SUMMARY
Estimated glomerular filtration rate (eGFR) is one of the best-performing methods in evaluating kidney function. There are limited data regarding the estimated glomerular filtration rate in children and young adults with hemophilia. The aim of this study was to determine the difference between three commonly used estimated glomerular filtration rate equations in the pediatric population in a cohort of patients with hemophilia. Our prospective study included 36 pediatric patients with moderate or severe hemophilia. eGFR was calculated for each patient using the original creatinine-based “bedside Schwartz” equation, the cystatin C-based equation and the creatinine-cystatin C-based equation. The difference between the equations, calculated using the one-way repeated ANOVA test, was statistically significant (p <0.001), and post hoc analysis found differences between each method. Correlation analysis showed the strongest positive correlation between the bedside Schwartz equation and creatinine-cystatin C-based equation (r=0.866) among the three methods examined. A correlation between the three eGFR methods was present, but with significant differences between them. Due to the observed differences between eGFR in pediatric patients with hemophilia, further research is needed to find the optimal measurement method for eGFR. Nevertheless, we recommend implementing eGFR equations in routine clinical monitoring of pediatric patients with hemophilia.
Key words: estimated glomerular filtration rate, hemophilia, children, cystatin C, bedside Schwartz equation, creatinine-cystatin C-based equation
Introduction
Hemophilia is a rare, X-linked recessive hemostatic disorder which occurs in 1:5000 new-born male children (1). It is a genetic disorder caused by a deficiency of clotting factor VIII (hemophilia A) or IX (hemophilia B) (1). According to the baseline level of deficient factor activity, clinical presentation can be mild (severe hemorrhages occurring only after serious trauma), moderate (rare spontaneous hemarthrosis) and severe (spontaneous deep tissue and joint bleedings) (1-3). The treatment involves a recombinant factor VIII/IX concentrates with a standard or prolonged half-life (4, 5) Today, we have comprehensive care for the patients with hemophilia, but uncertainties, i.e. kidney pathology, still arise (6). After Kulkarni’s publication on the topic, the myth of renal disease being a rare complication of hemophilia was overthrown (7). The relatively high rates of renal disease in adult patients with hemophilia were strongly associated with HIV infection, hypertension and kidney bleeding (7). It is important to note the higher-than-expected death rates than in adult patients with hemophilia and renal disease (8). Risk factors which may predispose the development of renal disease in younger patients with hemophilia are hematuria, diabetes, viral infection, and exposure to nephrotoxic agents (7).
Glomerular filtration rate (GFR) is the best indicator of kidney function in children and adolescents (9). Several methods estimating the glomerular filtration rate exist. The one commonly most used is the bedside Schwartz method, which uses anthropometric measurements and combines them with serum creatinine levels.
To the best of our knowledge, there are no publications regarding estimated glomerular filtration rate (eGFR) equations in children and young adults with hemophilia. Therefore, we hypothesized that it would be useful to evaluate eGFR equations in patients with hemophilia.
The aim of this study was to determine the differences between three commonly used estimated glomerular filtration rate equations in the pediatric population in a cohort of patients with hemophilia.
Patients and methods
The study included 36 male patients with moderate or severe hemophilia treated in a Croatian hemophilia referral center. The inclusion criteria were age between 2 and 19 years with moderate or severe hemophilia A or B and obtaining signed informed consent to participate in the study.
Routine blood samples for serum creatinine, urea and cystatin C measurements were collected simultaneously. Serum creatinine and urea levels were measured using the enzymatic photometry method, while serum cystatin C was measured using immunoturbidimetry (10). eGFR was calculated for each patient using the original creatinine-based bedside Schwartz equation, cystatin C-based equation and creatinine-cystatin C-based equation. We analyzed the correlation and agreement between the three eGFR equations (9, 11–14)
Original creatinine-based bedside Schwartz equation (2009):
 |
Cystatin C-based equation (2012):
 |
Creatinine-cystatin C-based equation (2012):
For male patients:
 |
For female patients:
 |
eGFR (estimated glomerular filtration rate) = mL/min/1.73 m2
ht (height) = meters
Scr (standardized serum creatinine) = mg/dL
cysC (cystatin C) = mg/L
BUN (blood urea nitrogen) = mg/dL
In addition to the analysis of the agreement between the results, we also investigate bias, calculated as:
 |
Data analysis
Statistical analysis was performed using the R (www.r-project.org) and MedCalc software. The numerical variables were descriptively summarized with arithmetic means and standard deviations if the data were normally distributed, and otherwise with medians and interquartile ranges. Normality testing was performed with the Kolmogorov Smirnov test. The correlation between the variables was examined using Pearson’s and Kendall’s τ correlation tests. Differences between numerical variables were tested with a one-way repeated measures analysis of variance (ANOVA). Bland-Altman analysis was performed to assess the agreement between the results of different eGFR methods. P values lower than 0.05 were considered statistically significant.
Results
Out of 36 patients included in the study, 27 had hemophilia A, while 9 had hemophilia B. The mean age was 11.20 ± 4.31 years, ranging from 2.80 to 18.30 years. Height was measured for all patients, with a mean value of 147 (IQR: 126.75-166.00) cm, and the calculated mean body mass index was 17.72 (IQR: 15.51-20.50) kg/m2. The median value for creatinine was 41.5 µmol/L (IQR: 36.5-52.0); 0.5 mg/dL (IQR: 0.40-0.59), the mean value for cystatin C was 0.87 ± 0.13 mg/L, 4.1 ± 0.86 mmol/L for urea and was 11.46 ± 2.41 mg/dL for BUN (Table 1).
Table 1. General patient characteristics and the most important laboratory values.
| mean ± SD | ||
|---|---|---|
| General characteristics | Age (years) | 11.2±4.31 |
| median (IQR) | ||
| Weight (kg) | 37.5 (26-56.5) | |
| Height (cm) | 147 (126.75-166) | |
| BMI (kg/m2) | 17.72 (15.51-20.5) | |
| mean ± SD | ||
| Laboratory values | Cystatin C (mg/L) | 0.87±0.13 |
| Urea - serum (mmol/L) | 4.1±0.86 | |
| BUN (mg/dL) | 11.46±2.41 | |
| median (IQR) | ||
| Creatinine - serum (µmol/L) Creatinine - serum (mg/dL) |
41.5 (36.5-52) 0.5 (0.4-0.59) |
|
| SD – standard deviation, IQR – interquartile range, BMI – body mass index BUN – blood urea nitrogen | ||
When calculating eGFR for all the subjects using the three equations, a statistically significant difference was found between the equations (p <0.001) using a one-way repeated ANOVA test, and post hoc analysis found differences between each method. Using the bedside Schwartz equation mean, eGFR was 119.94 ± 22.44 mL/min/1.73m2, while according to the cystatin C-based equation mean eGFR was 82 ± 10.73 mL/min/1.73m2, whereas mean eGFR calculated using the complex creatinine-cystatin C-based equation was 103 ± 12.5 mL/min/1.73m2, (Table 2).
Table 2. Overall differences between the estimated eGFR in the study sample.
| Equation | mean ± SD | p* |
|---|---|---|
| Bedside Schwartz | 119.94±22.44 | <0.001 |
| Cystatin C-based | 82±10.73 | |
| Creatinine-cystatin C-based | 103±12.5 | |
| *one-way repeated measures ANOVA, eGFR – estimated glomerular filtration rate | ||
Correlation analysis showed a strong positive correlation between the bedside Schwartz equation and creatinine-cystatin C-based equation (r=0.866), while a less strong positive correlation was observed between the cystatin C-based equation and creatinine-cystatin C-based equation (r=0.655). Additionally, the positive correlation between eGFR using the bedside Schwartz equation and cystatin C-based equation was not of statistical significance (r=0.305) (Table 3).
Table 3. Correlation coefficients between different eGFR equations.
| Equation | Schwartz | Cystatin C-based | Creatinine-cystatin C-based |
|---|---|---|---|
| Schwartz | 1.000 | ||
| Cystatin C-based | 0.305 | 1.000 | |
| Creatinine-cystatin C-based | 0.866* | 0.655* | 1.000 |
| *p<0.05 | |||
The results of the subgroup correlation analysis are presented in Table 4.
Table 4. Correlation analysis between different eGFR equations for patients with hemophilia A and B.
| Equation | Schwartz | Cystatin C-based | Creatinine-cystatin C-based | |
|---|---|---|---|---|
| Hemophilia A | Schwartz | 1 | ||
| Cystatin C-based | 0.230 | 1 | ||
| Creatinine-cystatin C-based | 0.574 | 0.617 | 1 | |
| Hemophilia B | Schwartz | 1 | ||
| Cystatin C-based | 0.841 | 1 | ||
| Creatinine-cystatin C-based | 0.886 | 0.783 | 1 | |
| *p<0.05 – Kendall’s 𝜏 | ||||
In the hemophilia A group, moderate positive correlations were observed between eGFR based on the bedside Schwartz and creatinine-cystatin C-based equation (τ=0.574) as well as between the cystatin C and creatinine-cystatin C-based equation (τ=0.617).
Within the hemophilia B group, statistically significant positive correlations were observed between all three eGFR methods: bedside Schwartz and cystatin C-based equations (τ=0.841), bedside Schwartz and creatinine-cystatin C-based equations (τ=0.886); and creatinine-cystatin C-based and cystatin C-based equations (τ=0.783).
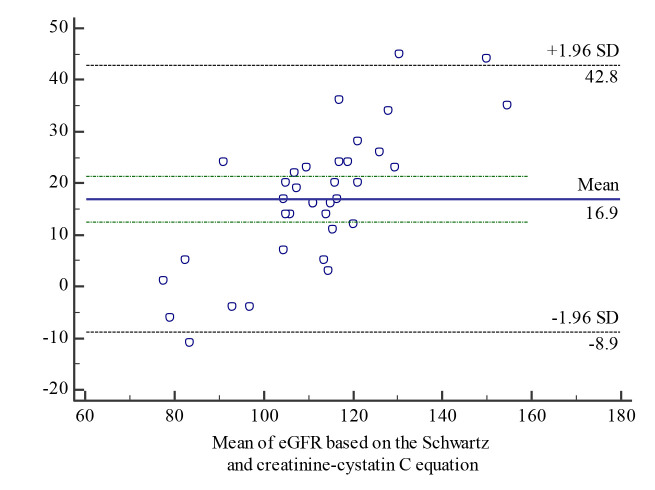
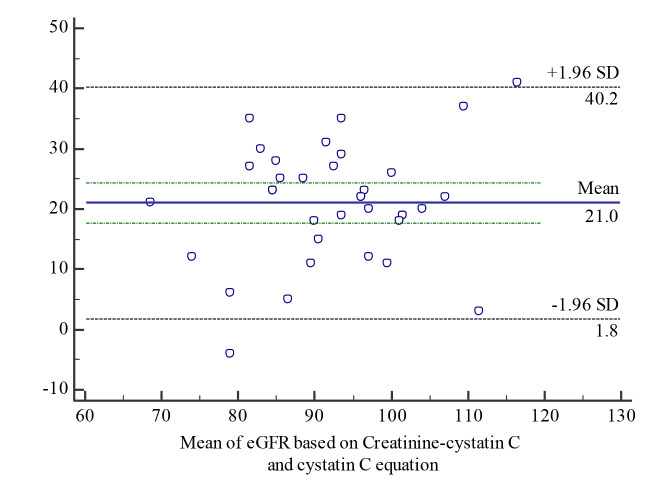
Bland-Altman analysis for all the equations is shown in the Fig. 1-3.
Fig. 1.
Bland-Altman analysis for eGFR based on the Schwartz and cystatin C equation
Fig. 2.
Bland-Altman eGFR agreement analysis between eGFR based on the Schwartz and creatinine-cystatin C equations
Fig. 3.
Bland-Altman agreement analysis between eGFR based on the creatinine-cystatin C and cystatin C equations
Significant disagreement was found when comparing estimated GFR calculations using all the tested methods, namely the creatinine-based bedside Schwartz equation, cystatin C-based equation and creatinine-cystatin C-based equation.
The results of the bias analysis showed that the bedside Schwartz equation overestimated the results of the cystatin C equation by 30.21% and the creatinine-cystatin C method by 14.41%, while the creatinine-cystatin C method overestimated the results of the cystatin C method by 20.32%.
Discussion
Estimated glomerular filtration rate (eGFR) is one of the best-performing methods in evaluating kidney function. Glomerular filtration rate cannot be directly measured; however, it can be determined by measuring the clearance of an ideal filtration marker or estimated using predictive formulas (15, 16). Routine use of an ideal filtration marker, e.g. inulin, is limited due to high cost and the duration of the procedure (16, 17). According to the National Kidney Disease Education Program (NKDEP), the bedside Schwartz equation is “currently the best equation for estimating GFR from serum creatinine in children” (18, 19). There have been many publications correlating estimated GFR in different pediatric patient groups. These included groups of healthy children, children with chronic kidney disease and children with malignant diseases (20-23).
However, to the best of our knowledge, studies including children with hemophilia have not been published so far. Therefore, the importance of the present study is regarding the estimation of GFR in young patients with hemophilia using three eGFR equations. In this study, we found strong positive correlation between the bedside Schwartz and creatinine-cystatin C-based equations, while the agreement between the bedside Schwartz and cystatin C-based equations was limited.
The name of the bedside Schwartz equation stems from its ease of use and the fact that it requires only the patient’s height and serum creatinine, while the other two equations used in our study are more complex also requiring serum cystatin C and BUN, respectively (9).
Hamed et al. published a study on 40 boys with hemophilia A, evaluating functional and structural renal abnormalities using standard laboratory testing and advanced renal scintigraphy methods to measure GFR (24). However, they did not use any of eGFR equations to evaluate GFR and compare it to the lowered GFR detected by 99mTc-DTPA. Therefore, their conclusion could not lead to further recommendations due to the lack of application of eGFR equations in their research (24).
Bernhardt et al. compared GFR by radioisotope measurement (99mTc-DTPA) to the estimated GFR equations (the original Schwartz, the revised bedside Schwartz and the Counahan-Barratt equation) in children with cancer (22). They found that differences between estimations and measurements in children with cancer were significant, and it was therefore not appropriate to use any of the tested formulas instead of radioisotope GFR measurement (22).
Gheissari et al. published a comparison of the updated Schwartz method, combining the Schwartz and the Grubb GFR equations in a large study sample of 712 children (20). They found high concordance and agreement between two 2009 Schwartz equations in estimating GFR, whereas there was a high inconsistency between the Grubb equation and the two Schwartz equations (20). Since pediatric patients with hemophilia do not receive nephrotoxic treatment, the results of this study could be applied to our population of patients. We should also note that the cystatin C-based equation was used in our study instead of the Grubb equation (20). Gheissari’s study results were similar to the results we obtained comparing bedside Schwartz and creatinine-cystatin C-based equation in patients with hemophilia. However, this comparison is a matter of debate since all the eGFR equations are based on patients with kidney disease and thus could not be extrapolated unconditionally to the general pediatric population (9, 20, 25).
It is known that hemophilia itself is a risk factor for developing kidney disease and could therefore lead to diminished eGFR, but much is still unknown and requires further multicenter studies.
Study limitations
The main limitations of our study were the lack of a gold standard and the small sample size. It was not possible to collect a larger group of patients with hemophilia who had moderate or severe hemophilia due to the small prevalence of the disease. The main reason for avoiding direct GFR measurement was that it would have resulted in exposure to radiation only for scientific purposes, without any clinical indications.
Conclusion
Male patients with moderate or severe hemophilia likely have higher incidence of renal disease. In our study, estimated GFR was calculated for each patient using the original creatinine-based bedside Schwartz equation, the cystatin C-based equation and the creatinine-cystatin C-based equation, examining the correlation and agreement between those three eGFR equations.
In conclusion, although a correlation between the three eGFR methods was found, significant differences were present as well. Given the lack of a recommended gold standard method to compare the eGFR methods, we cannot give clear recommendations on which method to apply.
However, we suggest implementing eGFR equations into routine clinical monitoring of patients with hemophilia. Since the bedside Schwartz equation is simpler to use and demands fewer data and less specific laboratory testing, we would suggest using this equation in the regular check-ups for pediatric patients with hemophilia without hematuria or other symptoms of renal disease, while the creatinine-cystatin C-based equation should be reserved for symptomatic cases, especially if the patient has gross or micro hematuria.
Due to the limitations mentioned above, further research is needed to evaluate which method of estimating GFR should be applied.
Ethical approval:
The study procedures were in accordance with the ethical standards of the institutional research committee of the UHC Zagreb at which the studies were conducted (IRB approval number 8.1-17/11-2) and with the 1975 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent:
Informed parental / patient consent was obtained from all individual participants included in the study.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- 1.Flood VH, Scott JP. Hereditary Clotting Factor Deficiencies (Bleeding Disorders). In: Nelson Textbook of Pediatrics. 21st ed. Philadelphia: Elsevier; 2019. p. 2594-7. [Google Scholar]
- 2.Zimmerman B, Valentino LA. Hemophilia: In review. Pediatr Rev. 2013;34(7):289–94. 10.1542/pir.34.7.289 [DOI] [PubMed] [Google Scholar]
- 3.Karim MA, Jamal CY. A Review on Hemophilia in Children. Bangladesh J Child Heal. 2013;37(1):27–40. 10.3329/bjch.v37i1.15349 [DOI] [Google Scholar]
- 4.Khair K. Management of haemophilia in children. Paediatr Child Heal (United Kingdom). 2019;29(8):334–8. 10.1016/j.paed.2019.05.002 [DOI] [Google Scholar]
- 5.Ljung RCR. Prevention and Management of Bleeding Episodes in Children with Hemophilia. Paediatr Drugs. 2018;20(5):455–64. 10.1007/s40272-018-0307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sørensen B, Auerswald G, Benson G, Elezovi ÊI, Felder M, Lambert T, et al. Rationale for individualizing haemophilia care. Blood Coagul Fibrinolysis. 2015;26(8):849–57. 10.1097/MBC.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni R, Soucie JM, Evatt B, Cicatello B, Jackson D, Hoffman R, et al. Renal disease among males with haemophilia. Haemophilia. 2003;9(6):703–10. 10.1046/j.1351-8216.2003.00821.x [DOI] [PubMed] [Google Scholar]
- 8.Ranta S, Valta H, Viljakainen H, Mäkitie O, Mäkipernaa A. Hypercalciuria and kidney function in children with haemophilia. Haemophilia. 2013;19(2):200–5. 10.1111/hae.12021 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Work DF. Measurement and Estimation of GFR in Children and Adolescents. Clin J Am Soc Nephrol. 2009;4:1832–43. 10.2215/CJN.01640309 [DOI] [PubMed] [Google Scholar]
- 10.Grubb A, Blirup-Jensen S, Lindström V, Schmidt C, Althaus H, Zegers I. First certified reference material for Cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619–21. 10.1515/CCLM.2010.318 [DOI] [PubMed] [Google Scholar]
- 11.Andersen TB, Eskild-Jensen A, Frøkiær J, Brøchner-Mortensen J. Measuring glomerular filtration rate in children; can cystatin C replace established methods? A review. Pediatr Nephrol. 2009;24(5):929–41. 10.1007/s00467-008-0991-y [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37. 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82(4):445–53. 10.1038/ki.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staples A, Leblond R, Watkins S, Wong C, Brandt J. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol. 2010;25(11):2321–6. 10.1007/s00467-010-1598-7 [DOI] [PubMed] [Google Scholar]
- 15.Pottel H. Measuring and estimating glomerular filtration rate in children. Pediatr Nephrol. 2017;32(2):249–63. 10.1007/s00467-016-3373-x [DOI] [PubMed] [Google Scholar]
- 16.Mian AN, Schwartz GJ. Measurement and Estimation of Glomerular Filtration Rate in Children. Adv Chronic Kidney Dis. 2017. November 1;24(6):348–56. 10.1053/j.ackd.2017.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traynor J, Mactier R, Geddes CC, Fox JG. How to measure renal function in clinical practice. BMJ. 2006;333(7571):733–7. 10.1136/bmj.38975.390370.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedside IDMS-traceable Schwartz GFR Calculator for Children. Cited 2020 Apr 24. Available from: https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate-calculators/children-conventional-units
- 19.Pottel H, Dubourg L, Goffin K, Delanaye P. Alternatives for the Bedside Schwartz Equation to Estimate Glomerular Filtration Rate in Children. Adv Chronic Kidney Dis. 2018;25(1):57–66. 10.1053/j.ackd.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 20.Gheissari A, Roomizadeh P, Kelishadi R, Abedini A, Haghjooy-Javanmard S, Abtahi SH, et al. Comparison of the performance of the updated Schwartz, combined Schwartz and the Grubb glomerular filtration rate equations in a general pediatric population. Saudi J Kidney Dis Transpl. 2014;25(5):1004–10. 10.4103/1319-2442.139890 [DOI] [PubMed] [Google Scholar]
- 21.Llanos-Paez CC, Staatz C, Lawson R, Hennig S. Comparison of methods to estimate glomerular filtration rate in paediatric oncology patients. J Paediatr Child Health. 2018;54(2):141–7. 10.1111/jpc.13752 [DOI] [PubMed] [Google Scholar]
- 22.Bernhardt MB, Moffett BS, Johnson M, Tam VH, Thompson P, Garey KW. Agreement among measurements and estimations of glomerular filtration in children with cancer. Pediatr Blood Cancer. 2015. January 1;62(1):80–4. 10.1002/pbc.25194 [DOI] [PubMed] [Google Scholar]
- 23.Safaei-Asl A, Enshaei M, Heydarzadeh A, Maleknejad S. Correlation between cystatin C-based formulas, Schwartz formula and urinary creatinine clearance for glomerular filtration rate estimation in children with kidney disease. J Renal Inj Prev. 2016;5(3):157–61. 10.15171/jrip.2016.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamed AA, Shalaby MH, El-Kinawy NS, Elamawy AA, Abd El-Ghany SM. Renal Abnormalities among Egyptian Children with Hemophilia A Using Renal Scintigraphy: Relation to Risk Factors and Disease Severity. Clin Appl Thromb Hemost. 2017;23(5):478–86. 10.1177/1076029615619484 [DOI] [PubMed] [Google Scholar]
- 25.Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L. Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol. 2011;6(3):552–60. 10.2215/CJN.04180510 [DOI] [PMC free article] [PubMed] [Google Scholar]