Abstract
Objective
To examine the association between the omicron adapted bivalent mRNA covid-19 booster vaccines received as a fourth dose and risk of adverse events.
Design
Nationwide cohort study.
Setting
Denmark.
Participants
2 225 567 adults aged ≥50 years who received three covid-19 vaccine doses during the study period, 1 January 2021 to 10 December 2022.
Main outcome measures
The main outcome measure was rates of hospital visits for 27 different adverse events in a 28 day main risk period after vaccination with a bivalent omicron adapted mRNA booster vaccine as a fourth dose compared with reference period rates from day 29 after the third or fourth vaccine dose and onward.
Results
1 740 417 adults (mean age 67.8 years, standard deviation 10.7 years) received a bivalent mRNA vaccine as a fourth dose. Fourth dose vaccination with a bivalent mRNA vaccine was not associated with a statistically significant increased rate of any of the 27 adverse outcomes within 28 days (eg, incidence rate ratio of 0.95, 95% confidence interval of 0.87 to 1.04 for ischaemic cardiac events based on 672 v 9992 events during the compared 28 day risk and reference period, respectively), nor when analysed according to age, sex, or vaccine type, or when using alternative analytical approaches. However, post hoc analysis detected signals for myocarditis (statistically significant in female participants), although the outcome was rare and findings were based on few cases. No risk of cerebrovascular infarction was found (incidence rate ratio 0.95, 95% confidence interval 0.87 to 1.05; 644 v 9687 events).
Conclusions
The use of bivalent mRNA vaccines as a fourth vaccine dose against covid-19 was not associated with an increased risk of 27 different adverse events in adults aged ≥50 years.
Introduction
Bivalent mRNA booster doses with either the Pfizer-BioNTech vaccine Comirnaty (BNT162b2, original strain and omicron variant BA.4-5 or BA.1), or the Moderna vaccine Spikevax (mRNA-1273, original and BA.1) that target the original (ancestral) strain of SARS-CoV-2 and omicron variant BA.4-5 or BA.1, were authorised for use in early autumn 2022.1 2 3 4 5 These bivalent omicron adapted mRNA booster vaccines have subsequently been recommended as single boosters after the completion of a primary vaccination series, with or without a (original) monovalent first booster (ie, a third vaccine dose).1 2 3 4 5
In Denmark, rollout of the bivalent mRNA boosters started on 15 September 2022 and were recommended and offered to all adults aged ≥50 years as well as to those considered at high risk of severe covid-19. As the vaccine coverage of the third dose (ie, first booster) has been high in Denmark (>90% for the targeted populations),6 the bivalent mRNA boosters have predominantly been administered as a fourth dose (ie, second booster) to the general population aged ≥50 years.
Regulatory safety evaluations of the adapted bivalent mRNA booster vaccines have primarily been based on available preauthorisation clinical trials, post-marketing safety data of the monovalent mRNA vaccines, and clinical data on immunogenicity and reactogenicity of the bivalent mRNA vaccines.5 7 8 9 10 11 12 13 14 One recent report from the US Centers for Disease Control and Prevention (CDC) of the early reported safety findings (mostly related to reactogenicity) through v-safe and the Vaccine Adverse Event Reporting System for the bivalent boosters containing BA.4-5 (ie, as ≥3 doses) appeared to be similar to those previously observed for monovalent vaccine boosters (not statistically analysed).15 Consequently, data to adequately inform about the potential risk of adverse events associated with the bivalent mRNA vaccines are warranted.
In a nationwide cohort study, we investigated the association between vaccination using bivalent mRNA booster vaccines as a fourth dose and the risk of 27 adverse events.
Methods
Setting and study population
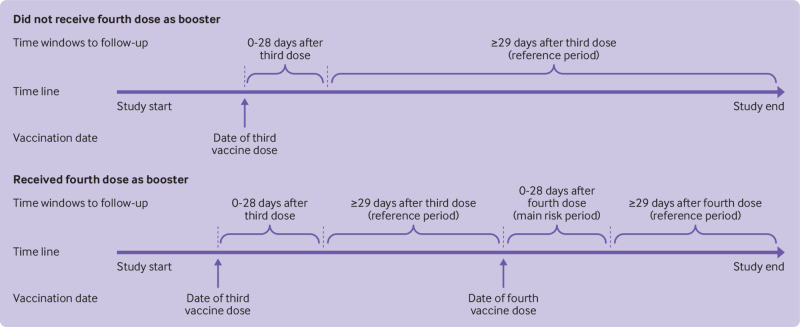
By use of the unique identifier assigned to all residents of Denmark at birth or immigration, we linked data on covid-19 vaccination, doctor assigned diagnoses, and other covariates from the nationwide healthcare registers, to construct a population based cohort representative of the general Danish population potentially eligible for a fourth dose of bivalent mRNA booster vaccine during autumn 2022.16 17 18 19 As such, to be included in our cohort an individual had to be born in 1972 or earlier (that is, turning 50 years or older in 2022; age was defined by 2022 minus birth year), to be a Danish resident at baseline 1 January 2021, and have received three previous vaccine doses with the monovalent Pfizer-BioNTech or Moderna vaccines, or both (or potentially AZD1222 vaccine (ChAdOx1 nCoV-19, Oxford-AstraZeneca) as part of primary vaccination) (see supplementary table S1). We excluded adults if they received the third dose within 90 days of the second dose (to ensure that the administered third dose was truly a first booster) or before 9 September 2021 (the national rollout date of the first booster). Similarly, censoring took account of those who had been vaccinated with a fourth dose if the vaccine was received before 15 September 2022 or within 90 days of the third dose. Vaccination status was classified in a time varying manner during the period from 1 January 2021 to 10 December 2022. The main risk period of interest was within 28 days of vaccination with the bivalent mRNA booster as a fourth dose, with the reference period from day 29 after a third or fourth dose and onward.
Outcomes
We included 27 different adverse events, adapted from prioritised lists of adverse events of special interest for the covid-19 vaccines.20 21 22 During follow-up, we identified outcome events as any hospital visits (that is, regardless of duration, including both inpatient, outpatient, and emergency contacts) where an outcome diagnosis was recorded as the primary reason for seeking hospital care (see supplementary table S2 for ICD-10 (international classification of diseases, 10th revision) codes).16 We included only incident events by excluding those with a history of the respective studied outcome during a washout period from 1 January 2018 to day 28 after the third dose. The date of admission served as the event date. Each of the 27 outcomes was studied separately.
Statistical analysis
Participants were followed from day 29 after the third dose until first outcome event, emigration or loss of contact, death, receipt of a fifth vaccine dose (such a dose was not implemented in the general Danish rollout strategy of covid-19 vaccines during the study period), or end of the study period. Individuals could contribute with person time during both the main 28 day risk period and the two reference periods; number of events and person time from the two reference periods were aggregated. We used Poisson regression to estimate adjusted incidence rate ratios, with corresponding 95% confidence intervals, comparing the outcome rates during the risk period with those during the reference periods. The model was adjusted for sex, age groups (50-64, 65-79, and ≥80 years), ethnicity (Nordic, western, non-western), region of residence, considered at high risk of severe covid-19 (yes or no), calendar time (three month bins), and number of comorbidities (defined as asthma, other chronic respiratory disorders, chronic cardiac disorders, renal disorders, diabetes, autoimmune related disorders, epilepsy, malignancies, and psychiatric disorders).
In secondary analysis, we analysed the associations by sex, age, and bivalent booster type; assessed the risk of severe outcome events, as defined by hospital contacts of ≥5 hours; included both the primary and the secondary outcome definition; and altered the main risk period to 7, 14, and 90 days after the fourth vaccine dose. The 28 day period after fourth dose vaccination was chosen as the main risk period for the primary analysis as this time interval offers a good balance between outcome sensitivity and outcome specificity (that is, also capturing medium term adverse events without too long follow-up that could potentially dilute any short term acute signals). The use of alternative risk periods was included to evaluate the sensitivity of our findings towards both shorter and longer risk periods. Furthermore, the robustness of the results from the primary analysis were evaluated using two alternative analytical approaches. Firstly, nested within our study cohort, a self-controlled case series analysis was performed23; in this design, only individuals experiencing an (incident) event were included, and we used conditional Poisson regression to estimate incidence rate ratios according to vaccination status. For the self-controlled case series analysis, we excluded the 14 days before the fourth vaccine dose from the reference period (to account for potential event dependent exposure bias). An advantage of the self-controlled case series design is that time periods are compared within study individuals and as such indirectly adjust for confounders that do not vary over the study period, such as many lifestyle and socioeconomic factors or comorbidities. Secondly, we compared the ratio between the observed rate of the studied outcome with the expected rate, with 95% confidence intervals, obtained from the Poisson distribution. The expected rates were estimated using indirect age standardisation and sex standardisation of the historical background rates within the Danish general population from 1 January 2018 to 31 December 2019 (with a washout period from 1 January 2015 to 31 December 2017). Moreover, in additional sensitivity analyses, we adjusted for a first SARS-CoV-2 infection, included as a time varying covariate, adjusted for calendar time on a monthly basis, and stratified analyses by the reference period of ≥29 days after a third and fourth vaccine dose.
In post hoc analysis, we specifically investigated the risk of cerebrovascular infarction, myocarditis, and pericarditis separately (owing to the particular scientific interest in these outcomes), including by sex and age subgroups as well as by type of bivalent booster vaccine. In addition, for the outcome of cerebrovascular infarction we stratified according to concurrent influenza vaccination. We used SAS version 9.4 and R version 4.0.2 for data management, and R version 4.0.2 for statistical analysis.
Patient and public involvement
No patients were involved in setting the research question, the outcome measures, or developing plans for design or implementation of the study owing to funding constraints. No patients were asked to advise on interpretation or writing up of results.
Results
Study population
The study cohort comprised a total of 2 225 567 adults (mean age 66.9 years, standard deviation (SD) 11.0 years, and 52.0% were female participants) who had received at least three covid-19 vaccine doses (91.2% received a monovalent Pfizer-BioNTech vaccine as the third dose), of whom 1 740 417 (78.2%, mean age 68.7 years, SD 10.7 years) received a fourth dose with a bivalent omicron adapted mRNA booster vaccine from 15 September 2022 to 10 December 2022 (fig 1 and table 1). The median time between receiving the third and fourth vaccine dose was 313 days (interquartile range 301-329 days); see supplementary figure S1 for the distribution of follow-up person time during the study period. Of the bivalent mRNA boosters, BNT162b2 with BA.4-5 (Pfizer-BioNTech) was the most commonly administered (1 157 754 vaccine recipients, 66.5%), followed by BNT162b2 with BA.1 and mRNA-1273 (Moderna) with BA.1 (526 397, 30.2% and 56 266, 3.3%, respectively). Except for chronic cardiac disorders (almost 10.0%), most comorbidities had a prevalence of ≤5.0% within the cohort.
Fig 1.
Overview of study design. The main risk period was days 0-28 after receipt of a bivalent covid-19 mRNA booster as fourth dose. The total reference period consisted of ≥29 days after a third dose (up to the day before the fourth dose (ie, day 0 minus 1 day)) and the fourth dose (second booster). See supplementary figure S1 for the distribution of reference period and main risk period person time during the study period
Table 1.
Characteristics of study participants vaccinated with a monovalent mRNA vaccine as a third dose and a bivalent omicron adapted vaccine as a fourth dose in Denmark. Values are number (percentage) unless stated otherwise
| Characteristics | Vaccine status | ||
|---|---|---|---|
| Three doses (entire cohort) | Fourth dose: bivalent booster | Fourth dose: no bivalent booster* | |
| Total No vaccinated | 2 225 567 | 1 740 417 | 485 150 |
| Mean (SD) age (years) | 66.9 (11.0) | 67.8 (10.7) | 63.7 (11.6) |
| Female | 1 158 395 (52.0) | 922 067 (53.0) | 236 328 (48.7) |
| Ethnicity: | |||
| Nordic countries | 2 081 076 (93.5) | 1 655 558 (95.1) | 425 518 (87.7) |
| Non-western countries | 89 347 (4.0) | 47 037 (2.7) | 42 310 (8.7) |
| Western countries | 55 179 (2.5) | 37 846 (2.2) | 17 333 (3.6) |
| Region of residency: | |||
| Northern Denmark Region | 240 019 (10.8) | 189 601 (10.9) | 50 418 (10.4) |
| Central Denmark Region | 503 569 (22.6) | 398 743 (22.9) | 104 826 (21.6) |
| Region of Southern Denmark | 500 917 (22.5) | 389 586 (22.4) | 111 331 (22.9) |
| Capital Region of Denmark | 622 056 (28.0) | 482 125 (27.7) | 139 931 (28.8) |
| Region Zealand | 359 006 (16.1) | 280 362 (16.1) | 78 644 (16.2) |
| Vaccination priority groups: | |||
| High risk of severe covid-19 | 107 983 (4.9) | 72 707 (4.2) | 35 276 (7.3) |
| Others | 2 117 584 (95.1) | 1 667 710 (95.8) | 449 874 (92.7) |
| Vaccine received as third dose: | |||
| BNT162b2 | 2 029 339 (91.2) | 1 587 589 (91.2) | 441 750 (91.1) |
| mRNA-1273 | 196 228 (8.8) | 152 828 (8.8) | 43 400 (8.9) |
| Vaccine received as fourth dose†: | |||
| Bivalent‡: | 1 740 417 (78.2) | 1 740 417 (100) | NA |
| BNT162b2 with BA.4-5 | 1 157 754 (52.0) | 1 157 754 (66.5) | NA |
| BNT162b2 with BA.1 | 526 397 (23.7) | 526 397 (30.2) | NA |
| mRNA-1273 with BA.1 | 56 266 (2.5) | 56 266 (3.3) | NA |
| Monovalent | 53 025 (2.4) | NA | 53 025 (10.9) |
| None | 432 125 (19.4) | NA | 432 125 (89.1) |
| Comorbidities: | |||
| Asthma | 31 236 (1.4) | 24 998 (1.4) | 6238 (1.3) |
| Chronic respiratory disorder | 52 497 (2.4) | 38 581 (2.2) | 13 916 (2.9) |
| Chronic cardiac disorder | 215 737 (9.7) | 170 772 (9.8) | 44 965 (9.3) |
| Renal disorder | 21 103 (0.9) | 13 664 (0.8) | 7439 (1.5) |
| Diabetes | 80 978 (3.6) | 62 290 (3.6) | 18 688 (3.9) |
| Autoimmune related disorder | 112 385 (5.0) | 84 719 (4.9) | 27 666 (5.7) |
| Epilepsy | 13 933 (0.6) | 10 723 (0.6) | 3210 (0.7) |
| Malignancy | 118 556 (5.3) | 81 375 (4.7) | 37 181 (7.7) |
| Psychiatric disorder | 86 531 (3.9) | 61 330 (3.5) | 25 201 (5.2) |
NA=not applicable; SD=standard deviation.
Percentages within a variable may not sum to 100 owing to rounding.
Not vaccinated with a bivalent booster as a fourth vaccine dose as of 10 December 2022 (end of study period).
BNT162b2 is manufactured by Pfizer-BioNTech and mRNA-1273 is manufactured by Moderna.
Those who received a bivalent vaccine as fourth dose were from the cohort that received three vaccine doses. The reference period consisted of person time of ≥29 days after third and fourth dose vaccination. Figure 1 provides an overview of the study design, and supplementary table S1 provides definitions of variables.
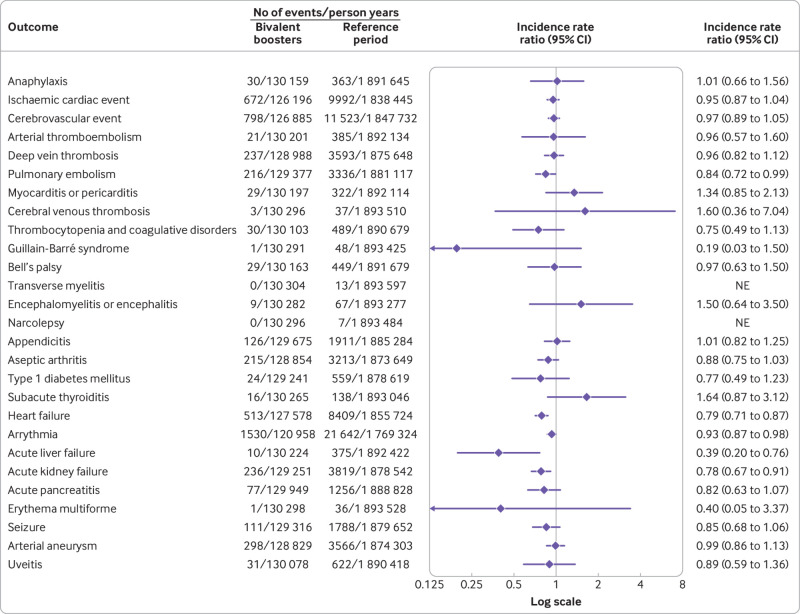
Risk of adverse events
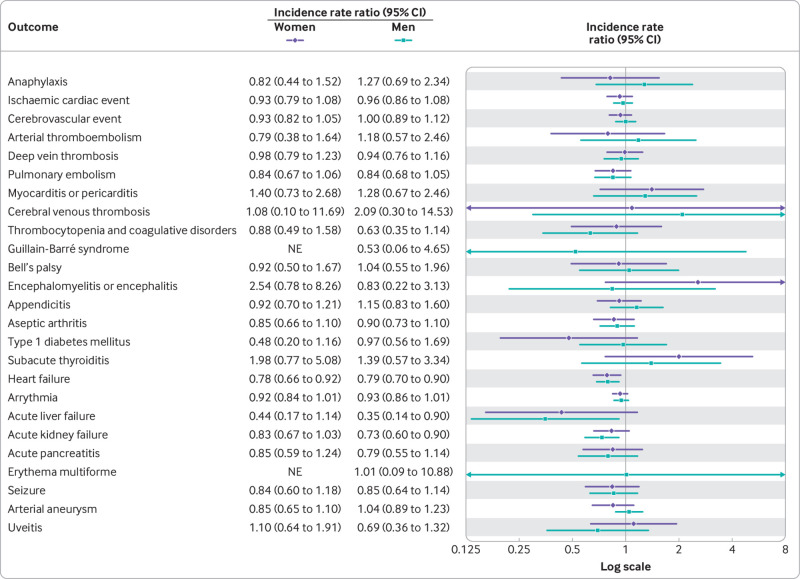
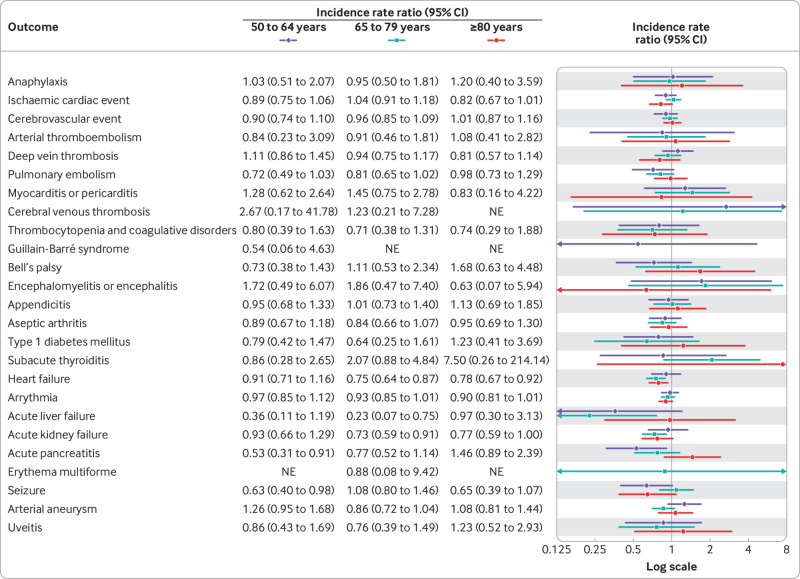
A fourth dose of a bivalent mRNA booster vaccine was not associated with a statistically significant increased rate of hospital visits for any of the 27 different adverse events within 28 days after vaccination compared with the reference period rates (fig 2). Similarly, no significant associations were found when stratifying by sex and age groups (fig 3, fig 4, and supplementary table S3) nor by type of bivalent mRNA booster received (fig 5 and supplementary table S4). Using the alternative analytical approaches of self-controlled case series (supplementary table S5) and observed versus expected (supplementary table S6), analyses showed comparable results to those of our main analysis (except for a rate ratio of 2.31 (95% confidence interval 1.32 to 3.75) for subacute thyroiditis based on 16 observed v 6.9 expected cases). When the risk of severe adverse events, defined as hospital visits of ≥5 hours, were compared, the results were almost similar to those of the main analysis (supplementary table S7; no cases of subacute thyroiditis requiring hospital admission for ≥5 hours were observed during the 28 day period after a fourth dose with a bivalent booster). Results did not change when both primary and secondary diagnoses were included in the outcome definitions (supplementary table S8). Our secondary analysis with use of different risk periods of 7, 14, and 90 days after a fourth dose with a bivalent booster showed an increased risk of myocarditis or pericarditis (incidence rate ratio 1.88, 95% confidence interval 1.13 to 3.11) within 14 days only (supplementary table S9); myocarditis and pericarditis were examined separately in post hoc analyses. The results of analyses with additional adjustment for a first SARS-CoV-2 infection (supplementary table S10), calendar time in monthly bins (supplementary table S11), and stratification of the reference period according to the period of ≥29 days after a third and fourth dose (ie, first or second booster; supplementary table S12) were all similar to those of the primary analysis.
Fig 2.
Risk of adverse events within 28 days of vaccination with a bivalent omicron adapted mRNA booster vaccine as fourth dose in Danish adults aged ≥50 years from 15 September 2022 to 10 December 2022. Each outcome was studied separately, explaining slight differences in denominators owing to different exclusions. NE=not estimable
Fig 3.
Risk of adverse events within 28 days of vaccination with a bivalent omicron adapted mRNA booster vaccine as fourth dose in Danish adults aged ≥50 years from 15 September 2022 to 10 December 2022 by sex. Each outcome was studied separately, explaining slight differences in denominators owing to different exclusions. NE=not estimable
Fig 4.
Risk of adverse events within 28 days of vaccination with a bivalent omicron adapted mRNA booster vaccine as fourth dose in Danish adults aged ≥50 years from 15 September 2022 to 10 December 2022 by age subgroups. Each outcome was studied separately, explaining slight differences in denominators owing to different exclusions. NE=not estimable
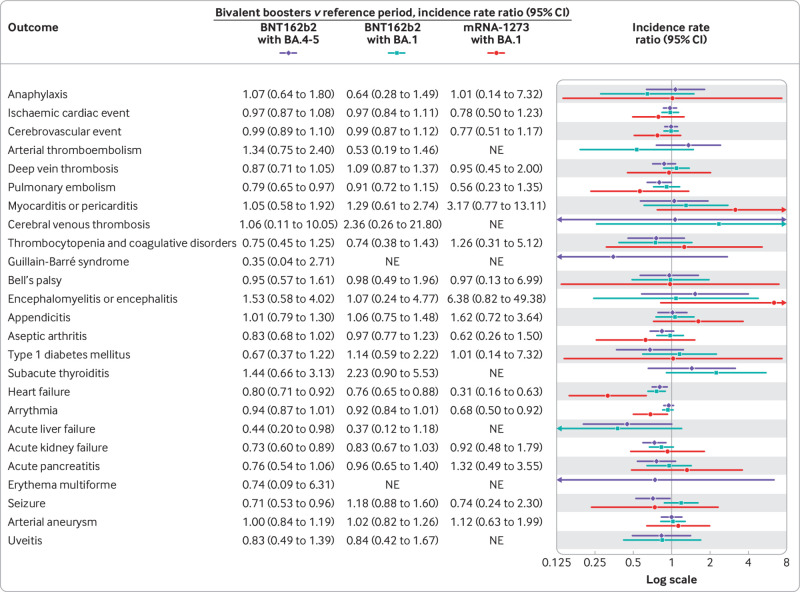
Fig 5.
Risk of adverse events within 28 days of vaccination with a bivalent omicron adapted mRNA booster vaccine as fourth dose in Danish adults aged ≥50 years from 15 September 2022 to 10 December 2022 by type of vaccine received. Each outcome was studied separately, explaining slight differences in denominators owing to different exclusions. *Corresponding number of events and person years are presented in supplementary table S3. NE=not estimable
Post hoc analysis
No association was observed between a fourth dose using a bivalent mRNA booster vaccine and risk of cerebrovascular infarction (incidence rate ratio 0.95, 95% confidence interval 0.87 to 1.05), including by sex, age, and type of bivalent vaccine (table 2 and supplementary table S13). We observed a total of nine events of myocarditis and 22 events of pericarditis within 28 days after a fourth dose with a bivalent mRNA vaccine (equal to incidence rates of 5.3 and 13.0 cases within 28 days per 1 000 000 vaccinated, respectively; reference period incidence rates were 2.6 and 10.7 per 28 days among 1 000 000 individuals, respectively). The corresponding incidence rate ratio was 2.65 (95% confidence interval 1.01 to 6.97) for myocarditis and 1.21 (0.72 to 2.03) for pericarditis. Statistically significant associations with myocarditis were observed for female participants and booster doses of BNT162b2 with BA.4-5 and mRNA-1273 with BA.1; the point estimate was similarly increased for a booster dose of BNT162b2 with BA.1, but this was not statistically significant. Examining the associated risk of myocarditis with the individual vaccine types by sex and age groups showed statistically significant associations in female participants for all three vaccine types and in individuals aged 50 to 64 years who had received the mRNA-1273 booster (supplementary table S13). All subgroup analyses of myocarditis risk were, however, based on six or fewer events among those who had received a bivalent mRNA booster as a fourth dose. No associations with pericarditis were found in any analysis. When a fourth dose of a bivalent mRNA vaccine was stratified according to concurrent influenza vaccination for the outcome of cerebrovascular infarction, the incidence rate ratio was 0.93 (0.83 to 1.04) among those vaccinated on the same day (supplementary table S14).
Table 2.
Risk of cerebrovascular infarction, myocarditis, and pericarditis after vaccination with a bivalent omicron adapted booster as fourth dose within 28 days in Danish adults aged ≥50 year during 15 September 2022 to 10 December 2022, including by sex, age, and vaccine type
| Outcome by subgroup | Bivalent booster | Reference period | Incidence rate ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Events/person years | Rate/1 000 000* | Events/person years | Rate/1 000 000* | |||||
| Cerebrovascular infarction (including TIA) | ||||||||
| All | 644/127 304 | 387.8 | 9687/1 853 451 | 400.7 | 0.95 (0.87 to 1.05) | |||
| Sex: | ||||||||
| Female | 290/67 794 | 327.9 | 4317/974 392 | 339.6 | 0.91 (0.79 to 1.05) | |||
| Male | 354/59 511 | 456.0 | 5370/879 059 | 468.3 | 0.99 (0.88 to 1.13) | |||
| Age group (years): | ||||||||
| 50-64 | 113/52 983 | 163.5 | 1910/858 398 | 170.6 | 0.96 (0.78 to 1.20) | |||
| 65-79 | 315/55 419 | 435.7 | 4426/728 948 | 465.5 | 0.94 (0.82 to 1.07) | |||
| ≥80 | 216/18 902 | 876.0 | 3351/266 105 | 965.4 | 0.98 (0.83 to 1.15) | |||
| Vaccine type†: | ||||||||
| BNT162b2 with BA.4-5 | 377/84 259 | 343.0 | 9687/1 853 451 | 400.7 | 0.97 (0.86 to 1.09) | |||
| BNT162b2 with BA.1 | 246/38 856 | 485.3 | 9687/1 853 451 | 400.7 | 0.98 (0.85 to 1.12) | |||
| mRNA-1273 with BA.1 | 21/4189 | 384.3 | 9687/1 853 451 | 400.7 | 0.83 (0.54 to 1.29) | |||
| Myocarditis | ||||||||
| All | 9/130 288 | 5.3 | 64/1 893 405 | 2.6 | 2.65 (1.01 to 6.97) | |||
| Sex: | ||||||||
| Female | 6/69 066 | 6.7 | 29/991 433 | 2.2 | 3.89 (1.05 to 14.50) | |||
| Male | 3/61 223 | 3.8 | 35/901 972 | 3.0 | 1.60 (0.36 to 7.07) | |||
| Age group (years): | ||||||||
| 50-64 | 4/53 520 | 5.7 | 30/866 339 | 2.7 | 3.29 (0.75 to 14.46) | |||
| 65-79 | 4/56 889 | 5.4 | 27/747 644 | 2.8 | 1.79 (0.45 to 7.08) | |||
| ≥80 | 1/19 880 | 3.9 | 7/279 421 | 1.9 | 10.13 (0.23 to 437.19) | |||
| Vaccine type†: | ||||||||
| BNT162b2 with BA.4-5 | 6/85 859 | 5.4 | 64/1 893 405 | 2.6 | 3.74 (1.16 to 12.05) | |||
| BNT162b2 with BA.1 | 2/40 146 | 3.8 | 64/1 893 405 | 2.6 | 2.59 (0.52 to 12.94) | |||
| mRNA-1273 with BA.1 | 1/4283 | 17.9 | 64/1 893 405 | 2.6 | 12.39 (1.46 to 105.47) | |||
| Pericarditis | ||||||||
| All | 22/130 215 | 13.0 | 265/1 892 351 | 10.7 | 1.21 (0.72 to 2.03) | |||
| Sex: | ||||||||
| Female | 11/69 036 | 12.2 | 104/991 018 | 8.0 | 1.20 (0.57 to 2.50) | |||
| Male | 11/61 179 | 13.8 | 161/901 332 | 13.7 | 1.22 (0.59 to 2.53) | |||
| Age group (years): | ||||||||
| 50-64 | 8/53 490 | 11.5 | 134/865 857 | 11.9 | 1.09 (0.48 to 2.48) | |||
| 65-79 | 13/56 856 | 17.5 | 96/747 218 | 9.8 | 1.47 (0.71 to 3.05) | |||
| ≥80 | 1/19 869 | 3.9 | 35/279 276 | 9.6 | 0.43 (0.05 to 3.67) | |||
| Vaccine type†: | ||||||||
| BNT162b2 with BA.4-5 | 14/85 811 | 12.5 | 265/1 892 351 | 10.7 | 0.85 (0.43 to 1.71) | |||
| BNT162b2 with BA.1 | 7/40 123 | 13.4 | 265/1 892 351 | 10.7 | 1.12 (0.48 to 2.64) | |||
| mRNA-1273 with BA.1 | 1/4281 | 17.9 | 265/1 892 351 | 10.7 | 1.82 (0.25 to 13.31) | |||
NE=not estimable; TIA=transient cerebral ischaemic attack.
Each outcome was studied separately, therefore slight differences exist in denominators owing to different exclusions. The sum of person years within different strata may not equal the total person years owing to rounding.
Incidence rate per 1 000 000 vaccinated individuals (or individuals for reference period) within 28 days. Supplementary table S13 shows the sex and age subgroup estimates by individual bivalent omicron adapted mRNA booster vaccine types.
BNT162b2 is manufactured by Pfizer-BioNTech and mRNA-1273 is manufactured by Moderna.
Discussion
In a nationwide cohort analysis, we evaluated the rate of 27 adverse events associated with the bivalent omicron adapted mRNA booster vaccines received as the fourth dose in 1 740 417 adults aged ≥50 years in Denmark. We found no support for an increased risk of adverse events after vaccination with a bivalent mRNA booster as a fourth dose.
Comparison with other studies
Our post hoc analysis did reveal an excess of hospital visits as a result of myocarditis in female patients. Myocarditis after vaccination with a bivalent mRNA booster was, however, rare (a total of nine events) during the 28 day post-vaccination period of follow-up. In absolute numbers, we observed a rate of 5.3 events in the 28 day post-vaccination period per 1 000 000 individuals vaccinated, compared with a reference period incidence rate of 2.6 events in 28 days per 1 000 0000 individuals. Previous reported rates of myocarditis in adults after vaccination with the monovalent mRNA vaccines were highest in younger male participants aged ≤39 years.24 25 26 Five reports of myocarditis events after booster vaccination with a bivalent vaccine (not statistically analysed) was received through the Vaccine Adverse Event Reporting System in the recent CDC report on 22.6 million booster doses of bivalent vaccine administered in the US to individuals aged ≥12 years.15
The CDC and Food and Drug Administration recently identified possible safety concerns of increased risk of cerebrovascular infarction after vaccination with the bivalent BNT162b2 vaccine with BA.4-5 (Pfizer-BioNTech) booster in adults aged ≥65 years owing to detected preliminary safety signal by the CDC Vaccine Safety Datalink surveillance system, and post hoc analysis raised additional concerns of increased risk associated with simultaneous influenza vaccination (adjusted rate ratio 2.00 (95% confidence interval 1.18 to 3.48), comparing day 1 to 21 with day 22 to 42 after the day of vaccination with a bivalent booster vaccine plus high dose or adjuvanted influenza vaccine).27 28 We found no support for this notion, including no associations in any age, sex, bivalent booster vaccine type, and simultaneous influenza vaccination subgroup analyses. Particularly, the upper bound of the 95% confidence interval from our analyses based on 1 157 754 individuals who had received a fourth dose of BNT162b2 with BA.4-5 were inconsistent with relative increased risks of cerebrovascular infarction larger than 9% (incidence rate ratio 0.97, 95% confidence interval 0.86 to 1.09).
Strengths and limitations of this study
The robustness of the results from our main analysis is supported by self-controlled case series analyses with time invariant confounding taken into account and comparisons with historical rates. Furthermore, the use of nationwide demography registers and health registers in a setting with universal access to free healthcare reduces concern about selection and information biases. However, one limitation of our study is that we cannot exclude differences in the ascertainment of adverse events between compared periods. The active comparative nature of our design, where we compare rates for the 28 day period after vaccination with a fourth vaccine dose with rates for the reference periods from day 29 after vaccination, mitigates larger differences in outcome ascertainment as opposed to a comparison with rates during a non-vaccinated period. However, ascertainment bias cannot be completely excluded; specifically, an increased awareness of any symptoms related to known adverse events (eg, myocarditis) in the weeks after a fourth vaccine dose relative to the reference periods with longer time since vaccination. However, this would bias the results towards increased risks in contrast with what we observed. Lastly, the probability of false positive findings was high given the extensive set of outcomes assessed and analyses conducted, and as we did not take into account multiple testing; however, no consistent increased risks were found.
A strength of our study is the high statistical precision of many of the evaluated associations. For 19 of the 27 adverse events analysed, the upper confidence interval bounds excluded 1.50 or higher, meaning that these results were incompatible with moderate-to-large relative risk increases. Despite analysis of a nationwide cohort, however, the statistical power was lower for some events that occurred rarely, such as cerebral venous thrombosis.
Owing to the nationwide population based design of this study, our findings are highly generalisable to similar populations. In contrast, these results may only indirectly support safety evaluations of bivalent mRNA booster vaccination in other scenarios such as in individuals younger than 50 years or other specific subgroups that were not studied.
Conclusion
Bivalent mRNA booster vaccines administered as a fourth dose were not associated with an increased risk of 27 different adverse events in a general population of adults aged ≥50 years. These results provide reassuring support for the safety of using bivalent mRNA as booster doses, and provide critical and timely insight for patients, clinicians, and regulatory authorities.
What is already known on this topic
The bivalent omicron adapted mRNA booster vaccines are now implemented in many covid-19 booster vaccination strategies around the world
Real world safety evaluations of the bivalent mRNA booster vaccines for rare but serious adverse events are lacking
A recent safety communication from the Centers for Disease Control and Prevention and Food and Drug Administration raised concerns about a preliminary safety signal of increased risk of cerebrovascular infarction after vaccination with the Pfizer-BioNTech bivalent BNT162b2 with BA.4-5 vaccine in adults aged ≥65 years
What this study adds
The findings of this Danish nationwide cohort study of >1.7 million adults who received a bivalent omicron adapted mRNA booster vaccine as a fourth dose, do not support an increased risk of serious adverse events after vaccination
No associations with cerebral infarction were found in any sex, age, or vaccine type subgroup
These results provide support for the safety of using bivalent mRNA vaccines
Web extra.
Extra material supplied by authors
Supplementary information: Additional tables S1-S14, figure S1, and references
Contributors: All authors conceptualised the study, interpreted the results, and critically reviewed the manuscript. NA drafted the manuscript, NA, ET, and JH carried out the statistical analyses. AH supervised the study. NA had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. NA is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: No specific funding.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Studied participants were anonymised in the utilised data sources, and therefore direct dissemination to study participants is not possible. The study results will be disseminated to the public and health professionals by a press release written using layman’s terms.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The analyses were performed as surveillance activities analyses as part of the advisory tasks of the governmental institution Statens Serum Institut (SSI) for the Danish Ministry of Health. SSI’s purpose is to monitor and fight the spread of disease in accordance with section 222 of the Danish Health Act. According to Danish law, national surveillance activities conducted by SSI do not require approval from an ethics committee. Both the Danish government law firm and the compliance department of SSI have approved that the study is fully compliant with all legal, ethical, and IT security requirements and there are no further approval procedures required for such studies.
Data availability statement
No additional data available. Owing to data privacy regulations in Denmark, the raw data cannot be shared.
References
- 1.Moderna FDA. COVID-19 Vaccines. Food and Drug Administration. 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccines (accessed 5 Jan 2023).
- 2.FDA. Pfizer-BioNTech COVID-19 Vaccines. Food and Drug Administration. 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccines (accessed 5 Jan 2023).
- 3.Spikevax EMA. (previously COVID-19 Vaccine Moderna). European Medicines Agency. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax (accessed 6 Jan 2023).
- 4.EMA. First adapted COVID-19 booster vaccines recommended for approval in the EU. European Medicines Agency. 2022. https://www.ema.europa.eu/en/news/first-adapted-covid-19-booster-vaccines-recommended-approval-eu (accessed 6 Jan 2023).
- 5.EMA. Adapted vaccine targeting BA.4 and BA.5 Omicron variants original SARS-CoV-2 recommended for approval. European Medicines Agency. 2022. https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omicron-variants-original-sars-cov-2-recommended-approval (accessed 6 Jan 2023).
- 6.Statens Serum Institut. Daglige opgørelser af tilslutningen til covid-19-vaccination. https://covid19.ssi.dk/overvagningsdata/arkiv-tidligere-overvaagningsdata/vaccinationstilslutning (accessed 15 Jan 2023).
- 7.FDA. Pfizer-BioNTech COVID-19 Vaccine EUA LOA reissued December 8, 2022. Food and Drug Administration. 2022. https://www.fda.gov/media/150386/download (accessed 15 Jan 2023).
- 8.Moderna FDA. COVID-19 Vaccine EUA Letter of Authorization 12082022. Food and Drug Administration. 2022. https://www.fda.gov/media/144636/download (accessed 15 Jan 2023).
- 9.Spikevax EMA. (COVID-19 mRNA Vaccine (nucleoside modified)). European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/overview/spikevax-previously-covid-19-vaccine-moderna-epar-medicine-overview_en.pdf (accessed 15 Jan 2023).
- 10. Chalkias S, Harper C, Vrbicky K, et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N Engl J Med 2022;387:1279-91. 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group . Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-15. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baden LR, El Sahly HM, Essink B, et al. COVE Study Group . Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 2021;384:403-16. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chalkias S, Whatley J, Eder F, et al. Safety and Immunogenicity of Omicron BA.4/BA.5 Bivalent Vaccine Against Covid-19. medRxiv 2022;2022.12.11.22283166. 10.1101/2022.12.11.22283166 [DOI]
- 14. Klein NP, Lewis N, Goddard K, et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021;326:1390-9. 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hause AM. Safety Monitoring of Bivalent COVID-19 mRNA Vaccine Booster Doses Among Persons Aged ≥12 Years — United States, August 31–October 23, 2022. MMWR Morb Mortal Wkly Rep 2022;71. 10.15585/mmwr.mm7144a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39(Suppl):30-3. 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563-91. 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011;39(Suppl):22-5. 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 19. Grove Krause T, Jakobsen S, Haarh M, Mølbak K. The Danish vaccination register. Euro Surveill 2012;17:20155. 10.2807/ese.17.17.20155-en. [DOI] [PubMed] [Google Scholar]
- 20.BC-coordinator. Priority list of adverse events of special interest: COVID-19. 2020. https://brightoncollaboration.us/priority-list-aesicovid/ (accessed 15 Oct 2021).
- 21.Center for Biologics Evaluation and Research Office of Biostatistics and Epidemiology. CBER Surveillance Program Background Rates of Adverse Events of Special Interest for COVID-19 Vaccine Safety Monitoring Protocol. 2021.https://www.bestinitiative.org/wp-content/uploads/2021/02/C19-Vaccine-Safety-AESI-Background-Rate-Protocol-FINAL-2020.pdf (accessed 10 Oct 2021).
- 22. Willame C, Dodd C, Durán C, et al. Background rates of 41 adverse events of special interest for COVID-19 vaccines in 10 European healthcare databases - an ACCESS cohort study. Vaccine 2023;41:251-62. 10.1016/j.vaccine.2022.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farrington P, Whitaker H, Weldeselassie YG. Self-controlled case series studies: a modelling guide with R. Chapman and Hall/CRC, 2018. 10.1201/9780429491313. [DOI] [Google Scholar]
- 24. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med 2021;385:2140-9. 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karlstad Ø, Hovi P, Husby A, et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol 2022;7:600-12. 10.1001/jamacardio.2022.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med 2021;385:2132-9. 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimabukuro TT, Klein N. COVID-19 mRNA bivalent booster vaccine safety. Vaccines and Related Biological Products Advisory Committee meeting. January 26, 2023. https://www.fda.gov/media/164811/download (accessed 10 Feb 2023).
- 28.CDC. COVID-19 Vaccination. CDC & FDA Identify Preliminary COVID-19 Vaccine Safety Signal for Persons Aged 65 Years and Older. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/bivalent-boosters.html (accessed 14 Jan 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional tables S1-S14, figure S1, and references
Data Availability Statement
No additional data available. Owing to data privacy regulations in Denmark, the raw data cannot be shared.