Abstract
Experiencing adversity in childhood and adolescence, including stressful life events (SLEs), may accelerate the pace of development, leading to adverse mental and physical health. However, most research on adverse early experiences and biological aging (BA) in youths relies on cross-sectional designs. In 171 youths followed for approximately 2 years, we examined if SLEs over follow-up predicted rate of change in two BA metrics: epigenetic age and Tanner stage. We also investigated if rate of change in BA was associated with changes in depressive symptoms over time. Youths aged 8–16 years at baseline self-reported Tanner stage and depressive symptoms at baseline and follow-up and provided saliva samples for DNA at both assessments. Horvath epigenetic age estimates were derived from DNA methylation data measured with the Illumina EPIC array. At follow-up, contextual threat interviews were administered to youths and caregivers to assess youths’ experiences of past-year SLEs. Interviews were objectively coded by an independent rating team to generate a SLE impact score, reflecting the severity of all SLEs occurring over the prior year. Rate of change in BA metrics was operationalized as change in epigenetic age or Tanner stage as a function of time between assessments. Higher objective SLE impact scores over follow-up were related to a greater rate of change in epigenetic age (β=0.21, p=.043). Additionally, among youths with lower—but not higher—Tanner stage at baseline, there was a positive association of SLE impact scores with rate of change in Tanner stage (Baseline Tanner Stage x SLE Impact Score interaction: β=−0.21, p=.011). A greater rate of change in epigenetic age was also associated with higher depressive symptom levels at follow-up, adjusting for baseline symptoms (β=0.15, p=.043). Associations with epigenetic age were similar, although slightly attenuated, when adjusting for epithelial (buccal) cell proportions. Whereas much research in youths has focused on severe experiences of early adversity, we demonstrate that more commonly experienced SLEs during adolescence may also contribute to accelerated BA. Further research is needed to understand the long-term consequences of changes in BA metrics for health.
Keywords: Adolescents, Adversity, Depression, DNA methylation age, Epigenetic age, Pubertal stage
1. Introduction
Environmental experiences in childhood and adolescence are strongly related to health over the lifespan. In particular, early-life adversity (ELA) has powerful associations with negative mental and physical health (Nelson et al., 2020). Accelerated development—although a potentially advantageous short-term adaptation to ELA—may be one mechanism by which ELA contributes to deleterious health outcomes (Belsky and Shalev, 2016; Callaghan and Tottenham, 2016). ELA has been linked to advanced biological aging (BA) across cellular and reproductive strategy metrics in youths, including shorter telomere length (Belsky and Shalev, 2016; Coimbra et al., 2017), advanced epigenetic age relative to chronological age (Jovanovic et al., 2017; Marini et al., 2020), and earlier pubertal timing and age at menarche (Belsky et al., 1991; Mendle et al., 2016). These BA metrics have been associated with all-cause mortality and various aging-related health risk factors and conditions, including cardiometabolic risk and cognitive decline (e.g., Chen et al., 2016; Levine et al., 2015; Prentice and Viner, 2013; Roetker et al., 2018; Wang et al., 2018). To date, much research linking early adversity with indicators of BA in youths has focused on more severe experiences of ELA, including abuse, neglect, and institutional rearing. Further, this work suggests that adversity characterized by threat (reflecting potential for physical harm, e.g., abuse)—and not deprivation (involving the absence of expected environmental inputs, e.g., neglect)—are particularly associated with advanced BA (Colich et al., 2020a; Colich et al., 2020b; Sumner et al., 2019; Sun et al., 2020; Tang et al., 2020b).
Less is known about whether less severe, but more common, adverse experiences such as stressful life events (SLEs) relate to BA in youths. Early SLEs—such as interpersonal conflicts and academic stressors—are strong predictors of adverse health (Grant et al., 2003; Jenness et al., 2019; Tang et al., 2020a; Wickrama et al., 2015), and these events become increasingly common during adolescence (Larson and Ham, 1993). Research in adults has linked a broad range of stressful life experiences with advanced BA (see Gassen et al., 2017 for a review). For example, greater SLEs and perceived stress have been associated with shorter telomere length (Epel et al., 2004; Parks et al., 2009; Schutte and Malouff, 2016) and advanced epigenetic age relative to chronological age (Zannas et al., 2015). Additionally, longitudinal research has demonstrated that SLEs early in life (e.g., prenatal stress exposure, discrimination during adolescence) predict epigenetic and telomere metrics of advanced BA in young adulthood (Brody et al., 2016; Entringer et al., 2011). However, less work has examined whether SLEs influence BA in youths, although maternal prenatal SLEs and adverse family contexts have been associated with earlier pubertal development (Bräuner et al., 2021; Pham et al., 2022). Furthermore, research in adolescent girls has linked greater diurnal cortisol production to advanced epigenetic age relative to chronological age (Davis et al., 2017), highlighting a potential mechanism by which SLEs may contribute to BA.
Many studies of ELA and BA in youths have been cross-sectional or included only one aging-related assessment. Accordingly, it is unclear whether early experiences are related to change in BA. Some longitudinal studies of adults have investigated how trauma and related psychopathology are associated with change in BA (e.g., greater rate of change of the epigenetic clock; Boks et al., 2015; Wolf et al., 2019). Recently, Copeland and colleagues (2022) examined whether a range of ELA, including dimensions of threat, material deprivation, loss, and unpredictability, related to change in epigenetic age from childhood to adulthood. Greater cumulative ELA was associated with accelerated epigenetic aging, but robust links between different ELA dimensions and change in epigenetic aging were not detected. However, research examining how SLEs influence the pace of BA in childhood and adolescence is largely lacking—a notable gap, as these BA indicators often exhibit substantial change during these developmental periods. For example, not only does pubertal development occur during this time, but the rate of change in the epigenetic clock is faster earlier in life compared to after puberty (Marioni et al., 2019; Raj and Horvath, 2020).
If SLEs relate to accelerated BA, this process could underlie links between stress and psychopathology. Substantial research indicates that earlier pubertal development predicts incident psychopathology in adolescence (Colich and McLaughlin, 2022), and emerging evidence in youths has revealed cross-sectional associations between advanced epigenetic age and depressive symptoms (Sumner et al., 2019) and internalizing disorders (Dammering et al., 2021). Additionally, in older adults, advanced epigenetic aging metrics partially accounted for the association of ELA with depressive symptoms (Klopack et al., 2022). However, longitudinal research investigating whether change in BA relates to psychopathology in youths—and whether accelerated aging may underlie associations of SLEs with psychopathology—is needed.
Additionally, although research in adults has identified relatively low concordance between different BA measures (Belsky et al., 2018), understanding of whether repeated measures of cellular and reproductive BA metrics relate to one another in youths is limited. Cross-sectional research has demonstrated a positive association between epigenetic age and pubertal stage in youths (Sumner et al., 2019), but evidence from longitudinal studies is mixed. In adolescent girls, greater epigenetic age advancement over puberty was related to younger age at menarche and a faster pubertal tempo (Binder et al., 2018). However, there was limited evidence that advanced epigenetic age at birth or in childhood or adolescence were related to pubertal development in a large birth cohort (Simpkin et al., 2017).
In this study, we addressed these limitations by examining rate of change in cellular and reproductive strategy BA measures—epigenetic age and pubertal stage—in children and adolescents followed over approximately 2 years. In 171 youths aged 8–16 years at baseline, we examined how BA metrics related to one another within and across time, and whether SLEs occurring between baseline and follow-up predicted differential change in these metrics. Additionally, we investigated whether changes in BA were related to changes in depressive symptoms. We hypothesized that experiences of SLEs would be positively associated with rate of acceleration in BA which, in turn, would be positively associated with change in depressive symptoms.
2. Materials and Methods
2.1. Participants and Procedure
Youths between 8 and 16 years of age and a caregiver (parent/guardian) were recruited to participate in a study of ELA, emotion regulation, and psychopathology (see Weissman et al., 2019 for details). Between January 2015 and January 2017, 262 children were enrolled from the community in Seattle, WA. Participants in the parent study completed a baseline assessment and were invited to return for a follow-up assessment approximately two years later (Weissman et al., 2019). Of the 262 youths enrolled in the parent study, 161 participants were included in a sub-sample that provided neuroimaging data and saliva samples for epigenetic analysis (Jenness et al., 2021); 121 youths had measures of epigenetic age at baseline and follow-up. Participants who were not in this sub-sample were still invited to complete measures of mental health symptoms and pubertal stage at the follow-up assessment; 140 youths in the parent study had reliable information on Tanner stage at both assessments (4 youths were excluded due to having unreliable reports indicating meaningful decreases in Tanner stage from baseline to follow-up). Study procedures were approved by the University of Washington Institutional Review Board, and research was carried out in accordance with the Declaration of Helsinki. Caregivers provided written informed consent and youths provided written assent. The analytic sample for the current study comprised 171 participants who had measures of at least one BA metric at both baseline and follow-up.
2.2. SLE Measure
We used a multi-informant contextual threat interview-based approach to measure SLEs that occurred between the baseline and follow-up assessments. SLEs occurring in the past year of the follow-up period were queried with the UCLA Life Stress Interview (LSI; Hammen, 1988, 1991), a semi-structured interview developed to characterize SLEs as objectively as possible. The UCLA LSI was administered to youths and a caregiver. This interview has been validated for use in youth and adult samples, and it is a gold-standard method for assessing SLEs (Monroe, 2008). The UCLA LSI utilizes a series of prompts to assess various domains of a youth’s life (e.g., school, peers, parents), and youths and caregivers each completed the interview and reported on events occurring in the past year. With this interview, an independent research team determines objective ratings indicating the likely impact of each event for a youth of that age and sex on a scale from 1 (no negative impact) to 5 (extremely severe negative impact). As in prior research (Hammen et al., 2000; Weissman et al., 2020), an objective SLE impact score was calculated by summing the impact scores for all events (excluding those scored as 1, as these have no likely negative impact). This total score provides a weighted average of the number and severity of SLEs in the past year. As in previous work in the parent study (Weissman et al., 2020), the highest score reported by youths or caregivers was used in analyses, reflecting the multi-informant method.
2.3. BA Measures
2.3.1. Epigenetic age.
Youths provided saliva samples at baseline and follow-up using Oragene® kits (DNA Genotek, Ontario, Canada). DNA extraction and methylation profiling were conducted by AKESOgen (Atlanta, GA). The Illumina Infinium MethylationEPIC array was used to assess DNA methylation levels at >850,000 cytosine-guanine dinucleotides across the genome at single-nucleotide resolution. Samples were plated such that a participant’s baseline and follow-up samples were on the same chip. Our plating strategy also minimized correlations between technical variables (chip, position, plate) with participant demographics and history of ELA. Additionally, two participants were randomly selected as technical replicates; duplicate baseline and follow-up samples for each individual were assayed on different chips. After measurement, data were cleaned following the pipeline of Illumina and processed using “minfi” (Fortin et al., 2017). CpGs with detection p-values >.01 in >5% of individuals were removed. Cross-hybridizing, genetically confounded, and sex chromosome probes were removed. DNA methylation data were pre-processed using the Illumina-type background correction, dye-bias adjustment, and normal-exponential out-of-band normalization.
We examined the pan-tissue Horvath clock trained on chronological age (Horvath, 2013) as our measure of epigenetic age in analyses. Given that most other DNA methylation age estimators have been constructed based on blood samples from adults, we selected this epigenetic clock because it was built and calibrated across various tissues, including saliva, and in younger individuals (Horvath, 2013). The CpG sites for the estimations of Horvath epigenetic age were retrieved from the whole epigenome data. Horvath epigenetic age estimates were calculated at baseline and follow-up based on raw (non-normalized) probe data according to instructions on the Horvath website (https://dnamage.genetics.ucla.edu). There was evidence of high reproducibility for the Horvath epigenetic age estimates for the technical replicates (intraclass correlation coefficient(3,1)=.88).
2.3.2. Pubertal stage.
At baseline and follow-up assessments, pubertal stage was determined using self-report Tanner staging (Marshall and Tanner, 1969, 1970; Morris and Udry, 1980). Using schematics of two secondary sex characteristics (pubic hair and breast/testes development), youths reported their developmental stage on a scale of 1–5. A Tanner stage of 1 indicates no pubertal development has begun, whereas a stage of 5 indicates adult levels of pubertal maturation. The ratings for these two secondary sex characteristics were averaged to generate a Tanner stage score at each assessment. Self-report Tanner stage scores correlate with physicians’ physical examinations of pubertal status (Chavarro et al., 2018; Coleman and Coleman, 2002; Morris and Udry, 1980).
2.4. Depressive Symptoms
At baseline and follow-up, youths completed the Children’s Depression Inventory-2, a widely used self-report measure of depressive symptoms in children and adolescents with sound psychometric properties (Kovacs, 1992; Reynolds, 1994). This scale has 27 items; for each item, individuals choose between 3 statements corresponding to a 3-point scale. Responses were summed to create a total depressive symptoms score; higher scores indicate greater symptom severity. Due to positive skew in the total scores (skewness=1.44 at baseline, 1.18 at follow-up), we analyzed natural log-transformed depressive symptom variables.
2.5. Covariates
Given the wide range in participants’ chronological age, analyses adjusted for chronological age at baseline, in addition to sex and race/ethnicity. Additionally, as poverty is a context that can increase the likelihood of experiencing stress, adversity, and other environmental risks that can influence BA (e.g., exposure to toxins, differences in parenting, crowding; Johnson et al., 2016), family income-to-needs ratio was included as a covariate. Caregivers reported household income at baseline and follow-up; income-to-needs ratio was calculated by dividing household income by the U.S. census-defined poverty line for their family size. As in prior longitudinal research in this cohort (Sumner et al., 2022), the income-to-needs ratio covariate was calculated by averaging baseline and follow-up reports to reflect circumstances over the study period.
2.6. Analytic Approach
We first calculated the correlations of 1) epigenetic age and 2) Tanner stage with chronological age at each time point. We then investigated the stability of epigenetic age and Tanner stage by calculating correlations across baseline and follow-up for each BA metric. We also examined the degree of correlation across the BA metrics by computing correlations of epigenetic age and Tanner stage both within and across time.
As in recent work by Wolf et al. (2019), we operationalized accelerated aging over time in terms of the rate of change in raw BA metrics. Although research has examined the residual of BA on chronological age as a measure of BA advancement relative to chronological age (e.g., Sumner et al., 2019), in the current longitudinal study, we were interested in the rate with which BA metrics themselves changed over time. Specifically, we computed the rate of epigenetic age change per calendar year by calculating the difference in epigenetic age estimates (follow-up minus baseline) as a function of the time in years between the baseline and follow-up assessments (time between assessments was calculated to the day rather than averaged up to a whole year value). A value of 1.0 suggests that for each intervening calendar year, epigenetic age increases by one year. Values greater than 1.0 indicate an accelerated rate of epigenetic age change relative to the intervening time, whereas values less than 1.0 indicate a slower rate of epigenetic age change relative to the intervening time. Similarly, we estimated the rate of Tanner stage change per calendar year by calculating the difference in Tanner stage scores (follow-up minus baseline) as a function of follow-up time. Although this does not have the same clear interpretation as change in epigenetic age per calendar year above, it does signal important information about pubertal tempo, or the rate of change in pubertal stage over time.
We examined if objective SLE impact scores over follow-up were associated with rate of change in our BA metrics using linear regression. Models adjusted for chronological age at baseline, sex, race/ethnicity, and average family income-to-needs ratio. Additionally, we tested for interactions of SLE impact scores with sex, as some research has identified sex differences in associations of early adverse experiences with BA (Tang et al., 2020b). Given that youths varied across the full Tanner stage 1–5 range at baseline, we also tested for an interaction of SLE impact scores with baseline Tanner stage to investigate whether SLEs might be more associated with rate of change in Tanner stage primarily among youths who had more of an opportunity to change. For this analysis examining rate of change in Tanner stage, the continuous Tanner stage and SLE impact score variables were z-scored and then multiplied to create the interaction term. Further, we did not include baseline chronological age as a covariate given its high positive correlation with Tanner stage at baseline (r=.82, p<.001).
To examine links between rate of change in BA metrics with change in depressive symptoms, we conducted linear regression models with follow-up depressive symptoms as the outcome. Models adjusted for chronological age at baseline, sex, race/ethnicity, average family income-to-needs ratio, length of follow-up in years, and baseline depressive symptoms. We also considered whether rate of change in BA might explain an association between SLEs and change in depressive symptoms. If a significant relation was observed between a BA rate of change measure and change in depressive symptoms, we first tested the total effect of objective SLE impact scores on change in depressive symptoms. We then utilized the PROCESS macro (Hayes, 2022) in SPSS Version 28.0 (IBM Corp, Armonk, NY) to test for an indirect effect using bootstrapping with percentile-based confidence intervals.
We conducted three sets of sensitivity analyses. First, given prior cross-sectional research in this sample linking threat-related ELA to advanced epigenetic age and Tanner stage relative to chronological age at baseline (Sumner et al., 2019), we conducted an analysis to examine whether associations between objective SLE impact scores and rate of change in BA remained when accounting for threat-related experiences at baseline. As described previously, a multi-informant, multi-method approach was used to assess youths’ lifetime threat-related experiences, including emotional, physical, and sexual abuse, domestic violence, and other forms of interpersonal violence (see Sumner et al., 2019 and Supplementary Methods for details). These experiences were summed to create a lifetime composite of threat-related ELA, which was included as a covariate in the sensitivity analysis.
Second, we conducted analyses that included the estimated proportion of epithelial (buccal) cells in each sample at baseline and follow-up as covariates in analyses of change in epigenetic age. The proportion of epithelial cells in saliva exhibits inter-individual variability and can influence DNA methylation levels (Smith et al., 2015). As with prior research (Jovanovic et al., 2017; Smith et al., 2015; Sumner et al., 2019), we estimated the proportion of epithelial (buccal) cells using the method of Houseman and colleagues (Houseman et al., 2012; Koestler et al., 2013) and a reference from buccal cells (GSE46573) from the Gene Expression Omnibus. The median proportion of epithelial (buccal) cells was 29.0% (interquartile range=22.6%–35.4%) at baseline and 31.3% (interquartile range=23.6%–37.8%) at follow-up. These ranges overlap with other estimates of epithelial (buccal) cells in saliva samples (Eipel et al., 2016; Theda et al., 2018).
Third, although longitudinal research has found that epigenetic aging metrics are positively associated with depressive symptoms (Klopack et al., 2022), other longitudinal research has found that depressive symptoms were positively associated with change in epigenetic aging (Copeland et al., 2022). Thus, we tested whether baseline depressive symptoms were related to rate of change in the BA metrics, adjusting for chronological age at baseline, sex, race/ethnicity, and average family income-to-needs ratio.
All analyses were performed with SPSS Version 28.0 (IBM Corp., Armonk, NY), and p-value <.05 indicated statistical significance.
3. Results
3.1. Participant Characteristics
Descriptive statistics for the analytic sample are presented in Table 1. Participants had a mean age of 12.5 years at baseline, and 44.4% of the analytic sample was female. Additionally, participants were diverse with respect to race/ethnicity and family income-to-needs ratio, and there was wide variability in past-year SLEs and depressive symptom levels. Consistent with research demonstrating that experiences of SLEs increase during adolescence relative to childhood (Ge et al., 2001; Larson and Lampman-Petraitis, 1989; Rudolph and Hammen, 1999), chronological age at baseline was positively associated with SLE impact scores at follow-up (r=.18, p=.049). Chronological age was also positively correlated with depressive symptoms at baseline (r=.21, p=.006) and follow-up (r=.34, p<.001). The average time between baseline and follow-up assessments was 1.82 years. Baseline demographics for participants in the analytic sample were very similar to those for the larger group of participants in the parent study (Supplementary Table 1).
Table 1.
Participant characteristics for the analytic sample (N=171).
| Characteristic | M (SD) | Range | % (n) | Valid n |
|---|---|---|---|---|
| Age at baseline, years | 12.50 (2.63) | 8.00–16.95 | 171 | |
| Female sex | 44.4 (76) | 171 | ||
| Race/ethnicity | 171 | |||
| White | 44.4 (76) | |||
| Black | 25.1 (43) | |||
| Latino | 11.1 (19) | |||
| Other | 19.3 (33) | |||
| Average family income-to-needs ratio | 3.58 (2.68) | 0.09–10.35 | 167 | |
| Time between baseline and follow-up, years | 1.82 (0.65) | 0.33–3.43 | 171 | |
| Stressful life events | ||||
| Objective stressful life events impact score, follow-up | 11.49 (9.98) | 0–44.0 | 126 | |
| Depressive symptoms | ||||
| Symptom levels, baseline | 8.94 (7.44) | 0–39 | 171 | |
| Symptom levels, follow-up | 8.41 (7.55) | 0–35 | 170 |
Note. M=mean; SD=standard deviation. Although we analyzed natural log-transformed depressive symptom variables due to positive skew, we present descriptive statistics based on the original scale.
3.2. BA Metrics Within and Across Time
Descriptive statistics for raw BA metrics and their correlations within and across time are presented in Table 2. Epigenetic age estimates and Tanner stage scores were strongly positively correlated with chronological age at baseline (epigenetic age: r=.70, Tanner stage: r=.82, ps<.001) and follow-up (epigenetic age: r=.69, Tanner stage: r=.78, ps<.001). There was also evidence of considerable stability over time for both epigenetic age (r=.73, p<.001) and Tanner stage (r=.86, p<.001). Additionally, the two BA metrics exhibited moderate positive correlations with one another both within and across time (Table 2).
Table 2.
Raw estimates of biological age and correlations within and across time.
| Descriptive Statistics | Correlations | ||||||
|---|---|---|---|---|---|---|---|
| Biological Age Metric | M (SD) | Range | n | 1. | 2. | 3. | 4. |
| 1. Baseline Horvath epigenetic age | 11.48 (3.53) | 4.02–20.89 | 121 | --- | |||
| 2. Follow-up Horvath epigenetic age | 13.24 (4.13) | 4.63–25.76 | 121 | .73 | --- | ||
| 3. Baseline Tanner stage | 3.19 (1.35) | 1–5 | 140 | .53 | .46 | --- | |
| 4. Follow-up Tanner stage | 3.85 (1.12) | 1–5 | 140 | .51 | .48 | .86 | --- |
Note. All correlations significant at p<.001. Sample size for correlations for the Horvath epigenetic age = 121 and for Tanner stage = 140. Sample size for correlations across Horvath epigenetic age and Tanner stage = 90. Abbreviations: M = mean, SD = standard deviation.
With respect to rate of change in BA metrics, epigenetic age estimates increased at a rate, on average, of 1.02 years per calendar year (SD=1.84, range= −3.90–6.15). The mean rate of change per calendar year for Tanner stage was 0.39 (SD=0.46, range= −0.39–2.99). Rates of change for epigenetic age and Tanner stage were not significantly correlated (r=.10, p=.338). Rate of change for Tanner stage was negatively correlated with chronological age at baseline (r=−.38, p<.001), but rate of change for epigenetic age was not significantly associated with chronological age (r=.05, p=.575).
3.3. SLEs and Change in BA
Higher objective SLE impact scores were significantly associated with a greater rate of change in epigenetic age per calendar year [b=0.04 (95% confidence interval [CI]: 0.00–0.08), β=0.21, p=.043, n=115; see Figure 1 and Supplementary Table 2 for results for full regression model]. There was no evidence of association between SLEs and rate of change in Tanner stage [b=0.01 (95% CI: −0.00–0.02), β=0.14, p=.187, n=95; see Supplementary Table 2 for results for full regression model]. However, there was a significant interaction of baseline Tanner stage and SLE impact score when examining rate of change in Tanner stage [b=−0.10 (95% CI: −0.18–0.02), β=−0.21, p=.011, n=95]. As shown in Figure 2, analyses of simple slopes indicated that higher objective SLE impact scores were associated with a greater rate of change in Tanner stage per calendar year for youths with lower—but not higher—Tanner stage scores at baseline.
Figure 1.
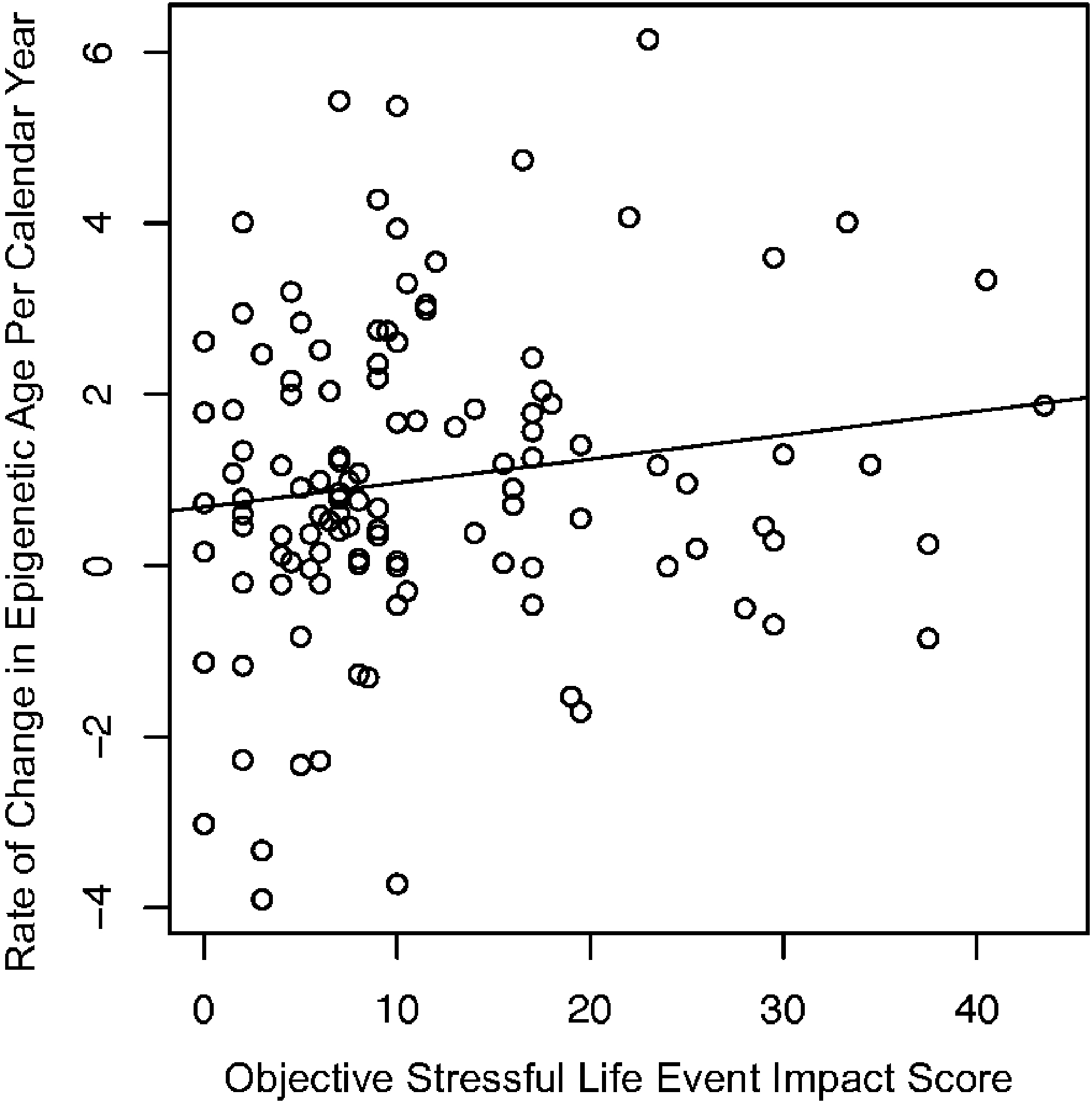
Scatter plot with regression line for the association of objective stressful life event impact scores with rate of change in epigenetic age per calendar year. For the rate of change in epigenetic age variable, a value of 1.0 suggests that for each intervening calendar year, epigenetic age increases by one year. Values greater than 1.0 indicate an accelerated rate of epigenetic age change relative to the intervening time, whereas values less than 1.0 indicate a slower rate of epigenetic age change relative to the intervening time.
Figure 2.
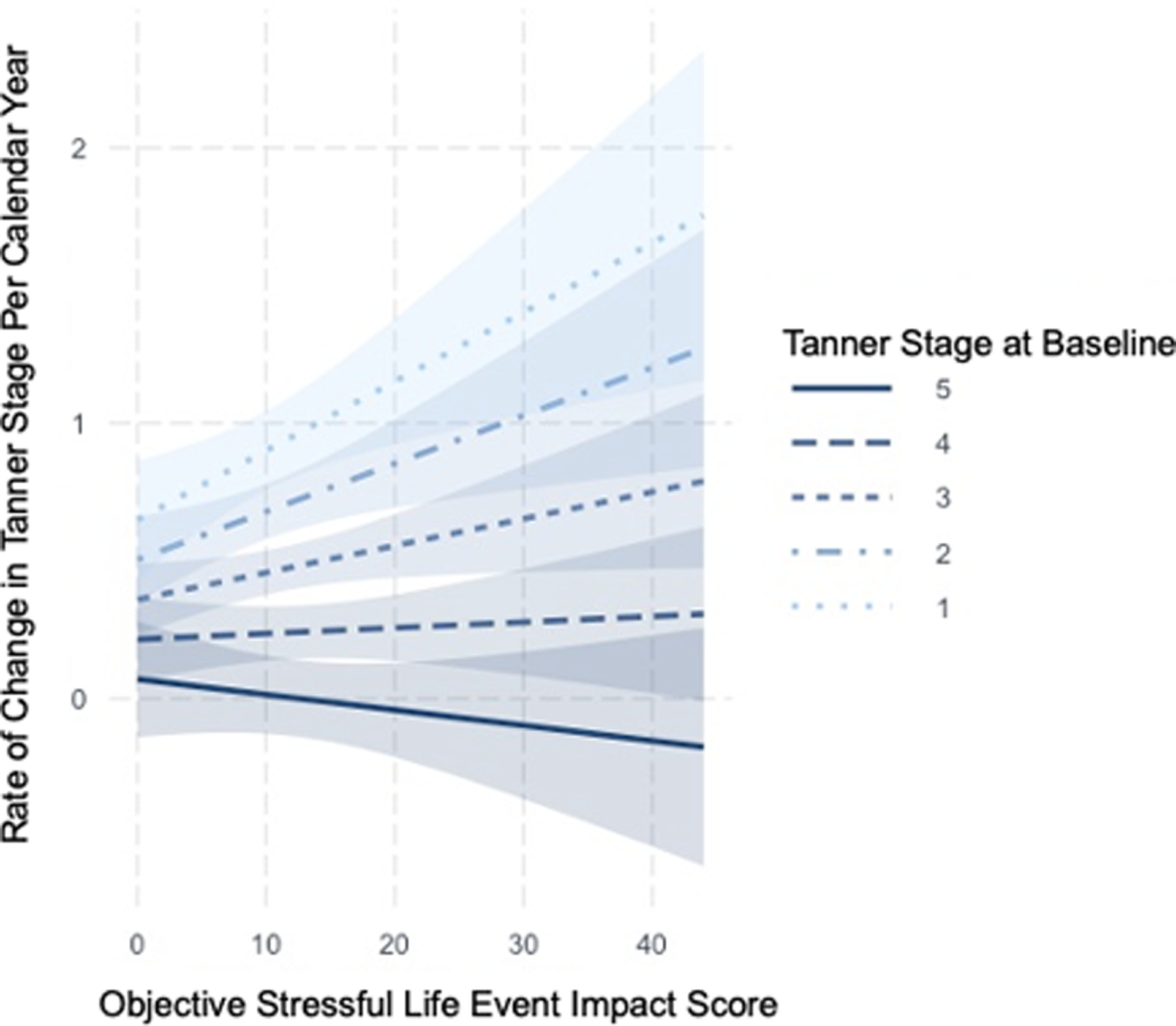
Moderation of the relation between objective stressful life event (SLE) impact scores and rate of change in Tanner stage per calendar year by Tanner stage at baseline; regression lines and 95% confidence intervals are presented in the plot. Past-year SLEs at follow-up were significantly positively associated with rate of change in Tanner stage, adjusting for sex, race/ethnicity, and average family income-to-needs ratio, in participants with lower—but not higher—Tanner stage at baseline. Objective stressful life event impact scores and Tanner stage at baseline were z-scored for testing the interaction but variables are plotted in their raw scale for ease of interpretability. Larger positive values for rate of change in Tanner stage indicate a faster pubertal tempo over time.
There were no statistically significant Sex x SLE Impact Score interactions for rate of change in epigenetic age or Tanner stage (ps>.191).
3.4. Rate of Change in BA and Change in Depressive Symptoms
A greater rate of change in epigenetic age per calendar year was associated with higher depressive symptom levels at follow-up, adjusting for depressive symptoms at baseline [b=0.07 (95% CI: 0.00–0.14), β=0.15, p=.043, n=119; see Supplementary Table 3 for results for full regression model]. In contrast, rate of change in Tanner stage was not significantly associated with depressive symptom levels from baseline to follow-up [b=0.10 (95% CI: −0.20–0.39), β=0.05, p=.525, n=136; see Supplementary Table 3 for results for full regression model]. In light of our finding that SLEs were associated with rate of change in Tanner stage only for youths with lower Tanner stage at baseline, we also tested the interaction of baseline Tanner stage with rate of change in Tanner stage when examining change in depressive symptoms over time. However, there was no evidence of significant moderation [b=0.05 (95% CI: −0.09–0.18), β=0.06, p=.490, n=136].
Given the significant relation between rate of change in epigenetic age and change in depressive symptoms over follow-up, we explored whether accelerated epigenetic aging was a potential pathway between SLEs and change in depressive symptoms. Objective SLE impact scores were positively associated with depressive symptoms at follow-up, adjusting for baseline symptom levels, but this association did not reach the level of statistical significance [b=0.01 (95% CI: −0.00–0.03), β=0.14, p=.079, n=124]. Furthermore, the indirect effect was small (0.003) and not statistically different from zero (95% CI: −0.00–0.01)].
3.5. Sensitivity Analyses
The mean of the composite score reflecting lifetime experiences of threat-related ELA at baseline in the analytic sample was 5.0 (SD=3.7, range= 0–14), and lifetime threat and objective SLE impact scores were positively correlated (r=.57, p<.001). Higher objective SLE impact scores remained associated with a greater rate of change in epigenetic age per calendar year when adjusting for lifetime threat-related ELA at baseline [b=0.04 (95% CI: 0.00–0.08), β=0.22, p=.050, n=115].
Additionally, although a similar pattern of results for associations of SLE impact scores with epigenetic aging was obtained when adjusting for the proportions of epithelial (buccal) cells at baseline and follow-up, results were slightly attenuated with respect to effect size and did not reach the threshold of statistical significance [b=0.04 (95% CI: −0.00–0.07), β=0.18, p=.057, n=115]. In addition, rate of change in epigenetic age per calendar year remained associated with higher depressive symptom levels at follow-up with a nearly identical effect size in the cell type-adjusted model, but it did not reach the level of statistical significance [b=0.07 (95% CI: −0.01–0.15), β=0.15, p=.072, n=119].
In analyses of baseline depressive symptoms and the rate of change in BA metrics, we did not find that depression at baseline predicted an accelerated rate of change in epigenetic age [b= −0.43 (95% CI: −0.87–0.01), β= −0.20, p=.053, n=119] or Tanner stage [b= −0.02 (95% CI: −0.11–0.07), β= −0.03, p=.701, n=137].
4. Discussion
In this study, we conducted a longitudinal examination of epigenetic and reproductive strategy indicators of BA in youths over approximately 2 years, and we investigated whether experiences of SLEs over follow-up related to changes in these metrics. There was evidence of stability over time for epigenetic age and Tanner stage, and both metrics were positively correlated with one another within and across time. Furthermore, exposure to greater stressful experiences over time was associated with differential rate of change in BA. Specifically, higher objective SLE impact scores reflecting past-year experiences at follow-up were significantly associated with a greater rate of change in epigenetic age per calendar year. SLE impact scores were also positively associated with rate of change in Tanner stage, but only for youths with lower Tanner stage at baseline who had more of an opportunity to change over follow-up. Furthermore, a greater rate of change in epigenetic age—but not in Tanner stage—was associated with an indicator of poor mental health, namely increased depressive symptom levels over follow-up. Results for epigenetic age were very similar in effect size, yet did not reach the threshold for statistical significance, when adjusting for cell types relevant to saliva, namely the estimated proportions of epithelial (buccal) cells. Given that DNA methylation differs across cell types, further research with larger samples is needed to ascertain the degree to which these associations are robust to cell type variation.
Our finding of correspondence between our two BA metrics (epigenetic age and Tanner stage), both within and across time, is consistent with prior literature demonstrating that greater average epigenetic age advancement is associated with faster pubertal development in adolescent girls (Binder et al., 2018). However, we did not always observe this same degree of concordance for other associations with measures of change in the two BA metrics. Indeed, rate of change in these two BA measures did not correlate significantly. Additionally, higher objective SLE impact scores were associated with a greater rate of change in epigenetic age and Tanner stage, but the latter relation was observed only among youths with lower Tanner stage at baseline who had more potential to change over the follow-up period. Furthermore, only rate of change in epigenetic age was related to change in depressive symptom levels. Together, these findings suggest that experiences of SLEs may relate to accelerated BA across cellular and reproductive strategy metrics. However, given the wide age range of youths at baseline in the current study, further testing is required in a larger sample of youths who have not yet undergone puberty at the start of follow-up. Including three or more assessments of pubertal stage, along with measures of adrenal and gonadal hormones that may capture more nuanced changes in pubertal status, will also permit investigations of potential nonlinear patterns of change.
To our knowledge, we are the first to demonstrate that SLEs are significantly associated with a greater rate of change in epigenetic age in youths. This finding is consistent with previous reports demonstrating that psychosocial stress is related to advanced epigenetic age in adults (Brody et al., 2016; Zannas et al., 2015). Furthermore, in the current study, we measured these experiences of intervening stress using a gold-standard interview and an objective rating system that integrates information about the frequency and severity of SLEs. This finding thus suggests that normative experiences of SLEs (e.g., an argument with a close friend, the break-up of a romantic relationship, failing an exam) may contribute to a greater rate of change in epigenetic age over time. This result is notable, as much research on early adversity and BA has focused on more severe forms of ELA, such as abuse, neglect, and institutional rearing (Colich et al., 2020b). Our work suggests that accelerated epigenetic aging may be a process that is influenced by less severe, yet more common, experiences of SLEs in addition to more severe forms of ELA. Furthermore, research suggests that accelerated development may result in greater experiences of ELA and SLEs; these findings are particularly strong for the associations between early menarche and sexual assault (Mendle et al., 2019). Thus, longitudinal research that examines bidirectional associations of change in SLEs and in BA over time is required to understand these relations more comprehensively.
We also examined the extent to which accelerated BA over time was associated with an indicator of mental health: change in depressive symptoms. Here, we extended our previous cross-sectional findings linking advanced epigenetic age to higher depressive symptom levels (Sumner et al., 2019) by demonstrating that a greater rate of change in epigenetic age predicted higher depressive symptom levels at follow-up, adjusting for baseline symptoms. In that baseline study, we also only detected an association between epigenetic age—and not Tanner stage—with depressive symptoms, which parallels our findings in the current study. The distinct methods for measuring epigenetic age (assayed from biological samples) and Tanner stage (self-reported by youths) may have resulted in differential measurement error for the BA metrics, and this may explain—in part—the differential associations observed with change in depressive symptoms over time. Nevertheless, our findings are consistent with previous research in adults linking advanced epigenetic age to depression diagnoses (Han et al., 2018) and symptoms (Klopack et al., 2022) and to reductions in the positive affect domain of a measure of depressive symptoms (Beydoun et al., 2019). However, there has been limited demonstration of predictions of change in depression over time (Beydoun et al., 2019), as we show here.
Mechanism-focused research is needed to better understand how accelerated BA may lead to increases in depressive symptoms over time. For example, accelerations in BA indicators may lead to neuronal changes such as neuroinflammation and neurodegeneration (either directly or through other physiological processes, including oxidative stress and mitochondrial dysfunction) in brain areas relevant to depression (e.g., hippocampus), and these brain changes may increase susceptibility to depression (Epel and Prather, 2018; Hawn et al., 2022; Wolf et al., 2021). Changes in systemic inflammation and behavioral risk factors (e.g., smoking) may also contribute to depression risk in the context of accelerated BA (Klopack et al., 2022; Miller et al., 2009). Preclinical studies utilizing experimental designs, in addition to further longitudinal research in humans, may help to elucidate key molecular and behavioral mechanisms.
Additionally, recent longitudinal research found that depressive symptoms during childhood partially explained the link between ELA and accelerated epigenetic aging from childhood to adulthood (Copeland et al., 2022). Even though we focused on the extent to which change in BA indicators was associated with mental health (here, depressive symptoms) over time, it is worth noting that mental health may also impact BA, although sensitivity analyses did not provide robust support for the notion that baseline depressive symptoms predicted an accelerated rate of change in the BA metrics. Further, we note that the timeframes for assessing BA and depressive symptoms were overlapping; thus, our analyses cannot address causality. Additional longitudinal research with multiple repeated assessments is needed to better understand the bidirectionality of these associations and how they may unfold over the life course. Furthermore, we did not observe that accelerated epigenetic aging was a potential path linking experiences of SLEs with change in depressive symptoms over time. This finding contrasts with a study in older adults indicating that epigenetic aging indicators partially accounted for associations of ELA with depressive symptoms (Klopack et al., 2022). Differences in the nature of the early-life experiences examined (self-reported ELA vs. objectively rated SLEs) and in the developmental stage of participants (older adults vs. youths) may explain the lack of concordance in results between the investigation by Klopack and colleagues and the current study. Moreover, numerous mechanisms spanning multiple levels of analysis underlie the link between early-life stressors and psychopathology (Grant et al., 2003; McLaughlin et al., 2020). Longitudinal research in large samples of youth is required to elucidate the most important mechanisms across these levels of analysis.
Several limitations need to be considered when interpreting the findings of the current investigation. We used saliva-based measures of DNA methylation, and we estimated Horvath epigenetic age, as this has been validated in saliva and in children and adolescents (Horvath, 2013). Numerous epigenetic age algorithms have been developed (e.g., Alisch et al., 2012; Hannum et al., 2013; Wu et al., 2019), but not all are available for saliva samples collected from younger individuals. Some clocks have also been trained on morbidity and mortality (e.g., Levine et al., 2018; Lu et al., 2019), which may not be as relevant for youths. Furthermore, this approach extended our prior cross-sectional research with Horvath epigenetic age in this sample (Sumner et al., 2019). Additionally, we used the MethylationEPIC array to assess DNA methylation levels, and the Horvath epigenetic clock was developed using the HumanMethylation27 and HumanMethylation450 arrays. However, even though 19 of the 353 CpG sites used to calculate Horvath epigenetic age are missing from the EPIC array, research suggests that this has a minimal effect on epigenetic age estimates (McEwen et al., 2018). In addition, although we used a multi-informant interview approach and an objective rating method for assessing SLE impact, experiences of SLEs were retrospectively reported, which has established limitations (Baldwin et al., 2019; Hardt and Rutter, 2004). We also examined self-reported Tanner stage. Despite high correlations between self-report and physical exam measures of pubertal development, variation exists in the accuracy of self-reporting (Chavarro et al., 2018; Coleman and Coleman, 2002; Dorn and Biro, 2011; Morris and Udry, 1980). We also acknowledge that Tanner stage (and pubertal development more broadly) is a developmentally constrained BA indicator. Although appropriate for youths, this marker loses value as a marker of BA in young adulthood and beyond. Additionally, our sample was diverse with respect to race/ethnicity. Although this increases the generalizability of our findings, race and ethnicity have been linked to differences in epigenetic age and pubertal stage (Herman‐Giddens, 2006; Horvath et al., 2016; Kaplowitz, 2006; Liu et al., 2019). However, we adjusted for self-reported race/ethnicity in our analyses. Even with these limitations, our study has a number of strengths that allow it to make a unique contribution to the literature, including 1) utilizing a longitudinal design to examine rate of change in cellular and reproductive strategy metrics of BA, and 2) including a gold-standard interview-based measure and objective rating of SLEs.
4.1. Conclusions
Despite considerable stability in measures of BA in youths over approximately 2 years, we found that greater exposure to objectively rated SLEs was significantly associated prospectively with accelerated epigenetic aging and—among those with lower Tanner stage at baseline—rate of change in Tanner stage. Additionally, a greater rate of change in epigenetic age predicted higher levels of depressive symptoms at follow-up, shedding light on how accelerated BA may impact mental health. Further research is needed to understand the long-term consequences of changes in these metrics of BA for mental and physical health, and whether the process of accelerated aging can be altered through psychosocial intervention.
Supplementary Material
Acknowledgements
This research was supported by the National Institutes of Health (R01MH103291; R01MH103291-S2 to KAM; K01HL130650; R01HL139614 to JAS). The funders had no role in the designing, collecting, managing, analyzing, interpreting, writing, reviewing, or approving the materials presented in this study. Funders had no role in the decision to submit the manuscript for publication.
Footnotes
Declarations of Interest
None
CRediT Authorship Contribution Statement
Jennifer A. Sumner: Conceptualization, Data curation, Funding acquisition, Analysis, Writing – original draft, Writing – review and editing; Xu Gao: Data curation, Writing – review and editing; Simone Gambazza: Data curation, Writing – review and editing; Christian K. Dye: Data curation, Writing – review and editing; Natalie L. Colich: Data curation, Writing – review and editing; Andrea A. Baccarelli: Writing – review and editing; Monica Uddin: Writing – review and editing; Katie A. McLaughlin: Conceptualization, Funding acquisition, Supervision, Writing – review and editing.
Supplementary Information: Supplementary Methods and 3 supplementary tables
Data Sharing
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Alisch RS, Barwick BG, Chopra P, Myrick LK, Satten GA, Conneely KN, Warren ST, 2012. Age-associated DNA methylation in pediatric populations. Genome Res. 22, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, Danese A, 2019. Agreement between prospective and retrospective measures of childhood maltreatment: a systematic review and meta-analysis. JAMA Psychiat. 76, 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, 2018. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am. J. Epidemiol 187, 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Shalev I, 2016. Contextual adversity, telomere erosion, pubertal development, and health: two models of accelerated aging, or one? Dev. Psychopathol 28, 1367–1383. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, Draper P, 1991. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 62, 647–670. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Hossain S, Chitrala KN, Tajuddin SM, Beydoun HA, Evans MK, Zonderman AB, 2019. Association between epigenetic age acceleration and depressive symptoms in a prospective cohort study of urban-dwelling adults. J. Affect. Disorders 257, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder AM, Corvalan C, Mericq V, Pereira A, Santos JL, Horvath S, Shepherd J, Michels KB, 2018. Faster ticking rate of the epigenetic clock is associated with faster pubertal development in girls. Epigenetics 13, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boks MP, van Mierlo HC, Rutten BP, Radstake TR, De Witte L, Geuze E, Horvath S, Schalkwyk LC, Vinkers CH, Broen JC, Vermetten E, 2015. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology 51, 506–512. [DOI] [PubMed] [Google Scholar]
- Bräuner E, Koch T, Juul A, Doherty D, Hart R, Hickey M, 2021. Prenatal exposure to maternal stressful life events and earlier age at menarche: the Raine Study. Hum. Reprod 36, 1959–1969. [DOI] [PubMed] [Google Scholar]
- Brody GH, Miller GE, Yu T, Beach SR, Chen E, 2016. Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: a replication across two longitudinal cohorts. Psychol. Sci 27, 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N, 2016. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci 7, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Watkins DJ, Afeiche MC, Zhang Z, Sanchez BN, Cantonwine D, Mercado-García A, Blank-Goldenberg C, Meeker JD, Téllez-Rojo MM, Peterson KE, 2018. Validity of self-assessed sexual maturation against physician assessments and hormone levels. J. Pediatr 186, 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai P-C, Roetker NS, Just AC, Demerath EW, Guan W, Bressler J, Fornage M, Studenski S, Vandiver AR, Moore AZ, Tanaka T, Kiel DP, Liang L, Vokonas P, Schwartz J, Lunetta KL, Murabito JM, Bandinelli S, Hernandez DG, Melzer D, Nalls M, Pilling LC, Price TR, Singleton AB, Gieger C, Holle R, Kretschmer A, Kronenberg F, Kunze S, Linseisen J, Meisinger C, Rathmann W, Waldenberger M, Visscher PM, Shah S, Wray NR, McRae AF, Franco OH, Hofman A, Uitterlinden AG, Absher D, Assimes T, Levine ME, Lu AT, Tsao PS, Hou L, Manson JE, Carty CL, LaCroix AZ, Reiner AP, Spector TD, Feinberg AP, Levy D, Baccarelli A, van Meurs J, Bell JT, Peters A, Deary IJ, Pankow JS, Ferrucci L, Horvath S, 2016. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging 8, 1844–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra BM, Carvalho CM, Moretti PN, Mello MF, Belangero SI, 2017. Stress-related telomere length in children: a systematic review. J. Psychiatr. Res 92, 47–54. [DOI] [PubMed] [Google Scholar]
- Coleman L, Coleman J, 2002. The measurement of puberty: a review. J. Adolesc 25, 535–550. [DOI] [PubMed] [Google Scholar]
- Colich NL, McLaughlin KA, 2022. Accelerated pubertal development as a mechanism linking trauma exposure with depression and anxiety in adolescence. Curr. Opin. Psychol 46, 101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Platt JM, Keyes KM, Sumner JA, Allen NB, McLaughlin KA, 2020a. Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls. Psychol. Med 50, 1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Rosen ML, Williams ES, McLaughlin KA, 2020b. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. Psychol. Bull 146, 721–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, McGinnis EW, Aberg KA, van den Oord EJ, 2022. Early adversities accelerate epigenetic aging into adulthood: a 10‐year, within‐subject analysis. J. Child Psychol. Psychiatry doi: 10.1111/jcpp.13575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammering F, Martins J, Dittrich K, Czamara D, Rex-Haffner M, Overfeld J, de Punder K, Buss C, Entringer S, Winter SM, Binder EB, Heim C, 2021. The pediatric buccal epigenetic clock identifies significant ageing acceleration in children with internalizing disorder and maltreatment exposure. Neurobiol. Stress 15, 100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E, Humphreys K, McEwen L, Sacchet M, Camacho M, MacIsaac J, Lin D, Kobor M, Gotlib I, 2017. Accelerated DNA methylation age in adolescent girls: associations with elevated diurnal cortisol and reduced hippocampal volume. Transl. Psychiatry 7, e1223-e1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Biro FM, 2011. Puberty and its measurement: a decade in review. J. Res. Adolesc 21, 180–195. [Google Scholar]
- Eipel M, Mayer F, Arent T, Ferreira MRP, Birkhofer C, Gerstenmaier U, Costa IG, Ritz-Timme S, Wagner W, 2016. Epigenetic age predictions based on buccal swabs are more precise in combination with cell type-specific DNA methylation signatures. Aging 8, 1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wüst S, Wadhwa PD, 2011. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl. Acad. Sci. USA 108, E513–E518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM, 2004. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 101, 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Prather AA, 2018. Stress, telomeres, and psychopathology: toward a deeper understanding of a triad of early aging. Annu. Rev. Clin. Psychol 14, 371–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J, Triche TJ, Hansen KD, 2017. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 33, 558–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen NC, Chrousos GP, Binder EB, Zannas AS, 2017. Life stress, glucocorticoid signaling, and the aging epigenome: implications for aging-related diseases. Neurosci. Biobehav. Rev 74, 356–365. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH, 2001. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev. Psychol 37, 404–417. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA, 2003. Stressors and child and adolescent psychopathology: moving from markers to mechanisms of risk. Psychol. Bull 129, 447–466. [DOI] [PubMed] [Google Scholar]
- Hammen C, 1988. Self-cognitions, stressful events, and the prediction of depression in children of depressed mothers. J. Abnorm. Child Psychol 16, 347–360. [DOI] [PubMed] [Google Scholar]
- Hammen C, 1991. Generation of stress in the course of unipolar depression. J. Abnorm. Psychol 100, 555–561. [DOI] [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE, 2000. Depression and sensitization to stressors among young women as a function of childhood adversity. J. Consult. Clin. Psychol 68, 782–787. [PubMed] [Google Scholar]
- Han LK, Aghajani M, Clark SL, Chan RF, Hattab MW, Shabalin AA, Zhao M, Kumar G, Xie LY, Jansen R, 2018. Epigenetic aging in major depressive disorder. Am. J. Psychiatry 175, 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan J-B, Gao Y, 2013. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49, 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M, 2004. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J. Child Psychol. Psychiatry 45, 260–273. [DOI] [PubMed] [Google Scholar]
- Hawn SE, Zhao X, Sullivan DR, Logue M, Fein-Schaffer D, Milberg W, McGlinchey R, Miller MW, Wolf EJ, 2022. For whom the bell tolls: psychopathological and neurobiological correlates of a DNA methylation index of time-to-death. Transl. Psychiatry 12, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2022. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach, third ed. Guilford Press, New York, NY. [Google Scholar]
- Herman‐Giddens ME, 2006. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. Int. J. Androl 29, 241–246. [DOI] [PubMed] [Google Scholar]
- Horvath S, 2013. DNA methylation age of human tissues and cell types. Genome Biol. 14, 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Gurven M, Levine ME, Trumble BC, Kaplan H, Allayee H, Ritz BR, Chen B, Lu AT, Rickabaugh TM, 2016. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 17, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT, 2012. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness JL, Peverill M, King KM, Hankin BL, McLaughlin KA, 2019. Dynamic associations between stressful life events and adolescent internalizing psychopathology in a multiwave longitudinal study. J. Abnorm. Psychol 128, 596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness JL, Peverill M, Miller AB, Heleniak C, Robertson MM, Sambrook KA, Sheridan MA, McLaughlin KA, 2021. Alterations in neural circuits underlying emotion regulation following child maltreatment: a mechanism underlying trauma-related psychopathology. Psychol. Med 51, 1880–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Riis JL, Noble KG, 2016. State of the art review: poverty and the developing brain. Pediatrics 137, e20153075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, Klengel T, Smith AK, 2017. Exposure to violence accelerates epigenetic aging in children. Sci. Rep 7, 8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz P, 2006. Pubertal development in girls: secular trends. Curr. Opin. Obstet. Gynecol 18, 487–491. [DOI] [PubMed] [Google Scholar]
- Klopack ET, Crimmins EM, Cole SW, Seeman TE, Carroll JE, 2022. Accelerated epigenetic aging mediates link between adverse childhood experiences and depressive symptoms in older adults: results from the Health and Retirement Study. SSM Popul. Health 17, 101071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koestler DC, Christensen BC, Karagas MR, Marsit CJ, Langevin SM, Kelsey KT, Wiencke JK, Houseman EA, 2013. Blood-based profiles of DNA methylation predict the underlying distribution of cell types: a validation analysis. Epigenetics 8, 816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, 1992. Children’s Depression Inventory Manual. Multi-Health Systems, North Tonawanda, NY. [Google Scholar]
- Larson R, Ham M, 1993. Stress and “storm and stress” in early adolescence: the relationship of negative events with dysphoric affect. Dev. Psychol 29, 130–140. [Google Scholar]
- Larson R, Lampman-Petraitis C, 1989. Daily emotional states as reported by children and adolescents. Child Dev. 60, 1250–1260. [DOI] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Bennett DA, Horvath S, 2015. Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging 7, 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S, 2018. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10, 573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen BH, Assimes TL, Ferrucci L, Horvath S, Levine ME, 2019. The role of epigenetic aging in education and racial/ethnic mortality disparities among older US women. Psychoneuroendocrinology 104, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Assimes TL, Ferrucci L, Horvath S, 2019. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini S, Davis KA, Soare TW, Zhu Y, Suderman MJ, Simpkin AJ, Smith AD, Wolf EJ, Relton CL, Dunn EC, 2020. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology 113, 104484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Suderman M, Chen BH, Horvath S, Bandinelli S, Morris T, Beck S, Ferrucci L, Pedersen NL, Relton CL, 2019. Tracking the epigenetic clock across the human life course: a meta-analysis of longitudinal cohort data. J. Gerontol. A Biol. Sci. Med. Sci 74, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM, 1969. Variations in pattern of pubertal changes in girls. Arch. Dis. Child 44, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM, 1970. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child 45, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen LM, Jones MJ, Lin DTS, Edgar RD, Husquin LT, MacIsaac JL, Ramadori KE, Morin AM, Rider CF, Carlsten C, Quintana-Murci L, Horvath S, Kobor M, 2018. Systematic evaluation of DNA methylation age estimation with common preprocessing methods and the Infinium MethylationEPIC BeadChip array. Clin. Epigenetics 10, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Colich NL, Rodman AM, Weissman DG, 2020. Mechanisms linking childhood trauma exposure and psychopathology: a transdiagnostic model of risk and resilience. BMC Med. 18, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Ryan RM, McKone KM, 2016. Early childhood maltreatment and pubertal development: replication in a population‐based sample. J. Res. Adolesc 26, 595–602. [DOI] [PubMed] [Google Scholar]
- Mendle J, Ryan RM, McKone KMP, 2019. Early menarche and internalizing and externalizing in adulthood: explaining the persistence of effects. J. Adolesc. Health 65, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Meltic V, Raison CL, 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, 2008. Modern approaches to conceptualizing and measuring human life stress. Annu. Rev. Clin. Psychol 4, 33–52. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR, 1980. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc 9, 271–280. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Scott RD, Bhutta ZA, Harris NB, Danese A, Samara M, 2020. Adversity in childhood is linked to mental and physical health throughout life. BMJ 371, m3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, Sandler DP, 2009. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol. Biomarkers Prev 18, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham HT, DiLalla LF, Corley RP, Dorn LD, Berenbaum SA, 2022. Family environmental antecedents of pubertal timing in girls and boys: a review and open questions. Horm. Behav 138, 105101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice P, Viner RM, 2013. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int. J. Obes 37, 1036–1043. [DOI] [PubMed] [Google Scholar]
- Raj K, Horvath S, 2020. Current perspectives on the cellular and molecular features of epigenetic ageing. Exp. Biol. Med 245, 1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds W, 1994. Assessment of depression in children and adolescents by self-report measures, in: Reynolds W, Johnston HF (Eds.), Handbook of Depression in Children and Adolescents. Plenum Press, New York, NY, pp. 209–234. [Google Scholar]
- Roetker NS, Pankow JS, Bressler J, Morrison AC, Boerwinkle E, 2018. Prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the ARIC study (Atherosclerosis Risk in Communities). Circ. Genom. Precis. Med 11, e001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, 1999. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 70, 660–677. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2016. The relationship between perceived stress and telomere length: a meta‐analysis. Stress Health 32, 313–319. [DOI] [PubMed] [Google Scholar]
- Simpkin AJ, Howe LD, Tilling K, Gaunt TR, Lyttleton O, McArdle WL, Ring SM, Horvath S, Smith GD, Relton CL, 2017. The epigenetic clock and physical development during childhood and adolescence: longitudinal analysis from a UK birth cohort. Int. J. Epidemiol 46, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Kilaru V, Klengel T, Mercer KB, Bradley B, Conneely KN, Ressler KJ, Binder EB, 2015. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am. J. Med. Genet. B Neuropsychiatr. Genet 168, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA, 2019. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol. Psychiatry 85, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Gambazza S, Gao X, Baccarelli AA, Uddin M, McLaughlin K, 2022. Epigenetics of early life adversity in youth: cross-sectional and longitudinal associations. Clin. Epigenetics 14, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Fang J, Wan Y, Su P, Tao F, 2020. Association of early-life adversity with measures of accelerated biological aging among children in China. JAMA Netw. Open 3, e2013588-e2013588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Wade M, Fox NA, Nelson CA, Zeanah CH, Slopen N, 2020a. The prospective association between stressful life events and inflammation among adolescents with a history of early institutional rearing. Dev. Psychopathol 32, 1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Howe LD, Suderman M, Relton CL, Crawford AA, Houtepen LC, 2020b. Adverse childhood experiences, DNA methylation age acceleration, and cortisol in UK children: a prospective population-based cohort study. Clin. Epigenetics 12, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theda C, Hwang SH, Czajko A, Loke YJ, Leong P, Criag JM, 2018. Quantitation of the cellular content of saliva and buccal swab samples. Sci. Rep 8, 6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S, 2018. Telomere length and all-cause mortality: a meta-analysis. Ageing Res. Rev 48, 11–20. [DOI] [PubMed] [Google Scholar]
- Weissman DG, Bitran D, Miller AB, Schaefer JD, Sheridan MA, McLaughlin KA, 2019. Difficulties with emotion regulation as a transdiagnostic mechanism linking child maltreatment with the emergence of psychopathology. Dev. Psychopathol 31, 899–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Lambert HK, Rodman AM, Peverill M, Sheridan MA, McLaughlin KA, 2020. Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depress. Anxiety 37, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickrama KA, Lee T-K, O’Neal CW, 2015. Stressful life experiences in adolescence and cardiometabolic risk factors in young adulthood. J. Adolesc. Health 56, 456–463. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Logue MW, Morrison FG, Wilcox ES, Stone A, Schichman SA, McGlinchey RE, Milberg WP, Miller MW, 2019. Posttraumatic psychopathology and the pace of the epigenetic clock: a longitudinal investigation. Psychol. Med 49, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Zhao X, Hawn SE, Morrison FG, Zhou Z, Fein-Schaffer D, Huber B, Traumatic Stress Brain Research Group, Miller MW, Logue MW, 2021. Gene expression correlates of advanced epigenetic age and psychopathology in postmortem cortical tissue. Neurobiol. Stress 15, 100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Chen W, Lin F, Huang Q, Zhong J, Gao H, Song Y, Liang H, 2019. DNA methylation profile is a quantitative measure of biological aging in children. Aging 11, 10031–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, 2015. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 16, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


