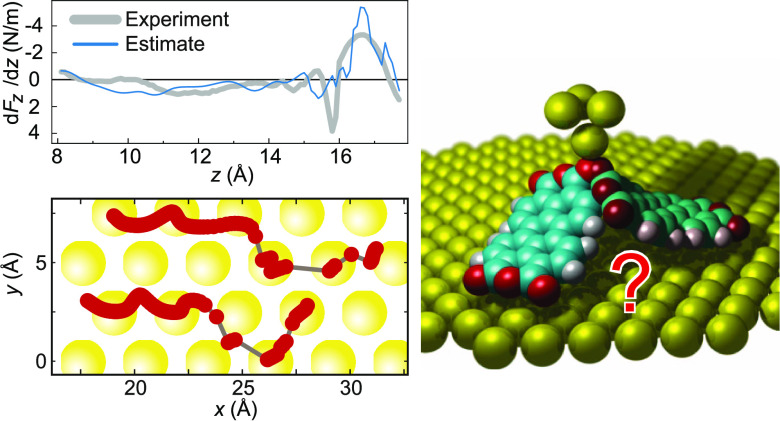
Abstract
A bold vision in nanofabrication is the assembly of functional molecular structures using a scanning probe microscope (SPM). This approach requires continuous monitoring of the molecular configuration during manipulation. Until now, this has been impossible because the SPM tip cannot simultaneously act as an actuator and an imaging probe. Here, we implement configuration monitoring using experimental data other than images collected during the manipulation process. We model the manipulation as a partially observable Markov decision process (POMDP) and approximate the actual configuration in real time using a particle filter. To achieve this, the models underlying the POMDP are precomputed and organized in the form of a finite-state automaton, allowing the use of complex atomistic simulations. We exemplify the configuration monitoring process and reveal structural motifs behind measured force gradients. The proposed methodology marks an important step toward the piece-by-piece creation of supramolecular structures in a robotic and possibly automated manner.
Introduction
The ability to handle single molecules as effectively as macroscopic building blocks would enable the fabrication of functional supramolecular structures that are not accessible by self-assembly.1−4 A way to approach this goal is the use of a low-temperature scanning probe microscope (SPM) which offers not only imaging but also manipulation capabilities with the tip as the actuator.5−10 So far, most SPM manipulation experiments address the rearrangement of atoms or molecules on the surface, typically in a dragging, pushing, or pick–transfer–drop approach.7,11−15 The problem of configuration monitoring is not urgent for such experiments since terminal configurations can be imaged, and, moreover, the tip-induced transitions between these configurations occur abruptly, often upon an inelastic excitation of the molecule, which is stochastic in nature. These lateral repositioning methods naturally limit the achievable (supra)molecular structures.
To add more degrees of freedom to the accessible configuration space, we have developed the concept of two-contact manipulation in which the tip forms a stable chemical bond with a single particularly reactive atom in a surface-adsorbed molecule (Figure 1a).1,3,6,10,18 This “handle” can then be used to apply a force to the molecule and thus manipulate it mechanically (lifting, deforming, translating, detaching) in a manner that is practically deterministic at the experimental temperature of 5 K and allows changing the molecular configuration along continuous trajectories, in contrast to abrupt jumps from one stable configuration to another. Importantly, two-contact manipulation also enables the step into the third dimension away from the surface. While this increases the possible manipulation options considerably,3,19,20 the monitoring of the molecular configurations during the manipulation process is very poor. In its dual role as imaging probe and guiding actuator, the SPM tip can only fulfill one task at a time, such that the manipulation happens “blindly”, with raw data from the few measurement channels of the SPM setup (typically force-gradient and conductance data) as the only available information. We have recently shown that even without explicit knowledge of the configuration, certain nanofabrication tasks can be achieved autonomously by using reinforcement learning with sparse feedback.3 However, to enable a broader scope of SPM-based robotic nanofabrication, it is indispensable to monitor the molecular configuration during manipulation.
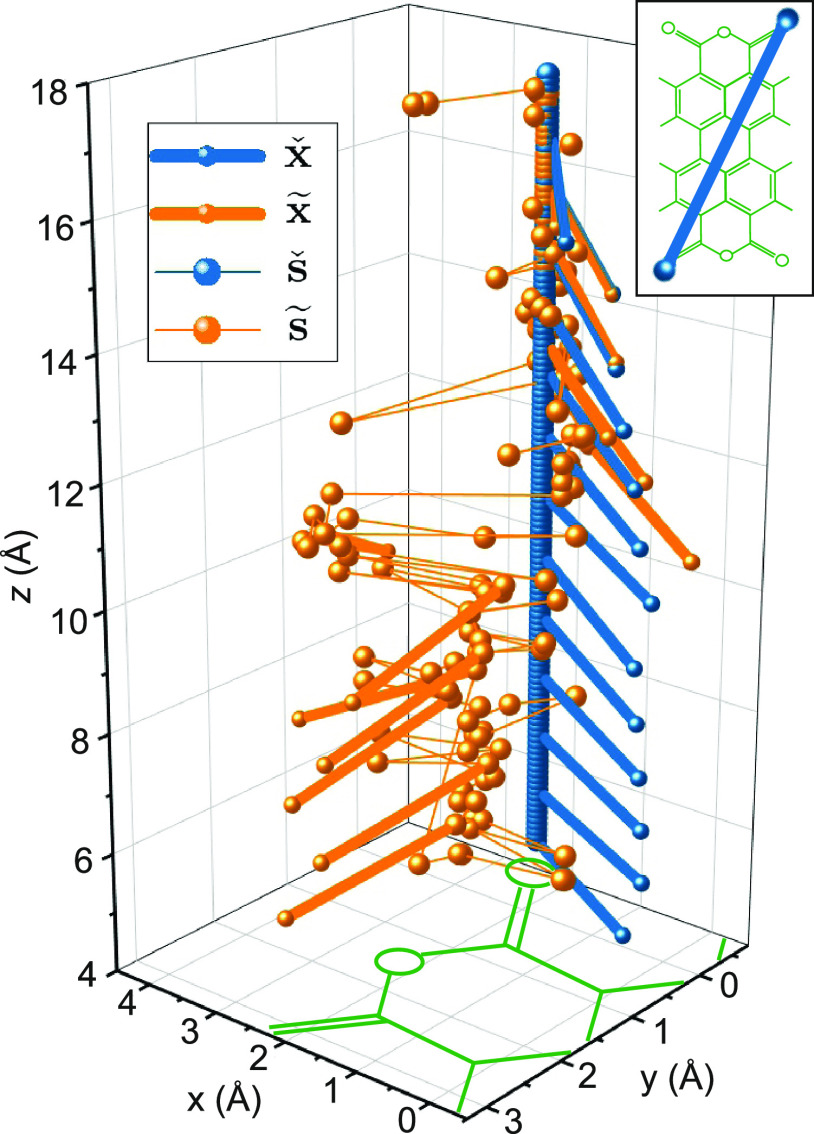
Figure 1.
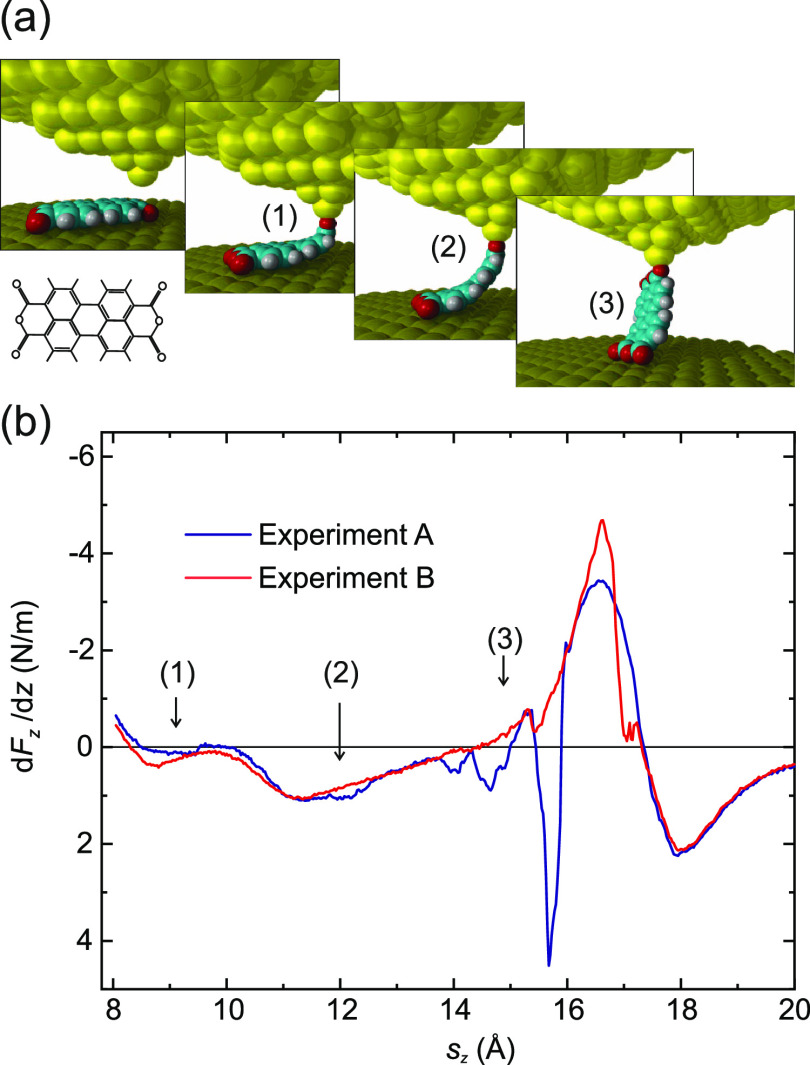
Lifting of a PTCDA molecule. (a) Two-contact manipulation process in which a PTCDA molecule is lifted from a Au(111) surface with the SPM tip. The tip is approached to one of the Ocarb atoms (left frame), where a chemical bond forms. When retracting the tip on a vertical trajectory (sz is the z coordinate of the tip apex), the molecule is gradually lifted up.6,16 (b) Two exemplary experimental dFz/dz(sz) curves were recorded for the lifting procedure shown in (a). The three stages of the lifting process (1)–(3) are indicated in both panels. Since PTCDA can take different paths across the surface, the dFz/dz(sz) curves differ, particularly in regions where PTCDA is vertical and the Ocarb atoms come close to the Au(111) surface. The zero of sz was determined as in ref (17).
Since imaging is not feasible, configuration monitoring must be based on data sets other than images. These could either originate from the available SPM channels or from additional experimental methods. Any method with single-molecule sensitivity, which is susceptible to the molecular configuration—and does not require scanning the tip—has the potential to provide useful information. Examples include current–voltage, Raman, or optical spectroscopy, as well as force gradient measurements.
How can the link between such data and the molecular configuration be established? On an abstract level, molecular manipulation can be described as a partially observable Markov decision process (POMDP). From this perspective, the tip–molecule–surface junction is a system that transitions from one hidden state, namely, the configuration x, into another by the actions of a decision maker, while the only information about these states can be obtained through observations u(x), which are the experimental data discussed above. In a POMDP, the acquired information forms the basis for autonomous decision-making aimed at reaching a target state. The policy that links observations and decisions is optimized through obtained rewards.3 Configuration monitoring thus provides the information on which rewards can be based or upon which an experimenter can choose actions. It is, hence, the key ingredient for molecular robotic nanofabrication. Here, we present an approach that aims at providing the best possible estimate of the hidden state x from the sequence of observations uj made throughout the manipulation process.
The task of configuration monitoring requires combining two strands of information, namely, the manipulation actions taken by the experimenter and the observations made in the simultaneous measurements. To interpret the actions, a state transition model is required, which can predict how the molecular configuration changes upon a certain action. Likewise, to interpret the measured data, an observation model is required that predicts which experimental observation(s) would be made in a given molecular configuration. Both models should, in principle, be statistical in nature since thermal fluctuations could affect state transitions in a statistical way, and experimental noise would affect the observations. Here, we nevertheless use a deterministic state transition model because our experiments were performed at T = 5 K. While our observation model is likewise deterministic, we include experimental uncertainty in our solution of the configuration monitoring task.
Because of unavoidable model limitations, experimental noise, and the fact that a molecular configuration is a high-dimensional vector x, while observations might only comprise a single scalar value u, a single observation is typically not sufficient to determine x with high confidence. This challenge can be understood and addressed in the framework of a Bayesian inference approach in which an initial (prior) configuration estimate is iteratively refined (posterior) as new pairs of actions and observations become available during the manipulation process. We realize this concept using a particle filter (PF) state estimation algorithm.21
Three aspects need to be considered when selecting a certain experimental technique or SPM data channel as the basis for configuration monitoring: (1) The method should be capable of producing measurement outcomes on the few-seconds time scale of a typical SPM manipulation experiment, should have single-molecule sensitivity, and a high signal-to-noise level. (2) It is advantageous if the experiment has a large number of possible outcomes (corresponding to different configurations) since this tends to increase the information content
| 1 |
of an individual measurement, where P(u) is the probability of measuring u (see the section on information gathering). Experiments measuring solely a spin orientation, for example, would be of limited use for configuration monitoring in a large configuration space. (3) Experimental quantities should be preferred for which the observation model can be obtained with limited effort. For a given molecular configuration x, the force F(x) acting on the SPM tip during manipulation is, for example, much easier to calculate than the conductance spectrum I(V) [x] or the vibrational spectrum A(ω) [x]. This is the reason why we abstain from including molecular junction conductance as a source of information in our approach. Note that we use Roman symbols to refer to the simulated (or estimated) data and italic symbols to denote experimental data.
Here, we describe a configuration monitoring approach in which
the observation u is the non-contact atomic force
microscope (NC-AFM) force gradient F′:= dFz/dz measured
during the manipulation process (Figure 1b). We use a qPlus tuning fork setup22 with an oscillation amplitude of 0.3 Å.
To avoid irregularities in the configuration space, we perform our
experiments in a region of the sample surface that is devoid of defects
or step edges and has thus the full symmetry of the Au(111) surface
lattice. The PF, which is used to infer the most likely molecular
configuration, samples the observation model at a limited number of
promising configurations, thus avoiding a search through the entire
configuration space 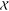 . For each sample, the PF compares the actual
current observation F′ with the predictions
F′ of the observation model and refines its state estimate x. The deviation between F′ and F′,
the action of the experimenter, and the state transition model determine
which points in configuration space will be sampled in the next PF
iteration that is carried out when a new manipulation action changes x and leads to a new F′
value.
. For each sample, the PF compares the actual
current observation F′ with the predictions
F′ of the observation model and refines its state estimate x. The deviation between F′ and F′,
the action of the experimenter, and the state transition model determine
which points in configuration space will be sampled in the next PF
iteration that is carried out when a new manipulation action changes x and leads to a new F′
value.
To be useful for the control of a manipulation experiment,
the
PF has to be able to follow the pace with which new action-observation
pairs become available, which happens on a time scale of seconds.
We achieve this performance by precalculating both the observation
and the state transition models for the entire configuration space 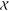 and mapping them onto a finite-state automaton
(FSA) for quick and structured access. A related important aspect
is the choice of the observation and state transition models themselves.
If they are based on an atomistic simulation, the computational costs
could be very high, rendering a computation of the entire configuration
space
and mapping them onto a finite-state automaton
(FSA) for quick and structured access. A related important aspect
is the choice of the observation and state transition models themselves.
If they are based on an atomistic simulation, the computational costs
could be very high, rendering a computation of the entire configuration
space 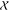 unfeasible. If, on the other hand, less
complex models are chosen, capturing certain details of the molecular
configurations could become impossible. Here, we use a molecular mechanics
approach that is atomistic and strikes a balance between accuracy
and speed.
unfeasible. If, on the other hand, less
complex models are chosen, capturing certain details of the molecular
configurations could become impossible. Here, we use a molecular mechanics
approach that is atomistic and strikes a balance between accuracy
and speed.
We demonstrate the functionality of our configuration monitoring on the example of lifting an isolated PTCDA (3,4,9,10-perylene-tetracarboxylic dianhydride) molecule from a Au(111) surface,8,16,17 as shown in Figure 1a. We have extensively studied the manipulation of PTCDA in the past,1,3,6,8,16−19,23,24 making it the ideal candidate for this proof-of-concept study. Moreover, we have an optimized force-field simulation for the manipulation of PTCDA on Au(111) at hand, which forms the basis for the observation and state transition models.8,17,25 Since this force field is fitted to experimental data, it properly captures the molecule–metal interaction, including many-body contributions.17 In this model, the tip is described by its apex atom alone. This is a reasonable simplification since the oxygen atom prefers to bind on top of the tip apex atom and not to a bridge or hollow site.20 Moreover, the rest of the tip apex beyond the metal–oxygen bond plays only a minor role in the behavior of the tip–molecule junction.26
Methods
Primary and Duplicate Configurations
The atomic lattice of the Au(111) surface constitutes the natural frame of reference relative to which all tip–molecule configurations are defined. For simplicity, we neglect the small uniaxial compression of the surface layer which arises from the Au(111) surface reconstruction and assume a strictly hexagonal surface lattice with a periodicity of 2.884 Å, endowed with the respective translational, rotational, and mirror symmetries. This implies that from any given primary tip–molecule configuration x, an infinite number of translated, rotated, and mirrored duplicates can be created, all of which are equivalent with respect to the atomic lattice of the surface. If, furthermore, a discrete grid of tip positions s is used, the number of primary tip–molecule configurations, which are the ones that need to be computed, becomes finite. This holds under the reasonable assumption of a finite number of relaxed molecular configurations r for each s, the latter of which is safeguarded by a surface corrugation with distinct minima.
For the discretization of s space, we used a hexagonal grid with a step size of 0.120167 Å in the x,y plane, thus sampling the rhombic Au(111) unit cell at 24 × 24 positions. In the z direction, we performed calculations in the interval 5.3 ≤ sz ≤ 17.7 Å, i.e., the range between a flat-lying and a fully vertical molecule, with a grid step size of 0.1 Å. Altogether, this led to a total of 72,000 allowed tip positions s.
Similarity between Molecular Configurations
When creating the FSA, it is necessary to determine whether a new configuration obtained by relaxation with the molecular mechanics model is already included in the FSA or whether it has been attained for the first time. Two simulated configurations obtained by different manipulation steps will never be precisely the same because the relaxation is terminated by a set of thresholds and, therefore, always incomplete. Since configuration similarity checks are frequently required when the FSA is created, their computational efficiency is important. We use the positions of four atoms for this purpose. Two configurations are considered identical if (1) their tip positions on the discretization grid are the same, (2) the positions of their bottom Ocarb atoms are the same within an accuracy of 0.2 Å, and (3) the bending direction of the molecule between its anchor points at the tip and in the surface is the same. The third aspect is relevant because the bending is the only remaining degree of freedom of the planar PTCDA molecule once three of its corners are fixed in space. Usually, the molecule would bend toward the surface because of attractive vdW forces, but there are configurations in which PTCDA is bent upwards. They can be reached from the vertical molecular configuration by pushing the tip toward the surface. Subsequently, rotating the bent molecule around the bottom Ocarb–Ocarb axis toward the direction into which it is not bent preserves the bent.
Reversibility and Surface Corrugation
When analyzing the aspect of reversibility, it must be remembered that the states and transitions in the FSA depend on the underlying atomistic model. In particular, the model employed for the local Au–Ocarb interaction has a strong impact on reversibility. Simply speaking, the stronger these bonds are, the more stringent the anchor concept and its consequences are because intermediate states in which the Ocarb atoms are located between Au atoms (i.e., hollow or bridge sites) will then be suppressed.
In our molecular mechanics approach, we model the Au–Ocarb interaction for each of the four Ocarb atoms by a lateral cosine potential18
| 2 |
with c = 2.884 Å and a z-dependent amplitude Vz(z). Note that the minima are centered on the Au atoms. The chosen z dependence is a simple representation of (weak) chemical bonding; it follows the Fermi-type function Vz(z) = 40 meV (1 + e(z – 2.95Å)/0.22Å)−1. As a consequence, close to the surface, the Ocarb atoms feel a stronger corrugation. This happens, for example, in an inclined molecular orientation (last panel of Figure 1a).
Roulette Wheel Selection
We used the so-called roulette wheel selection27 for redistributing the particles according to importance weights Wl. The algorithm creates a set of G intervals, one for each particle xl, with normalized widths wl = Wl/∑l=1GWl that are proportional to the importance weights of each particle. For l′ = 1, ··· G, the intervals are given by Il′ = [∑l=0wl,∑l=0l′wl], where we defined w0 ≡ 0. Thus, I1 = [0,w1], I2 = [w1, w1 + w2], I3 = [w1 + w2, w1 + w2 + w3], and so on. Next, a random value between 0 and 1 is assigned to each of the particles, which places them in one of the intervals. Finally, each particle is relocated into the vicinity (up to three neighboring FSA states) of the particle from which the respective interval was created.
K-Medoid Clustering
The K-medoid clustering technique28 is a robust algorithm to cluster points in a metric space. To keep the computation speed high, we do not use as our metric the Cartesian distance in the high-dimensional vector space in which configuration vectors x live but define a distance based on fewer quantities which are, in contrast to the components of x, largely uncorrelated. Specifically, given two configuration vectors xA and xB, we use the distance between the tip positions sA and sB and the differences between the azimuthal angles of the anchor–tip position vectors ∠A and ∠B as a measure of the metric distance between xA and xB,
| 3 |
Note that ∠A – ∠B is multiplied by a constant Caz, which, on average, increases the importance of the azimuthal angles to the same level as sA – sB.
Results and Discussion
Concept
Bayes’ Rule
In our proof-of-concept study, configuration
monitoring essentially means solving an inverse problem in which the
unknown high-dimensional molecular configuration  , i.e., the positions of all n atoms of the molecule, has to be inferred from the measured z component of the NC-AFM force gradient F′ (r, s) (Figure 1b) which depends on both r and the
SPM tip position
, i.e., the positions of all n atoms of the molecule, has to be inferred from the measured z component of the NC-AFM force gradient F′ (r, s) (Figure 1b) which depends on both r and the
SPM tip position 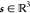 in a way that is described by our observation
model. In fact, configuration monitoring has to include the tip position s into the sought-after quantity
in a way that is described by our observation
model. In fact, configuration monitoring has to include the tip position s into the sought-after quantity 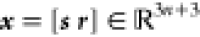 because, in the experiment, s is typically only known relative to the other tip
positions in a trajectory but not relative to the atomic surface lattice
that constitutes the fixed reference frame.
because, in the experiment, s is typically only known relative to the other tip
positions in a trajectory but not relative to the atomic surface lattice
that constitutes the fixed reference frame.
In the context of
a POMDP, the actions of the experimenter are not based on the knowledge
of configuration x but on their belief
about the nature of this configuration. In its Bayesian interpretation,
this belief is manifested by a probability distribution over all N possible configurations in the configuration space  . For a given current experimental observation u, the conditional probability that the molecule is in a
given configuration xk is
given by Bayes’ rule
. For a given current experimental observation u, the conditional probability that the molecule is in a
given configuration xk is
given by Bayes’ rule
| 4 |
in which the denominator is simply the probability P(u). All P(u|xi) values, which measure the probability of observing u when the molecule is in configuration xi, have to be obtained from the observation model, and P(xk) measures the prior probability of xk, that is, the probability prior to observing u. If the tip–molecule bond has just been established, the prior could be obtained from an SPM scan recorded before the start of the manipulation. Another example is a prior based on the estimated tip height. If no prior information is available or considered, then P(xk) = 1/N. Equation 4 expresses a single belief update step in which the probability P(xk) is refined from its unconditional prior to its posterior value P(xk|u).
Belief Update along a Trajectory
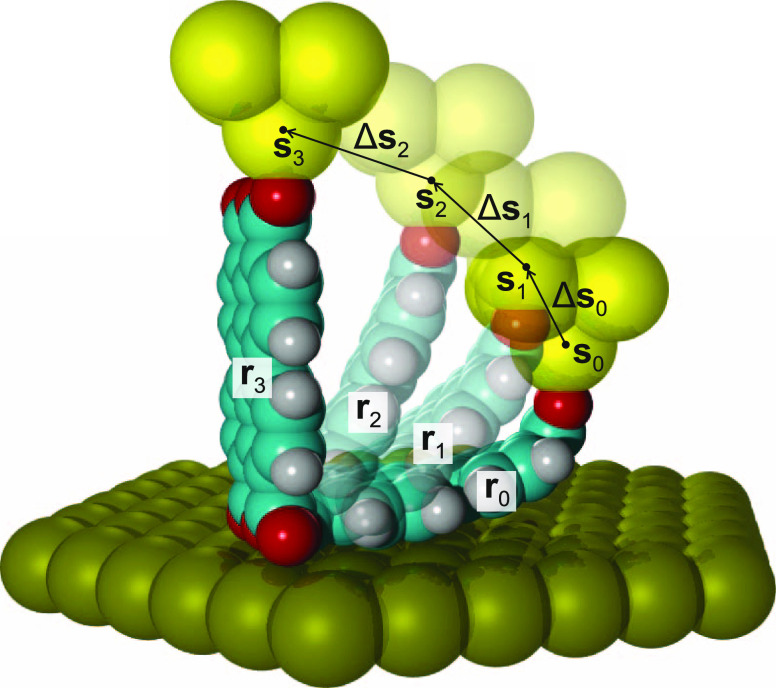
Since configuration monitoring means predicting the high-dimensional state, that is, the tip–molecule configuration x from a low-dimensional observation u, it is likely that even substantially different configurations will result in very similar observations. This would lead to a wide probability distribution P(xi|u) over many xi (eq 4), which does not favor a single, highly probable configuration. This is unsatisfactory for configuration monitoring. To improve the situation, further belief update steps can be performed based on observations along the entire past trajectory of the manipulation. We represent this trajectory, in accordance with the actual experimental procedure, as a time series of M tip translation steps Δsj–1 for j = 1, ··· M (i.e., Δs0, ··· ΔsM–1) at discrete times t0, ··· tM–1, carried out between M + 1 tip positions sj and associated observations uj, both for j = 0, ··· M (Figure 2). Given such a trajectory, the fully updated belief that xk is the first state of the trajectory becomes under the Markov assumption
 |
5 |
In the third transformation, we have used
Bayes’ rule P(uj|xk,j)
= P(xk,j|uj)P(uj)/P(xk,j). While xi runs over all configurations
in the configuration space 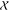 , the configurations xi,j are obtained stepwise
from xi,0 = xi and the (deterministic) state transition
model S as xi,j+1 = S(xi,j, Δsj), where Δsj is the action taken
at step j. Since the state transition model links
all configurations in the numerator of eq 5 into a deterministic sequence {xk,0, ···, xk,M}, the probability P(xk|u0, ···, uM) in eq 5 is, in fact, the probability for the entire trajectory. Hence, it
is the probability that xk,0 is the first configuration, xk,1 is the second configuration, etc., and xk,M is the current configuration
in the trajectory. This is relevant because the current configuration xM is naturally
the object of interest in configuration monitoring.
, the configurations xi,j are obtained stepwise
from xi,0 = xi and the (deterministic) state transition
model S as xi,j+1 = S(xi,j, Δsj), where Δsj is the action taken
at step j. Since the state transition model links
all configurations in the numerator of eq 5 into a deterministic sequence {xk,0, ···, xk,M}, the probability P(xk|u0, ···, uM) in eq 5 is, in fact, the probability for the entire trajectory. Hence, it
is the probability that xk,0 is the first configuration, xk,1 is the second configuration, etc., and xk,M is the current configuration
in the trajectory. This is relevant because the current configuration xM is naturally
the object of interest in configuration monitoring.
Figure 2.
Example of a manipulation trajectory. For this exemplary tip trajectory, the bottom Ocarb atoms do not move on the surface, such that each of the manipulation steps Δs0 to Δs2 is reversible in terms of r.
Computational Complexity
The probability distribution eq 5 for all 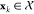 represents a complete solution to the configuration
monitoring problem. However, this approach will typically fail because
it is computationally intractable to evaluate eq 5 for a large configuration space. In particular,
the denominator of eq 5 requires N × M evaluations
of the state transition model, where N and M are on the order of 500,000 and 100, respectively, in
the examples discussed below.
represents a complete solution to the configuration
monitoring problem. However, this approach will typically fail because
it is computationally intractable to evaluate eq 5 for a large configuration space. In particular,
the denominator of eq 5 requires N × M evaluations
of the state transition model, where N and M are on the order of 500,000 and 100, respectively, in
the examples discussed below.
Additionally, each evaluation of the state transition model requires the structural relaxation of the molecular configuration, accounting for the new tip position sj+1 ≡ sj + Δsj, which is done here via a molecular mechanics model. For the PTCDA molecule with n = 38 atoms, this means solving a nonlinear optimization problem in a 114-dimensional space. Although the optimization problem is effectively less complex because the individual atoms’ degrees of freedom are coupled by constraints that follow from the chemical structure of the molecule, performing all optimizations within the few seconds of time tj+1 – tj elapsing between two steps along the manipulation trajectory is nevertheless unfeasible. This situation calls for an approximation of the probability distribution, which we will describe below.
We take two measures to counter the tremendous computational effort resulting from a brute-force application of eq 5. First, we use a PF to approximate a solution to eq 5, and second, we represent the observation and the state transition models by an FSA, which contains precalculated configurations and corresponding predicted measurement values u from the underlying molecular mechanics model.
Particle Filter
Particle filters are one of the methods
that can be employed for belief updates in large POMDPs.29−31 A prominent application is the localization of mobile robots in
a complex environment,31−33 based on sensor data and a map as the basis for the
observation and state transition models. Two aspects of the PF are
central for simplifying the belief update: (1) The probability distribution P(xi) over all
configurations, which is needed to evaluate eq 5, is never computed in its entirety but instead
sampled only for a small subset 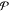 of the entire configuration space
of the entire configuration space 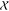 , in our case G = 1500
configurations xl, l = 1 ··· G, which are called
particles. (2) Since even a single evaluation of eq 5 would require a summation across all of
, in our case G = 1500
configurations xl, l = 1 ··· G, which are called
particles. (2) Since even a single evaluation of eq 5 would require a summation across all of  , the PF replaces the formally exact calculation
of the conditional probabilities P(xl,j|uj) in eq 5 by a relative measure of probability which is based
on the likeness between predicted and measured observations Fl,j′ and Fj for all xl,j, where Fl,j′ is obtained from the (deterministic)
observation model U as Fl,j = U(xl,j). Here, relative probability simply means that a
sampled configuration xl,j with a high correspondence between Fj′ and Fl,j has a higher
probability of resembling the unknown configuration xj than a configuration xl′,j with
a low correspondence. The calculation of this correspondence requires
the definition of a metric over the space of all observations.
, the PF replaces the formally exact calculation
of the conditional probabilities P(xl,j|uj) in eq 5 by a relative measure of probability which is based
on the likeness between predicted and measured observations Fl,j′ and Fj for all xl,j, where Fl,j′ is obtained from the (deterministic)
observation model U as Fl,j = U(xl,j). Here, relative probability simply means that a
sampled configuration xl,j with a high correspondence between Fj′ and Fl,j has a higher
probability of resembling the unknown configuration xj than a configuration xl′,j with
a low correspondence. The calculation of this correspondence requires
the definition of a metric over the space of all observations.
The aspect that the prior probability of step j +
1 is the posterior probability of step j, which is
implicitly contained in the considerations that lead from eqs 4 to 5, is contained in the PF via a weighted distribution of sampling
points xl at which the prior
is evaluated. Regions in 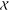 , which have a higher prior probability,
are sampled more densely. In this way, one only obtains a probability
distribution over likely configurations and not across the entirety
of
, which have a higher prior probability,
are sampled more densely. In this way, one only obtains a probability
distribution over likely configurations and not across the entirety
of 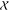 , but this is no principal shortcoming if
the task is to find the best representation of the actual molecular
configuration xj in the experiment. However, since the sampling steps of the
PF involve some randomness, the PF does not necessarily find the globally
best estimate of xj but only a good approximation. Below, we will exemplify this
aspect using experimental data.
, but this is no principal shortcoming if
the task is to find the best representation of the actual molecular
configuration xj in the experiment. However, since the sampling steps of the
PF involve some randomness, the PF does not necessarily find the globally
best estimate of xj but only a good approximation. Below, we will exemplify this
aspect using experimental data.
Finite-State Automaton
To follow a given tip trajectory, the PF needs information on how the molecular configurations xl,j transform into each other upon any single tip displacement step Δs. This information is provided by the state transition model. While our molecular mechanics model could directly serve as the state transition model, it would be too slow for configuration monitoring, as detailed above. We circumvent this problem by precomputing all possible transitions in the entire configuration space. To manage this data set, we insert a layer of abstraction between the atomistic simulation and the PF, namely, an FSA. After discretizing the state space, the transition model can be represented by a directed graph in which edges equal transitions and nodes equal states. Since our model is deterministic, the graph is rather sparse with, at each node, only one edge for each possible tip translation step Δs. In a probabilistic model, on the other hand, the graph would be edge-weighted and much denser because a tip step could induce a transition to several different states xk with transition probabilities P(xk) > 0. Given these properties, the task of generating and storing the transition model can be performed by an FSA.34 Since the calculation of the force gradients F′(xk) for each configuration xk is a byproduct of the molecular mechanics relaxation procedure, the observation model is generated and stored alongside the transition model by the FSA.
Information Gathering
As we have discussed above, a single action-observation pair is typically not sufficient to determine a given configuration xk with high certainty, such that the entire trajectory needs to be taken into account. This poses a problem in situations where no such trajectory is available (yet), although a decision about the next steps of the manipulation process nevertheless requires knowledge of xk. Hence, the question arises whether a sufficiently long trajectory can be generated by manipulation with the sole purpose of information gathering before returning to the initial (and previously unknown) configuration xk at the end.
Information-gathering policies are a recurring topic in POMDPs.35,36 Given the FSA, one could, in principle, compute the optimal information-gathering trajectory, in which the choice of each next step Δs would be based on the sequence of F′ values measured so far. However, computing this strategy would lead to a combinatorial explosion since there exist 8l possible trajectories of length l for a single starting configuration alone. Moreover, the resulting strategy would only be valid for the specific FSA it has been developed for and not transferable. Therefore, we take a more general approach here and calculate the number of steps K that would, on average, be required to identify a molecular configuration unambiguously, resorting to our specific FSA only for parameterization. The knowledge of K, together with the aspect of reversibility discussed below, can then form the basis for the design of appropriate information-gathering trajectories.
Following the concepts pioneered by Shannon,37 we equate the information necessary for unambiguous identification of the configuration with the information content of force gradient measurements along a sufficiently long trajectory (on average K steps). Modeling the configuration as a discrete random variable C for which the uniform probability of each of the N configurations is PC = 1/N, the information content in natural units (nats) of knowing the configuration is I(C) = ln(1/PC) = ln(N) (cf. eq 1). The measurement process, on the other hand, involves continuous distributions of a measured quantity Y (here F′) and the inherent noise Z. The true signal X and the noise Z add up to the measured signal, Y ≡ X + Z. To calculate K, we need to determine the mutual information I(X, Y) of X and Y, that is, how much is learned about X from knowing Y.38 Using the concept of differential entropy to analyze the information content in continuous distributions, we obtain the simple relation I(X, Y) = ln(σY/σZ),38 which depends only on the standard deviations of Y and Z (see Appendix).
If this information is obtained at each step of the information-gathering trajectory, we simply get K = I(C)/I(X, Y). However, in practice, more steps will be required since the true values, X1, X2, ···, at subsequent steps are typically correlated. As a consequence, the additional information gained from every but the first measurement is less than I(X1, Y1) because, for all i > 1, some information about Xi, specifically I(Xi, Yi–1), was already known from the Yi–1 measured at the previous step. Since for all i > 1 only the reduced information ΔI(Xi, Yi) = I(Xi, Yi) – I(Xi, Yi–1) is obtained from measuring Yi, a larger number of steps is needed to obtain the information I(C) required for unambiguous identification. In the Appendix, we derive the relation
| 6 |
which depends on the standard deviations of the involved distributions and on the Pearson correlation coefficient ρ between subsequent Xi along the trajectory. Knowing ΔI(X, Y), the required length of an information-gathering trajectory can be computed as
| 7 |
since the first measurement yields the full information I(X1, Y1), while all subsequent measurements only yield the difference ΔI. As we will later show in the section on information gathering, values of up to K = 60 are possible in our experiments, raising the question of whether an information-gathering trajectory of such a length can be successfully reversed, thus safely returning to the initial configuration xk, for the determination of which the trajectory was performed in the first place.
Reversibility
The large number of stable molecular configurations at a single tip position in Figure 3b illustrates that returning the tip to its original position, sk, alone does not guarantee that r and, thus, x return to their initial values as well. On the contrary, after a sufficiently long random manipulation trajectory, the probability of returning to rk is rather small, as is shown in the Supporting Information. On the other hand, intuition tells us that a sufficiently small tip translation step Δs should usually be reversible with respect to r. If all individual steps Δsj–1 = sj – sj–1 of a manipulation trajectory were reversible in such a way, the molecule could be returned to its initial state by reversing the entire trajectory step by step. We will address this aspect in the Supporting Information.
Figure 3.
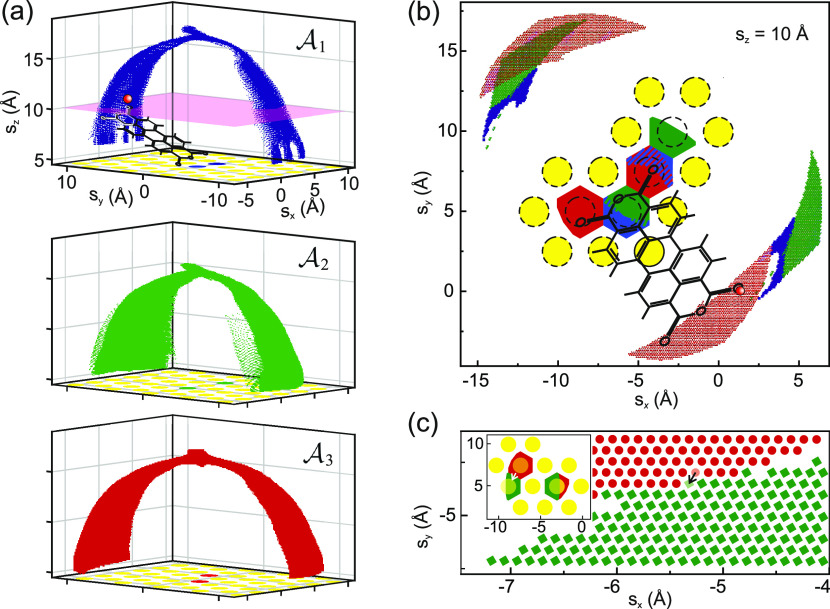
Atomistic
simulation. (a) Visualization of a single stable molecular
configuration at sz =
10 Å. (b) Visualization of a subset of 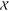 , which contains all 47 stable molecular
configurations resulting from our molecular mechanics state transition
model for a given exemplary SPM tip position at sz = 10 Å. The primary degree of
freedom is the azimuthal angle of the molecule.
, which contains all 47 stable molecular
configurations resulting from our molecular mechanics state transition
model for a given exemplary SPM tip position at sz = 10 Å. The primary degree of
freedom is the azimuthal angle of the molecule.
To answer the question of which configurations and which trajectories allow reversibility in practice, we also have to consider some specific properties of the molecule–surface system for which the manipulation is performed. Two-contact manipulation of PTCDA proceeds by approaching the tip to one of the carboxylic oxygen atoms (Ocarb) of the molecule, whereupon a tip–Ocarb bond forms spontaneously due to the reactivities of the oxygen atom and the undercoordinated Au tip apex atom (Figure 1a). Ocarb is also expected to form bonds with the Au atoms in the close-packed Au(111) surface if the Au–Ocarb distance is not too large. While these bonds will be weaker due to the higher coordination of the Au atoms in the surface,26 they still have a considerable impact on the measured force gradients (Figure 1b). Such molecule–surface bonding is more prominently observed on the Ag(111) surface, where it leads to a distortion of PTCDA, with the Ocarb atoms bending toward the surface to a distance of 2.66 Å above the topmost atomic plane of the substrate.39 In its adsorbed state on Au(111), the Ocarb atoms of PTCDA are, on the other hand, found at a larger distance from the surface,39 such that we expect considerable local bonding to appear only once the Ocarb atoms approach the surface when the PTCDA molecule is inclined in the lifting process (right panel in Figure 1a).
Based on this assumption
of a chemical molecule–surface
interaction via Ocarb atoms, we can postulate a simple
model of reversible manipulation. We assume that any manipulation
step Δsj is reversible in terms of r if
the bonds between the two bottom Ocarb atoms (the ones
at the side of PTCDA to which the tip is not attached) and two Au
atoms in the surface stay intact. An example of such reversible steps
is shown in Figure 2. If, however, an Ocarb atom changes its binding partner
to another Au surface atom as a result of a tip translation Δsj, the respective
change from rj to rj+1 is assumed to be ratchet-like and discontinuous and thus irreversible
(because of being hysteretic) upon returning the tip to sj. We will test the
validity of this assumption when discussing the fundamental properties
of the entire state space 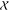 of all simulated tip–molecule configurations.
For a set of exemplary trajectories designed for information gathering,
we moreover will determine the probability of returning to the starting
configuration xj=0 upon trajectory
reversal (Supporting Information). This
requires an analysis of the generic structure of the directed graph
of the FSA.
of all simulated tip–molecule configurations.
For a set of exemplary trajectories designed for information gathering,
we moreover will determine the probability of returning to the starting
configuration xj=0 upon trajectory
reversal (Supporting Information). This
requires an analysis of the generic structure of the directed graph
of the FSA.
Finite-State Automaton
Motivation and Objective
Coming back to the issue of
computational complexity in configuration monitoring, we analyze the
situation in more detail. The fastest atomistic simulations available
are force-field methods. They are based on rapidly computable analytical
potential energy functions and are often used in molecular dynamics
(MD) simulations. Recent advances in hardware and broadband communication
have enabled new applications of such MD simulations: animations based
on precomputed MD trajectories can be streamed online,40 and virtual reality environments have been combined
with interactive MD to enable intuitive, user-controlled ad hoc simulations.41 In the latter case, the equations of motion
have to be solved fast enough to allow all atoms to follow the user
input adiabatically. At first sight, this is similar to the requirements
of configuration monitoring. However, the PF applied in configuration
monitoring would require running hundreds of force-field simulations
in parallel (one for each particle) with the external input of a constraint,
for example, the SPM tip position, changing at a rate similar to the
user input in interactive MD. It is currently unfeasible to realize
this computational effort in a lab environment. This holds even when
applying the substantial simplification of zero temperature, which
turns MD into pure structural optimization, as done in this work.
The task would become utterly impossible if a more sophisticated ab-initio
method were chosen to map out the space  of possible molecular geometries and calculate
the force gradients F′. For each relaxed tip–molecule
configuration, the respective density functional theory calculation
would take minutes to hours on a supercomputer. We note that machine-learned
force fields (e.g., ref (42)) may be an alternative along these lines for future studies.
As a radical solution to this dilemma, we therefore completely detach
the simulations from the PF state prediction and instead precalculate
and store simulation data.
of possible molecular geometries and calculate
the force gradients F′. For each relaxed tip–molecule
configuration, the respective density functional theory calculation
would take minutes to hours on a supercomputer. We note that machine-learned
force fields (e.g., ref (42)) may be an alternative along these lines for future studies.
As a radical solution to this dilemma, we therefore completely detach
the simulations from the PF state prediction and instead precalculate
and store simulation data.
Reachable Configurations and Anchor Sets
We continue
with some initial thoughts on the FSA. Since it is not known a priori
at which points the PF will sample the state space  , it is required that all reachable tip–molecule
configurations in
, it is required that all reachable tip–molecule
configurations in 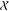 are computed in advance and stored in a
rapidly accessible form. Here, reachable denotes all configurations
that can be accessed from a given starting configuration x0 in which the molecule is flat on the surface via any
possible sequence of tip translation steps, Δs0, Δs1, ···, and
subsequent structure relaxations. Relaxation is required at each step
because a change in s changes the potential energy landscape
of the entire tip–molecule–surface systems.
are computed in advance and stored in a
rapidly accessible form. Here, reachable denotes all configurations
that can be accessed from a given starting configuration x0 in which the molecule is flat on the surface via any
possible sequence of tip translation steps, Δs0, Δs1, ···, and
subsequent structure relaxations. Relaxation is required at each step
because a change in s changes the potential energy landscape
of the entire tip–molecule–surface systems.
Two notable facts about reachable configurations should be stressed. First, it cannot be excluded that there are stable configurations that cannot be reached starting from a molecule that is flat on the surface. Since, however, all manipulation experiments considered here start by contacting a flat molecule, we assume that configurations that cannot be reached by our discovery procedure will also not be obtained in an experiment. Second, reaching a configuration in the described way is not trivial. For example, the chirality of the tip–molecule–surface system, which arises since the Ocarb atom bound to the tip is located on neither of the two mirror axes of PTCDA, can only be switched by rotating the molecule around the bottom Ocarb–Ocarb axis onto its other side via a configuration in which the molecule is vertical on the surface.
The criterion
of being reachable defines an infinite continuum
of configurations. To cope with this problem, we discretize the space
of tip positions s and take advantage of the symmetry
and periodicity of the Au(111) surface lattice by defining a finite
set of primary configurations and, correspondingly, an infinite set
of symmetry-equivalent duplicates (see Methods). Since 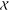 can thus be divided into subsets, each
containing infinitely many symmetry-equivalent configurations, it
is possible to freely select one member of each subset as its primary
configuration. These primary configurations form a subset
can thus be divided into subsets, each
containing infinitely many symmetry-equivalent configurations, it
is possible to freely select one member of each subset as its primary
configuration. These primary configurations form a subset 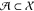 with the property that no two elements
of
with the property that no two elements
of 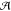 can be transformed into each other by a
symmetry operation.
can be transformed into each other by a
symmetry operation.
Given the freedom to configure 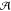 , we opted for building the notion of reversibility
directly into
, we opted for building the notion of reversibility
directly into  . For that purpose, we choose
. For that purpose, we choose 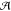 such that transitions between its elements
should, whenever possible, be reversible according to the model of
Au–Ocarb bonds developed above (Figure 2). Hence, we form
such that transitions between its elements
should, whenever possible, be reversible according to the model of
Au–Ocarb bonds developed above (Figure 2). Hence, we form 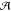 by including all configurations in which
the two bottom Ocarb atoms of PTCDA bind to a specific
pair of Au atoms in the surface; the latter we refer to as the anchor.
Since there will be molecular configurations for which such bonds
are not well defined or absent, we stipulate that an Ocarb atom is always considered as “bound” to the Au atom
in the 2D Voronoi cell43 (Wigner-Seitz
cell) in which it is located (Figure 4). Thereby, the vertical Au–Ocarb distance is not taken into account. Using this convention, we identify
in the simulation three nonequivalent anchors that PTCDA can bind
to (Figure 4a). Consequently,
by including all configurations in which
the two bottom Ocarb atoms of PTCDA bind to a specific
pair of Au atoms in the surface; the latter we refer to as the anchor.
Since there will be molecular configurations for which such bonds
are not well defined or absent, we stipulate that an Ocarb atom is always considered as “bound” to the Au atom
in the 2D Voronoi cell43 (Wigner-Seitz
cell) in which it is located (Figure 4). Thereby, the vertical Au–Ocarb distance is not taken into account. Using this convention, we identify
in the simulation three nonequivalent anchors that PTCDA can bind
to (Figure 4a). Consequently,  consists of three anchor sets,
consists of three anchor sets, 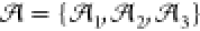 . With this definition of
. With this definition of 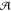 , we have specified which states should
be included in the FSA. The final step is, therefore, the construction
of the FSA itself. Note that the anchors do not have to be specified
a priori but are discovered during the creation of the FSA. In practical
terms, the anchor also provides a reference frame for the definition
of the relative xy coordinates of s and r, which simplifies transformations among symmetry-equivalent
configurations.
, we have specified which states should
be included in the FSA. The final step is, therefore, the construction
of the FSA itself. Note that the anchors do not have to be specified
a priori but are discovered during the creation of the FSA. In practical
terms, the anchor also provides a reference frame for the definition
of the relative xy coordinates of s and r, which simplifies transformations among symmetry-equivalent
configurations.
Figure 4.
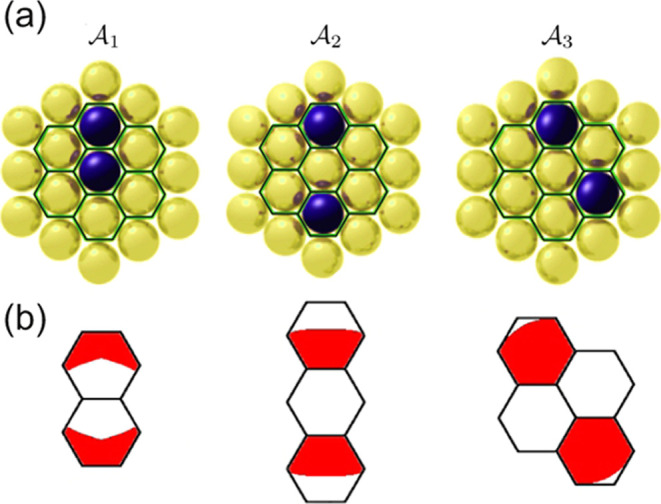
Anchor configurations. (a) The three unique anchors found for PTCDA on Au(111), including their respective Voronoi diagrams. (b) The reachable areas for the three anchors. Red indicates possible lateral positions of the two Ocarb atoms when PTCDA is bound to the respective anchor (compare Figure 6b). These reachable positions are primarily defined by the intrinsic separation of the two Ocarb atoms (4.5 Å).
Implementation of the FSA
As discussed above, the states  and the transitions between these states
form a directed graph, which in turn can be built up by the FSA.34 An FSA is a mathematical model of computation
that operates on a sequence of input symbols. This input determines
a transition from one state of the automaton to another. In our case,
tip–molecule configurations x are the states of
the FSA, and tip steps to neighboring points on the grid of discrete
tip positions represent the transitions τ ≡ (x, x*), with s stepping to s* and r changing to r*. Using the original notation of finite-state transducers
by Roche and Schabes,34 we can describe
our FSA as M ≡ (Σ1, Σ2,
and the transitions between these states
form a directed graph, which in turn can be built up by the FSA.34 An FSA is a mathematical model of computation
that operates on a sequence of input symbols. This input determines
a transition from one state of the automaton to another. In our case,
tip–molecule configurations x are the states of
the FSA, and tip steps to neighboring points on the grid of discrete
tip positions represent the transitions τ ≡ (x, x*), with s stepping to s* and r changing to r*. Using the original notation of finite-state transducers
by Roche and Schabes,34 we can describe
our FSA as M ≡ (Σ1, Σ2,  , x1,
, x1,  ) where the input alphabet Σ1 = {a1, ···, a8} contains all possible actions, i.e., all tip translations ad, where d is one of the eight directions along which the SPM tip can move
on the hexagonal grid (see Methods).
) where the input alphabet Σ1 = {a1, ···, a8} contains all possible actions, i.e., all tip translations ad, where d is one of the eight directions along which the SPM tip can move
on the hexagonal grid (see Methods).
Σ2 is the output alphabet. In our case, we return the complete state x for the updated tip position together with the simulated F′ = U(x) value.
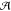 is the set of states consisting of the
primary configurations as defined in the previous section. Initially,
the size of this set is unknown because
is the set of states consisting of the
primary configurations as defined in the previous section. Initially,
the size of this set is unknown because 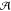 will be gradually filled up during the
building of the FSA.
will be gradually filled up during the
building of the FSA.
 is the initial state, which, in our case,
is an arbitrarily chosen configuration in which the molecule rests
flat on the surface.
is the initial state, which, in our case,
is an arbitrarily chosen configuration in which the molecule rests
flat on the surface.
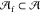 is the set of final states. In our case,
there are two types of final states. The first type is encountered
when the tip position s moves out of the allowed range
of z coordinates. The second type of final state
represents those states x* that belong to a different
anchor set from that of x.
is the set of final states. In our case,
there are two types of final states. The first type is encountered
when the tip position s moves out of the allowed range
of z coordinates. The second type of final state
represents those states x* that belong to a different
anchor set from that of x.
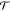 is the set of transitions
is the set of transitions
| 8 |
from a state x to a neighboring state x*, where an input from Σ1 is used in the state transition model S:x × Σ1 → x*. Note that it is impossible to have a transition τ = (x, x) after applying an input ad.
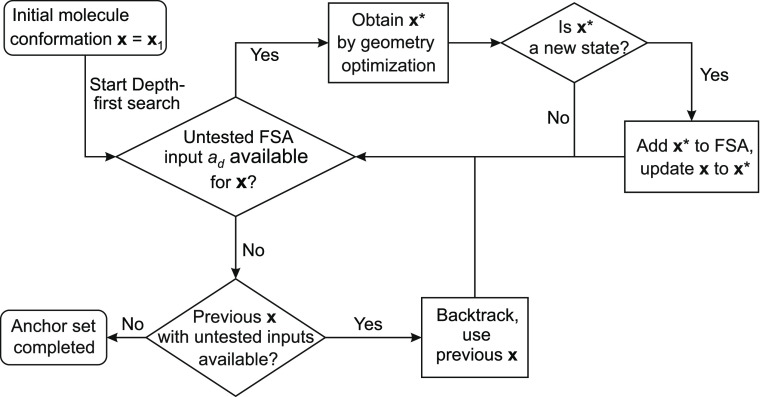
The algorithm builds the FSA and its underlying graph by sequentially applying all tip translation steps in a depth-first search44 (Figure 5), starting from state x1. Applying an input ad, the tip moves from s to s*. At s*, the respective molecular configuration r* is computed by geometry optimization with our molecular mechanics model, starting from the previous configuration r. The resulting tip–molecule configuration x* found in this way is added to the FSA (or the graph, respectively) as a new state. All eight tip translation steps ad are applied sequentially in this manner. Since there are several r and several inputs ad for any given s, each point s on the grid of tip positions must be visited multiple times. If a state is encountered which has been visited before (see Methods) and to which all possible inputs have already been applied the algorithm backtracks, that is, it continues with the last visited state that still has untested inputs. When all inputs to all states have been tested, the algorithm stops since the FSA is completed.
Figure 5.
Flowchart
of the algorithm that creates the FSA and its underlying
graph. This simplified scheme shows the creation of a single anchor
set 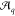 . Once the depth-first search is complete,
the algorithm will repeat with a new initial state x1 from the next anchor set.
. Once the depth-first search is complete,
the algorithm will repeat with a new initial state x1 from the next anchor set.
A final state 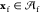 normally stops the algorithm, but in our
case, it only stops the current branch of the depth-first search through
state space, resulting in backtracking to a new branch or, ultimately,
in the completion of the FSA and its underlying graph. Whenever a
final state of the second type, i.e., a state in a different anchor
set, is encountered, the algorithm not only backtracks but also stores
the new state x* to a separate branch of the depth-first
search at the root level. Only after the current anchor set
normally stops the algorithm, but in our
case, it only stops the current branch of the depth-first search through
state space, resulting in backtracking to a new branch or, ultimately,
in the completion of the FSA and its underlying graph. Whenever a
final state of the second type, i.e., a state in a different anchor
set, is encountered, the algorithm not only backtracks but also stores
the new state x* to a separate branch of the depth-first
search at the root level. Only after the current anchor set 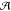 q has been completed,
the algorithm continues with the states x* that generate
new anchor sets, thus filling all anchor sets
q has been completed,
the algorithm continues with the states x* that generate
new anchor sets, thus filling all anchor sets  sequentially. For these states x*, the FSA also stores additional information on the translation
and rotation from the anchor set
sequentially. For these states x*, the FSA also stores additional information on the translation
and rotation from the anchor set  to the anchor set
to the anchor set 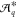 , as this is required to transform the tip
position s relative to the old anchor to the tip position s* relative to the new anchor. Hence, the second type of final
states yields information on how and where in s space
the various anchor sets are connected and which transformations switch
between them.
, as this is required to transform the tip
position s relative to the old anchor to the tip position s* relative to the new anchor. Hence, the second type of final
states yields information on how and where in s space
the various anchor sets are connected and which transformations switch
between them.
The obtained FSA (graph) for PTCDA on Au(111)
contains a total
of 504.432 states x in three anchor sets (branches) and
4.029.635 transitions τ. The tip
positions of all states are displayed in Figure 6a for each anchor set separately. The arch-like structure
of these positions arises because, in all configurations, the lower
oxygen atoms of the molecule bind to their two respective anchor atoms,
which thus act like hinges (cf. Figures 2 and 7). As a consequence,
the arch of 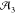 is rotated by 30° compared to
is rotated by 30° compared to 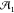 and
and 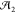 because the respective anchor is rotated
likewise (see colored anchor atoms in Figure 6a). The spatial relations between tip positions,
anchor atoms, and their reachable areas are depicted in Figure 6b for the tip height sz = 10 Å. An example of
a final state
because the respective anchor is rotated
likewise (see colored anchor atoms in Figure 6a). The spatial relations between tip positions,
anchor atoms, and their reachable areas are depicted in Figure 6b for the tip height sz = 10 Å. An example of
a final state 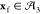 and the respective transition into the
anchor set
and the respective transition into the
anchor set 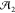 is visualized in Figure 6c.
is visualized in Figure 6c.
Figure 6.
Anchor sets. (a) Graphic representation of all
tip positions s in the three primary anchor sets 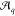 . The lateral positions of Au atoms are
displayed in yellow, with the anchor atoms in the corresponding color.
As exemplified in the upper panel, each point in the displayed cloud
of tip positions stands for a full configuration x of
the tip–molecule–surface junction. (b) Horizontal cut
through the s cloud of the three anchor sets at sz = 10 Å (the pink plane in panel (a)).
Au atoms are shown in yellow, and the reachable positions of the two
bottom Ocarb atoms (see Figure 4) are shown in the color of the respective
anchor set. An exemplary molecule in
. The lateral positions of Au atoms are
displayed in yellow, with the anchor atoms in the corresponding color.
As exemplified in the upper panel, each point in the displayed cloud
of tip positions stands for a full configuration x of
the tip–molecule–surface junction. (b) Horizontal cut
through the s cloud of the three anchor sets at sz = 10 Å (the pink plane in panel (a)).
Au atoms are shown in yellow, and the reachable positions of the two
bottom Ocarb atoms (see Figure 4) are shown in the color of the respective
anchor set. An exemplary molecule in 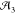 is drawn in black, with a red sphere marking
the corresponding tip position. (c) A tip displacement step (black
arrow) causes one anchor atom to change (white arrow in the inset,
length exaggerated for better visibility), such that the molecule
configuration moves from
is drawn in black, with a red sphere marking
the corresponding tip position. (c) A tip displacement step (black
arrow) causes one anchor atom to change (white arrow in the inset,
length exaggerated for better visibility), such that the molecule
configuration moves from 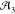 to
to 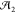 . Note that in panel (c), duplicate anchor
sets are displayed, which are rotated and translated with respect
to the primary anchor sets shown in panels (a) and (b).
. Note that in panel (c), duplicate anchor
sets are displayed, which are rotated and translated with respect
to the primary anchor sets shown in panels (a) and (b).
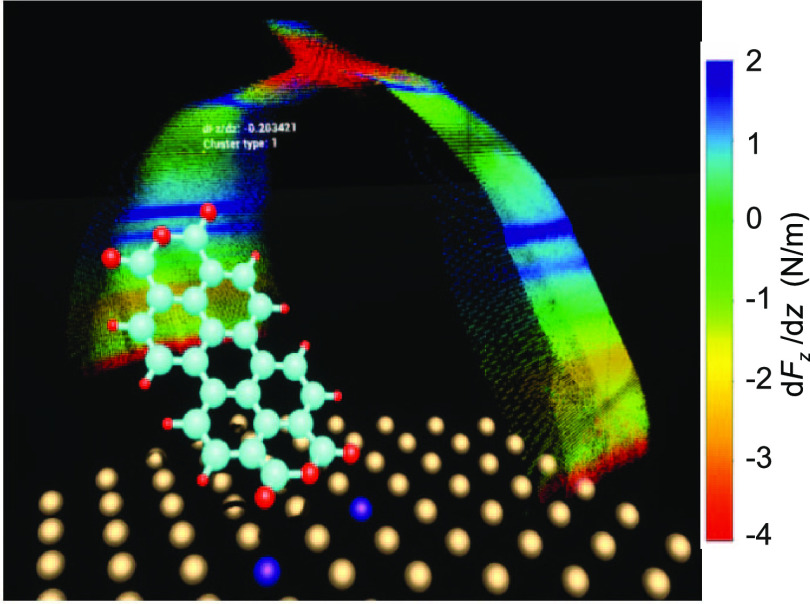
Figure 7.
Virtual reality representation of the FSA. Screenshot
of the custom-made
VR software that allows browsing the FSA and performing simulated
manipulations in an interactive manner. Each tip position in the selected
anchor set (here 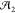 ) is represented by a dot, the color of
which encodes the force gradient in the respective configuration.
Motion capture of the operator hand is used to control the tip position s for which the molecular configuration r is
displayed. Anchor atoms are shown in violet.
) is represented by a dot, the color of
which encodes the force gradient in the respective configuration.
Motion capture of the operator hand is used to control the tip position s for which the molecular configuration r is
displayed. Anchor atoms are shown in violet.
Once the complete FSA has been built, it serves as our observation and state transition model. Providing a state x and a tip displacement step ad, we can retrieve the corresponding next state x* = S(x, ad) and its force gradient F′ = U(x*). The latter is obtained by taking the experimental SPM tip oscillation amplitude into account. By discretizing a given experimental tip trajectory into accepted input steps Δsj belonging to the input alphabet Σ1, we obtain a sequence of molecular configurations rj and force gradients Fj′ for the entire tip trajectory in a stepwise manner. Since this type of query is explicitly supported by the structure of the FSA, it only takes a millisecond to traverse a trajectory consisting of 100,000 states.
We have coupled the FSA to a fully immersive virtual reality display, which allows us to inspect the molecular configuration data interactively in three dimensions (Figure 7). Specifically, we can visualize simulated manipulation processes for which the input, i.e., the desired tip trajectory, is generated by a motion capture device that records the motion of human hands.23,45 This interactive exploration of molecular manipulation is crucial since it provides an extremely efficient way to access the large amount of information in the FSA. In this way, an intuition for the manipulation process itself can be developed effortlessly.
Information Gathering
In this section, we analyze the FSA regarding the aspect of information gathering. Above, we outlined a concept for calculating the average length K (eq 7) that an information-gathering trajectory must have in order to uniquely identify the initial state. Using eqs 6 and 7, we can compute K after extracting the required parameters from the FSA.
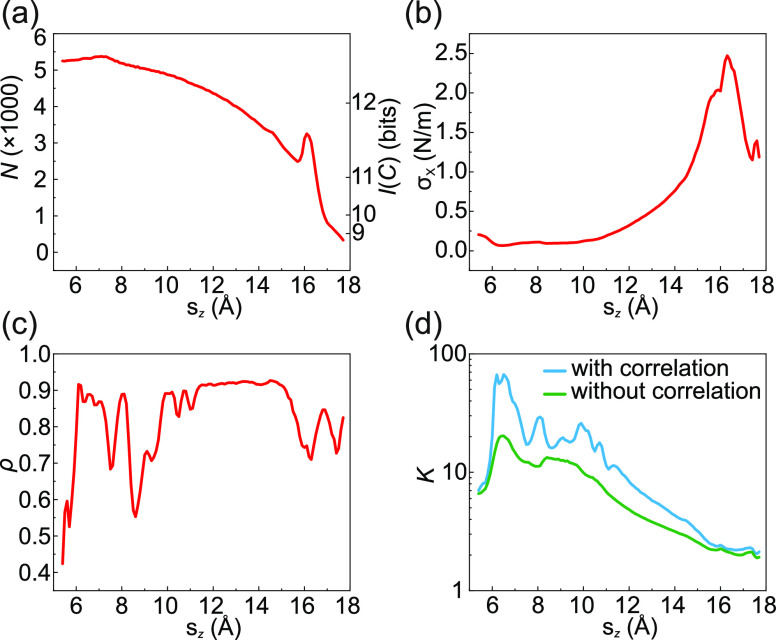
It is insightful to calculate K as a function of tip height instead of giving one number
for the entire configuration space 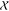 . From the FSA, we obtain both the standard
deviation σX(sz) of all F′ values and the number of configurations N(sz) in a 0.1 Å wide sz interval. Figure 8a reveals that the information required to
discern the configurations in a fixed interval around a given value
sz drops from 12.5 bits to 8.5 bits (8.5
nats to 6 nats) with increasing tip height. In parallel, the distribution
of possible F′ values as specified by σX becomes wider as sz increases
(Figure 8b). For the
limiting case of a sequence of uncorrelated F′ values along
the information-gathering trajectory, the average number of required
steps K = I(C)/I(X, Y) drops from 20
to 2 (green curve in Figure 8d).
. From the FSA, we obtain both the standard
deviation σX(sz) of all F′ values and the number of configurations N(sz) in a 0.1 Å wide sz interval. Figure 8a reveals that the information required to
discern the configurations in a fixed interval around a given value
sz drops from 12.5 bits to 8.5 bits (8.5
nats to 6 nats) with increasing tip height. In parallel, the distribution
of possible F′ values as specified by σX becomes wider as sz increases
(Figure 8b). For the
limiting case of a sequence of uncorrelated F′ values along
the information-gathering trajectory, the average number of required
steps K = I(C)/I(X, Y) drops from 20
to 2 (green curve in Figure 8d).
Figure 8.
Information gathering. (a) The number of configurations N and respective information content I(C) in Δsz = 0. 1 Å wide intervals. (b) Variance σX of the F′ values stored in the FSA. (c) Correlation ρ between the F′ values of all states between which a direct transition exists. (d) The approximate number of steps K that would be required to identify a molecular configuration unambiguously with correlation (blue curve) and without correlation (ρ(sz) = 0, green curve). All quantities are calculated for a series of sz intervals of 0.1 Å width.
While this result describes the tendency correctly, in practice, more steps will be required because the F′ values of neighboring states are typically correlated. As discussed above, we quantify this aspect via the Pearson correlation coefficient ρ, which is obtained from the data in the FSA (Figure 8c). Using eqs 6 and 7, we can now compute K(sz) for the more general case of correlated X values. The result is a strong increase in K compared to the case of uncorrelated X values (Figure 8d). Particularly at smaller sz values, up to 60 steps are now required for unambiguous identification of the molecular configuration. For large sz values, the effect becomes less dramatic since the variance σX increases and the correlation ρ drops. Having obtained an estimate for the length of a trajectory, the next question to address is that of reversibility.
Reversibility
Reversibility is crucial for information gathering since the manipulation process has to return the tip and the molecule to their original state x0 once its configuration has been identified. As mentioned above, we expect individual manipulation steps to be reversible if they occur within one and the same anchor set, that is, if the bottom Ocarb atoms do not change their Au bonding partner. Using the FSA, we are able to investigate reversibility at the fundamental level of FSA transitions τ, which represent individual tip displacements.
We classify each of the approximately 4 × 106 transitions τ in the FSA as either reversible or irreversible in the following way: a transition τ = (x, x*) is reversible if the transition τ′ = (x*, x) also exists in the FSA; otherwise, it is irreversible. As a result, we find that only 2.1% of all transitions are irreversible (Tab. 1). In addition, we label each transition τ = (x, x*) with respect to its connectivity as either an external transition, if it connects states x and x* in two different anchor sets (including symmetry equivalents), or as an internal transition, if it connects states within the same anchor set. Combining the two properties of connectivity and reversibility, as done in Table 1, we find that 99.8% of the internal transitions, but only approximately 75% of the external transitions, are reversible. Thus, we can conclude that a reconfiguration of the Au–Ocarb bonds at the lower end of the molecule indeed has a 25% chance of introducing irreversibility into the molecular manipulation process. In Figure 9, this has been visualized: the lateral tip positions (sx, sy) of states, which have at least one irreversible (outgoing) transition, are practically always located on the outer hull of their anchor set. Irreversibility within an anchor set (found for 0.2% of all internal transitions) can, for example, result from an abrupt inversion of the bending direction of PTCDA: while being concave when lifted (Figure 1a), it might bend away from the surface in a convex metastable configuration when lowered under tension from the vertical. Finally, we note that a different atomistic simulation approach may influence the precise percentages in Table 1 (see Methods), but the general conclusion regarding reversibility is expected to be robust.
Table 1. Percentage of FSA Transitions Which Belong to the Reversible/Irreversible and Internal/External (Connectivity) Categories.
| external | internal | |
|---|---|---|
| reversible | 5.6% | 92.3% |
| irreversible | 1.9% | 0.2% |
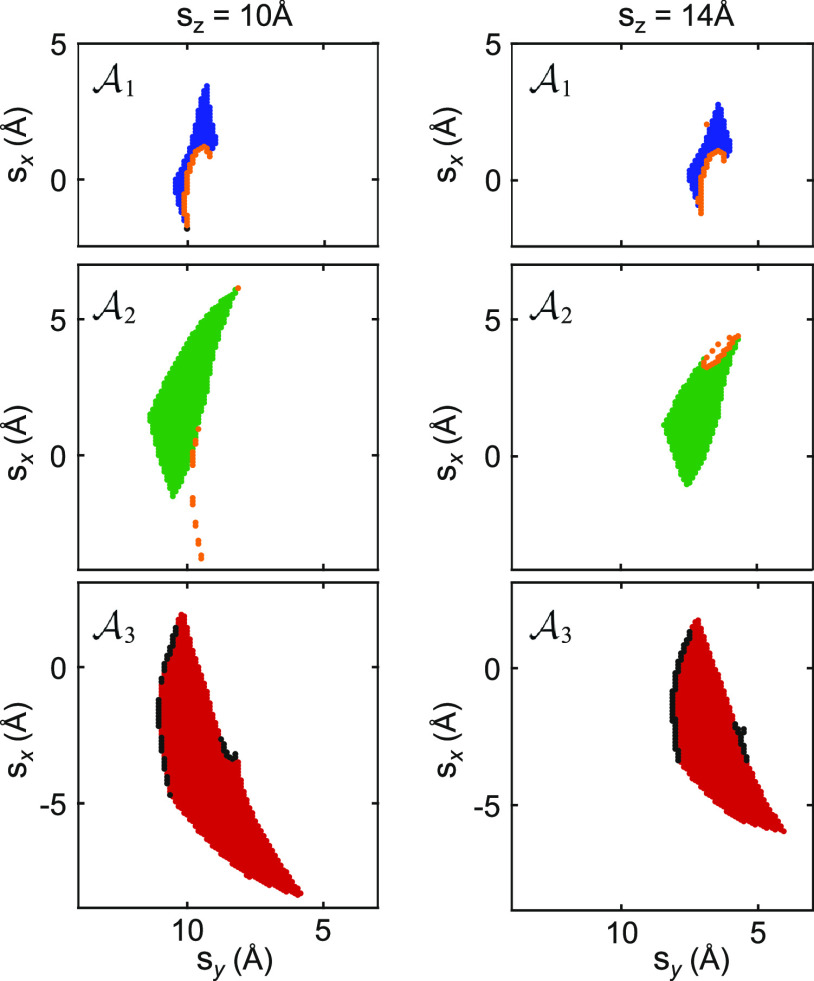
Figure 9.
Reversible and irreversible states. Horizontal cuts through
the
left branch of the s clouds of all three anchor sets
in Figure 6a at two
different heights sz. The tip positions
of states with at least one irreversible transition are colored orange
(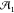 and
and 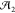 ) or black (
) or black (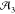 ). Isolated states (prominent in
). Isolated states (prominent in 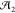 ) can only be reached from other (duplicate)
anchor sets (compare Figure 6c).
) can only be reached from other (duplicate)
anchor sets (compare Figure 6c).
With the results of Table 1 and Figure 9, we have obtained the simple rule of thumb that all states within a single anchor set can safely be visited for information gathering in order to identify a given initial state x0. Note, however, that as long as the boundaries of individual anchor sets are not known in the experiment, this rule is only of limited value. In the Supporting Information, we analyze the reversibility of full manipulation trajectories in a simulated experiment. We find that for the given molecule–surface system, the chances of returning to a state other than x0 increase at a rate of approximately 2.5% per Angstrom trajectory length. Information gathering must therefore be balanced between the amount of obtainable information and the risk of irreversibility, both of which increase with trajectory length.
Particle Filter
Objectives
With the basic functionality of the PF outlined above, we formulate the following two objectives for its practical application in configuration monitoring:
-
1.
In a manipulation process in which, starting from the initial configuration x0 with observation F0′, M action-observation pairs (Δsj–1, Fj) are recorded for j = 1, ···, M sequentially, we want to determine the best ad hoc estimate x̃j for the configuration xj (which yields the observation Fj′) at each step j of the trajectory.
-
2.
Further, we want to determine the consistent manipulation trajectory x̌j–1 with j = 1, ···, M + 1 that agrees best with F0′ and the entire sequence j = 1, ··· M of action-observation pairs (Δsj–1, Fj).
In the second objective, the term consistent means that
the sequence of configurations x̌0 ··· x̌M should evolve from the
initial configuration x̌0 as dictated
by the individual tip displacement steps Δsj–1, j = 1, ···, M, and the state transition
model S as x̌j = S(x̌j–1, Δsj–1). In contrast, the sequence x̃0 ··· x̃M of the best ad hoc configurations from objective
(1) does not have to fulfill this consistency requirement, such that
subsequent configurations x̃j–1 and x̃j do not necessarily have to be related at all. This distinction between x̃ and x̌ is necessitated by the
probabilistic nature of the PF (see below) and the fact that, given
the limited available information that is particularly sparse at the
beginning of the manipulation trajectory, several entirely different
regions in the configuration space 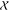 can provide good estimates for xj. Consequently, the
PF may locate x̃j–1 and x̃j in two different
regions of
can provide good estimates for xj. Consequently, the
PF may locate x̃j–1 and x̃j in two different
regions of 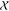 , between which Δsj–1 does not provide a
transition, i.e., x̃j ≠ S(x̃j–1, Δsj–1), while for the consistent trajectory,
we enforce a sequence of configurations x̌j which (in the spirit of eq 5) follows deterministically from x̌0 and the experimental tip translation
steps Δsj–1. Specifically, when determining the consistent trajectory,
all measured data points F0′, ··· FM are taken into account on an equal footing, such that
even the value FM′ obtained at the very end
of the manipulation process contributes to the determination of the
configuration x̌0 at its very beginning.
, between which Δsj–1 does not provide a
transition, i.e., x̃j ≠ S(x̃j–1, Δsj–1), while for the consistent trajectory,
we enforce a sequence of configurations x̌j which (in the spirit of eq 5) follows deterministically from x̌0 and the experimental tip translation
steps Δsj–1. Specifically, when determining the consistent trajectory,
all measured data points F0′, ··· FM are taken into account on an equal footing, such that
even the value FM′ obtained at the very end
of the manipulation process contributes to the determination of the
configuration x̌0 at its very beginning.
Since the above objectives exceed the typical functionality of a PF, we will first describe the basic operation principle and algorithm of a PF and subsequently discuss the additionally required modifications to achieve configuration monitoring.
Operation Principle
The PF neither provides a probability
distribution across all states in 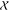 nor a single most promising state estimate x̃j, but a set of G more or less likely estimates of the current experimental
configuration xj. These estimates are the set of particles
nor a single most promising state estimate x̃j, but a set of G more or less likely estimates of the current experimental
configuration xj. These estimates are the set of particles  . At each step j–1
of the PF, the prior information gained from F0′ and all
action-observation pairs (Δs0, F1), ···, (Δsj–2, Fj–1′) is contained exclusively in the distribution
of these particles in
. At each step j–1
of the PF, the prior information gained from F0′ and all
action-observation pairs (Δs0, F1), ···, (Δsj–2, Fj–1′) is contained exclusively in the distribution
of these particles in 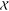 . To update the prior according to the action
Δsj–1 in step j, the entire particle cloud xl,j–1 is relocated
according to the state transition model to xl,j = S(xl,j–1, Δsj–1),
∀l. Then, the particles are redistributed
such that a higher particle density is created in regions of state
space where particles have just been evaluated comparatively positively
(Figure 10). In our
case, this means a good correspondence between simulated and measured
force gradient values Fl,j and Fj′ at this step. A high local density of the particle
cloud thus indicates a cluster of estimates which have performed particularly
well over the last couple of iterations. The rate at which particles
agglomerate, as well as other hyperparameters of the PF, have to be
adjusted to the problem at hand. The stepwise operation of the PF
is preceded by an initialization in which the G particles
are randomly placed in
. To update the prior according to the action
Δsj–1 in step j, the entire particle cloud xl,j–1 is relocated
according to the state transition model to xl,j = S(xl,j–1, Δsj–1),
∀l. Then, the particles are redistributed
such that a higher particle density is created in regions of state
space where particles have just been evaluated comparatively positively
(Figure 10). In our
case, this means a good correspondence between simulated and measured
force gradient values Fl,j and Fj′ at this step. A high local density of the particle
cloud thus indicates a cluster of estimates which have performed particularly
well over the last couple of iterations. The rate at which particles
agglomerate, as well as other hyperparameters of the PF, have to be
adjusted to the problem at hand. The stepwise operation of the PF
is preceded by an initialization in which the G particles
are randomly placed in 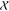 . If available, prior knowledge, such as
an approximate height of the tip or an approximate azimuthal angle
of the molecule in the experiment, can be included by biasing the
initial distribution of sampling points (particles) accordingly.
. If available, prior knowledge, such as
an approximate height of the tip or an approximate azimuthal angle
of the molecule in the experiment, can be included by biasing the
initial distribution of sampling points (particles) accordingly.
Figure 10.
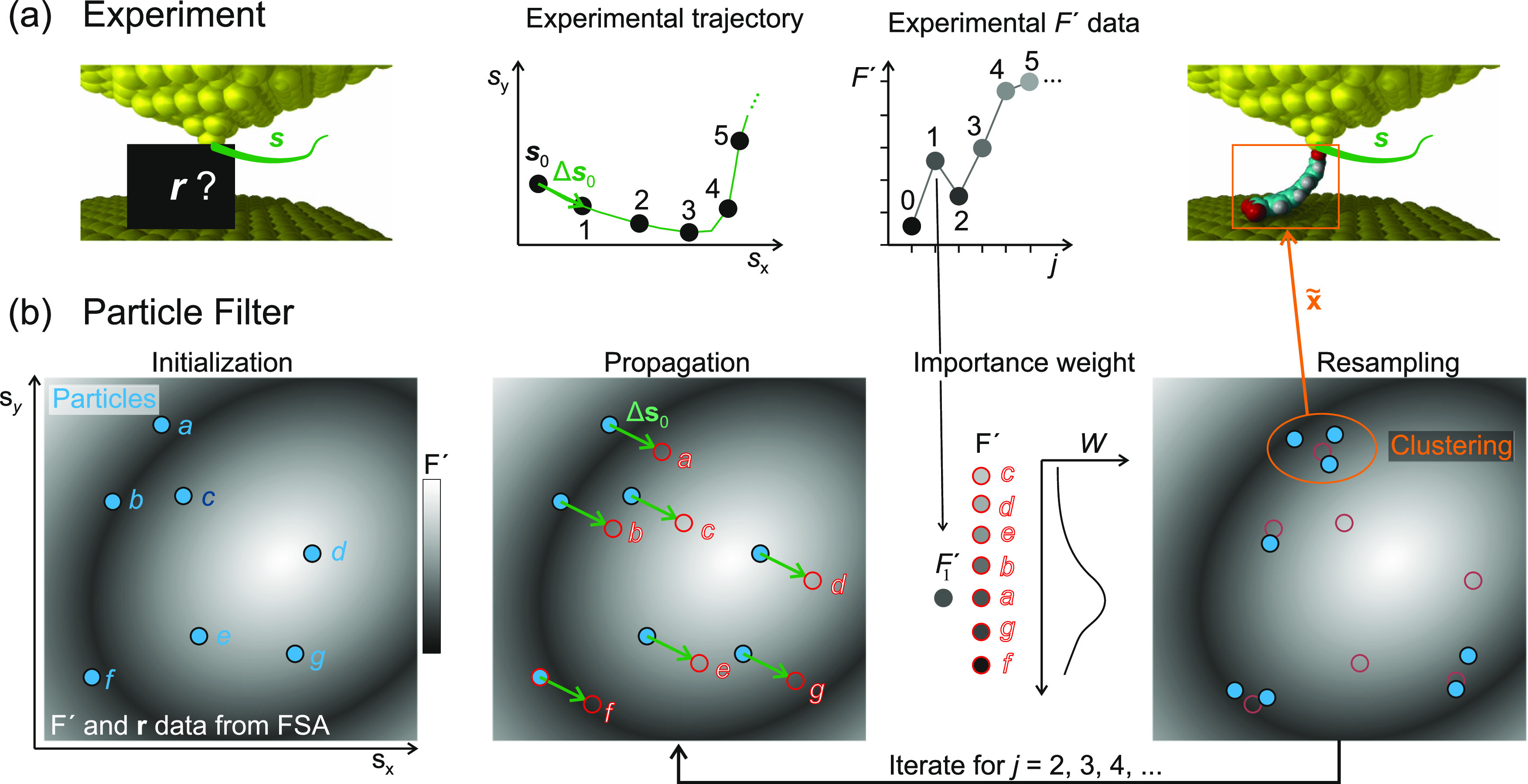
Operation
principle of the particle filter. (a) Experiment. Starting
from an unknown molecule configuration r0, the tip is moved along a trajectory sj–1, j = 1, ···, 5 in the x,y plane (green), and force gradients F′ (sj–1) are
recorded. (b) Particle filter. Initialization. Particles (G = 7) are dispersed in  at random tip–molecule configurations xl, l = 1, ···,
7 (blue). The gray background symbolizes the observation model F′
= U(x) stored in the FSA. (1) Propagation.
All particles are displaced according to the experimental tip displacement
step Δs0 and the state
transition model as xl,1 = S(xl,0, Δs0). Synthetic noise in the displacement
is omitted here. Each particle l has a distinct Fl′ (background greyscale). (2) Importance weight. According to the
agreement between their Fl value and the experimental F′, the particles receive individual importance weights Wl (eq 9). (3) Resampling. All particles are randomly
relocated to the proximity of previous particle locations (faint red),
favoring the original locations of particles with high Wl (here: particles a, b, g, and f).
Exploration places a fraction ϵ of the particles in completely
random locations (not shown). (4) Clustering. Regions with high particle
density are identified because they represent the PF’s best
estimates of the actual molecular configuration r1, which is the property of interest. The PF
will iterate through steps (1)–(3) for j =
2, 3, ···, converging the particle locations further
onto good configuration estimates for xj. Step (4) is only required when an
ad hoc conformation estimate x̃ is requested.
at random tip–molecule configurations xl, l = 1, ···,
7 (blue). The gray background symbolizes the observation model F′
= U(x) stored in the FSA. (1) Propagation.
All particles are displaced according to the experimental tip displacement
step Δs0 and the state
transition model as xl,1 = S(xl,0, Δs0). Synthetic noise in the displacement
is omitted here. Each particle l has a distinct Fl′ (background greyscale). (2) Importance weight. According to the
agreement between their Fl value and the experimental F′, the particles receive individual importance weights Wl (eq 9). (3) Resampling. All particles are randomly
relocated to the proximity of previous particle locations (faint red),
favoring the original locations of particles with high Wl (here: particles a, b, g, and f).
Exploration places a fraction ϵ of the particles in completely
random locations (not shown). (4) Clustering. Regions with high particle
density are identified because they represent the PF’s best
estimates of the actual molecular configuration r1, which is the property of interest. The PF
will iterate through steps (1)–(3) for j =
2, 3, ···, converging the particle locations further
onto good configuration estimates for xj. Step (4) is only required when an
ad hoc conformation estimate x̃ is requested.
Algorithm
In detail, at each step j, the PF performs the following three actions to update its estimate of the molecule configuration xj in the experiment (Figure 10):
(1) Propagation. The particles are propagated using the tip translation step Δsj–1 and the state transition model stored in the FSA. This propagation forces each particle to replicate the movement of the tip and to transform its configuration from xl,j–1 to xl,j accordingly (see above). If one of the particles xl,j–1 indeed matched the experimental molecular configuration xj–1, the propagation step ensures that also xl,j and xj match. If the length of the tip translation step Δsj–1 exceeds the nearest-neighbor distance in the dense discretization grid of the FSA, xl,j–1 and xl,j will be FSA states between which no direct (one-step) transition exists. In this case, a path-finding algorithm46 is used to calculate the required intermediate transitions. Subsequently, each particle receives an additional random displacement which scales with the length of Δsj–1 but never exceeds three FSA transitions. This randomization accounts for the fact that a certain degree of stochasticity is present in the experiment, even when performed at cryogenic temperatures.
(2) Importance weight. The second step is the calculation of an importance weight Wl,j for each particle l. It quantifies how well the simulated Fl,j′ value of the particle matches the measured value Fj at this step. As discussed above, the importance weight approximates P(xl,j|Fj′) in eq 5, defined as the conditional probability that the configuration is xl,j if Fj is measured. Here, it is assumed that the probability that the specific configuration xl,j of particle l matches the actual configuration xj in the experiment increases with the importance weight Wl,j of xl,j. We calculate the importance weight as
| 9 |
where C is the constant importance weight factor, one of the hyperparameters of the PF.
(3) Resampling. Finally, the particles are redistributed.
A small
fraction ϵ ≪ 1 of all particles is randomly placed in  , with the exploration factor ϵ being
a hyperparameter of the PF. All other (1 – ϵ) G particles are relocated into the proximity of states for
which large importance weights have been computed in (2). Since placement
probabilities scale with the respective importance weights Wl,j of each
location (see Methods), configurations with
high Wl,j will accumulate particles in their vicinity, that is, within a (randomly
chosen) distance of up to three neighboring FSA states for the purpose
of exploration. This updates the points at which the configuration
space
, with the exploration factor ϵ being
a hyperparameter of the PF. All other (1 – ϵ) G particles are relocated into the proximity of states for
which large importance weights have been computed in (2). Since placement
probabilities scale with the respective importance weights Wl,j of each
location (see Methods), configurations with
high Wl,j will accumulate particles in their vicinity, that is, within a (randomly
chosen) distance of up to three neighboring FSA states for the purpose
of exploration. This updates the points at which the configuration
space  is sampled in the following trajectory
step j + 1. The agglomeration of particles in promising
regions of the configuration space updates the prior information for
this following step, similar in spirit to the repeated use of eq 4 (or a single evaluation
of eq 5).
is sampled in the following trajectory
step j + 1. The agglomeration of particles in promising
regions of the configuration space updates the prior information for
this following step, similar in spirit to the repeated use of eq 4 (or a single evaluation
of eq 5).
Three hyperparameters determine the behavior of the PF:
The number of particles G used for sampling the configuration space
 . More particles result in a higher chance
of finding a configuration that matches the unknown configuration
in the experiment.
. More particles result in a higher chance
of finding a configuration that matches the unknown configuration
in the experiment.The exploration factor ϵ ≪ 1 defines the fraction of particles that are randomly initialized during each step instead of being relocated to the vicinity of a particle position from the previous generation of particles.
The importance weight factor C is a multiplier in the importance weight function (eq 9), which is used to control the convergence of the PF. Large C values mean fast convergence onto the most promising particle positions.
Configuration Estimation with the PF
The iterative
procedure described above covers the basic functionality of the PF.
However, to obtain an estimate of the current most likely configuration x̃j, as desired for objective
(1) above, further steps are required. To obtain x̃j, we have to identify regions with a
particularly high particle density. Since particles with large (relative)
importance weight Wl attract
more particles to their vicinity, a considerable number of particles
will accumulate in regions of 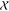 where they maintain a large Wl over several PF iterations. Unlike
any single particle which happens to have the largest Wl,j in a particular
step j, such clusters indicate groups of particles
with a high prior probability, that is, particles that have performed
particularly well in the past and that will typically be very similar
in terms of their tip–molecule configurations x and force gradients F′. In this case, the entire cluster
represents a single good estimate of the experimental molecular configuration xj.
where they maintain a large Wl over several PF iterations. Unlike
any single particle which happens to have the largest Wl,j in a particular
step j, such clusters indicate groups of particles
with a high prior probability, that is, particles that have performed
particularly well in the past and that will typically be very similar
in terms of their tip–molecule configurations x and force gradients F′. In this case, the entire cluster
represents a single good estimate of the experimental molecular configuration xj.
To
identify x̃j, we search,
at the respective step j, for the largest cluster
of particles in the entire particle distribution. To this end, we
use the K-medoid clustering technique28 (see Methods), which splits the complete set of particles  into K subsets
into K subsets  . Following the arguments above, we assume
the largest cluster, as a whole, to represent the best estimate for xj. Since a specific
configuration x̃j has
to be determined, we retrieve the medoid (center) particle as a representative
member of that cluster. The computational effort for the clustering
is quadratic in the number of particles G and, therefore,
typically more demanding than the plain PF, which scales linearly
with G. Hence, one can seek a trade-off between accuracy
(more particles) and frequent updates of the configuration estimate x̃ by clustering.
. Following the arguments above, we assume
the largest cluster, as a whole, to represent the best estimate for xj. Since a specific
configuration x̃j has
to be determined, we retrieve the medoid (center) particle as a representative
member of that cluster. The computational effort for the clustering
is quadratic in the number of particles G and, therefore,
typically more demanding than the plain PF, which scales linearly
with G. Hence, one can seek a trade-off between accuracy
(more particles) and frequent updates of the configuration estimate x̃ by clustering.
As pointed out above, configuration estimates x̃j–1 and x̃j of consecutive steps may turn out to be completely unrelated to each other, such that the sequence of all x̃j, with j = 0, ···, M will typically not represent a valid manipulation trajectory: the tip–molecule configuration x̃j instead varies discontinuously with j, as exemplified in Figure 12 and in two supplementary animations. As mentioned in objective (2) above, a solution to this problem is the computation of a consistent manipulation trajectory x̌j, j = 0, ··· M. We select promising consistent trajectories using a greedy algorithm inspired by beam search.47 Specifically, we calculate M consistent trajectories x̌j,γ, with γ = 1, ···, M, based on the previously found M ad hoc configurations x̃j, j = 1, ···, M, which are considered to be good configuration estimates. To this end, we propagate each x̃j forward and backward in time, using the state transition model and the sequence of tip steps Δs0, ···, ΔsM–1, such that each consistent manipulation trajectory by construction passes through one of the configurations x̃j; for the consistent trajectory γ, this crossing occurs at x̌γ,γ = x̃γ. Subsequently, we select the consistent trajectory that best matches the experimental F′ values. The respective deviation is computed over the entire length of each trajectory as
| 10 |
Based on their χ2 values, we can judge which of the underlying configurations x̃j was indeed a good estimate and which only performed well on a small segment of the entire set of action-observation pairs (Δsj–1, Fj′). Note that the best estimate for the consistent trajectory is not necessarily found by the PF close to γ = M because, due to simulation uncertainties, noise, and the stochasticity of its operation principle, the PF can, in spite of the correct propagation (1), loose a once found good estimate as the manipulation progresses. In the following, we will, for simplicity, denote the best consistent trajectory x̌j,γ with the lowest χγ as x̌j.
Figure 12.
Ad hoc and consistent trajectories. Example of the x̃j and x̌j configuration estimates in a PF run on a synthetic data set that starts at low sz values. Large spheres mark tip positions, thin lines connect subsequent s̃j points, and thick lines and small spheres visualize the azimuthal orientation of the molecule (see inset) for selected j values. The molecule (green) at the bottom of the main panel is drawn to scale.
Results of PF Application
We now continue with benchmarking the PF by applying it to synthetic as well as experimental data sets from refs (8, 17).
PF Performance on Synthetic Data
We first use synthetic (xj, Fj′) trajectories sampled from the FSA to assess the performance of the PF for various combinations of hyperparameters. Note that in this case, ground-truth molecular configurations rj are available. Since vertical tip trajectories with fixed (sx, sy) were used in the experiments that will be analyzed in the following section, we also limit the synthetic data to vertical tip trajectories. We assess the quality of each configuration estimate x̃j by comparing the actual tip positions, sj, with the predicted ones, s̃j, using the distance
| 11 |
as a measure of the PF performance (Figure 11). We note that the azimuthal angles of the molecule are implicitly also compared to the ground-truth data because sj and s̃j are defined relative to the mid-point between the two anchor atoms of the corresponding anchor, thus effectively including information on the azimuthal angle in the tip position.
Figure 11.
Grid search over PF parameters. The plot shows the average distance d (eq 11) between the predicted and the actual (ground truth) tip positions s̃j and s for PF runs with various combinations of C and ϵ.
To optimize the hyperparameters of the PF, we create a data set of 10 vertical tip trajectories sampled from the FSA at random lateral tip positions (sx, sy) and random azimuthal orientations of the molecule. We introduce noise to the synthetic F′ data by randomly offsetting the tip at each point of the trajectory by up to three steps in every direction of the FSA (that is, by about 0.3 Å) and choosing the F′ value at this location. While the number of particles (G = 1500) that we employ is dictated by the required speed of the PF, the exploration rate ϵ and the importance weight factor C can be chosen freely. We explore these parameters in a grid search with the values ϵ ∈ {0.01, 0.02, 0.05, 0.07, 0.1, 0.2, 0.3} and C ∈ {0.25, 0.5, 0.75, 1, 2, 4, 6, 7, 8, 10}. Since the PF is stochastic in nature, we have performed 10 PF runs with different random initializations on each vertical trajectory, resulting in a total of 100 different tests for each of the 70 different parameter combinations.
The result of the performance evaluation is displayed in Figure 11, where we plotted the distance d (eq 11) averaged over all M points of each trajectory and over the 100 PF runs at each (ϵ, C) combination. It shows that the PF performs poorly if set to slow convergence and high exploration. In this regime, the particles remain almost entirely randomly distributed throughout all steps of the PF. This renders the outcome of the K-medoid clustering and thereby x̃ completely arbitrary, which explains the poor PF performance. In contrast, for C ≥ 5 and ϵ ≤ 0.1, the PF performs well. For the application of the PF to experimental data, we, therefore, select C = 5 and ϵ = 0.05, which are values well inside the range of our grid search (Figure 11).
In Figure 12, we visualize the qualitative difference
between the two configuration estimates x̃j and x̌j, using one of the synthetic data sets as an example. As expected,
the tip position s̃ and, with it, the azimuthal
molecular orientation often jump erratically between subsequent configurations x̃j and x̃j+1, particularly in the first half of
the trajectory (sz ≲
11 Å). In contrast, the consistent trajectory exhibits a constant
lateral tip position and a molecular orientation that evolves smoothly.
In fact, the consistent trajectory x̌ matches the
ground-truth configuration x perfectly,
which was therefore omitted from the plot. Finally, the plot shows
that the ad hoc estimates x̃ improve substantially
and become more consistent during the manipulation process; this is
to be expected as more F′ data becomes available
and the particles have the chance to cluster in configuration space 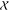 . The analysis of information gathering
above revealed that 20–60 steps are required to identify a
configuration unambiguously at sz < 10 Å, while (on average) only 2 steps are required
at large tip height. This is consistent with the better performance
of the FSA at larger sz in Figure 12.
. The analysis of information gathering
above revealed that 20–60 steps are required to identify a
configuration unambiguously at sz < 10 Å, while (on average) only 2 steps are required
at large tip height. This is consistent with the better performance
of the FSA at larger sz in Figure 12.
The speed of the PF can be tuned in a wide range
by adjusting the
number of particles G and the frequency of K-medoid
clustering, which requires 90% of the computational effort at G = 1500. In the present example, we clustered after each
PF step. This, however, would usually not be necessary. In a plausible
scenario, one could perform clustering steps less frequently and instead
increase the number of particles, thus keeping the PF speed constant.
More particles allow a denser sampling of 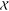 , which would, on average, improve the quality
of the obtained configuration estimates and reduce the random scattering
of the quality between individual PF runs (see also Figures 13a and 14a). On the downside, however, this would also reduce the frequency
of explicit ad hoc configuration estimates x̃ (by
clustering) and thus also the number of configurations from which
a consistent trajectory x̌ can be constructed.
, which would, on average, improve the quality
of the obtained configuration estimates and reduce the random scattering
of the quality between individual PF runs (see also Figures 13a and 14a). On the downside, however, this would also reduce the frequency
of explicit ad hoc configuration estimates x̃ (by
clustering) and thus also the number of configurations from which
a consistent trajectory x̌ can be constructed.
Figure 13.

Ex-post configuration monitoring of experiment A. (a) Deviation χ2 (eq 10) between F′ (sz) on the one hand and F̌j′ (blue) or F̃j (orange) on the other, for 50 PF runs. The χ2 value for the best possible consistent trajectory (brute-force search) is shown in green. Blue and orange stars mark χ2 values for the PF run with the median χ2 value for F̌′(sz). The black star marks the run with the lowest value for F̌′(sz). (b)–(e) Comparison of the experimental F′(sz) curve (gray) and exemplary PF results. Panels (b) and (c) show F̃j′ and F̌j curves with the median χ2 value from panel (a). Panels (d) and (e) show F̂j′ compared to the experiment and to the best F̌j curve found by the PF [black star in panel (a)]. (f) The plot of predicted tip height for the ad hoc and the consistent PF results, s̃z,j and šz,j. (g) Traces of the two bottom Ocarb atoms of the brute-force trajectory x̂j (green) and the best consistent trajectory x̌j (blue) found by the PF. Initial positions are color-coded in pink, and subsequent positions at specific sz heights are in red. The lateral tip position is marked by an encircled cross (black), and Ocarb atom jumps related to specific features in the F′(sz) curves (see the text) are marked by black arrows.
Figure 14.

Ex-post configuration monitoring of experiment B. For a detailed description of the panels, see Figure 13. (a) The χ2 plot for F̌j′ (blue) and F̃j (orange). (b)–(e) Comparison of F′(sz) from experiment B to F̃j′, F̂j and F̌j′. (f) Predicted tip heights s̃z, and šz. (g) Traces of both bottom Ocarb atoms of the brute-force trajectory x̂j and the best consistent trajectory x̌j.
Retrieving Molecule Configurations from Experimental Data
Having optimized its hyperparameters on synthetic data, which provide the ground truth as a benchmark, we now apply the PF to actual experiments. Specifically, we selected two vertical lifting experiments from ref (8). As Figure 1b reveals, these two experiments exhibit distinctly different F′(sz) curves. For each of the two, we perform and analyze 50 PF runs. Here, each run represents a possible outcome of a hypothetical scenario in which the PF is used in real time alongside the experiment. With G = 1500 particles, the speed requirements for such an application can indeed be met since the execution of a single PF step takes approximately 1.8 s. To offer an unbiased impression of the PF performance, we have not considered any prior information in our examples.
Testing the PF on experimental
data is conceptually different from tests on synthetic trajectories
because for experimental data, i.e., action-observation pairs (Δsj–1, Fj′), the ground-truth configurations xj are not known.
We can, therefore, only assess the capability of the PF to reproduce
the individual experimental Fj → F′(sz) curves as well as possible. Importantly, the capability of any
search method aimed at finding the correct experimental trajectory
is intrinsically limited by the observation and the state transition
models. If the models cannot reproduce parts of the measured F′(sz) data for any configuration  , no search algorithm will be able to match
the F′(sz) curve in its entirety. This turns out to be the case in the
examples discussed below.
, no search algorithm will be able to match
the F′(sz) curve in its entirety. This turns out to be the case in the
examples discussed below.
We determine the discrepancy between
the experimental and the simulated
force-gradient curves with the help of eq 10 and M = 96 individual tip
translation steps per curve. To obtain a reference against which the
PF can be benchmarked, we additionally perform an FSA-based brute-force
search across all N conceivable manipulation trajectories xi,0··· xi,M, i = 1 ··· N, each of which starts
at a different configuration  and follows the experimental tip displacement
steps Δs0···
ΔsM–1. Since the tip is retracted vertically in the experiments analyzed
here, those steps are simply Δsz translations of constant length. The brute-force
search requires 3.5 h, while a PF run takes about 3 min.
and follows the experimental tip displacement
steps Δs0···
ΔsM–1. Since the tip is retracted vertically in the experiments analyzed
here, those steps are simply Δsz translations of constant length. The brute-force
search requires 3.5 h, while a PF run takes about 3 min.
The results of the 50 PF runs for experiments A and B are summarized in Figures 13 and 14, respectively. Each PF run yields two sequences of configurations x̃j and x̌j, with j = 0, ··· 96, both of which have their associated F̃j′ and F̌j sequences. Examples of the latter are compared to the experimental F′ curve in panels (b) and(c), while some properties of the configurations x̃j and x̌j are presented in panels (f) and (g). Finally, the brute-force search yields a sequence x̂j with its associated F̂j′ (panels (d) and (e)). Figures 13a and 14a show that the χ2 values (eq 10) of both the F̃j and F̌j′ sequences scatter substantially, varying by almost a factor of four.
While only the best consistent trajectories x̌j (blue) come close to the χ2 values of the brute-force trajectories x̂j (green), it is remarkable that many of the χ2 values for the ad hoc configurations x̃j (orange) are smaller than the brute-force value, in particular for experiment B. This is a consequence of our specific molecular mechanics observation model, which cannot fully reproduce the measured F′(sz) curves. Hence, even the best possible consistent simulation (brute force) must have a χ2 well above zero. The sequence of ad hoc configurations x̃j, on the other hand, may also yield impossible manipulation trajectories in which both the tip height and the azimuthal molecular orientation jump discontinuously. This becomes clear when the tip heights of one selected ad hoc and one selected consistent trajectory are compared in Figures 13f and 14f. By construction, šz,j increases by Δsz for each step j → j + 1, but s̃z,j exhibits several large jumps. Particularly for the (consistent) tip heights in the range 9.5 < sz < 11.5 Å (15 < j < 35), the simulation is apparently not capable of reproducing the measured F′(sz), which are in fact close to zero in this region. The PF, therefore, returns configurations x̃j with tip heights s̃z,j ≥ 13 Å, for which F′ values close to zero are indeed possible in the simulation, but disregarding the fact that these values of sz,j are incompatible with the overall manipulation process. It is likely that this effect contributes to the substantial performance variations between different PF runs that we observe in Figures 13a and 14a because, tracing the entire F′(sz) curve, the best solutions were found in disparate particle clusters between which the PF had to jump. Importantly, this is no deficiency of the PF but expresses its expected (and desired) flexibility. This example, in retrospect, also confirms the necessity to extend the PF to the computation of the best consistent trajectory x̌j.
To offer a realistic impression of the average performance of the PF, we choose the PF run with the median χ2 value for F̌′(sz) and plot the obtained F̌j′ and F̃j sequence in panels (b) and (c) of Figures 13 and 14 (blue and orange stars). To illustrate also the best PF performance, the F̌j′ sequence with the lowest χ2 value (black star) is compared to the brute-force sequence F̂j in panel (e). For both experiments A and B, the comparisons show that F̌j′ sequences with low χ2 values capture all the characteristic features of the experimental F′(sz) curves to the same degree as they are reproduced in the brute-force solution. This is reassuring with regard to the real-time application of the PF for the control of a live experiment. In such a scenario, a brute-force search would not be possible.
In the final step of our analysis, we turn to salient features of the predicted tip–molecule configurations x̌j and x̂j themselves. As mentioned before, the positions of both the SPM tip and the lower Ocarb atoms relative to the surface lattice largely define the manipulation process. Therefore, we plotted the lateral paths of the two lower Ocarb atoms across the Au(111) surface in Figures 13g and 14g for x̂j (green) and x̌j (blue). For the latter, the trajectory with the lowest χ2 (black star) was chosen. In these plots, the Ocarb positions are shown as filled circles for each of the points j = 0, ···, M, with pink and red symbols representing reference positions at j = 0, 20, 40, 60, 80 that are also highlighted by markers in panel (e). The tip position is indicated by an encircled cross. As expected from the model of a nearly stiff molecule,10,18 the bottom oxygen atoms initially move slowly on the surface until the molecule is oriented almost vertically, whence a small change in the tip height leads to a strong lateral shift of the Ocarb positions. Our results also show that the Ocarb atoms prefer sites close to on-top positions, between which they tend to jump abruptly, particularly in the second half of the lifting process when the molecule is inclined substantially. These jumps are both a manifestation and a confirmation of the anchor concept that we employed in our FSA.
A careful analysis of the Ocarb trails on the surface proves the eminent role of local Au–Ocarb bonds. We exemplify this with the help of three prominent features in the experimental F′(sz) curves, namely, the sharp dip found in experiment A at sz = 15.8 Å, the small spike in experiment B at sz = 17.3 Å (marked by open arrows in Figures 13b and 14b, respectively), and the differences in height and shape of both main F′ peaks around sz = 16.7 Å. Since these features are also found in the respective F̂j′ (green) and F̌j (blue) sequences in panel (e), their origin can be analyzed on the basis of x̂j and x̌j—a first showcase of configuration monitoring as proposed in this work (albeit still ex-post). Configuration monitoring indeed reveals that both sharp dip and sharp spike are directly preceded by an abrupt relaxation of both Ocarb atoms together. The respective jumps are marked by black arrows in Figures 13g and 14g and in the F̌j′ and F̂j plots in Figures 13e and 14e; they are also visible in the visualizations of the lifting process (Supporting Information).
Unlike the spike and dip, the main peak at sz = 16.7 Å is a generic feature of the force gradient curves F′(sz) in all experiments in which the molecule is removed from the surface. Overall, the potential energy curve of the molecule on the surface exhibits two rounded steps to lower binding energies,10 one connected with overcoming the extended bond of the flat-lying molecule to the surface (i.e., peeling the molecule off the surface), the other associated with overcoming the local Ocarb–Au bonds at the bottom of the molecule (i.e., finally detaching the molecule from the surface). The main peak in F′(sz) occurs at the point of maximum potential curvature after the first plateau when, in the wake of breaking the extended bond, the attack on the local bonds begins. At the zero crossing of the F′(sz) curve to the right of the force gradient peak (around sz = 17.7 Å), the slope of the potential reaches its maximum, indicating that the Ocarb–Au bonds are essentially broken. Thus, the main peak in the force gradient curve at around sz = 16.7 Å is the signature of finally detaching the molecule from the surface. Its specific shape encodes how precisely this process proceeds down to the atomic level. We have in the past observed a great variability of its peak shape,16 and also, in the present experiments A and B, they look markedly different. In experiment B, the peak appears sharp, while in experiment A, it is broader, less intense, and more flat-topped (cf. also Figure 1b).
With the help of configuration monitoring, we can now chart these differences back to distinctive atomic configurations involving the Ocarb atoms. To this end, we concentrate on the terminal phase of their traces in Figures 13g and 14g, starting with the last pair of red symbols at sz = 16 Å. In this phase, the two bottom Ocarb atoms are detached sequentially from the surface, the last one being the one diagonally opposite the tip. The respective molecular configurations are shown in Figure 15, where they are projected onto the surface plane in panel (a) and onto a plane approximately aligned with the plane of the relevant vertical molecules [dotted line in panel (a)].
Figure 15.
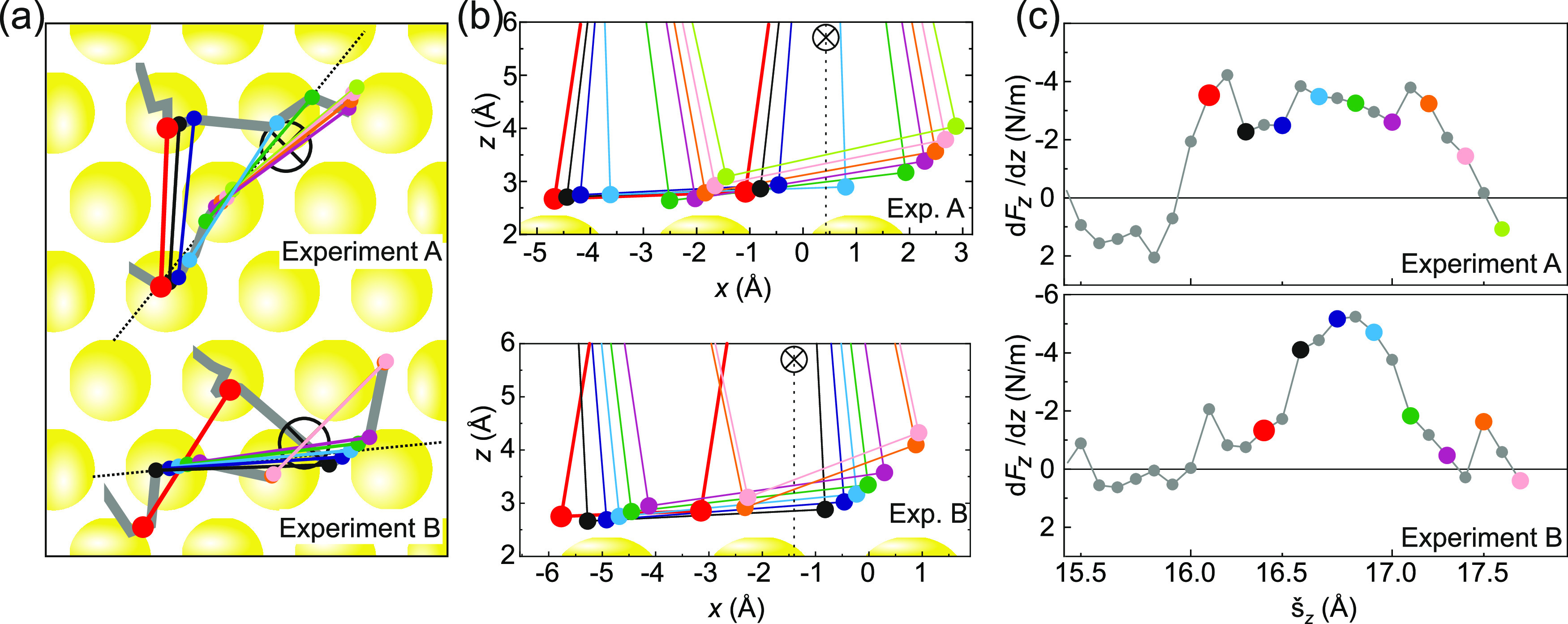
Configuration analysis of vertical molecule. (a) Lateral positions of the two bottom Ocarb atoms for every second x̌j in the interval 80 ≤ j ≤ 96 (cf. Figures 13g and 14g). The tip position is marked by an encircled cross. The dotted line marks the projection plane used in panel (b). (b) Projection of all PTCDA configurations marked in panel (a) onto a plane that is perpendicular to the surface plane and intersects it along the dotted line in panel (a). PTCDA is abstracted as a quadrangle formed by its four Ocarb atoms, where only the lower part is shown. The indicated Au atoms are the ones closest to the intersecting planes and likewise projected as is the tip position (dashed line). (c) The best F̌′ curves from Figures 13e and 14e, respectively. Every second F̌j′ value with 80 ≤ j ≤ 96 is marked using the color code from panels (a) and (b). Experiment B reaches the limit of the FSA z-range already at j = 94, such that the last two points are omitted.
In experiment A, the Ocarb atoms are in energetically favorable positions right on top of Au atoms when being detached. In the first three configurations in Figure 15 (red, black, dark blue), both Ocarb are bound to Au atoms which are rather far from the tip such that there is already a substantial stress on these bonds even though the molecule is not fully upright yet. Consequently, the main peak starts already at a tip height of sz = 16 Å. After a jump, the first Ocarb atom is detached from an on-top position (cyan), and after another jump of the molecular configuration, the second Ocarb atom is detached from an on-top position as well (violet, orange, rose, light green). This sequence of detachments from strongly bound locations leads to the wide peak of experiment A, as shown in Figure 15c.
For experiment B, the atomic mechanism is different. Here, the red configuration has only one Ocarb atom in an on-top position. After the first jump, the bottom Ocarb atom on the side of the tip is detached from a less favorable bridge position (black, dark blue, cyan). At this point, the second Ocarb atom diagonally opposite of the tip is still on top of a Au atom, but once its detachment starts, it moves away from that position (green, purple). Only at the very end of this process does the lower Ocarb jump again to the neighboring on-top position (orange, rose), leading to the small spike to the right of the main peak that was discussed above. The peak in experiment B is more intense since the detachment of the first Ocarb atom happens while the second one is forced to leave its favorable on-top position as well. In experiment A, on the other hand, this detachment happens while the second Ocarb atom moves into a favorable position.
We note that the two atomic configurations at the bottom end of the molecule in the vertical orientation represent two generic situations that are expected to occur in many lifting experiments. In our view, it is a remarkable success of configuration monitoring that, with nothing else than the two qualitatively different force gradient curves at hand, it has been possible to identify these generic atomic configurations and assign them consistently to either of the two curves.
However, our analysis also illustrates the limits of the configuration monitoring on the basis of force gradient data F′(sz). In spite of overall similarity, there clearly is a visible discrepancy between the x̂j and x̌j traces for z < 14 Å, both in experiments A and B (Figures 13g and 14g). Yet, a comparison of the corresponding Fj′ sequences in panel (e) reveals that there is only a very small difference between F̂j and F̌j′. We can thus conclude that it would barely be possible for the PF to distinguish between both configuration estimates based solely on the measured F′(sz) data in this region. This can in fact be understood as a consequence of the comparatively weak Ocarb–metal interaction on the Au(111) surface. Unlike on the Ag(111) surface, where the oxygen–metal bonds are relatively strong and therefore deform the flat-lying PTCDA molecule by reducing the Ag–Ocarb distance,39 on Au(111) flat-lying PTCDA remains undistorted, with a relatively large oxygen–metal distance. This will reduce the lateral corrugation felt by the Ocarb atoms, which in turn means a weak dependence of the potential energy landscape on the molecular configuration48 at small sz—hence the difficulty of the PF to differentiate between different xj. In contrast, at larger sz, the Ocarb atoms may approach the Au surface further because the entire molecule–surface vdW attraction is balanced only by the repulsion between the Ocarb atoms and the surface (Figure 1a). As a consequence, the potential energy landscape becomes more corrugated, and details of molecular configurations have a stronger impact on F′, as can be seen when comparing experiments A and B (Figure 1b).
Conclusions
The future utility of two-contact single-molecule manipulation depends heavily on our capability to monitor molecular configurations. This is true for both fundamental and applied research: known configurations substantially improve our ability to interpret, for example, charge transport experiments through single molecules, and they thoroughly upgrade our control over molecular nanofabrication processes. Here, we presented and benchmarked an approach that turns configuration monitoring into reality. It overcomes the most important challenges which in the past have proved to be showstoppers: the need for accurate yet fast simulations, the disparity between a scalar observation quantity and a high-dimensional unknown molecular configuration, the vast configuration space to be searched, and the need to operate on the few-minutes time scales of typical experiments. Our approach counters these challenges by combining a finite-state automaton (FSA) to store and rapidly access the results of atomistic molecular simulations and a particle filter (PF) to search for likely manipulation trajectories, given an input sequence of observations.
A particular strength of our concept is its generic nature, which allows for flexibility and modularity when applied in a wide range of scenarios: most notably, it works irrespective of the physical observable(s) that is (are) used for configuration monitoring. While the force gradient F′ is an obvious choice because it is frequently available in scanning probe microscopy instrumentation, the use of conductance, vibrational spectra, or any other observable with a link to the monitored configurations is also feasible if a corresponding observation model is available. Moreover, since the FSA allows separating the computation of the state transition and observation models from the configuration monitoring itself, the nature of these models is not restricted to a specific choice. While we utilized a tailor-made molecular mechanics simulation in this work, particularly promising future option are machine-learned models trained on high-quality DFT data. Strong research efforts are currently dedicated toward the development of such models; they promise an unmatched combination of accuracy and speed and might ultimately be able to run in real time in a supercomputing facility to react to unexpected events like chemical reactions. Finally, also the particle filter offers a high degree of flexibility. Its performance can be adjusted broadly to different application scenarios by choosing targeted hyperparameters, the most important one being the number of particles. Our specific implementation of the particle filter combines the capability to find good solutions in far-away regions of configuration space with the ability to find a consistent manipulation trajectory. In summary, we have developed and implemented a strategy that firmly establishes molecular configuration monitoring in real time with the limited computational resources typically available in a lab environment.49
Based on this advance, we have been able to assign systematic differences in generic features of a common observable (force gradient) to well-defined atomic configurations for the first time in an unbiased statistical evaluation of the experimental data against an exhaustive database of possible structures. This proves the opportunities for configuration monitoring. In the future, such information could provide a much-needed input in the field of molecular electronics, where the interpretation of conductance spectra would benefit enormously from an objective determination of the atomic structure of the molecule–metal interface.
An aspect that we have only briefly touched upon is the automation of molecular manipulation in a robotic manner.3,50,51 The concept of a partially observable Markov decision process (POMDP) includes autonomous decision-making in which the policy for selecting an action is based on the agent’s belief regarding the configuration of the molecule, which can be obtained from the particle filter. In the future, configuration monitoring as demonstrated here and decision-making can thus be integrated seamlessly, such that a learning agent takes over the role of the experimenter who, in turn, sets up the rewards which control the agent’s behavior, thereby steering it toward a desired target (manipulation goal). In the past, we have demonstrated this concept in combination with reinforcement learning, albeit operating on very sparse information about the configuration of the molecule.3 In synergy with configuration monitoring, such an approach would become much more versatile and efficient.
Acknowledgments
J.S., A.D., M.M., and C.W. acknowledge funding through the European Research Council (ERC-StG 757634 “CM3”). C.W., J.S., and K.-R.M. acknowledge funding by the Helmholtz Association (Helmholtz-AI project ZI-I-PF-5-29 “MomoNano”). F.S.T., C.W., and K.-R.M. acknowledge support from the Institute for Pure and Applied Mathematics (IPAM) at the UCLA (program “Understanding Many-Particle Systems with Machine Learning”), as well as helpful discussion with Marina Meila, University of Washington. F.S.T. acknowledges financial support from the Deutsche Forschungsgemeinschaft through SFB 1083 (project ID 223848855), project A12. This work was supported in part by the German Ministry for Education and Research under Grant Nos. 01IS14013A-E, 01GQ1115, 01GQ0850, 01IS18025A, 031L0207D, and 01IS18037A. K.-R.M. was partly supported by the Institute of Information & Communications Technology Planning & Evaluation (IITP) grants funded by the Korea government (MSIT) (No. 2019-0-00079, Artificial Intelligence Graduate School Program, Korea University and No. 2022-0-00984, Development of Artificial Intelligence Technology for Personalized Plug-and-Play Explanation and Verification of Explanation).
Appendix
Information Gathering
Above, we outline a universal formalism to calculate the average length of a successful information-gathering trajectory. In order to minimize the number of required parameters, we use a model in which the true signal X, the additive noise Z, and the actually measured value Y = X + Z are continuous, normally distributed random variables with zero mean. Since X, Y, and Z are continuously distributed, the mutual information I(X, Y) has to be determined from the differential entropy h of the respective distributions, which is defined for a function f(y) as38
| 12 |
Here, Y describes a distribution and y denotes individual values of the distributed variable. In our specific case where X and Z are uncorrelated, this mutual information is I(X, Y) = h(Y) – h(Z).38 The differential entropy of a normal distribution Y with standard deviation σY equals37
| 13 |
such that the information content of a single measurement becomes
| 14 |
Here, σY and σZ denote the standard deviations
of the distribution of (noisy) force gradients F′
and of the additive noise Z, respectively. Since Y is a sum over two uncorrelated normal distributions, σY can be expressed as 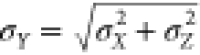 , where σX is a parameter obtained from the FSA (Figure 8b) and σZ is a parameter characteristic of the experimental setup. We find
a value of σZ = 0.04 N/m in our
data by analyzing F′(sz) curves with an empty junction.
, where σX is a parameter obtained from the FSA (Figure 8b) and σZ is a parameter characteristic of the experimental setup. We find
a value of σZ = 0.04 N/m in our
data by analyzing F′(sz) curves with an empty junction.
To incorporate the aspect of correlation into our formalism, we model the two true F′ values at neighboring tip positions, choosing i = 2 without loss of generality, as two normally distributed random variables X1 and X2 with a Pearson correlation coefficient ρ. To compute I(Y1, X2) in this more complex case, we use the relation38
| 15 |
where h(X2, Y1) is the differential entropy of the bivariate normal distribution (X2, Y1). This entropy is given by52
| 16 |
with |Σ| denoting the determinant of the covariance matrix of (X2, Y1)
| 17 |
The off-diagonal elements of Σ require the calculation of the covariance cov(X2, Y1), which is not known but can be obtained from known quantities as
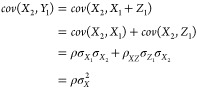 |
18 |
Here, we have taken advantage of the fact that the true signal X and the noise Z are uncorrelated, such that ρXZ = 0. The covariance matrix of (X2, Y1) is thus
| 19 |
Consequently, the expression for the differential entropy (eq 16) is
| 20 |
and the corresponding expression for the mutual information content is (eqs 13 and 15)
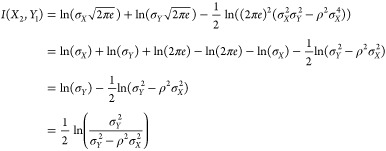 |
21 |
In the limit of ρ = 1, X1 and X2 are identical and, consequently, the simpler relation in eq 14 is obtained in this limit. Finally, the expression for the additional information ΔI(X2, Y2) = I(X2, Y2) – I(X2, Y1) gained from observing Y2 is
| 22 |
Having obtained this final result, the number of required steps can be computed as K = 1 + (I(C) – I(X1,Y1))/ΔI(X, Y) because the first measurement yields the full information I(X1,Y1), while all subsequent measurements only yield ΔI(Xi, Yi) = ΔI(X2, Y2).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpcc.3c02072.
Ad hoc, brute force, and consistent configuration estimates x̃, x̂, and x̌ of the PF change as the tip is lifted up (Video S1) (MP4)
Ad hoc, brute force, and consistent configuration estimates x̃, x̂, and x̌ of the PF change as the tip is lifted up (Video S2) (MP4)
Detailed analysis of the reversibility of manipulation trajectories, which is relevant for information gathering (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Green M. F. B.; Esat T.; Wagner C.; Leinen P.; Grötsch A.; Tautz F. S.; Temirov R. Patterning a Hydrogen-Bonded Molecular Monolayer with a Hand-Controlled Scanning Probe Microscope. Beilstein J. Nanotechnol. 2014, 5, 1926–1932. 10.3762/bjnano.5.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocić N.; Blank D.; Abufager P.; Lorente N.; Decurtins S.; Liu S. X.; Repp J. Implementing Functionality in Molecular Self-Assembled Monolayers. Nano Lett. 2019, 19, 2750–2757. 10.1021/acs.nanolett.8b03960. [DOI] [PubMed] [Google Scholar]
- Leinen P.; Esders M.; Schütt K. T.; Wagner C.; Müller K.-R.; Tautz F. S. Autonomous Robotic Nanofabrication with Reinforcement Learning. Sci. Adv. 2020, 6, eabb6987 10.1126/sciadv.abb6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q.; Ihle A.; Ahles S.; Wegner H. A.; Schirmeisen A.; Ebeling D. Constructing Covalent Organic Nanoarchitectures Molecule by Molecule via Scanning Probe Manipulation. Nat. Chem. 2021, 13, 1133–1139. 10.1038/s41557-021-00773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salapaka S. M. Scanning Probe Microscopy. IEEE Control Syst. Mag. 2008, 28, 65–83. 10.1109/MCS.2007.914688. [DOI] [Google Scholar]
- Temirov R.; Lassise A.; Anders F. B.; Tautz F. S. Kondo Effect by Controlled Cleavage of a Single-Molecule Contact. Nanotechnology 2008, 19, 065401. 10.1088/0957-4484/19/6/065401. [DOI] [PubMed] [Google Scholar]
- Custance O.; Perez R.; Morita S. Atomic Force Microscopy as a Tool for Atom Manipulation. Nat. Nanotechnol. 2009, 4, 803–810. 10.1038/nnano.2009.347. [DOI] [PubMed] [Google Scholar]
- Wagner C.; Fournier N.; Tautz F. S.; Temirov R. Measurement of the Binding Energies of the Organic-Metal Perylene-teracarboxylic-dianhydride/Au(111) Bonds by Molecular Manipulation Using an Atomic Force Microscope. Phys. Rev. Lett. 2012, 109, 076102. 10.1103/PhysRevLett.109.076102. [DOI] [PubMed] [Google Scholar]
- Findeisen R.; Grover M. A.; Wagner C.; Maiworm M.; Temirov R.; Tautz F. S.; Salapaka M. V.; Salapaka S.; Braatz R. D.; Moheimani S. O. R.. Control on a Molecular Scale: a Perspective, American Control Conference (ACC), Boston, MA, USA, 2016; pp 3069–3082.
- Wagner C.; Temirov R.; Tautz F. S.. Perspectives of Molecular Manipulation and Fabrication. In Molecular Architectonics The Third Stage of Single Molecule Electronics, Ogawa T., Ed.; Springer Nature: Cham, Switzerland, 2017; pp 253–319. [Google Scholar]
- Eigler D. M.; Lutz C. P.; Rudge W. E. An Atomic Switch Realized with the Scanning Tunnelling Microscope. Nature 1991, 352, 600–603. 10.1038/352600a0. [DOI] [Google Scholar]
- Jung T. A.; Schlittler R. R.; Gimzewski J. K.; Tang H.; Joachim C. Controlled Room-Temperature Positioning of Individual Molecules: Molecular Flexure and Motion. Science 1996, 271, 181–184. 10.1126/science.271.5246.181. [DOI] [Google Scholar]
- Weiss P. S. A Molecular Four-Wheel Drive. Nature 2011, 479, 187–188. 10.1038/479187a. [DOI] [PubMed] [Google Scholar]
- Khajetoorians A. A.; Wegner D.; Otte A. F.; Swart I. Creating Designer Quantum States of Matter Atom-by-Atom. Nat. Rev. Phys. 2019, 1, 703–715. 10.1038/s42254-019-0108-5. [DOI] [Google Scholar]
- Tewari S.; Bakermans J.; Wagner C.; Galli F.; van Ruitenbeek J. M. Intuitive Human Interface to a Scanning Tunnelling Microscope: Observation of Parity Oscillations for a Single Atomic Chain. Beilstein J. Nanotechnol. 2019, 10, 337–348. 10.3762/bjnano.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier N.; Wagner C.; Weiss C.; Temirov R.; Tautz F. S. Force-Controlled Lifting of Molecular Wires. Phys. Rev. B 2011, 84, 035435. 10.1103/PhysRevB.84.035435. [DOI] [Google Scholar]
- Wagner C.; Fournier N.; Ruiz V. G.; Li C.; Müllen K.; Rohlfing M.; Tkatchenko A.; Temirov R.; Tautz F. S. Non-Additivity of Molecule-Surface van der Waals Potentials From Force Measurements. Nat. Commun. 2014, 5, 5568 10.1038/ncomms6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C.; Fournier N.; Tautz F. S.; Temirov R. The Role of Surface Corrugation and Tip Oscillation in Single-Molecule Manipulation with a Non-Contact Atomic Force Microscope. Beilstein J. Nanotechnol. 2014, 5, 202–209. 10.3762/bjnano.5.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esat T.; Friedrich N.; Tautz F. S.; Temirov R. A Standing Molecule as a Single-Electron Field Emitter. Nature 2018, 558, 573–576. 10.1038/s41586-018-0223-y. [DOI] [PubMed] [Google Scholar]
- Knol M.; Arefi H. H.; Corken D.; Gardner J.; Tautz F. S.; Maurer R. J.; Wagner C. The Stabilization Potential of a Standing Molecule. Sci. Adv. 2021, 7, eabj9751 10.1126/sciadv.abj9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon N.; Salmond D.; Smith A. Novel Approach to Nonlinear/Non-Gaussian Bayesian State Estimation. IEEE Proc. F 1993, 140, 107–113. 10.1049/ip-f-2.1993.0015. [DOI] [Google Scholar]
- Giessibl F. J. The qPlus Sensor, a Powerful Core for the Atomic Force Microscope. Rev. Sci. Instrum. 2019, 90, 011101. 10.1063/1.5052264. [DOI] [PubMed] [Google Scholar]
- Leinen P.; Green M. F. B.; Esat T.; Wagner C.; Tautz F. S.; Temirov R. Virtual Reality Visual Feedback for Hand-Controlled Scanning Probe Microscopy Manipulation of Single Molecules. Beilstein J. Nanotechnol. 2015, 6, 2148–2153. 10.3762/bjnano.6.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temirov R.; Green M. F. B.; Friedrich N.; Leinen P.; Esat T.; Chmielniak P.; Sarwar S.; Rawson J.; Kögerler P.; Wagner C.; et al. Molecular Model of a Quantum Dot Beyond the Constant Interaction Approximation. Phys. Rev. Lett. 2018, 120, 206801. 10.1103/PhysRevLett.120.206801. [DOI] [PubMed] [Google Scholar]
- Ruiz V. G.; Wagner C.; Maaß F.; Arefi H. H.; Stremlau S.; Tegeder P.; Tautz F. S.; Tkatchenko A.. Accurate Quantification of the Stability of the Perylene-tetracarboxylic dianhydride on Au(111) Molecule-Surface Interface Commun. Chem. 2023, 6, 136. [DOI] [PMC free article] [PubMed]
- Arefi H. H.; Corken D.; Tautz F. S.; Maurer R. J.; Wagner C. Design Principles for Metastable Standing Molecules. J. Phys. Chem. C 2022, 126, 6880–6891. 10.1021/acs.jpcc.2c01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipowski A.; Lipowska D. Roulette-Wheel Selection via Stochastic Acceptance. Phys. A 2012, 391, 2193–2196. 10.1016/j.physa.2011.12.004. [DOI] [Google Scholar]
- Kaufman L.; Peter J.. Statistical Data Analysis Based on the L1-Norm and Related Methods, Dodge Y., Ed.; North Holland: Amsterdam, 1987; pp 405–416. [Google Scholar]
- Bai H.; Hsu D.; Lee W. S.; Ngo V. A.. Algorithmic Foundations of Robotics IX—Selected Contributions of the Ninth International Workshop on the Algorithmic Foundations of Robotics. In Springer Tracts in Advanced Robotics; Hsu D.; Isler V.; Latombe J.-C.; Lin M. C., Eds.; Springer-Verlag: Berlin, Heidelberg, 2010; Vol. 68, pp 175–191. [Google Scholar]
- Silver D.; Veness J.. Monte-Carlo Planning in Large POMDPs. In Advances in Neural Information Processing Systems 23; Vancouver, Canada, 2010; pp 2164–2172. [Google Scholar]
- Pajarinen J.; Kyrki V. Robotic Manipulation of Multiple Objects as a POMDP. Artif. Intell. 2017, 247, 213–228. 10.1016/j.artint.2015.04.001. [DOI] [Google Scholar]
- Thrun S.; Fox D.; Burgard W.; Dellaert F. Robust Monte Carlo Localization for Mobile Robots. Artif. Intell. 2001, 128, 99–141. 10.1016/S0004-3702(01)00069-8. [DOI] [Google Scholar]
- Kurniawati H. Partially Observable Markov Decision Processes and Robotics. Annu. Rev. Control Robot. Auton. Syst. 2022, 5, 253–277. 10.1146/annurev-control-042920-092451. [DOI] [Google Scholar]
- Finite-State Language Processing, Roche E.; Schabes Y., Eds.; MIT Press: Cambridge, Massachusetts; London, England, 1997. [Google Scholar]
- Theocharous G.; Kaelbling L. P.. Approximate Planning in POMDPs with Macro-Actions, Proceedings of the Seventeenth Annual Conference on Neural Information Processing Systems (NIPS 2003), MIT Press, 2004; pp 775–782.
- Lauri M.; Pajarinen J.; Peters J.. Information Gathering in Decentralized POMDPs by Policy Graph Improvement, Proceedings of the International Conference on Autonomous Agents and Multiagent Systems (AAMAS) Montreal, Canada, 2019; pp 1143–1151.
- Shannon C. E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. , 623–656 10.1002/j.1538-7305.1948.tb00917.x. [DOI] [Google Scholar]
- Cover T. M.; Thomas J. A.. Elements of Information Theory; 2nd ed.; Wiley & Sons: Hoboken, New Jersey, 2006. [Google Scholar]
- Hauschild A.; Temirov R.; Soubatch S.; Bauer O.; Schöll A.; Cowie B. C. C.; Lee T.-L.; Tautz F. S.; Sokolowski M. Normal-Incidence X-Ray Standing-Wave Determination of the Adsorption Geometry of PTCDA on Ag(111): Comparison of the Ordered Room-Temperature and Disordered Low-Temperature Phases. Phys. Rev. B 2010, 81, 125432. 10.1103/PhysRevB.81.125432. [DOI] [Google Scholar]
- Tiemann J. K. S.; Guixà-González R.; Hildebrand P. W.; Rose A. S. MDsrv: Viewing and Sharing Molecular Dynamics Simulations on the Web. Nat. Methods 2017, 14, 1123–1124. 10.1038/nmeth.4497. [DOI] [PubMed] [Google Scholar]
- O’Connor M. B.; Bennie S. J.; Deeks H. M.; Jamieson-Binnie A.; Jones A. J.; Shannon R. J.; Walters R.; Mitchell T. J.; Mulholland A. J.; Glowacki D. R. Interactive Molecular Dynamics in Virtual Reality from Quantum Chemistry to Drug Binding: An Open-Source Multi-Person Framework. J. Chem. Phys. 2019, 150, 220901. 10.1063/1.5092590. [DOI] [PubMed] [Google Scholar]
- Unke O. T.; Chmiela S.; Gastegger M.; Schütt K. T.; Sauceda H. E.; Müller K.-R. SpookyNet: Learning Force Fields with Electronic Degrees of Freedom and Nonlocal Effects. Nat. Commun. 2021, 12, 7273 10.1038/s41467-021-27504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrough P. A.; McDonnell R. A.. Principles of Geographical Information Systems; Oxford University Press: Oxford, 1998. [Google Scholar]
- Tarjan R. Depth-First Search and Linear Graph Algorithms. SIAM J. Comput. 1972, 1, 146–160. 10.1137/0201010. [DOI] [Google Scholar]
- Leinen P.; Green M. F. B.; Esat T.; Wagner C.; Tautz F. S.; Temirov R. Hand Controlled Manipulation of Single Molecules via a Scanning Probe Microscope with a 3D Virtual Reality Interface. J. Visualized Exp. 2016, e54506 10.3791/54506-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong-Kui L. The Generation of Straight Lines on Hexagonal Grids. Comput. Graph. Forum 1993, 12, 27–31. 10.1111/1467-8659.1210027. [DOI] [Google Scholar]
- Lowerre B. T.The HARPY Speech Recognition System. Ph.D. Dissertation, Carnegie-Mellon University, 1976. [Google Scholar]
- Kilian L.; Umbach E.; Sokolowski M. A Refined Structural Analysis of the PTCDA Monolayer on the Reconstructed Au(111) Surface-“Rigid or Distorted Carpet?”. Surf. Sci. 2006, 600, 2633–2643. 10.1016/j.susc.2006.03.049. [DOI] [Google Scholar]
- Ziatdinov M.; Ghosh A.; Wong C. Y.; Kalinin S. V. AtomAI Framework for Deep Learning Analysis of Image and Spectroscopy Data in Electron and Scanning Probe Microscopy. Nat. Mach. Intell. 2022, 4, 1101–1112. 10.1038/s42256-022-00555-8. [DOI] [Google Scholar]
- Chen I. J.; Aapro M.; Kipnis A.; Ilin A.; Liljeroth P.; Foster A. S. Precise Atom Manipulation Through Deep Reinforcement Learning. Nat. Commun. 2022, 13, 7499 10.1038/s41467-022-35149-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer B.; Simpson G. J.; Cartus J. J.; Jeindl A.; García-López V.; Tour J. M.; Grill L.; Hofmann O. T. Autonomous Single-Molecule Manipulation Based on Reinforcement Learning. J. Phys. Chem. A 2023, 127, 2041–2050. 10.1021/acs.jpca.2c08696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N. A.; Gokhale D. V. Entropy Expressions and Their Estimators for Multivariate Distributions. IEEE Trans. Inf. Theory 1989, 35, 688–692. 10.1109/18.30996. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.