SUMMARY
Osteoarthritis (OA) can be treated using either a pharmacological or non-pharmacological approach, or a combination of both. The purpose of the present study was to investigate the efficacy of crystalline glucosamine sulfate (CGS) in patients with knee OA. This open-label prospective study (with a 12-month follow-up) included 111 patients of both genders suffering from knee OA, who attended the Special Hospital for Rheumatic Diseases in Novi Sad, Serbia during the 2011-2013 period. Patients were divided into the experimental (n=52) and the control (n=59) group. While the former was prescribed CGS 1500 mg/day, the latter was treated with nonsteroidal anti-inflammatory drugs (NSAIDs) according to the standard protocol. The efficacy of both treatment modes was assessed using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and Lequesne index, along with the radiological findings which involved knee joint space width (JSW) measurements. One year following the initial assessment, all patients reported pain intensity reduction; however, those in the CGS group experienced significantly lower pain intensity when compared with controls. At the end of the study, no reduction in the progression of joint structure damage (p>0.5) was noted in either group. Thus, while CGS demonstrated symptomatic efficacy, it failed to delay the progression of knee OA.
Key words: arthritis, chondroitin, disease modifying drugs, rehabilitation
Introduction
Osteoarthritis (OA) is a painful degenerative condition manifesting as swollen and stiff joints. It presents a major burden for modern society due to decreased mobility, increased pain, and thus increased disability rates among adults and the elderly (1, 35). OA treatment based on nonsteroidal anti-inflammatory drugs (NSAIDs) alleviates the disease symptoms in most cases. However, such therapy cannot delay the disease progression or prevent its final stage, which necessitates surgery (2-4). Cartilage plays an important role as soft tissue, providing a cushion between the bones that meet at a joint, allowing them to smoothly move without rubbing against each other. In individuals suffering from OA, the protective cartilage begins to break down and the bones start grinding together. Chondroitin and glucosamine are the key structural components of cartilage. Both are produced naturally in the body, but can also be administered as dietary supplements. Researchers have studied the effects of various dietary supplements on OA, both in combination or individually.
A new generation of drugs, the so-called disease modifying drugs (DMDs) or chondroprotectives (2), are increasingly being administered as a part of modern pharmacological therapy. However, the action mechanisms of their constituent substances are presently unclear. Nonetheless, empirical evidence indicates that N-acetyl glucosamine and proteoglycan-containing supplements are effective in relieving knee pain and swelling in patients with OA, while improving knee function in terms of walking or climbing stairs, bending, and stretching (27). Moreover, Sterzi (34) recently reported that combining CartiJoint Forte supplement intake with physical therapy may alleviate knee pain and help improve the functional score in patients with OA.
According to the latest European Guidelines (5, 6), crystalline glucosamine sulfate (CGS) is a drug with the highest level of evidence for structure-modifying effects in knee OA (7). Glucosamine acts as a preferred substrate for the biosynthesis of glycosaminoglycan chains, and thus for the production of aggrecan and other cartilage-specific proteoglycans (27). Several extant short-term studies have shown symptomatic efficacy of this drug (8-10) when compared with placebo (11) and drugs in the NSAID group (7, 12). Previous findings indicate that daily oral intake of 1500 mg glucosamine sulfate is more effective than placebo in treating knee OA symptoms (10). Nonetheless, all traditional pharmacological treatments are aimed solely at the management of OA symptoms.
However, as a part of several recent randomized controlled studies, plain radiography was performed to monitor joint space narrowing over time and to evaluate the structure-modifying effect of chondroitin sulfate and glucosamine sulfate. The results yielded by these investigations indicate that glucosamine sulfate (but not glucosamine hydrochloride) and chondroitin sulfate have small-to-moderate symptomatic efficacy in OA. Although the reliability of this data is still unclear, from the structure-modifying point of view, there is compelling evidence that glucosamine sulfate and chondroitin sulfate may interfere with OA progression (32). For several years, great efforts have been dedicated to the study of CGS, resulting it its approval for clinical use, owing to the evidence provided by previous studies, demonstrating its structure-modifying effect, i.e., its ability to delay the disease progression (6, 13). The Long-term Evaluation of Glucosamine Sulfate (LEGS) study findings further indicate that glucosamine sulfate, chondroitin sulfate, or their combination, are effective in alleviating chronic knee pain in patients with OA (33). Still, it is worth noting that evidence has emerged indicating that none of the aforementioned treatment modes reduce joint pain or hinder joint space narrowing (29). In sum, the available evidence is inconsistent, possibly due to inadequate allocation concealment, use of different glucosamine preparations, etc (31).
Thus, the aim of the present study was to establish if there are any long-term benefits of CGS treatment in patients with knee OA.
Methods
Participants
The study sample comprised 111 participants of both genders (92 women and 19 men, aged 60.24±5.8years; body mass index [BMI] 27.7 ± 2.1kg/m2) who signed informed consent to voluntarily participate in the study, which was approved by the local Institutional Review Board at the University of Novi Sad and was conducted in accordance with the Declaration of Helsinki. Patients fulfilling the inclusion criteria were randomly assigned to Group 1 (n=52; age 59.04±6.71 years; BMI 27.86±2.11 kg/m2) or Group 2 (n=59; age 61.44±5.07 years; BMI 27.70±2.11 kg/m2). The inclusion criteria were: diagnosis of knee OA, age 40-65 years, knee pain lasting for at least a month, radiological results of the knee corresponding to Level 2 or 3 according to the Kellgren-Lawrence (KL) score (14), and the Laquesne index (15) in the 5-13 range. The exclusion criteria were: presence of blood test indicators of inflammatory processes, obesity (BMI ≥29.9 kg/m2), history of previous knee trauma, presence of an inflammatory rheumatic disease, presence of comorbidities (malignity, hematologic disease, liver, or kidney disease), and recent history of local or systemic corticosteroid therapy (within the last three months). In order to assess the patients according to the aforementioned criteria and diagnose knee OA, a review of medical history was conducted, along with a physical examination of the joint (knee circumference measurement, measurement of knee movement range and measurement of m. quadriceps strength – m.QPS, according to the manual muscle test), anthropometric measurements (body weight and body height), radiological examination of the knee (anteroposterior direction), and routine blood and urine tests. All patients gave permission for the inclusion of their medical data in this study.
Experimental intervention
All 111 patients that took part in the study attended the five scheduled follow-up appointments at 1, 3, 6, 9, and 12 months. Those in Group 1, i.e., the experimental group (n=52), were prescribed 1500 mg/day crystalline glucosamine sulfate (CGS) to be taken once a day as a powder for oral solution, based on the following protocol: daily intake for the first 6 and last 3 months, with a 3-month pause (months 7 to 9). Every patient could further reduce knee pain by taking acetaminophen (500 mg tablets) up to the maximum daily dose of 3 g. During each check-up, the patients reported drug intake (the number of tablets taken) since the last appointment. Intake of acetaminophen or any other analgesic during the week preceding the appointment was prohibited. The patients assigned to Group 2, i.e., the control group (n=59), were prescribed ibuprofen 400 mg tablets, to be taken three or four times a day, or diclofenac sodium, one 75 mg capsule a day. Their intake regime commenced with continuous use of the drug for the first 15 days, after which medication was only allowed when pain occurred, and the intake period was limited to 5 days. At each follow-up, patients were required to report on the number of NSAID doses taken. Patients in whom risk for stomach ulcer was noted could take the proton pump inhibitor (omeprazole 20 mg/day).
Fig. 1 depicts the number of patients that started (111) and completed (80) the study. Initially, 52 patients were assigned to the CGS group, two of whom did not attend the 3-month check-up for undisclosed reasons. Between the 2nd and 3rd check-up (i.e., in the 3-6-month study period), three patients (6% of the CGS group) experienced side-effects and were excluded from the study (hypertension was noted in one individual, while two reported gastrointestinal [GIT] problems). Exclusion of the two patients due to adverse GIT events was based on the assessment of the medical practitioner leading the study. Thus, patients reporting abdominal pain + nausea + dyspepsia, as well as those experiencing cardiovascular irregularities (hypertension), were excluded from the study. The last (12-month) check-up was not attended by a further three participants (5.77% of the CGS group) due to their inability to afford the medication. As a result, 44 CGS group participants completed the study. However, the WOMAC questionnaire responses provided by one individual at the last check-up were incomplete or illegible, and were thus excluded from analyses.
Fig 1.
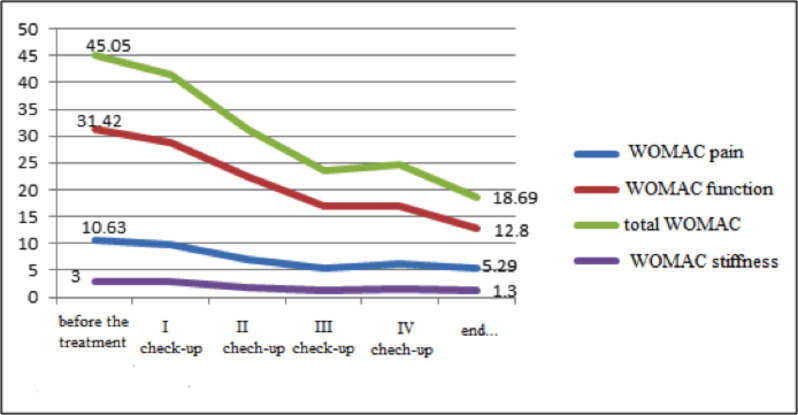
WOMAC pain, WOMAC stiffness, WOMAC function, and total WOMAC.
The NSAID group initially comprised 59 patients, one of whom withdrew from the study the next day for personal reasons. By the second (3-month) check-up, six participants were excluded: one due to adverse GIT events, one as a result of comorbidities, and four owing to protocol non-adherence (NSAID medication was taken for longer than prescribed). The third (6-month) check-up was not attended by one individual for undisclosed reasons, and five patients were excluded due to side-effects (four due to adverse GIT events and one due to hypertension). In the last three months of the study, a further four participants were excluded owing to adverse GIT events. Six patients failed to attend the last (12-month) check-up, three of whom stated different reasons, while three cited dissatisfaction with the treatment. As a result, 36 NSAID group participants completed the study.
Study design
The study was conducted at the Special Hospital for Rheumatic Diseases in Novi Sad, Serbia, as an open-label prospective clinical study with a 12-month follow-up period. The investigation had prior approval from the Ethics Committee of the Special Hospital for Rheumatic Diseases and from the Ethics Committee of the Faculty of Medicine at the University of Novi Sad, Serbia under number 01-2019-VII/2. The study was a part of a doctoral dissertation titled “Glucosamine sulfate in the treatment of osteoarthritis of the knee” conducted by one of the authors, Karmela Filipovic. As a part of the investigation, structural efficacy of medications was monitored using radiological findings of the knee, by measuring the joint space width (JSW) at the medial compartment of the tibiofemoral joint in the affected knee. In both groups, structural changes were measured at the start of the study and after one year. Two validated questionnaires were used In order to assess the clinical symptom intensity and monitor the clinical efficacy of the prescribed medications, the Lequesne index (15) and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (16).
a). Lequesne index
The first part of the questionnaire, comprising five questions, examined perceived pain and discomfort levels related to night-time pain; pain upon waking; pain after prolonged standing; pain when shifting positions; and morning joint stiffness. Patients were required to rate perceived pain on a 0-2 scale, with 0 signifying absence of pain and 2 indicating intense pain.
The second part of the questionnaire comprised two questions pertaining to maximum walking distance. The first question examined joint pain in relation to distance covered (rated on a 0-6 scale, with 0 indicating no limitations and 6 an ability to walk <100 m). The second question related to the use of walking aids (rated on a 0-2 scale, with 0 denoting no need for aids and 2 indicating need for two walking sticks/crutches).
The second part of the questionnaire contained four questions related to functional joint limitations, focusing on the ability to climb up/down the stairs, walk on uneven surfaces, and squat. Each question was rated on a 0-2 scale, with 0 signifying no limitations and 2 indicating inability to perform the stated activity.
As the lowest Lequesne index score is 24; patients with an overall score of <4 and >13 were excluded from the study. When completing the questionnaire, the patient selected the most appropriate response after receiving an explanation from the lead medical investigator. Each patient completed the questionnaire at the start of the study as well as at the five follow-up appointments at 1, 3, 6, 9, and 12 months.
b). Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
The first part of the questionnaire consisted of five questions pertaining to the severity of knee joint pain while walking on even surfaces, while climbing up/down stairs, at night-time, when shifting positions (while sitting or lying down), and while standing upright.
The second part of the questionnaire included two questions examining joint stiffness immediately upon waking and later during the day.
The second part of the questionnaire, comprising 17 questions, assessed the patient’s functional status, i.e., difficulties in performing activities of daily living (ADL). The respondents were required to indicate difficulties in climbing up/down stairs; raising from seated position; when getting into bed; while standing, bending, resting; performing lighter or heavier housework; getting in/out of a car or bus; getting in/out of a bathtub; during shopping; putting on/taking off socks, etc.
The WOMAC questionnaire was filled by selecting one of the five available options that best matched the patient’s condition during the preceding 48 hours, corresponding to None (0), Mild (1), Moderate (2), Severe (3), and Extreme (4). For each part of the questionnaire, scores were added up before calculating the average for that section. The questionnaire was completed at the start of the study, as well as at the 1-, 3-, 6-, 9-, and 12-month check-ups.
Statistical analyses
Wilcoxon test was performed to compare the mean values between the groups, with p<0.05 indicating statistical significance. A nonparametric test for the comparison of ordinary variables (scales), i.e., the Mann-Whitney U test (p≤0.05), was adopted for comparison of the observed results pertaining to the two groups. Differences in the structural changes were assessed by independent-samples t test at the p≤0.05 level of significance.
Results
Both groups comprised predominantly female patients (about 2/3), and were matched in terms of age, BMI and functional status, as well as Kellgren-Lawrence (KL) score. According to the radiographic findings, most participants were at the KL Level 2 (Table 1).
Table 1. Demographic characteristics of both groups of patients before the beginning of the study.
| Demographic characteristics | CGS group (n=52)¶ | NSAID group (n=59)** |
|---|---|---|
| Female | 88.50% | 78.0% |
| Average age* (years) | 59.04±6.71 | 61.44±5.07 |
| Localization of sites | ||
| Left | 14 (26.92%) | 16 (27.19%) |
| Right | 19 (36.63%1) | 27 (45.76%) |
| Both | 19 (36.93%) | 16 (27.19%) |
| Knee girth | 39.22±3.62 | 40.12±4.20 |
| Movement volume (flexion, extension) | 70-120-0 | 60-130-0 |
| Strength in Qps (by MMT3)† | 39 (54.20%) | 39 (52.0%) |
| (by MMT4)† | 32 (44.4%) | 36 (48.0%) |
| BMI*‡ | 27.86±2.11 | 27.70±2.11 |
| Kellgren Lawrence score§ | ||
| Level 2 | 40 (70.92%) | 42 (71.18%) |
| Level 3 | 12 (23.07%) | 17 (28.81%) |
| WOMAC OA Index|| | ||
| Total* | 45.05±18.41 | 36.93±13.96 |
| Pain* | 10.63±4.34 | 9.21±3.82 |
| Stiffness | 3.0±2.09 | 2.41±1.84 |
*Values shown as mean values with SD; †MMT=Manual muscle test; ‡BMI=Body mass index (kg/m2); §Kellgren Lawrence score- classification of radiological changes in knee joint; ||WOMAC OA Index - Western Ontario and McMaster Universities Osteoarthritis Index; ¶CGS – crystalline glucosamine sulfate; **NSAID - nonsteroidal anti-inflammatory drug
As previously noted, patients in the experimental (i.e., CGS) group were allowed to use acetaminophen. The majority (55.8%) of these patients took acetaminophen in the first three months of the study and during the period in which CGS was not administered (i.e., months 7-9). While 18 (34.60%) patients took acetaminophen during the first month only, only three (5.8%) individuals relied on this analgesic for the entire 3-month period at the start of the study. On the other hand, acetaminophen was taken by 24 (52.17%) patients during the break from CGS (months 7-9). While none of the patients took analgesics in the 7th month, 14 (30.4%) relied on acetaminophen during the 9th month. A comparison of these two periods when the largest number of acetaminophen tablets was taken (the first three months of the study and the period when the patients did not take the CGS) failed to reveal statistically significant differences in acetaminophen intake (t=0.84, p>0.05). As previously noted, the symptomatic effects of CGS were monitored using the WOMAC and Lequesne index (14, 15). The findings indicated that, at all check-ups (p<0.01), the experimental group reported pain reduction when completing the WOMAC (results shown in Fig. 1).
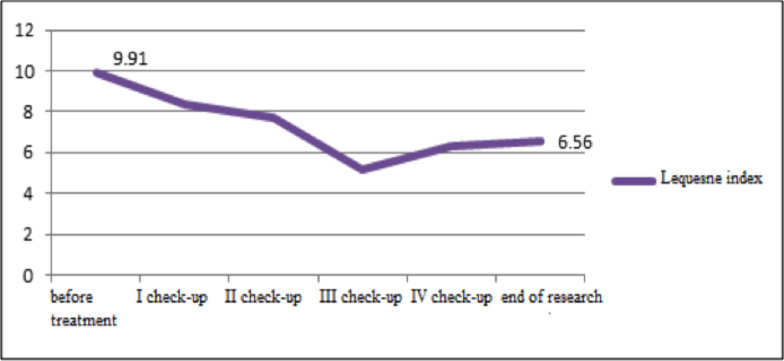
At the first check-up, no reduction in stiffness according to WOMAC was noted (p>0.5), but statistically significant improvements were reported at all subsequent appointments (p<0.01). As shown in Fig. 1 using WOMAC, a statistically significant improvement in knee function was recorded at all follow-up appointments (p<0.01). Lequesne index scores also indicated that all patients reported marked improvements (p<0.01) (Fig. 2).
Fig 2.
Lequesne index before the treatment and at check-ups.
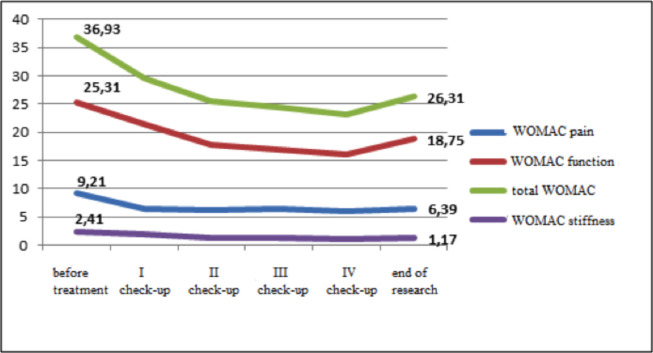
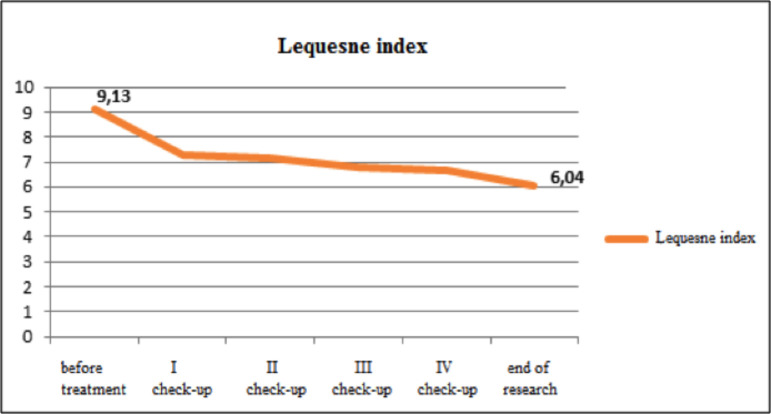
Symptomatic effects of the NSAID drugs. In patients assigned to the control (i.e., NSAID) group, WOMAC scores at all check-ups indicated reduction in pain, stiffness, function, and overall OA score (p<0.01), as shown in Fig. 3, and these findings corresponded to those obtained using the the Lequesne index (Fig. 4).
Fig. 3.
WOMAC pain, WOMAC stiffness, WOMAC function. and WOMAC score.
Fig 4.
Lequesne index before treatment and at check-ups.
Comparison between groups. At the first (1-month) check-up, the patients in the NSAID group had lower WOMAC OA index, indicating significant reduction in pain and stiffness, as well as joint function improvement, compared with the CGS group (p<0.05, values not tabulated). At the 3-month check-up, lower values of the WOMAC index and similar average reduction in the scores (p>0.05) were registered for both groups, as indicated in Table 2.
Table 2. Reduction of WOMAC index in the first 3 months in both groups.
| WOMAC OA † index in first 3 months | N | change X- bar with min-max | ||
|---|---|---|---|---|
| WOMAC pain | CGS‡ | 50 | 3.73 (-3.0-16.0) | U=1160.0 p>0.05* |
| NSAID§ | 52 | 2.88 (-3.0-11.0) | ||
| total | 102 | 3.3 (-3.0-16.0) | ||
| WOMAC stiffness | CGS | 50 | 1.22 (-2.0-7.0) | U=1233.0 p>0.05* |
| NSAID | 52 | 1.17 (-4.0-5.0) | ||
| total | 102 | 1.2 (-2.0-7.0) | ||
| WOMAC function | CGS | 50 | 9.62 (-19.0-38.0) | U=1100.0 p>0.05* |
| NSAID | 52 | 7.71 (-7.0-36.0) | ||
| total | 102 | 8,65 (-19.0-38.0) | ||
| total WOMAC score | CGS | 50 | 14.57 (-22.0-42.0) | U=1107.0 p>0.05* |
| NSAID | 52 | 11. 77 (-5.0-47.0) | ||
| total | 102 | 13.14 (-22.0-42.0) | ||
| Lequesne index before beginning of treatment until 2nd check-up | CGS | 50 | 2.35 (-6.5-10.5) | U=1190.0 p>0.05* |
| NSAID | 52 | 2.09 (-2.0-7.5) | ||
| total | 102 | 2.2 (-6.5-10.0) | ||
*U=Mann Whitney test; †WOMAC OA Index – Western Ontario and McMaster Universities Osteoarthritis Index; ‡CGS – crystalline glucosamine sulfate; §NSAID – nonsteroidal anti-inflammatory drug
At the 6-month check-up, the CGS group had a significant reduction in the WOMAC pain score (p<0.01), while improvement in stiffness was not statistically significant. Compared with the control group, the CGS group reported a more significant improvement in knee function (based on both WOMAC and Lequesne index), as indicated in Table 3.
Table 3. Reduction of WOMAC pain, WOMAC stiffness, WOMAC function, WOMAC score, and Lequesne index in patients after the 6th month.
| WOMAC OA † after the 6th month | N | change x̄ and MIN-MAX | ||
|---|---|---|---|---|
| WOMAC pain | CGS‡ | 47 | 5.29 (-5.0-16.0) | U=722, * p<0.01 |
| NSAID§ | 46 | 2.78 (-5.0-11.0) | ||
| total | 93 | 4.05 (-5.0-16.0) | ||
| WOMAC stiffness | CGS | 46 | 2.13 (-4.0-7.0) | U=877, * p>0.05 |
| NSAID | 45 | 1.56 (-3.0-6.0) | ||
| total | 91 | 1.85 (-4.0-7.0) | ||
| WOMAC function | CGS | 47 | 15.62 (-7.0-41.0) | U=749, * p<0.01 |
| NSAID | 46 | 8.72 (-8.0-34) | ||
| total | 93 | 12.2 (-8.0-41.0) | ||
| total WOMAC score | CGS | 46 | 23.23 (-2.0-58.0) | U=699, * p<0.01 |
| NSAID | 45 | 13. 29 (-6.0-41.0) | ||
| total | 91 | 18.31 (-6.0-58.0) | ||
| Lequesne after the 6th month | CGS | 46 | 5.16 (0.5-10.5) | U=541, * p<0.01 |
| NSAID | 46 | 2.41 (-6.0-9.5) | ||
| Total | 92 | 3.78 (-6.0-9.5) | ||
*U=Mann Whitney test; †WOMAC OA Index – Western Ontario and McMaster Universities Osteoarthritis Index; ‡CGS – crystalline glucosamine sulfate; §NSAID – nonsteroidal anti-inflammatory drug
At the final (12-month) check-up, a statistically significant reduction in OA intensity (p<0.01) as well as in the Lequesne index (p<0.05) was registered in the CGS group, as reported in Table 4.
Table 4. Reduction of WOMAC OA index and Lequesne index over a period of one year.
| WOMAC OA † index for the period of one year | N | change x̄ and MIN-MAX | ||
|---|---|---|---|---|
| WOMAC pain | CGS‡ | 43 | 5.29 (-5.0-16.0) | U=430.0* p<0.01 |
| NSAID§ | 36 | 2.78 (-5.0-11.0) | ||
| Total | 79 | 4.05 (-5.0-16.0) | ||
| WOMAC stiffness | CGS | 43 | 1.84 (-2.0-6.0) | U=652.0* p>0.05 |
| NSAID | 36 | 1.39 (-2.0-5.0) | ||
| Total | 79 | 1.63 (-2.0-6.0) | ||
| WOMAC function | CGS | 43 | 19.98 (0-47.0) | U=267.5* p<0.01 |
| NSAID | 36 | 6.19 (10.0-25.0) | ||
| Total | 79 | 13.7 (0-47.0) | ||
| total WOMAC score | CGS | 43 | 28.06 (14.0-67.0) | U=301.0* p<0.01 |
| NSAID | 36 | 10.17 (14.0-39.0) | ||
| Total | 79 | 19.91 (14.0-67.0) | ||
| Lequesne index before treatment after the 12th month (end of treatment) | CGS | 44 | 3.75 (-2.0-10.0) | U=652.5* p<0.05 |
| NSAID | 36 | 2.9 (-2.5-9.5) | ||
| Total | 80 | 3.37 (-2.0-10.0) | ||
*U=Mann Whitney test; †WOMAC OA Index – Western Ontario and McMaster Universities Osteoarthritis Index; ‡CGS – crystalline glucosamine sulfate; §NSAID – nonsteroidal anti-inflammatory drug
As noted previously,, the experimental group did not take CGS during the 7-9-month study period. Thus, it was not surprising that, at the 9-month check-up, more intense pain was reported compared with the levels noted at the preceding appointments. In the control group, NSAID intake led to a slight pain reduction, but the improvement was not statistically significant relative to the levels reported at the previous check-up. Moreover, no statistically significant differences between the two groups were noted in any of the observed parameters (p>0.05). In the CGS group, an exacerbation of joint stiffness was registered at the 9-month check-up compared to the previous check-up, while the patients in the NSAID group had similar values. Nonetheless, the difference between the groups was not statistically significant (p>0.05). The CGS group WOMAC function score also worsened compared with the previous check-up, while that of the NSAID group slightly improved, but the difference between the groups was not statistically significant (p>0.05). At the 9-month check-up, the NSAID group also exhibited a slight improvement in the Lequesne index compared with the previous appointment, but the change was not statistically significant and no statistically significant differences were noted between the two groups (p>0.05). Structure-modifying effects of drugs were also monitored by examining any changes in the tibiofemoral (TF) joint space width (JSW) (17) (Table 5).
Table 5. Measure of JSW* of the tibiofemoral joint of medial compartment of right and left knee at the beginning of the research in all patients.
| Groups | min-max | |||
|---|---|---|---|---|
| JWS | CGS† | Right | 4.31±0.91 | 2.36-6.55 |
| Left | 4.45±0.95 | 2.23-6.33 | ||
| NSAID‡ | Right | 4.26±0.973 | 2.3-6.1 | |
| Left | 4.33±1.2 | 2.1-7.8 | ||
| TOTAL (mm) | 4.28±0.94 | 2.3-6.65 | ||
| 4.39±1.088 | 2.1-7.8 | |||
*JSW – joint space width; †CGS – crystalline glucosamine sulfate; ‡NSAID – nonsteroidal anti-inflammatory drug
In patients who had pain in both knees, the JSW change was monitored at the knee in which pain was more severe. In some cases, where the JSW difference was ≤0.15 mm, the values related to both knees were considered in the analyses. At the start of the study, similar knee JSW was obtained for the two groups (right t=0.29, p>0.05; left t=0.55, p>0.05) and a reduction in knee JSW was noted in all patients at the end of the study (p<0.01), as reported in Table 6.
Table 6. Values of JSW before the beginning of the treatment and at the end of the study.
| Groups | n | |||
|---|---|---|---|---|
| CGS | JWS right | Before treatment | 25 | 4.08 ± 0. 91 |
| After one year | 25 | 3.79 ± 0.90 | ||
| JWS left | Before treatment | 19 | 3.98 ± 0.87 | |
| After one year | 19 | 3.68 ± 0.84 | ||
| NSAID | JWS right | Before treatment | 22 | 4.16 ± 1.02 |
| After one year | 22 | 3.93 ± 1.04 | ||
| JWS left | Before treatment | 20 | 3.57 ± 0.84 | |
| After one year | 20 | 3.33 ± 0.82 | ||
*JSW – joint space width; †CGS – crystalline glucosamine sulfate; ‡NSAID – nonsteroidal anti-inflammatory drug
At 12-month follow-up, similar reductions in JSW were registered for the two groups (p>0.05), as indicated in Table 7.
Table 7. Change of JSW values in both groups of patients compared with the initial values.
| Group | n | After one year | reduction |
|---|---|---|---|
| CGS | 43 | 3.74 ± 0.87 | 0.29 ± 0.26 (0.26-1.13) |
| NSAID | 41 | 3.63 ± 0.93 | 0.24 ± 0.22 (0.10-1.10) |
*JSW – joint space width; †CGS – crystalline glucosamine sulfate; ‡NSAID – nonsteroidal anti-inflammatory drug
The experimental group initially included 52 patients, 44 of whom completed the investigation. In the control group, 36 of the original 59 patients finished the treatment. In both groups, attrition was due to dissatisfaction with the treatment, adverse gastrointestinal tract events (dyspepsia, nausea), hypertension, non-compliance with the study protocol, personal reasons, and/or medication unaffordability.
Discussion
One year following the initial assessment, all patients reported pain intensity reduction; however, those in the CGS group experienced significantly lower pain intensity when compared with controls. At the end of the study, no reduction in the progression of joint structure damage (p>0.5) was noted in either group. Thus, while CGS demonstrated symptomatic efficacy, it failed to delay the progression of knee OA.
After the 1st follow-up, significant improvements in the disease symptoms (pain, stiffness, and functional status WOMAC scores, as well as total WOMAC score and Lequesne index) were noted in the NSAID compared with the CGS group. These findings differ from the results obtained in previous short-term studies (7, 13) where CGS and NSAID had equal efficacy. This discrepancy can be ascribed to the differences in the study design and the choice of parameters adopted for monitoring the disease progression. At the 3-month follow-up, similar average reductions in the observed parameters were registered in both groups. Lequesne index reduction was reported in a previous 12-month placebo-controlled study involving 319 patients where the control group used NSAID (piroxicam 20 mg/day) (9). In the present study, lower average reductions in the Lequesne index were noted in the CGS group. In line with the previous data, we demonstrated good CGS safety, as indicated by the lower frequency of side-effects (gastric problems in particular) in the experimental group. We posit that fewer patients suffered from gastritis because they were permitted to take ibuprofen and diclofenac sodium, both of which have low ulcerogenic potential, while piroxicam has high ulcerogenic potential (17). In addition, patients at risk of developing these issues were advised to take a gastroprotective drug (omeprazole). At the 6-month follow-up, daily CGS intake resulted in a reduction in pain and stiffness WOMAC scores, as well as the total WOMAC score, along with improvements in the functional status and the Lequesne index. Although the same trend was observed in the NSAID group, greater benefits were noted in the CGS group. Unfortunately, these results cannot be compared with the findings reported by other authors, since no studies based on the same protocol have been conducted. In the long-term studies in which the control group received placebo, CGS efficacy was monitored for 1-3 years (3, 13, 18). In the only study for which six-month data is available, i.e., the GUIDE study (10), the analgetic effect of a CGS 1.5 g daily dose was compared with the acetaminophen (3 g/day) and the placebo group. In this study, the patients were allowed to use ibuprofen for pain reduction, and their status was assessed via the Lequesne and the WOMAC OA index. While the Lequesne index values declined in both the treatment and the control groups, the reduction was statistically significant for the CGS group only, but not for the acetaminophen group. In contrast, our results show statistically significant reduction in the Lequesne index value in the CGS group compared with the NSAID group. In addition, in the GUIDE study, the total WOMAC score reduction was statistically significantly lower compared with that of the placebo group. On the other hand, no improvements in the total WOMAC score were registered in the acetaminophen group. This is in line with current study findings, where the CGS group experienced improvements in the total WOMAC score, even though a more positive trend in the WOMAC pain scale was reported in the GUIDE study. At the 6-month follow-up, a significant reduction in pain was reported by the CGS group. Even though different comparators were utilized in the present and the GUIDE study, the results are similar and the CGS analgetic effects can be compared for a 6-month period. In another study, daily ibuprofen intake (1200-1600 mg/day) was shown to produce primarily analgetic effects (19). Similarly, previous study found that diclofenac sodium (75 mg/day) achieved the desired analgetic effect, even though it took longer to attain the maximum concentration in plasma, but with a better compliance and better tolerance by the gastrointestinal tract when taken for a longer period (20). In the present study,, reduction in pain and joint stiffness and the total WOMAC scores, as well as functional status improvement, at 12-month follow-up compared with the initial values were noted in both groups. However, greater reductions in all aforementioned measures as well as in the Lequesne index were recorded for the CGS group compared with the NSAID group. These results show that CGS intake can bring about significant reduction in the disease symptoms one year later, whereas NSAID administration can only maintain the initial treatment benefits. GAIT is the only long-term study comparing the efficacy of glucosamine in the form of chloride salts (GhCl) with NSAID (celecoxib, 200 mg/day) over a 24-month period (21). For this purpose, participants were divided into five groups (I − placebo; II − GhCl; III − HS; IV − GhCl+HS; and V − celecoxib) and their WOMAC pain index was monitored, with a reduction of at least 20 mm compared with the initial value signifying drug efficacy. In this study, 60% of patients assigned to the placebo group reported pain reduction meeting the aforementioned criterion. On the other hand, GhCL and HS, administered as a mono or combined therapy, did not result in a significant pain reduction. These results might be attributed to the fact that the participants were given glucosamine in the form of chloride salt, which is a dietary product rather than a drug. In addition, GhCL was administered in single doses (22-24), even though the total dose corresponded to the currently recommended dose for both products. On the other hand, in the studies conducted by Pavelka (3) and Reginster (13), the chondroprotective CGS effect was monitored, rather than drug efficacy. Pavelka reported pain reduction and joint function improvement (as indicated by lower Lequesne and WOMAC index) in all patients who completed the treatment, even though reductions were greater in the CGS compared with the placebo group (from 20% to 25%) (3). This is in line with the findings obtained by our study, as the improvements in the WOMAC and Lequesne index were more pronounced in the CGS compared with the NSAID group. Reginster reported similar results (13) in their study, in which 40-60% of the patients in both groups took analgesics or NSAID drugs for pain relief.
In our study, CGS efficacy was also assessed by radiographic findings, focusing on changes in the TF JSW in the affected knee. Changes in the JSW served as an indirect indicator of the disease improvement. Available evidence indicates that JSW or osteophyte measurements taken before and at least one year after the treatment are the most reliable means of evaluating the structural effects of some drugs (25). Even though only 80 of the 111 patients completed the study, 84 knees were included in the JSW analysis, as the JSW values in four patients had been taken for both knees (as the difference between the knees was ≤0.15 mm). One of the study inclusion criteria was a KL score Level 2 or 3, as this is necessary for a clear radiological diagnosis of OA and thus for monitoring drug efficacy. (5) At the end of the study, no statistically significant differences in the JSW were noted between the groups. It should be noted that the knee which had a lower initial JSW value was chosen for monitoring purposes. At the fourth follow-up at end of the 7-9 month period, when the experimental group did not take CGS and could only use acetaminophen when knee pain occurred, no statistically significant differences in the WOMAC OA or Lequesne index values between the two groups were noted. In addition, the CGS group reported minimal exacerbation of the disease symptoms compared with the previous (6-month) follow-up. Moreover, the NSAID group had better Lequesne index values compared with the CGS group, suggesting that CGS has not demonstrated prolonged symptomatic efficacy over a 3-month period. As a result, 52.1% of patients took acetaminophen for pain management. It is important to emphasize that none of the patients took pain medication in the 7th month, indicating that a certain CGS therapeutic response was initially maintained, as previously reported (8, 9).
When interpreting these findings, it is important to consider the study limitations, one of which was small sample size. Moreover, as JSW was measured via conventional radiography, which predominantly visualizes bones, it would be advantageous to use MRI in future research, owing to its ability to directly visualize all the joint structures, including soft tissue and cartilage and subchondral bone marrow lesions.
Conclusion
Crystalline glucosamine sulfate administration resulted in pain reduction and joint function improvement, but the drug did not demonstrate structural efficacy, as it failed to delay knee OA progression.
References
- 1.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–46. 10.7326/0003-4819-133-8-200010170-00016 [DOI] [PubMed] [Google Scholar]
- 2.Brayer O, Compere S, Lowati LS, Giacovelli G, Deroisy R, Reginster JY. Five years follow up of patients with previous 3 year randomised controlled trial of glucosamine sulfate in knee osteoarthritis. Arthritis Rheum. 2003;48:80. [Google Scholar]
- 3.Pavelka K, Gatterova J, Giacovelli G, Olejarova M, Rovati LC. Glucosamine sulfate prevents total joint replacement in the long-term follow-up of knee osteoarthritis patients. Arthritis Rheum. 2004;50(9) Suppl:S251. [Google Scholar]
- 4.Bruyere O, Pavelka K, Rovati LC, Gatterová J, Giacovelli G, Olejarová M, et al. Total joint replacement after glucosamine sulfate treatment in knee osteoarthritis: results of a mean 8-years observation of patients in two previous 3-years, randomised placebo controlled trials. Osteoarthritis Cartilage. 2008;16(2):254–60. 10.1016/j.joca.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(4):476–99. 10.1016/j.joca.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 6.Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR Recommendations 2003: an evidence-based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145–55. 10.1136/ard.2003.011742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Setnikar I, Cereda R, Pacine MA, Revel R. Antireactive properties of glucosamine sulfate. Arzneimittelforschung. 1991;41(2):157–61. [PubMed] [Google Scholar]
- 8.Müller-Fassbender H, Bach GL, Haase W, Rovati LC, Setnikar I. Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthritis Cartilage. 1994;2(1):61–9. 10.1016/S1063-4584(05)80007-X [DOI] [PubMed] [Google Scholar]
- 9.Rovati LC. The clinical profile of glucosamine sulfate as a selective symptom modifying drug in osteoarthritis: current data and perspectives. Osteoarthritis Cartilage. 1997;5 Suppl A:S72.
- 10.Herrero-Beaumont G, Ivorra JA, Del Carmen Trabado M, Blanco FJ, Benito P, Martín-Mola E, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: a randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum. 2007;56(2):555–67. 10.1002/art.22371 [DOI] [PubMed] [Google Scholar]
- 11.Persiani S, Rotini R, Trisolino G, Rovati LC, Locatelli M, Paganinin N, et al. Synovial and plasma glucosamine concentrations in osteoarthritic patients following oral crystaline glucosamine sulphate at therapeutic dose. Osteoarthritis Cartilage. 2007;15(7):764–72. 10.1016/j.joca.2007.01.019 [DOI] [PubMed] [Google Scholar]
- 12.Lopes Vaz A. Double-blind clinical evaluation of the relative efficacy of ibuprofen and glucosamine sulphate in the management of osteoarthrosis of the knee in out-patients. Curr Med Res Opin. 1982;8(3):145–9. 10.1185/03007998209112375 [DOI] [PubMed] [Google Scholar]
- 13.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Brayer O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357(9252):251–6. 10.1016/S0140-6736(00)03610-2 [DOI] [PubMed] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lequesne MG. The algofunctional indices for hip and knee osteoarthritis. J Rheumatol. 1997;24(4):779–81. [PubMed] [Google Scholar]
- 16.Bellamy N, Buchana WW, Goldsmith CH, Campel J, Stitt LW. Validation study of WOMAC: health status instrument for measuring clinically important patient relevant outcomes to antireumatic drug therapy in patients with osteoarthritis hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 17.Doyle G, Furey S, Berlin R, Cooper S, Jayawardena S, Ashraf E, et al. Gastrointestinal safety and tolerance of ibuprofen at maximum over-the-counter dose. Aliment Pharmacol Ther. 1999;13(7):897–906. 10.1046/j.1365-2036.1999.00539.x [DOI] [PubMed] [Google Scholar]
- 18.Bruyere O, Pavelka K, Rovati LC, Deroisy R, Olejarova M, Gatterova J, et al. Glucosamine sulfate reduces osteoarthritis progression in postmenopausal women with knee osteoarthritis: evidence from two 3-year studies. Menopause. 2004;11(2):138–43. 10.1097/01.GME.0000087983.28957.5D [DOI] [PubMed] [Google Scholar]
- 19.Moore N, Gance EV, Parc JM, Schneid H, Wall R, Farhan M, et al. The PAIN Study: paracetamol, aspirin and ibuprofen new tolerability study a large-scale randomised clinical trial comparing the tolerability of aspirin, ibuprofen and paracetamol for short-term analgesia. Clin Drug Investig. 1999;18(2):89–98. 10.2165/00044011-199918020-00001 [DOI] [Google Scholar]
- 20.Todd PA, Sorkin EM. Diclofenac sodium. A reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy. Drugs. 1988;35(3):244–85. 10.2165/00003495-198835030-00004 [DOI] [PubMed] [Google Scholar]
- 21.Sawitzke AD, Shi H, Finco MF, Dunlop DD, Bingham CO, 3rd, Harris CL, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin intervention trial. Arthritis Rheum. 2008;58(10):3183–91. 10.1002/art.23973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate and two combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. 10.1056/NEJMoa052771 [DOI] [PubMed] [Google Scholar]
- 23.Altman RD, Cheung H. Glucosamine sulfate on cartilage: lapine study. Arthritis Rheum. 2001;44(9):1535. 10.2217/thy.10.67 [DOI] [Google Scholar]
- 24.Jackson CG, Plaas AH, Sandy JD, Hua C, Kim-Rolands S, Barnhil JG, et al. The human pharmacokinetics of oral ingestion of glucosamine and chondroitin sulfate taken separately or in combination. Osteoarthritis Cartilage. 2010;18(3):297–302. 10.1016/j.joca.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reginster JY, Reiter-Niesert S, Bruyère O, Berenbaum F, Brandi ML, Branco J, et al. Recommendations for an update of the 2010 European regulator guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis and reflections about related clinically relevant outcomes: expert consensus statement. Osteoarthritis Cartilage. 2015;23(12):2086–93. 10.1016/j.joca.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 26.Setnikar I, Cereda R, Pacini MA, Revel L. Antireactive properties of glucosamine sulfate. Arzneimittelforschung. 1991;41(2):157–61. [PubMed] [Google Scholar]
- 27.Naraoka Y, Harada H, Katagiri M, Yamamura H, Shirasawa T. N-acetyl glucosamine and proteoglycan containing supplement improves the locomotor functions of subjects with knee pain. Drug Discov Ther. 2017;11(3):140–5. 10.5582/ddt.2017.01019 [DOI] [PubMed] [Google Scholar]
- 28.Runhaar J, Rozendaal RM, van Middelkoop M, Bijlsma HJW, Doherty M, Dziedzic KS, et al. Subgroup analyses of the effectiveness of oral glucosamine for knee and hip osteoarthritis: a systematic review and individual patient data meta-analysis from the OA trial bank. Ann Rheum Dis. 2017;76(11):1862–9. 10.1136/annrheumdis-2017-211149 [DOI] [PubMed] [Google Scholar]
- 29.Wandel S, Juni P, Tendal B, Nuesch E, Villiger PM, Welton NJ, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341(9):c4675. 10.1136/bmj.c4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrero-Beaumont G, Ivorra JA, Del Carmen Trabado M, Blanco FJ, Benito P, Martín-Mola E, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: a randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum. 2007;56(2):555–67. 10.1002/art.22371 [DOI] [PubMed] [Google Scholar]
- 31.Vlad SC, LaValley MP, McAlindon TE, Felson DT. Glucosamine for pain in osteoarthritis: why do trial results differ? Arthritis Rheum. 2007;56(7):2267–77. 10.1002/art.22728 [DOI] [PubMed] [Google Scholar]
- 32.Bruyere O, Reginster JY. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging. 2007;24(7):573–80. 10.2165/00002512-200724070-00005 [DOI] [PubMed] [Google Scholar]
- 33.Fransen M, Agaliotis M, Nairn L, Votrubec M, Bridgett L, Su S, et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis. 2015;74(5):851–8. 10.1136/annrheumdis-2013-203954 [DOI] [PubMed] [Google Scholar]
- 34.Sterzi S, Giordani L, Morrone M, Lena E, Magrone G, Scarpini C, et al. The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: a randomized, double-blind, placebo-controlled study. Eur J Phys Rehabil Med. 2016;52(3):321–30. [PubMed] [Google Scholar]
- 35.Golob M, Marković I, Zovko N, Šakić D, Gudelj-Gračanin A, Morović-Vergles J. Do We Pay Enough Attention to Neuropathic Pain in Knee Osteoarthritis Patients? Acta Clin Croat. 2018;57(1):16–21. 10.20471/acc.2018.57.01.02 [DOI] [PMC free article] [PubMed] [Google Scholar]