Abstract
Our planet teeters on the brink of massive ecosystem collapses, and arid regions experience manifold environmental and climatic challenges that increase the magnitude of selective pressures on already stressed ecosystems. Ultimately, this leads to their aridification and desertification, that is, to simplified and barren ecosystems (with proportionally less microbial load and diversity) with altered functions and food webs and modification of microbial community network. Thus, preserving and restoring soil health in such a fragile biome could help buffer climate change's effects. We argue that microorganisms and the protection of their functional properties and networks are key to fight desertification. Specifically, we claim that it is rational, possible and certainly practical to rely on native dryland edaphic microorganisms and microbial communities as well as dryland plants and their associated microbiota to conserve and restore soil health and mitigate soil depletion in newly aridified lands. Furthermore, this will meet the objective of protecting/stabilizing (and even enhancing) soil biodiversity globally. Without urgent conservation and restoration actions that take into account microbial diversity, we will ultimately, and simply, not have anything to protect anymore.
By considering recent literature, we discuss how dryland microorganisms could notably be used in the protection of the soil and, in general, the dryland environment and improve world food production. More specifically, we expose our vision on why and how drylands—among the most dominant terrestrial biome surface‐wise—are expanding under the threat of climate change, and their indigenous microbiota could be used (i) to design desertification mitigation strategies and (ii) to improve dryland agriculture.
BACKGROUND
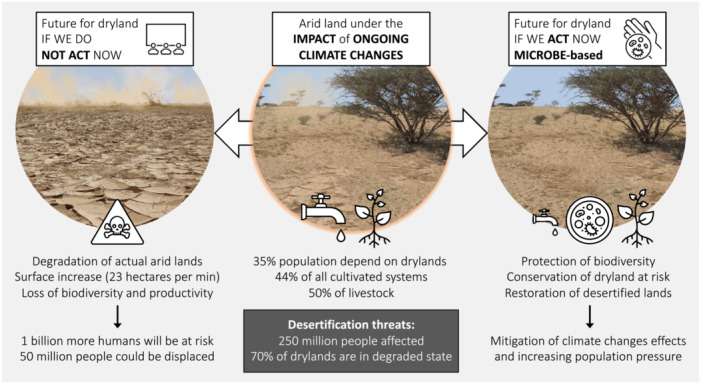
Terrestrial surfaces are defined as drylands when the aridity index (AI), the ratio of precipitation (P) over potential evapotranspiration (PET), is below 0.65 (Prăvălie, 2016). Drylands cover more than 45% of the Earth's emerged surface, and hence, their biomes are a main component of the overall terrestrial biome. Despite a false perception that tends to categorize biomes of arid ecosystems as poor in terms of biodiversity and with low global economic contribution, almost 3 billion humans and ~half of our global food production (45% of all cultivated surfaces and 50% of livestock) live in and originate from drylands, respectively (IPCC, 2019). To this, we must add the huge diversity of wild and endemic life forms adapted to survive in these areas by (co)evolving with natural modifications (Maestre et al., 2021). However, the ongoing temperature and weather pattern fluctuations—caused by the anthropogenic‐mediated rise of the concentration of greenhouse gases, such as methane (CH4), nitrous oxide (N2O) and carbon dioxide (CO2)—entail an intensification in global aridity. The consequences are an expansion of dryland surfaces (more than 20% of the terrestrial surface; Huang et al., 2016) and abrupt changes in ecosystem functionality, that is, soil fertility and plant productivity and diversity (Berdugo et al., 2020). If we assume that “It takes up to 1000 years to form one centimetre of topsoil, but this one centimetre can be lost with just one heavy rainfall if such soil is not covered/protected” (quote from Mr. Mansur, FAO's Director of the Land and Water Division, 2019), it is clear that losing (top)soil due to degradation, aridification or desertification is a critical issue for ecosystem sustainability in the Anthropocene because to recover from this damage the environment needs much longer than a human lifetime. Furthermore, desertification not only drives the formation of new arid lands, but it also threatens existing ones. More specifically, it negatively affects the biodiversity, cover and stability of Biological Soil Crusts (BSCs)—the living skin of many dryland biomes (Weber et al., 2022)—, soil microbial diversity and abundances (Maestre et al., 2015), xerophytic vegetation (Berdugo et al., 2020), and in many cases increases soil salinity (IPCC, 2019). Biodiversity decline and ecosystem instability are intensified by improper land management, overgrazing, inadequate agricultural practices, and over‐exploitation of aquifers (Maestre et al., 2022; Martínez‐Valderrama et al., 2020). As a result, dryland food production yields are declining, with dramatic long‐term effects in prone‐to‐famine arid regions of Africa and Asia, with 250 million people affected globally and 1 billion people at risk within the next ten years (IPCC, 2019).
To adapt to the new environmental conditions imposed by global changes, natural and managed arid lands (e.g. for food production) need to reach a new steady state. In this opinion, we argue that the microbiota of drylands—already adapted to xeric‐stressed and oligotrophic conditions—has the potential to be exploited for supporting the short‐ and long‐term sustainability and survival of plant species under increasing water scarcity. BSCs and xerophytic desert plant microbiomes are unique microbiological (eco)systems that can offer a vast range of natural solutions to drought and aridity. We discuss that they should be exploited and applied at the forefront of conservation‐restoration‐management projects of dryland soil to (i) fight soil erosion, (ii) promote revegetation and (iii) improve plant‐crop cultivation.
Why does soil in arid lands are under the threat of degradation? The impact on edaphic microbial communities under the ongoing climate change
Soils and their (micro)biosphere are critical for human livelihood and food production (i.e. crops and livestock). We rely on soil health and productivity and thus—as a corollary—on healthy, productive and functioning edaphic microbial communities that support them (Timmis & Ramos, 2021). Recent studies contend that aridification/desertification detrimentally affects the soil's micronutrient composition and physicochemical properties (Moreno‐Jiménez et al., 2023), as well as edaphic microbial community composition, diversity, functioning, and ecosystem service delivery (Guerra et al., 2022). Altogether, such studies indicate that climate change is detrimentally impacting the (micro)biology, chemistry and stability of soils globally and that dryland soils and their endemic microbial communities are among the most vulnerable in terrestrial biomes. This is further emphasized by the fact that these fragile ecosystems harbour distinct edaphic microbial assemblages generally with low alpha‐diversity, leading to the recent identification of dryland soils as nature conservation hotspots. The newly barren soils, which lack plants and/or BSCs, further constitute human and environmental threats via their newly acquired dust emission potentials. Indeed, desert dust plumes, which can have intercontinental ranges, transport highly resilient alien particle‐bound microorganisms that could be potential invaders of sensitive and/or pristine sink environments and potential pathogens for food crops or humans (Behzad et al., 2018). These “invisible atmospheric travellers” are also projected to survive longer with warming atmospheric temperatures and thus be able to travel longer distances than is currently possible (Archer & Pointing, 2020), exacerbating such concerns.
Supporting Albert Einstein's maxim, “a problem without a solution is a poorly stated problem”, dryland microbial communities are usually ignored in global‐scale desertification mitigation strategies despite dominating dryland ecosystem biology and service delivery. This is surprising as microorganisms have developed many ingenious strategies to cope with and function under quasi‐constant xeric‐stressed conditions, including, among others, UV tolerance and the capabilities of colonizing specific substrates, adapting their genome G + C content and developing heat shock responses (Jordaan et al., 2020). Therefore, we advocate using dryland microorganisms to support desert farming production yields and alleviate soil erosion. Indeed, the seeding and better management of endemic existing BSCs can enhance dryland soil stability (Reeve et al., 2023) and, if used in combination with beneficial microorganisms (e.g. plant growth promoting, PGP), have the potential to be at the forefront of dryland revegetation strategies, and even enhance desert farming production.
How do edaphic microorganisms improve soil stability and quality? Protecting and restoring the “living skin” of soil in arid lands is important
Edaphic microbial populations are pivotal for dryland soils' health and productivity/fertility, but their abundance and diversity are inversely proportional to the extent of abiotic stresses, resource limitations and aridity (Maestre et al., 2015). In drylands, microorganisms can form complex and structured topsoil communities, namely BSCs, composed of a phototrophic fraction, a rich fungal component, and often small plants and mosses in the more mature developmental stages (Weber et al., 2022). BSCs are essential biotic elements to arid habitats as they drive important ecosystem services mainly related to biogeochemical cycling (e.g. carbon and nitrogen fixation, phosphorous mobilization and bioavailability), mediate gaseous exchanges with the atmosphere, support dryland hydrological cycles and limit topsoil erosion (Pointing & Belnap, 2012). Where they are present in undisturbed steady states, BSCs are a stable component of dryland ecosystems and are essential to reach the climax in ecological successions, that is, the state in which the biotic ecosystem components are in equilibrium with each other and with the environment. However, exacerbation of aridity will make the cover and distribution of such BSC communities patchier, compromising soil fertility and enhancing the release of dust into the atmosphere.
Since most of the biological activities in arid soils occur in the uppermost layers, the enhancement of soil erosion tolerance provided by BSCs should, in our opinion, be considered a pivotal aspect of preserving dryland ecosystem functioning and multi‐functionality. The preservation of BSCs also has positive feedback on maintaining the soil's nutrient pool and water retention properties. Indeed, alterations of BSC physicochemical properties make the soil nutrient pool quickly depleted by lateral soil transfer due to wind and water erosion and accelerate soil‐atmosphere water exchanges through evaporation thus affecting the soil water balance. That is why mechanical (e.g. straw checkerboards), chemical (fixing chemical agents) or biological soil stabilization (e.g. through microorganisms bioinoculation) are increasingly studied as approaches to control sand stability and vegetation restoration, as reported by many studies conducted in China. Straw checkerboards are under study as an engineering measure for ecosystem rehabilitation, because they increase the threshold friction velocity of wind erosion, that is, the minimum friction velocity required to detach soil particles and exclude the disturbance agent(s). In this way, the efficacy of further biotechnological interventions (e.g. bioinoculation of microorganisms) is increased.
Given the essential role of BSCs in achieving a stable ecosystem steady state, it is important to predict how physical disturbance and climate change, two crucial drivers of BSC degradation and loss, will impact BSC cover, their community structure and functioning, and how their devastating outcomes, in turn, will be reflected on ecosystem processes. Physical disturbances (e.g. vehicle and human trampling, animal grazing) are conducive to a regression from later‐stage BSCs (“mature” moss/lichen‐dominated) to incipient cyanobacteria‐dominated BSCs (or worse barren soil without BSCs) with net‐negative incidence on water retention, carbon balance and nitrogen cycling. This owes to the extent that ecosystem services provided by BSCs are positively related to their maturation stage and the presence of mosses and lichens. Indeed, the loss of BSCs has significant drawbacks in terms of a decrease in the stored TOC and long‐term effects on C storage. Moreover, disturbances and modifications of the BSC autotrophic components will drastically impact the CO2‐sequestration potential of the arid/semi‐arid biomes (Rossi et al., 2015), which accounts for a substantial fraction of the total C sequestered by terrestrial ecosystems per year, resulting in positive carbon‐climate feedback (Dacal et al., 2022). The corollary is that the restoration and protection of BSCs would maintain the balance of nutrients in arid systems and increase C‐storage and C‐sequestration in drylands.
Ecosystem functioning and multifunctionality are strictly related to the richness of the BSC community, so its preservation must be regarded as paramount by restoration practitioners. Therefore, understanding how the BSC community will respond in the long term to climate change scenarios, both in cold and hot drylands, is an urgent need. Biotechnological approaches are emerging for rehabilitating disturbed BSCs and/or their ex‐novo introduction. Such interventions, in any case, must be accompanied by raising awareness and education on the ecological significance of BSCs, especially for those in charge of land management. This can be useful to develop valid/effective policies to reduce physical damages to BSCs, which may include restricting/removing livestock grazing, off‐road vehicle circulation, and recreational activities. Removing existing disturbances is an important prerequisite to elicit passive BSC recovery and increase the effectiveness of any possible restoration approach. While physical disturbances of BSCs can be mitigated, the future changes in precipitation patterns and temperature—considered unavoidable for many dryland ecosystems—can only be predicted. It advocates for the elaboration of updated/novel predictive models deriving from controlled microcosms and field experiments to anticipate—and maybe mitigate—the magnitude of the impact of climate change on BSC communities. At the same time, forecasting possible alternative steady states of the ecosystem (Zaneveld et al., 2017) and devising target restoration/rehabilitation plans for the more fragile areas (Coban et al., 2022) are important goals to evaluate the overall impact of loss/gain in BSCs and define ad hoc strategies of action.
How do plants and agricultural systems in arid lands survive under climate change and desertification? The plant microbiome is an ally to revegetate and cultivate arid lands
It has been estimated that in drylands, up to 23 hectares of vegetation per minute are lost due to drought and desertification (UNCCD). The worst‐case scenario is that aridity will lead to systemic and abrupt changes in ecosystem multi‐functionality, which ultimately will not be able to support/sustain plant productivity and will significantly negatively impact soil fertility and plant cover (Berdugo et al., 2020). This terrifying scenario is becoming more realistic every day if we consider that “more than 20% of the Earth's terrestrial surface will cross one or several of these aridification thresholds by 2100” (IPCC, 2019) without having enough time to evolve and adapt to such new environmental conditions as happened for the historical drylands (Maestre et al., 2021).
In this context, authorities and scientists—warned about desertification since at least the early 1980s—along with the general public, farmers and “stakeholders” have to consider the complexity of each organism, including plants. A plant is not a standalone organism that only interacts with the living soil (eco)system, but it is itself an ecosystem—or, better defined, as a metaorganism/holobiont—since plants have co‐evolved with complex and heterogeneous microbial communities (their associated microbiome) that drive their health/fitness and expedite adaptation (Trivedi et al., 2022). For this reason, the plant microbiome is also depicted and considered as a second plant genome that participates in the adaptation of the meta‐organisms (i.e. plant and microbiome) to (rapid) environmental changes (Angulo et al., 2022). Even though replacing the classic view of “organismal adaptation” with a “microbe‐mediated organismal adaptation” is still challenging, we cannot ignore the latest research in our field. We have to understand and exploit this holistic view to take advantage of the adaptation strategies/processes of both components, that is, the plant and its microbiome. Their cooperation and co‐evolution are the keys to conserve, restore and improve plant‐metaorganism resilience (ecological and evolutionary adaptation), growth and production in natural and managed systems (Mueller et al., 2020), especially in a warming and drying world (Trivedi et al., 2022). This implies that we can/should explore the microbial functional reservoir to improve dryland plant crop growth. Furthermore, it entails that we—the scientific community—should educate more extensively and deliver the above‐messages on the role of microbiomes to the relevant practitioners involved in soil/plant management; for instance, we could question why do agriculture practitioners add nitrogen to improve crop production to the soil—which can further have dramatic effects such as watershed eutrophication—when N‐fixing microorganisms could be used?
Whereas advances in genomic technologies have laid the path towards a better understanding of environmental microbial communities (Marasco et al., 2022), further efforts are necessary to expand our knowledge of the diversity of microbial communities associated with xerophytic plants in desert/arid ecosystems (Which microorganisms are there?), their functional role (What are they doing?), the process in place to drive their selection and recruitment (Why are they there?), and possibly to establish Nature‐Based‐Solutions that can proficiently implement the “microbiome services”. To explore and exploit the benefits of microbial (rescue) effects, a rewilding plant microbiome hypothesis has recently been proposed (Raaijmakers & Kiers, 2022): plant health can be improved by reinstating key members of the ancestral microbiota that were lost through domestication.
This innovative and natural approach underlines the importance of moving from “cultivated/crop” to “wild/ancestors” to identify beneficial‐microbial players that have long co‐evolved with plants in native ecosystems rather than by using synthetic microbial communities. We envisage that this type of study can be widened to microbial communities associated with “close sister plants” living in arid ecosystems. The available literature confirms that, even if the characterization of microbial diversity is the first step to dig into the ecological service carried by the plant microbiomes, we still lack a comprehensive understanding of the entire communities' components (among others, bacteria, archaea, and fungi), especially for non‐crop plants, and thus the network of the potential ecological services mediated by the rescue/beneficial microorganisms remain still elusive in their functional aspects.
How do we choose and use probiotic microorganisms to promote (re)vegetation and restore degraded lands?
Like any perturbation, climate change will unpredictably (i.e. with high variability) affect soil ecosystems, their indigenous life forms, the microbial ecology and the ecosystem services and multi‐functionality. In this context, and given all the above considerations, we need to conserve the existing microbial biodiversity of healthy ecosystems, especially in drylands, because it is the “key to soil survival”. Furthermore, it is pivotal to incorporate the microbial component into the ecosystem restoration planning to rebuild the disturbed ecosystem microbiome and take it into account within the land management practices (Averill et al., 2022).
Soil microbiome and its manipulation through inoculation‐based techniques are increasingly a focus of scientific research. Currently, available methods differ mainly in goals and methodology, encompassing actions aimed at soil productivity and fertility and techniques apt to conserve or restore soil ecosystem structure and microbial diversity. Methods can be distinguished between inoculation‐based approaches (soil addition of beneficial microbial strains or their bio‐products) and microbiome transplant‐based approaches (transfer of entire microbial communities collected from a healthy “sacrificial area” to a dysbiotic area). A critical point is that these techniques act by manipulating environmental microbial communities, which are complex entities composed of many interacting members whose interactions are largely unknown (De Roy et al., 2013). We are not yet able to comprehensively predict how microbial communities respond to external perturbations (e.g. climate change), nor are we able to determine with certainty how native communities respond to additive manipulations. It implies questioning if we are able (or not) to surgically create a change in the way we want (Li et al., 2021). Therefore, fine‐tuning the inoculation approach is not an easy task.
Ecosystem processes are related to the diversity of functional microbial groups and not simply to soil species richness. Hence, acting on the abundance and the activity of random microbial groups that are only theoretically beneficial could not meet the desired long‐lasting effects and/or may even be counterproductive for microbial community balance. For example, concern has been raised about commercial biofertilizers containing mixtures of mycorrhizal fungi spores because of possible invasive effects on the native mycorrhizal community, with effects similar to that of invasive plants (Koch et al., 2011). Therefore, upstream preparatory work is necessary, optimizing molecular approaches to identify and monitor the taxa more sensitive to disturbances whose responses are critical to environmental processes. A proposed approach is the determination of the richness, alpha diversity, evenness and phylogeny‐related trait dissimilarity of key functional groups (De Roy et al., 2013) and not, as it is commonly done, of the whole microbiome (Li et al., 2021). In this way, it is possible to act on those taxa whose abundance and activity are under threat and therefore become privileged targets of monitoring.
Both the inoculation of specific microbial strains/consortia and microbiome transplants currently present advantages and weaknesses. Regarding the former, while it is more accessible to manage, even by small farmers, the workflow to inoculum preparation and validation requires a significant investment of resources and time (Bashan et al., 2014). The steps in the process (strain isolation, screening for functional traits, selection of the most suitable culture mode, formulation) and the choice of delivery method (i.e. modality of inoculum distribution on/in the soil) are critical, as they can affect the fitness and performance of the inoculum to varying extents. The envisaged inoculants, cultured under the optimal conditions of the laboratory, are introduced in a largely challenging hostile environment, the target soil (Rossi et al., 2022). The inoculum must be treated and formulated to sustain the most complicated hurdle: surviving the competition with the native microflora that has undergone a long‐lasting selection by environmental conditions. This is a reason why the use of strains isolated from the target site is preferable. Besides avoiding the introduction of alien species—with unpredictable consequences—using native inoculants increases the chances of an effective adaptation to the local environmental conditions. However, such an approach alone does not guarantee the success of the treatment: finely tuning the inoculum preparation for specific inoculant types can be extremely important. For example, in the case of cyanobacteria, good results were obtained by a “hardening” process, that is, subjecting the inoculant to several wet‐dry cycles and increasing light intensities before inoculation (Giraldo‐Silva et al., 2019). The standardization of protocols designed for specific microbial groups and sharing these with the scientific community can help to enhance the optimization of the chances of adaptation of the inoculants. In this framework, fundamental studies on the physiological characteristics and inoculum potential of the more important inoculant species are to be encouraged to consolidate the technology. We believe that creating strains/consortia‐trait database fed and enlarged by multiple independent studies on the protocol of their application and effects on soil would represent important support for any effective experimental design for large‐scale applications.
The microbiome‐transplant approach may reduce such upstream workload as it relies on already assembled microbial communities from healthy “donor” areas proximal to the degraded soil considered for the recovery intervention. While “synthetic microbial community” inoculants can only include cultivable species, the transplanted natural communities harness the contribution of both cultivable and uncultivable members, already adapted to the local environmental (stressful) conditions. Microbiome transplant has been employed notably to restore BSCs, using off‐site BSC fragments or slurries on disturbed BSCs as a natural fertilizer (Maestre et al., 2006). Although the approach showed good promise, the drawback of the limited amount of inoculum that can be obtained should be considered as well as the risk of transforming healthy ecosystems into “sacrificial areas”.
The implementation of inoculation‐based techniques currently relies mainly on microcosm‐ and mesocosm‐level studies. On the other hand, large‐scale inoculation approaches exist but are rare and limited to specific microbial groups (Lan et al., 2014). This is where cost/benefit considerations are more pressing as the need arises for facilities for massive biomass cultivation, equipment suitable for inoculum distribution (e.g. modified irrigation systems). The knowledge gaps and uncertainties regarding the long‐term return for the treated ecosystems represent a current hurdle. Once the basic technology is established, investments in practical and economically sustainable methods in the field should follow. Present valid suggestions may be the use of open‐raceway ponds for culturing photosynthetic microorganisms, direct sunlight as an energy source and cost‐effective growth media to reduce cultivation costs. Another aspect of being considered is the selection of inoculants that can grow with reduced amount of water to limit the cost related to the transportation or movement of large water masses, especially when the areas to be treated are remote and far from any facility.
CONCLUSION
The United Nations have declared the decade starting with 2020 as the “UN Decade of Ecosystem Restoration” and even highlighted the role of BSCs in this pursuit. However, the recent outcome of COP27 shows that there is still much work to do to convince political leaders to take effective decisions against climate change.
In this context of the political status quo, very little attention is given to drylands, which constitute one of the largest and certainly among the most vulnerable biomes on Earth, and even less to the application of their adapted/selected edaphic microbial communities to prevent and mitigate soil erosion and fertility loss. Here, we put forward an opinion, guided by an important set of recent advancements in understanding the dryland systems and in the potential applications for restorations and by the need for nature‐based solutions in our fight against climate change. We consider that the endemic dryland soil microbiota is revealed to be a concrete and viable solution for protecting vulnerable soil ecosystems under the threat of climate change and for sustaining dryland agriculture and food production.
As discussed above, desertification and soil erosion should be counteracted by applying microbial Nature‐Based solutions. There is evidence that microorganisms already adapted to arid conditions, microbial communities and metaorganisms, such as BSCs and xerophytic plants, have the potential to be exploited for such nature‐based‐solutions and should be at the forefront of the strategies implemented in ecosystem restoration of degraded landscapes. The biodiversity and life‐history traits of arid/desert ecosystems and their biota is paramount and offer a natural advantage ahead of introducing foreign microorganisms or man‐made processes.
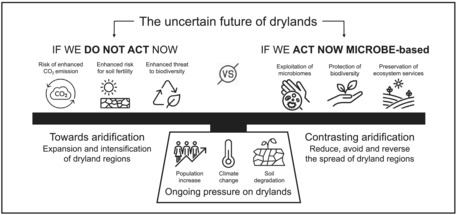
We have described microbes‐centred strategies that could be used to mitigate the effect of aridification in drylands: (i) the protection and conservation of edaphic dryland microbiome from total disruption (complete desertification) as well as the restoration of its biodiversity (e.g. BSCs and plants) when already impacted, (ii) support adaptation of crops in drylands by using beneficial microbes (probiotics). Combined, these approaches will limit soil erosion and they should be used to improve current practices of arid land management and have the potential to be used either individually or in concert. In other words, if we do not act now, we will not have anything left to protect in the future as arid lands are expanding and therefore, their BSCs and vegetation risk disappearing (Figure 1). Restoring microbial biodiversity and multifunctionality in the already lost soil is critical for ecologically and socially responsible landscape restoration attempts, particularly as plants accelerate their biomass production when their endemic soil microbiome is healthy. Thus, we strongly advocate for an urgent awareness that we no longer have the time to “wait and see”. Actions should be made, and decisions should be taken. Within the scale of ecological catastrophes, it is indeed more pressing to conserve and restore drylands than to monitor off‐target effects while BSC cover erodes globally and 2 billion people are on the verge of starvation.
FIGURE 1.
The need to modify our perspective on global drylands and their protection. With climate change, drylands are expanding and becoming drier. In this context, it is necessary to “act now”, and we argue that dryland conservation, restoration and management can—and must—be done using “microbe‐based solutions”. Indeed, with probably one more billion human beings living in drylands in the next decade, and to conserve their diversity and food production potential, we have to consider the entire dryland ecosystem, including their indigenous microorganisms. We advocate for the use of dryland microbial communities in any environmental initiatives strategies and global diversity framework designed to mitigate the effects of climate change in drylands globally, giving them the attention deserve.
AUTHOR CONTRIBUTIONS
Ramona Marasco: Conceptualization (lead). Jean‐Baptiste Ramond: Conceptualization (equal). Marc W. Van Goethem: Conceptualization (equal). Federico Rossi: Conceptualization (equal). Daniele Daffonchio: Conceptualization (equal).
FUNDING INFORMATION
No funding information provided.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
ACKNOWLEDGEMENTS
This study is supported by KAUST WDRC CCF funding FCC/1/1971‐44‐01. We thank Dr Marco Fusi for critically reviewing the manuscript and the graphic designer Claudia Andreotti for drawing the images of the figure.
Marasco, R. , Ramond, J.‐B. , Van Goethem, M.W. , Rossi, F. & Daffonchio, D. (2023) Diamonds in the rough: Dryland microorganisms are ecological engineers to restore degraded land and mitigate desertification. Microbial Biotechnology, 16, 1603–1610. Available from: 10.1111/1751-7915.14216
REFERENCES
- Angulo, V. , Beriot, N. , Garcia‐Hernandez, E. , Li, E. , Masteling, R. & Lau, J.A. (2022) Plant–microbe eco‐evolutionary dynamics in a changing world. The New Phytologist, 234, 1919–1928. [DOI] [PubMed] [Google Scholar]
- Archer, S.D.J. & Pointing, S.B. (2020) Anthropogenic impact on the atmospheric microbiome. Nature Microbiology, 5, 229–231. [DOI] [PubMed] [Google Scholar]
- Averill, C. , Anthony, M.A. , Baldrian, P. , Finkbeiner, F. , van den Hoogen, J. , Kiers, T. et al. (2022) Defending Earth's terrestrial microbiome. Nature Microbiology, 7, 1717–1725. [DOI] [PubMed] [Google Scholar]
- Bashan, Y. , De‐Bashan, L.E. , Prabhu, S.R. & Hernandez, J.‐P. (2014) Advances in plant growth‐promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant and Soil, 378, 1–33. [Google Scholar]
- Behzad, H. , Mineta, K. & Gojobori, T. (2018) Global ramifications of dust and sandstorm microbiota. Genome Biology and Evolution, 10, 1970–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdugo, M. , Delgado‐Baquerizo, M. , Soliveres, S. , Hernández‐Clemente, R. , Zhao, Y. , Gaitán, J.J. et al. (2020) Global ecosystem thresholds driven by aridity. Science, 367, 787–790. [DOI] [PubMed] [Google Scholar]
- Coban, O. , De Deyn, G.B. & van der Ploeg, M. (2022) Soil microbiota as game‐changers in restoration of degraded lands. Science, 375, abe0725. [DOI] [PubMed] [Google Scholar]
- Dacal, M. , Delgado‐Baquerizo, M. , Barquero, J. , Berhe, A.A. , Gallardo, A. , Maestre, F.T. et al. (2022) Temperature increases soil respiration across ecosystem types and soil development, but soil properties determine the magnitude of this effect. Ecosystems, 25, 184–198. [Google Scholar]
- De Roy, K. , Marzorati, M. , Negroni, A. , Thas, O. , Balloi, A. , Fava, F. et al. (2013) Environmental conditions and community evenness determine the outcome of biological invasion. Nature Communications, 4, 1383. [DOI] [PubMed] [Google Scholar]
- Giraldo‐Silva, A. , Nelson, C. , Barger, N.N. & Garcia‐Pichel, F. (2019) Nursing biocrusts: isolation, cultivation, and fitness test of indigenous cyanobacteria. Restoration Ecology, 27, 793–803. [Google Scholar]
- Guerra, C.A. , Berdugo, M. , Eldridge, D.J. , Eisenhauer, N. , Singh, B.K. , Cui, H. et al. (2022) Global hotspots for soil nature conservation. Nature, 610, 693–698. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Yu, H. , Guan, X. , Wang, G. & Guo, R. (2016) Accelerated dryland expansion under climate change. Nature Climate Change, 6, 166–171. [Google Scholar]
- IPCC . (2019) Special Report. Climate Change and Land.
- Jordaan, K. , Lappan, R. , Dong, X. , Aitkenhead, I.J. , Bay, S.K. , Chiri, E. et al. (2020) Hydrogen‐oxidizing bacteria are abundant in desert soils and strongly stimulated by hydration. mSystems, 5, e01131‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, A.M. , Antunes, P.M. , Kathryn Barto, E. , Cipollini, D. , Mummey, D.L. & Klironomos, J.N. (2011) The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biological Invasions, 13, 1627–1639. [Google Scholar]
- Lan, S. , Zhang, Q. , Wu, L. , Liu, Y. , Zhang, D. & Hu, C. (2014) Artificially accelerating the reversal of desertification: cyanobacterial inoculation facilitates the succession of vegetation communities. Environmental Science & Technology, 48, 307–315. [DOI] [PubMed] [Google Scholar]
- Li, H. , Chen, Y. , Yu, G. , Rossi, F. , Huo, D. , De Philippis, R. et al. (2021) Multiple diversity facets of crucial microbial groups in biological soil crusts promote soil multifunctionality. Global Ecology and Biogeography, 30, 1204–1217. [Google Scholar]
- Maestre, F.T. , Benito, B.M. , Berdugo, M. , Concostrina‐Zubiri, L. , Delgado‐Baquerizo, M. , Eldridge, D.J. et al. (2021) Biogeography of global drylands. The New Phytologist, 231, 540–558. [DOI] [PubMed] [Google Scholar]
- Maestre, F.T. , Delgado‐Baquerizo, M. , Jeffries, T.C. , Eldridge, D.J. , Ochoa, V. , Gozalo, B. et al. (2015) Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proceedings of the National Academy of Sciences of the United States of America, 112, 15684–15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre, F.T. , Le Bagousse‐Pinguet, Y. , Delgado‐Baquerizo, M. , Eldridge, D.J. , Saiz, H. , Berdugo, M. et al. (2022) Grazing and ecosystem service delivery in global drylands. Science, 378, 915–920. [DOI] [PubMed] [Google Scholar]
- Maestre, F.T. , Martín, N. , Díez, B. , López‐Poma, R. , Santos, F. , Luque, I. et al. (2006) Watering, fertilization, and slurry inoculation promote recovery of biological crust function in degraded soils. Microbial Ecology, 52, 365–377. [DOI] [PubMed] [Google Scholar]
- Marasco, R. , Fusi, M. , Ramond, J.‐B. , Van Goethem, M.W. , Seferji, K. , Maggs‐Kölling, G. et al. (2022) The plant rhizosheath–root niche is an edaphic “mini‐oasis” in hyperarid deserts with enhanced microbial competition. ISME Communications, 2, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Valderrama, J. , Guirado, E. & Maestre, F.T. (2020) Desertifying deserts. Nature Sustainability, 3, 572–575. [Google Scholar]
- Moreno‐Jiménez, E. , Maestre, F.T. , Flagmeier, M. , Guirado, E. , Berdugo, M. , Bastida, F. et al. (2023) Soils in warmer and less developed countries have less micronutrients globally. Global Change Biology, 29, 522–532. [DOI] [PubMed] [Google Scholar]
- Mueller, E.A. , Wisnoski, N.I. , Peralta, A.L. & Lennon, J.T. (2020) Microbial rescue effects: how microbiomes can save hosts from extinction. Functional Ecology, 34, 2055–2064. [Google Scholar]
- Pointing, S.B. & Belnap, J. (2012) Microbial colonization and controls in dryland systems. Nature Reviews. Microbiology, 10, 551–562. [DOI] [PubMed] [Google Scholar]
- Prăvălie, R. (2016) Drylands extent and environmental issues. A global approach. Earth‐Science Reviews, 161, 259–278. [Google Scholar]
- Raaijmakers, J.M. & Kiers, E.T. (2022) Rewilding plant microbiomes. Science, 378, 599–600. [DOI] [PubMed] [Google Scholar]
- Reeve, S. , Palmer, B. , Cobb, P. , Pietrasiak, N. & Lipson, D.A. (2023) Facilitating restoration of degraded biological soil crusts using mixed culture inoculation. Journal of Arid Environments, 208, 104876. [Google Scholar]
- Rossi, F. , Mugnai, G. & De Philippis, R. (2022) Cyanobacterial biocrust induction: a comprehensive review on a soil rehabilitation‐effective biotechnology. Geoderma, 415, 115766. [Google Scholar]
- Rossi, F. , Olguín, E.J. , Diels, L. & De Philippis, R. (2015) Microbial fixation of CO2 in water bodies and in drylands to combat climate change, soil loss and desertification. New Biotechnology, 32, 109–120. [DOI] [PubMed] [Google Scholar]
- Timmis, K. & Ramos, J.L. (2021) The soil crisis: the need to treat as a global health problem and the pivotal role of microbes in prophylaxis and therapy. Microbial Biotechnology, 14, 769–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi, P. , Batista, B.D. , Bazany, K.E. & Singh, B.K. (2022) Plant–microbiome interactions under a changing world: responses, consequences and perspectives. The New Phytologist, 234, 1951–1959. [DOI] [PubMed] [Google Scholar]
- Weber, B. , Belnap, J. , Büdel, B. , Antoninka, A.J. , Barger, N.N. , Chaudhary, V.B. et al. (2022) What is a biocrust? A refined, contemporary definition for a broadening research community. Biological Reviews, 97, 1768–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneveld, J.R. , McMinds, R. & Vega Thurber, R. (2017) Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nature Microbiology, 2, 17121. [DOI] [PubMed] [Google Scholar]


