Abstract
Objective
To estimate the effectiveness of the bivalent mRNA booster vaccines containing the original SARS-CoV-2 and omicron BA.4-5 or BA.1 subvariants as the fourth dose against severe covid-19.
Design
Nationwide cohort analyses, using target trial emulation.
Setting
Denmark, Finland, Norway, and Sweden, from 1 July 2022 to 10 April 2023.
Participants
People aged ≥50 years who had received at least three doses of covid-19 vaccine (that is, a primary course and a first booster).
Main outcome measures
The Kaplan-Meier estimator was used to compare the risk of hospital admission and death related to covid-19 in people who received a bivalent Comirnaty (Pfizer-BioNTech) or Spikevax (Moderna) BA.4-5 or BA.1 mRNA booster vaccine as a fourth dose (second booster) with three dose (first booster) vaccinated people and between four dose vaccinated people.
Results
A total of 1 634 199 people receiving bivalent BA.4-5 fourth dose booster and 1 042 124 receiving bivalent BA.1 fourth dose booster across the four Nordic countries were included. Receipt of a bivalent BA.4-5 booster as a fourth dose was associated with a comparative vaccine effectiveness against admission to hospital with covid-19 of 67.8% (95% confidence interval 63.1% to 72.5%) and a risk difference of –91.9 (95% confidence interval –152.4 to –31.4) per 100 000 people at three months of follow-up compared with having received three doses of vaccine (289 v 893 events). The corresponding comparative vaccine effectiveness and risk difference for bivalent BA.1 boosters (332 v 977 events) were 65.8% (59.1% to 72.4%) and –112.9 (–179.6 to –46.2) per 100 000, respectively. Comparative vaccine effectiveness and risk difference against covid-19 related death were 69.8% (52.8% to 86.8%) and –34.1 (–40.1 to –28.2) per 100 000 for bivalent BA.4-5 booster (93 v 325 events) and 70.0% (50.3% to 89.7%) and –38.7 (–65.4 to –12.0) per 100 000 for BA.1 booster (86 v 286) as a fourth dose. Comparing bivalent BA.4-5 and BA.1 boosters as a fourth dose directly resulted in a three month comparative vaccine effectiveness and corresponding risk difference of –14.9% (–62.3% to 32.4%) and 10.0 (–14.4 to 34.4) per 100 000 people for admission to hospital with covid-19 (802 v 932 unweighted events) and –40.7% (–123.4% to 42.1%) and 8.1 (–3.3 to 19.4) per 100 000 for covid-19 related death (229 v 243 unweighted events). The comparative vaccine effectiveness did not differ across sex and age (</≥70 years) and seemed to be sustained up to six months from the day of vaccination with modest waning.
Conclusion
Vaccination with bivalent BA.4-5 or BA.1 mRNA booster vaccines as a fourth dose was associated with reduced rates of covid-19 related hospital admission and death among adults aged ≥50 years. The protection afforded by the bivalent BA.4-5 and BA.1 boosters did not differ significantly when directly compared, and any potential difference would most likely be very small in absolute numbers.
Introduction
Fourth dose (that is, second booster) vaccination to improve protection against severe and fatal covid-19 outcomes in target populations are now recommended in many countries. To combat the attenuated efficacy of the original monovalent BNT162b2 (Comirnaty; Pfizer-BioNTech) and mRNA-1273 (Spikevax; Moderna) mRNA covid-19 vaccines observed against the omicron variants compared with other variants,1 2 3 bivalent mRNA booster vaccines, containing spike sequences from the original (ancestral) SARS-CoV-2 strain and omicron subvariants (BA.4-5 or BA.1), were authorised for use in autumn 2022 and subsequently implemented in booster vaccination programmes including in the Nordic countries.
Although some clinical studies have shown that the bivalent BA.4-5 and BA.1 mRNA booster vaccines increase neutralising antibody responses against omicron compared with the original monovalent mRNA covid-19 vaccines, others have not.4 5 6 7 8 Data on the effectiveness of the bivalent mRNA booster vaccines to protect against severe covid-19 outcomes are scarce,9 10 11 12 13 14 15 and previous knowledge on the effectiveness of a fourth covid-19 vaccine dose is mostly based on studies of the monovalent vaccines.16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 In nationwide cohort analyses in Denmark, Finland, Norway, and Sweden, we assessed the comparative effectiveness of the bivalent BA.4-5 or BA.1 mRNA booster vaccines received as the fourth dose against covid-19 related hospital admission and death among adults aged ≥50 years.
Methods
Data sources and source populations
All four Nordic countries hold nationwide demography and healthcare registers with individual level data that can be linked using the country specific unique identifiers assigned to all residents. With linkage of these registers, we obtained information on covid-19 vaccinations and laboratory confirmed SARS-CoV-2 infections and hospital admissions, as well as the presence or absence of selected comorbidities (chronic pulmonary disease, cardiovascular conditions and diabetes, autoimmunity related conditions, cancer, and renal disorders), and demographic variables (age, sex, residency, healthcare occupation, and vital status) (see supplementary tables S1-S2 for further details on registers and definitions of variables). Within each country, we established a source population of people who were known residents and had received at least three vaccine doses (that is, a primary two dose vaccination course and one booster) with the AZD1222 (Vaxzevria; Oxford-AstraZeneca; as part of the primary course only), monovalent (original) BNT162b2, and/or monovalent (original) mRNA-1273 vaccines from 27 December 2020 to 10 April 2023 in Denmark, 7 April 2023 in Finland, 1 April 2023 in Norway, and 31 December 2022 in Sweden (last day of data availability within each country at time of analysis). See supplementary table S3 for a description of ethical approvals/exemptions.
Study cohorts
To be included in our study, people must not have received the third or fourth vaccine dose within 90 days after the second or third dose (to ensure that the received third or fourth doses were truly first or second booster doses), respectively; be younger than a country specific lower age limit of 50 years in Denmark and Sweden, 60 in Finland, and 65 in Norway; or have received the fourth dose before 1 September 2022 in Denmark, 18 July 2022 in Finland, and 1 July 2022 in Norway and Sweden (the last two criteria were defined according to respective health authorities’ fourth dose rollout strategy for the general target population). Omicron BA.5 was the predominant sublineage until late autumn 2022; since then, multiple sublineages have been circulating, primarily sublineages of BQ, BF, and XBB. We classified any fourth vaccine dose according to whether it was a bivalent BA.4-5, bivalent BA.1, or monovalent (original) mRNA booster vaccine regardless of vaccine brand. We considered any comparison that included monovalent fourth dose vaccination as an additional comparison analysis.
Outcomes
We defined covid-19 related hospital admission as admission to hospital as an inpatient with a registered covid-19 related diagnosis and a positive polymerase chain reaction (PCR) test for SARS-CoV-2 (tested positive within 14 days before to two days after the day of admission) (see supplementary table S4 for country specific outcome definitions). We defined covid-19 related death as death within 30 days of a positive PCR test for SARS-CoV-2 in Denmark, Finland, and Sweden, whereas we used covid-19 specific diagnoses registered as the main cause of death in Norway (owing to data availability). The day of admission or death served as the respective event date.
Comparisons
Fourth dose compared with third dose vaccinated
We used a matched design to assess the effectiveness of receipt of a bivalent BA.4-5 or BA.1 mRNA booster vaccine as the fourth dose compared with previous receipt of three monovalent vaccine doses. We matched people who received a fourth dose during follow-up on this vaccination day with those who had not yet received a fourth dose. We matched people (exactly; without replacement) on age (5 year bins), the calendar month of third dose vaccination, and a propensity score including sex, region of residence, vaccination priority groups (that is, people at high risk of severe covid-19 or healthcare workers), selected comorbidities, and previous history of SARS-CoV-2 infection (supplementary table S2). The day the fourth dose was administered within each matched pair served as the index date for both individuals. If people who were included as a matched three dose vaccinated (that is, a reference) individual received a fourth dose later than the assigned index date, they were allowed to potentially re-enter as a fourth dose recipient in a new matched pair on that date. See supplementary figure S1 for a graphical illustration of our matched four versus three dose study design.
Fourth dose comparisons by type of vaccine
We compared the effectiveness of fourth dose vaccination with the bivalent BA.4-5 and BA.1 boosters directly while using stabilised inverse probability of treatment weights to take into account the covariates of calendar month of fourth dose vaccination, age, sex, region of residence, vaccination priority groups, selected comorbidities, and previous history of SARS-CoV-2 infection (supplementary table S2). The day of vaccination with the fourth dose served as the index date. See supplementary figure S2 for a graphical illustration of our weighted four versus four dose study design.
Statistical analysis
For the four versus three dose matched analyses, we used logistic regression to estimate the propensity score of receiving the fourth dose under study given covariates as predictors, with matching on age and calendar month of third dose vaccination and with a calliper width of 0.01 on the propensity score. For the four versus four dose weighted analyses, we used logistic regression to calculate stabilised inverse probability of treatment weights as ((1−p0)/(1−pc))/(p0/pc), with p0 being the crude probability of receiving a bivalent BA.4-5 booster and pc being the same probability given covariates.
We followed people from day 8 after the index date (to ensure full immunisation among fourth dose recipients) up until the day of an outcome event, 90 days after the index date, death, emigration, or end of the study period, whichever occurred first. Additionally, we censored people with a positive PCR test for SARS-CoV-2 in our follow-up period after 14 and 30 days after the test (as a positive test was part of the outcome ascertainment) for the covid-19 related hospital admission and death outcome analyses, respectively. Moreover, we did not allow inclusion of people with recent SARS-CoV-2 infection (≤12 weeks) before the index date (to avoid outcome misclassification). Similarly, for the covid-19 related hospital admission outcome analysis, we did not allow inclusion of people admitted to hospital with covid-19 any time before the index date. For the four versus three dose matched analyses, we also right censored matched pairs if the reference third dose vaccinated person received a fourth dose (on that day) during follow-up.33 34 We used the Kaplan-Meier estimator to estimate cumulative incidences, and from these we calculated the relative (that is, comparative vaccine effectiveness: 1–risk ratio) and absolute risk differences at day 90. We calculated the corresponding 95% confidence intervals by using the delta method. Upper 95% confidence intervals for the comparative vaccine effectiveness estimates were truncated at 100% if higher. We combined country specific estimates by random effects meta-analyses implemented using the mixmeta package in R. Counts smaller than five but not zero could not be reported owing to privacy regulations.
Additional analyses
Additional analyses included assessing the effectiveness of a fourth dose with a monovalent mRNA vaccine compared with three dose vaccination and with four dose bivalent booster vaccination, subgrouping according to sex and age (</≥70 years), and extending follow-up to day 180. In sensitivity analyses of the BA.4-5 versus BA.1 four dose comparison, we firstly included calendar month of third dose vaccination in the weights (to test for residual confounding due to differential waning of the third dose; evaluated in Denmark only) and secondly restricted analysis to people vaccinated after 1 October 2022 (to test for any early vaccinee selection bias not fully captured), as the BA.1-booster was introduced a few weeks before the BA.4-5 booster. Also, the Nordic fourth dose vaccination rollouts initially prioritised older and vulnerable people, and, as the Danish rollout was initiated in September, this coincided with the authorisation of use of bivalent boosters. In Finland, Norway, and Sweden, these risk groups were prioritised for vaccination before the respective study periods (during spring 2022).
Patient and public involvement
No patients or members of the public were formally involved in defining the research question, study design, or outcome measures, or in the conduct of the study owing to privacy constrains, funding restrictions, and the short timeline during which the study was conducted. However, we received feedback from one layperson as part of the peer review process.
Results
Study populations
The study cohorts comprised 2 676 323 people who received fourth dose vaccination with a bivalent booster across the four countries; 1 634 199 (61%) had received a bivalent BA.4-5 mRNA booster vaccine and 1 042 124 (39%) a bivalent BA.1 mRNA booster vaccine (an additional 911 731 had received a monovalent mRNA booster vaccine). Denmark contributed a relatively larger sample of bivalent mRNA booster vaccinees (a total of 1 697 078; 63% of all included people vaccinated with a bivalent booster) than Finland (235 813; 9%), Norway (221 502; 8%), and Sweden (521 930; 20%) (table 1; supplementary figures S3-S4 and table S5). Slightly more than half of all people who had received a bivalent BA.4-5 or BA.1 booster vaccine within each country cohort were women, with mean ages of approximately 72 years, except in Sweden (approximately 61 years) and for the bivalent BA.4-5 booster vaccinated in Denmark (66 years). The distribution of comorbidities and history of previous SARS-CoV-2 infection was relatively similar between people vaccinated with bivalent BA.4-5 and BA.1 booster within each country. Across countries, the proportion of people with a medical history of cardiovascular disease or diabetes in the overall cohorts was larger in Finland (29%) and Norway (28%) than in Denmark (10%) and Sweden (15%). The matched four versus three dose cohorts consisted of a total of 1 233 741 and 932 846 matched pairs for the bivalent BA.4-5 and BA.1 booster comparisons (corresponding to 75% and 90% of all included bivalent boosted vaccinees), respectively, and characteristics were overall similar to those of the entire bivalent boosted cohorts.
Table 1.
Baseline characteristics of study cohorts in four versus three dose and four versus four dose comparisons for estimating effectiveness of bivalent BA.4-5 or BA.1 mRNA booster vaccination as fourth vaccine dose in four Nordic countries. Values are numbers (percentages) unless stated otherwise
| Characteristic | Four v three dose vaccinated comparison | Four v four dose vaccinated comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| Matched | Matched | |||||||
| BA.4-5 booster as fourth dose | Third dose | BA.1 booster as fourth dose | Third dose | BA.4-5 booster as fourth dose | BA.1 booster as fourth dose | |||
| No of individuals | ||||||||
| Total | 1 233 741 | 123 3741 | 932 846 | 932 846 | 1 634 199 | 1 042 124 | ||
| Denmark | 748 648 | 748 648 | 471 810 | 471 810 | 1 130 045 | 567 033 | ||
| Finland | 146 007 | 146 007 | 74 690 | 74 690 | 156 480 | 79 333 | ||
| Norway | 87 481 | 87 481 | 128 833 | 128 833 | 89 670 | 131 832 | ||
| Sweden | 251 605 | 251 605 | 257 513 | 257 513 | 258 004 | 263 926 | ||
| Mean (SD) age, years | ||||||||
| Denmark | 64.5 (10) | 64.4 (10.1) | 71.3 (10.3) | 71.2 (10.4) | 65.8 (10) | 72.5 (10.5) | ||
| Finland | 71.6 (7.2) | 71.5 (7.2) | 71.3 (7.1) | 71.2 (7.1) | 71.9 (7.4) | 71.5 (7.3) | ||
| Norway | 72.5 (6.4) | 72.5 (6.5) | 72 (6) | 72.1 (6.1) | 72.6 (6.5) | 72.1 (6) | ||
| Sweden | 60.8 (7.8) | 60.8 (7.8) | 60.8 (8) | 60.8 (8) | 60.9 (7.9) | 60.9 (8.1) | ||
| Female sex | ||||||||
| Denmark | 383 413 (51.2) | 378 736 (50.6) | 261 828 (55.5) | 254 519 (53.9) | 582 724 (51.6) | 316 979 (55.9) | ||
| Finland | 79 063 (54.2) | 78 338 (53.7) | 39 760 (53.2) | 39 896 (53.4) | 85 151 (54.4) | 42 322 (53.3) | ||
| Norway | 43 966 (50.3) | 44 958 (51.4) | 65 619 (50.9) | 66 113 (51.3) | 45 123 (50.3) | 67 155 (50.9) | ||
| Sweden | 130 651 (51.9) | 124 228 (49.4) | 138 942 (54) | 132 471 (51.4) | 134 233 (52) | 142 887 (54.1) | ||
| Calendar period, min to max | ||||||||
| Denmark | 23/09/22 to 12/04/23 | 23/09/22 to 12/04/23 | 19/09/22 to 12/04/23 | 19/09/22 to 12/04/23 | 16/09/22 to 31/03/23 | 12/09/22 to 30/03/23 | ||
| Finland | 11/08/22 to 07/04/23 | 11/08/22 to 07/04/23 | 30/08/22 to 07/04/23 | 30/08/22 to 07/04/23 | 04/08/22 to 30/03/23 | 23/08/22 to 30/03/23 | ||
| Norway | 20/09/22 to 10/04/23 | 20/09/22 to 10/04/23 | 13/09/22 to 10/04/23 | 13/09/22 to 10/04/23 | 13/09/22 to 01/04/23 | 06/09/22 to 27/03/23 | ||
| Sweden | 25/07/22 to 31/12/22 | 25/07/22 to 31/12/22 | 11/08/22 to 31/12/22 | 11/08/22 to 31/12/22 | 18/07/22 to 23/12/22 | 04/08/22 to 23/12/22 | ||
| Vaccination priority groups | ||||||||
| Severe covid-19 risk group: | ||||||||
| Denmark | 26 395 (3.5) | 30 362 (4.1) | 33 641 (7.1) | 39 104 (8.3) | 46 171 (4.1) | 53 082 (9.4) | ||
| Finland | 15 502 (10.6) | 14 974 (10.3) | 8186 (11.0) | 7782 (10.4) | 16 598 (10.6) | 8677 (10.9) | ||
| Norway | 715 (0.8) | 977 (1.1) | 566 (0.4) | 576 (0.4) | 826 (0.9) | 598 (0.5) | ||
| Sweden | 327 (0.1) | 327 (0.1) | 808 (0.3) | 639 (0.2) | 405 (0.2) | 914 (0.3) | ||
| Healthcare workers: | ||||||||
| Denmark | 57 048 (7.6) | 67 321 (9.0) | 25 484 (5.4) | 25 163 (5.3) | 88 933 (7.9) | 27 418 (4.8) | ||
| Finland | 3562 (2.4) | 3109 (2.1) | 1605 (2.1) | 1258 (1.7) | 4260 (2.7) | 1879 (2.4) | ||
| Norway | 3287 (3.8) | 3906 (4.5) | 4703 (3.7) | 5678 (4.4) | 3367 (3.8) | 4794 (3.6) | ||
| Sweden | 26 013 (10.3) | 28 989 (11.5) | 31 176 (12.1) | 33 888 (13.2 | 26 977 (10.5) | 32 332 (12.3) | ||
| Comorbidities | ||||||||
| Autoimmune related condition: | ||||||||
| Denmark | 27 983 (3.7) | 27 193 (3.6) | 20 702 (4.4) | 20 812 (4.4) | 43 542 (3.9) | 25 353 (4.5) | ||
| Finland | 5848 (4.0) | 5223 (3.6) | 3237 (4.3) | 2651 (3.5) | 6318 (4.0) | 3451 (4.4) | ||
| Norway | 2362 (2.7) | 2453 (2.8) | 3317 (2.6) | 3629 (2.8) | 2425 (2.7) | 3403 (2.6) | ||
| Sweden | 11 915 (4.7) | 11 180 (4.4) | 11 787 (4.6) | 11 807 (4.6) | 12 271 (4.8) | 12 115 (4.6) | ||
| Cancer: | ||||||||
| Denmark | 31 023 (4.1) | 30 438 (4.1) | 26 920 (5.7) | 30 123 (6.4) | 50 491 (4.5) | 34 407 (6.1) | ||
| Finland | 14 690 (10.1) | 13 293 (9.1) | 7144 (9.6) | 6758 (9.0) | 15 877 (10.1) | 7588 (9.6) | ||
| Norway | 3343 (3.8) | 3209 (3.7) | 4892 (3.8) | 4774 (3.7) | 3422 (3.8) | 5028 (3.8) | ||
| Sweden | 12 544 (5.0) | 11 600 (4.6) | 12 711 (4.9) | 12 156 (4.7) | 12 967 (5.0) | 13 111 (5.0) | ||
| Chronic pulmonary disease: | ||||||||
| Denmark | 20 900 (2.8) | 20 495 (2.7) | 20 291 (4.3) | 18 928 (4.0) | 33 419 (3.0) | 25 267 (4.5) | ||
| Finland | 2860 (2.0) | 2932 (2.0) | 1488 (2.0) | 1456 (1.9) | 3078 (2.0) | 1578 (2.0) | ||
| Norway | 9254 (10.6) | 9253 (10.6) | 13 392 (10.4) | 13 471 (10.5) | 9471 (10.6) | 13 702 (10.4) | ||
| Sweden | 8730 (3.5) | 7525 (3.0) | 8932 (3.5) | 8109 (3.1) | 9014 (3.5) | 9230 (3.5) | ||
| Cardiovascular condition or diabetes: | ||||||||
| Denmark | 57 485 (7.7) | 58 302 (7.8) | 54 570 (11.6) | 55 353 (11.7) | 93 538 (8.3) | 69 277 (12.2) | ||
| Finland | 40 780 (27.9) | 40 961 (28.1) | 22 543 (30.2) | 20 652 (27.7) | 44 259 (28.3) | 24 211 (30.5) | ||
| Norway | 25 217 (28.8) | 24 825 (28.4) | 36 235 (28.1) | 35 826 (27.8) | 25 932 (28.9) | 37 096 (28.1) | ||
| Sweden | 37 559 (14.9) | 35 974 (14.3) | 39 131 (15.2) | 37 798 (14.7) | 38 811 (15.0) | 40 446 (15.3) | ||
| Renal disease: | ||||||||
| Denmark | 6781 (0.9) | 7463 (1.0) | 7003 (1.5) | 7910 (1.7) | 11 415 (1.0) | 9202 (1.6) | ||
| Finland | 1746 (1.2) | 1666 (1.1) | 866 (1.2) | 818 (1.1) | 1939 (1.2) | 957 (1.2) | ||
| Norway | 621 (0.7) | 668 (0.8) | 918 (0.7) | 968 (0.8) | 650 (0.7) | 938 (0.7) | ||
| Sweden | 2599 (1.0) | 2514 (1.0) | 2859 (1.1) | 2729 (1.1) | 2712 (1.1) | 2971 (1.1) | ||
| Previous SARS-CoV-2 infection | ||||||||
| After third vaccine dose: | ||||||||
| Denmark | 270 003 (36.1) | 241 282 (32.2) | 141 474 (30.0) | 128 299 (27.2) | 394 282 (34.9) | 168 084 (29.6) | ||
| Finland | 20 613 (14.1) | 13 767 (9.4) | 7483 (10.0) | 6895 (9.2) | 22 613 (14.5) | 8138 (10.3) | ||
| Norway | 3637 (4.2) | 3362 (3.8) | 5463 (4.2) | 5378 (4.2) | 3742 (4.2) | 5617 (4.3) | ||
| Sweden | 17 716 (7.0) | 16 658 (6.6) | 19 849 (7.7) | 20 430 (7.9) | 18 225 (7.1) | 20 570 (7.8) | ||
| Before third vaccine dose: | ||||||||
| Denmark | 41 807 (5.6) | 34 872 (4.7) | 17 381 (3.7) | 14 085 (3.0) | 53 468 (4.7) | 19 609 (3.5) | ||
| Finland | 4093 (2.8) | 2389 (1.6) | 1262 (1.7) | 1141 (1.5) | 4359 (2.8) | 1347 (1.7) | ||
| Norway | 1254 (1.4) | 851 (1.0) | 864 (0.7) | 627 (0.5) | 1300 (1.4) | 890 (0.7) | ||
| Sweden | 34 109 (13.6) | 33 642 (13.4) | 31 322 (12.2) | 30 895 (12.0) | 34 751 (13.5) | 31 833 (12.1) | ||
| No previous infection before third vaccine dose: | ||||||||
| Denmark | 436 838 (58.4) | 472 494 (63.1) | 312 955 (66.3) | 329 426 (69.8) | 682 295 (60.4) | 379 340 (66.9) | ||
| Finland | 121 301 (83.1) | 129 851 (88.9) | 65 945 (88.3) | 66 654 (89.2) | 129 508 (82.8) | 69 848 (88.0) | ||
| Norway | 82 590 (94.4) | 83 268 (95.2) | 122 506 (95.1) | 122 828 (95.3) | 84 628 (94.4) | 125 325 (95.1) | ||
| Sweden | 199 780 (79.4) | 201 305 (80.0) | 206 342 (80.1) | 206 188 (80.1) | 205 028 (79.5) | 211 523 (80.1) | ||
| Omicron infection: | ||||||||
| Denmark | 260 681 (34.8) | 233 387 (31.2) | 136 388 (28.9) | 123 628 (26.2) | 380 263 (33.7) | 161 958 (28.6) | ||
| Finland | 21 064 (14.4) | 14 066 (9.6) | 7615 (10.2) | 7006 (9.4) | 23 056 (14.7) | 8277 (10.4) | ||
| Norway | 3337 (3.8) | 3070 (3.5) | 4917 (3.8) | 4810 (3.7) | 3427 (3.8) | 5040 (3.8) | ||
| Sweden | 24 237 (9.6) | 21 345 (8.5) | 24 940 (9.7) | 23 627 (9.2) | 24 813 (9.6) | 25 693 (9.7) | ||
| No previous omicron infection: | ||||||||
| Denmark | 487 967 (65.2) | 515 261 (68.8) | 335 422 (71.1) | 348 182 (73.8) | 749 782 (66.3) | 405 075 (71.4) | ||
| Finland | 124 943 (85.6) | 131 941 (90.4) | 67 075 (89.8) | 67 684 (90.6) | 133 424 (85.3) | 71 056 (89.6) | ||
| Norway | 84 144 (96.2) | 84 411 (96.5) | 123 916 (96.2) | 124 023 (96.3) | 86 243 (96.2) | 126 792 (96.2) | ||
| Sweden | 227 368 (90.4) | 230 260 (91.5) | 232 573 (90.3) | 233 886 (90.8) | 233 191 (90.4) | 238 233 (90.3) | ||
SD=standard deviation.
Variable definitions are shown in supplementary table S2.
Effectiveness of bivalent booster as fourth dose
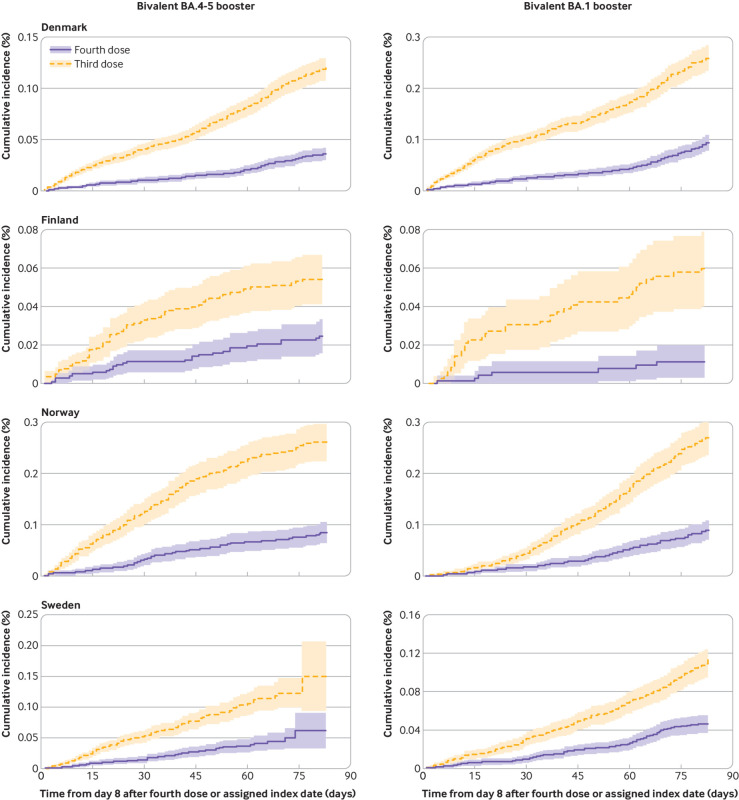
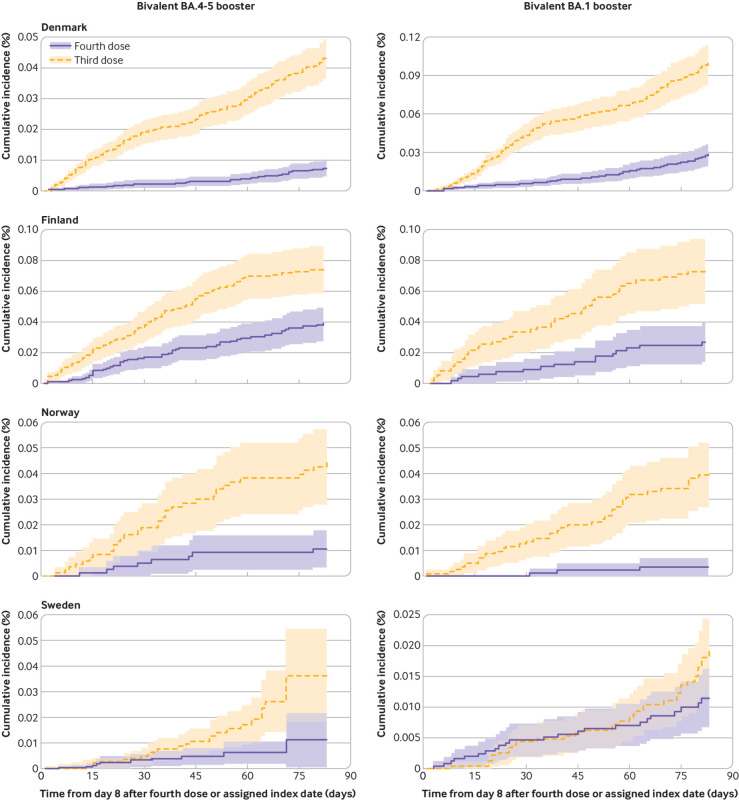
The cumulative incidences of covid-19 related hospital admission and death within 90 days of follow-up comparing four dose with three dose vaccinated people were very low (fig 1 and fig 2). Receipt of a fourth vaccine dose with a bivalent BA.4-5 booster was associated with a lower risk of admission to hospital with covid-19 than previous receipt of three vaccine doses (289 v 893 events); the comparative vaccine effectiveness was 67.8% (95% confidence interval 63.1% to 72.5%) and the risk difference was –91.9 (95% confidence interval –152.4 to –31.4) per 100 000 people (table 2). A fourth dose with a bivalent BA.1 booster was similarly associated with lower risks of admission to hospital with covid-19 (332 v 977 events), corresponding to a comparative vaccine effectiveness of 65.8% (59.1% to 72.4%) and a risk difference of –112.9 (–179.6 to –46.2) per 100 000 people. For covid-19 related death, the comparative vaccine effectiveness and risk difference were 69.8% (52.8% to 86.8%) and –34.1 (–40.1 to –28.2) per 100 000 people for bivalent BA.4-5 (93 v 325 events) and 70.0% (50.3% to 89.7%) and –38.7 (–65.4 to –12.0) per 100 000 people for BA.1 boosters (86 v 286 events). The comparative vaccine effectiveness did not differ by sex or age, but the risk difference was greater among people aged ≥70 years than among those who were younger (for example, risk differences for BA.4-5 booster against admission to hospital with covid-19 were –210.4 (–361.9 to –58.8) and –29.1 (–36.0 to –22.2) per 100 000 people among those aged ≥70 years and those who were younger, respectively) (supplementary figure S5-S8). Extending follow-up to day 180 resulted in comparative vaccine effectiveness against admission to hospital with covid-19 of 54.9% (49.0% to 60.8%) and 63.5% (49.5% to 77.5%) for the BA.4-5 and BA.1 boosters, respectively (similar for covid-19 related death; supplementary figures S9-S10 and table S6).
Fig 1.
Cumulative incidence curves of admission to hospital with covid-19, comparing people vaccinated with bivalent BA.4-5 or BA.1 mRNA booster vaccine as fourth dose with those who had received three vaccine doses only in each of four Nordic countries. Matched four dose and three dose vaccinated pairs were followed from day 8 to day 90 after four dose vaccinated people received bivalent booster as fourth dose
Fig 2.
Cumulative incidence curves of covid-19 related death comparing people vaccinated with bivalent BA.4-5 or BA.1 mRNA booster vaccine as a fourth dose with those who had received three vaccine doses only in each of four Nordic countries. Matched four dose and three dose vaccinated pairs were followed from day 8 to day 90 after four dose vaccinated people received bivalent booster as fourth dose
Table 2.
Risk of covid-19 related hospital admission and death comparing people vaccinated with bivalent mRNA booster vaccine received as fourth dose with those vaccinated with only three doses in four Nordic countries*
| Contributing countries | Events/person years | Risk difference (95% CI) per 100 000 people | Comparative vaccine effectiveness, % (95% CI) | ||
|---|---|---|---|---|---|
| Four dose vaccinated | Three dose vaccinated | ||||
| Hospital admission | |||||
| Bivalent BA.4-5 booster: | |||||
| All | DK, FI, NO, SE | 289/157 024.6 | 893/155 560.2 | −91.9 (−152.4 to −31.4) | 67.8 (63.1 to 72.5) |
| Female | DK, FI, NO, SE | 123/81 201.4 | 421/77 934.4 | −83.8 (−150.0 to −17.5) | 71.4 (64.3 to 78.4) |
| Male | DK, FI, NO, SE | 166/75 823.2 | 472/77 625.7 | −92.9 (−156.5 to −29.4) | 65.0 (55.1 to 74.9) |
| Age <70 years | DK, FI, NO, SE | 73/111 745.4 | 227/110 941.2 | −29.1 (−36.0 to −22.2) | 68.4 (59.6 to 77.1) |
| Age ≥70 years | DK, FI, NO, SE | 216/45 279.2 | 666/44 619.0 | −210.4 (−361.9 to −58.8) | 67.4 (61.8 to 73.1) |
| Bivalent BA.1 booster: | |||||
| All | DK, FI, NO, SE | 332/121 229.6 | 977/120 173.2 | −112.9 (−179.6 to −46.2) | 65.8 (59.1 to 72.4) |
| Female | DK, FI, NO, SE | 130/65 260.7 | 459/62 392.8 | −111.3 (−171.1 to −51.5) | 75.6 (61.9 to 89.3) |
| Male | DK, FI, NO, SE | 202/55 969.0 | 518/57 780.5 | −110.6 (−184.4 to −36.8) | 58.4 (51.3 to 65.6) |
| Age <70 years | DK, FI, NO, SE | 92/77 391.8 | 199/77 062.0 | −31.8 (−49.3 to −14.3) | 54.2 (42.5 to 66.0) |
| Age ≥70 years | DK, FI, NO, SE | 240/43 837.8 | 778/43 111.3 | −251.7 (−379.8 to −123.6) | 71.4 (59.8 to 83.1) |
| Death | |||||
| Bivalent BA.4-5 booster: | |||||
| All | DK, FI, NO, SE | 93/159 012.0 | 325/157 163.0 | −34.1 (−40.1 to −28.2) | 69.8 (52.8 to 86.8) |
| Female | DK, FI, NO, SE | 40/82 066.3 | 159/78 647.7 | −28.9 (−41.3 to −16.6) | 74.1 (53.9 to 94.4) |
| Male | DK, FI, NO, SE | 53/76 945.7 | 166/78 515.2 | −32.6 (−41.6 to −23.7) | 68.0 (54.5 to 81.6) |
| Age <70 years | DK, FI, SE | 13/106 242.7 | 36/105 162.2 | −3.7 (−10.1 to 2.7) | 62.9 (3.8 to 100.0) |
| Age ≥70 years | DK, FI, NO, SE | 80/45 994.1 | 287/45268.3 | −104.2 (−169.3 to −39.1) | 74.4 (58.4 to 90.3) |
| Bivalent BA.1 booster: | |||||
| All | DK, FI, NO, SE | 86/122 646.3 | 286/121 509.9 | −38.7 (−65.4 to −12.0) | 70.0 (50.3 to 89.7) |
| Female | DK, FI, NO, SE | 34/65 840.5 | 137/62 815.9 | −37.2 (−61.8 to −12.5) | 79.8 (70.3 to 89.2) |
| Male | DK, FI, SE | 52/46 271.6 | 127/48 331.3 | −35.4 (−79.8 to 8.9) | 54.7 (30.6 to 78.7) |
| Age <70 years | DK, FI, SE | 10/69 358.3 | 23/68.905.0 | −5.6 (−15.3 to 4.1) | 66.8 (39.0 to 94.7) |
| Age ≥70 years | DK, FI, NO, SE | 76/44 509.2 | 262/43 770.0 | −86.9 (−124.9 to −48.8) | 70.9 (52.6 to 89.3) |
CI=confidence interval; DK=Denmark; FI=Finland; NO=Norway; SE=Sweden.
Matched pairs were followed up from day 8 after fourth dose vaccination until day 90, an outcome event, three dose reference individual received fourth dose, 14 (for hospital admission) or 30 (for death) days after positive polymerase chain reaction test for SARS-CoV-2, emigration, death, or end of study period, whichever occurred first. Comparisons were matched to take into account year of birth (5 year bins), calendar month of receipt of third vaccine dose, sex, region of residence, vaccination priority groups, selected comorbidities, and previous SARS-CoV-2 infection.
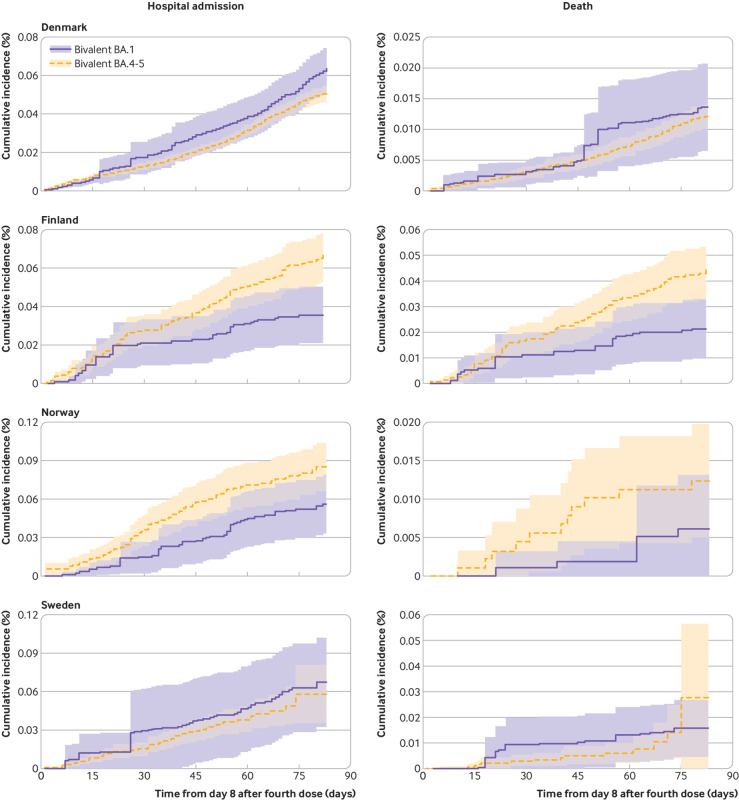
Comparative effectiveness between bivalent BA.4-5 and BA.1 boosters as fourth vaccine dose
When comparing bivalent BA.4-5 versus BA.1 boosters directly, the comparative vaccine effectiveness and corresponding risk difference at day 90 were –14.9% (–62.3% to 32.4%) and 10.0 (–14.4 to 34.4) per 100 000 people for admission to hospital with covid-19 (802 v 932 unweighted events) and –40.7% (–123.4% to 42.1%) and 8.1 (–3.3 to 19.4) per 100 000 people for covid-19 related death (229 v 243 unweighted events) (table 3 and fig 3). The direction of the estimates was not uniform across countries (supplementary table S7). Findings were similar with stratification according to sex and age and when follow-up was extended to day 180 (supplementary figures S11-S13 and table S8). Also, the results did not change when we included adjustment for calendar month of third dose vaccination and when we excluded early fourth dose vaccine adopters (supplementary tables S9 and S10).
Table 3.
Risk of covid-19 related hospital admission and death comparing people vaccinated with bivalent BA.4-5 mRNA booster vaccine received as fourth dose with those vaccinated with bivalent BA.1 mRNA booster vaccine received as fourth dose in four Nordic countries*
| Contributing countries | Events/person years | Risk difference (95% CI) per 100 000 people | Comparative vaccine effectiveness, % (95% CI) | ||
|---|---|---|---|---|---|
| Fourth dose bivalent BA.4-5 booster | Fourth dose bivalent BA.1 booster | ||||
| Hospital admission | |||||
| All | DK, FI, NO, SE | 802/333 522.2 | 932/229 594.7 | 10.0 (−14.4 to 34.4) | −14.9 (−62.3 to 32.4) |
| Female | DK, FI, NO, SE | 355/173 218.5 | 389/125 445.2 | 10.9 (−7.1 to 28.9) | −5.3 (−32.1 to 21.6) |
| Male | DK, FI, NO, SE | 447/160 303.6 | 543/104 149.5 | 8.7 (−31.3 to 48.7) | −1.2 (−54.4 to 52.1) |
| Age <70 years | DK, FI, NO, SE | 165/215 129.7 | 165/119 986.2 | −3.3 (−10.9 to 4.3) | 28.0 (5.6 to 50.3) |
| Age ≥70 years | DK, FI, NO, SE | 637/118 392.5 | 767/109 608.5 | 17.2 (−38.5 to 73.0) | −17.4 (−71.5 to 36.6) |
| Death | |||||
| All | DK, FI, NO, SE | 229/335 719.6 | 243/231 679.9 | 8.1 (−3.3 to 19.4) | −40.7 (−123.4 to 42.1) |
| Female | DK, FI, NO, SE | 101/174 193.8 | 106/126 399.7 | 6.5 (−10.8 to 23.8) | 13.3 (−45.7 to 72.3) |
| Male | DK, FI, NO, SE | 128/161 525.8 | 137/105 280.1 | 7.7 (−5.5 to 20.9) | 1.9 (−44.7 to 48.6) |
| Age <70 years | DK, FI, SE | 24/208 233.3 | 17/108 554.2 | 2.3 (−3.0 to 7.6) | 8.7 (−67.8 to 85.3) |
| Age ≥70 years | DK, FI, NO, SE | 205/119 525.1 | 225/110 887.5 | 9.8 (−12.3 to 31.8) | 6.4 (−38.7 to 51.4) |
CI=confidence interval; DK=Denmark; FI=Finland; NO=Norway; SE=Sweden.
Each person was followed up from day 8 after fourth dose vaccination until day 90, an outcome event, 14 (for hospital admission) or 30 (for death) days after positive polymerase chain reaction test for SARS-CoV-2, emigration, death, or end of study period, whichever occurred first. Comparisons used stabilised inverse probability of treatment weights to take into account year of birth (5 year bins), calendar month of receipt of fourth vaccine dose, sex, region of residence, vaccination priority groups, comorbidities, and previous SARS-CoV-2 infection. Comparative vaccine effectiveness and risk difference point estimates in sex and age subgroup analyses of covid-19 related death are in opposite directions (insignificantly so) to overall estimates because of lack of statistical precision due to few events in country specific analyses and lack of overlap between comparison groups (represented by corresponding wide 95% CIs; see supplementary table S7).
Fig 3.
Cumulative incidence curves of covid-19 related hospital admission and death comparing people vaccinated with bivalent BA.4-5 mRNA booster vaccine as fourth dose with those vaccinated with bivalent BA.1 mRNA booster vaccine as fourth dose in each of four Nordic countries. Each person was followed from day 8 to day 90 after receiving bivalent booster as fourth dose
Comparative effectiveness of monovalent vaccine as fourth dose
Among three dose vaccinated people, at day 90 after receipt of a fourth dose with monovalent vaccine the comparative vaccine effectiveness and risk difference were 57.4% (44.6% to 70.1%) and –86.8 (–152.9 to –20.7) per 100 000 for admission to hospital with covid-19 (326 v 737 events) and 65.4% (36.0% to 94.7%) and –27.8 (–48.5 to –7.0) per 100 000 for covid-19 related death (90 v 254 events) (supplementary figures S14 and S15 and table S11). Sex, age, and extended follow-up analyses were similar to those for the bivalent boosters. Comparing bivalent versus monovalent booster as the fourth dose at day 90 did not result in significant differences in the risk of the severe covid-19 outcomes (for example, comparative vaccine effectiveness for hospital admission was 33.8% (–2.7% to 70.3%) for BA.4-5 and 0.8% (–49.0% to 50.7%) for BA.1 booster versus monovalent vaccination) (supplementary figure S16 and table S12).
Discussion
This study found that fourth dose vaccination (that is, second booster) with the bivalent BA.4-5 or BA.1 mRNA booster vaccines was associated with lower rates of covid-19 related hospital admission and death (comparative vaccine effectiveness of ≥65%) among people from the Nordic countries aged ≥50 years who had previously been vaccinated with three doses of monovalent vaccine (that is, a primary course and a first booster dose). Furthermore, we observed no significant difference in the protection afforded by the bivalent BA.4-5 and BA.1 mRNA booster vaccines as a fourth dose when we compared them directly. Specifically, although severe covid-19 was a very rare event in our Nordic cohorts including 2.68 million people vaccinated with bivalent booster, the 95% confidence interval of the main analysis comparing BA.4-5 with BA.1 bivalent booster vaccines allowed us to conclude that any risk differences in covid-19 hospital admissions would be small, between –14.4 and 34.4 per 100 000 people vaccinated.
Comparison with other studies
Covid-19 vaccination policies recommending the bivalent mRNA boosters as a fourth vaccine dose are mainly supported by studies on immunogenicity, some of which have shown induction of higher antibody concentrations against omicron subvariants compared with monovalent boosters.4 5 6 7 8 Previous observational studies of the effectiveness of fourth dose monovalent vaccination also lend some indirect support to these recommendations.16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32
Although the protection afforded by the bivalent boosters seems to be modest against SARS-CoV-2 infection, with estimates of vaccine effectiveness ranging between 14% and 52%,35 36 37 the protection against severe covid-19 outcomes is likely higher, but data are sparse.9 10 11 12 13 14 15 Available estimates from the US, Israel, Canada, and England of the effectiveness of the bivalent BA.4-5 and BA.1 boosters against covid-19 related hospital admission, death, or both seem to be compatible with our findings (table 4).9 10 11 12 13 14 15 The size of our population enabled the estimation of three month comparative effectiveness with high statistical precision. Moreover, our estimates at six months of follow-up indicated that the additional protection provided by a fourth dose was well preserved with little waning. The estimates of the effectiveness against severe covid-19 of BA.4-5 boosters in North Carolina and BA.1 boosters in the UK was 38.4% (13.4% to 56.1%) after 20 weeks and 35.9% (31.4% to 40.1%) after ≥10 weeks, respectively,12 15 and results from Canada indicate a similar modest waning of vaccine effectiveness during 119 days of follow-up (for example, vaccine effectiveness of 76% (66% to 83%) for BA.1 boosters).14 Additionally, as both the BA.4-5 and BA.1 bivalent boosters were implemented in the Nordic vaccination strategies, we were able to compare these head to head. We found no consistent support for large differences in effectiveness between the two vaccine types. Notably, the precision of our combined Nordic estimates suggests that any potential difference is likely to be small in absolute numbers. For example, the 95% confidence interval for admission to hospital with covid-19 is incompatible with >14 fewer events and >34 excess events per 100 000 among people vaccinated with BA.4-5 compared with BA.1 booster during the first three months after vaccination.
Table 4.
Identified relatable studies on vaccine effectiveness of bivalent BA.4-5 and BA.1 booster vaccination
| Country | Bivalent booster population size and age | Design | Severe covid-19 outcome | Comparative vaccine effectiveness, % (95% CI) | |
|---|---|---|---|---|---|
| Denmark, Finland, Norway, and Sweden* | 2 676 323 BA.4-5 or BA.1 boosted (fourth dose) people aged ≥50 years | Cohort analyses | Hospital admission | BA.4-5, 3 months | 67.8 (63.1 to 72.5) |
| Death | 69.8 (52.8 to 86.8) | ||||
| Hospital admission | BA.1, 3 months | 65.8 (59.1 to 72.4) | |||
| Death | 70.0 (50.3 to 89.7) | ||||
| United States9 | 884 BA.4-5 boosted (any dose) people aged ≥18 years | Test negative case-control study | Hospital admission | BA.4-5, ≥7 days | ≥42 (20 to 58); 59 (44 to 70)† |
| United States10 | 79 BA.4-5 boosted (any dose) people aged ≥65 years | Test negative case-control study | Hospital admission | BA.4-5, ≥7 days | ≥73 (52 to 85); 84 (64 to 93)† |
| United States‡11 12 | 1 279 802 BA.4-5 boosted (any dose) people aged ≥12 years | Cohort study | Hospital admission or death§ | BA.4-5, 2 weeks | 67.4 (46.2 to 80.2)† |
| BA.4-5, 20 weeks | 38.4 (13.4 to 56.1)† | ||||
| Israel13 | 134 215 BA.4-5 boosted (any dose) people aged ≥65 years | Cohort study | Hospital admission | BA.4-5, 4 months | 72 (60 to 81)** |
| Death | 68 (45 to 82)** | ||||
| Canada14 | Numbers of included BA.4-5 and BA.1 boosted (≥fourth dose) people aged ≥50 years uncertain¶ | Test negative case-control study | Hospital admission or death§ | BA.4-5, day 7-29 | 83 (77 to 88)† |
| BA.4-5, day 60-89 | 81 (72 to 87)† | ||||
| Hospital admission or death§ | BA.1, day 7-29 | 86 (82 to 90)† | |||
| BA.1, day 90-119 | 76 (66 to 83)† | ||||
| England15 | 29 954 BA.1 boosted (any dose) people aged ≥50 years | Test negative case-control study | Hospital admission | BA.1, week 2-4 | 53.0 (47.9 to 57.5) |
| BA.1, ≥10 weeks | 35.9 (31.4 to 40.1) | ||||
CI=confidence interval.
This study. Comparative vaccine effectiveness at 6 months of follow-up for BA.4-5 boosted was 54.9% (49.0% to 60.8%) for hospital admission and 61.3% (35.5% to 87.1%) for death; for BA.1 boosted, estimates were 63.5% (49.5% to 77.5%) and 67.4% (47.7% to 87.2%), respectively.
Not comparative vaccine effectiveness as booster vaccinated people were compared with unvaccinated people.
Initial study from North Carolina in US11 included 1 070 136 BA.4-5 boosted (any dose) people with vaccine effectiveness of 61.8% (48.2% to 71.8%) for hospital admission or death compared with unvaccinated people from day 15-99 after bivalent booster vaccination.
Combined outcome reported.
Specific number of bivalent boosted people across cases and control could not be found; however, study reported 636 cases among bivalent booster vaccinated people.
Reported as hazard ratio of 0.28 (0.19 to 0.40) for hospital admission and 0.32 (0.18 to 0.58) for death.
Strengths and limitations of study
A main limitation of this observational study is the lack of controlled randomisation. Therefore, we cannot fully exclude the possibility of residual unmeasured confounding factors being unevenly distributed between compared groups to the extent that such factors were not indirectly adjusted for by the set of included covariates (that is, proxies). To the best of our knowledge, the assignment of the type of booster as the fourth dose was unselective during the study period, and, except for the initial prioritisation of older and vulnerable people during the study period in Denmark (results did not change when we excluded this period), the analyses should reflect a time when fourth dose vaccination was offered to the general public. Although people were required to fulfil a restrictive set of pre-specified criteria to be considered cases admitted to hospital with covid-19, we cannot exclude the possibility that our outcome definition captured cases in which the infection with SARS-CoV-2 only partly contributed to or coincided with the timing of admission. Similarly, our definition of covid-19 related death as any death occurring within 30 days of a positive SARS-CoV-2 PCR test used in Denmark, Finland, and Sweden was most likely subject to some outcome misclassification. Also, we had no information on at-home antigen testing for SARS-CoV-2 infection, and, although confirmatory PCR testing has generally been advised and people at risk of severe covid-19 have been recommended to have PCR testing if presenting with symptoms, we did not capture all SARS-CoV-2 infections in each country. We reassuringly observed no major differences in the effect estimates for covid-19 related death between Norway (where a covid-19 cause specific definition of death was used) and other countries. In addition, our comparative population based design would tend to mitigate larger differences in this potential outcome misclassification between compared groups as opposed to, for example, analyses using unvaccinated people as a reference group, which would be more prone to bias owing to differences in outcome misclassifications (for example, greater differences in healthcare contact patterns) and healthy vaccinee bias; also, unvaccinated people do not reflect the targeted population for fourth dose booster vaccination. Moreover, the booster vaccination uptake among ≥50 year olds has been high (>90%) in the Nordic countries. Nevertheless, we cannot fully exclude a relative healthy vaccinee bias; our effect estimates with the longer follow-up of 180 days for the four versus three dose vaccinated comparisons would be most susceptible to any such influence. If so, this would suggest that the waning effects we estimated would tend towards being conservative relative to the true waning effects.
Owing to the nature of the healthcare registers in the four Nordic countries, we were able to consider potential confounders on an individual level, which we included by matching or by stabilised inverse probability of treatment weights. However, our direct head-to-head comparison between bivalent and monovalent boosters as a fourth dose was limited by the poorer overlap of calendar periods of use and the need for control thereof (owing to otherwise potential confounding by, for example, differences in waning of third dose, background population infection rates, and circulating variants), resulting in lower statistical precision for some of these analyses. Although fourth dose vaccination with a monovalent vaccine seemed to offer similar protection to the bivalent boosters when we compared the results from the four versus three dose comparisons indirectly (the 95% confidence intervals broadly overlap), these abovementioned factors limit the interpretability of such indirect comparisons of the association estimates. Moreover, given that we controlled for potential confounders through either matching or weighting, results from the individual comparisons should primarily be interpreted separately.
Our results likely have a high degree of generalisability to other similar populations. However, because we assessed the comparative effectiveness against covid-19 related hospital admission and death associated with the bivalent mRNA booster vaccines given as a fourth dose, our results may only indirectly support any evaluation of the effectiveness of these vaccines in other covid-19 vaccination schedule scenarios. As per the study design, we did not examine, and thus our results cannot directly help to inform on, the vaccine effectiveness among people younger than 50; similarly, our estimates may not compare to those in populations with a demographically different composition or in other specific clinical subgroups that were not studied.
Conclusions
Among people aged ≥50 years who had received three doses of covid-19 vaccine, vaccination with the bivalent BA.4-5 or BA.1 mRNA boosters as a fourth vaccine dose (that is, second booster) was associated with lower rates of covid-19 related hospital admission and death at three months of follow-up. The effectiveness remained high when we extended follow-up to six months. In these combined analyses of data from four Nordic countries, the effectiveness of BA.4-5 and BA.1 boosters as a fourth vaccine dose did not differ, and the results indicate that any potential differences in the risk of severe covid-19 outcomes are most likely to be very small in absolute numbers.
What is already known on this topic
The omicron adapted bivalent mRNA booster vaccines containing the original SARS-CoV-2 and omicron BA.4-5 or BA.1 subvariants are now implemented in many covid-19 booster vaccination programmes
Data to evaluate the clinical effectiveness of the bivalent BA.4-5 and BA.1 mRNA booster vaccines against severe covid-19 are limited, and studies on immunogenicity have shown diverging results
What this study adds
Vaccination of adults aged ≥50 years with a bivalent booster vaccine as a fourth dose was associated with lower rates of covid-19 related hospital admission and death at three months of follow-up
Effectiveness was well preserved at six months of follow-up
Any potential difference between the protection afforded by the BA.4-5 and the BA.1 mRNA boosters against severe covid-19 seem to be very small in absolute numbers
Acknowledgments
We thank Anja Bråthen Kristoffersen for the statistical support in relation to the Norwegian data analysis during the revision process.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary appendix
Contributors: NWA, EMT, and AH conceptualised the study. NWA drafted the manuscript. EMT, UB, JS, and NP did the statistical analyses. All authors interpreted the results and critically reviewed the manuscript. AH supervised the study. NWA and AH are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research was supported by the European Medicines Agency. The funders had no role in considering the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. This document expresses the opinion of the authors of the paper and may not be understood or quoted as being made on behalf of, or reflecting the position of, the European Medicines Agency or one of its committees or working parties.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the European Medicines Agency; EP has received a grant from the Finnish Medical Foundation; RL has received grants from Sanofi Aventis paid to his institution and personal fees from Pfizer (all outside the submitted work); no other relationships or activities that could appear to have influenced the submitted work.
The lead authors (the manuscript’s guarantors) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Studied participants were anonymised in the data sources used; therefore, direct dissemination to study participants is not possible. The study results will be disseminated to the public and health professionals by a press release written using layman’s terms.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
According to Danish, Finish, Norwegian, and Swedish law, ethics approval is exempt for such research.
Data availability statement
Owing to data privacy regulations in each country, the raw data cannot be shared.
References
- 1. Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139-45. 10.15585/mmwr.mm7104e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edara V-V, Manning KE, Ellis M, et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep Med 2022;3:100529. 10.1016/j.xcrm.2022.100529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lyke KE, Atmar RL, Islas CD, et al. DMID 21-0012 Study Group . Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med 2022;3:100679. 10.1016/j.xcrm.2022.100679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalkias S, Feng J, Chen X, et al. Neutralization of Omicron Subvariant BA.2.75 after Bivalent Vaccination. N Engl J Med 2022;387:2194-6. 10.1056/NEJMc2212772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chalkias S, Harper C, Vrbicky K, et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N Engl J Med 2022;387:1279-91. 10.1056/NEJMoa2208343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou J, Kurhade C, Patel S, et al. Neutralization of BA.4-BA.5, BA.4.6, BA.2.75.2, BQ.1.1, and XBB.1 with Bivalent Vaccine. N Engl J Med 2023;388:854-7. 10.1056/NEJMc2214916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis-Gardner ME, Lai L, Wali B, et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N Engl J Med 2023;388:183-5. 10.1056/NEJMc2214293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Q, Bowen A, Valdez R, et al. Antibody Response to Omicron BA.4-BA.5 Bivalent Booster. N Engl J Med 2023;388:567-9. 10.1056/NEJMc2213907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tenforde MW, Weber ZA, Natarajan K, et al. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults - VISION Network, Nine States, September-November 2022. MMWR Morb Mortal Wkly Rep 2023;71:1637-46. 10.15585/mmwr.mm7153a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surie D, DeCuir J, Zhu Y, et al. IVY Network . Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19-Associated Hospitalization Among Immunocompetent Adults Aged ≥65 Years - IVY Network, 18 States, September 8-November 30, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1625-30. 10.15585/mmwr.mm715152e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin D-Y, Xu Y, Gu Y, et al. Effectiveness of Bivalent Boosters against Severe Omicron Infection. N Engl J Med 2023;388:764-6. 10.1056/NEJMc2215471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin D-Y, Xu Y, Gu Y, Zeng D, Sunny SK, Moore Z. Durability of Bivalent Boosters against Omicron Subvariants. N Engl J Med 2023;388:1818-20. 10.1056/NEJMc2302462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arbel R, Peretz A, Sergienko R, et al. Effectiveness of a bivalent mRNA vaccine booster dose to prevent severe COVID-19 outcomes: a retrospective cohort study. Lancet Infect Dis 2023;S1473-3099(23)00122-6. 10.1016/S1473-3099(23)00122-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grewal R, Buchan SA, Nguyen L, et al. Effectiveness of mRNA COVID-19 monovalent and bivalent vaccine booster doses against Omicron severe outcomes among adults aged ≥50 years in Ontario, Canada. medRxiv 2023. 10.1101/2023.04.11.23288403. [DOI] [PMC free article] [PubMed]
- 15. Kirsebom FCM, Andrews N, Stowe J, et al. Long-term duration of protection of ancestral-strain monovalent vaccines and effectiveness of the bivalent BA.1 boosters against COVID-19 hospitalisation during a period of BA.5, BQ.1, CH.1.1. and XBB.1.5 circulation in England. medRxiv 2023. 10.1101/2023.03.31.23288018. [DOI] [PubMed]
- 16. Muhsen K, Maimon N, Mizrahi AY, et al. Association of Receipt of the Fourth BNT162b2 Dose With Omicron Infection and COVID-19 Hospitalizations Among Residents of Long-term Care Facilities. JAMA Intern Med 2022;182:859-67. 10.1001/jamainternmed.2022.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen MJ, Oster Y, Moses AE, Spitzer A, Benenson S, Israeli-Hospitals 4th Vaccine Working Group . Association of Receiving a Fourth Dose of the BNT162b Vaccine With SARS-CoV-2 Infection Among Health Care Workers in Israel. JAMA Netw Open 2022;5:e2224657. 10.1001/jamanetworkopen.2022.24657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nordström P, Ballin M, Nordström A. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: A nationwide, retrospective cohort study in Sweden. Lancet Reg Health Eur 2022;21:100466. 10.1016/j.lanepe.2022.100466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arbel R, Sergienko R, Friger M, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med 2022;28:1486-90. 10.1038/s41591-022-01832-0 [DOI] [PubMed] [Google Scholar]
- 20. Magen O, Waxman JG, Makov-Assif M, et al. Fourth Dose of BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med 2022;386:1603-14. 10.1056/NEJMoa2201688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N Engl J Med 2022;386:1712-20. 10.1056/NEJMoa2201570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gazit S, Saciuk Y, Perez G, Peretz A, Pitzer VE, Patalon T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ 2022;377:e071113. 10.1136/bmj-2022-071113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grewal R, Kitchen SA, Nguyen L, et al. Effectiveness of a fourth dose of covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ 2022;378:e071502. 10.1136/bmj-2022-071502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Intawong K, Chariyalertsak S, Chalom K, et al. Reduction in severity and mortality in COVID-19 patients owing to heterologous third and fourth-dose vaccines during the periods of delta and omicron predominance in Thailand. Int J Infect Dis 2023;126:31-8. 10.1016/j.ijid.2022.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brosh-Nissimov T, Hussein K, Wiener-Well Y, et al. Hospitalized Patients With Severe Coronavirus Disease 2019 During the Omicron Wave in Israel: Benefits of a Fourth Vaccine Dose. Clin Infect Dis 2023;76:e234-9. 10.1093/cid/ciac501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiss Z, Wittmann I, Polivka L, et al. Nationwide Effectiveness of First and Second SARS-CoV2 Booster Vaccines During the Delta and Omicron Pandemic Waves in Hungary (HUN-VE 2 Study). Front Immunol 2022;13:905585. 10.3389/fimmu.2022.905585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lau JJ, Cheng SMS, Leung K, et al. Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat Med 2023;29:348-57. 10.1038/s41591-023-02219-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Link-Gelles R, Levy ME, Gaglani M, et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA Vaccine Doses Among Immunocompetent Adults During Periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 Sublineages Predominated - VISION Network, 10 States, December 2021-June 2022. MMWR Morb Mortal Wkly Rep 2022;71:931-9. 10.15585/mmwr.mm7129e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jara A, Cuadrado C, Undurraga EA, et al. Effectiveness and duration of a second COVID-19 vaccine booster. medRxiv 2022. 10.1101/2022.10.03.22280660. [DOI]
- 30. Britton A, Embi PJ, Levy ME, et al. Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults During SARS-CoV-2 Omicron Predominance - VISION Network, 10 States, December 2021-August 2022. MMWR Morb Mortal Wkly Rep 2022;71:1335-42. 10.15585/mmwr.mm7142a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tseng HF, Ackerson BK, Bruxvoort KJ, et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat Commun 2023;14:189. 10.1038/s41467-023-35815-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Surie D, Bonnell L, Adams K, et al. IVY Network . Effectiveness of Monovalent mRNA Vaccines Against COVID-19-Associated Hospitalization Among Immunocompetent Adults During BA.1/BA.2 and BA.4/BA.5 Predominant Periods of SARS-CoV-2 Omicron Variant in the United States - IVY Network, 18 States, December 26, 2021-August 31, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1327-34. 10.15585/mmwr.mm7142a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021;398:2093-100. 10.1016/S0140-6736(21)02249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ioannou GN, Bohnert ASB, O’Hare AM, et al. COVID-19 Observational Research Collaboratory (CORC) . Effectiveness of mRNA COVID-19 Vaccine Boosters Against Infection, Hospitalization, and Death: A Target Trial Emulation in the Omicron (B.1.1.529) Variant Era. Ann Intern Med 2022;175:1693-706. 10.7326/M22-1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Link-Gelles R, Ciesla AA, Roper LE, et al. Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5- and XBB/XBB.1.5-Related Sublineages Among Immunocompetent Adults - Increasing Community Access to Testing Program, United States, December 2022-January 2023. MMWR Morb Mortal Wkly Rep 2023;72:119-24. 10.15585/mmwr.mm7205e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huiberts AJ, de Gier B, Hoeve CE, et al. Effectiveness of bivalent mRNA booster vaccination against SARS-CoV-2 Omicron infection, the Netherlands, September to December 2022. Euro Surveill 2023;28:2300087. 10.2807/1560-7917.ES.2023.28.7.2300087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chemaitelly H, Ayoub HH, AlMukdad S, et al. Bivalent mRNA-1273.214 vaccine effectiveness in Qatar. medRxiv 2023. 10.1101/2023.04.15.23288612. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary appendix
Data Availability Statement
Owing to data privacy regulations in each country, the raw data cannot be shared.