Abstract
The stimuli-responsive nanofibers prepared by electrospinning have become an ideal stimuli-responsive material due to their large specific surface area and porosity, which can respond extremely quickly to external environmental incitement. As an intelligent drug delivery platform, stimuli-responsive nanofibers can efficiently load drugs and then be stimulated by specific conditions (light, temperature, magnetic field, ultrasound, pH or ROS, etc.) to achieve slow, on-demand or targeted release, showing great potential in areas such as drug delivery, tumor therapy, wound dressing, and tissue engineering. Therefore, this paper reviews the recent trends of stimuli-responsive electrospun nanofibers as intelligent drug delivery platforms in the field of biomedicine.
Keywords: Stimuli-responsive, Nanofibers, Electrospinning, Drug delivery, Biomedicine
Introduction
Stimuli-responsive materials are a new class of intelligent materials developed based on the concept of bionics, which can respond to small changes in the environment, such as temperature, pH, magnetic field, light, and ultrasound, through significant changes in their chemical or physical properties [1, 2]. Due to their functional properties, stimuli-responsive materials have a wide range of applications in the field of biomedicine [3–5]. Researchers have developed a large number of stimuli-response materials, including hydrogels [6], microspheres [7], micelles [8], and nanofibers [9], to enhance the efficacy of drug treatment or to confer special biological functions [10]. The stimuli-responsive hydrogels with bulk structures respond slowly to external stimuli, because the large size of the hydrogels makes it difficult for external stimuli to diffuse throughout the materials. Although microspheres and micelles dispersed in water in the form of nanoparticles have a fast stimulus response speed, their application in living organisms is limited due to their limited stability and lack of macroscopic shape and mechanical strength. Nanofiber membranes formed by random or ordered arrangement of nanofibers not only have macroscopic shape and mechanical strength, but also respond quickly to external stimuli, so they have broader application prospects in the field of biomedicine [11, 12].
Electrospinning can prepare polymer fibers nanometer diameter, and possesses the advantages of simple operation, extensive application range and high production efficiency [13, 14]. Electrospun nanofibers have high specific surface area, diverse structures, wide sources of preparation materials, unique physicochemical properties, and flexibility in surface modification [15, 16]. In addition, electrospun nanofibers can mimic the structure of the extracellular matrix (ECM), which can promote cell adhesion, proliferation, differentiation, and guide tissue repair and regeneration [17, 18]. These unique properties appealed to researchers to endow electrospun nanofibers with the ability to respond to stimuli. Stimuli-responsive electrospun nanofibers have extremely fast response speed to external stimuli due to their large specific surface area and porosity [19]. More importantly, they can efficiently load drugs, thereby realizing active drug release in vivo by controlling changes in environmental conditions [20]. In addition, the preparation materials of stimuli-responsive electrospun nanofibers have a wide range of sources, and the production process is simple and fast, which is promising to solve the problem of scale-up production of stimuli-responsive materials [21].
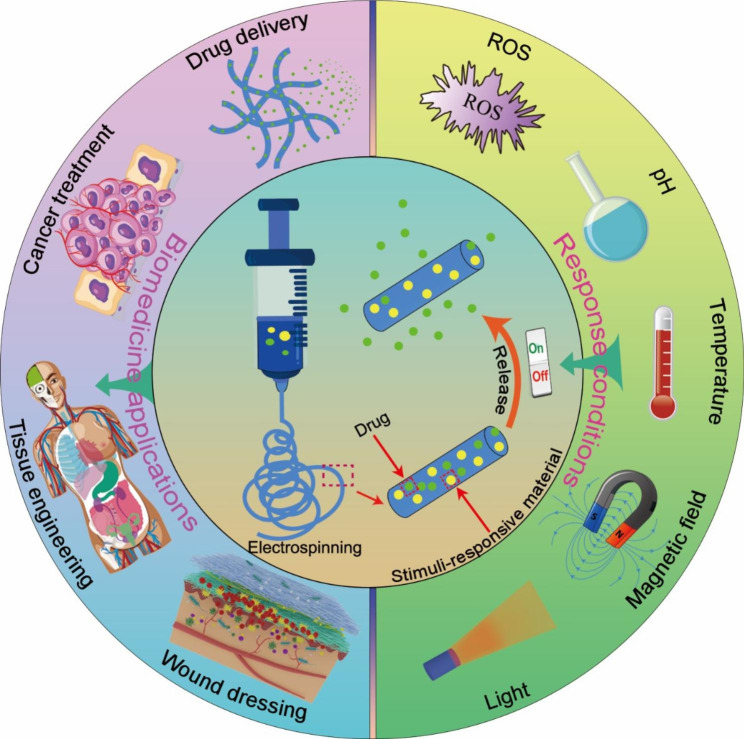
Therefore, this paper reviews the recent trends of intelligent drug delivery systems constructed from stimuli-responsive electrospun nanofibers in the biomedical field (Scheme 1). Firstly, the preparation methods of stimuli-response electrospun nanofibers were summarized. Then the design strategies of various types of stimuli-responsive electrospun nanofibers were discussed. In addition, the research progress of stimuli-responsive electrospun nanofibers in drug delivery, tumor therapy, wound dressing and tissue regeneration were introduced. Finally, the challenges and future development of stimuli-responsive electrospun nanofibers in clinical application were presented.
Scheme 1.
Schematic illustration of stimuli-responsive electrospun nanofibers as intelligent drug delivery platform in biomedicine field
Preparation method of stimuli-responsive electrospun nanofibers
Principle of electrospinning
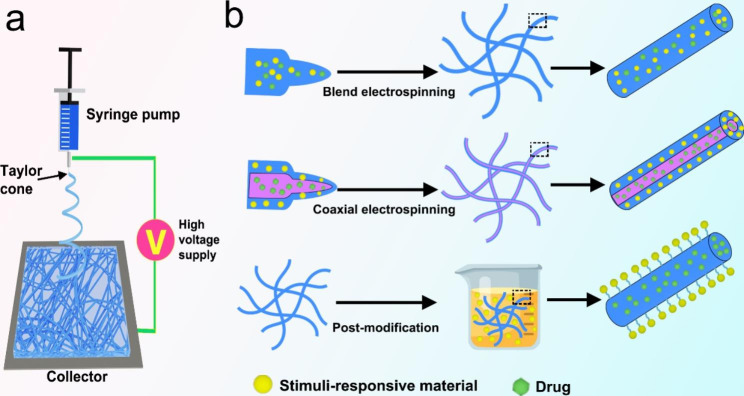
Electrospinning is a process in which the charged polymer solution is stretched and deformed by the electric field force generated by high-voltage static electricity, and then the nanofibers are obtained by volatilization of the solvent [22, 23]. The electrospinning equipment is mainly composed of high voltage power supply, syringe pump and collector (Fig. 1a) [24]. During the spinning process, the spinning liquid droplets in the syringe pump are subjected to two opposite forces: surface tension and electric field force. As the electric field force increases, the droplet is stretched into a cone shape, called a Taylor cone [25]. The droplet is sprayed from the tip of the Taylor cone onto the collector when the electric field force overcomes the surface tension of the droplet. The jet is stretched and lengthened, and the solvent is volatilized continuously [26]. Finally, the jet solidifies on the collector to form nanofibers [27]. The electrospinning process is controlled by numerous parameters, mainly involving solution parameters (e.g., viscosity, concentration, conductivity of the spinning solution, and evaporation rate of the solvent), process parameters (e.g., applied voltage, a flow rate of the polymer solution, and collection distance), and environmental parameters (temperature and humidity) [28, 29].
Fig. 1.
(a) Schematic of a conventional electrospinning equipment. (b) Schematic illustration of three common methods for preparing stimuli-responsive electrospun nanofibers
Materials and solvents
The materials used to prepare nanofibers by electrospinning are generally polymers. At present, more than 100 kinds of polymers have been successfully prepared as nanofibers, which can be divided into synthetic polymers and natural polymers. Synthetic polymers such as polyvinyl alcohol (PVA), polycaprolactone (PCL), polylactic acid (PLA), polyethylene oxide (PEO), polyurethane (PU) and other materials have controllable mechanical strength and physical properties, and excellent spinning performance [30, 31]. Due to the absence of biological properties of synthetic polymers, natural polymers are more appealing as biomedical materials. Natural polymers such as collagen, silk fibroin, gelatin, chitosan (CS), and hyaluronic acid (HA) have good biocompatibility and biodegradability, and can be better recognized by cell surface receptors in the process of tissue repair, thus triggering cell adhesion and proliferation [32]. However, the spinnability and mechanical properties of a single natural polymer are poor, so many researchers have developed natural/synthetic polymer composite fibers to take into account the advantages of two different polymers at the same time, so as to have better performance [33].
Common solvents utilized in electrospinning include formic acid, dichloromethane, hexafluoroisopropanol (HFIP), dimethylformamide (DMF), acetic acid, ethanol, trifluoroacetic acid, tetrahydrofuran and distilled water [34]. The properties of the solvent such as the dielectric constant, conductivity, volatility, and solubility of the polymer will have a significant effect on the electrospinning process and the morphology of nanofibers [35]. The effect of spinning with a single solvent is often not ideal, notably for the preparation of composite nanofibers. Therefore, it is frequently essential to use blend solvents to improve spinnability and nanofiber morphology.
Preparation methods of stimuli-responsive electrospun nanofibers
Electrospun nanofibers have a large specific surface area and high porosity and can simulate the complex three-dimensional micro/nanofiber structure in the extracellular matrix (ECM) to facilitate cell adhesion and proliferation, which is very attractive in the field of biomedicine. More importantly, electrospun nanofibers can efficiently load a variety of drugs (e.g., small molecules, Chinese medicines, proteins, and nucleic acids), and then release the drugs through specific sites, specific doses, and timed to achieve better efficacy and lower toxic side effects. Further, by adding stimulus-responsive materials that can respond to external or internal stimuli, the nanofibers can be complexly regulated to release drugs. At present, the preparation of stimuli-responsive electrospun nanofibers mainly includes blend electrospinning, coaxial electrospinning and post-modification of electrospun nanofibers (Fig. 1b) (Figs. 2, 3).
Fig. 2.
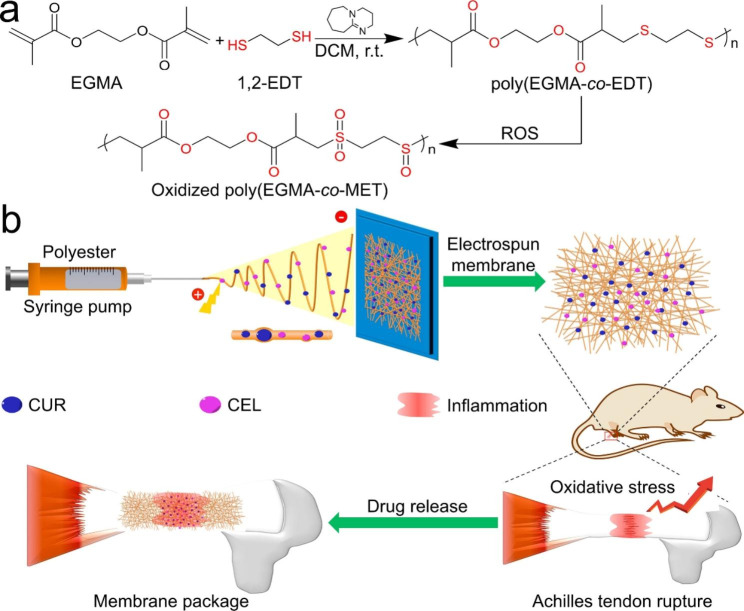
(a) Synthesis of ROS-responsive oxidized poly(EGMA-co-EDT). (b) Schematic illustrating of preparing drugs-loaded ROS-responsive nanofibers for postoperative tendon anti-adhesion. Reprinted with permission from ref [99]., Elsevier, 2021
Fig. 3.
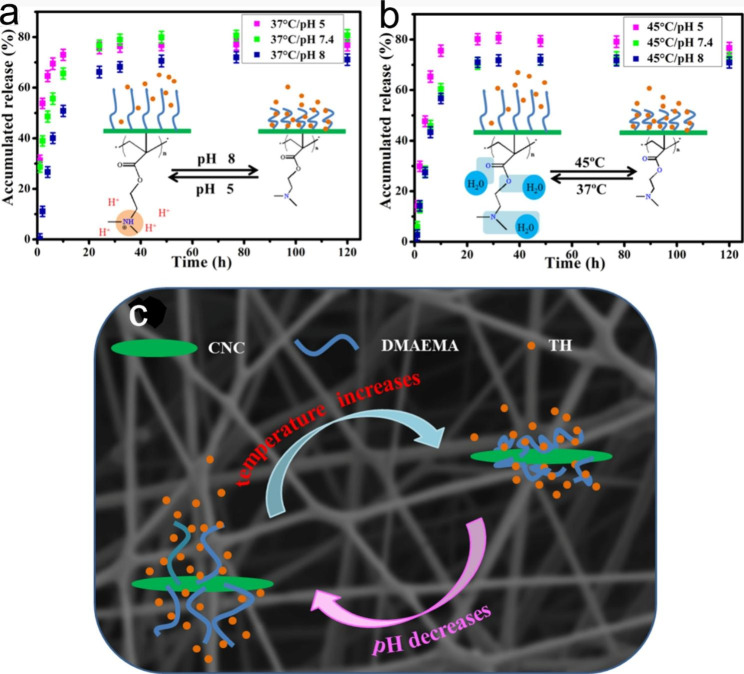
In vitro drug release profiles of double stimuli-responsive cellulose nanocrystals reinforced electrospun PHBV composites membrane at (a) 37 °C and (b) 45 °C with different pH. (c) Schematic diagram of possible drug release mechanism of composite membranes at different physiological pH and temperature. Reprinted with permission from ref [109]., Elsevier, 2020
Blend electrospinning is to add components with specific stimulus response to the spinning solution and form stimuli-responsive nanofibers by electrospinning with a single nozzle [36]. For example, Pham-Nguyen et al. added photosensitizers (chlorin e6, Ce6) to polyoxalates (POX) solution by blend electrospinning to prepare POX/Ce6 nanofibers membrane for cell culture [37]. The nanofibers membrane was excited by the red laser (660 nm) to accelerate the release of ROS and induce the degradation of the nanofibers, thereby promoting the lateral migration of cells. The drug can be directly added to the spinning solution with the stimulus response component to prepare the drug-loaded stimulus response nanofibers by blend electrospinning. However, it is important to note that drugs in nanofibers may burst release, especially water-soluble drugs.
Coaxial electrospinning is to spray two spinning solutions through a coaxial nozzle, and finally form core-shell nanofibers under the action of a high-voltage electric field [38]. The addition of stimuli-responsive substances into the shell can rapidly respond to changes in external conditions. Encapsulating bioactive factors or drugs in the nanofiber core can protect the bioactive factors from being degraded by the in vivo environment and can significantly prolong the drug release time [39]. Zheng et al. fabricated light-responsive nanofibers with a core-shell structure by coaxial electrospinning. Gold nanorods (GNRs) with photothermal properties were uniformly distributed within the shell layer of the nanofibers, and plasmid DNA (pDNA) encoding basic fibroblast growth factor was encapsulated in the core layer. Under near-infrared (NIR) light irradiation, the release of pDNA from the nanofiber cores were increased, and transient pores were created in the membrane of attached cells, facilitating intracellular delivery and pDNA transfection [40].
Stimuli-responsive electrospun nanofibers can also be obtained by post-modification on the surface of nanofibers. Ordinary electrospun nanofibers can immobilize stimuli-responsive substances on the surface of nanofibers through post-modification techniques [41]. Nanofibers surface modification technologies mainly include physical adsorption, layer-by-layer assembly, and chemical immobilization [42]. For example, Schoeller et al. assembled chitosan and sodium alginate layer by layer on PLGA nanofibers to form polyelectrolyte complexes to introduce PH-responsive control of ibuprofen release. Due to the interaction between the drug and the coating, ibuprofen is released more slowly at acidic pH (1.0) than at neutral pH [43].
Characterization techniques of electrospun nanofibers
Researchers typically utilize a range of techniques to characterize the structure and properties of nanofibers. Scanning electron microscope (SEM) has been widely applied to observe the morphology of nanofibers, including a smooth surface, beaded structure, banded structure, multilayer structure, and rough surface [44]. Furthermore, nanofibers with complicated structures, such as multi-layer coaxial nanofibers and shell-core nanofibers, can be observed by transmission electron microscope (TEM) [45]. For some drugs with fluorescent properties, the distribution of drugs in nanofibers can be observed by fluorescence microscope and confocal laser scanning microscope (CLSM) [38, 46]. The crystal structure and chemical composition of nanofibers can be determined by X-ray diffractometer (XRD) and Fourier transform infrared spectrometer (FTIR) [47]. In addition, nanofibers generally require better mechanical properties when used in tissue engineering, and their mechanical properties can be measured by universal testing machines. For stimuli-responsive electrospun nanofibers loaded with drugs, the study of in vitro release is essential. The release models and kinetics of these drug delivery systems were characterized by varying the stimulus-response conditions in vitro release experiments (e.g., near-infrared light, temperature, magnetic field, electric field, and dissolving media of diverse pH) [10]. These release models and kinetics are important for drug dose prediction in vivo trials.
Types of stimuli-responsive electrospun nanofibers
Current stimuli-responsive electrospun nanofibers can respond to stimuli including light, temperature, magnetic field, pH, or ROS [10]. Table 1 lists the details of some stimuli-responsive electrospun nanofibers and their applications in the field of biomedicine. Most stimuli-responsive electrospun nanofibers can only respond to one of these stimuli, which limits their application in complex environments. Researchers have developed multiple stimuli-responsive electrospun nanofibers that can respond to two or more stimuli, which can respond to external stimuli more quickly and efficiently, and thus have a wider range of applications [48].
Table 1.
Stimuli-responsive electrospun nanofibers as intelligent drug delivery platform in biomedicine field
| Responsive material | Type of stimulus | Other polymers | Drug | Electrospinning method | Biomedicine | Ref |
|---|---|---|---|---|---|---|
| ICG | NIR light | CS/PVA | Doxorubicin | Blend | Treatment of cervical cancer | [54] |
| MoS2 | NIR light | CS/PVA | Doxorubicin | Blend | Inhibit the postoperative tumor reoccurrence | [55] |
| Pyrrole | NIR light | PCL/PLGA | Doxorubicin | Post-modification | Local cancer treatment following surgical resection | [56] |
| PNLA | Temperature | PLLA | Rifampicin | Blend | Controllable drug delivery | [57] |
| Fe3O4 | Magnetic field | PCL/gelatin | Ciprofloxacin | Coaxial | Wound dressing | [58] |
| ZIF-8 | pH | PVA | Vancomycin | Blend | Infected bone repair | [59] |
| Chi-AO | Electro | PVA | Dexamethasone | Blend | Tissue regeneration | [60] |
| GO/Au NRs and CS | NIR light/pH | PTMG-PU | Paclitaxel | Coaxial | Treatment of lung cancer | [61] |
| Iron oxide and Poly(NIPAAm-coHMAAm) | Magnetic field/ Temperature | / | Curcumin | Blend | Treatment of melanoma cancer | [62] |
| CTS-g-PNIPAAm | Temperature/pH | PEO | BSA | Blend | Drug delivery and tissue engineering | [63] |
| P(NIPAAm-co-AAc) | Temperature/pH | RSF | Rhodamine B | Blend | Controllable drug delivery | [64] |
Abbreviations: Au NRs, Gold nanorods; BSA, Bovine serum albumin; Chi-AO, Chitosan-aniline oligomer; CS, Chitosan; CTS-g-PNIPAAm, Chitosan-graft-poly(N-isopropylacrylamide); GO, Graphene oxide; ICG, Indocyanine green; NIR, Near-infrared; P(NIPAAm-co-AAc), poly(N-isopropylacrylamide-co-acrylic acid); PCL, Poly(ε-caprolactone); PEO, Poly(ethylene oxide); PLGA, Poly (D,L-lactic-co-glycolic acid); PLLA, Poly(L-lactide); PNLA, Poly(N-isopropylacrylamide)-b-poly(L-lactide); PTMG-PU, Poly (tetramethylene ether) glycol based-polyurethane; PVA, Poly(vinyl alcohol); Poly(NIPAAm-coHMAAm), Copolymer of N-isopropylacrylamide (NIPAAm) and N-hydroxymethylacrylamide (HMAAm); RSF, Regenerated silk fibroin; ZIF-8, Zeolitic imidazolate framework-8
Light-responsive electrospun nanofibers
Light-responsive electrospun nanofibers can generate heat or release drugs in response to irradiation at specific wavelengths (ultraviolet, visible, or NIR region), with the possibility of non-invasive and remote spatiotemporal control [49]. The preparation of such stimuli-responsive nanofibers is usually obtained by incorporating light-responsive materials into a polymer matrix [50]. Light-responsive materials mainly include graphene [51], carbon nanotubes [52], indocyanine green [53], black phosphorus [65], gold nanorods [66], and gold nanocages [67]. Li et al. incorporated black phosphorus nanosheets with photothermal properties into electrospun nanofibers. The prepared nanofibers exhibited excellent photothermal properties, and its temperature was increased by 15.26 ℃ under NIR (808 nm, 2.5 W/cm2) irradiation, which was able to significantly kill HepG2 cancer cells in vitro [68]. Light-responsive electrospun nanofibers can be loaded with drugs to construct an intelligent drug delivery system to control drug release. For example, Park et al. prepared poly(ε-caprolactone) (PCL) nanofibers loaded with photothermal agent gold nanocages (AuNCs) by electrospinning. The core was loaded with anti-tumor drugs, and the shell was loaded with phase-changeable fatty acid. When irradiated by NIR light, AuNCs generate heat to melt phase-changeable fatty acid, resulting in rapid release of drugs from nanofibers [69].
Temperature-responsive electrospun nanofibers
Temperature-responsive electrospun nanofibers require the addition of thermosensitive polymers to the nanofibers [70, 71]. Thermosensitive polymers usually have a lower critical solution temperature (LCST) at which they exhibit hydrophilicity, and gradually transition from hydrophilicity to hydrophobicity when the temperature increases beyond their LCST [72]. Poly-N-isopropylacrylamide (PNIPAAm) is the most widely studied temperature-responsive polymer [73]. It is soluble in water at temperatures lower than its LCST (32 °C) and precipitates at higher temperatures. However, PNIPAAm electrospun nanofibers are easily dispersed in water because PNIPAAm is difficult to be cross-linked. Therefore, it can be copolymerized with crosslinkable comonomers to obtain stable nanofibers in aqueous media. Kim et al. polymerized N-isopropylacrylamide (NIPAAm) and crosslinkable N-hydroxymethylacrylamide (HMAAm) and then prepared nanofibers by electrospinning. The hydroxyl groups of HMAAm are then cross-linked by thermal curing. The resulting cross-linked nanofibers exhibited rapid and reversible volume changes with temperature cycling in aqueous media. This dextran-loaded temperature-responsive nanofibers exhibited an “on-off” switchable release of dextran. After six heating cycles, almost glucan was released from the nanofibers, while only a very small amount of glucan was released during cooling [74].
Magnetic field-responsive electrospun nanofibers
Magnetic field-response electrospun nanofibers move or generate heat when stimulated by a magnetic field [75]. The superparamagnetic nanoparticles are added to the electrospun nanofibers, and these magnetic particles can interact with the external magnetic field to endow the electrospun nanofibers with magnetic field response characteristics [76]. Among various functional superparamagnetic nanoparticles, Co, Fe, Ni, iron oxide and some ferrites are the most popular [77, 78]. Veres et al. prepared polysuccinimide (PSI) nanofibers by electrospinning and crosslinking, and then synthesized magnetic iron oxide nanoparticles (MIONs) inside and between the electrospun nanofibers by co-precipitation. This MIONs-loaded electrospun nanofibers have a remarkable magnetocaloric efficacy, with the temperature of the nanofiber rising over 8 °C within five minutes at a given alternating current magnetic field [79]. Therefore, magnetic field-responsive nanofibers show great potential in hyperthermia-assisted cancer therapy.
pH-responsive electrospun nanofibers
pH-responsive electrospun nanofibers have a broad prospect in disease treatment because abnormal pH changes in human tissue sites are closely related to pathological conditions [80, 81]. pH-responsive electrospun nanofibers require adding pH-responsive materials to the nanofibers or immobilizing pH-responsive materials on the surface of the nanofibers [82, 83]. pH-responsive materials are usually weak electrolytes, such as alginate [84], chitosan [85], sodium carboxymethyl cellulose [86], polylysine [87], carboxymethyl chitosan [88], polyacrylic acid (PAAc) and so on [89]. Among them, PAAc has a simple structure, obvious pH response and low cost, and is a pH-responsive polymer that has been studied more [90]. For example, Miranda-Calderon et al. prepared three antibiotic-loaded electrospun nanofibers using methacrylic acid copolymer Eudragit® L100-55 (which dissolves at pH > 5.5), Eudragit® S100 (which dissolves at pH > 7) and methacrylic ester copolymer Eudragit® RS100 (which is pH independent). These three pH-responsive wound dressings were able to modulate drug release kinetics in response to changes in the pH of the wound site at different stages of wound healing [91]. In addition, some chemical bonds also have pH-response properties, such as imine bond, 2-propionate 3-methylmaleic anhydride (CDM) bond and phenyl borate bond [92]. For example, the CDM bond is unstable under acidic conditions and breaks. Chen et al. grafted camptothecin (CPT) and α-tocopherol succinate (TOS) onto hyaluronic acid (HA) and then linking the HA conjugates to poly (DL-lactide) (PLA) via CDM bond, prepared short nanofibers by electrospinning. CDM disruption in the weakly acidic microenvironment of tumor tissues (such as pH 6.8) results in the release of HA conjugates and self-assembly into drug-loaded micelles for aggregation to the tumor site, improving therapeutic efficacy and reducing the toxic side effects of chemotherapy drugs [93].
ROS-responsive electrospun nanofibers
Oxidative stress plays an important role in the pathogenesis of various diseases, including tumors, chronic wounds, myocardial infarction (MI), and postoperative tissue adhesions [94]. During oxidative stress, ROS levels increase dramatically, causing severe damage to cells. Therefore, ROS-responsive electrospun nanofibers are developed to consume ROS or control drug release at specific sites [95]. ROS-responsive nanofibers can be obtained by adding ROS-responsive polymers into the electrospinning solution [96]. ROS-responsive polymers usually have groups such as thioether and ketal in the main or side chain of the polymer [97, 98]. These groups can be cleaved in response to overexpressed ROS in the microenvironment, leading to polymer dissociation and drug release. For example, Zhang et al. synthesized the polymer Poly (ethylene glycol dimethacrylate- co-1,2-ethanedithiol) with ethylene ether groups. The synthetic polymers were then electrospun to prepare ROS-responsive nanofibers loaded with curcumin/celecoxib (CUR/CEL) to prevent peritenendal adhesions. The thioether group in the nanofibers can react with ROS to become hydrophilic sulfoxide or sulfoxide to accelerate the release rate of drugs and intelligently regulate the level of oxidative stress at the inflammatory site (Fig. 2) [99]. Yao et al. synthesized ROS-responsive biodegradable elastic polyurethane containing thioacetone (PUTK) bond, and then the synthetic materials were prepared into glucocorticoid-loaded methylprednisolone electrospun fiber membrane for the treatment of myocardial infarction. The thioketone-containing (PUTK) bond in polyurethane membrane can consume excessive ROS, reduce the damage to heart tissue, and has the potential to prevent and treat cardiovascular diseases. In addition, ROS can trigger the degradation of synthetic polyurethane and accelerate the release of drugs [100].
Multiple stimuli-responsive nanofibers
Many researchers are designing electrospun nanofibers into multiple stimuli-responsive systems, such as light/temperature [101], pH/ROX [102], pH/temperature [103], light/ magnetic field [104], pH/temperature/ magnetic field [105]. These multiple stimuli-responsive electrospun nanofibers can respond to external stimuli simultaneously or sequentially, and better control drug release through the synergistic interaction between different stimulation behaviors, so as to improve the therapeutic effect [106, 107]. Tiwari et al. modified the surface of doxorubicin (DOX)-loaded PCL nanofibers with multiple stimuli-responsive polydopamine (PDA) to obtain nanofibers with dual pH- and light-responsive properties. Compared with physiological pH conditions (pH 7.4), the acidic medium showed improved drug release. In addition, NIR laser irradiation at 808 nm will lead to a significant increase in local temperature, thus accelerating the release of DOX [108]. This delivery platform is based on dual-responsive properties of pH and light with adjustable drug release properties can be used for local treatment of cancer and other diseases. Some researchers have developed electrospun nanofibers based on the dual response of the internal microenvironment to the specific pH and temperature of certain diseases. In some pathological processes, such as inflammation and tumors, there is a slight increase in local temperature (2–5 °C) or a decrease in pH (1-2.5 pH units). Chen et al. developed pH/temperature responsive electrospun nanofiber membranes as drug delivery carriers based on this feature. The prepared material shows a pH-dependent swelling property and miscibility gap with respect to temperature. When stimulated by internal temperature and pH, the electrospun nanofibers can achieve accelerated drug release at targeted pathological sites, thus providing superior therapeutic effects and less toxic side effects (Fig. 3) [109].
Application of stimuli-responsive electrospun nanofibers in biomedicine
Drug delivery
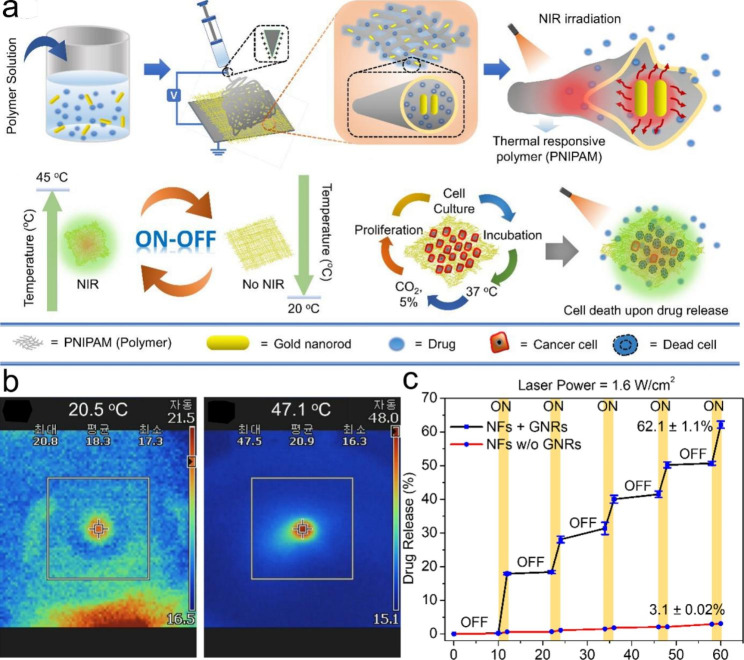
The stimuli-responsive electrospun nanofibers can directly encapsulate drugs in the fibers, which have the characteristics of large loading capacity and high encapsulation efficiency [110, 111]. In addition, they can achieve slow, on-demand or targeted release of drugs under different stimuli [10]. Therefore, stimuli-responsive electrospun nanofibers exhibit great potential for drug delivery. For example, Singh et al. designed poly(N-isopropylacrylamide) (PNIPAM) nanofibers containing gold nanorods (GNRs) as an on-demand drug delivery system. Upon NIR irradiation, the heat generated by GNRs ensures that the nanofibers shrink due to the thermal response of PNIPAM, resulting in the on-off release behavior of the drug (Fig. 4) [112]. Stimuli-responsive electrospun nanofibers can also control the targeted release of drugs at the disease site, thus enhancing the therapeutic effect. For example, the gastrointestinal tract of the human body has a specific pH value, and the release of drugs in different parts of the gastrointestinal tract can be achieved by preparing pH-responsive electrospun nanofibers. Coban et al. prepared niclosamide loaded electrospun nanofibers using pH-responsive polymer (Eudragit®L100). The Eudragit®L100 dissolves at pH values above 6, whereas the colonic pH ranges from 5.7 to 7.7. Therefore, the prepared electrospun nanofibers will not dissolve in the acidic environment of the stomach, and only begin to dissolve and release drugs when they reach the colon, so as to realize the targeted delivery of drugs in the colon [113].
Fig. 4.
(a) Schematic illustration of stimuli-responsive nanofibers containing GNRs for on-demand drug delivery platform. (b) Infrared camera images of the nanofibers without GNRs (Left) and with GNRs (Right). (c) Pulsatile drug release from the nanofibers through the cyclic on–off of NIR light irradiation at different time intervals. Reprinted with permission from ref [95]., MDPI, 2021
Cancer therapy
At present, the commonly used clinical cancer treatment methods include surgical resection, chemotherapy, radiotherapy and gene therapy, among which chemotherapy is still the main method for the clinical treatment of various cancers [114–116]. Due to the lack of targeting, chemotherapy can cause damage to normal tissue cells while killing cancer cells, resulting in serious toxic side effects [117, 118]. Therefore, some researchers have developed stimuli-responsive electrospun nanofibers used in cancer treatment, which can respond within the tumor microenvironment of stimulus (such as pH and ROS) or an external stimulus (such as magnetic field and light) to the tumor site targeted release drugs, improve the ability of drugs to kill tumor cells, reduce the harm of human body normal tissue at the same time [119, 120]. Samadzadeh et al. prepared magnetic field-responsive nanofibers by electrospinning a mixture of iron oxide (II, III) magnetic nanoparticles, temperature-responsive copolymers, and mesoporous silica nanoparticles (MSNs) loaded with metformin. Under the action of alternating magnetic field, the nanofibers can be induced to generate heat, promote the release of drugs, thereby increasing the concentration of intracellular drugs and improving the efficacy of chemotherapy. At the same time, hyperthermia can make cancer cells temporarily more sensitive to the destructive effect of anti-cancer drugs, leading to the synergistic effect of magnetic hyperthermia/chemotherapy [121, 122]. Hypoxia and lactic acid accumulation around or within solid tumors lead to decreased pH in tumor tissues, which enables acid-responsive targeted drug delivery in the tumor environment [123, 124]. Yuan et al. loaded sodium bicarbonate (NaHCO3) and the anticancer drug doxorubicin (DOX) in mesoporous silica and then blended with polylactic acid (PLLA) to obtain acid-responsive electrospun nanofibers. When nanofibers are delivered to the acidic environment of the tumor, NaHCO3 rapidly reacts with acid (H+) to produce carbon dioxide gas, and forms a channel inside the fiber to promote the release of DOX, which can effectively kill cancer cells [125].
Wound dressing
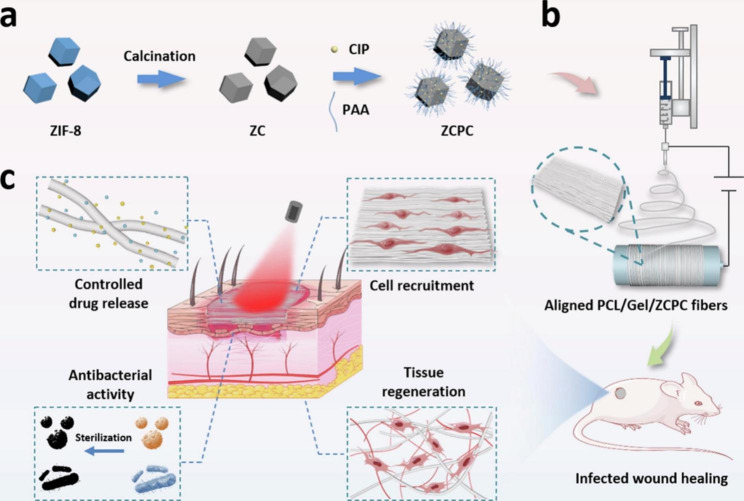
During wound healing, colonization of the wound site by bacteria and other microorganisms leads to inflammation and delayed healing. While most wound infections will heal on their own, untreated or poorly treated severe wounds can persist and become life-threatening. As a result, many wound dressings with antimicrobial properties have been developed [126, 127]. But there are problems with these dressings, such as premature drug release or poor treatment response due to bacterial resistance [128]. In order to solve these problems, an effective strategy is to develop stimuli-responsive electrospun nanofibers, which can be induced to release a large amount of antimicrobial drugs at a specific time through certain stimuli to improve the antibacterial effect [129, 130]. For example, Chen et al. designed a NIR light-responsive electrospun nanofiber loaded with the antibacterial agent ciprofloxacin hydrochloride (CIP), in which zeolitic imidazolate framework-8 (ZIF-8)-derived nanocarbon were added as the NIR laser trigger. The electrospun fiber membrane generates a lot of heat under near-infrared light irradiation, which accelerates the release of CIP to kill bacteria. The high temperature generated at the same time also destroys the structure of bacterial biofilms, inactivating their active substrates such as nucleic acids and proteins. In addition, it is difficult for bacteria to develop resistance by blocking absorption, increasing metabolism, and drug excretion (Fig. 5) [131]. Photothermally responsive electrospun nanofibers can significantly improve the bactericidal effect through photothermal and bacteriostatic synergistic antibacterial, and avoid the generation of bacterial drug resistance, showing great potential in the field of wound treatment. In addition to infection treatment, real-time monitoring of wound status is also the development direction of stimuli-responsive electrospun nanofibers [132]. Truskewycz et al. synthesized novel fluorescent magnesium hydroxide nanosheets with potent antimicrobial properties, which were then added into PCL/PEO nanofibers as wound dressing. This dressing not only has strong antimicrobial activity, but also has pH-dependent fluorescent properties that can be used to monitor the acidified microenvironment that represents healthy wound healing [133].
Fig. 5.
(a) Schematic illustration of synthesis of photothermal-chemotherapeutic nanoagent ZCPC. (b) Schematic illustration of electrospun aligned PCL/Gel/ZCPC fibers. (c) Schematic illustration of the healing mechanism of a full-thickness wound infection model in SD rats. Reprinted with permission from ref [131]., Elsevier, 2022
Tissue engineering
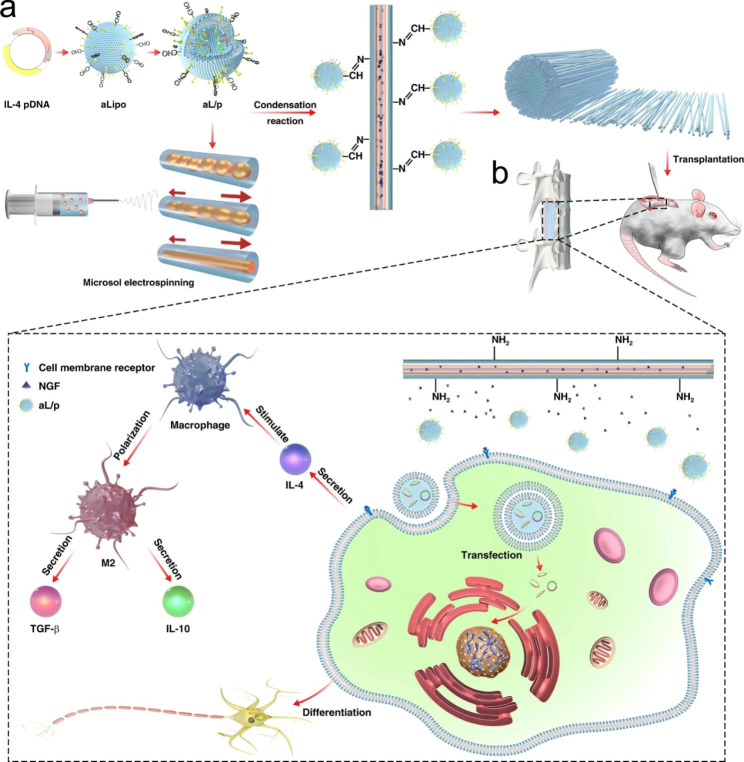
The development of tissue engineering scaffolds is of great significance for the repair and regeneration of damaged tissues or organs. Nanofibers prepared by electrospinning have good mechanical properties, high surface-volume ratio and morphological characteristics similar to ECM, which are widely used for tissue regeneration such as nerves, blood vessels, bone and heart. To enhance the therapeutic effect of electrospun nanofibers, researchers endowed them with intelligent properties to develop stimuli-responsive electrospun nanofibers, which can intelligently release drugs and accelerate tissue regeneration [134]. For example, severe immune inflammation occurs in acute spinal cord injury, which leads to the failure of ordinary electrospun nanofibers to repair nerve tissue. Inspired by the acidic microenvironment at the site of acute spinal cord injury, Xi et al. constructed a functional pH-responsive immunomodulatory electrospun nanofibrous scaffold for assisting nerve regeneration. The stimuli-responsive scaffolds were prepared by grafting the aldehyde-dissociated cationic liposomes loaded with IL-4 plasmid (pDNA) onto the surface of amino-modified directional microsol electrospun nanofibers through Schiff base bonds that would break under acidic conditions. This electrospun nanofiber can directly respond to the local acidic microenvironment and stimulate IL-4 plasmid liposome release in vitro, inhibit the release of inflammatory cytokines, and promote neural differentiation of mesenchymal stem cells, providing an alternative for the treatment of acute spinal cord injury (Fig. 6) [135].
Fig. 6.
Scheme illustration of (a) the construction of bioinspired composite scaffold for the treatment of spinal cord injury along with (b) its microenvironment-responsive immune regulation and nerve regeneration effect. Reprinted with permission from ref [135], Springer Nature, 2020
Rapid endothelialization and long-term anticoagulation are a key challenge in the development of tissue-engineered small-diameter vascular grafts [136]. Therefore, it is necessary to add adhesion factors and antithrombotic factors to electrospun nanofiber scaffolds to promote endothelial cell affinity and antithrombotic property. However, the two factors may be released explosively in nanofibers, which cannot achieve a long-term therapeutic effect. Therefore, Wang et al. coated the electrospun nanofibers with gelatin, polylysine and heparin nanoparticles. This coating can be cleaved in response to matrix metalloproteinase-2 dynamically secreted by the vascular extracellular matrix, long-term release of adhesion and antithrombotic factors without burst release, and successfully induce vascular endothelialization and long-term anticoagulation [137].
Some studies have demonstrated that mild photothermal stimulation can effectively promote osteogenesis and bone repair. Therefore, Ma et al. designed PCL/molybdenum disulfide (MoS2) nanofibers membrane with photothermal properties for bone regeneration, in which MoS2 nanosheets were used as osteogenic enhancer and near-infrared photothermal agent [138]. This nanofibers membrane can enhance cell growth and osteogenic properties. PCL/MoS2 nanofibers membrane produced mild photoheat induced by near-infrared laser, which promoted the growth of bone mesenchymal stem cells in vitro, repaired the tibial bone defect in vivo, and promoted osteogenesis and bone healing in rats. This nanofibers membrane can be used as a light-responsive bone scaffold for the treatment of bone defects.
Clinical status of stimuli-responsive electrospun nanofibers
Stimulation-responsive electrospun nanofibers for drug delivery are a very intriguing and effective way to regulate the release of drugs from the fibers at a specific time or site and are particularly suitable for the treatment of intractable diseases. Compared with systemic drug delivery methods such as intravenous injections, stimulus-response nanofibers can effectively ensure a relatively high drug concentration around local disease tissues, and decrease the distribution of drugs in other normal tissues, thereby reducing the toxic side effects of drugs. Although much work has described the remarkable effects of stimuli-responsive electrospun nanofibers in vitro or in vivo models, they have predominantly remained in the preclinical phase, and data from clinical trials are still very limited. Zhang et al. prepared light-responsive nanofibers loaded with doxorubicin by electrospinning. In vitro cell experiment results showed that the nanofibers could significantly induce cancer cell death. At the same time, the in vivo experiment confirmed that the nanofibers had an obvious inhibitory effect on tumor growth of tumor-bearing mice [139]. While this work demonstrates that stimulus-response nanofibers have good potential for tumor therapy, there are still no clinical trials to confirm the effectiveness of such systems in patients. On the one hand, this drug delivery system requires a substantial amount of external stimulation (for example, electric, magnetic, light, and heat) which can cause damage to healthy tissues. On the other hand, the response parameters are difficult to accurately control and individual differences may lead to a lot of drug leakage and threaten the lives of patients [1]. It has been very challenging to obtain regulatory approval for clinical studies of stimuli-responsive electrospun nanofibers, as their safety and toxicity have not been fully demonstrated. Excitingly, a new electrospun nanofiber scaffold called ReDura™ has been accredited by the China Food and Drug Administration (CFDA) and Conformite Europeenne (CE) for the successful clinical repair of ruptured meninges [140]. Clinical trials of this electrospun nanofiber scaffold can provide clinical data for the development of stimuli-responsive electrospun nanofibers. It is believed that as the safety of stimuli-responsive electrospinning nanofibers is fully verified, they will soon enter the clinical trial stage.
Conclusions and future perspectives
Electrospun nanofibers can respond to external stimuli at a very high speed due to their great surface area and porosity, making them an ideal stimuli-responsive material. Furthermore, stimuli-responsive nanofibers can efficiently load drugs, and then stimulated by specific conditions (light, temperature, magnetic field, ultrasound, pH or ROS, etc.) to achieve slow, on-demand or targeted release, showing great potential in areas such as drug delivery, tumor therapy, wound dressing, and tissue engineering.
Although several in vitro proof-of-concept of stimuli-responsive electrospun nanofibers have been reported, no stimuli-responsive electrospun nanofibers have made it to the clinic. The toxicity of these smart drug delivery systems is uncertain and is influenced by a variety of factors, including composition, physicochemical properties, route of administration, and dosage. Their composition and drug release mechanisms are more complex than those of ordinary formulations, making their toxicity more difficult to determine accurately. In addition, the clinical efficacy of stimuli-responsive electrospun nanofibers is uncertain, and the difference in response to these agents implanted in each individual may be significant. For example, the depth of the implant site may affect the intensity of light and magnetic field response, and the microenvironment (temperature, pH, and ROS) at the site of the disease is different for different people. It should be noted that the complexity of the architecture design leads to the high difficulty and cost of scale-up production of stimuli-responsive electrospun nanofibers, which limits their development into clinical practice. Ensuring the industrial production and security of electrospun nanofibers in the human body is crucial to promoting their clinical trials. Although stimuli-responsive electrospun nanofibers have great clinical problems, their potential in disease treatment cannot be denied. In particular, stimuli-responsive electrospun nanofibers have unique advantages in targeting intractable diseases such as cancer and tissue repair.
In the future, stimulus-responsive electrospinning nanofibers will evolve in the direction of multi-function and intelligence. For instance, the combination therapy of tumors can inoculate immune cells into stimulus-responsive electrospun nanofibers, and achieve a controlled release of drugs and immune cells at the same time after implantation in the body to jointly kill tumor cells [141]. In addition, disease diagnostic imaging technology and stimulus-responsive electrospinning nanofiber drug delivery systems are combined to enable precision therapy by monitoring pathological processes in deep tissues and then triggering the quantitative release of drugs [142]. In conclusion, stimuli-responsive electrospun nanofibers may be a promising drug delivery strategy to control drug release in response to different stimuli, especially disease-specific signals, but their clinical application is still a long way off.
Acknowledgements
Not applicable.
Authors’ contributions
Kai Chen wrote the main manuscript text. Yingshuo Liu, Youbin Li and Yinfeng Tan prepared figures. Yonghui Li and Weisan Pan contributed to the conception of the study. Guoxin Tan contributed significantly to analysis and manuscript preparation. All authors reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported financially by the Open research projects of Hainan provincial key laboratory of R&D on tropical herbs (grant number: KF202202).
Data Availability
Not applicable.
Declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have read and approved the final version of the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kai Chen, Email: chenkai19910726@163.com.
Guoxin Tan, Email: tanguoxintgx@163.com.
References
- 1.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 2.Puiggali-Jou A, Del Valle LJ, Aleman C. Cell responses to Electrical pulse stimulation for Anticancer Drug Release. Mater (Basel) 2019, 12. [DOI] [PMC free article] [PubMed]
- 3.Tang J, Zhang X, Cheng L, Liu Y, Chen Y, Jiang Z, Liu J. Multiple stimuli-responsive nanosystem for potent, ROS-amplifying, chemo-sonodynamic antitumor therapy. Bioact Mater. 2022;15:355–71. doi: 10.1016/j.bioactmat.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amarsy I, Papot S, Gasser G. Stimuli-Responsive Metal Complexes for Biomedical Applications. Angew Chem Int Ed Engl. 2022;61:e202205900. doi: 10.1002/anie.202205900. [DOI] [PubMed] [Google Scholar]
- 5.Tapeinos C, Gao H, Bauleth-Ramos T, Santos HA. Progress in Stimuli-Responsive Biomaterials for treating Cardiovascular and Cerebrovascular Diseases. Small. 2022;18:e2200291. doi: 10.1002/smll.202200291. [DOI] [PubMed] [Google Scholar]
- 6.Karimi S, Bagher Z, Najmoddin N, Simorgh S, Pezeshki-Modaress M. Alginate-magnetic short nanofibers 3D composite hydrogel enhances the encapsulated human olfactory mucosa stem cells bioactivity for potential nerve regeneration application. Int J Biol Macromol. 2021;167:796–806. doi: 10.1016/j.ijbiomac.2020.11.199. [DOI] [PubMed] [Google Scholar]
- 7.Yang F, Wang J, Song S, Rao P, Wang R, Liu S, Xu L, Zhang F. Novel controlled release Microspheric Soil Conditioner based on the temperature and pH dual-stimuli response. J Agric Food Chem. 2020;68:7819–29. doi: 10.1021/acs.jafc.0c01825. [DOI] [PubMed] [Google Scholar]
- 8.Jimaja S, Varlas S, Foster JC, Taton D, Dove AP, O’Reilly RK. Stimuli-responsive and core cross-linked micelles developed by NiCCo-PISA of helical poly(aryl isocyanide)s. Polym Chem. 2022;13:4047–53. doi: 10.1039/D2PY00397J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croitoru AM, Karacelebi Y, Saatcioglu E, Altan E, Ulag S, Aydogan HK, Sahin A, Motelica L, Oprea O, Tihauan BM et al. Electrically triggered drug delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin fibrous scaffolds for wound dressing applications. Pharmaceutics 2021, 13. [DOI] [PMC free article] [PubMed]
- 10.Chen M, Li YF, Besenbacher F. Electrospun nanofibers-mediated on-demand drug release. Adv Healthc Mater. 2014;3:1721–32. doi: 10.1002/adhm.201400166. [DOI] [PubMed] [Google Scholar]
- 11.Mane PP, Ambekar RS, Kandasubramanian B. Electrospun nanofiber-based cancer sensors: a review. Int J Pharm. 2020;583:119364. doi: 10.1016/j.ijpharm.2020.119364. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y, Sun Y, Shi W, Wan B, Zhang H. Dual-functional shikonin-loaded quaternized chitosan/polycaprolactone nanofibrous film with pH-sensing for active and intelligent food packaging. Food Chem. 2023;399:133962. doi: 10.1016/j.foodchem.2022.133962. [DOI] [PubMed] [Google Scholar]
- 13.Becelaere J, Van Den Broeck E, Schoolaert E, Vanhoorne V, Van Guyse JFR, Vergaelen M, Borgmans S, Creemers K, Van Speybroeck V, Vervaet C, et al. Stable amorphous solid dispersion of flubendazole with high loading via electrospinning. J Control Release. 2022;351:123–36. doi: 10.1016/j.jconrel.2022.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Thangadurai M, Ajith A, Budharaju H, Sethuraman S, Sundaramurthi D. Advances in electrospinning and 3D bioprinting strategies to enhance functional regeneration of skeletal muscle tissue. Biomater Adv. 2022;142:213135. doi: 10.1016/j.bioadv.2022.213135. [DOI] [PubMed] [Google Scholar]
- 15.Kiss K, Hegedus K, Vass P, Vari-Mezo D, Farkas A, Nagy ZK, Molnar L, Tovari J, Mezo G, Marosi G. Development of fast-dissolving dosage forms of curcuminoids by electrospinning for potential tumor therapy application. Int J Pharm. 2022;611:121327. doi: 10.1016/j.ijpharm.2021.121327. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Chen JN, Peng ZX, Chen NB, Liu CB, Zhang P, Zhang X, Chen GQ. Multifunctional Electrospinning Polyhydroxyalkanoate Fibrous Scaffolds with Antibacterial and Angiogenesis Effects for accelerating Wound Healing. ACS Appl Mater Interfaces. 2023;15:364–77. doi: 10.1021/acsami.2c16905. [DOI] [PubMed] [Google Scholar]
- 17.Kong Y, Xu J, Han Q, Zheng T, Wu L, Li G, Yang Y. Electrospinning porcine decellularized nerve matrix scaffold for peripheral nerve regeneration. Int J Biol Macromol. 2022;209:1867–81. doi: 10.1016/j.ijbiomac.2022.04.161. [DOI] [PubMed] [Google Scholar]
- 18.He C, Yu B, Lv Y, Huang Y, Guo J, Li L, Chen M, Zheng Y, Liu M, Guo S et al. Biomimetic asymmetric composite dressing by electrospinning with aligned nanofibrous and micropatterned structures for severe burn Wound Healing. ACS Appl Mater Interfaces 2022. [DOI] [PubMed]
- 19.Han D, Yu X, Chai Q, Ayres N, Steckl AJ. Stimuli-responsive self-immolative polymer nanofiber membranes formed by Coaxial Electrospinning. ACS Appl Mater Interfaces. 2017;9:11858–65. doi: 10.1021/acsami.6b16501. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Li R, Li X, Xie J. Electrospinning: an enabling nanotechnology platform for drug delivery and regenerative medicine. Adv Drug Deliv Rev. 2018;132:188–213. doi: 10.1016/j.addr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Serio F, da Cruz AF, Chandra A, Nobile C, Rossi GR, D’Amone E, Gigli G, Del Mercato LL, de Oliveira CC. Electrospun polyvinyl-alcohol/gum arabic nanofibers: biomimetic platform for in vitro cell growth and cancer nanomedicine delivery. Int J Biol Macromol. 2021;188:764–73. doi: 10.1016/j.ijbiomac.2021.08.069. [DOI] [PubMed] [Google Scholar]
- 22.Chen K, Hu H, Zeng Y, Pan H, Wang S, Zhang Y, Shi L, Tan G, Pan W, Liu H. Recent advances in electrospun nanofibers for wound dressing. Eur Polymer J 2022, 178.
- 23.Nadaf A, Gupta A, Hasan N, Fauziya, Ahmad S, Kesharwani P, Ahmad FJ. Recent update on electrospinning and electrospun nanofibers: current trends and their applications. RSC Adv. 2022;12:23808–28. doi: 10.1039/D2RA02864F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan G, Wang L, Pan W, Chen K. Polysaccharide Electrospun Nanofibers for Wound Healing Applications. Int J Nanomedicine. 2022;17:3913–31. doi: 10.2147/IJN.S371900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madruga LYC, Kipper MJ. Expanding the repertoire of Electrospinning: New and emerging biopolymers, techniques, and applications. Adv Healthc Mater. 2022;11:e2101979. doi: 10.1002/adhm.202101979. [DOI] [PubMed] [Google Scholar]
- 26.Gao C, Zhang L, Wang J, Jin M, Tang Q, Chen Z, Cheng Y, Yang R, Zhao G. Electrospun nanofibers promote wound healing: theories, techniques, and perspectives. J Mater Chem B. 2021;9:3106–30. doi: 10.1039/D1TB00067E. [DOI] [PubMed] [Google Scholar]
- 27.Shi S, Si Y, Han Y, Wu T, Iqbal MI, Fei B, Li RKY, Hu J, Qu J. Recent progress in protective membranes fabricated via Electrospinning: Advanced Materials, Biomimetic Structures, and functional applications. Adv Mater. 2022;34:e2107938. doi: 10.1002/adma.202107938. [DOI] [PubMed] [Google Scholar]
- 28.Krysiak ZJ, Stachewicz U. Electrospun fibers as carriers for topical drug delivery and release in skin bandages and patches for atopic dermatitis treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15:e1829. doi: 10.1002/wnan.1829. [DOI] [PubMed] [Google Scholar]
- 29.Yu DG, Wang M, Li X, Liu X, Zhu LM, Annie Bligh SW. Multifluid electrospinning for the generation of complex nanostructures. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:e1601. doi: 10.1002/wnan.1601. [DOI] [PubMed] [Google Scholar]
- 30.Farokhi M, Mottaghitalab F, Reis RL, Ramakrishna S, Kundu SC. Functionalized silk fibroin nanofibers as drug carriers: advantages and challenges. J Control Release. 2020;321:324–47. doi: 10.1016/j.jconrel.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Song J, Lin X, Ee LY, Li SFY, Huang M. A review on Electrospinning as Versatile supports for diverse nanofibers and their applications in Environmental Sensing. Adv Fiber Mater. 2023;5:429–60. doi: 10.1007/s42765-022-00237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen K, Li Y, Li Y, Pan W, Tan G. Silk Fibroin combined with Electrospinning as a Promising Strategy for tissue regeneration. Macromol Biosci 2022:e2200380. [DOI] [PubMed]
- 33.Ding Y, Li W, Zhang F, Liu Z, Zanjanizadeh Ezazi N, Liu D, Santos HA. Electrospun Fibrous Architectures for Drug Delivery, tissue Engineering and Cancer Therapy. Adv Funct Mater 2019, 29.
- 34.Xue J, Wu T, Dai Y, Xia Y. Electrospinning and Electrospun Nanofibers: methods, materials, and applications. Chem Rev. 2019;119:5298–415. doi: 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luraghi A, Peri F, Moroni L. Electrospinning for drug delivery applications: a review. J Control Release. 2021;334:463–84. doi: 10.1016/j.jconrel.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 36.Bonadies I, Di Cristo F, Valentino A, Peluso G, Calarco A, Di Salle A. pH-Responsive resveratrol-loaded Electrospun membranes for the Prevention of Implant-Associated Infections. Nanomaterials (Basel) 2020, 10. [DOI] [PMC free article] [PubMed]
- 37.Pham-Nguyen OV, Lee JW, Park Y, Jin S, Kim SR, Park J, Park JH, Jung YM, Yoo HS. Light-triggered structural modulation of Nanofibrous meshes to promote deep penetration of cultured cells. Macromol Biosci. 2022;22:e2100530. doi: 10.1002/mabi.202100530. [DOI] [PubMed] [Google Scholar]
- 38.Cui J, Yu X, Yu B, Yang X, Fu Z, Wan J, Zhu M, Wang X, Lin K. Coaxially Fabricated Dual-Drug Loading Electrospinning Fibrous Mat with programmed releasing behavior to Boost Vascularized Bone Regeneration. Adv Healthc Mater. 2022;11:e2200571. doi: 10.1002/adhm.202200571. [DOI] [PubMed] [Google Scholar]
- 39.Kalani MM, Nourmohammadi J, Negahdari B, Rahimi A, Sell SA. Electrospun core-sheath poly(vinyl alcohol)/silk fibroin nanofibers with Rosuvastatin release functionality for enhancing osteogenesis of human adipose-derived stem cells. Mater Sci Eng C Mater Biol Appl. 2019;99:129–39. doi: 10.1016/j.msec.2019.01.100. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Wu Y, Zhou Y, Wu J, Wang X, Qu Y, Wang Y, Zhang Y, Yu Q. Photothermally activated Electrospun Nanofiber Mats for high-efficiency surface-mediated gene transfection. ACS Appl Mater Interfaces. 2020;12:7905–14. doi: 10.1021/acsami.9b20221. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Smith L, Li W, Jiang L, Zhou F, Davies GL, Williams GR. Polydopamine-coated nanocomposite theranostic implants for localized chemotherapy and MRI imaging. Int J Pharm. 2022;615:121493. doi: 10.1016/j.ijpharm.2022.121493. [DOI] [PubMed] [Google Scholar]
- 42.Miguel SP, Figueira DR, Simoes D, Ribeiro MP, Coutinho P, Ferreira P, Correia IJ. Electrospun polymeric nanofibres as wound dressings: a review. Colloids Surf B Biointerfaces. 2018;169:60–71. doi: 10.1016/j.colsurfb.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Schoeller J, Itel F, Wuertz-Kozak K, Gaiser S, Luisier N, Hegemann D, Ferguson SJ, Fortunato G, Rossi RM. pH-Responsive Chitosan/Alginate Polyelectrolyte Complexes on Electrospun PLGA Nanofibers for Controlled Drug Release. Nanomaterials (Basel) 2021, 11. [DOI] [PMC free article] [PubMed]
- 44.Yang G, Li X, He Y, Ma J, Ni G, Zhou S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog Polym Sci. 2018;81:80–113. doi: 10.1016/j.progpolymsci.2017.12.003. [DOI] [Google Scholar]
- 45.Kabay G, Meydan AE, Eom T, Shim BS, Mutlu M, Kaleli-Can G. Stimuli-responsive nanoparticle-nanofiber hybrids for drug delivery and photodynamic therapy. Int J Pharm. 2023;630:122442. doi: 10.1016/j.ijpharm.2022.122442. [DOI] [PubMed] [Google Scholar]
- 46.Altinbasak I, Kocak S, Colby AH, Alp Y, Sanyal R, Grinstaff MW, Sanyal A. pH-Responsive nanofiber buttresses as local drug delivery devices. Biomater Sci. 2023;11:813–21. doi: 10.1039/D2BM01199A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang GZ, Li JJ, Yu DG, He MF, Yang JH, Williams GR. Nanosized sustained-release drug depots fabricated using modified tri-axial electrospinning. Acta Biomater. 2017;53:233–41. doi: 10.1016/j.actbio.2017.01.069. [DOI] [PubMed] [Google Scholar]
- 48.Huang C, Soenen SJ, Rejman J, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Stimuli-responsive electrospun fibers and their applications. Chem Soc Rev. 2011;40:2417–34. doi: 10.1039/c0cs00181c. [DOI] [PubMed] [Google Scholar]
- 49.Zarate IA, Aguilar-Bolados H, Yazdani-Pedram M, Pizarro GDC, Neira-Carrillo A. In Vitro Hyperthermia evaluation of Electrospun Polymer Composite fibers loaded with reduced Graphene Oxide. Polym (Basel) 2020, 12. [DOI] [PMC free article] [PubMed]
- 50.Sun J, Song L, Fan Y, Tian L, Luan S, Niu S, Ren L, Ming W, Zhao J. Synergistic photodynamic and photothermal antibacterial nanocomposite membrane triggered by single NIR Light source. ACS Appl Mater Interfaces. 2019;11:26581–9. doi: 10.1021/acsami.9b07037. [DOI] [PubMed] [Google Scholar]
- 51.Cardano F, Frasconi M, Giordani S. Photo-Responsive Graphene and Carbon Nanotubes to control and tackle Biological Systems. Front Chem. 2018;6:102. doi: 10.3389/fchem.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee HP, Gaharwar AK. Light-responsive Inorganic Biomaterials for Biomedical Applications. Adv Sci (Weinh) 2020;7:2000863. doi: 10.1002/advs.202000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Z, Liu J, Tian L, Li J, Gao Y, Xing Y, Yan W, Hua C, Xie X, Liu C, Liang C. Insights into stimuli-responsive diselenide bonds utilized in drug delivery systems for cancer therapy. Biomed Pharmacother. 2022;155:113707. doi: 10.1016/j.biopha.2022.113707. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Wang L, Zong S, Qiu R, Liu S. Use of multifunctional composite nanofibers for photothermalchemotherapy to treat cervical cancer in mice. Biomater Sci. 2019;7:3846–54. doi: 10.1039/C9BM00756C. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J, Zhu Y, Ye C, Chen Y, Wang S, Zou D, Li Z. Photothermal transforming agent and chemotherapeutic co-loaded electrospun nanofibers for tumor treatment. Int J Nanomedicine. 2019;14:3893–909. doi: 10.2147/IJN.S202876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khanom J, A IR, Park CH, Kim CS. Near-Infrared Responsive Synergistic Chemo-Phototherapy from Surface-Functionalized poly(epsilon-caprolactone)-Poly(d,l-lactic-co-glycolic acid) composite nanofibers for Postsurgical Cancer Treatment. Biomacromolecules. 2022;23:3582–92. doi: 10.1021/acs.biomac.2c00351. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Peng C, Wang Z, Ren J. Preparation of thermo-responsive drug-loaded nanofibrous films created by electrospinning. RSC Adv. 2018;8:17551–7. doi: 10.1039/C8RA02442A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao C, Zhang L, Wang J, Cheng Y, Chen Z, Yang R, Zhao G. Coaxial structured drug loaded dressing combined with induced stem cell differentiation for enhanced wound healing. Biomater Adv. 2022;134:112542. doi: 10.1016/j.msec.2021.112542. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y, Wang H, Zou X, Wang D, Fan Y, Zhao X, Li M, Yang L, Liang C. Antibacterial Vancomycin@ZIF-8 loaded PVA Nanofiber membrane for infected bone repair. Int J Mol Sci 2022, 23. [DOI] [PMC free article] [PubMed]
- 60.Bagheri B, Zarrintaj P, Samadi A, Zarrintaj R, Ganjali MR, Saeb MR, Mozafari M, Park OO, Kim YC. Tissue engineering with electrospun electro-responsive chitosan-aniline oligomer/polyvinyl alcohol. Int J Biol Macromol. 2020;147:160–9. doi: 10.1016/j.ijbiomac.2019.12.264. [DOI] [PubMed] [Google Scholar]
- 61.Azerbaijan MH, Bahmani E, Jouybari MH, Hassaniazardaryani A, Goleij P, Akrami M, Irani M. Electrospun gold nanorods/graphene oxide loaded-core-shell nanofibers for local delivery of paclitaxel against lung cancer during photo-chemotherapy method. Eur J Pharm Sci. 2021;164:105914. doi: 10.1016/j.ejps.2021.105914. [DOI] [PubMed] [Google Scholar]
- 62.Wei W, Zarghami N, Abasi M, Ertas YN, Pilehvar Y. Implantable magnetic nanofibers with ON-OFF switchable release of curcumin for possible local hyperthermic chemotherapy of melanoma. J Biomed Mater Res A. 2022;110:851–60. doi: 10.1002/jbm.a.37333. [DOI] [PubMed] [Google Scholar]
- 63.Yuan H, Li B, Liang K, Lou X, Zhang Y. Regulating drug release from pH- and temperature-responsive electrospun CTS-g-PNIPAAm/poly(ethylene oxide) hydrogel nanofibers. Biomed Mater. 2014;9:055001. doi: 10.1088/1748-6041/9/5/055001. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Zhu J, Jia L, Ma Y, Wu H. Aqueous-based electrospun P(NIPAAm-co-AAc)/RSF medicated fibrous mats for dual temperature- and pH-responsive drug controlled release. RSC Adv. 2019;10:323–31. doi: 10.1039/C9RA08832F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qing Y, Li R, Li S, Li Y, Wang X, Qin Y. Advanced Black Phosphorus Nanomaterials for Bone Regeneration. Int J Nanomedicine. 2020;15:2045–58. doi: 10.2147/IJN.S246336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zong Q, Dong N, Yang X, Ling G, Zhang P. Development of gold nanorods for cancer treatment. J Inorg Biochem. 2021;220:111458. doi: 10.1016/j.jinorgbio.2021.111458. [DOI] [PubMed] [Google Scholar]
- 67.Li C, Zhao T, Li L, Hu X, Li C, Chen W, Hu Y. Stimuli-Responsive Gold Nanocages for Cancer diagnosis and treatment. Pharmaceutics 2022, 14. [DOI] [PMC free article] [PubMed]
- 68.Li X, Zhou J, Wu H, Dai F, Li J, Li Z. Electrospun Silk Fibroin/Polylactic-co-glycolic Acid/Black Phosphorus Nanosheets Nanofibrous membrane with Photothermal Therapy potential for Cancer. Molecules 2022, 27. [DOI] [PMC free article] [PubMed]
- 69.Park JH, Seo H, Kim DI, Choi JH, Son JH, Kim J, Moon GD, Hyun DC. Gold Nanocage-Incorporated Poly(epsilon-Caprolactone) (PCL) Fibers for Chemophotothermal Synergistic Cancer Therapy. Pharmaceutics 2019, 11. [DOI] [PMC free article] [PubMed]
- 70.Oh HH, Ko YG, Uyama H, Park WH, Cho D, Kwon OH. Fabrication and characterization of thermoresponsive polystyrene nanofibrous mats for cultured cell recovery. Biomed Res Int. 2014;2014:480694. doi: 10.1155/2014/480694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Safari S, Ehsani M, Zandi M. Stimuli-responsive electrospun nanofibers based on PNVCL-PVAc copolymer in biomedical applications. Prog Biomater. 2021;10:245–58. doi: 10.1007/s40204-021-00168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-Husseiny HM, Mady EA, Hamabe L, Abugomaa A, Shimada K, Yoshida T, Tanaka T, Yokoi A, Elbadawy M, Tanaka R. Smart/stimuli-responsive hydrogels: cutting-edge platforms for tissue engineering and other biomedical applications. Mater Today Bio. 2022;13:100186. doi: 10.1016/j.mtbio.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang X, Zhang C, Deng D, Gu Y, Wang H, Zhong Q. Multiple stimuli-responsive MXene-Based hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small. 2022;18:e2104368. doi: 10.1002/smll.202104368. [DOI] [PubMed] [Google Scholar]
- 74.Kim YJ, Ebara M, Aoyagi T. Temperature-responsive electrospun nanofibers for ‘on-off’ switchable release of dextran. Sci Technol Adv Mater. 2012;13:064203. doi: 10.1088/1468-6996/13/6/064203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song C, Wang XX, Zhang J, Nie GD, Luo WL, Fu J, Ramakrishna S, Long YZ. Electric Field-Assisted in situ precise deposition of Electrospun gamma-Fe2O3/Polyurethane nanofibers for magnetic hyperthermia. Nanoscale Res Lett. 2018;13:273. doi: 10.1186/s11671-018-2707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duran-Guerrero JG, Martinez-Rodriguez MA, Garza-Navarro MA, Gonzalez-Gonzalez VA, Torres-Castro A, De La Rosa JR. Magnetic nanofibrous materials based on CMC/PVA polymeric blends. Carbohydr Polym. 2018;200:289–96. doi: 10.1016/j.carbpol.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 77.Longo R, Gorrasi G, Guadagno L. Electromagnetically Stimuli-Responsive Nanoparticles-Based Systems for Biomedical Applications: recent advances and future perspectives. Nanomaterials (Basel) 2021, 11. [DOI] [PMC free article] [PubMed]
- 78.Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio. 2022;14:100223. doi: 10.1016/j.mtbio.2022.100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Veres T, Voniatis C, Molnar K, Nesztor D, Feher D, Ferencz A, Gresits I, Thuroczy G, Markus BG, Simon F et al. An implantable magneto-responsive poly(aspartamide) based Electrospun Scaffold for Hyperthermia Treatment. Nanomaterials (Basel) 2022, 12. [DOI] [PMC free article] [PubMed]
- 80.Tyo KM, Duan J, Kollipara P, Dela Cerna MVC, Lee D, Palmer KE, Steinbach-Rankins JM. pH-responsive delivery of Griffithsin from electrospun fibers. Eur J Pharm Biopharm. 2019;138:64–74. doi: 10.1016/j.ejpb.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 81.He CW, Parowatkin M, Mailander V, Flechtner-Mors M, Ziener U, Landfester K, Crespy D. Sequence-controlled delivery of peptides from hierarchically structured Nanomaterials. ACS Appl Mater Interfaces. 2017;9:3885–94. doi: 10.1021/acsami.6b13176. [DOI] [PubMed] [Google Scholar]
- 82.Kim S, Traore YL, Ho EA, Shafiq M, Kim SH, Liu S. Design and development of pH-responsive polyurethane membranes for intravaginal release of nanomedicines. Acta Biomater. 2018;82:12–23. doi: 10.1016/j.actbio.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Sayin S, Tufani A, Emanet M, Genchi GG, Sen O, Shemshad S, Ozdemir E, Ciofani G. Ozaydin Ince G: Electrospun Nanofibers with pH-Responsive Coatings for Control of Release Kinetics. Front Bioeng Biotechnol. 2019;7:309. doi: 10.3389/fbioe.2019.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aguero L, Zaldivar-Silva D, Pena L, Dias ML. Alginate microparticles as oral colon drug delivery device: a review. Carbohydr Polym. 2017;168:32–43. doi: 10.1016/j.carbpol.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 85.Shikhi-Abadi PG, Irani M. A review on the applications of electrospun chitosan nanofibers for the cancer treatment. Int J Biol Macromol. 2021;183:790–810. doi: 10.1016/j.ijbiomac.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 86.Yan M, Chen T, Zhang S, Lu T, Sun X. A core-shell structured alginate hydrogel beads with tunable thickness of carboxymethyl cellulose coating for pH responsive drug delivery. J Biomater Sci Polym Ed. 2021;32:763–78. doi: 10.1080/09205063.2020.1866350. [DOI] [PubMed] [Google Scholar]
- 87.Manouchehri S, Zarrintaj P, Saeb MR, Ramsey JD. Advanced Delivery Systems based on lysine or lysine polymers. Mol Pharm. 2021;18:3652–70. doi: 10.1021/acs.molpharmaceut.1c00474. [DOI] [PubMed] [Google Scholar]
- 88.Jing H, Du X, Mo L, Wang H. Self-coacervation of carboxymethyl chitosan as a pH-responsive encapsulation and delivery strategy. Int J Biol Macromol. 2021;192:1169–77. doi: 10.1016/j.ijbiomac.2021.10.072. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Zhang M, Zhang L, Li L, Li S, Wang C, Su Z, Yuan Y, Pan W. Designed synthesis of lipid-coated polyacrylic Acid/Calcium phosphate nanoparticles as dual pH-Responsive Drug-Delivery Vehicles for Cancer Chemotherapy. Chemistry. 2017;23:6586–95. doi: 10.1002/chem.201700060. [DOI] [PubMed] [Google Scholar]
- 90.Xu C, Ma J, Liu Z, Wang W, Liu X, Qian S, Chen L, Gu L, Sun C, Hou J, Jiang Z. Preparation of shell-core fiber-encapsulated Lactobacillus rhamnosus 1.0320 using coaxial electrospinning. Food Chem. 2023;402:134253. doi: 10.1016/j.foodchem.2022.134253. [DOI] [PubMed] [Google Scholar]
- 91.Miranda-Calderon L, Yus C, Landa G, Mendoza G, Arruebo M, Irusta S. Pharmacokinetic control on the release of antimicrobial drugs from pH-responsive electrospun wound dressings. Int J Pharm. 2022;624:122003. doi: 10.1016/j.ijpharm.2022.122003. [DOI] [PubMed] [Google Scholar]
- 92.Liang Y, Li M, Yang Y, Qiao L, Xu H, Guo B. pH/Glucose dual responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic bonding for athletic Diabetic Foot Wound Healing. ACS Nano. 2022;16:3194–207. doi: 10.1021/acsnano.1c11040. [DOI] [PubMed] [Google Scholar]
- 93.Chen Z, Zhang Z, Chen M, Xie S, Wang T, Li X. Synergistic antitumor efficacy of hybrid micelles with mitochondrial targeting and stimuli-responsive drug release behavior. J Mater Chem B. 2019;7:1415–26. doi: 10.1039/C8TB02843E. [DOI] [PubMed] [Google Scholar]
- 94.Yuan Y, Nie T, Fang Y, You X, Huang H, Wu J. Stimuli-responsive cyclodextrin-based supramolecular assemblies as drug carriers. J Mater Chem B. 2022;10:2077–96. doi: 10.1039/D1TB02683F. [DOI] [PubMed] [Google Scholar]
- 95.Federico S, Martorana A, Pitarresi G, Palumbo FS, Fiorica C, Giammona G. Development of stimulus-sensitive electrospun membranes based on novel biodegradable segmented polyurethane as triggered delivery system for doxorubicin. Biomater Adv. 2022;136:212769. doi: 10.1016/j.bioadv.2022.212769. [DOI] [PubMed] [Google Scholar]
- 96.Lee H, Woo J, Son D, Kim M, Choi WI, Sung D. Electrospinning/Electrospray of Ferrocene containing Copolymers to fabricate ROS-Responsive particles and fibers. Polym (Basel) 2020, 12. [DOI] [PMC free article] [PubMed]
- 97.Jo YJ, Gulfam M, Jo SH, Gal YS, Oh CW, Park SH, Lim KT. Multi-stimuli responsive hydrogels derived from hyaluronic acid for cancer therapy application. Carbohydr Polym. 2022;286:119303. doi: 10.1016/j.carbpol.2022.119303. [DOI] [PubMed] [Google Scholar]
- 98.Sia CS, Lim HP, Tey BT, Goh BH, Low LE. Stimuli-responsive nanoassemblies for targeted delivery against tumor and its microenvironment. Biochim Biophys Acta Rev Cancer. 2022;1877:188779. doi: 10.1016/j.bbcan.2022.188779. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J, Xiao C, Zhang X, Lin Y, Yang H, Zhang YS, Ding J. An oxidative stress-responsive electrospun polyester membrane capable of releasing anti-bacterial and anti-inflammatory agents for postoperative anti-adhesion. J Control Release. 2021;335:359–68. doi: 10.1016/j.jconrel.2021.04.017. [DOI] [PubMed] [Google Scholar]
- 100.Yao Y, Ding J, Wang Z, Zhang H, Xie J, Wang Y, Hong L, Mao Z, Gao J, Gao C. ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials. 2020;232:119726. doi: 10.1016/j.biomaterials.2019.119726. [DOI] [PubMed] [Google Scholar]
- 101.Nakielski P, Pawlowska S, Rinoldi C, Ziai Y, De Sio L, Urbanek O, Zembrzycki K, Pruchniewski M, Lanzi M, Salatelli E, et al. Multifunctional platform based on Electrospun Nanofibers and Plasmonic Hydrogel: a Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl Mater Interfaces. 2020;12:54328–42. doi: 10.1021/acsami.0c13266. [DOI] [PubMed] [Google Scholar]
- 102.Wu Y, Wang Y, Long L, Hu C, Kong Q, Wang Y. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J Control Release. 2022;341:147–65. doi: 10.1016/j.jconrel.2021.11.027. [DOI] [PubMed] [Google Scholar]
- 103.Wei Z, Lin Q, Yang J, Long S, Zhang G, Wang X. Fabrication of novel dual thermo- and pH-sensitive poly (N-isopropylacrylamide-N-methylolacrylamide-acrylic acid) electrospun ultrafine fibres for controlled drug release. Mater Sci Eng C Mater Biol Appl. 2020;115:111050. doi: 10.1016/j.msec.2020.111050. [DOI] [PubMed] [Google Scholar]
- 104.Yu QA-O, Zhang YM, Liu YA-OX, Xu X, Liu YA-O. Magnetism and photo dual-controlled supramolecular assembly for suppression of tumor invasion and metastasis. [DOI] [PMC free article] [PubMed]
- 105.Amini Z, Rudsary SS, Shahraeini SS, Dizaji BF, Goleij P, Bakhtiari A, Irani M, Sharifianjazi F. Magnetic bioactive glasses/Cisplatin loaded-chitosan (CS)-grafted- poly (epsilon-caprolactone) nanofibers against bone cancer treatment. Carbohydr Polym. 2021;258:117680. doi: 10.1016/j.carbpol.2021.117680. [DOI] [PubMed] [Google Scholar]
- 106.Song B, Wu C, Chang J. Ultrasound-triggered dual-drug release from poly(lactic-co-glycolic acid)/mesoporous silica nanoparticles electrospun composite fibers. Regen Biomater. 2015;2:229–37. doi: 10.1093/rb/rbv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ashrafizadeh M, Delfi M, Zarrabi A, Bigham A, Sharifi E, Rabiee N, Paiva-Santos AC, Kumar AP, Tan SC, Hushmandi K, et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: pre-clinical & clinical approaches. J Control Release. 2022;351:50–80. doi: 10.1016/j.jconrel.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 108.Tiwari AP, Bhattarai DP, Maharjan B, Ko SW, Kim HY, Park CH, Kim CS. Polydopamine-based Implantable Multifunctional Nanocarpet for highly efficient photothermal-chemo therapy. Sci Rep. 2019;9:2943. doi: 10.1038/s41598-019-39457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Y, Abdalkarim SYH, Yu HY, Li Y, Xu J, Marek J, Yao J, Tam KC. Double stimuli-responsive cellulose nanocrystals reinforced electrospun PHBV composites membrane for intelligent drug release. Int J Biol Macromol. 2020;155:330–9. doi: 10.1016/j.ijbiomac.2020.03.216. [DOI] [PubMed] [Google Scholar]
- 110.Contreras-Caceres R, Cabeza L, Perazzoli G, Diaz A, Lopez-Romero JM, Melguizo C, Prados J. Electrospun Nanofibers: recent applications in Drug Delivery and Cancer Therapy. Nanomaterials (Basel) 2019, 9. [DOI] [PMC free article] [PubMed]
- 111.Shetty K, Bhandari A, Yadav KS. Nanoparticles incorporated in nanofibers using electrospinning: a novel nano-in-nano delivery system. J Controlled Release. 2022;350:421–34. doi: 10.1016/j.jconrel.2022.08.035. [DOI] [PubMed] [Google Scholar]
- 112.Singh B, Shukla N, Kim J, Kim K, Park MH. Stimuli-Responsive Nanofibers Containing Gold Nanorods for On-Demand drug delivery platforms. Pharmaceutics 2021, 13. [DOI] [PMC free article] [PubMed]
- 113.Coban O, Aytac Z, Yildiz ZI, Uyar T. Colon targeted delivery of niclosamide from beta-cyclodextrin inclusion complex incorporated electrospun Eudragit(R) L100 nanofibers. Colloids Surf B Biointerfaces. 2021;197:111391. doi: 10.1016/j.colsurfb.2020.111391. [DOI] [PubMed] [Google Scholar]
- 114.Ribeiro S, Soares M, Hermenegildo B, Correia V, Diez AG, Lanceros-Mendez S, Ribeiro C. Electroactive functional microenvironments from bioactive polymers: a new strategy to address cancer. Biomater Adv. 2022;137:212849. doi: 10.1016/j.bioadv.2022.212849. [DOI] [PubMed] [Google Scholar]
- 115.Hsu MY, Hsieh CH, Huang YT, Chu SY, Chen CM, Lee WJ, Liu SJ. Enhanced Paclitaxel Efficacy to Suppress Triple-Negative breast Cancer Progression using metronomic chemotherapy with a controlled release system of Electrospun Poly-d-l-Lactide-co-glycolide (PLGA) nanofibers. Cancers (Basel) 2021, 13. [DOI] [PMC free article] [PubMed]
- 116.Chen K, Pan H, Yan Z, Li Y, Ji D, Yun K, Su Y, Liu D, Pan W. A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int J Biol Macromol. 2021;182:1339–50. doi: 10.1016/j.ijbiomac.2021.05.074. [DOI] [PubMed] [Google Scholar]
- 117.Ding C, Chen C, Zeng X, Chen H, Zhao Y. Emerging strategies in Stimuli-Responsive Prodrug Nanosystems for Cancer Therapy. ACS Nano. 2022;16:13513–53. doi: 10.1021/acsnano.2c05379. [DOI] [PubMed] [Google Scholar]
- 118.Hu L, Xiong C, Wei G, Yu Y, Li S, Xiong X, Zou JJ, Tian J. Stimuli-responsive charge-reversal MOF@polymer hybrid nanocomposites for enhanced co-delivery of chemotherapeutics towards combination therapy of multidrug-resistant cancer. J Colloid Interface Sci. 2022;608:1882–93. doi: 10.1016/j.jcis.2021.10.070. [DOI] [PubMed] [Google Scholar]
- 119.Graham-Gurysh EG, Moore KM, Schorzman AN, Lee T, Zamboni WC, Hingtgen SD, Bachelder EM, Ainslie KM. Tumor responsive and tunable polymeric platform for optimized delivery of Paclitaxel to treat Glioblastoma. ACS Appl Mater Interfaces. 2020;12:19345–56. doi: 10.1021/acsami.0c04102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang J, Zhuang C, Chen J, Chen X, Li X, Zhang T, Wang B, Feng Q, Zheng X, Gong M, et al. Targeted Drug/Gene/Photodynamic therapy via a stimuli-responsive dendritic-polymer-based Nanococktail for treatment of EGFR-TKI-Resistant non-small-cell Lung Cancer. Adv Mater. 2022;34:e2201516. doi: 10.1002/adma.202201516. [DOI] [PubMed] [Google Scholar]
- 121.Samadzadeh S, Babazadeh M, Zarghami N, Pilehvar-Soltanahmadi Y, Mousazadeh H. An implantable smart hyperthermia nanofiber with switchable, controlled and sustained drug release: possible application in prevention of cancer local recurrence. Mater Sci Eng C Mater Biol Appl. 2021;118:111384. doi: 10.1016/j.msec.2020.111384. [DOI] [PubMed] [Google Scholar]
- 122.Zhang J, Lin Y, Lin Z, Wei Q, Qian J, Ruan R, Jiang X, Hou L, Song J, Ding J, Yang H. Stimuli-Responsive Nanoparticles for Controlled Drug Delivery in Synergistic Cancer Immunotherapy. Adv Sci (Weinh) 2022;9:e2103444. doi: 10.1002/advs.202103444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu HS, Lee ES. Honeycomb-like pH-responsive gamma-cyclodextrin electrospun particles for highly efficient tumor therapy. Carbohydr Polym. 2020;230:115563. doi: 10.1016/j.carbpol.2019.115563. [DOI] [PubMed] [Google Scholar]
- 124.Zeng Y, Zhang C, Du D, Li Y, Sun L, Han Y, He X, Dai J, Shi L. Metal-organic framework-based hydrogel with structurally dynamic properties as a stimuli-responsive localized drug delivery system for cancer therapy. Acta Biomater. 2022;145:43–51. doi: 10.1016/j.actbio.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Yuan Z, Wu W, Zhang Z, Sun Z, Cheng R, Pan G, Wang X, Cui W. In situ adjuvant therapy using a responsive doxorubicin-loaded fibrous scaffold after tumor resection. Colloids Surf B Biointerfaces. 2017;158:363–9. doi: 10.1016/j.colsurfb.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 126.Chen K, Pan H, Ji D, Li Y, Duan H, Pan W. Curcumin-loaded sandwich-like nanofibrous membrane prepared by electrospinning technology as wound dressing for accelerate wound healing. Mater Sci Eng C Mater Biol Appl. 2021;127:112245. doi: 10.1016/j.msec.2021.112245. [DOI] [PubMed] [Google Scholar]
- 127.Xiong Y, Xu Y, Zhou F, Hu Y, Zhao J, Liu Z, Zhai Q, Qi S, Zhang Z, Chen L. Bio-functional hydrogel with antibacterial and anti-inflammatory dual properties to combat with burn wound infection. Bioeng Transl Med. 2023;8:e10373. doi: 10.1002/btm2.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu Y, Li H, Lv X, Xu Y, Xie Y, Yuwen L, Song Y, Li S, Shao J, Yang D. Stimuli-responsive therapeutic systems for the treatment of diabetic infected wounds. Nanoscale. 2022;14:12967–83. doi: 10.1039/D2NR03756D. [DOI] [PubMed] [Google Scholar]
- 129.Li H, Kang Z, He E, Wu X, Ma X, Yang DP, Diao Y, Chen X. A fish-scale derived multifunctional nanofiber membrane for infected wound healing. Biomater Sci. 2022;10:5284–300. doi: 10.1039/D2BM00646D. [DOI] [PubMed] [Google Scholar]
- 130.Huang Y, Zou L, Wang J, Jin Q, Ji J. Stimuli-responsive nanoplatforms for antibacterial applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022;14:e1775. doi: 10.1002/wnan.1775. [DOI] [PubMed] [Google Scholar]
- 131.Chen L, Zhang D, Cheng K, Li W, Yu Q, Wang L. Photothermal-responsive fiber dressing with enhanced antibacterial activity and cell manipulation towards promoting wound-healing. J Colloid Interface Sci. 2022;623:21–33. doi: 10.1016/j.jcis.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 132.Bazbouz MB, Tronci G. Two-layer Electrospun System Enabling Wound Exudate Management and visual infection response. Sens (Basel) 2019, 19. [DOI] [PMC free article] [PubMed]
- 133.Truskewycz A, Truong VK, Ball AS, Houshyar S, Nassar N, Yin H, Murdoch BJ, Cole I. Fluorescent Magnesium Hydroxide Nanosheet Bandages with tailored Properties for Biocompatible Antimicrobial Wound Dressings and pH monitoring. ACS Appl Mater Interfaces. 2021;13:27904–19. doi: 10.1021/acsami.1c05908. [DOI] [PubMed] [Google Scholar]
- 134.Johnson CDL, Ganguly D, Zuidema JM, Cardinal TJ, Ziemba AM, Kearns KR, McCarthy SM, Thompson DM, Ramanath G, Borca-Tasciuc DA, et al. Injectable, magnetically orienting Electrospun Fiber conduits for Neuron Guidance. ACS Appl Mater Interfaces. 2019;11:356–72. doi: 10.1021/acsami.8b18344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xi K, Gu Y, Tang J, Chen H, Xu Y, Wu L, Cai F, Deng L, Yang H, Shi Q, et al. Microenvironment-responsive immunoregulatory electrospun fibers for promoting nerve function recovery. Nat Commun. 2020;11:4504. doi: 10.1038/s41467-020-18265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rafique M, Wei T, Sun Q, Midgley AC, Huang Z, Wang T, Shafiq M, Zhi D, Si J, Yan H, et al. The effect of hypoxia-mimicking responses on improving the regeneration of artificial vascular grafts. Biomaterials. 2021;271:120746. doi: 10.1016/j.biomaterials.2021.120746. [DOI] [PubMed] [Google Scholar]
- 137.Wang D, Wang X, Li X, Jiang L, Chang Z, Li Q. Biologically responsive, long-term release nanocoating on an electrospun scaffold for vascular endothelialization and anticoagulation. Mater Sci Eng C Mater Biol Appl. 2020;107:110212. doi: 10.1016/j.msec.2019.110212. [DOI] [PubMed] [Google Scholar]
- 138.Ma K, Liao C, Huang L, Liang R, Zhao J, Zheng L, Su W. Electrospun PCL/MoS2 nanofiber membranes combined with NIR-Triggered Photothermal Therapy to accelerate bone regeneration. Small. 2021;17:e2104747. doi: 10.1002/smll.202104747. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Z, Liu S, Xiong H, Jing X, Xie Z, Chen X, Huang Y. Electrospun PLA/MWCNTs composite nanofibers for combined chemo- and photothermal therapy. Acta Biomater. 2015;26:115–23. doi: 10.1016/j.actbio.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 140.Kong B, Liu R, Guo J, Lu L, Zhou Q, Zhao Y. Tailoring micro/nano-fibers for biomedical applications. Bioact Mater. 2023;19:328–47. doi: 10.1016/j.bioactmat.2022.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Agarwalla P, Ogunnaike EA, Ahn S, Froehlich KA, Jansson A, Ligler FS, Dotti G, Brudno Y. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. Nat Biotechnol. 2022;40:1250–8. doi: 10.1038/s41587-022-01245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang SL, Zhang LL, Zhao JJ, He M, Huang Y, Zhao SL. A tumor microenvironment-induced absorption red-shifted polymer nanoparticle for simultaneously activated photoacoustic imaging and photothermal therapy. Sci Adv 2021, 7. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.