Abstract
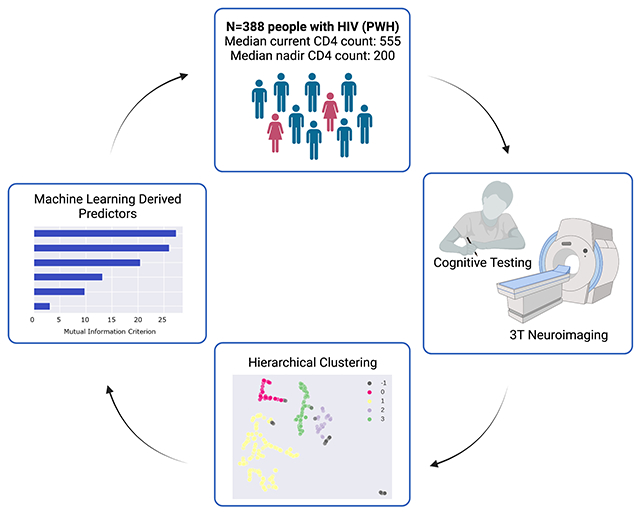
The current study applied data-driven methods to identify and explain novel cognitive phenotypes of HIV. Methods: 388 people with HIV (PWH) with an average age of 46 (15.8) and median plasma CD4+ T-cell count of 555 copies/mL (79% virally suppressed) underwent cognitive testing and 3T neuroimaging. Demographics, HIV disease variables, and health comorbidities were recorded within three months of cognitive testing/neuroimaging. Hierarchical clustering was employed to identify cognitive phenotypes followed by ensemble machine learning to delineate the features that determined membership in the cognitive phenotypes. Hierarchical clustering identified five cognitive phenotypes. Cluster 1 (n=97) was comprised of individuals with normative performance on all cognitive tests. The remaining clusters were defined by impairment on action fluency (Cluster 2; n=46); verbal learning/memory (Cluster 3; n=73); action fluency and verbal learning/memory (Cluster 4; n=56); and action fluency, verbal learning/memory, and tests of executive function (Cluster 5; n=114). HIV detectability was most common in Cluster 5. Machine learning revealed that polysubstance use, race, educational attainment, and volumes of the precuneus, cingulate, nucleus accumbens, and thalamus differentiated membership in the normal vs. impaired clusters. The determinants of persistent cognitive impairment among PWH receiving suppressive treatment are multifactorial nature. Viral replication after ART plays a role in the causal pathway, but psychosocial factors (race inequities, substance use) merit increased attention as critical determinants of cognitive impairment in the context of ART. Results underscore the need for comprehensive person-centered interventions that go beyond adherence to patient care to achieve optimal cognitive health among PWH.
Keywords: HIV, Cognition, Substance use, Machine learning
Graphical Abstract
Introduction
Approximately 1 in every 2 people with HIV (PWH) experience cognitive difficulties (Antinori et al. 2007; Heaton et al. 2015; Kore et al. 2015; Paul et al. 2017). Among those with evidence of cognitive symptoms, the manifestation and severity of impairment varies substantially from one person to the next. While cases of severe cognitive impairment (i.e., dementia) are rare in the era of antiretroviral therapy (ART), mild to moderate cognitive difficulties persist among individuals with chronic HIV who are receiving stable suppressive ART. Understanding the combination of factors that underlie the expression of cognitive difficulties in the context of HIV treatment is important to facilitate the development and implementation of clinical care strategies necessary to optimize health outcomes among the global population of PWH.
Uncertainty exists as to whether cognitive difficulties among ART-experienced individuals reflect pre-morbid factors, brain injury before the onset of ART (i.e., legacy effects), deleterious effects of co-morbid health conditions (e.g., hepatitis C) that emerge during HIV treatment, psychosocial factors (e.g., racial inequities, substance use), and/or ongoing HIV neuropathogenesis. Results from prior studies offer modest support for each explanation. For example, a recent study by Kallianpur et al. (2020) reported that higher levels of P-selectin glycoprotein ligand-1 (PSGL-1)-expressing total monocytes and PSGL-1-expressing inflammatory (CD14CD16) monocytes at the time of ART onset predicted atrophy of the caudate and putamen, respectively, during the first two years of ART commenced during acute infection. Alternatively, findings from CHARTER revealed that cognitive decline among PWH was most common among individuals with comor-bid psychiatric histories and/or substance use histories (Grant et al. 2014). Furthermore, work from members of our team has recently shown that racial inequities embedded in the assessment and norming of cognitive performance have potential to artificially inflate the severity of cognitive impairment among racially diverse PWH (Paul et al. 2021).
Little is understood about how the convergence of these factors contribute to individual differences in cognitive performance among PWH. This is an important knowledge gap because risk factors for incident cognitive impairment tend to cluster together (e.g., higher rates of substance use, lower educational attainment, worse adherence to ART). Traditional analytic methods are not designed to model the contribution of multiple, interactive predictors of complex clinical phenotypes such as HIV-associated cognitive symptoms (Paul et al. 2020a, b). This is especially true when key explanatory variables are latent (i.e., not observable) and/or nonlinear, and when diagnostic categories (e.g., the research criteria for HIV-Associated Neurocognitive Disorders; HAND; Antinori et al. 2007) serve as the primary outcomes of interest.
Data-driven methods offer an alternative approach to identify novel cognitive phenotypes, as well as potential explanatory features that differentiate membership in each cognitive subgroup. The current study employed a combination of data-driven and inferential analytic strategies to discover novel, distinct cognitive subgroups of HIV. We employed hierarchical clustering using HDBScan (dos Santos et al. 2015) with the UMAP variant (McInnes et al. 2018) to determine the presence of distinct cognitive phenotypes. Additionally, we employed gradient boosted multivariate regression (GBM), a form of ensemble machine learning, to determine a predictive model that distinguished membership in normal vs. impaired cognitive subgroups.
Methods
Study Participants
Study participants included 388 PWH (mean age=46; SD=15.8) enrolled in studies between 2006 and 2020 at the Washington University Saint Louis Infectious Disease Clinic and AIDS Clinical Trial Unit. Inclusion criteria were: 1) ≥18 years of age, 2) confirmed HIV, 3) ≥8 years of education, 4) ≥3 months of ART, 5) English as the primary language, and 6) voluntary informed consent. Exclusion criteria were: 1) history of head injury with loss of consciousness >30 minutes, 2) untreated psychiatric disorder, and/or 3) active opportunistic infection. The parent studies were approved by the participating institutional review boards. Participants received reimbursement for their time and transportation costs. Study participants were on a stable ART regimen (no change) for at least 6 months prior to completing the assessments (most were receiving a DTG-based regimen). No participants underwent a change in their regimen during the study protocol.
Primary Outcome Variable
Cognitive Performance
Participants completed a standardized battery of cognitive tests that covered the following domains: Attention and Psychomotor Speed: Trail Making Test A (Trails A; Army U.S. 1944) Digit-Symbol (Wechsler 1997), Symbol Search (Wechsler 1997), 2) Executive function: Color Word Interference Test trial 3 (CWIT3; Golden and Freshwater 1978), Trail Making Test B (Army U.S. 1944), action fluency (verbs; Piatt et al. 1999), and Letter Number Sequencing (LNS; Wechsler 1997). Motor speed and dexterity: Grooved Pegboard dominant and non-dominant hands (Matthews and Klove 1964), Learning and Memory: Hopkins Verbal Learning Test-Revised (HVLT-R; Brandt and Benedict 2001), Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict 1997), and Language: animal fluency (Tombaugh et al. 1999), and letter fluency (FAS; Tombaugh et al. 1999). Raw scores were converted to demographically adjusted z scores using normative data derived from test manuals (i.e., D-KEFS (Delis et al. 2001) for Color Word Interference Test trial 3, and WAIS-III (Wechsler 1997) for Digit Symbol, Symbol Search, and Letter Number Sequencing) and from published sources (i.e., Heaton 2004; Norman et al. 2011; Benedict et al. 1998; Piatt et al. 2004; Woods et al. 2005b; Gladsjo et al. 1999; Lucas et al. 2005). Impairment at the individual test level was defined as a Z score < −1.0. Similarly, impairment for a domain was defined by an average z score for all tests within the domain < −1.0.
Cognitive testing was completed by certified psychometricians with extensive experience in test administration and scoring. The tests were administered and scored in accord with standardized instructions described in the test manuals. Study participants did not undergo the cognitive assessment if they exhibited signs of acute substance use intoxication.
Candidate Explanatory Variables
Demographic Variables
Age, sex, educational attainment, and self-identified race were included as demographic indices.
HIV Clinical Indices
Current and nadir CD4+ T-cell count as well as viral load (for those with detectable virus) were included in the analysis.
Co-Morbidities
History of hepatitis C and total score on the affective subscale of the Beck Depression Inventory-II (BDI-II; Beck et al. 1996) were included as potential predictors. The BDI-II affective score was utilized to minimize the overlap in somatic and cognitive symptoms that are independently associated with HIV and depression. Use of the affective scale to measure mood symptoms in the context of neuroimmune dysregulation is supported by factor analytic and clinical validation studies, previous publications focused on neuroHIV (Paul et al. 2018; O’Halloran et al. 2019; Paul et al. 2020a).
Substance use was examined using a multi-dimensional approach. First, we assessed lifetime substance use using the Kreek-McHugh-Schluger-Kellogg (KMSK) lifetime scale (Kellogg et al. 2003). The KMSK incorporates self-reported frequency, amount, and duration of alcohol, cannabis, tobacco, opioids, and hallucinogens. Total scores for each drug class were examined vis-a-vis hierarchical clustering (see Statistical Analysis section for further detail) to identify individuals who shared similar profiles of lifetime substance use, including polysubstance use. This preliminary analysis revealed four discrete subgroups of lifetime substance use. Results from urine toxicology acquired at the time of study participation were then examined to characterize current use of cocaine, amphetamine, methamphetamine, cannabis, methadone, opiates, phencyclidine, benzodiazepines, and tricyclic antidepressants for each of the clusters; current alcohol use was not available from the urine toxicology results.
Neuroimaging Variables
Neuroimaging was acquired using high resolution 3T MR (Siemens Tim Trio; Siemens AG, Erlangen Germany). For all scanning parameters, a 12-channel head coil was applied. All participants completed 3T MRI using the same scan hardware and software. The T1 structural scans were derived from 3-dimensional, sagittal, magnetization-prepared rapid gradient echo (MP-RAGE) sequence with repetition time (TR)=2400ms, echo time (TE)=3.16ms, flip angle=8°, inversion time=1000ms, voxel size=1x1x1mm3 voxels, 256x256x176 acquisition matrix, 162 slices. Brain segmentation and parcellation of the structural images were obtained using the FreeSurfer software suite (v5.3) (Martinos Center, Harvard University, Boston, Massachusetts, USA). All volumetric regions of interest (ROIs) from the FreeSurfer pipeline were included in the analyses.
Statistical Analysis
First, we examined the data for missing values, outliers, and skewness to confirm that values were within acceptable ranges. Second, we utilized HDBScan (dos Santos et al. 2015) with the UMAP variant (McInnes et al. 2018) to identify individuals with similar substance use and, separately, cognitive profiles. HDBScan is a hierarchical clustering method that utilizes a proximal distance to the nearest neighbor approach. In contrast to common clustering methods, HDBScan with the UMAP variant does not require a priori determination of the expected number of clusters, and outliers are defined as a separate cluster rather than forcing the data into an existing and unrelated cluster. The UMAP variant defines the cluster structure using both linear and nonlinear associations. Z scores for each cognitive test were included in the cluster analysis.
Third, MANOVA and Chi Square tests were implemented to determine if the cognitive clusters differed on demographic, psychosocial, and/or HIV disease variables. Fourth, gradient boosted multivariate regression (GBM), a form of ensemble machine learning, was implemented to identify the combination of features that classified individuals into either the cognitively normal (Cluster 1) or the cognitively impaired (Cluster 5) phenotypes. GBM is generally robust to restricted sample sizes, differences in base rates of outcome variables, multi-collinearity, and overfitting (Miller et al. 2016). Feature selection utilized an in-house program based on SciKit (Pedregosa et al. 2011) and PDPBox (Jiangchun 2019). Cognitive phenotype subgroup membership was determined using a probability score based on the sigmoid function (1/(1+e^(−x))), 0.5 decision boundary, and gradient descent to minimize prediction error. Highly correlated features (r >.65) were managed by selecting the feature with the highest mutual information criterion (MIC) value. Consistent with our prior work (Paul et al. 2020a, b, 2021), predictors were included into the models up to the point of saturation, when the inclusion of an additional feature resulted in minimal improvement in classification accuracy (defined by an increase in average AUC > 1SD from the base model). For the current analysis, model saturation was achieved with 6 input features. Interactive features were described based on the directionality of each contributing variable, with variables sharing the same directionality (i.e., both high or low values) to the outcome represented by the multiplier symbol, and variables with inverse relationships to the outcome classification represented by the division symbol. The average AUC served as the final metric of model performance.
Validity of the GBM models was examined using five-fold cross validation repeated five times. Each partition was randomly re-sampled, yielding 25 validation trials in total. Results from the GBM models were compared to multiple logistic regression, a common statistical method for testing the relative contribution of a given set of predictors to differentiate a binary outcome (e.g., cognitively normal vs. impaired; Lemon et al. 2003).
Results
Demographic and Clinical Characteristics of the Total Sample
Study participants were between 18 and 85 years of age (M=46 years old, SD=15.8 years). The sample was 78% male (n=301) and had completed an average of 13 years of education. The median blood CD4+ T-cell count for the overall sample was 555 (IQR=383 to 777) cells/mL. Plasma viral suppression (≤50 copies/mL) was observed in 79% of the sample. Among participants with detectable virus (n=77), the median viral load was 2110 (IQR=104 to 30,108). Participants reported an average time since HIV diagnosis of 15.9 (SD = 9.3) years. Average duration of ART was 13.6 (SD = 8.5) years. 49% of the sample identified as heterosexual, 37% were men who have sex with men (MSM), and 14% identified as bisexual. See Table 1 for additional demographic and clinical information.
Table 1.
Demographic and Clinical Characteristics and Differences between Cognitive Phenotypes
| Cluster 1 n=97 | Cluster 2 n=46 | Cluster 3 n=73 | Cluster 4 n=56 | Cluster 5 n=116 | Test | Eta2 | |
|---|---|---|---|---|---|---|---|
| Age: M (SD) | 50.4 (15.0)b | 45.8 (17.8) | 44.8 (16.5) | 43.9 (16.1) | 43.9 (14.6)a | F=2.8* | .03 |
| Sex: n (%) | χ2=9.53* | .02 | |||||
| Male | 77 (79.4%) | 43 (93.5%) | 56 (76.7%) | 42 (75%) | 83 (71.6%) | ||
| Female | 20 (20.6%)a | 3 (6.5%)b | 17 (23.3%)a | 14 (25%)a | 33 (28.4%)a | ||
| Race: n (%) | χ2=52.6** | .06 | |||||
| Multi-racial | 0 (%) | 1 (2.2%) | 3 (4.1%) | 0 (%) | 1 (.9%) | ||
| American Indian | 1 (1.0%) | 0 (%) | 0 (%) | 0 (%) | 0 (%) | ||
| Asian | 1 (1.0%) | 0 (%) | 0 (%) | 0 (%) | 0 (%) | ||
| Black | 47 (48.5%) | 29 (63%) | 46 (63%) | 47 (83.9%) | 96 (82.8%) | ||
| White | 48 (49.5%) | 16 (34.8%) | 23 (31.5%) | 9 (16.1%) | 19 (16.4%) | ||
| Other | 0 (%) | 0 (%) | 1 (1.4%) | 0 (%) | 0 (%) | ||
| Education: M (SD) | 14.2 (2.5)b | 13.6 (2.5) | 13.3 (2.6) | 12 (2.1)a | 12.4 (2.1)a | F=11.7** | .11 |
| HIV Disease | |||||||
| Duration of HIV Infection | 167 (124) | 195 (102) | 139 (110) | 170 (119) | 161 (98) | F=.68 | .01 |
| Undetectable viral load (<50 copies/ml), % | 86 (90%)a | 33 (81%) | 57 (80%) | 33 (65%)b | 86 (76%)b | χ2=13.6** | .04 |
| Current CD4 count, median (IQR) | 556 (407) | 541 (316) | 556 (362) | 563 (446) | 492 (354) | F=1.07 | .01 |
| Nadir CD4 count, median (IQR) | 196 (327) | 185 (270) | 219 (299) | 174 (325) | 222 (301) | F=.49 | .007 |
| Health Comorbidities | |||||||
| Affective BDI-II Subscale Score: M (SD) | 4.8 (5.8)b | 3.1 (2.9)b | 5.6 (7) | 6.5 (7.3) | 7.8 (7.2)a | F=4.3** | .05 |
| Hepatitis-C Positive: n (%) | 6 (6.2%) | 6 (13%) | 7 (9.6%) | 5 (8.9%) | 9 (7.8%) | χ2=.72 | .005 |
| Substance Use KSMK Lifetime: M (SD) | |||||||
| Tobacco Total Score | 6 (5.1) | 6.9 (4.9) | 7.8 (4.2) | 7.2 (4.6) | 7 (5.0) | F=1.6 | .018 |
| Alcohol Total Score | 7.2 (3.9) | 7.8 (3.9) | 7.2 (4.2) | 7.1 (4.1) | 6 (4.3) | F=1.3 | .021 |
| Marijuana Total Score | 5.1 (4.2) | 5.1 (3.7) | 5.3 (3.7) | 6.2 (5) | 6.4 (4.6) | F=.68 | .019 |
| Cocaine Total Score | 2.9 (4.7) | 3 (4.9) | 3.4 (5.4) | 3.3 (5.3) | 3.1 (5.2) | F=.11 | .002 |
| Opiates Total Score | .14 (.92) | .25 (.75) | .11 (.50) | .33 (1.3) | .54 (1.9) | F=1.4 | .019 |
All values are reported as z scores. Descriptive statistics are reported in favor of full statistical test results in order to focus on clinically meaningful (i.e., Z-score < −1) results
M Mean, SD Standard Deviation
Hierarchical Clustering of Cognitive Performance
Hierarchical clustering revealed five cognitive subgroups. The largest subgroup (Cluster 5: n=114; 29%) was composed of study participants who performed in the impaired range (>1SD below normative values) on action fluency, verbal learning/memory, and tests of executive function. The second largest subgroup (Cluster 1: n = 97; 25%) included individuals with normative performance on all test measures. Two of the remaining clusters exhibited impairment on one test: action fluency (Cluster 2: n=46; 12%) and verbal learning/memory (Cluster 3: n = 73; 19%). Cluster 4 exhibited impairment on both action fluency and verbal learning/memory (Cluster 4: n = 56; 14%). Table 2 provides the means and standard deviations for each test by cognitive cluster.
Table 2.
Average standardized performance on the cognitive tests by cluster group
| Cluster 1 n=97 | Cluster 2 n=46 | Cluster 3 n=73 | Cluster 4 n=56 | Cluster 5 n=116 | |
|---|---|---|---|---|---|
| BVMT Total Learning: M (SD) | .30 (1.14) | .62 (1.09) | .26 (1.34) | .38 (.92) | −.11 (1.11) |
| BVMT Delay Recall: M (SD) | .45 (1.11) | .83 (1.15) | .42 (1.56) | .63 (1.17) | .08 (1.24) |
| HVLT-R Total Learning: M (SD) | −.91 (1.20) | .29 (.63) | −1.08 (1.00) | −1.32 (.54) | −1.50 (.82) |
| HVLT-R Delay Recall: M (SD) | −.84 (1.04) | .03 (.95) | −1.06 (1.07) | −1.04 (.89) | −1.31 (.79) |
| Trails A: M (SD) | .54 (1.04) | .28 (1.09) | −.16 (1.10) | .27 (.82) | −.62 (1.02) |
| Trails B: M (SD) | .62 (.91) | .78 (.97) | −.04 (1.14) | .51 (.39) | −.90 (.64) |
| Grooved Pegboard Dominant: M (SD) | −.21 (.89) | −.83 (.76) | −.51 (1.28) | −.14 (.79) | −.78 (1.01) |
| Grooved Pegboard Nondominant: M (SD) | −.07 (1.05) | −.99 (.67) | −.56 (1.32) | −.11 (.84) | −.82 (.91) |
| Color Word Interference Test: M (SD) | .15 (.89) | −.18 (1.00) | −.22 (1.03) | −.74 (1.01) | −1.23 (1.19) |
| Digit Symbol: M (SD) | .66 (.81) | .23 (1.06) | −.32 (.98) | −.53 (.67) | −.77 (.85) |
| Symbol Search: M (SD) | .57 (.86) | .15 (.82) | .08 (.88) | −.11 (.71) | −.52 (.73) |
| Letter Number Sequencing: M (SD) | .05 (.87) | .03 (.94) | −.32 (1.04) | −.49 (.93) | −1.06 (.96) |
| Action Fluency: M (SD) | .05 (.91) | −1.19 (.75) | −.53 (.81) | −1.43 (.68) | −1.51 (.70) |
| Category Fluency (Animals): M (SD) | .40 (1.13) | .36 (.85) | −.05 (1.15) | .03 (.92) | −.21 (1.06) |
| Letter Fluency: M (SD) | .31 (1.01) | .08 (.76) | .06 (.82) | −.15 (.85) | −.68 (.88) |
All values are reported as z scores. Descriptive statistics are reported in favor of full statistical test results in order to focus on clinically meaningful (i.e., Z-score < −1) results identified by the GBM rather than statistically significant (i.e., p < .05) results that may not be as readily interpretable
M Mean, SD Standard Deviation
Demographic and Psychosocial Comparisons between all Cognitive Clusters
The subgroup with normal performance on all tests (Cognitive Cluster 1) was 5-6 years older than the other Clusters, (p<.05). Cognitive Cluster 2 included significantly fewer females (6.5%) compared to all other subgroups (p<.05). Cognitive Clusters 4 and 5 were more likely to include Black individuals compared to Clusters 1, 2, and 3 (p<.01). Clusters 4 and 5 achieved 1-2 fewer years of education than clusters 1, 2, and 3 (p<.01). The normative subgroup (Cognitive Cluster 1) was significantly more likely to be virally suppressed compared to the other subgroups (p<.01). The impaired subgroup (Cognitive Cluster 5) reported more affective symptoms of depression on the BDI-II (p< .01) compared to the other subgroups. Duration of HIV infection, current CD4 count, nadir CD4 count, and KMSK lifetime substance use scores did not differ by subgroup.
Comparisons between the two Clusters representing the largest percentage of the sample (Cognitive Cluster 1; normal performance and Cognitive Cluster 5; impaired performance) revealed that individuals in the impaired group were younger (p<.05), more likely to identify as Black (p<.01), reported fewer years of educational attainment (p<.05), were more likely to have detectable HIV viral load (p<.01), and had a positive urine toxicology result for stimulants when compared to individuals in the Cognitive Cluster 1. The impaired group also had lower amygdala volume on the left and lower volume in the posterior middle frontal gyrus (ps<.01). Duration of HIV infection (p=.71), total years on ART (p=.35), nadir CD4 (p=.49), and sex (p=.22) did not differ between Cognitive Cluster 1 and Cognitive Cluster 5.
Data-derived Substance Clusters from Urine Toxicology Results
The percentage of positive urine toxicology results per drug class is provided in Online Resource 1. The drug use clusters included: 1) Substance Cluster 1: cannabis and prescription drugs (e.g., benzodiazepines and tricyclics); Substance Cluster 2: stimulants, cannabis, and prescription drugs; Substance Cluster 3); cannabis and benzodiazepines; Substance Cluster 4: cannabis and cocaine.
Machine Learning Derived Determinants of Normal vs. Impaired Cognitive Subgroups
The univariate GBM classifying individuals into the cognitively normal vs. impaired subgroups achieved an average AUC of 79% (Accuracy=70%, F1 score=67%, Precision=70%, and Recall=64%; Fig. 1, left panel). The features in the model included: 1) lower volume in the left amygdala; 2) lower volume in the left posterior middle frontal gyrus; 3) lower educational attainment; 4) non-White race; 5) Substance Cluster 1 (positive urine toxicology for cannabis, benzodiazepines, and tricyclics); and 6) Black race. Figure 2 illustrates the features in the classification model and the MIC value for each predictor. Brain volumes that were identified as top classifiers are illustrated in Fig. 3.
Fig. 1.
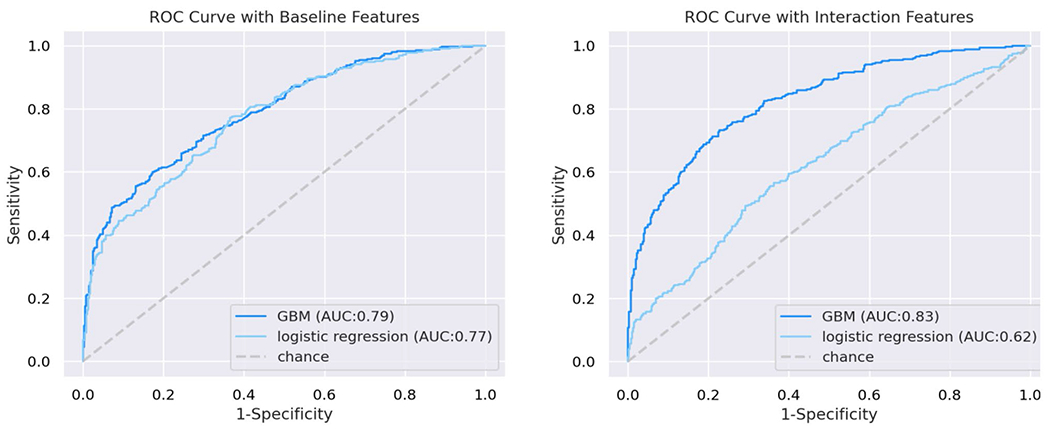
Receiver operator curves demonstrating area under the curve derived from the gradient boosted multivariate regression model (GBM) and logistic regression; left panel: univariate features; right panel: two-way interactive features
Fig. 2.
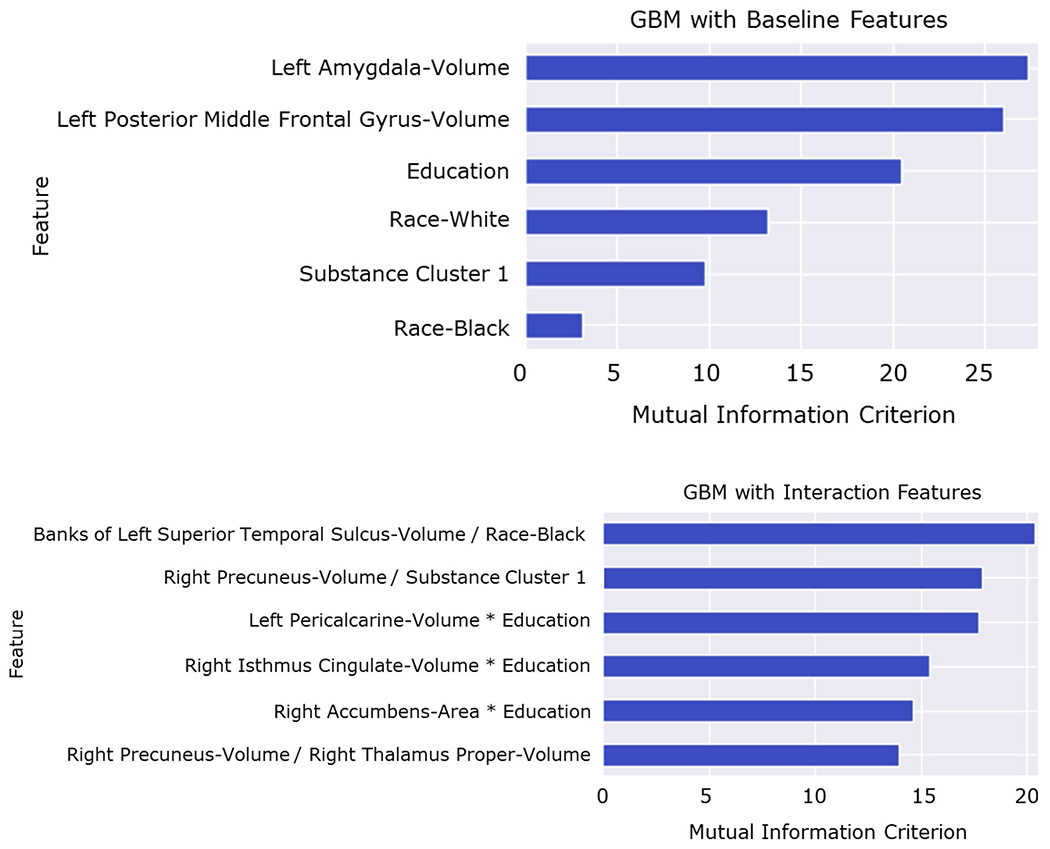
Univariate (top) and two-way interactive (bottom) predictors of cognitive subgroups; features are listed in descending order according to mutual information criterion (MIC), a metric that describes the relative contribution of each feature to the model; interaction terms capture both linear and nonlinear combinations (see Online Resources 3 and 4); results suggest prominent contributions of brain volumetrics, demographics, and substance use in the delineation of cognitive subgroups
Fig. 3.
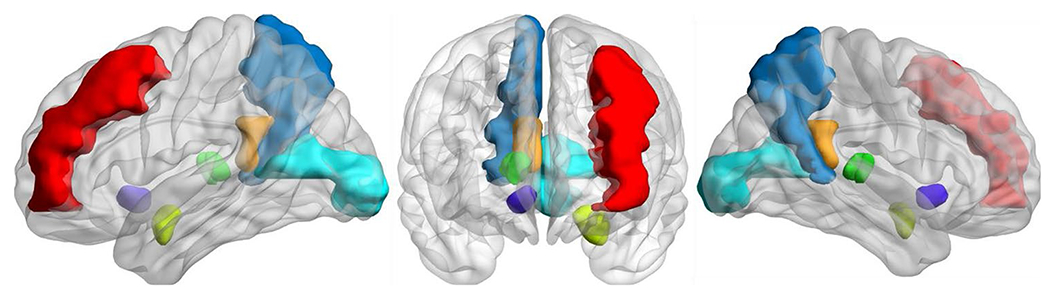
Volumetric brain regions identified by the GBM as significant predictors of cognitive phenotype are represented in a 3-dimensional visualization; many of these regions have been implicated in prior research on substance use craving, withdrawal, and relapse; brain regions identified by the univariate GBM included the left middle frontal gyrus (red) and left amygdala (light green); the interactive GBM included the right precuneus (blue), left pericalcarine gyrus (teal), right isthmus cingulate (orange), right thalamus (bright green), and the right nucleus accumbens (purple)
The GBM with two-way interactions yielded an average AUC of 83% (Accuracy=75%, F1 score=73%, Precision=74%, and Recall=72%; Fig. 1, right panel). The model included the following features: 1) lower volume of the left superior temporal banks combined with Black race; 2) lower right precuneus volume combined with Substance Cluster 1; 3) lower left peri-calcarine volume combined with lower educational attainment; 4) lower right cingulate isthmus volume combined with lower educational attainment; 5) lower right nucleus accumbens area combined with lower educational attainment; and 6) lower volume of the right precuneus combined with lower volume of the thalamus proper. Figure 2 provides the MIC value for the interactive predictors of the impaired cognitive phenotype. See Online Resource 2 for complete model performance metrics, Online Resource 3 for partial dependency plots depicting top features differentiating membership in normative vs. impaired cognitive performance clusters identified by the univariate GBM, and Online Resource 4 for heatmaps depicting color-coded probabilities of membership in normative vs. impaired cognitive performance clusters identified by the interactive GBM.
Discussion
This is the first study to leverage the analytic strengths of hierarchical clustering and ensemble machine learning to identify novel cognitive phenotypes of HIV and the combination of explanatory variables that distinguish cognitively normal vs. impaired profiles. Study findings revealed five discrete cognitive subgroups that differed in the presence and severity of impairment. Our data-driven approach identified a gradient of cognitive status that ranged from normal cognitive performance to impairment in multiple domains. Verb fluency and verbal learning/memory differentiated individuals into one of the four cognitive impaired subgroups. Machine learning revealed that the strongest classifiers of cognitive status included psychosocial measures and social determinants of health (e.g., race, education), as well as history of substance use, and volumes of brain regions that are implicated in addiction. Additionally, individuals with impairment in multiple cognitive domains were most likely to have detectable HIV.
Hierarchical clustering, with the UMAP variant revealed a larger number of distinct cognitive subgroups compared to results from prior studies (Fazeli et al. 2014; Ham et al. 2019). The larger number of subgroups identified in the current study likely reflects the analytic strengths of our clustering approach, a method that allowed for detection of nonlinear patterns in the data (e.g., U-shaped and/or multinomial distributions). Additionally, the clustering method did not force poorly fitting data observations into unrelated clusters, as is the case with other cluster methods (e.g., K-means; Raykov et al. 2016). We believe the methodological strengths of the clustering model utilized in the present study provides a robust approach to interrogate and explain the heterogeneity in cognitive performance that exists among PWH, though additional studies are needed in separate cohorts to confirm the clinical utility of the finding.
Twenty-five percent of the sample exhibited normal performance on each cognitive test, whereas nearly the same percentage of individuals exhibited impairment on multiple tests. This finding underscores the heterogeneity in cognitive status among PWH receiving ART. Tests of action fluency, verbal learning/memory, and executive function differentiated membership across the cognitive subgroups, with a clear gradient of impairment evident across the groups. Verb fluency is sensitive to frontal-subcortical dysfunction (Piatt et al. 1999), and numerous studies demonstrate worse verb fluency among PWH when compared to normative data (Woods et al. 2005a, 2006; Iudicello et al. 2007). Action fluency can be administered and scored in less than 5 minutes, and the test does not require equipment or proprietary materials. Furthermore, descriptions of actions are familiar to individuals from a wide variety of cultures and geographic regions. Collectively, these attributes make action fluency a good candidate for inclusion in international test protocols aimed at establishing a common data element approach to support neuroHIV research.
An important contribution from the current study is the delineation of distinct cognitive subgroups of PWH. Using a data-driven approach, our findings underscore the heterogeneity of cognitive performance across PWH. This finding is relevant for efforts focused on updating the Frascati criteria for HAND, which currently does not include information on cognitive phenotypes. Based on our results, HAND may manifest as one of several combinations of impairment in specific cognitive domains. This differs from other neurologic disorders that are characterized by a dominant cognitive phenotype, such as amnestic memory impairment in Alzheimer’s disease (Albert 1996; Salmon and Bondi 2009; Milanini and Valcour 2017) and executive dysfunction in subcortical ischemic vascular disease (Kramer et al. 2002; Ljubenkov and Geschwind 2016; Paul et al. 2022).
In addition to identifying unique cognitive subgroups, the current study explored putative determinants of cognitive subgroup membership. Results from the machine learning analysis revealed that the strongest classifiers of membership in Cluster 5 (cognitively impaired) vs. Cluster 1 (cognitively normal) included a combination of psychosocial factors and social determinants of health rather than traditional clinical metrics of HIV disease. This is not to say that viral detectability is irrelevant, as individuals in Cognitive Cluster 5 (impaired group) were more likely to have detectable virus. However, viral detectability did not emerge as a top classification feature in either the univariate or the interactive GBM. It is possible that the relevance of viremia was masked by other features in the models. Nevertheless, the results from the current study emphasize the multifactorial nature of risk for cognitive impairment among PWH receiving ART.
Substance use also emerged as an important classifier of cognitive subgroup membership. Furthermore, the brain regions selected by the classification models (i.e., nucleus accumbens, amygdala, superior temporal gyrus, hippocampus, precuneus, cingulate, and thalamus) have been implicated in substance use craving, withdrawal, and relapse (Goldstein and Volkow 2002; Rippeth et al. 2004; Soontornniyomkij et al. 2016; Tannous et al. 2019). Work by Zhang and Volkow suggest that these brain regions are linked to substance use behavior through subsystems of the default mode network (DMN; Zhang and Volkow 2019). This may explain the interaction between Substance Cluster 1 and volume of the precuneus volume, which is a key brain region for DMN connectivity.
It is well established from our team and others that stimulant users experience difficulties achieving and maintaining viral suppression. As such, it is not surprising that individuals in this study with the most severe cognitive impairment (Cluster 5) included a higher proportion of substance users and individuals with detectable viral load (Carrico et al. 2019; Feelemyer et al. 2020; Fulcher et al. 2021). While viremia is understood as an important risk factor for persistent cognitive symptoms among PWH receiving ART, results from the current investigation highlight the relative contribution of neurobehavioral factors that may confer increased risk for viremia. Longitudinal studies are needed to examine the potential bidirectional associations between neurobehavioral alterations and unsuppressed viral load in stimulant users with HIV. Understanding the neurobehavioral vulnerabilities that possibly confer amplified risk for viremia in the context of suppressive treatment has implications for the deployment of comprehensive approaches to improve health outcomes (e.g., treatment as prevention efforts).
The present study explored cognitive phenotypes using a traditional HIV-centric protocol. The battery of tests administered in the current investigation was sufficient to identify distinct cognitive subgroups. Nevertheless, it is possible that alternative measures could offer additional explanatory information related to discrete cognitive subgroups. For example, Martin et al. (2019) reported differential sensitivity to HIV serostatus using a measure of probability learning vs. substance use using delayed discounting. These results suggest that in addition to action fluency, measures sensitive to the cognitive sequelae of substance use such as delayed discounting, merit consideration as a common data element for use in neuroHIV research.
Educational attainment was a prominent feature in both the univariate and the interactive GBM models. Education and race are well known social determinants of performance on standardized cognitive tests (Manly et al. 2004; Mindt et al. 2014). Results from prior studies demonstrate that educational experience (both years of attainment and quality of education) account for a significant degree of variability in cognitive performance between White and non-White individuals regardless of HIV status (Manly et al. 1998; Byrd et al. 2006; Amariglio et al. 2020; Eng et al. 2021). Failure to account for these effects in studies of HIV-associated cognition has potential to inflate the frequency and/or severity of cognitive impairment among racially diverse samples (Paul et al. 2021).
Additional studies are needed to examine cognitive trajectories. Dastgheyb et al. (2019) applied principal component analysis and K-means clustering to define subgroups of PWH who exhibited longitudinal decline on select cognitive tests. Results revealed four clusters with specific cognitive decline on: 1) verbal fluency, 2) learning and recall, 3) executive function, or 4) motor function. The four clusters did not differ on measures of HIV disease severity (e.g., CD4+ T-cell count, viral detectability), age, education, or distribution of sex. However, the subgroup demonstrating decline on tests of verbal fluency included a disproportionate percentage of non-White participants, a finding that bolsters the results of the current analysis.
Limitations of the study merit attention. The current investigation was cross-sectional in nature, and therefore, causal pathways cannot be established. Further, the study sample included individuals with variable degrees of viral suppression and heterogeneous histories of substance use. As such, the results may not generalize to clinical cohorts with sustained viral suppression or cohorts without a history of substance use. Additionally, detailed information related to prior exposure to cognitive testing was not available. While it is plausible that some individuals benefited from practice effects based on prior exposure to the cognitive tests, this would not significantly influence the current findings given that the impaired group performed below expectations on multiple measures. There did also exist some variability in the latency between MRI and cognitive/clinical/health data collection (i.e., ≤ 3 months), but we did not examine this directly in analyses. Finally, it is plausible that acute intoxication from illicit substances negatively affected performance on the cognitive tests for individuals with reactive urine screens. However, the observation that brain metrics relevant to the addiction network emerged as salient classifiers provides additional assurance that impairment on the cognitive tests for the participants in cluster 5 were not due to acute intoxication.
In summary, we identified cognitive impairment in nearly one-third of individuals with HIV receiving ART. Variables that differentiated individuals with normal vs. impaired cognitive performance included regional brain volumes involved in addiction, substance use history, Black race, lower educational, and detectable viral load. Interventions aimed at bolstering cognitive reserve and reducing the burden of substance are mandatory to achieve global health initiatives for PWH. Results from the current study support the inclusion of verb fluency, verbal learning and memory, and possibly delayed discounting as common data set elements to facilitate the harmonization of neuroHIV data across studies. Finally, more attention is needed to develop cognitive tests that do not artificially inflate the frequency and/or severity of cognitive impairment in racially and ethnically diverse populations.
Supplementary Material
Funding
BA received funding from the National Institute of Mental Health (R01MH118031; K23MH08175) and National Institute of Nursing Research (R01NR015738; R01NR012907; R01NR012657; R01NR014449) and National Institute of Drug Abuse (R01DA054009). RP received funding from the National Institute of Mental Health (R01MH113560).
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11481-021-10045-0.
Ethics Approval The research was approved by the participating institutions.
Consent to Participate Participants provided written informed consent prior to enrollment in the parent studies.
Consent for Publication Not applicable
Conflicts of Interest/Competing Interests The authors report no conflicts of interest.
Availability of Data and Material
Data are available upon request and execution of an authorized and signed data sharing agreement.
References
- Albert MS (1996) Cognitive and neurobiologic markers of early Alzheimer disease. Proc Natl Acad Sci U S A 93:13547–13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio RE, Buckley RF, Rabin JS, Papp KV, Quiroz YT, Mormino EC, Sparks KP, Johnson KA, Rentz DM, Sperling RA (2020) Examining Cognitive Decline Across Black and White Participants in the Harvard Aging Brain Study. J Alzheimers Dis 75:1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Army U.S (1944) Army individual test battery. Manual of directions and scoring [Google Scholar]
- Beck AT, Steer RA, Brown G (1996) Beck depression inventory–II. Psychological Assessment [Google Scholar]
- Benedict RH (1997) Brief visuospatial memory test-revised: PAR [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J (1998) Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 12:43–55 [Google Scholar]
- Brandt J, Benedict RH (2001) Hopkins verbal learning test--revised: professional manual: Psychological Assessment Resources [Google Scholar]
- Byrd DA, Walden Miller S, Reilly J, Weber S, Wall TL, Heaton RK (2006) Early environmental factors, ethnicity, and adult cognitive test performance. Clin Neuropsychol 20:243–260 [DOI] [PubMed] [Google Scholar]
- Carrico AW, Hunt PW, Neilands TB, Dilworth SE, Martin JN, Deeks SG, Riley ED (2019) Stimulant Use and Viral Suppression in the Era of Universal Antiretroviral Therapy. J Acquir Immune Defic Syndr 80:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastgheyb RM, Sacktor N, Franklin D, Letendre S, Marcotte T, Heaton R, Grant I, McArthur JC, Rubin LH, Haughey NJ (2019) Cognitive Trajectory Phenotypes in Human Immunodeficiency Virus-Infected Patients. J Acquir Immune Defic Syndr 82:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001) Delis-Kaplan Executive Function System: Examiner’s Manual. The Psychological Corporation, San Antonio, TX [Google Scholar]
- dos Santos JA, Syed TI, Naldi MC, Campello RJ, Sander J (2015) Hierarchical density-based clustering using MapReduce. Journal of latex class files 14 [Google Scholar]
- Eng CW, Glymour MM, Gilsanz P, Mungas DM, Mayeda ER, Meyer OL, Whitmer RA (2021) Do the Benefits of Educational Attainment for Late-life Cognition Differ by Racial/Ethnic Group?: Evidence for Heterogenous Treatment Effects in the Kaiser Healthy Aging and Diverse Life Experience (KHANDLE) Study. Alzheimer Dis Assoc Disord 35:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PL, Crowe M, Ross LA, Wadley V, Ball K, Vance DE (2014) Cognitive Functioning in Adults Aging with HIV: A Cross-Sectional Analysis of Cognitive Subtypes and Influential Factors. J Clin Res HIV AIDS Prev 1:155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelemyer J, Arasteh K, Huong DT, Oanh KTH, Khue PM, Giang HT, Thanh NTT, Moles JP, Vinh VH, Vallo R, Quillet C, Rapoud D, Le SM, Michel L, Laureillard D, Nagot N, Des Jarlais DC, Drive Study T (2020) Associations between methamphetamine use and lack of viral suppression among a cohort of HIV-positive persons who inject drugs in Hai Phong. Vietnam. AIDS 34:1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher JA, Javanbakht M, Shover CL, Ragsdale A, Brookmeyer R, Shoptaw S, Gorbach PM (2021) Comparative impact of methamphetamine and other drug use on viral suppression among sexual minority men on antiretroviral therapy. Drug and Alcohol Dependence 221:108622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK (1999) Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment 6:147–178 [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM (1978) Stroop color and word test [Google Scholar]
- Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I et al. (2014) Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 82:2055–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham L, Snow J, Agan BK, Smith B, Nath A, Won SH, Chu X, Horne EF, Norato G, Mburu T (2019) Cluster analysis of cognitive functioning in HIV+ and HIV− subjects. Abstract, CROI, Seattle, Washington [Google Scholar]
- Heaton RK (2004) Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults, professional manual: Psychological Assessment Resources [Google Scholar]
- Heaton RK et al. (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 60:473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Parsons TD, Moran LM, Carey CL, Grant I (2007) Verbal fluency in HIV infection: a meta-analytic review. J Int Neuropsychol Soc 13:183–189 [DOI] [PubMed] [Google Scholar]
- Jiangchun L (2019) SauceCat/PDPbox. In [Google Scholar]
- Kallianpur KJ, Jahanshad N, Sailasuta N, Benjapornpong K, Chan P, Pothisri M, Dumrongpisutikul N, Laws E, Ndhlovu LC, Clifford KM (2020) Regional brain volumetric changes despite 2 years of treatment initiated during acute HIV infection. AIDS 34:415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ (2003) The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend 69:137–150 [DOI] [PubMed] [Google Scholar]
- Kore I, Ananworanich J, Valcour V, Fletcher JL, Chalermchai T, Paul R, Reynolds J, Tipsuk S, Ubolyam S, Rattanamanee S, Jagodzinski L, Kim J, Spudich S, Group RSS (2015) Neuropsychological Impairment in Acute HIV and the Effect of Immediate Antiretroviral Therapy. J Acquir Immune Defic Syndr 70:393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Reed BR, Mungas D, Weiner MW, Chui HC (2002) Executive dysfunction in subcortical ischaemic vascular disease. J Neurol Neurosurg Psychiatry 72:217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W (2003) Classification and regression tree analysis in public health: methodological review and comparison with logistic regression [DOI] [PubMed] [Google Scholar]
- Ljubenkov PA, Geschwind MD (2016) Dementia. Semin Neurol 36:397–404 [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Ferman TJ, Willis FB, Petersen RC, Graff-Radford NR (2005) Mayo’s older African Americans normative studies: Norms for Boston naming test, controlled oral word association, category fluency, animal naming, token test, wrat-3 reading, trail making test, Stroop test, and judgment of line orientation. Clin Neuropsychol 19:243–269 [DOI] [PubMed] [Google Scholar]
- Manly JJ, Byrd DA, Touradji P, Stern Y (2004) Acculturation, reading level, and neuropsychological test performance among African American elders. Appl Neuropsychol 11:37–46 [DOI] [PubMed] [Google Scholar]
- Manly JJ, Miller SW, Heaton RK, Byrd D, Reilly J, Velasquez RJ, Saccuzzo DP, Grant I (1998) The effect of African-American acculturation on neuropsychological test performance in normal and HIV-positive individuals. The HIV Neurobehavioral Research Center (HNRC) Group. J Int Neuropsychol Soc 4:291–302 [PubMed] [Google Scholar]
- Martin EM, Gonzalez R, Vassileva J, Bechara A (2019) Double dissociation of HIV and substance use disorder effects on neurocognitive tasks dependent on striatal integrity. AIDS 33:1863–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C, Klove K (1964) Instruction Manual for the Adult Neuropsychology Test Battery. University of Wisconsin Medical School, Madison, WI [Google Scholar]
- McInnes L, Healy J, Melville J (2018) UMAP: Uniform manifold approximation and projection for dimension reduction. arXiv preprint arXiv:180203426 [Google Scholar]
- Milanini B, Valcour V (2017) Differentiating HIV-Associated Neurocognitive Disorders from Alzheimer’s Disease: an Emerging Issue in Geriatric NeuroHIV. Curr HIV/AIDS Rep 14:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller PJ, Lubke GH, McArtor DB, Bergeman C (2016) Finding structure in data using multivariate tree boosting. Psychol Methods 21:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindt MR, Miranda C, Arentoft A, Byrd D, Monzones J, Fuentes A, Arias F, Rentería MA, Rosario A, Morgello S (2014) Aging and HIV/AIDS: neurocognitive implications for older HIV-positive Latina/o adults. Behav Med 40:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr, Cysique L, Ake C, Lazarretto D, Vaida F, Heaton RK, Group H (2011) Demographically corrected norms for African Americans and Caucasians on the Hopkins verbal learning test–revised, brief visuospatial memory test–revised, Stroop color and word test, and Wisconsin card sorting test 64-card version. J Clin Exp Neuropsychol 33:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Halloran JA, Cooley SA, Strain JF, Boerwinkle A, Paul R, Presti RM, Ances BM (2019) Altered neuropsychological performance and reduced brain volumetries in people living with HIV on integrase strand transfer inhibitors. AIDS 33:1477–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Rhee G, Baker LM, Vaida F, Cooley SA, Ances BM (2017) Effort and neuropsychological performance in HIV-infected individuals on stable combination antiretroviral therapy. J Neurovirol 23:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Cooley SA, Garcia-Egan PM, Ances BM (2018) Cognitive Performance and Frailty in Older HIV-Positive Adults. J Acquir Immune Defic Syndr 79:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Cho KS, Luckett P, Strain JF, Belden AC, Bolzenius JD, Navid J, Garcia-Egan PM, Cooley SA, Wisch JK, Boerwinkle AH, Tomov D, Obosi A, Mannarino JA, Ances BM (2020a) Machine Learning Analysis Reveals Novel Neuroimaging and Clinical Signatures of Frailty in HIV. J Acquir Immune Defic Syndr 84:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH et al. (2020b) Machine-learning classification of neurocognitive performance in children with perinatal HIV initiating de novo antiretroviral therapy. AIDS 34:737–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Tsuei T, Cho K, Belden A, Milanini B, Bolzenius J, Javandel S, McBride J, Cysique L, Lesinski S, Valcour V (2021) Ensemble machine learning classification of daily living abilities among older people with HIV. EClinicalMedicine 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Salminen LE, McBride JAD (2022) Everyday functioning in vascular cognitive impairment. In: Marcotte TD (ed) Neuropsychology of everyday functioning, 2nd edn. Guilford Press, New York. In press [Google Scholar]
- Pedregosa FVG, Michel V et al. (2011) Scikit-learn: machine learning in Python. J Mach Learn Res 12:2825–2830 [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Tröster AI (1999) Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia 37:1499–1503 [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Tröster AI (2004) Action verbal fluency normative data for the elderly. Brain and language 89:580–583 [DOI] [PubMed] [Google Scholar]
- Raykov YP, Boukouvalas A, Baig F, Little MA (2016) What to Do When K-Means Clustering Fails: A Simple yet Principled Alternative Algorithm. PLoS One 11:e0162259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I (2004) Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc 10:1–14 [DOI] [PubMed] [Google Scholar]
- Salmon DP, Bondi MW (2009) Neuropsychological assessment of dementia. Annu Rev Psychol 60:257–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soontornniyomkij V, Kesby JP, Morgan EE, Bischoff-Grethe A, Minassian A, Brown GG, Grant I (2016) Effects of HIV and Methamphetamine on Brain and Behavior: Evidence from Human Studies and Animal Models. J Neuroimmune Pharmacol 11:495–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous J, Mwangi B, Hasan KM, Narayana PA, Steinberg JL, Walss-Bass C, Moeller FG, Schmitz JM, Lane SD (2019) Measures of possible allostatic load in comorbid cocaine and alcohol use disorder: Brain white matter integrity, telomere length, and anti-saccade performance. PLoS One 14:e0199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L (1999) Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 14:167–177 [PubMed] [Google Scholar]
- Wechsler D (1997) WAIS-III administration and scoring manual. The Psychological Corporation, San Antonio, TX [Google Scholar]
- Woods SP, Carey CL, Troster AI, Grant I, Group HIVNRC (2005a) Action (verb) generation in HIV-1 infection. Neuropsychologia 43:1144–1151 [DOI] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Tröster AI, Group HNRC (2005b) Action (verb) fluency: Test–retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc 11:408–415 [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Dawson M, Cobb Scott J, Grant I, Group HIVNRC (2006) Action (verb) fluency predicts dependence in instrumental activities of daily living in persons infected with HIV-1. J Clin Exp Neuropsychol 28:1030–1042 [DOI] [PubMed] [Google Scholar]
- Zhang R, Volkow ND (2019) Brain default-mode network dysfunction in addiction. Neuroimage 200:313–331 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request and execution of an authorized and signed data sharing agreement.


