors was conducted between February 2016 and March 2017. A total of 174 herds comprising of 2,754 dairy cattle in the cities of Gondar, Hawassa and Mekelle were tested using the Single Intradermal Comparative Cervical Tuberculin (SICCT) test. Data on herd structure, animal origin, body condition, housing condition, farm hygiene, management and biosecurity practices were collected using a pre-tested structured questionnaire. Generalized Linear Models (GLM) and Generalized Linear Mixed Models (GLMM) were used to analyze the herd and animal level risk factors, respectively. The herd prevalence was 22.4% (95% CI: 17–29%) while the animal prevalence was 5.2% (95% CI: 4–6%) at the cut-off > 4 mm. The herd prevalence rose to 65.5% (95% CI: 58–72%) and the animal prevalence rose to 9% (95% CI: 8–10%) when the severe interpretation of > 2 mm cut-off was applied. The mean within-herd prevalence in positive farms at the cut-off > 4 mm was 22.7% (95% CI: 15–31%). At the herd level, the analysis showed that herd size, farm hygiene, feeding condition and biosecurity were significantly associated with BTB status, while new cattle introductions showed only borderline significance and that age of farm, housing condition, farmers’ educational status and animal health care practice were not significant. At the animal level, the results showed that age and animal origin were identified as significant predictors for BTB positivity but sex and body condition score were not related to BTB status. Descriptive analysis revealed that herds having ‘BTB history’ showed slightly higher likelihood of being BTB positive compared to farms having no previous BTB exposure. In conclusion, this study showed relatively lower average prevalence in the emerging dairy regions as compared to the prevalence observed in and around Addis Ababa City, warranting for implementation of control program at this stage to reduce or possibly stop further transmission of BTB.
Keywords: Bovine tuberculosis, Cattle, Prevalence, Risk factors, Emerging dairy sector, Ethiopia
1. Introduction
Bovine tuberculosis (BTB), caused mainly by Mycobacterium bovis (M. bovis), is a chronic progressive disease characterized by the development of tubercles in different tissues of the infected host. BTB is a zoonotic disease with notable economic significance (WHO et al., 2017), and remains a source of concern for livestock, wildlife and human health. Economic losses from BTB accrue from its impact on international trade, livestock productivity (e.g., reduced milk yields and meat production, reduced fertility), restrictions to trade, compensation from control programs, and the cost to human health (Cosivi et al., 1998; Olea-Popelka et al., 2016; WHO et al., 2017).
Geographically, BTB is distributed worldwide. According to the Worldwide Animal Health Information Database of OIE (OIE-WAHID Interface, 2018), 91 out of 182 countries reported the presence of BTB infection in cattle during 2015–2017 period.. In Africa, 30 of 59 countries reported the presence of the disease in the same period. The disease is also widespread in Central and South America, parts of Asia and Middle East countries. While BTB has been controlled successfully in most developed countries through the implementation of test-and-slaughter schemes, meat inspection at abattoirs and pasteurisation of milk, BTB remains a problem in some developed countries in the face of extensive control programs (e.g., UK, Ireland, New Zealand) (Allen et al., 2018; Humblet et al., 2009), and in most developing countries where practice of control programs are either at early stage or non-existent (Teppawar et al., 2018).
In Ethiopia, BTB is endemic with prevalence varying among regional states. Studies conducted by various investigators in central Ethiopia recorded prevalence ranging between 22% and 47% in intensive dairy herds (Ameni et al., 2007, 2003; Elias et al., 2008; Firdessa et al., 2012; Tsegaye et al., 2009). These dairy herds keep mainly exotic breeds (Jersey and Holstein-Friesian) or their crosses with the indigenous zebu breed, due to the considerably smaller milk yield from zebus. In contrast, in the peripheral areas of the country including pastoral areas where intensive dairy farming is less developed, the reported prevalence has been lower than 9% (Admasu et al., 2014; Ameni et al., 2010; Gumi et al., 2012; Nega et al., 2012; Regassa et al., 2010; Tschopp et al., 2010b, 2009). However, since the start of the new millennium the dairy sector has been expanding from the central part of the country to the different regional states with establishment of many dairy farms in and around regional cities. Given the concentration of dairy cattle around the capital Addis Ababa, the demand for genetically improved cattle in the regional states is likely to be met by purchasing dairy cattle from this central region with high BTB prevalence. As there are no BTB control policies, animal identification and traceability system in Ethiopia, animals can be traded freely without health certification. These centrifugal trades of dairy cattle from areas of higher prevalence are risky for transmission between regions and expected to create new hotspots of BTB breakouts in the newly emerging dairy areas of regional cities. Despite this risk of disease transmission, it is assumed that hundreds of dairy cattle move every year between the central dairy areas and regional cities for dairy development purpose. The objective of this study was therefore, to estimate the prevalence of BTB in the emerging dairy areas and determine associated risk factors for BTB infection at the animal and herd level.
2. Materials and methods
2.1. Study sites
The study was conducted in three selected regional administrative cities namely Gondar, Hawassa and Mekelle (Fig. 1). These sites were purposively selected in light of the Ethiopian government plan for dairy expansion and are representative of the modern dairy industry managed under the intensive and semi-intensive systems in the southern, northwestern and northern parts of Ethiopia, respectively. Their respective distances from the capital, Addis Ababa, are 273, 738 and 783 km. The majority of the dairy herds involved in the study were owned by private owners and the remainder was government owned.
Fig. 1.
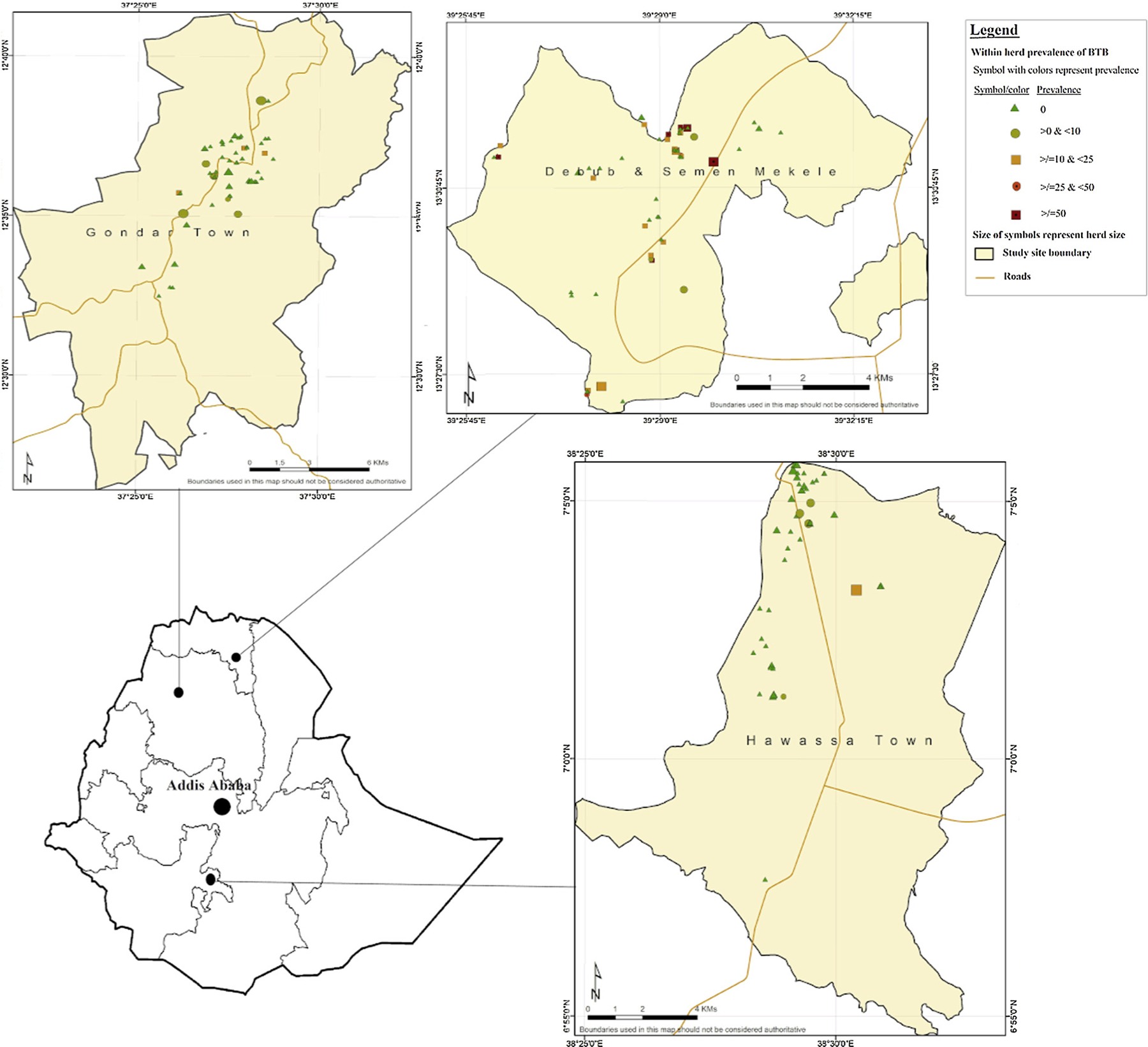
Study sites and selected farms distributions. Shape of symbols and colours represent within-herd prevalence, while size of symbols represent size of the investigated herds.
2.2. Study population
The study population was dairy cattle managed in the selected intensive or semi-intensive herds of the study sites, i.e. cattle in the emerging dairy areas of the country. Dairy cattle in the selected herds were study units and their breed compositions were either of the following: crosses of Holstein Friesian and Zebu, crosses of Jersey and Zebu, or pure Zebu. The husbandry and farm setting differed somewhat from one site to the other depending on the level of awareness, educational status of farmers and access of extension services. All cattle except calves younger than four weeks, clinically sick cattle with disease not suggestive of BTB and cows in the last month of pregnancy, were included for tuberculin testing and sampling. These inclusion criteria were set to avoid possible interference with the action of tuberculin test (De la Rua-Domenech et al., 2006; Goodchild and Clifton-Hadley, 2001).
2.3. Study design and sampling strategy
A cross-sectional study design with a one-stage cluster sampling strategy was used. This choice was informed by historical testing data collected by the National Animal Health Diagnostic and Investigation Centre (NAHDIC) Ethiopia (Alehegne et al., 2015) that suggested there was considerable variability in prevalence both between regions and between herds in the same region. Herds therefore represent both a unit of convenience for sampling effort and an important source of biological variation that must be included in sample size calculations. In this situation, where the variability between clusters is high while the variability within-cluster is negligible, the required number of groups can be calculated by the formula recommended by Thrusfield (2007) and Ahmed (2009): g= Z2 [nVc + q(1-q)]/nd2, where, g = number of clusters to be sampled; Z = 1.96 (critical value for 95% confidence level, Z distribution); n = predicted average number of animals per cluster; d = desired absolute precision (0.05), Vc = between-cluster variance and q = within-herd prevalence. The historical test data from NAHDIC was used as pilot data to estimate the within-herd prevalence and between-herd variance for Gondar, Hawassa and Mekelle as 11% and 0.018, 7% and 0.03, 6.6% and 0.01, respectively.
The number of herds calculated for the respective study sites were 54 for Hawassa, 61 for Mekelle and 59 for Gondar. Herds with number of animals greater than 20 were selected without any prerequisite in all the sites as they were few in number, while herds with fewer than 20 animals were recruited using random selection method among the list of dairy herds obtained from local agricultural agents. Within the selected herds all animals fulfilling the inclusion criteria were subjected to tuberculin testing.
2.4. Skin testing
The Single Intradermal Comparative Cervical Tuberculin (SICCT) test method was used to differentiate between animals infected with M. bovis and those sensitized to tuberculin due to exposure to other mycobacteria or related genera. Two sites at the middle of the neck were shaved and cleaned 12–15 cm apart on the same side of the neck parallel to the shoulder for larger cattle while for calves the opposite sides of the neck were used because of the limited space. The skin of the neck and nearby lymph nodes were checked for any visible lesion or swelling before measuring the skin fold thickness at the two sites with a digital caliper. Animals were then injected with 0.1 ml (2500 IU/ml) avian PPD and 0.1 ml (3000 IU/ml) bovine PPD (Lelystad B.V., The Netherlands) intradermally using insulin syringes at the respective sites. The injections sites were examined and the skin thicknesses measured 72 h post-injection. The difference in the increase of skin thickness measurements at the bovine and avian sites before and after inoculation was considered for interpretation. A reaction was considered positive if the increase in skin thickness at the bovine site of injection was more than 4 mm greater than the reaction shown at the site of the avian injection. The reaction was interpreted as inconclusive if the increase was from 1 to 4 mm (OIE, 2009), or negative if the increase was less than 1 mm. A severe cut-off value of > 2 mm was also applied to re-estimate the prevalence to compare with that of the standard cut-off (4 mm) (Ameni et al., 2008; Downs et al., 2013; Goodchild et al., 2015).
2.5. Data collection
Information on herd level risk factors was collected from cattle owners using a pretested questionnaire. The questionnaire used contained open ended and closed questions and was filled in by the researcher on the farm at the same occasion as tuberculin testing. Administration of the questionnaire was based on translation into the local language and in a way that the respondents would feel comfortable. The objective and possible outcomes of the study were described to each respondent and the respondents were told to discontinue responding whenever they felt not to do so.
Data on animal level risk factors such as sex, age, breed, animal origin, body condition score, pregnancy and lactation were collected during the skin testing. Cattle were categorized as calves (≤1 year of age), juvenile (> 1 and < 3 years), young adults (≥3 and < 5 years), adults (≥5 and < 7 years), mature adults (≥7 and < 10 years), and old adults (≥10 years). Body condition scoring was categorized into three scales: poor, medium and good, a modification from the five scales described by Kellogg (2010) to better reflect the assessment in field conditions. Necessary training was given to the survey team at the beginning of the study to reduce discrepancy on subjective measures.
2.6. Ethical considerations
Ethical approval to implement the research was granted by the Institutional Review Board (IRB) of Aklilu Lemma Institute of Pathobiology, Addis Ababa University (Reference number IRB/ALIPB/2018). This study was supported by the Ethiopian Ministry of Livestock and Fisheries. Skin testing was based on the international standards (OIE, 2009), and all skin testing and data collections were reliant on the willingness of herd owners and/ or managers following elaboration of the study purpose, adverse effect and benefits of the research. As part of the survey, the BTB testing team also treated sick animals in the herds with antibiotics, anthelminthic drugs and wound spray to incentivise participation and the team also advised owners to seek further advice from the local veterinary clinic for close follow up and further medication of their sick animals.
2.7. Data analysis
Herd, animal and within-herd prevalence both at standard and severe interpretations were calculated using proportion (summarized in Table 1). Herd level predictor variables collected by the questionnaire survey were initially selected after data screening for presence of outliers, lack of variability, small number of observations in each categories (merged where possible biologically), effect of missing values, and existence of correlation among predictors. The remaining number of variables was further reduced by creating indices through grouping related predictors based on the perceived importance and weight for their contribution according to Dohoo et al. (2003) and Anderson et al. (2007) (Supplementary Table S2). Weights were assigned based on expert opinion and other evidence wherever possible (operational definitions and determination of categories are provided in Supplementary Table S1). The strength of association between herd level risk factors and BTB status (binary response: positive or negative) were analyzed by Generalized Linear Models (GLM, binomial family with, logit link) using ‘glm2′ package (Marschner and Donoghoe, 2017). Animal level risk factors analysis was conducted using a Generalized Linear Mixed Model approach (GLMM) using maximum likelihood (Adaptive Gauss-Hermite Quadrature) with the logit link of the binomial family using ‘lme4’ package (Bates et al., 2017). In this study herd ID nested in study site was considered as group level random effect to account for clustering. The random effect was tested by comparing the likelihood ratios of the models with and without the random effects.
Table 1.
Herd, animal and within-positive herd animal prevalence of BTB using SICCT test at the standard and severe interpretations.
| Site | Level | n | > 4 mm cut-off |
> 2 mm cut-off |
||
|---|---|---|---|---|---|---|
| n positive | Prevalence (95% CI) | n positive | Prevalence (95% CI) | |||
| Gondar | Herd | 59 | 10 | 17 (9, 28) | 23 | 73 (60, 83) |
| animal | 976 | 14 | 1.4 (0.9, 2) | 42 | 4.3 (3, 6) | |
| Animal within-positive herdδ | 302 (549)* | 14 | 8.2 (4, 12) | 42 | 11 (7, 14) | |
| Hawassa | Herd | 54 | 6 | 11 (5, 22) | 24 | 67 (53, 78) |
| animal | 960 | 29 | 3 (2, 4) | 66 | 7.4 (5, 9) | |
| Animal within-positive herd | 212 (600)* | 29 | 8.7 (2, 15) | 66 | 9.2 (7, 12) | |
| Mekelle | Herd | 61 | 23 | 38 (27, 50) | 32 | 57 (45, 69) |
| animal | 818 | 100 | 12 (10, 15) | 141 | 17.2 (15, 20) | |
| Animal within-positive herd | 393 (581)* | 100 | 32.7 (20, 45) | 141 | 30.5 (20, 41) | |
| Total | Herd | 174 | 39 | 22.4 (17, 29) | 79 | 65.5 (58, 72) |
| animal | 2754 | 142 | 5.2 (4, 6) | 248 | 9 (8, 10) | |
| Animal within-positive herd | 903 (1730)* | 142 | 22.7(15, 31) | 248 | 18.3 (13, 23) | |
Numbers outside brackets shows number of animals within positive herds by standard interpretation while numbers inside brackets are number of animals within positive herds by severe interpretation.
Within-herd prevalence was average of animal level prevalence in the positive herds based on the respective cutoff.
In both the herd and animal level risk factor analyses, variable selection for the multivariable analysis was made based on p value in the univariate regression, i.e. variables with p value less than 0.20 were considered in the multivariable regression (Sperandei, 2014). Intra-cluster coefficients were calculated based on Killip et al. (2004). Absence of interactions between variables was tested using ‘MASS’ package (Ripley et al., 2018). Multi-collinearity among predictor variables was checked using variance inflation factors (VIF) using ‘car’ package (Fox et al., 2017) and confirmed to be less than 2 for all variables. The Hosmer and Lemeshow goodness-of-fit test was calculated using the ‘resourceSelection’ package (Lele et al., 2017). The discrimination ability of the model was checked using the receiver operating characteristic curve (ROC) using ‘pROC’ package (Robin et al., 2017).
Software used for the statistical analysis was R statistical software (version 3.5.1) (R Core Team) with R Studio editor. Package ‘aod’ (Lesnoff and Lancelot, 2012) and ‘questioner’ (Barnier et al., 2017) were used to calculate odds ratio (OR) and confidence intervals in the GLMM and GLM, respectively. Confidence intervals for prevalence were calculated using ‘EpiTools epidemiological calculators’ with Wilson methods (Sergeant, 2019). In all cases, 95% confidence level and significance level of 5% were used to determine statistical significance.
3. Results
3.1. Animal and herd level prevalence
The prevalence of BTB at herd and individual animal levels are stratified by study sites and shown in. The overall herd and animal prevalence were 22.4% and 5.2%, respectively, as determined by SICCT at > 4 mm cut-off. This prevalence increased to 65.5% and 9%, respectively, when > 2 mm cut-off (severe interpretation) was used. Variation in prevalence was observed among the study sites. Compared to Gondar and Hawassa, Mekelle showed higher herd and animal prevalence at both the standard and severe interpretations. Considering only positive farms in both standard and severe interpretations, higher within-herd prevalence was observed in Mekelle, while the findings in Hawassa and Gondar were comparable to each other. Overlap of BTB and Mycobacterium avium Complex (MAC) positivity was observed in 2% of the tested cattle based on the standard interpretations of SICCT for BTB and > 4 mm cut-off for MAC (considering only the reaction to avian PPD); however, 3.2% and 12.7% of them reacted to BTB and MAC alone, respectively Table 1.
3.2. Herd level risk factors
A questionnaire survey with twelve potential herd level risk factors (defined in Supplementary Table S1) were summarized. Outputs of the univariate and multivariable final model GLM analysis for the selected herd level risk factors are summarized in Table 2.
Table 2.
GLM point estimates of the herd univariate and multivariable models for BTB positivity at standard interpretations (n = 170, with missing values from four herds).
| Risk factor | Class | % positive | Univariate |
Multivariable final model |
||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) | P value | Adjusted OR(95% CI) | P value | |||
| Herd size | ≤10 animal/herd | 16.2 (12/74) | – | – | – | – |
| > 10 & ≤ 20 animal/ herd | 15.8 (9/57) | 0.8 (0.3, 2) | 0.645 | 1.3 (0.5,3.7) | 0.627 | |
| > 20 animal/herd | 41 (16/39) | 2.8 (1.2, 6.6) | 0.016 | 10.5 (3.5, 36) | < 0.001 | |
| Farm age | ≥20 years | 20.5 (9/44) | – | – | – | – |
| 10–20 years | 27.5 (14/51)8 | 1.5 (0.6,4) | 0.425 | – | – | |
| < 10 years | 18.7 (14/75) | 0.9 (0.4,2) | 0.804 | – | – | |
| Housing condition | Poor | 19.8 (17/86) | – | – | – | |
| Good | 23.8 (20/84) | 1.1 (0.5,2.2) | 0.779 | – | – | |
| Educational level | Schooling ≥ 15yrs | 29.4 (10/34) | – | – | – | – |
| Schooling 11–15yrs | 16 (11/71) | 0.49 (0.2,1.3) | 0.150 | – | – | |
| Schooling for ≤10 | 25 (16/65) | 0.9 (0.4,2.3) | 0.853 | – | – | |
| Hygiene | Modest | 17 (15/88) | – | – | – | – |
| Meager | 26.8 (22/82) | 2.1 (1,4.3) | 0.048 | 3.3 (1.3,9) | 0.014 | |
| Animal health care | Case based | 18.7 (17/91) | – | – | – | – |
| Regular | 25.3 (20/79) | 1.2 (0.6,2.5) | 0.541 | – | – | |
| Feeding condition | Excellent | 11.8 (8/68) | – | – | – | – |
| Moderate | 30 (8/27) | 3.3 (1,10) | 0.034 | 5.5 (1.6, 21) | 0.010 | |
| Deprived | 28 (21/75) | 3 (1.3,8) | 0.014 | 3.3 (1.3,10) | 0.021 | |
| New cattle introduction | No | 14.3 (12/84) | – | – | – | – |
| Yes | 29 (25/86) | 2.3 (1.1, 5) | 0.023 | 2.3 (1, 6) | 0.054 | |
| Biosecurity | Modest | 12.5 (10/80) | – | – | – | – |
| Poor | 30 (27/90) | 2.4 (1.1, 5.3) | 0.03 | 3.3 (1.3, 9) | 0.015 | |
| Study site* | Hawassa | 11 (6/54) | – | – | – | – |
| Gondar | 17 (10/59) | 1.6 (0.6, 5) | 0.370 | – | – | |
| Mekelle | 38 (23/61) | 4.8 (2, 14) | 0.001 | – | – | |
| Stress conditions¥ | Stressed | 13.6 (14/103) | – | – | – | – |
| Less stressed | 34 (26/76) | 3.3 (1.6, 7) | 0.002 | – | – | |
Study site was not significant in the global model thus not presented in the table.
Stress condition was not considered for multivariable models due to collinearity issues.
In the univariate analysis, seven out of eleven variables (namely herd size, hygiene, feeding conditions, new cattle introduction, biosecurity, study site and stress condition) demonstrated statistical significance (p < 0.05). However, age of farms, housing conditions, educational level and animal health care practices were not significant. The remaining variable from the survey, ‘BTB history’, was not considered in the regression analysis due to lower sample size within their classes. However, descriptive analysis of this variable showed that 41 herds among the recruited herds for the study were previously tested for BTB, of which 11 herds had infection history while 30 herds had not. The odds of BTB positivity in herds with infection history was higher compared to herds with no BTB history (OR 1.6, 95% CI; 0.4–6.4) although the difference was not significant. Cumulative stress conditions of a herd, assessed based on various indicators (Supplementary Table S2) and defined in Supplementary Table S1, was significantly related with herd BTB positivity (p < 0.05) in the univariate regression. Herds in stressful conditions showed higher odds of BTB positivity than herds with less stressed conditions.
Multivariable final model showed that herd size, hygiene, feeding condition and biosecurity were significant predictors (p < 0.05) for herd BTB positivity, while new cattle introduction was only borderline significant (p = 0.054) (Table 2). Fifty one percent of the investigated herds introduced at least one new cattle in the duration of the last two to three years and the prevalence of BTB in these herds was 29% while, on the other hand, the prevalence was only 14.3% in the remaining 49% that did not introduce new cattle.
3.3. Animal level risk factors
The breed compositions of cattle involved in the study were 96% Holstein-Friesian – Zebu crosses (HZ), 2% Jersey – Zebu crosses (JZ) and 2% Zebus. Skin test data by the standard interpretation revealed 5% BTB positivity in the HZ crosses, 2% in the JZ crosses and 0% in Zebu breeds. Due to fewer numbers of JZ and Zebu cattle, we excluded breed composition in the GLMM regression analysis.
Intra-cluster coefficients (ICC) demonstrated a higher inter-cluster variability than within-cluster variability. The ICC calculated using the pooled dataset was 0.78 while site specific ICC values were 0.91, 0.77 and 0.69 for Gondar, Hawassa and Mekelle, respectively.
The results of GLMM univariate and multivariable final model outputs for the other animal level risk factors are presented in Table 3. Age group and animal origin were identified in the multivariable final model as significant predictors for BTB positivity (p < 0.05). A non-linear relationship between BTB positivity and age was observed, with positivity increasing with age until mature adults and then declining for older animals. We also found that about 3% (20/674) BTB reactors among calves (≤1 year old), and 25% of them were younger than six months.
Table 3.
GLMM analysis for animal level risk factors of BTB based on standard interpretations; herd ID nested in study site was considered as random effect (p value for the difference between likelihood ratios was less than 0.001 by chi-square test); n total = 2715 with 39 missing values, n matured female = 1607.
| Risk factor | Class | % positive | Univariate | Multivariable final model | ||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |||
| Sex* | Male | 2.9 (9/315) | – | – | – | – |
| Female | 5.6 (134/2415) | 2.4 (1, 6.4) | 0.05 | – | – | |
| Age groupδ | Calves | 3.0 (20/674) | – | – | – | – |
| Juvenile | 4.4 (27/617) | 2 (0.9, 4.6) | 0.085 | 2 (1,4.8) | 0.096 | |
| Young adults | 6.4 (32/497) | 4.1 (1.9, 9) | < 0.001 | 3.7 (1.7, 8.8) | 0.002 | |
| Adults | 7.7 (30/391) | 4.9 (2.3, 11) | < 0.001 | 3.7 (1.6, 9) | 0.002 | |
| Matured adults | 7.3 (30/409) | 3.8 (1.7, 8.8) | 0.001 | 3 (1.3, 7.2) | 0.010 | |
| Old adults | 3.1 (4/127) | 1.5 (0.3, 6) | 0.56 | 1.3 (0.2, 5) | 0.760 | |
| Animal origin | Same herd | 4.9 (116/2375) | – | – | – | – |
| Other herd | 7.6 (26/340) | 2.6 (1.3, 5) | 0.005 | 2.4 (1.2, 4.8) | 0.017 | |
| BCS | Poor | 4.6 (12/261) | – | – | – | – |
| Moderate | 6.2 (122/1975) | 0.8 (0.4, 1.8) | 0.624 | – | – | |
| Good | 1.9 (9/479) | 0.5 (0.2, 1.4) | 0.199 | – | – | |
| PregnancyŜ | No | 6.9 (71/1033) | – | – | – | – |
| Yes | 4.4 (25/574) | 0.9 (0.5, 1.6) | 0.70 | – | – | |
| LactationŜ | No | 5 (28/556) | – | – | – | – |
| Yes | 6.5 (68/1051) | 1.6 (0.9,3) | 0.147 | – | – | |
analysis was made using data specific to matured females.
sex as a variable did not show significance in the global model, thus not presented in the table.
age category: calves: ≤1 year of age, juvenile: > 1 and < 3 years, young adults: ≥ 3 and < 5 years, adults: ≥ 5 and < 7 years, matured adults: ≥ 7 and < 10 years, old adults: ≥ 10 years.
4. Discussion
In the present study, the prevalence of BTB was estimated among dairy farms in three Ethiopian cities with an emerging dairy sector, namely in Gondar, Hawassa and Mekelle. A total of 174 herds comprising of 2,754 dairy cattle were sampled from these three emerging dairy centers, and tested for BTB using the SICCT test. Additionally, the study explored risk factors at herd and animal levels to inform possible control policies of the disease. Our results demonstrated that herd prevalence was high but with considerable differences between the study sites. At the standard interpretation and in order of magnitude, Mekelle showed the highest prevalence followed by Gondar and then Hawassa. The overall animal prevalence was 5.2% at the standard cutoff > 4 mm of SICCT and was comparable with the pooled prevalence estimate (5.8%) from a recent meta-analysis of national level data from Ethiopia (Sibhat et al., 2017). Our estimated animal level prevalence in Mekelle was higher than published in previous reports (Romha et al., 2013; Zeru et al., 2014; Zeweld, 2014; Zeweld et al., 2013), in Gondar it was similar to estimates by Mengistu et al. (2015) and Nuru et al. (2015), while in Hawassa it was slightly lower than what previous reports had found in that area (Alehegne et al., 2015; Romha et al., 2014). However, estimates from all of our ‘emerging’ study sites are much lower than the average prevalence of 16.6% estimated in intensive and /or semi intensive herds of the central areas of the country (Sibhat et al., 2017) where the dairy sector has been established for much longer. The difference of prevalence between this study and previous reports could be associated with differences in sampling design, environment, breed compositions, husbandry practices, subject measuring the skin test and nature of the tuberculin itself.
Since Ethiopia has not implemented a control strategy for BTB, the disease has established endemicity in most part of the country with higher prevalence in the central and some peripheral areas (Ameni et al., 2007, 2003; Elias et al., 2008; Firdessa et al., 2012; Tsegaye et al., 2009). The Ethiopian Government has initiated various dairy programs to expand the dairy production to the peripheral areas sourcing cattle mainly from the central part of the country to respond to the increasing demand of milk related to the rapidly growing human population in these peripheral ‘emerging’ areas, ranging between 1.7–3.3% (Central Statistical Agency (CSA, 2013). However, the risk of BTB transmission, as can be deduced from the comparison of our findings with previous report, in particular that of Mekelle, has increased because the dairy development plans implemented so far have not considered the need for a control strategy for BTB.
Results presented in this study suggest that introduction of new cattle into apparently BTB free herds is one of the major risk factors for transmission of BTB. As of the time of writing, the country has no legislation or regulation in place for BTB control including traceability, accountability and animal movement. Therefore, animals moved from one region to another without any regulatory checks such as animal health certification, might insidiously promote the BTB dissemination into wider areas. The present data demonstrate that movement of animals onto herds is associated with increased risk of BTB positivity at both herd (OR 2.3, 95% CI: 1–6) and animal (OR 2.4, 95% CI: 1.2–4.8) levels. Continuing to allow the free movement of animals without any precaution could increase the risk of BTB spreading into wider areas. Similar findings have been reported in UK, Italy, Tanzania and Michigan, USA (Dejene et al., 2016; Gopal et al., 2006; Johnston et al., 2011; Kaneene et al., 2002; Marangon et al., 1998; Shirima et al., 2003). Sourcing animals from BTB free herds, reducing cattle trade in general, and prioritizing trade of young animals before adults have previously been suggested as effective steps to reduce the spread of BTB (Reilly and Courtenay, 2007). Our data supports the prompt implementation of regulation on the trade of animals in Ethiopia, should arresting the spread of BTB truly be a priority for the nation.
The present study has identified herd size as one of the herd level risk factors for BTB spread which concurs with previous studies carried out in several parts of the world. It has been shown that BTB positivity is higher in larger herds than smaller ones (Cleaveland et al., 2007; Firdessa et al., 2012; Griffin et al., 1996; Inangolet et al., 2008; Kaneene et al., 2002; Munroe et al., 1999; Munyeme et al., 2008; Olea-Popelka et al., 2004; Porphyre et al., 2008). This may be related to the increased chance of BTB transmission in larger herds, possibly due to high stocking density in combination with poor ventilation (Ameni et al., 2006; Dejene et al., 2016; Huang et al., 2013; Reilly and Courtenay, 2007; van Arendonk and Liinamo, 2003). However, other confounding factors in the management, trading and grazing practices between large and small herds may also contribute to this so-called density dependence in transmission (Begon et al., 2002; Skuce et al., 2012).
Cumulative stress conditions happening as the result of overcrowding due to disproportionately housed herds; recurrence of immunosuppressive diseases; under fed conditions, have all been shown to contribute to BTB positivity of a herd (Humblet et al., 2009). Here we show that herds managed under stressful conditions showed higher odds of BTB positivity (OR 3.3, 95% CI: 1.6–7), implicating the deleterious effect of stress on the animals’ resistance for the disease. Our data on feeding practice, as assessed by type and frequency of supplement feeding, indicated that herds fed with poor to moderate level were at higher risk of being BTB positive compared to well-nourished herds. In line with this, a study conducted in the UK on feeding practice revealed that supplement feeding could diminish the risk of transient BTB outbreaks in UK (Reilly and Courtenay, 2007). Stressful conditions created as the result of poor housing conditions and overcrowding have been reported as a potential cause for the increased BTB positivity (Ameni et al., 2006; Costello et al., 1998; Elias et al., 2008). When the housing/barn is poorly ventilated, aerosols carrying the M. bovis bacilli remain for long time within the congested air so that cattle can inhale concentrated dose of the pathogen, which will be worsened when close contact between animals is high due to overcrowding (Ameni et al., 2006).
Hygiene - as assessed in terms of the method in use for manure disposal, drainage conditions of the floor and frequency of waste cleaning of the house in a day - has been found to be important for the health of dairy cattle. The present data revealed that herds managed in farms with poor hygiene have a higher risk of being BTB positive compared to herds managed in farms with modest hygiene. Poor hygiene condition in a farm may allow M. bovis to remain for a longer period and potentially to proliferate (Humblet et al., 2009).
Biosecurity practices in farm management are important to prevent BTB introduction into a herd. In the present study we assessed biosecurity practices by evaluating relevant indicators such as awareness level, access to wildlife, mixing with neighboring herds, sharing of bulls with neighboring herds, farm enclosure and sharing of livestock extension services. Linear combination of values obtained for these indicators were considered to estimate the biosecurity level. Accordingly, farms practicing poor biosecurity measures showed higher likelihood of herd BTB positivity (OR 3.3, 95% CI: 1.3–9) compared to those which practiced modest levels of biosecurity. Potential contacts of dairy cattle with other species of domestic animals and wildlife have been demonstrated to breach the biosecurity of dairy farms. Of the domestic animals, goats have been demonstrated to be susceptible to M. bovis infection and can maintain infection in the absence of cattle though reported only occasionally (Alvarez et al., 2008; Crawshaw et al., 2008); while sheep are less susceptible and thus reported only rarely (Houlihan et al., 2008a; Malone et al., 2003). Previous studies revealed that wild life plays major role in the transmission of M. bovis (Munyeme et al., 2008; Smith et al., 2009). In the present study certain studied herds had frequent contacts with one or more species of wildlife, namely, hyenas, bush duiker [Midaqua], common warthog, and honey badger. Studies in other countries showed that M. bovis has been isolated from spotted hyenas (Vathsala et al., 2007), warthog (Miller et al., 2016) and badger (Fitzgerald and Kaneene, 2013). A study from Ethiopia revealed that no M. bovis was detected in wildlife species from national parks and sanctuaries (Tschopp et al., 2010a); however, wildlife species that have close contact with dairy cattle managed in the intensive management system may play role in BTB transmission requiring further study.
Previous studies suggest that older cattle are more likely to be positive for BTB than young ones (Cleaveland et al., 2007; Griffin et al., 1996; Inangolet et al., 2008; Munyeme et al., 2008). Our study also concurs with these observed patterns, with the proportion of BTB reactors increasing to a peak at five to seven years of age. The increase with age could be linked to longer exposure time to the M. bovis. The apparent decay at later stage in life might be due to development of an anergic state or excess mortality of infected animals. It has been reported that chronically infected animals with severe pathology may be unresponsive to the tuberculin test (Houlihan et al., 2008b; OIE, 2009), thus might be more likely to give a false negative result. However, at what level of the infection or period of time after infection such potential anergic state might develop has not been well characterised.
It is not uncommon to observe overlap of BTB and MAC positivity in cattle due to either antigenic cross reactivity and/ or co-infection (Mamo et al., 2013; Tschopp et al., 2010b). There exists no clear evidence on the immunological relationship between these two but studies have shown that co-infection with MAC compromises BTB skin test results by negatively influencing the sensitivity of the tuberculin test (Alvarez et al., 2008; Aranaz et al., 2006; Walravens et al., 2002). Further evidence (Amadori et al., 2002) suggests that cattle sensitized by MAC might conceal M. bovis for a period of time. However, it is not clear to what extent this disease could jeopardize the detection of BTB with SICCT test, thus requiring future research.
5. Conclusions
This study showed relatively low average prevalence in the emerging dairy regions as compared to the prevalence observed in the established dairy belt in the central parts of Ethiopia, especially in and around Addis Ababa City. Herd size, feeding, biosecurity, introduction of new cattle from other herd, age and animal origin are important risk factors identified here. Implementation of control program in these cities at this stage could be effective to reduce or possibly stop further BTB transmission between cattle and to reduce the likely zoonotic impact.
Supplementary Material
Acknowledgements
This work was funded by the Biotechnology and Biological Sciences Research Council, the Department for International Development, the Economic & Social Research Council, the Medical Research Council, the Natural Environment Research Council and the Defence Science & Technology Laboratory, under the Zoonoses and Emerging Livestock Systems (ZELS) programme, ref: BB/L018977/1. The authors are indebted to the management of the National Animal Health Diagnostic and Investigation Center (NAHDIC) and its technical staff who conducted the field testing and data collection, Regional Livestock Agencies, Organizations, Dairy co-operations and Dairy farmers who collaborate or allow this work to be done. The authors would also like to extend their appreciation to the editors and reviewers for their contribution to shape the manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.prevetmed.2019.04.010.
References
- Admasu P, Berihun W, Niguse A, 2014. Prevalence of bovine tuberculosis in dairy cattle of Yeki District, Southern Ethiopia. Afr. J. Basic App. Sci 6, 135–140. 10.5829/idosi.ajbas.2014.6.5.86219. [DOI] [Google Scholar]
- Ahmed S, 2009. Methods in Sample Surveys Johns Hopkins Bloomberg School of Public Health., United States. [Google Scholar]
- Alehegne W, Gizat A, Tafesse K, Mekedes T, Mesfin S, Getnet AT, Gebiru L, Girma A, Meseret A, Ketema B, Asinake F, Tsegaye A, Fekadu B, Wubishet Z, 2015. Bovine Tuberculosis Study in Farmed Dairy Cattle and Cattle Genetic Improvement Centers in Selected Areas of Ethiopia. Poster Abstracts - Epidemiology - British Cattle Veterinary Association (Accessed 20 January 2018). https://www.bcva.eu/system/files/resources/Epidemiology.
- Allen AR, Skuce RA, Byrne AW, 2018. Bovine tuberculosis in Britain and Ireland - a perfect storm? The confluence of potential ecological and epidemiological impediments to controlling a chronic infectious disease. Front. Vet. Sci 5, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, de Juan L, Bezos J, Romero B, Sáez JL, Reviriego Gordejo FJ, Briones V, Moreno MA, Mateos A, Domínguez L, Aranaz A, 2008. Interference of paratuberculosis with the diagnosis of tuberculosis in a goat flock with a natural mixed infection. Vet. Microbiol 128, 72–80. [DOI] [PubMed] [Google Scholar]
- Amadori M, Tagliabue S, Lauzi S, Finazzi G, lombard i G, Teló P, Pacciarini L, Bonizzi L, 2002. Diagnosis of Mycobacterium bovis infection in calves sensitized by mycobacteria of the avium/intracellulare group. J. Vet. Med. Ser. B 49, 89–96. [DOI] [PubMed] [Google Scholar]
- Ameni G, Bonnet P, Tibbo M, 2003. A cross-sectional study of bovine tuberculosis in selected dairy farms in Ethiopia. Int. J. App. Res. Vet. Med 1 (4), 253–258. [Google Scholar]
- Ameni G, Aseffa A, Engers H, Young D, Hewinson G, Vordermeier M, 2006. Cattle husbandry in Ethiopia is a predominant factor affecting the pathology of bovine tuberculosis and gamma interferon responses to mycobacterial antigens. Clin. Vaccine Immunol 13, 1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameni G, Aseffa A, Engers H, Young D, Gordon S, Hewinson G, Vordermeier M, 2007. High prevalence and increased severity of pathology of bovine tuberculosis in holsteins compared to zebu breeds under field cattle husbandry in Central Ethiopia. Clin. Vaccine Immunol 14 (10), 1356–1361. 10.1128/CVI.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameni G, Hewinson G, Aseffa A, Young D, Vordermeier M, 2008. Appraisal of interpretation criteria for the comparative intradermal tuberculin test for diagnosis of tuberculosis in cattle in central Ethiopia. Clin. Vaccine Immunol 15, 1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameni G, Desta F, Firdessa R, 2010. Molecular typing of Mycobacterium bovis isolated from tuberculosis lesions of cattle in north eastern Ethiopia. Vet. Rec 167, 138–141. 10.1136/vr.b4881. [DOI] [PubMed] [Google Scholar]
- Anderson D, Feldblum S, Modlin C, Schirmacher D, Schirmacher E, Thandi N, 2007. A Practitioner’s Guide to Generalized Linear Models, 3rd edition. (Accessed 20 March 2019. https://www.casact.org/pubs/dpp/dpp04/04dpp1.pdf. [Google Scholar]
- Aranaz A, De Juan L, Bezos J, Alvare ZJ, Romero B, Lozano F, Paramio JL, López-sánchez J, Mateos A, Do Mínguez L, 2006. Assessment of diagnostic tools for eradication of bovine tuberculosis in cattle co-infected with Mycobacterium bovis and M avium subsp paratuberculosis. Vet. Res 37, 593–606. [DOI] [PubMed] [Google Scholar]
- Barnier J, Briatte F, Larmarange J, 2017. Functions to Make Surveys Processing Easier. Package ‘questionr’, Version 0.6.2
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHH, Singmann H, Dai B, Scheipl F, Grothendieck G, Green P, 2017. Linear Mixed-effects Models Using’ Eigen’ and S4. Package ‘lme4’, Version 1.1–13
- Begon M, Bennett M, Bpwers RG, French NP, Hazel SM, Turner J, 2002. A clarification of transmission terms in host-microparasite models: numbers, densities and areas. Epidemiol. Infect 129, 147–153. 10.1017/S0950268802007148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Statistical Agency (CSA), 2013. Population Projection of Ethiopia for All Regions at District Level From pp. 2014–2017. (Accessed May 30, 2015). http://www.csa.gov.et/images/general/news/pop_pro_wer_2014-2017_final.
- Cleaveland S, Shaw DJ, Mfinanga SG, Shirima G, Kazwala RR, Eblate E, Sharp M, 2007. Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations. Tuberculosis 87, 30–43. [DOI] [PubMed] [Google Scholar]
- Cosivi O, Grange J, Daborn C, Raviglione M, Fujikura T, Cousins D, Robinson RA, Huchzermeyer HFAK, de Kantor I, Meslin FX, 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis 4, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E, Doherty ML, Monaghan ML, Quigley FC, O’Reilly PF, 1998. A study of cattle-to-cattle transmission of Mycobacterium bovis infection. Vet. J 155, 245–250. [DOI] [PubMed] [Google Scholar]
- Crawshaw T, Daniel R, Clifton-Hadley R, Clark J, Evans H, Rolfe S, de la Rua-Domenech R, 2008. TB in goats caused by Mycobacterium bovis. Vet. Rec 163, 127. [DOI] [PubMed] [Google Scholar]
- De la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS, 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res. Vet. Sci 81, 190–210. [DOI] [PubMed] [Google Scholar]
- Dejene SW, Heitkönig IMA, Prins HHT, Lemma FA, Mekonnen DA, Alemu ZE, Kelkay TZ, de Boer WF, 2016. Risk factors for bovine tuberculosis in cattle in Ethiopia. PLoS One 11 (7), e0159083. 10.1371/journal.pone.0159083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohoo IR, Martin W, Stryhn HE, 2003. Veterinary Epidemiologic Research. University of Prince Edward Island, Charlottetown, P.E.I, pp. 438–454.
- Downs SH, Clifton-Hadley RS, Upton PA, Milne IC, Ely ER, Gopal R, Goodchild AV, Sayers AR, 2013. Tuberculin manufacturing source and breakdown incidence rate of bovine tuberculosis in British cattle, 2005–2009. Vet. Rec 172, 98–98. [DOI] [PubMed] [Google Scholar]
- Elias K, Hussein D, Asseged B, Wondwossen T, Gebeyehu M, 2008. Status of bovine tuberculosis in Addis Ababa dairy farms. Rev. Sci. Technol 27, 915–923. [DOI] [PubMed] [Google Scholar]
- Firdessa R, Tschopp R, Wubete A, Sombo M, Hailu E, Erenso G, Kiros T, Yamuah L, Vordermeier M, Hewinson RG, Young D, Gordon SV, Sahile M, Aseffa A, Berg S, 2012. High prevalence of bovine tuberculosis in dairy cattle in Central Ethiopia: implications for the dairy industry and public health. PLoS One 7, 12. 10.1371/journal.pone.0052851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald SD, Kaneene JB, 2013. Wildlife reservoirs of bovine tuberculosis worldwide: hosts, pathology, surveillance, and control. Vet. Pathol 50 (3), 488–499. 10.1177/0300985812467472. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, Firth D, Friendly M, Gorjanc G, Graves S, Heiberger R, Laboissiere R, Monette G, Murdoch D, Nilsson H, Ogle D, Ripley B, Venables W, Winsemius D, Zeileis A, 2017. Companion to Applied Regression. Package ‘car’, Version 2 pp. 1–5.
- Goodchild AV, Clifton-Hadley RS, 2001. Cattle-to-cattle transmission of Mycobacterium bovis. Tuberculosis (Edinb) 81 (1–2), 23–41. [DOI] [PubMed] [Google Scholar]
- Goodchild AV, Downs SH, Upton P, Wood JLN, De La Rua-Domenech R, 2015. Specificity of the comparative skin test for bovine tuberculosis in Great Britain. Vet. Rec 177, 258. 10.1136/vr.10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal R, Goodchild A, Hewinson G, De la Rua-Domenech R, Clifton-Hadley R, 2006. Introduction of bovine tuberculosis to north-east England by bought in cattle. Vet. Rec 159, 265–271. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Martin SW, Thorburn MA, Eves JA, Hammond RF, 1996. A case-control study on the association of selected risk factors with the occurrence of bovine tuberculosis in the Republic of Ireland. Prev. Vet. Med 27, 75–87. [Google Scholar]
- Gumi B, Schelling E, Firdessa R, Erenso G, Biffa B, Aseffa A, Tschopp T, Yamuah L, Young D, Zinsstag J, 2012. Low prevalence of bovine tuberculosis in Somali pastoral livestock, southeast Ethiopia. Trop. Anim. Health Prod 44, 1445–1450. 10.1007/s11250-012-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan MG, Williams SJ, Poff JD, 2008a. Mycobacterium bovis isolated from a sheep during routine surveillance. Vet. Rec 163, 94–95. [DOI] [PubMed] [Google Scholar]
- Houlihan MG, Dixon FW, Page NA, 2008b. Outbreak of bovine tuberculosis featuring anergy to the skin test, udder lesions and milk-borne disease in young calves. Vet. Rec 163, 357–361. 10.1136/vr.163.12.357. [DOI] [PubMed] [Google Scholar]
- Huang ZYX, de Boer WF, van Langevelde F, Xu C, Jebara KB, Berlingieri F, Prins HHT, 2013. Dilution effect in bovine tuberculosis: risk factors for regional disease occurrence in Africa. Proc. R. Soc. B 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humblet MF, Boschiroli ML, Saegerman C, 2009. Classification of worldwide bovine tuberculosis risk factors in cattle: a stratified approach. Vet. Res 40, 50. 10.1051/vetres/2009033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inangolet FO, Demelash B, Oloya J, Opuda-Asibo J, Skjerve E, 2008. A cross-sectional study of bovine tuberculosis in the transhumant and agro-pastoral cattle herds in the border areas of Katakwi and Moroto districts, Uganda. Trop. Anim. Health Prod 40, 501–508. [DOI] [PubMed] [Google Scholar]
- Johnston WT, Vial F, Gettinby G, Bourne FJ, Clifton-Hadley RS, Cox DR, Crea P, Donnelly CA, McInerney JP, Mitchell AP, Morrison WI, Woodroffe R, 2011. Herd-level risk factors of bovine tuberculosis in England and Wales after the 2001 foot-and-mouth disease epidemic. Int. J. Infect. Dis 15 (12), e833–40 2011. [DOI] [PubMed] [Google Scholar]
- Kaneene JB, Bruning-Fann CS, Granger LM, Miller R, Porter-Spalding A, 2002. Environmental and farm management factors associated with tuberculosis on cattle farms in northeastern Michigan. JAVMA 221, 837–842. [DOI] [PubMed] [Google Scholar]
- Kellogg DW, 2010. Body Condition Scoring With Dairy Cattle, Agriculture and Natural Resources Vol. 4008. FSA (University of Arkansas (System), Cooperative Extension Service, pp. P6. [Google Scholar]
- Killip S, Mahfoud Z, Pearce K, 2004. What is an intra-cluster correlation coefficient? Crucial concepts for primary care researchers. Ann. Fam. Med 2, 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele SR, Keim JL, Solymos P, 2017. Resource Selection (Probability) Functions for Use-availability Data. Package ‘ResourceSelection’, Version 0.3–2
- Lesnoff M, Lancelot R, 2012. Analysis of Overdispersed Data, Package ‘aod’ package, version1.3
- Malone FE, Wilson EC, Pollock JM, Skuce A, 2003. Investigations into an outbreak of tuberculosis in a flock of sheep in contact with tuberculous cattle. J. Vet. Med. B Infect. Dis. Vet. Public Health 50, 500–504. [DOI] [PubMed] [Google Scholar]
- Mamo G, Abebe F, Worku Y, Hussein N, Legessw M, Tilahun G, Medhin G, Bjune G, Ameni G, 2013. Bovine tuberculosis and its associated risk factors in pastoral and agro-pastoral cattle herds of Afar Region, Northeast Ethiopia. J. Vet. Med. Anim. Health 5 (6), 171–179. [Google Scholar]
- Marangon S, Martini M, Dalla Pozza M, Ferreira Neto J, 1998. A case-control study on bovine tuberculosis in the Veneto Region (Italy). Prev. Vet. Med 34, 87–95. [DOI] [PubMed] [Google Scholar]
- Marschner I, Donoghoe MW, 2017. Fitting Generalized Linear Models. Package ‘glm2’, Version 1.1.3
- Mengistu A, Enqusselasie F, Aseffa A, Beyene D, 2015. Bovine tuberculosis in rural Ethiopia: a comparative cross-sectional study on cattle owned by households with and without tuberculosis. Int. J. Lepr. Mycobact. Dis 5, 4. 10.4172/2161-1068.1000191. [DOI] [Google Scholar]
- Miller M, Buss P, de Klerk-Lorist LM, Hofmeyr J, Hausler G, Lyashchenko K, Lane EP, Botha L, Parsons S, van Helden P, 2016. Application of rapid serologic tests for detection of Mycobacterium bovis infection in free-ranging warthogs (Phacochoerus africanus)-Implications for antemortem disease screening. J. Wildl. Dis 52 (1), 180–182. 10.7589/2015-07-186. [DOI] [PubMed] [Google Scholar]
- Munroe FA, Dohoo IR, McNab WB, Spangler L, 1999. Risk factors for the between-herd spread of Mycobacterium bovis in Canadian cattle and cervids between 1985 and 1994. Prev. Vet. Med 41, 119–133. [DOI] [PubMed] [Google Scholar]
- Munyeme M, Muma JB, Skjerve E, Nambota AM, Phiri IG, Samui KL, Dorny P, Tryland M, 2008. Risk factors associated with bovine tuberculosis in traditional cattle of the livestock/wildlife interface areas in the Kafue basin of Zambia. Prev. Vet. Med 85, 317–328. 10.1016/j.prevetmed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Nega M, Mazengia H, Mekonen G, 2012. Prevalence and zoonotic implications of bovine tuberculosis in Northwest Ethiopia. Int. J. Med. Med. Sci 2, 182–192. [Google Scholar]
- Nuru A, Mamo G, Teshome L, Zewdie A, Medhin M, Pieper R, Ameni G, 2015. Bovine tuberculosis and its risk factors among dairy cattle herds in and around Bahir Dar City, Northwest Ethiopia. Eth. Vet. J 19 (2), 27–40. 10.4314/evj.vJ9i2.3. [DOI] [Google Scholar]
- OIE, 2009. Terrestrial Manual: Bovine Tuberculosis World Organization for Animal Health press, Paris. [Google Scholar]
- OIE-WAHID Interface, 2018. http://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/statusdetail (Accessed 2 August 2018).
- Olea-Popelka FJ, White PW, Collins JD, O’Keeffe J, Kelton DF, Martin SW, 2004. Breakdown severity during a bovine tuberculosis episode as a predictor of future herd outbreaks in Ireland. Prev. Vet. Med 63, 163–172. [DOI] [PubMed] [Google Scholar]
- Olea-Popelka F, Muwonge A, Perera A, Dean AS, Mumford E, Erlacher-Vindel E, Forcella S, Silk BJ, Ditiu L, El Idrissi A, Raviglione M, Cosivi O, LoBue P, Fujiwara PI, 2016. Zoonotic tuberculosis in human beings caused by Mycobacterium bovis - a call for action. Lancet Infect. Dis 16, 30139–30146. [DOI] [PubMed] [Google Scholar]
- Porphyre T, Stevenson MA, McKenzie J, 2008. Risk factors for bovine tuberculosis in New Zealand cattle farms and their relationship with possum control strategies. Prev. Vet. Med 86, 93–106. [DOI] [PubMed] [Google Scholar]
- Regassa A, Tassew A, Amenu K, Megersa B, Abunna F, Mekibib B, Marcotty T, Ameni G, 2010. A cross-sectional study on bovine tuberculosis in Hawassa town and its surroundings, Southern Ethiopia. Trop. Anim. Health Prod 42, 915–920. 10.1007/s11250-009-9507-4. [DOI] [PubMed] [Google Scholar]
- Reilly LA, Courtenay O, 2007. Husbandry practices, badger sett density and habitat composition as risk factors for transient and persistent bovine tuberculosis on UK cattle farms. Prev. Vet. Med 80, 129–142. [DOI] [PubMed] [Google Scholar]
- Ripley B, Venables B, Bates DM, Hornik K, Gebhardt A, Firth D, 2018. Support Functions and Datasets for Venables and Ripley’s MASS. Package ‘MASS’, Version 7 pp. 3–51 1.
- Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M, Siegert S, 2017. Display and Analyze ROC Curves. Package ‘pROC’, Version 1.10.0
- Romha G, Ameni G, Berhe G, Mamo G, 2013. Epidemiology of mycobacterial infections in cattle in two districts of Western Tigray Zone, northern Ethiopia. Afr. J. Microbiol. Res 7 (31), 4031–4038. 10.5897/AJMR12.1951. [DOI] [Google Scholar]
- Romha G, Gebre egziabher G, Ameni G, 2014. Assessment of bovine tuberculosis and its risk factors in cattle and humans, at and around Dilla town, Southern Ethiopia. Anim. Vet. Sci 2 (4), 94–100. 10.11648/j.avs.20140204.12. [DOI] [Google Scholar]
- Sergeant, E.S.G., 2019. Epitools Epidemiological Calculators Ausvet Pty Ltd; (Accessed 20 January 2019). http://epitools.ausvet.com.au. [Google Scholar]
- Shirima GM, Kazwala RR, Kambarage DM, 2003. Prevalence of bovine tuberculosis in cattle in different farming systems in the eastern zone of Tanzania. Prev. Vet. Med 57, 167–172. [DOI] [PubMed] [Google Scholar]
- Sibhat B, Asmare K, Demissie K, Ayelet G, Mamo G, Ameni G, 2017. Bovine tuberculosis in Ethiopia: a systematic review and meta-analysis. Prev. Vet. Med 147, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuce RA, Allen AR, McDowell SWJ, 2012. Herd-level risk factors for bovine tuberculosis: a literature review. Vet. Med. Int 2012, 621210. 10.1155/2012/621210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, White PCL, Marion G, Hutchings MR, 2009. Livestock grazing behavior and inter- vs. Intra-specific disease risk via the faecal oral route. Behav. Ecol 20, 426–432. [Google Scholar]
- Sperandei S, 2014. Understanding logistic regression analysis. Biochem. Med 24 (1), 12–18. 10.11613/BM.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppawar RN, Chaudhari SP, Moon SL, Shinde SV, Khan WA, Patil AR, 2018. Zoonotic tuberculosis: a concern and strategies to combat. In: Enany S (Ed.), Basic Biology and Applications of Actinobacteria. IntechOpen 10.5772/intechopen.76802. [DOI] [Google Scholar]
- Thrusfield M, 2007. Veterinary Epidemiology, third ed Blackwell science Ltd, Cambridge. [Google Scholar]
- Tschopp R, Schelling E, Hattendorf J, Aseffa A, Zinsstag J, 2009. Risk factors of bovine tuberculosis in cattle in rural livestock production systems of Ethiopia. Prev. Vet. Med 10.1016/j.prevetmed.2009.02.006. [DOI] [PMC free article] [PubMed]
- Tschopp R, Berg S, Argaw K, Gadisa E, Habtamu M, Schelling E, Young D, Aseffa A, Zinsstag J, 2010a. Bovine tuberculosis in Ethiopian wildlife. J. Wildl. Dis 46 (3), 753–762. 10.7589/0090-3558-46.3.753. [DOI] [PubMed] [Google Scholar]
- Tschopp R, Schelling E, Hattendorf J, Young D, Aseffa A, Zinsstag J, 2010b. Repeated cross-sectional skin testing for bovine tuberculosis in cattle kept in a traditional husbandry system in Ethiopia. Vet. Rec 167, 250–256. 10.1136/vr.c3381. [DOI] [PubMed] [Google Scholar]
- Tsegaye W, Aseffa A, Mache A, Mengistu Y, Berg S, Ameni G, 2009. Conventional and molecular epidemiology of bovine tuberculosis in dairy farms in Addis Ababa city, the capital of Ethiopia. J. App. Res. Vet. Med 8, 143–151. [Google Scholar]
- van Arendonk JAM, Liinamo AE, 2003. Dairy cattle production in Europe. Theriogenology 59, 563–569. [DOI] [PubMed] [Google Scholar]
- Vathsala M, Swathika MC, Jayathangaraj MG, Gomathinayagam S, Ramesh S, Abdul Basith S, 2007. Diagnosis of bovine tuberculosis in wild animals by polymerase chain reaction. Indian J. Anim. Res 41 (4), 282–285. [Google Scholar]
- Walravens K, Marché S, Rosseels V, Wellemans V, Boelaert F, Huygen K, Godfroid J, 2002. IFN-gamma diagnostic tests in the context of bovine mycobacterial infections in Belgium. Vet. Immunol. Immunopathol 87, 401–406. [DOI] [PubMed] [Google Scholar]
- WHO, OIE, FAO, The Union, 2017. The roadmap for zoonotic tuberculosis. A Call to Action ISBN 978 92 4 151304 3. https://www.who.int/tb/publications/2017/zoonotic_TB/en/ (Accessed 21 January 2019).
- Zeru F, Romha G, Berhe G, Mamo G, Sisay T, Ameni G, 2014. Prevalence of bovine tuberculosis and assessment of Cattle owners’ awareness on its public health implication in and around Mekelle, Northern Ethiopia. J. Vet. Med. Anim. Health 6 (6), 160–167. [Google Scholar]
- Zeweld SW, 2014. Cultural and molecular detection of zoonotic tuberculosis and its public health impacts in selected districts of Tigray region. Ethiopia. Sokoto J. Vet. Sci 12 (1), 1–12. [Google Scholar]
- Zeweld WS, Reta DH, Abay AG, Zelelew YB, 2013. Detection of human and bovine tuberculosis using an existing diagnostic practice in residential districts of Tigray Region, Northern Ethiopia. J. Environ. Occup. Sci 2 (2), 77–88. 10.5455/jeos.20130912032146. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


