Abstract
Background/Objective:
Patients with juvenile idiopathic arthritis (JIA) often present with signs and symptoms suggestive of serious bacterial infection (SBI). Procalcitonin (PCT) is a biomarker that is elevated in SBI. We conducted a comparative cohort study to test the hypothesis that PCT levels will differ between active JIA, quiescent JIA, and bacteremic patients and healthy controls.
Methods:
From October 2016 to May2018, consecutive children 6 months to 18 years of age with (a) active untreated JIA, (b) quiescent JIA, and (c) healthy elective presurgical candidates were recruited from clinics at a musculoskeletal specialty hospital. Juvenile idiopathic arthritis was defined according to the International League of Associations for Rheumatology criteria. Clinical data and serum samples meeting the same criteria were included from a prior study. Consecutive bacteremic patients were identified over the same period. Procalcitonin and other common measures of inflammation were measured. Descriptive statistics and univariate logistic analyses were performed.
Results:
Ninety-two study subjects were recruited. Erythrocyte sedimentation rate, C-reactive protein (CRP), and PCT levels were all elevated in bacteremic patients in comparison to the other groups. Erythrocyte sedimentation rate and CRP both had wide ranges that overlapped between groups; however, the PCT concentration was 0.15 μg/mL or greater in 1 of 59 patients with JIA, whereas it was 0.15 μg/mL or less in only 1 bacteremic patient.
Conclusions:
Our study indicates that serum erythrocyte sedimentation rate, CRP, and PCT levels are all biomarkers that can be used to distinguish SBI versus active JIA at presentation. However, PCT is the most accurate, with the least overlap between patients with infection and noninfectious inflammatory arthritis. This finding can help clinicians direct therapy.
Keywords: infection, JIA, juvenile arthritis, juvenile idiopathic arthritis, procalcitonin
Juvenile idiopathic arthritis (JIA) is a chronic autoimmune disease that affects approximately 200,000 children in the United States.1 Monitoring of disease activity relies on history, physical examination, and laboratory data. Expeditious control of disease exacerbations is crucial to optimize long-term outcomes and prevent disruptions to quality of life. However, differentiating disease flares from infections can be challenging due to the similarities in clinical presentation. Many studies have been done with the goal of identifying new biomarkers for the monitoring of disease activity in JIA.2–4 In particular differentiating disease flares from infection has important clinical implications, as the treatments for each are diametrically opposed. Differentiating infection from disease flare is often hampered by diagnostic uncertainty, patient age, and administration of chronic immunosuppressive therapy. The evaluation and management of a suspected infection can involve significant morbidity, including lumbar puncture and arthroscopic lavage, and often delay the initiation of appropriate antirheumatic treatment.5,6 However, to date, no accurate diagnostic biomarkers have been identified.
Procalcitonin (PCT) is typically secreted by C cells of the thyroid and then cleaved to calcitonin.7 During stress, PCT secretion is elevated through multiple mechanisms: (1) excessive production, (2) decreased cleavage to calcitonin, and (3) extrathyroidal transcription and synthesis. It is known to be consistently elevated in bacterial infection.8,9 In addition, some data suggest this elevation occurs exclusively in bacterial infections.10,11
The utility of PCTas a diagnostic biomarker has been evaluated in Kawasaki disease and Henoch-Schönlein purpura.12,13 However, whether PCT is able to distinguish between JIA disease activity and bacterial infection is unknown. Therefore, this comparative cohort study was designed to test the hypotheses that (1) PCT levels will differ between active disease in JIA and infection, and (2) PCT will differentiate between active and quiescent disease in children with JIA.
METHODS
Patient Population
Between October 2016 and May 2018, 4 groups of children aged 1 to 18 years who presented for care at a single academic center were identified for this study. The 4 groups of children recruited were (1) consecutive patients with active JIA during a standard-of-care visit, (2) consecutive patients with quiescent JIA during a standard-of-care visit, (3) consecutive patients without rheumatic disease who presented for elective musculoskeletal surgery, and (4) children with bacteremia admitted to the pediatric intensive care unit or pediatric inpatient unit.
Juvenile idiopathic arthritis was defined according to the International League of Associations for Rheumatology criteria, and JIA disease activity was assessed.14 Active JIAwas defined as untreated with anything besides nonsteroidal anti-inflammatory drugs (NSAIDs) and 4 or more inflamed joints; quiescent JIA was defined as treated cases, with fewer than 4 inflamed joints, and stable treatment for 1 month or more. Patients with JIA were excluded if the patient/parent was non–English speaking, because some of the disease assessment tools utilized in this study are validated only in English; however, no patients were excluded based on this criterion. Further exclusion criteria were (1) patients with oligoarticular orsystemic-type JIA, as these are distinctly different diseases from the JIA subtypes that were included, and systemic JIA was exceedingly rare at the center where this study was conducted; (2) patients with active JIA despite immunosuppression, to minimize confounding by treatment; (3) patients with other rheumatologic diagnoses; (4) patients with primary renal disease or patients taking any stimulant medication, as these have been shown to affect PCT levels15,16; and (5) JIA patients with current infection, as this would affect PCT levels. Inclusion and exclusion criteria were similar for surgical controls and bacteremic patients. Exclusion criteria for surgical controls were as follows: (1) non–English speaking, (2) patients with any rheumatologic diagnoses, (3) patients with primary renal disease or patients taking stimulant medications, and (4) patients with current infection or known congenital or acquired immunodeficiency.
Data Collection
Collected data for all study subjects included the following:
demographic information, including gender, race/ethnicity, address, telephone number, and email address;
medical information, including JIA subtype, disease duration, medication information, physical examination data, and serologies; and
laboratory data including complete blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and PCT. All laboratory tests were processed at a single study laboratory.
In addition, the following data were collected for JIA study subjects:
The Juvenile Arthritis Disease Activity Score in 71 joints (JADAS-71) score for JIA activity.17 Scores range from 0 to 101, with higher scores indicating more severe disease activity. Components of the JADAS-71 include swollen joint count, physician global health assessment, patient global health assessment, and modified ESR.
The Childhood Health Assessment Questionnaire.18 This includes more than 30 questions that are focused on physical function. This measure has a final score of 0 to 3, with higher scores indicating more physical disability.
This study was approved by the Hospital for Special Surgery and Weill Cornell Medicine Institutional Review Boards.
Statistical Analysis
Procalcitonin, ESR, CRP, and hemoglobin levels were compared between JIA patients with active disease, JIA patients with quiescent disease, nonrheumatic disease patients undergoing elective musculoskeletal surgery (chosen as “healthy controls”), and children in the intensive care unit with bacterial infections. Continuous variables were compared using analysis of variance or Wilcoxon rank-sum test, and categorical variables were compared using Fisher exact test.
RESULTS
Patient characteristics of all 4 groups are summarized in Table 1. Bacteremic patients were younger than the other groups and more racially diverse. Juvenile idiopathic arthritis characteristics are further characterized in Table 2. Both the active and quiescent groups had a mix of JIA subtypes. Because study subjects enrolled in the active group were untreated with anything besides NSAIDs, disease duration was significantly shorter than that in the children with quiescent treated JIA. The JADAS-71 scores reveal that disease activity was relatively low in this population; however, it was higher in the active group compared with the quiescent group. The Childhood Health Assessment Questionnaire scores were low in all study subjects. All of the study subjects with active JIA were treated with NSAIDs, whereas only 40.6% of quiescent cases were treated with NSAIDs. In addition, 34.4% of study subjects with quiescent JIA were treated with methotrexate, and the remaining 65.6% were treated with anti–tumor necrosis factor therapy.
TABLE 1.
Patient Characteristics
| Active Untreated JIA (n = 27) | Quiescent Treated JIA (n = 32) | Healthy Controls (n = 16) | Bacteremic Patients (n = 17) | |
|---|---|---|---|---|
| Age, median (interquartile range), y | 13.0 (7.0, 16.0) | 14.0 (10.8, 15.4) | 14.4 (13.9, 15.5) | 2.1 (0.8, 8.8) |
| Sex Male |
11 (40.7%) | 5 (15.6%) | 6 (37.5%) | 10 (58.8%) |
| Race White |
20 (74.1%) | 30 (93.8%) | 12 (75.0%) | 5 (29.4%) |
| African American/black | 3 (11.1%) | 0 (0.0%) | 3 (18.8%) | 4 (23.5%) |
| Asian | 3 (11.1%) | 1 (3.1%) | 0 (0.0%) | 3 (17.7%) |
| Other | 1 (3.7%) | 1 (3.1%) | 1 (6.3%) | 5 (29.4%) |
| Ethnicity Hispanic |
0 (0%) | 4 (12.5%) | 1 (6.3%) | 1 (5.9%) |
TABLE 2.
JIA Disease Characteristics
| Active Untreated and Quiescent Treated JIA (n = 59) | Active Untreated JIA (n = 27) | Quiescent Treated JIA (n = 32) | |
|---|---|---|---|
| JIA category Polyarticular |
14 (23.7%) | 6 (22.2%) | 8 (25.0%) |
| Psoriatic | 12 (20.3%) | 6 (22.2%) | 6 (18.8%) |
| Spondyloarthropathy | 33 (56.0%) | 15 (55.6%) | 18 (56.2%) |
| Disease duration (mean) | 6–12 mo | 3–6 mo | 2–3 y |
| JADAS-71 scores, median (interquartile range) | 5.5 (2.5, 12) | 12 (6.4, 18.5) | 3 (0.5, 5) |
| Medications NSAIDs |
40 (67.8%) | 27 (100.0%) | 13 (40.6%) |
| Methotrexate | 11 (18.6%) | 0 (0.0%) | 11 (34.4%) |
| Anti–tumor necrosis factor | 21 (35.6%) | 0 (0.0%) | 21 (65.6%) |
| ANA+ | 28 (47.4%) | 10 (37.0%) | 17 (53.1%) |
| B27+ | 11 (18.6%) | 4 (14.8%) | 16 (50.0%) |
| RF+ | 4 (6.8%) | 2 (7.4%) | 13 (40.6%) |
| CCP+ | 8 (13.5%) | 4 (14.8%) | 11 (34.4%) |
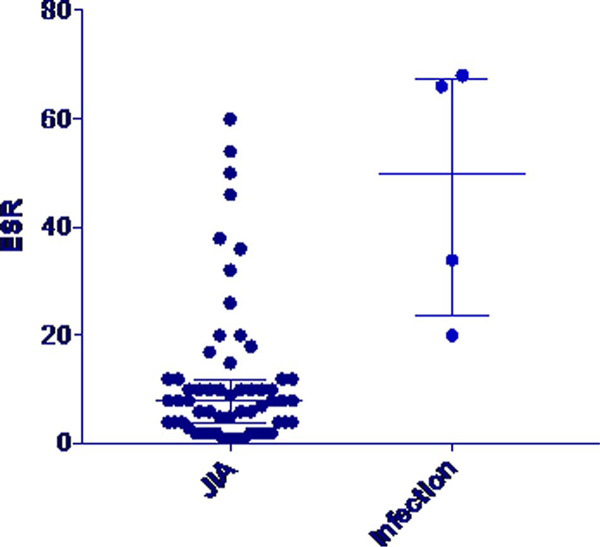
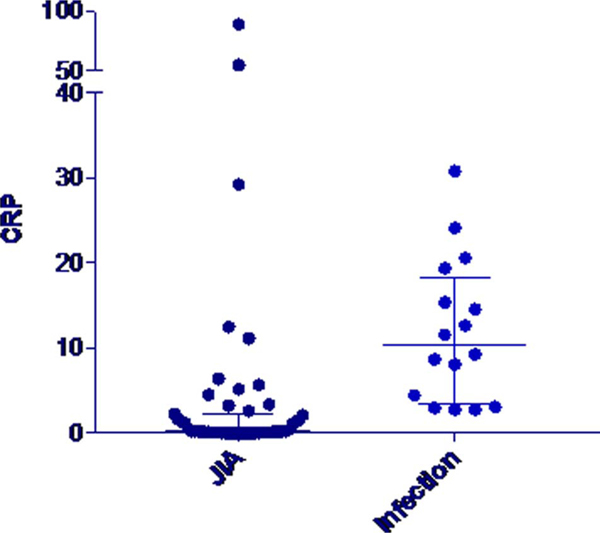
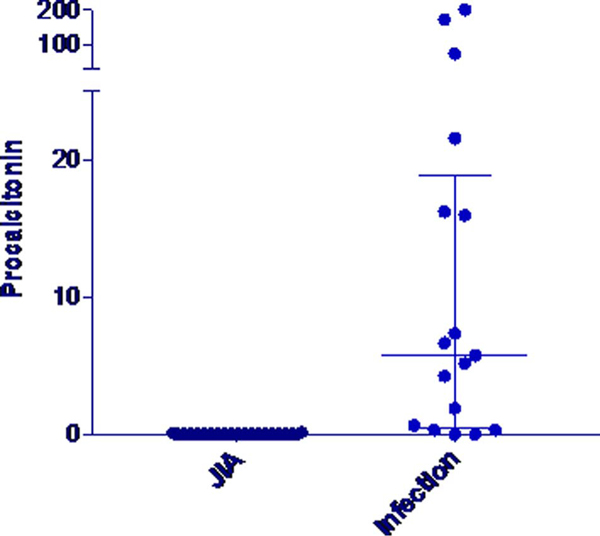
Erythrocyte sedimentation rate, CRP, and PCT were all elevated in bacteremic patients in comparison to the other groups (Table 3). Erythrocyte sedimentation rate and CRP both had wide ranges that overlapped between all groups (FIGURE 1 and 2). The PCT concentration did not exceed 0.15 μg/mL, the standard cutoff for suspicion for infection, in any of the patients with active JIA and in only 1 of 32 patients with quiescent JIA. In contrast, PCT concentrations exceeded this level in 16 of 17 bacteremic patients (Fig. 3). Procalcitonin did not differ between patients with active and quiescent JIA. Based on these data, we compared children with active and quiescent JIA to those with bacteremia, and sensitivity and specificity for PCT and infection were 94.1% and 98.3%, respectively (Table 4), and positive and negative predictive values were 94.1% and 98.3%, respectively.
TABLE 3.
Laboratory Values
| Active Untreated JIA (n = 27) | Quiescent Treated JIA (n = 32) | Healthy Controls (n = 16) | Bacteremic Patients (n = 17) | p value | |
|---|---|---|---|---|---|
| White blood cells, ×109/L | 7.0 (5.6, 8.2) | 7.4 (6.2, 8.2) | 6.9 (5.3, 8.4) | 7.7 (4.9, 13.0) | 0.72 |
| Hemoglobin, g/dL | 12.6 (11.9, 13.3) | 13.1 (12.4, 13.9) | 14.0 (12.5, 14.3) | 9.9 (9.3, 11.1) | <0.001 |
| Platelets, ×109/L ESR, mm/h |
288 (248, 353) 10 (6, 20) |
273 (218, 308) 6 (2, 10) |
293 (243, 316) 8 (5, 10) |
200 (148, 259) 50 (27, 67) |
0.004 <0.001 |
| CRP, mg/dL | 1.40 (0.12, 5.20) | 0.18 (0.06, 0.57) | 0.42 (0.14, 1.56) | 10.45 (3.80, 17.40) | <0.001 |
| PCT, μg/mL | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 6.68 (1.93, 21.58) | <0.001 |
Values are presented as median (interquartile range).
FIGURE 1.
Erythrocyte sedimentation rate values in JIA versus infection.
FIGURE 2.
C-reactive protein values in JIA versus infection.
FIGURE 3.
Procalcitonin values in JIA versus infection.
TABLE 4.
Sensitivity, Specificity, and Positive and Negative Predictive Values for PCT
| Point Estimate | Confidence Interval | |
|---|---|---|
| Sensitivity | 94.1% | 71.3%–99.9% |
| Specificity | 98.3% | 90.9%–99.9% |
| Positive predictive value | 94.1% | 69.5%–99.1% |
| Negative predictive value | 98.3% | 89.7%–99.7% |
DISCUSSION
To our knowledge, this is the first study to examine PCT specifically in children with JIA, for its utility in differentiating infection and noninfectious inflammatory disease activity. Korczowski et al.19 evaluated PCT, CRP, and ESR in 28 children with autoimmune disease, 9 of whom had juvenile arthritis. They found that while ESR and CRP are sometimes elevated in children with autoimmune disease, PCT remains low in this population. This study concluded that PCT may be useful for differentiation of infection and inflammatory disease activity; however, sample size was small, and findings were not disease specific.
Recently, multiple studies have been done evaluating PCT and additional biomarkers for differentiation of infection and inflammatory disease activity in systemic autoimmune diseases, especially systemic lupus erythematosus.20–22 These studies have repeatedly shown that PCT, in conjunction with other biomarkers, can reliably differentiate infection from an inflammatory disease flare. A meta-analysis performed by Serio et al.23 in 2014 confirmed this finding.
Here, we evaluated PCTas a marker for infectionversus noninfectious inflammation in children with JIA specifically. Our study indicates that serum ESR, CRP, and PCT levels are all biomarkers that can be used to distinguish serious bacterial infection (SBI) from active JIA at presentation. However, PCT is the most accurate, with the least overlap between patients with infection and noninfectious inflammatory arthritis and a high sensitivity and specificity. Although 1 child with quiescent JIA had a mildly elevated PCT level, we hypothesize that this patient may have had a subclinical infection or that this result was due to laboratory error. This finding can help clinicians direct therapy more accurately and swiftly at disease onset. However, PCT is not a useful biomarker to assess disease activity in JIA, as PCT levels did not differ between patients with active untreated JIA and quiescent treated JIA.
It is important to note that a majority of our study subjects had spondyloarthritis. However, we believe that these results are generalizable, as no difference was seen between children with spondyloarthritis compared with other JIA subtypes.
Some strengths of this study include recruitment of multiple groups of patients for comparison, including children with active JIA, quiescent JIA, healthy presurgical candidates, and children with SBI. Some limitations of our study are that this was performed at a single center with a limited sociodemographic population. It is important to note that the age and race differed between the group of patients with infection and the other 3 groups; however, there are currently no data to support that PCT differs by age or race. In addition, children with SBI were utilized as an infectious comparator group; however, our findings may not be able to be extrapolated to less severe infections. We also utilized unique definitions of active and quiescent disease for 2 reasons: (1) in order to minimize confounding by treatment, as no studies exist that describe the effects of arthritis medications on PCT levels; and (2) because many children have low disease activity, few children meet the standard criteria for inactive disease. Finally, we were not able to evaluate children with JIA with active infection, as these children typically present to their pediatricians, making capturing these patients exceedingly difficult.
This study is the first step toward establishing a biomarker panel that can differentiate infection and inflammatory disease activity in children with JIA. We have shown that in this population of patients PCT remains reliably low, even when disease is active and other more commonly used inflammatory markers are elevated, which is a new finding in JIA. Further studies are needed to assess additional biomarkers in conjunction with PCT for distinction of infection and disease flare, throughout the disease course. Further evaluation and measurement of serum PCT for differentiating JIA flares from less severe infections are also required.
Acknowledgments
This study was funded by a grant received from the Inflammatory Arthritis Center of Excellence at Hospital for Special Surgery.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Sacks JJ, Helmick CG, Luo YH, et al. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001–2004. Arthritis Rheum. 2007;57:1439–1445. [DOI] [PubMed] [Google Scholar]
- 2.Funk RS, Chan MA, Becker ML. Cytokine biomarkers of disease activity and therapeutic response after initiating methotrexate therapy in patients with juvenile idiopathic arthritis. Pharmacotherapy. 2017;37: 700–711. [DOI] [PubMed] [Google Scholar]
- 3.Bojko J. Measurement of blood calprotectin (MRP-8/MRP-14) levels in patients with juvenile idiopathic arthritis. Reumatologia. 2017;55: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashaad NI, Fawzy RM, Elazem AA, et al. Serum calreticulin as a novelbiomarker of juvenile idiopathic arthritis disease activity. Eur J Rheumatol. 2017;4:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward DT, Hansen EN, Takemoto SK, et al. Cost and effectiveness of postoperative fever diagnostic evaluation in total joint arthroplasty patients. J Arthroplasty. 2010;25(Suppl 6):43–48. [DOI] [PubMed] [Google Scholar]
- 6.Becerra Nakayo EM, García Vicente AM, Soriano Castrejón AM, et al. Analysis of cost-effectiveness in the diagnosis of fever of unknown origin and the role of (18)F-FDG PET-CT: a proposal of diagnostic algorithm [in Spanish]. Rev Esp Med Nucl Imagen Mol. 2012;31:178–186. [DOI] [PubMed] [Google Scholar]
- 7.Pathobiochemistry Meisner M. and clinical use of procalcitonin. Clin Chim Acta. 2002;323:17–29. [DOI] [PubMed] [Google Scholar]
- 8.Milcent K, Faesch S, Gras-Le Guen C, et al. Use of procalcitonin assays to predict serious bacterial infection in young febrile infants. JAMA Pediatr. 2016;170:62–69. [DOI] [PubMed] [Google Scholar]
- 9.Hu R, Gong Y, Wang Y. Relationship of serum procalcitonin levels to severity and prognosis in pediatric bacterial meningitis. Clin Pediatr. 2015; 54:1141–1144. [DOI] [PubMed] [Google Scholar]
- 10.Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and c-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004;39:206–217. [DOI] [PubMed] [Google Scholar]
- 11.Hoshina T, Nanishi E, Kanno S, et al. The utility of biomarkers in differentiating bacterial from non-bacterial lower respiratory tract infection in hospitalized children: difference of the diagnostic performance between acute pneumonia and bronchitis. J Infect Chemother. 2014;20: 616–620. [DOI] [PubMed] [Google Scholar]
- 12.Teng X, Wang Y, Lin N, et al. Evaluation of serum procalcitonin and C-reactive protein levels as biomarkers of Henoch-Schönlein purpura in pediatric patients. Clin Rheumatol. 2016;35:667–671. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez SR, Martin B, Heizer H, et al. Procalcitonin (PCT) and Kawasaki disease: does PCT correlate with IVIG-resistant disease, admission to the intensive care unit, or development of coronary artery lesions? J Pediatr Infect Dis Soc. 2016;5:297–302. [DOI] [PubMed] [Google Scholar]
- 14.Ruperto N, Ravelli A, Pistorio A, et alPaediatric Rheumatology International Trials Organisation (PRINTO); Pediatric Rheumatology Collaborative Study Group (PRCSG). The Provisional Paediatric Rheumatology International Trials Organisation/American College of Rheumatology/European League Against Rheumatism Disease Activity Core Set for the Evaluation of Response to Therapy in Juvenile Dermatomyositis: a prospective validation study. Arthritis Rheum. 2008;59: 4–13. [DOI] [PubMed] [Google Scholar]
- 15.El-Sayed D, Grotts J, Golgert WA, et al. Sensitivity and specificity of procalcitonin in predicting bacterial infections in patients with renal impairment. Open Forum Infect Dis. 2014;1:ofu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovas A, Agoston Z, Késmárky K, et al. Extreme procalcitonin elevation without proven bacterial infection related to amphetamine abuse. Case Rep Crit Care. 2014;2014:179313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consolaro A, Ruperto N, Bazso A. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:658–666. [DOI] [PubMed] [Google Scholar]
- 18.Pouchot J, Ecosse E, Coste J, et al. Validity of the Childhood Health Assessment Questionnaire is independent of age in juvenile idiopathic arthritis. Arthritis Rheum. 2004;51:519–526. [DOI] [PubMed] [Google Scholar]
- 19.Korczowski B, Kowalczyk JR, Bijak M, et al. Concentration of procalcitonin and C-reactive protein in serum and erythrocyte sedimentation rate in active autoimmune diseases in children [in Polish]. Pol Merkur Lekarski. 2003;15:155–157. [PubMed] [Google Scholar]
- 20.Chen DY, Chen YM, Ho WL, et al. Diagnostic value of procalcitonin for differentiation between bacterial infection and non-infectious inflammation in febrile patients with active adult-onset Still’s disease. Ann Rheum Dis. 2009;68:1074–1075. [DOI] [PubMed] [Google Scholar]
- 21.Quintana G, Medina YF, Rojas C, et al. The use of procalcitonin determinations in evaluation of systemic lupus erythematosus. J Clin Rheumatol. 2008;14:138–142. [DOI] [PubMed] [Google Scholar]
- 22.Beça S, Rodríguez-Pintó I, Alba MA, et al. Development and validation ofa risk calculator to differentiate flares from infections in systemic lupus erythematosus patients with fever. Autoimmun Rev. 2015;14:586–593. [DOI] [PubMed] [Google Scholar]
- 23.Serio I, Arnaud L, Mathian A, et al. Can procalcitonin be used to distinguish between disease flare and infection in patients with systemic lupus erythematosus: a systematic literature review. Clin Rheumatol. 2014;33:1209–1215. [DOI] [PubMed] [Google Scholar]