Abstract
Expression of the catalase-peroxidase of Caulobacter crescentus, a gram-negative member of the α subdivision of the Proteobacteria, is 50-fold higher in stationary-phase cultures than in exponential cultures. To identify regulators of the starvation response, Tn5 insertion mutants were isolated with reduced expression of a katG::lacZ fusion on glucose starvation. One insertion interrupted an open reading frame encoding a protein with significant amino acid sequence identity to TipA, a helix-turn-helix transcriptional activator in the response of Streptomyces lividans to the peptide antibiotic thiostrepton, and lesser sequence similarity to other helix-turn-helix regulators in the MerR family. The C. crescentus orthologue of tipA was named skgA (stationary-phase regulation of katG). Stationary-phase expression of katG was reduced by 70% in the skgA::Tn5 mutant, and stationary-phase resistance to hydrogen peroxide decreased by a factor of 10. Like the wild type, the skgA mutant exhibited starvation-induced cross-resistance to heat and acid shock, entered into the helical morphology that occurs after 9 to 12 days in stationary phase, and during exponential growth induced katG in response to hydrogen peroxide challenge. Expression of skgA increased 5- to 10-fold in late exponential phase. skgA is the first regulator of a starvation-induced stress response identified in C. crescentus. SkgA is not a global regulator of the stationary-phase stress response; its action encompasses the oxidative stress-hydrogen peroxide response but not acid or heat responses. Moreover, SkgA is not an alternative ς factor, like RpoS, which controls multiple aspects of starvation-induced cross-resistance to stress in enteric bacteria. These observations raise the possibility that regulation of stationary-phase gene expression in this member of the α subdivision of the Proteobacteria is different from that in Escherichia coli and other members of the γ subdivision.
Bacterial stationary phase, a growth arrest that can be initiated by nutrient limitation, is a state of stress readiness (12, 27). In response to starvation, cultures become resistant to multiple stresses, including heat, acidity, hyperosmolarity, and hydrogen peroxide (21). In addition, patterns of gene expression that lead to secondary metabolites, changes in morphology, and acquisition of virulence traits are initiated in stationary phase (15). In Escherichia coli and Salmonella spp., RpoS, the ςS subunit of RNA polymerase, is a central regulator of stationary-phase gene expression. RpoS levels increase during exit from exponential growth and entrance into stationary phase. In E. coli, the rpoS regulon includes genes involved in stress response, metabolic changes, and morphological alterations (12, 18). In Salmonella sp., rpoS is a pathogenicity factor (26). Variations on the enteric bacterial rpoS paradigm are found in Yersinia enterocolitica, where rpoS controls starvation-induced stress but not stationary-phase expression of two virulence factors.
Given the diversity of bacterial responses in stationary phase, regulators other than rpoS might be anticipated. It is estimated that expression of ≈20% of all genes increases in E. coli stationary phase but that only ≈10% of the increases are dependent on rpoS (32). Homologues of rpoS have been identified for 18 gram-negative species in the γ subdivision of the Proteobacteria (purple bacteria), which includes E. coli and other enteric bacteria. No homologues have been reported for the α subdivision. Caulobacter crescentus, a gram-negative organism in the α subdivision, acquires resistance to heat, hydrogen peroxide, alkali, and acid in stationary phase in a fashion very similar to that of starvation-induced resistance to stress in enteric bacteria (41). After 9 to 12 days of starvation, C. crescentus enters a new morphological pathway, forming helical structures 15 to 20 times longer than the stalked and swarmer cells found in exponential cultures. Attempts to clone an rpoS homologue in C. crescentus by complementation of an E. coli null strain containing an rpoS-dependent lacZ fusion have been unsuccessful (37). No nucleic acid sequence homologue of rpoS is present in the C. crescentus genome being sequenced by The Institute for Genomic Research.
We initiated a search for regulators of the C. crescentus stationary-phase response based on our prior studies on katG encoding C. crescentus catalase-peroxidase. During exponential growth and entrance into stationary phase, katG is expressed at a constant level. During the ensuing 24 to 72 h, katG expression increases 10- to 90-fold as determined by LacZ activity of a katG::lacZ translational fusion and by measurement of peroxidase activity in extracts (33, 38). We identified transposon-insertion mutants with attenuated expression of a katG::lacZ fusion on glucose starvation. One mutant contained an insertion in a gene encoding a putative helix-turn-helix transcriptional regulator named skgA (stationary-phase regulation of katG). Characterization of the growth-stage dependence of skgA expression and phenotypes of the skgA::Tn5 mutant strain indicate that SkgA is a regulator of the starvation-induced resistance to H2O2 but not a master regulator of the starvation-induced stress response in the manner of rpoS. C. crescentus and by extension other members of the α subdivision may not conform to the rpoS paradigm of enteric bacteria in which a single regulator controls a broad spectrum of starvation-induced responses.
MATERIALS AND METHODS
Media and growth conditions.
E. coli DH5α was grown in Luria-Bertani broth at 37°C (34). C. crescentus was grown at 30°C in peptone-yeast extract (PYE) medium containing 0.5 mM CaCl2 and 0.8 mM MgSO4. M2 salts for C. crescentus contained 0.5 mM CaCl2 and 0.5 mM MgSO4 (8, 17). Drug concentrations were as follows: for E. coli, 50 μg of streptomycin sulfate per ml and 100 μg of sodium ampicillin per ml; for C. crescentus, 2 μg of tetracycline hydrochloride per ml, 5 μg of kanamycin sulfate per ml, 25 μg of streptomycin sulfate per ml, and 20 μg of nalidixic acid per ml. Strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference(s) or source |
|---|---|---|
| Strains | ||
| E. coli S17-1 (λpir) | recA::RP4-2-Tc::Mu Km::Tn7 λpir | 5, 13, 35 |
| C. crescentus | ||
| CB15N | Synchronizable mutant of CB15; syn-1000 (= NA1000) | 9 |
| LS800 | CB15N recA526 with pKM3001 | 24, 28 |
| SGC103 | CB15N recA526 katG+ katG::lacZ translational fusion with pKM3001 | 38 |
| SGC109 | CB15N recA526 katG+ katG::lacZ translational fusion | 38 |
| SGC111 | CB15N katG17::(Spcr/Smr) katG null | 38 |
| SGC125 | SGC109 random Tn5 (Spcr/Smr); control Tn5 insertion strain | This study |
| SGC126 | SGC109 skgB::Tn5 | This study |
| SGC127 | SGC109 skgA::Tn5 | This study |
| SGC130 | CB15N recA526 skgA+ skgA144::lacZ translational fusion | This study |
| Plasmids | ||
| pJBZ 282 | Kmr; ori ColE1; for construction of translational lacZ fusions | 2 |
| pUT-mini-Tn5 Spc/Sm | Apr Spcr Smr; ori R6K; mob RP4 tnp* of Tn5-IS50R | 5 |
Transposon mutagenesis.
The translational fusion strain SGC109 katG::lacZ (38) was mutagenized with Tn5 (Spcr/Smr) introduced by conjugation (22). The donor was E. coli S17-1 λpir with pUT-mini-Tn5 (Spcr/Smr), an ori R6K plasmid which fails to replicate in C. crescentus (5). Overnight cultures from 12 isolated colonies of SGC109 (0.5 ml each) were mixed with 12 mid-exponential-phase cultures of the S17-1 λpir donor from 12 isolated colonies (0.5 ml each) and incubated for 4 h at 30°C on 0.22-μm-pore-size filters on PYE plates (8, 38). Plating on PYE-nalidixic acid-streptomycin yielded a library of 12,000 Tn5 insertion mutants. The frequency of transposition based on Smr was 10−6.
Screening for mutants with reduced katG induction in stationary phase.
The Tn5 insertion library was plated on M2 minimal medium with 0.02% glucose (1/15 of the standard concentration), X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 1 mg/plate), and streptomycin. Similar glucose starvation plates were used when E. coli rpoS was identified as a starvation-inducible gene fusion following transposon mutagenesis with promoterless lacZ (19). Unmutagenized strain SGC109 formed minute dark blue colonies (0.1 to 0.2 mm) on this medium without streptomycin. White or light blue Tn5 insertion mutants were picked from the Tn5 insertion library after 3 days at 30°C and then 3 days at room temperature and confirmed by restreaking. Dark blue Tn5 insertion mutants, with an intensity similar to that of unmutagenized SGC109, were chosen as control strains.
Transduction.
Published protocols were used for preparation of transducing lysates and transduction with phage φ CR30 (8). Plate lysates of the donor strain (≥109 PFU/ml) were irradiated by using the germicidal lamp of a tissue culture hood. Infections were performed with a multiplicity of infection of 0.2 to 2:1 based on the titer before irradiation. Drug-resistant transductants were obtained at a frequency of 10−6 to 10−7 per infected cell.
Transduction was used to determine if white mutants arose from Tn5 (Spcr/Smr) insertion in the katG::lacZ fusion. The katG::lacZ fusion strain was constructed by chromosomal integration of a nonreplicating Kmr 6.9-kbp ColE1 plasmid containing 5.7 kbp of C. crescentus DNA from the katG locus and its upstream region. Wild-type C. crescentus was transduced to Smr with φ CR30 lysates prepared from individual Tn5 insertion mutants and scored for Kmr. All Smr transductants were Kms. Since the packaging size for φ CR30 (100 to 200 kbp) far exceeds the 12.6 kbp of the integrated plasmid, it is highly unlikely that white mutants were attributable to Tn5 insertion in the lacZ reporter.
Tn5 insertion sites were transduced back into the original katG::lacZ fusion strain to establish linkage between the phenotypes of Tn5 (Spcr/Smr) insertion mutants and the transposon insertion. The fusion strain that was mutagenized, SGC109, had been made recA to minimize recombination between the tandem wild-type katG and the katG::lacZ fusion allele. Since recA+ is required for transduction, the following strategy was used to obtain the desired transductants. Strain SGC103 contains the katG::lacZ fusion, is chromosomally recA, and contains pKM3001 with recA+ of Pseudomonas aeruginosa on Tcr plasmid pCP13 (28, 38). Strain SGC103 was transduced to Smr Tcr by using lysates from the Tn5 insertion mutants. Drug-resistant transductants were grown overnight in the absence of tetracycline to favor loss of pCP13 recA+, and Tcs recA colonies were isolated (24, 38). These strains, isogenic with the katG::lacZ fusion strain SGC109 except for the Tn5 insertion, were used for physiological characterizations. The control strain was made by transducing SGC103 to Smr with a lysate from a randomly picked dark blue colony in the initial screen, followed by loss of the recA+ plasmid as described above.
Cloning the Tn5 insertion sites of the skgA and skgB mutations.
Genomic Southern blots were probed with the Ω (Spcr/Smr) cassette of Tn5 to determine the sizes of restriction fragments containing the insertion sites. Fragments of the desired size were isolated following agarose gel electrophoresis of genomic DNA digested with EagI and SalI for skgA and skgB, respectively. Clones in pBluescript, identified by Smr, contained about 0.5 and 1 kbp of C. crescentus genomic DNA adjacent to Tn5.
Cloning the wild-type skgA allele.
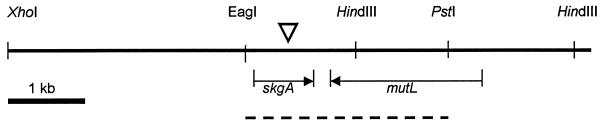
The wild-type allele was cloned from a cosmid library of 20- to 30-kb inserts in pLAFR5 represented by ≈1,340 colonies in grids on 28 plates (2). Southern blots, probed with a restriction fragment containing ≈600 bp of the skgA 5′ sequence, were used to identify the plate and then the colony containing the skgA cosmid. Two fragments, identified by hybridization to oligonucleotides from sequenced regions, were subcloned from the cosmid into pBluescript. One contained the skgA gene and in addition ≈3 kb of upstream sequence and 0.5 kb of downstream sequence in a XhoI-HindIII fragment. The other contained the adjacent HindIII-HindIII fragment with ≈3 kb of downstream sequence (see Fig. 4).
FIG. 4.
Map of the C. crescentus skgA locus. The XhoI-HindIII and HindIII-HindIII fragments were subcloned from the original cosmid. EagI and PstI sites mark the ends of the sequenced region (dashed line) submitted to GenBank (accession no. AF170912) and are not unique sites. The Tn5 insertion site is designated by an arrowhead. The 5′ end of mutL was placed by assuming equal lengths for the C. crescentus and E. coli homologues.
Construction of skgA::lacZ chromosomal fusion strain.
A fragment ending with the codon for the eighth amino acid of SkgA and containing ≈700 nucleotides of upstream sequence was amplified by PCR and cloned into the EcoRI and SalI sites of pJBZ282 (2), producing a translational fusion with lacZ. The amino-terminal sequence of SkgA was changed from VDLSVYTV to VDLSVDTV (see Fig. 3) as a result of introducing the SalI site. The 5′ PCR primer was CGGTGGAATTCCGCGCCGACCTCGTTGCGC (EcoRI site underlined), and the 3′ primer was GACCGTGTCGACGCTCAAATCAACCTTCCC (SalI site underlined). The chromosomal skgA::lacZ fusion strain was constructed by homologous integration as described before (38). Plasmid pJBZ282 is a Kmr ColE1 vector that fails to replicate in C. crescentus. Strain LS800 recA526(pKM3001) was transformed to Kmr with the pJBZ282 PskgA construct and grown overnight in the absence of tetracycline to favor loss of pKM3001 (Tcr recA+). Strain SGC130 recA skgA::lacZ was obtained as a Tcs isolate.
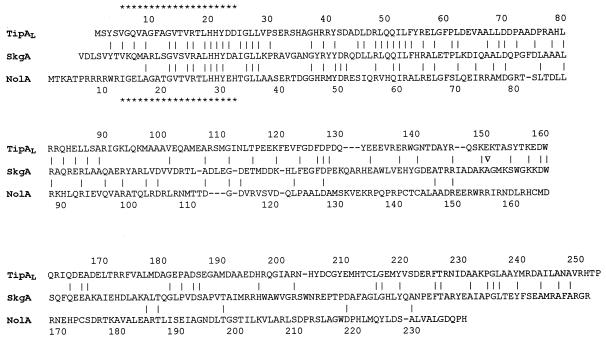
FIG. 3.
Amino acid sequence alignment of SkgA with known transcriptional regulators. Asterisks designate putative helix-turn-helix DNA binding motifs in TipAL from S. lividans and NolA from Bradyrhizobium sp. Identities with SkgA are indicated by vertical lines. TipAS, lacking the amino-terminal DNA binding domain and containing the carboxyl-terminal ligand binding domain, corresponds to residues 110 to 253 of TipAL. The Tn5 insertion in skgA occurs within the codon for the Ala residue designated by the arrowhead. The amino terminus of SkgA was located by homology and may be Val-1 or Val-5.
Assay of β-galactosidase.
Assays with o-nitrophenyl-β-d-galactosidase (ONPG) as substrate were performed as described before; activity was expressed in Miller units (23). Assays of β-galactosidase with chlorophenol red β-galactoside (CPRG), a substrate which is ≈10-fold more sensitive than ONPG, were performed by the protocol of Matin and coworkers (20). The respective extinction coefficients for assays with ONPG and CPRG were ɛM405 = 3.5 × 103 and ɛM574 = 7.5 × 104 cm−1 M−1. Frozen cell pellets were resuspended in 0.2 ml of M2 salts and brought to 1 ml with 0.1 M potassium phosphate–1 mM magnesium chloride, pH 7, and then mixed with 5 μl of 0.1% sodium dodecyl sulfate and 10 μl of chloroform and vortexed. After 5 min at 37°C in the dark, the sample was centrifuged and the supernatant was mixed with 20 μl of CPRG, 30 mg per ml in water. The change in A574 was monitored spectrophotometrically at 37°C. Activity was expressed as nanomoles of CPRG hydrolyzed per minute divided by optical density at 600 nm (OD600) of the original culture.
Resistance to heat and acid exposure.
Exponential or 24-h stationary-phase cultures were washed with and resuspended in an equal volume of M2 salts. For heat shock, exponential and overnight cultures were diluted 1:10 and 1:50, respectively, in M2 salts, and 1-ml volumes (to 108 and 107 to 108 CFU per ml, respectively) were heated at 50°C. For acid shock, exponential- and stationary-phase cultures were resuspended in an equal volume of M2 salts adjusted to pH 4.0 with HCl and 1-ml volumes were incubated at 30°C. For H2O2 challenge, after resuspension in an equal volume of M2 salts, H2O2 was added and 1-ml volumes were incubated at 30°C on a drum. Aliquots were removed, diluted into neutral M2 salts, and spread on PYE plates (16, 41).
Other methods.
Recombinant DNA methods have been described before (3, 38). The source for most enzymes was New England Biolabs (Beverly, Mass.). Resistance to H2O2 was measured by zone of inhibition. Whatman 3MM disks (0.7-cm diameter with 10 μl of 1 M H2O2) were laid on top of 3 ml of PYE top agar containing 0.1 ml of culture on PYE plates without drugs. Cell extracts prepared by sonication (36) were assayed for catalase activity by monitoring the decomposition of 10 mM H2O2 at pH 6.4 (1). Specific activity units were micromoles of H2O2 decomposed per minute per milligram of cell protein, determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Analyses of nucleotide and amino acid sequences were performed with GCG and BLAST packages. Preliminary sequence data from the C. crescentus genome were obtained from The Institute for Genomic Research website (40a).
Nucleotide sequence accession number.
The nucleotide sequence determined in this work has been assigned GenBank accession no. AF170912.
RESULTS
Isolation of mutants with diminished stationary-phase induction of katG.
To screen for regulators of the stationary-phase response, a chromosomal katG::lacZ translational fusion strain was randomly mutagenized with Tn5 (Spcr/Smr) and mutants impaired in the starvation-induced expression of katG were isolated. Carbon starvation was used because carbon was most likely the nutrient depleted in our prior studies with PYE medium (38). Following plating with X-Gal, 11 white or light blue mutants (corresponding to a frequency of 1 of 500 Tn5 insertions) were picked. Transduction was used to establish that white mutants did not represent Tn5 insertions in the katG::lacZ fusion (see Materials and Methods). The mutations, presumed to be in genes directly or indirectly regulating stationary-phase expression of katG, were named skg for stationary-phase regulation of katG.
Strains for further characterization were constructed by transducing the skg::Tn5 mutations back into the original katG::lacZ fusion strain SGC109 (see Materials and Methods). Characterization of two mutant strains, SGC127 skgA::Tn5 and SGC126 skgB::Tn5, is described here. Comparisons were made to a control strain, SGC125, constructed similarly from a colony in the initial screen whose blue color was the intensity of unmutagenized katG::lacZ fusion strain SGC109. Control strain SGC125 behaved identically to the original fusion strain SGC109 in all characterizations examined.
Growth stage induction of katG::lacZ fusion in Tn5 insertion mutants.
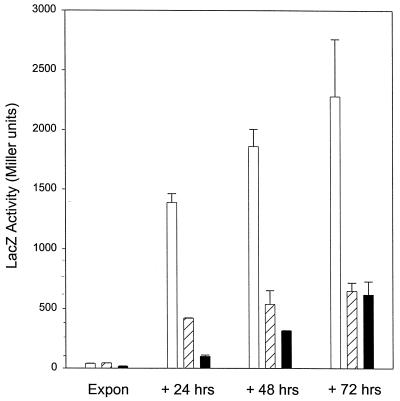
We previously showed that expression of the katG::lacZ fusion was constant (±20%) during exponential growth and increased 50 times postexponentially (38). Expression of the fusion in the control Tn5 strain paralleled that in the original strain (Fig. 1 and data not shown). In the skgA and skgB mutant strains, lacZ activity was reduced by 70 and 70 to 90%, respectively, during 1 to 3 days in stationary phase (Fig. 1). Assay of KatG catalase activity in cell extracts by H2O2 decomposition confirmed that lacZ activity reflected the enzymatic activity of the KatG catalase-peroxidase (data not shown).
FIG. 1.
Growth stage dependence of katG expression in skg mutants. LacZ activity from a chromosomal katG::lacZ translational fusion was measured with ONPG as substrate at the indicated times during growth in PYE. Bars: open, Tn5 control strain SGC125; hatched, skgA::Tn5 strain SGC127; filled, skgB::Tn5 strain SGC126. Values of OD600 for exponential cultures of SGC125, SGC127, and SGC126 were 0.44, 0.55, and 0.29, respectively. We previously showed for wild-type C. crescentus a low and constant expression of katG::lacZ during the entire period of exponential growth in katG fusion strain SGC109. The parental strain for the Tn5 mutants was SGC109. Expon, exponential.
Response to hydrogen peroxide and survival of skgA and skgB mutants in stationary phase.
KatG is the sole catalase-peroxidase in C. crescentus (38). A katG-null strain is more sensitive than wild type to H2O2 challenge in zone-of-inhibition studies (38). By this approach, the reduced expression of katG in stationary phase (Fig. 1) was shown to correlate with increased sensitivity to H2O2 in skgA and skgB mutant strains (Table 2).
TABLE 2.
Resistance to hydrogen peroxidea
| Strain | Diam of clearing (cm) at time after exponential phase:
|
|
|---|---|---|
| + 24 h | + 48 h | |
| Control | 3.75 ± 0.04 | 4.15 ± 0.07 |
| skgA::Tn5 | 4.95 ± 0.07 | 4.60 ± 0.14 |
| skgB::Tn5 | 4.75 ± 0.21 | 4.33 ± 0.46 |
Cultures at the indicated growth stage were plated in top agar and exposed to H2O2 as indicated in Materials and Methods.
Several observations argue that this increased sensitivity to H2O2 relates to the decreased expression of katG in the stationary phase of growth and not to differences in exponential phase. (i) The doubling times of the mutants were identical to those of wild type: 97 ± 12 and 104 ± 1 min for skgA and skgB mutants, respectively, compared to 99 ± 10 min for the Tn5 control and wild type. By this criterion, the skg mutations were not detrimental to exponentially growing cells. (ii) Expression of katG in exponential cultures was comparable for the skg mutants and control strains (Fig. 1). This suggested that changes in the H2O2 resistance of skg mutants were likely to be present during stationary phase. (iii) Resistance to H2O2 was identical with that of the control when exponential cultures were used in the zone-of-inhibition test, 3.3 versus 3.35 ± 0.07 cm for skgA mutant and control strains, respectively.
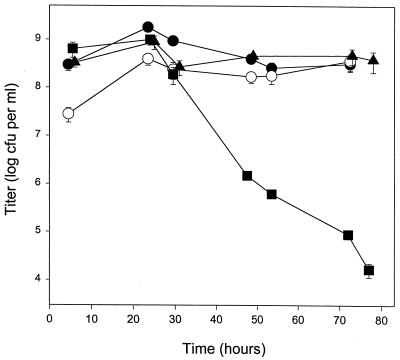
We previously showed that during the first 3 days of stationary phase, survival of the katG-null mutant decreases by 5 orders of magnitude (Fig. 2) (38). During this time, the titer of wild-type C. crescentus remains constant. The stationary-phase survival of the skgA and skgB mutants and the Tn5 control was comparable to that of wild type. There were no significant decreases in titer over 3 days (Fig. 2). These results suggest that the reduced levels of KatG in stationary-phase cultures of skgA and skgB mutants were sufficient to maintain stationary-phase survival but inadequate to protect against exogenous H2O2.
FIG. 2.
Stationary-phase survival. Cultures in PYE were sampled at the indicated times, and titers were determined on PYE plates without antibiotics. The OD600 values at 0 h were 0.06 to 0.11 and for exponential-phase samples (4.5 to 6 h) were 0.29 to 0.65. Saturation OD600 values for skgA and skgB strains were slightly lower than those for the katG-null and Tn5 control strains, 1.0 to 1.2 versus 1.2 to 1.5. Strains used were SGC111 katG (■), SGC125 Tn5 control (●), SGC127 skgA::Tn5 (○), and SGC126 skgB::Tn5 (▴).
Site of Tn5 insertion in the skgA mutant.
The sequence adjacent to Tn5 in the skgA mutant showed a high degree of nucleotide and amino acid sequence identity at homologous positions to the TipA transcriptional activator of Streptomyces lividans. Significant but lesser identity to other transcriptional regulators in the MerR family, e.g., NolA, a regulator of nodulation in Rhizobium species; BltR; MerD; and SoxR, was shown. Alignment of SkgA with TipA (34% identical) and NolA (19% identical) is shown in Fig. 3.
TipA was initially purified as a protein induced in S. lividans by nontoxic, subnanomolar levels of the peptide antibiotic thiostrepton. Subsequently, TipA was shown to bind to its own promoter in a thiostrepton-dependent manner in vitro and to be an autogenous activator of PtipA (14, 25). The amino acid sequence of TipA is homologous to helix-turn-helix regulators in the MerR family in which N-terminal domains are involved in DNA binding and C-terminal domains are involved in ligand binding (40). The C-terminal domain of TipA is known to bind thiostrepton (25).
The wild-type skgA locus was subcloned from a C. crescentus cosmid library. Downstream of skgA and transcribed in the opposing direction is a C. crescentus homologue of mutL (Fig. 4), a gene in the E. coli mismatch repair system that is critical for mismatch repair in stationary phase (11). The wild-type skgA allele was used to construct a complementing plasmid. Introduction of the plasmid into the skgA::Tn5 strain restored the growth-stage-dependent expression of katG::lacZ to that of wild type (data not shown), thus demonstrating that this phenotype is attributable to inactivation of skgA.
Site of Tn5 insertion in the skgB mutant.
The sequence adjacent to Tn5 in the skgB mutant was identified in the preliminary C. crescentus genome sequence data available from The Institute for Genomic Research. Surrounding the skgB Tn5 insertion site was an open reading frame (ORF) of ≈290 amino acids. This ORF had significant amino acid sequence identity to hypothetical proteins of comparable lengths in the sequenced genomes of five bacterial species (percent identity/amino acid overlap): E. coli (36%/283 residues), Haemophilus influenzae (31%/285 residues), Synechocystis sp. (strain PCC 6803; 28%/264 residues), Aquifex aeolicus (26%/281 residues), and Bacillus subtilis (24%/288 residues). These hypothetical proteins may play a role in the response to starvation or oxidative stress. Our further characterizations focused on the skgA mutant.
Characterizations of the skgA mutant: response to exogenous H2O2.
When exponential cultures of C. crescentus are challenged with H2O2, a 10- to 20-fold increase in katG expression is observed either by direct assay of catalase activity in cell extracts or by using a katG::lacZ fusion (38). This adaptive response to H2O2 is similar to that seen in E. coli and controlled by E. coli oxyR (31, 39). To determine if this response was dependent upon skgA, induction of katG::lacZ was measured following an H2O2 challenge during exponential growth. The magnitude and time course of the responses for skgA::Tn5 and control strains were comparable to one another and to those for the katG::lacZ fusion strain SGC109 (reference 38 and data not shown). Thus, skgA is not required for this adaptive response to H2O2 stress in rapidly growing cells.
Growth stage dependence of skgA expression.
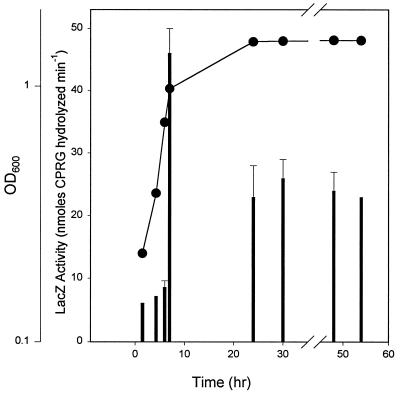
The growth-stage dependence of skgA expression was assessed by using a chromosomal skgA::lacZ fusion (Fig. 5). Expression was relatively constant during early and mid-exponential growth and then increased 5- to 10-fold in late exponential phase. Steady-state levels during stationary phase were three- to fivefold higher than those in mid-exponential phase. These fusion data were consistent with the proposal that skgA is a regulator of postexponential phenomena, playing a major role in postexponential katG induction and hydrogen peroxide resistance.
FIG. 5.
Growth stage dependence of skgA expression. LacZ activity in SGC130 skgA+ skgA::lacZ was measured by hydrolysis of CPRG during growth in PYE. Thick bars indicate LacZ activity as nanomoles of CPRG hydrolyzed per minute divided by OD600 of the culture sampled; solid circles indicate OD600.
Cross-resistance to stress in the skgA mutant.
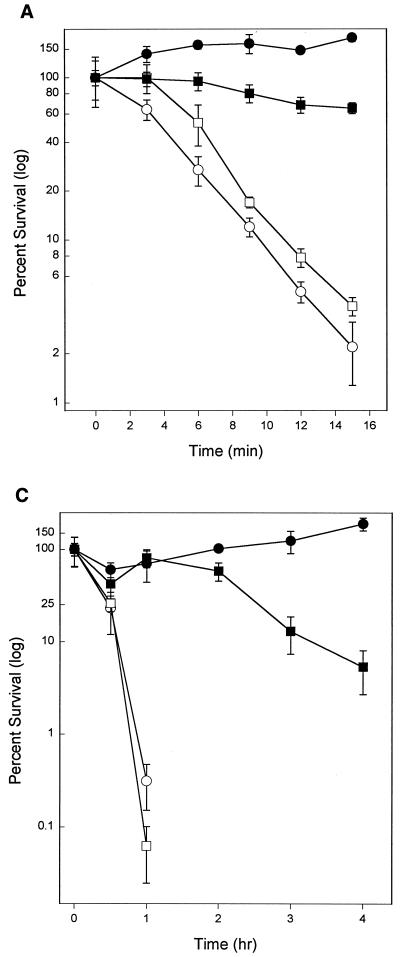
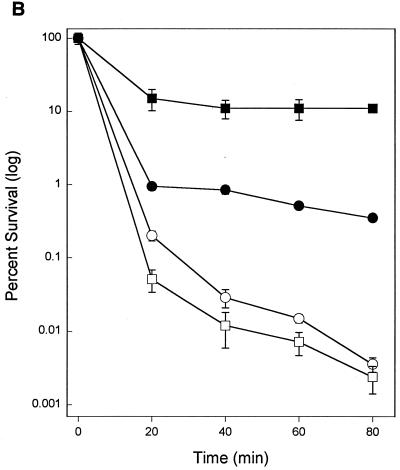
Stationary-phase cultures of C. crescentus are more resistant to heat, acid, alkali, and H2O2 than exponentially growing cultures (41). This cross-resistance to stress is similar to that seen in stationary-phase cultures of E. coli and other species where rpoS is a central regulator of the cross-resistance. In E. coli, null mutations of rpoS have pleiotropic effects on stationary-phase phenotypes. Resistance of stationary-phase cultures to heat and H2O2 is reduced by several orders of magnitude compared to that of wild type (12, 16, 19, 21). The skgA mutant was similar to the control strain in the increased resistance of stationary-phase cultures to 50°C heat and pH 4 compared to that of exponential cultures (Fig. 6A and B). Resistance of the skgA mutant to H2O2 was reduced in stationary phase (Fig. 6C), consistent with the reduced stationary-phase expression of katG (Fig. 1), but still significantly greater than resistance of exponential cultures. These data indicate that the skgA::Tn5 mutation does not have a pleiotropic effect on stationary-phase cross-resistance to stress as do null mutations of rpoS in E. coli.
FIG. 6.
Resistance to heat and acid in exponential and stationary phase. (A) 50°C heat shock. (B) pH 4 shock. (C) 5 mM H2O2. See Materials and Methods for details. Circles, Tn5 control (strain SGC125). Squares, skgA::Tn5 (strain SGC127). Open symbols, exponential phase (OD600 of 0.36 to 0.76). Filled symbols, stationary phase (20 h later). In panel C (5 mM H2O2), no CFU were seen for either exponential culture at time points beyond 1 h.
DISCUSSION
The function of skgA in regulation of the starvation response in C. crescentus.
We identified the first regulator of the starvation response to H2O2 stress in C. crescentus. Genes in the response to heat shock and alkylation have been characterized previously, but their regulation was studied only in the context of the swarmer-stalked cell cycle in exponentially growing cultures (4, 30). A starvation-induced cross-resistance to heat, acid, alkali, and H2O2 has been demonstrated and a novel helical morphology has been identified for C. crescentus cultures starved for 9 to 12 days (41). However, the regulation of these stationary-phase responses was not investigated.
SkgA plays a major role in the induction of katG and in resistance to H2O2 in carbon starvation. Several observations support the contention that skgA acts predominantly in stationary phase. Expression of skgA is induced in late exponential phase. The skgA::Tn5 mutation reduces stationary-phase katG induction and diminishes H2O2 resistance in stationary phase. Finally, the skgA mutation is without effect on doubling time or the induction of katG by H2O2 added to exponential cultures.
SkgA is not a functional correlate of E. coli rpoS because SkgA is not a master regulator of the starvation response. In E. coli, rpoS controls the cross-resistance to heat, acid shock, and H2O2 in stationary phase as well as metabolic and morphological aspects of the starvation response. In the C. crescentus skgA mutant, stationary-phase resistance to heat and acid shock was maintained. The resistance to H2O2 was reduced, as expected from reduced stationary-phase expression of katG. In addition, the skgA mutant did not show a stationary-phase survival deficit, which is a phenotype of rpoS-null mutants of E. coli (12, 15). Finally, entrance into the helical morphology of late stationary phase was not altered in the skgA mutant (29).
The presence of rpoS in C. crescentus remains an open question. Our data show that there must be regulators other than skgA which control cross-resistance to heat and acid in stationary phase. RpoS may be one of those. In addition, other regulators of katG stationary-phase induction are likely, because in the skgA::Tn5 mutant katG induction is still 30% of the wild-type level. It appears that C. crescentus katG is controlled by three regulatory networks: one for adaptive induction to H2O2 in exponential cultures (38); skgA, the major regulator of postexponential induction; and a third regulator responsible for residual postexponential katG induction in the skgA mutant. RpoS may act in the last process. The degree of overall similarity of stationary-phase gene regulation in enteric bacteria and C. crescentus remains to be determined. However, it is noteworthy that SkgA, a major regulator of starvation-induced resistance to H2O2, is not an alternate ς factor and does not control cross-resistance to heat and acid shock in stationary phase. Both of these traits distinguish SkgA from RpoS, the master regulator of stationary-phase gene expression in enteric bacteria.
Identification of genes other than katG under the control of skgA will provide additional information about the C. crescentus starvation response. Genetic approaches used with E. coli for expanding the list of genes in the rpoS regulon (10, 32) can be adapted to C. crescentus. The phenotypes of the skgB mutant were similar to those of the skgA mutant, and further studies of the skgB mutant are merited. Having significant amino acid similarities to hypothetical proteins in the genomes of E. coli and four other species, SkgB may lead to new insights on the role of catalase-peroxidase in stationary phase and regulation of the starvation response in bacterial species other than C. crescentus. SkgA had no significant sequence similarity to ORFs in E. coli or other sequenced genomes.
Inferences about structure-function relationships in SkgA.
SkgA is inferred to be a helix-turn-helix transcriptional regulator from similarities in its amino acid sequence to those of known bacterial regulators of that type. Although similar in sequence, SkgA may not share structure-function relationships with such regulators. With MerR and SoxR, DNA binding and changes in the activity of the transcriptional regulator are influenced by cysteine residues that are sites for binding of mercury and formation of an iron-sulfur cluster, respectively (6, 7, 40). Since SkgA lacks half-cystine residues, redox or metal binding changes involving protein sulfhydryl groups must be absent. Redox changes or metal binding to non-sulfhydryl groups in SkgA are possible, however.
Several directions for future study of SkgA are suggested by its primary structural similarities to the TipA regulator. Two proteins are synthesized from the TipA ORF, TipAL (Fig. 3 and its legend) and the C-terminal 144 residues, TipAS. The C-terminal fragment, TipAS is found in 20-fold excess over TipAL in S. lividans. The fact that TipAS binds thiostrepton and is present in excess suggested that it may regulate the availability of thiostrepton for TipAL. Binding of thiostrepton to TipAL increases the binding of TipAL to PtipA 10-fold. Therefore, sequestration of thiostrepton by TipAS could limit transcriptional activation by TipAL (14, 25). A potential ribosome binding site is present in the SkgA ORF in a position homologous to the start of TipAS. This raises the possibility that the C-terminal regulatory domain may be transcribed and translated and play a role in skgA regulation in C. crescentus.
Amino acid sequence identities between SkgA and TipA in both DNA and ligand binding domains raise the possibility of structural homologies between the ligand-inducer of SkgA and the peptide thiostrepton, the inducer of TipA. Little is known about the signal(s) or inducer(s) of the E. coli starvation response. Internal pH, UDP-glucose, ppGpp, and acyl homoserine lactone(s) have all been implicated (12). If the C-terminal domain of SkgA binds ligands-inducers, as is the case for other members of the MerR family of transcriptional regulators, then it may be possible to perform in vitro binding screens for candidate ligands-inducers of the starvation response. In conclusion, identifying a helix-turn-helix regulator in contrast to the alternate ς factors implicated in the starvation response in other bacterial species has far-ranging implications for understanding structure-function relationships in the starvation response of C. crescentus.
ACKNOWLEDGMENTS
This work was supported in part by National Science Foundation grant MCB 9513706 (to H.M.S.) and an REU supplement to that grant.
We thank Laurie Weinstein for expert technical assistance; A. C. Matin (Stanford University) for the CPRG protocol, Yves Brun (University of Indiana) for the pLAFR5 cosmid library and strain S17-1 λpir (Tn5), Ellen M. Quardokus (University of Indiana) for analysis of the helical morphology in the skgA-null mutant, and the DNA Sequencing/Synthesis Services Facility at Albert Einstein College of Medicine for DNA sequencing and synthetic oligonucleotides.
REFERENCES
- 1.Aebi H. Catalase in vitro. Methods Enzymol. 1984;108:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Alley M R K, Gomes S L, Alexander W, Shapiro L. Genetic analysis of a temporally transcribed chemotaxis gene cluster in Caulobacter crescentus. Genetics. 1991;129:333–342. doi: 10.1093/genetics/129.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandyopadhyay P, Steinman H M. Legionella pneumophila catalase-peroxidases: cloning of the katB gene and studies of KatB function. J Bacteriol. 1998;180:5369–5374. doi: 10.1128/jb.180.20.5369-5374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombi D, Gomes S L. An alkB gene homolog is differentially transcribed during the Caulobacter crescentus cell cycle. J Bacteriol. 1997;179:3139–3145. doi: 10.1128/jb.179.10.3139-3145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demple B. A bridge to control. Science. 1998;279:1655–1656. doi: 10.1126/science.279.5357.1655. [DOI] [PubMed] [Google Scholar]
- 7.Ding H, Demple B. Thiol-mediated disassembly and reassembly of [2Fe-2S] clusters in the redox-regulated transcription factor SoxR. Biochemistry. 1998;37:17280–17286. doi: 10.1021/bi980532g. [DOI] [PubMed] [Google Scholar]
- 8.Ely B. Genetics of Caulobacter crescentus. Methods Enzymol. 1991;204:372–384. doi: 10.1016/0076-6879(91)04019-k. [DOI] [PubMed] [Google Scholar]
- 9.Evinger M, Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977;132:294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang F C, Chen C Y, Guiney D G, Xu Y. Identification of sigma S-regulated genes in Salmonella typhimurium: complementary regulatory interactions between sigma S and cyclic AMP receptor protein. J Bacteriol. 1996;178:5112–5120. doi: 10.1128/jb.178.17.5112-5120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris R S, Feng G, Ross K J, Sidhu R, Thulin C, Longerich S, Szigety S K, Winkler M E, Rosenberg S M. Mismatch repair protein MutL becomes limiting during stationary-phase mutation. Genes Dev. 1997;11:2426–2437. doi: 10.1101/gad.11.18.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1497–1512. [Google Scholar]
- 13.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes D J, Caso J L, Thompson C J. Autogenous transcriptional activation of a thiostrepton-induced gene in Streptomyces lividans. EMBO J. 1993;12:3183–3191. doi: 10.1002/j.1460-2075.1993.tb05987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huisman G W, Siegele D A, Zamgrano M A, Kolter R. Morphological and physiological changes during stationary phase. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1672–1682. [Google Scholar]
- 16.Jenkins D E, Schultz J E, Matin A. Starvation-induced cross-protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988;170:3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson R C, Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977;86:25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange R, Hengge-Aronis R. The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 19.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 20.Lomovskaya O L, Kidwell J P, Matin A. Characterization of the sigma 38-dependent expression of a core Escherichia coli starvation gene, pexB. J Bacteriol. 1994;176:3928–3935. doi: 10.1128/jb.176.13.3928-3935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991;5:3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 22.Meisenzahl A C, Shapiro L, Jenal U. Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J Bacteriol. 1997;179:592–600. doi: 10.1128/jb.179.3.592-600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. [Google Scholar]
- 24.Mohr C D, Jenal U, Shapiro L. Flagellar assembly in Caulobacter crescentus: a basal body P-ring null mutation affects stability of the L-ring protein. J Bacteriol. 1996;178:675–682. doi: 10.1128/jb.178.3.675-682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami T, Holt T G, Thompson C J. Thiostrepton-induced gene expression in Streptomyces lividans. J Bacteriol. 1989;171:1459–1466. doi: 10.1128/jb.171.3.1459-1466.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickerson C A, Curtiss R., III Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect Immun. 1997;65:1814–1823. doi: 10.1128/iai.65.5.1814-1823.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nystrom T. To be or not to be: the ultimate decision of the growth-arrested bacterial cell. FEMS Microbiol Rev. 1998;21:283–290. [Google Scholar]
- 28.O’Neill E A, Hynes R H, Bender R A. Recombination deficient mutant of Caulobacter crescentus. Mol Gen Genet. 1985;198:275–278. doi: 10.1007/BF00383006. [DOI] [PubMed] [Google Scholar]
- 29.Quardokus, E. M. 1998. Personal communication.
- 30.Reisenauer A, Mohr C D, Shapiro L. Regulation of a heat shock ς32 homolog in Caulobacter crescentus. J Bacteriol. 1996;178:1919–1927. doi: 10.1128/jb.178.7.1919-1927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosner J L, Storz G. Regulation of bacterial responses to oxidative stress. Curr Top Cell Regul. 1997;35:163–177. doi: 10.1016/s0070-2137(97)80007-6. [DOI] [PubMed] [Google Scholar]
- 32.Schellhorn H E, Audia J P, Wei L I, Chang L. Identification of conserved, RpoS-dependent stationary-phase genes of Escherichia coli. J Bacteriol. 1998;180:6283–6291. doi: 10.1128/jb.180.23.6283-6291.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnell S, Steinman H M. Function and stationary-phase induction of periplasmic copper-zinc superoxide dismutase and catalase/peroxidase in Caulobacter crescentus. J Bacteriol. 1995;177:5924–5929. doi: 10.1128/jb.177.20.5924-5929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 35.Simon R, Priefer U, Puhler A. A broad host-range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negaive bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 36.Steinman H M. Bacteriocuprein superoxide dismutases in pseudomonads. J Bacteriol. 1985;162:1255–1260. doi: 10.1128/jb.162.3.1255-1260.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinman, H. M. 1998. Unpublished results.
- 38.Steinman H M, Fareed F, Weinstein L. Catalase-peroxidase of Caulobacter crescentus: function and role in stationary-phase survival. J Bacteriol. 1997;179:6831–6836. doi: 10.1128/jb.179.21.6831-6836.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storz G, Altuvia S. The OxyR regulon. Methods Enzymol. 1994;234:217–223. doi: 10.1016/0076-6879(94)34088-9. [DOI] [PubMed] [Google Scholar]
- 40.Summers A O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992;174:3097–3101. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a.The Institute for Genomic Research. 5 October 1998, release date. Sequence data. [Online.] http://www.tigr.org/tdb/mdb/mdb.html. [19 April 1999, last date accessed.]
- 41.Wortinger M A, Quardokus E M, Brun Y V. Morphological adaptation and inhibition of cell division during stationary phase in Caulobacter crescentus. Mol Microbiol. 1998;29:963–973. doi: 10.1046/j.1365-2958.1998.00959.x. [DOI] [PubMed] [Google Scholar]