Abstract
Background
Complete mesocolic excision (CME) for right colonic cancer is a more complex operation than standard right hemicolectomy but evidence to support its routine use is still limited. This prospective multicentre study evaluated the effect of CME on long-term survival in colorectal cancer centres in Germany (RESECTAT trial). The primary hypothesis was that 5-year disease-free survival would be higher after CME than non-CME surgery. A secondary hypothesis was that there would be improved survival of patients with a mesenteric area greater than 15 000 mm2.
Methods
Centres were asked to continue their current surgical practices. The surgery was classified as CME if the superior mesenteric vein was dissected; otherwise it was assumed that no CME had been performed. All specimens were shipped to one institution for pathological analysis and documentation. Clinical data were recorded in an established registry for quality assurance. The primary endpoint was 5-year overall survival for stages I–III. Multivariable adjustment for group allocation was planned. Using a primary hypothesis of an increase in disease-free survival from 60 to 70 per cent, a sample size of 662 patients was calculated with a 50 per cent anticipated drop-out rate.
Results
A total of 1004 patients from 53 centres were recruited for the final analysis (496 CME, 508 no CME). Most operations (88.4 per cent) were done by an open approach. Anastomotic leak occurred in 3.4 per cent in the CME and 1.8 per cent in the non-CME group. There were slightly more lymph nodes found in CME than non-CME specimens (mean 55.6 and 50.4 respectively). Positive central mesenteric nodes were detected more in non-CME than CME specimens (5.9 versus 4.0 per cent). One-fifth of patients had died at the time of study with recorded recurrences (63, 6.3 per cent), too few to calculate disease-free survival (the original primary outcome), so overall survival (not disease-specific) results are presented. Short-term and overall survival were similar in the CME and non-CME groups. Adjusted Cox regression indicated a possible benefit for overall survival with CME in stage III disease (HR 0.52, 95 per cent c.i. 0.31 to 0.85; P = 0.010) but less so for disease-free survival (HR 0.66; P = 0.068). The secondary outcome (15 000 mm2 mesenteric size) did not influence survival at any stage (removal of more mesentery did not alter survival).
Conclusion
No general benefit of CME could be established. The observation of better overall survival in stage III on unplanned exploratory analysis is of uncertain significance.
Some 1004 patients with right-sided colonic cancer were recruited from 53 centres. There were no differences in short-term survival and complications after CME versus non-CME surgery. Long-term multivariate survival analysis suggested a survival benefit for CME in stage III disease. CME should be offered to low- and medium-risk patients with right-sided colonic cancer if stage III disease cannot be excluded.
Introduction
The concept of complete mesocolic excision (CME) for right colonic cancer was published in 20091 followed by population-based observations from Denmark2,3,4 and Sweden5, case–control studies6, retrospective series7, and meta-analyses of these reports8,9,10,11. Several randomized trials12,13,14 comparing D2 and D3 dissections are under way, with short-term results showing no clinically important difference. Designing a randomized trial for the evaluation of CME in right-sided colonic cancer is challenging. The control group should reflect current practice but a ‘standard’ right hemicolectomy may vary; a clear description of CME15 ranges from partial superior mesenteric vein (SMV) dissection to D3 clearance with central vascular ligation. Therefore, it was decided to undertaken a pragmatic, prospective, multicentre, non-randomized, quasi-experimental16, registry-based study (RESECTAT) allowing comparison of predefined groups (regression discontinuity design16) and centralized specimen evaluation17.
Methods
This prospective multicentre, open, quasi-experimental (regression discontinuity design16), registry-based study included patients with stage I–III right-sided colonic adenocarcinoma who underwent elective surgery with curative intent. The protocol for the study has been described previously17. The participating centres were either certified or preparing for certification in colorectal cancer according to the German Cancer Society18.
Groups
To generate comparable groups, surgeons were asked to continue their current surgical treatment. The extent of resection was noted by the operating surgeon. If the SMV was dissected, the operation was classified as CME (CME group). Otherwise, it was assumed that there had been no CME (non-CME group). This (regression discontinuity16) criterion was predefined but not communicated to the participants to avoid confounding.
Specimens
Photographic documentation of the fresh specimen was performed in a standardized fashion. Suture markings were placed on the ileocolic and middle colic arteries. An additional suture was inserted 4 cm peripheral to the ileocolic artery stalk, irrespective of the level of division. All specimens were fixed in formalin and shipped to the pathology laboratory. Assessment of the dissection plane19 and planimetry of the mesocolic surface area was performed.
Data management
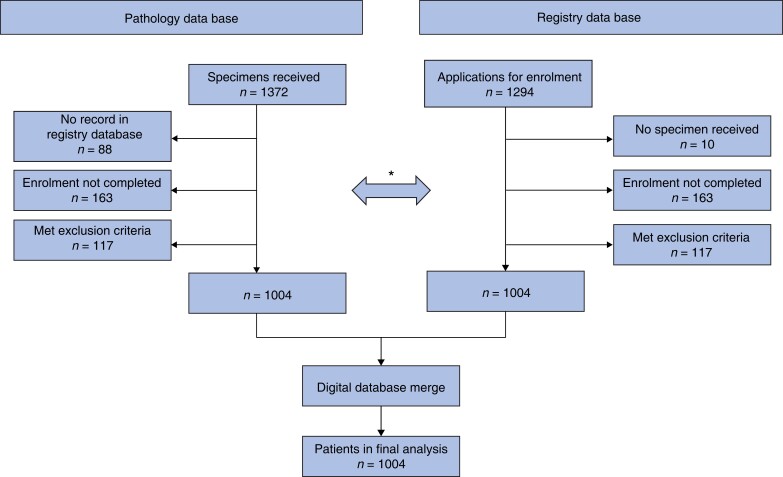
Perioperative and follow-up data were recorded using an established online registry for quality assurance in colorectal surgery (AN-Institut für Qualitätssicherung in der operativen Medizin; Universität Magdeburg, Germany)20,21. Follow-up was undertaken mainly by contacting family doctors, patients, and their relatives. Hospital reports, including operation notes, were reviewed independently, and compared with database records from the recruiting centres. For the final analysis, all data were merged into an IBM® SPSS® database (Fig. 1).
Fig. 1.
Study flow diagram
*Manual cross-check of databases during recruitment. Data in the pathology database and the registry were checked continuously for consistency and the following exclusion criteria: carcinomas distal to the hepatic flexure, polyposis syndromes, Lynch syndrome, second malignant tumour, chronic inflammatory bowel disease, emergency operation, previous operations on right colon except appendicectomy, inability to provide informed consent.
Hypotheses
The primary hypothesis was that the 5-year disease-free survival (DFS) rate after CME would be higher than that after non-CME surgery in stages I–III. However, as detailed below, overall survival was eventually chosen as the primary endpoint. A secondary hypothesis was that there would be improved survival of patients with a mesocolic area (MA) of the specimen greater than 15 000 mm2.
Statistical analysis
Using the primary hypothesis of an increase in DFS from 60 to 70 per cent, an overall sample size of 662 patients was calculated. Because of the pragmatic design, a high primary drop-out rate of approximately 50 per cent was anticipated.
Confounding may have occurred because the patients were not assigned randomly to the groups. To determine which variables were relevant confounders, a causal directed acyclic graph was created (Fig. S1). This type of graph shows the causal influences of different variables on each other. Using this graph and Pearl’s backdoor criterion22, the relevant confounders for assessing the causal effect of CME or MA on survival were determined. The adjustment set consisted of BMI, age, centre, T category, tumour location, ASA fitness grade, and operative approach.
To investigate the effect of CME on survival as a first step, a simple Cox regression analysis was performed without adjustment. Another Cox regression analysis was then carried out in which all relevant confounders were included: Cox regression with multiple imputations for missing values, inverse probability treatment weighing, and generalized boosted models23,24 (Figs S2 and S3). Kaplan–Meier survival curves were calculated for each treatment group. Survival probabilities were obtained using the multivariate Cox regression models to perform direct standardization. The corresponding confidence intervals were obtained using a normal approximation, where the asymptotic variance was estimated using the influence function25.
To analyse the influence of MA on survival, the same regression models and sensitivity analyses were used (Table S1). In each case, MA was included once as a dichotomous variable (less than 15 000 mm2versus 15 000 mm2 or more) and once as a continuous variable. All P values are two-sided.
All analyses were performed using the R programming language version 4.0 (R Foundation for Statistical Computing, Vienna, Austria). Continuous data were compared using students T-test. Missing values were removed before analysis using listwise deletion. To assess the impact of this procedure, analysis of multivariable Cox regression models was repeated using multiple imputations. The multiple imputation by chained equations algorithm with predictive mean matching was used to create 15 data sets. The analysis was performed on all of these and subsequently pooled using Rubin’s rule26.
Registration and ethics
The trial was registered according to the International Committee of Medical Journal Editors guidelines at Deutsches Register für klinische Studien (registration number DRKS0001240027) and approved by the ethics board of the Landesärztekammer Baden-Württemberg (2009–118-f), Germany.
Results
Patient characteristics
Between February 2012 and October 2016, 1131 patients who underwent right hemicolectomy for stage I–III adenocarcinoma were registered in the database. After checking for exclusion criteria and matching with the Institute for Pathology Bochum databases, 1004 patients from 53 centres with intraoperative, histological, and survival data were included in this study (Fig. 1). Group allocation and demographics are shown in Table 1. Long-term follow-up (closed November 2020) was available for 987 patients (98.3 per cent) with a mean follow up of 50.6 months. At data cut-off, 196 patients (19.5 per cent) had died but recurrence was recorded at a low frequency (63 patients, 6.3 per cent), forcing a switch to overall survival in this study. It should be noted that this was not accounted for in sample size considerations and the potential for bias is apparent (overall survival is not cancer-specific). Data for DFS are included in Table 4. Age and the proportion of women were significantly higher in the non-CME group. All other patient and tumour characteristics, especially administration of adjuvant chemotherapy, were well balanced. Most operations were done in open technique (88.4%).
Table 1.
Demographic and clinical data
| All patients (n = 1004) |
No CME (n = 508) |
CME (n = 496) |
P* | |
|---|---|---|---|---|
| Sex ratio (M : F) | 444 : 560 | 208 : 300 | 236 : 260 | 0.040 |
| Age (years), mean(s.d.) | 72.2 (10.9) | 73.0 (1.3) | 71.4 (11.3) | 0.020† |
| 0–40 | 12 (1.2) | 6 (50.0) | 6 (50.0) | 0.024† |
| 41–60 | 130 (12.9) | 51 (39.2) | 79 (60.8) | |
| 61–80 | 635 (63.2) | 324 (51.0) | 311 (49.0) | |
| >80 | 227 (22.6) | 127 (55.9) | 100 (44.1) | |
| BMI (kg/m2), mean(s.d.) | 26.7 (4.9) | 26.8 (4.9) | 26.6 (5.0) | 0.570 |
| −18.5 | 13 (1.3) | 4 (30.8) | 9 (69.2) | 0.188 |
| 18–25 | 382 (38.0) | 195 (51.0) | 187 (49.0) | |
| 25–30 | 359 (35.8) | 170 (47.4) | 189 (52.6) | |
| 30- | 194 (19.3) | 107 (55.2) | 87 (44.8) | |
| Not available | 56 (5.6) | 32 (57.1) | 24 (42.9) | |
| Adjuvant chemotherapy | ||||
| UICC stage I | 1.000 | |||
| Yes | 2 (0.7) | 1 (50.0) | 1 (50.0) | |
| No | 235 (77.8) | 109 (46.4) | 126 (53.6) | |
| Not available | 65 (21.5) | 41 (63.1) | 24 (36.9) | |
| UICC stage II | 0.225 | |||
| Yes | 38 (9.6) | 21 (55.3) | 17 (44.7) | |
| No | 265 (67.3) | 117 (44.2) | 148 (55.8) | |
| Not available | 91 (23.1) | 61 (67.0) | 30 (33.0) | |
| UICC stage III | 0.888 | |||
| Yes | 180 (59.2) | 88 (48.9) | 92 (51.1) | |
| No | 70 (23.0) | 33 (47.1) | 37 (52.9) | |
| Not available | 54 (17.8) | 36 (66.7) | 18 (33.3) | |
| Tumour location | 0.601 | |||
| Ascending colon | 512 (51.0) | 256 (50.0) | 256 (50.0) | |
| Hepatic flexure | 72 (7.2) | 33 (45.8) | 39 (54.2) | |
| Caecum | 414 (41.2) | 215 (51.9) | 199 (48.1) | |
| Not available | 6 (0.6) | 4 (66.7) | 2 (33.3) | |
| Surgical approach | 0.148 | |||
| Laparoscopic | 204 (20.3) | 103 (50.5) | 101 (49.5) | |
| Open | 764 (76.1) | 379 (49.6) | 385 (50.4) | |
| Converted | 13 (1.3) | 10 (76.9) | 3 (23.1) | |
| Not available | 23 (2.3) | 16 (69.6) | 7 (30.4) | |
| ASA fitness grade | 0.226 | |||
| I | 63 (6.3) | 39 (61.9) | 24 (38.1) | |
| II | 554 (55.2) | 269 (48.6) | 285 (51.4) | |
| III | 333 (33.2) | 168 (50.5) | 165 (49.5) | |
| IV | 14 (1.4) | 6 (42.9) | 8 (57.1) | |
| Not available | 40 (4.0) | 26 (65.0) | 14 (35.0) |
Values are n (%) unless otherwise indicated. Rates of chemotherapy were 62.5 per cent in the complete mesocolic excision (CME) group and 56.0 per cent in non-CME group in stage III, and 8.7 and 10.5 per cent respectively in stage II. *χ2test, except †? test.
Table 4.
Multivariable analyses of survival
| Unadjusted Cox regression | Adjusted Cox regression | |||
|---|---|---|---|---|
| HR | P | HR | P | |
| Complete mesocolic excision | ||||
| Stages I–III | 0.78 (0.59, 1.03) | 0.081 | 0.81 (0.60, 1.10) | 0.176 |
| Stage I | 0.87 (0.52, 1.47) | 0.608 | 0.85 (0.49, 1.48) | 0.566 |
| Stage II | 0.92 (0.55, 1.54) | 0.746 | 1.13 (0.65, 1.95) | 0.667 |
| Stage III | 0.61 (0.39, 0.95) | 0.031 | 0.52 (0.31, 0.85) | 0.010 |
| Complete mesocolic excision (DFS*) | ||||
| Stages I–III | 0.83 (0.63, 1.08) | 0.164 | 0.86 (0.65, 1.15) | 0.306 |
| Stage I | 0.88 (0.53, 1.47) | 0.631 | 0.88 (0.51, 1.52) | 0.654 |
| Stage II | 0.91 (0.55, 1.49) | 0.711 | 1.09 (0.65, 1.82) | 0.752 |
| Stage III | 0.75 (0.50, 1.13) | 0.167 | 0.66 (0.42, 1.03) | 0.068 |
| Mesocolic plane (continuous variable) | ||||
| Stages I–III | 1.01 (0.99, 1.04) | 0.441 | 1.00 (0.97, 1.03) | 0.867 |
| Stage I | 1.04 (0.99, 1.08) | 0.114 | 0.99 (0.52, 1.87) | 0.970 |
| Stage II | 1.03 (0.98, 1.07) | 0.181 | 0.73 (0.40, 1.31) | 0.291 |
| Stage III | 0.97 (0.93, 1.01) | 0.179 | 0.53 (0.32, 0.88) | 0.015 |
| Mesocolic plane (using prespecified cut-off) | ||||
| Stages I–II | 1.26 (0.91, 1.73) | 0.164 | 1.15 (0.82, 1.63) | 0.418 |
| Stage I | 1.45 (0.78, 2.69) | 0.237 | 1.41 (0.75. 2.68) | 0.289 |
| Stage II | 1.41 (0.78, 2.54) | 0.262 | 1.23 (0.65, 2.31) | 0.533 |
| Stage III | 1.04 (0.63, 1.69) | 0.889 | 0.95 (0.55. 1.64) | 0.862 |
Values in parentheses are 95% confidence intervals. Results are shown for overall survival unless otherwise indicated. *For disease-free survival (DFS) see main text. Adjustments were made for the following variables: BMI, age, centre, T category, tumour location, ASA grade, and operative approach.
Short-term outcomes
Short-term outcomes and complications are summarized in Table 2. Intraoperative and overall postoperative complications were comparable. Anastomotic leakage was noted in 3.4 per cent of the CME group and 1.6 per cent of the non-CME group (P = 0.071). Postoperative 30-and 90-day mortality rates as well as hospital stay were comparable.
Table 2.
Short-term outcomes and complications
| All patients | No CME | CME | P* | |
|---|---|---|---|---|
| No. of deaths | ||||
| Within 30 days | 1 (0.1) | 1 (0.2) | 0 (0) | 1.000 |
| Within 90 days | 13 (1.3) | 9 (1.8) | 4 (0.8) | 0.26 |
| No. of intraoperative complications | 12 (1.2) | 6 (1.2) | 6 (1.2) | 1.000 |
| Tumour laceration | 1 (0.1) | 1 (0.2) | 0 (0) | 1.000 |
| Blood loss > 500 ml | 2 (0.2) | 0 (0) | 2 (0.4) | 0.499 |
| Pancreatic injury | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Intestinal injury | 6 (0.6) | 5 (1.0) | 1 (0.2) | 0.217 |
| Ureteral injury | 1 (0.1) | 0 (0) | 1 (0.2) | 1.000 |
| Anastomotic complications | 1 (0.1) | 0 (0) | 1 (0.2) | 1.000 |
| Injury to SMV | 1 (0.1) | 0 (0) | 1 (0.2) | 1.000 |
| No. of general postoperative complications | 181 (18.0) | 92 (18.1) | 89 (17.9) | 0.780 |
| Pulmonary embolism | 3 (0.3) | 2 (0.4) | 1 (0.2) | 1.000 |
| Pneumonia | 35 (3.5) | 21 (4.1) | 14 (2.8) | 0.305 |
| Other pulmonary | 14 (1.4) | 6 (1.2) | 8 (1.6) | 0.601 |
| Urinary tract infection | 25 (2.5) | 13 (2.6) | 12 (2.4) | 1.000 |
| Fever | 13 (1.3) | 4 (0.8) | 9 (1.8) | 0.174 |
| Cardiac | 29 (2.9) | 15 (3.0) | 14 (2.8) | 1.000 |
| Multiple organ failure | 1 (0.1) | 0 (0) | 1 (0.2) | 0.449 |
| Deep vein thrombosis | 1 (0.1) | 1 (0.2) | 0 (0) | 1.000 |
| Renal | 15 (1.5) | 10 (2.0) | 5 (1.0) | 0.299 |
| Other | 45 (4.5) | 20 (3.9) | 25 (5.0) | 0.449 |
| No. of surgical postoperative complications | 249 (24.8) | 120 (23.6) | 129 (26.0) | 0.290 |
| Bleeding | 7 (0.7) | 3 (0.6) | 4 (0.8) | 0.723 |
| Wound abscess | 6 (0.6) | 3 (0.6) | 3 (0.6) | 1.000 |
| Sepsis | 7 (0.7) | 5 (1.0) | 2 (0.4) | 0.452 |
| Anastomotic leakage | 25 (2.5) | 8 (1.6) | 17 (3.4) | 0.071 |
| Aseptic wound complication | 12 (1.2) | 5 (1.0) | 7 (1.4) | 0.575 |
| Wound infection | 42 (4.2) | 23 (4.5) | 19 (3.8) | 0.690 |
| Intra-abdominal abscess | 8 (0.8) | 4 (0.8) | 4 (0.8) | 1.000 |
| Mechanical ileus | 5 (0.5) | 2 (0.4) | 3 (0.6) | 0.634 |
| Peritonitis | 12 (1.2) | 5 (1.0) | 7 (1.4) | 0.575 |
| Postoperative functional | 72 (7.2) | 38 (7.5) | 34 (6.9) | 0.807 |
| Abdominal wall dehiscence | 19 (1.9) | 8 (1.6) | 11 (2.2) | 0.496 |
| Other | 34 (3.4) | 16 (3.1) | 18 (3.6) | 0.730 |
| No. of patients who had relaparotomies | 0.490 | |||
| 1 | 61 (6.1) | 30 (5.9) | 31 (6.1) | |
| 2 | 7 (0.7) | 3 (0.6) | 4 (0.8) | |
| > 2 | 15 (1.5) | 5 (1.0) | 10 (2.0) | |
| Not available | 20 (2.0) | 16 (3.1) | 4 (0.8) | |
| No. of patients with complications | 289 (28.8) | 141 (27.8) | 148 (29.8) | 0.466 |
| Clavien–Dindo grade I–IIIa | 202 (20.1) | 101 (19.9) | 101 (20.4) | 0.878 |
| Clavien–Dindo grade IIIb–IV | 87 (8.7) | 40 (7.9) | 47 (9.5) | 0.435 |
| Duration of hospital stay (days), mean(s.d.) | 14.6 (9.6) | 13.9 (11.4) | 13.1 (8.3) | 0.206† |
Values are n (%) unless otherwise indicated. Leakage refers to grade C (need for relaparotomy). No chronic anastomotic sinuses were reported. Two intraoperative complications (bleeding and superior mesenteric vein (SMV) injury) occurred in one patient in the CME group. CME, complete mesocolic excision. *χ2test, except †? test.
Pathological outcomes
There were no differences in the distribution of stages, suggesting that CME did not alter staging, despite there being slightly more lymph nodes in CME specimens (mean 55.0 versus 50.4) (Table 3). The plane of surgery was classified as mesocolic in nearly all specimens, with no difference between groups. Mean mesocolic surface of the specimens could be assessed in 809 specimens (80.5 per cent), with considerable variability (high standard deviation). The prespecified cut-off of at least 15 000 mm2 was met in 36.5 and 27.8 per cent of patients in the CME and non-CME groups respectively. CME was associated with a larger MA in the unadjusted and adjusted linear regression analyses. Positive lymph nodes were detected in the central mesentery in 30 of 508 patients (5.9 per cent) in the non-CME and 20 of 496 (4.0 per cent) in the CME group. In stage III alone, central nodes were positive in 19.1 per cent of non-CME and 13.1 per cent of CME specimens.
Table 3.
Pathological outcomes
| All patients | No CME | CME | P* | |
|---|---|---|---|---|
| T category | 0.920 | |||
| T1 | 63 (6.3) | 34 (54.0) | 29 (46.0) | |
| T2 | 278 (27.7) | 142 (51.1) | 136 (48.9) | |
| T3 | 619 (61.7) | 312 (50.4) | 307 (49.6) | |
| T4 | 40 (4.0) | 19 (47.5) | 21 (52.5) | |
| Not available | 4 (0.4) | 1 (25.0) | 3 (75.0) | |
| N category | 0.950 | |||
| N0 | 697 (69.4) | 351 (50.4) | 346 (49.6) | |
| N1 | 180 (17.9) | 93 (51.7) | 87 (48.3) | |
| N2 | 125 (12.5) | 64 (51.2) | 61 (48.8) | |
| Not available | 2 (0.2) | 0 (0) | 2 (100) | |
| Tumour grade | 0.499 | |||
| 1 | 34 (33.9) | 16 (47.1) | 18 (52.9) | |
| 2 | 706 (70.3) | 366 (51.8) | 340 (48.2) | |
| 3 | 261 (26.0) | 125 (47.9) | 136 (52.1) | |
| Not available | 3 (0.3) | 1 (33.3) | 2 (66.7) | |
| Lymphatic invasion | 0.344 | |||
| L0 | 252 (25.1) | 121 (48.0) | 131 (52.0) | |
| L1 | 750 (74.7) | 387 (51.6) | 363 (48.4) | |
| Not available | 2 (0.2) | 0 (0) | 2 (100) | |
| Venous invasion | 0.377 | |||
| V0 | 483 (48.1) | 252 (52.2) | 231 (47.8) | |
| V1 | 519 (51.7) | 256 (49.3) | 263 (50.7) | |
| Not available | 2 (0.2) | 0 (0) | 2 (100) | |
| Perineural invasion | 0.049 | |||
| Pn0 | 885 (88.1) | 459 (51.9) | 426 (48.1) | |
| Pn1 | 117 (11.7) | 49 (41.9) | 68 (58.1) | |
| Not available | 2 (0.2) | 0 (0) | 2 (100) | |
| UICC stage | 0.960 | |||
| I | 302 (30.1) | 151 (50.0) | 151 (50.0) | |
| II | 394 (39.2) | 199 (50.5) | 195 (49.5) | |
| III | 304 (30.3) | 157 (51.6) | 147 (48.4) | |
| IV | 2 (0.2) | 1 (50.0) | 1 (50.0) | |
| Not available | 2 (0.2) | 0 (0) | 2 (100) | |
| R classification | – | |||
| R0 | 1001 (99.7) | 507 (50.6) | 494 (49.4) | |
| R1 | 0 (0) | 0 (0) | 0 (0) | |
| Not available | 3 (0.3) | 1 (33.3) | 2 (66.7) | |
| Tumour location | 0.588 | |||
| Caecum | 410 (40.8) | 213 (51.9) | 197 (48.0) | |
| Ascending colon | 500 (49.8) | 248 (49.6) | 252 (50.4) | |
| Hepatic flexure | 72 (7.2) | 33 (45.8) | 39 (54.2) | |
| Right transverse colon | 4 (0.4) | 3 (75.0) | 1 (25.0) | |
| Overlapping | 18 (1.8) | 11 (61.1) | 7 (38.9) | |
| Histological type | 0.570 | |||
| Adenocarcinoma | 699 (69.6) | 353 (50.5) | 346 (49.5) | |
| Partial mucinous | 253 (25.2) | 131 (51.8) | 122 (48.2) | |
| Mucinous | 52 (5.2) | 24 (46.2) | 28 (53.8) | |
| CME grade19 | 0.119 | |||
| I | 996 (99.2) | 507 (50.9) | 489 (49.1) | |
| II | 6 (0.6) | 1 (16.7) | 5 (83.3) | |
| Not available | 2 (0.2) | 0 (0) | 2 (100) | |
| Mesocolic area (mm2), mean(s.d.) | 9947 (7327) | 11 444 (7967) | 0.002† | |
| < 15 000 | 548 (54.6) | 288 (52.6) | 260 (47.4) | 0.013 |
| ≥ 15 000 | 261 (26.0) | 111 (42.5) | 150 (57.5) | |
| Not available | 195 (19.4) | 109 (55.9) | 86 (44.1) | |
| No. of lymph nodes, mean(s.d.) | 50.4 (16.8) | 55.6 (17.6) | <0.001† |
Values are n (%) unless otherwise indicated. CME, complete mesocolic excision. *χ2test, except †? test.
Survival
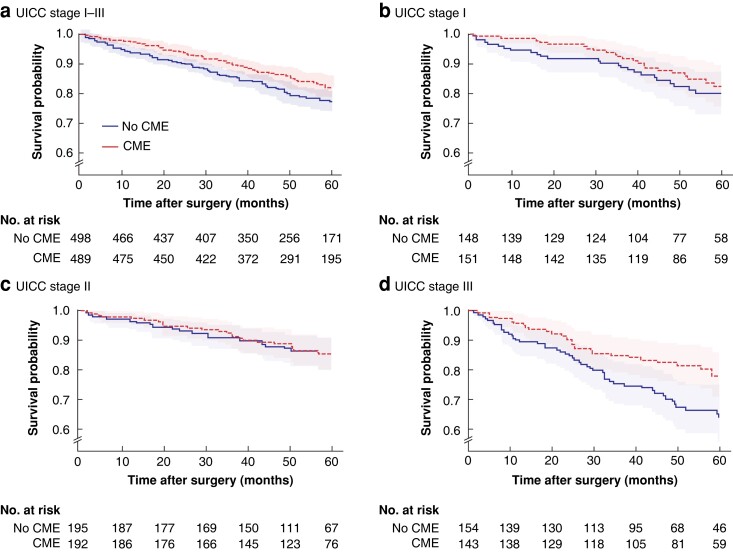
For the primary outcome variable on which the statistical power calculation was based, Kaplan–Meier and multivariable analyses found no difference in survival for CME in any stage (Table 4). For reasons stated above OAS had to be used as main outcome measure instead of DFS (Fig. 2). A cut-off of 15 000 mm2 for MA (secondary endpoint) did not discriminate survival in any stage.
Fig. 2.
Kaplan–Meier overall survival curves according to UICC stage and whether complete mesocolic excision was performed
a Stage I–III, b stage I, c stage II, and d stage III. Shaded areas represent 95% confidence intervals. The stage I–III curves represent unadjusted analysis. For adjusted P values see Table 4. CME, complete mesocolic excision.
In a separate assessment of stage III in an exploratory analysis (overall survival, not cancer-specific), CME had an unadjusted HR of 0.61 (95 per cent c.i. 0.39 to 0.95; P = 0.031) and an adjusted HR of 0.52 (0.31 to 0.85; P = 0.01) in Cox regression (Table 4). Sensitivity analysis of this result confirmed HRs below 0.66 (Table S1). The 5-year overall survival probability for CME in stage III was 0.783 (95 per cent c.i. 0.707 to 0.858) compared with 0.650 (0.565 to 0.735) in the non-CME group (Table 5). Furthermore, a larger MA, treated as a continuous variable, was associated with better overall (not cancer-specific) survival in stage III in adjusted Cox regression (HR 0.53, 0.32 to 0.88) (Table 4). In sensitivity analysis, HRs ranged from 0.95 to 0.97 (Table S1).
Table 5.
Five-year overall survival probabilities according to UICC stage
| 5-year survival probability | |
|---|---|
| Stage I–III | |
| No CME | 0.778 (0.740, 0.819) |
| CME | 0.814 (0.777, 0.851) |
| Difference | 0.035 (−0.016, 0.08) |
| Stage I | |
| No CME | 0.791 (0.717, 0.865) |
| CME | 0.832 (0.771, 0.893) |
| Difference | 0.041 (−0.047, 0.128) |
| Stage II | |
| No CME | 0.856 (0.807, 0.906) |
| CME | 0.838 (0.782, 0.894) |
| Difference | 0.018 (−0.086, 0.049) |
| Stage III | |
| No CME | 0.650 (0.565, 0.735) |
| CME | 0.783 (0.707, 0.858) |
| Difference | 0.133 (0.023, 0.242) |
Values in parentheses are 95% confidence intervals. The survival probabilities were obtained using multivariate Cox regression models to perform direct standardization. The corresponding confidence intervals were obtained using a normal approximation, where the asymptotic variance was estimated using the influence function. CME, complete mesocolic excision.
Discussion
No difference was found for the primary and secondary outcomes of this study; CME did not influence survival. The subanalysis of stage III describing a risk reduction is reported cautiously, and interpreted in the context of overall (not disease-specific) survival and limited statistical power. The association between MA as a continuous variable (rather than the threshold value in the secondary outcome) with overall survival in stage III was weaker. Although the evidence from this trial suggests no general survival benefit from CME, stage III disease needs further evaluation.
The study had a pragmatic registry-based design with a high rate of follow-up, but the assessment of recurrences did not reach the required level to calculate cancer-specific survival. The design of the study relied on variation in the accomplishment of wide mesocolic excision. At the time of protocol development (2008–2010), the authors considered that at least half of the centres would perform only limited resections. The mesocolic surface area of the non-CME group was larger than the older data upon which it was based28. The CME group had a larger mesocolic surface but did not quite achieve the values published by West et al.28 (16 769 mm2) and others (open 15 788 mm2, laparoscopic 15 097 mm2)29 for CME.
It could also be argued that a clearer difference was not detected because this was not a randomized trial. Confounding was reduced by the fact that every surgeon was to pursue their current operative standard and selection bias was addressed by applying advanced statistical methods (to adjust for potentially uneven group allocation)30. The similar patient and pathological characteristics indicated that balanced grouping was largely accomplished in this way. The identical overall survival in stages I and II underscores this notion.
Limiting complication risks is essential in routine implementation of surgery3–12. The borderline significant higher rate of anastomotic leakage in the CME group is of some concern. This may be related to an impairment of perfusion of the left transverse colon by inadvertent ligation of the middle colic artery. Using the critical view concept31, with reliable preservation of the left branch of the middle colic artery, is important. Current developments in standardization and didactics31 and visualization32 may help. Only 20 per cent of operations were performed laparoscopically (including conversions), which reflects the rate of laparoscopic surgery in Germany at the time33. The study was not powered to detect differences between laparoscopic and open CME, so only a univariable analysis to exclude unexpected effects was undertaken (data not shown).
A strength of the study was the prospective multicentre registry-based design, yet pathology being evaluated at one institution using mesocolic planimetry, documentation of the plane of surgery, and exhaustive lymph node counting. Limitations were that follow-up could not be performed meticulously for recurrence, and disease-specific survival could not be assessed for the intended endpoints. This presumably led to reduced discrimination and internal validity. It cannot be excluded that additional unknown factors influenced group allocation and outcomes.
Not all studies have reported a beneficial effect of CME34 so the story is not complete. More data from ongoing and future trials are needed. Until then, outside the context of expected stage III disease (for which the authors cautiously propose an advantage), CME should be performed only within clinical trials or quality assurance programmes because no general advantage was observed in the present study.
Supplementary Material
Acknowledgements
The authors acknowledge the support of S. Rhode and F. Otto, AN-Institut Magdeburg, for database management and follow-up. They thank the following centres for participating and supporting the trial: Ameos Klinikum Aschersleben, Zollernalb Klinikum Albstadt, Klinikum St Marien Amberg, Klinikum Ansbach, Lukas-Krankenhaus Bünde, Darmzentrum Berlin-Buch, Klinikum Brandenburg, Klinikum Uelzen, Krankenhaus Buchholz und Winsen, Malteser-Krankenhaus Bonn, Sozialstiftung Bamberg, St Agnes-Hospital Bocholt, Fürst-Stirum-Klinik Bruchsal, St-Josef-Hospital, Bochum, Krankenhaus St Joseph Stift Dresden, Vincenz-Hospital Datteln, Evangelisches Krankenhaus, Düsseldorf, Marien Hospital, Düsseldorf, Evangelisches Krankenhaus, Dinslaken, Werner Forssmann Krankenhaus, Barnim Kliniken an der Paar Friedberg, Klinikum Robert Koch Gehrden, Klinikum Gifhorn, Klinik am Eichert Göppingen, Main Kinzig Kliniken, Gelnhausen, Klinikum Herford, St Barbara Hamm-Heessen, KRH Klinikum Siloah Hannover, Klinikum Heidenheim, Vinzenz Krankenhaus Hannover, Sana Klinikum Hof, Westküstenklinikum Heide, St Anna Hospital Herne, Klinikum Hanau, St Bonifatius-Hospital Lingen, Krankenhaus Leonberg, Krankenhaus Lichtenfels, Klinikum Dritter Orden München, Krankenhaus Bethanien Moers, St Walburga-Krankenhaus Meschede, Klinken Nagold, Klinikum Neumarkt, St Martinus Hospital Olpe, Pius-Hospital Oldenburg, Klinikum Pinneberg, GPR-Klinikum Rüsselsheim, Helios Klinikum Siegburg, Kreiskrankenhaus Sigmaringen, Ameos Klinikum Schönebeck, Kliniken Villingen, Sofien- und Hufelandklinikum Weimar, Krankenhaus Winsen, Marien-Hospital Witten, St Marien-Hospital Buer, Gelsenkirchen.
Contributor Information
Stefan R Benz, Klinik für Allgemein-, Viszeral-, Thorax- und Kinderchirurgie Kliniken Boeblingen, Boeblingen, Germany.
Inke S Feder, Institut für Pathologie der Ruhr-Universität Bochum, Bochum, Germany.
Saskia Vollmer, Klinik für Allgemein-, Viszeral-, Thorax- und Kinderchirurgie Kliniken Boeblingen, Boeblingen, Germany.
Yu Tam, Institut für Pathologie der Ruhr-Universität Bochum, Bochum, Germany.
Anke Reinacher-Schick, Hämatologie und Onkologie mit Palliativmedizin, Ruhruniversität Bochum, Bochum, Germany.
Robin Denz, Abteilung für medizinische Informatik, Biometrie und Epidemiologie der Rur-Universität Bochum, Bochum, Germany.
Werner Hohenberger, Chirurgische Universitätsklinik Erlangen, Erlangen, Germany.
Hans Lippert, AN-Institut für Qualitätssicherung in der operativen Medizin, Magdeburg, Germany.
Andrea Tannapfel, Institut für Pathologie der Ruhr-Universität Bochum, Bochum, Germany.
Ingo Stricker, Institut für Pathologie der Ruhr-Universität Bochum, Bochum, Germany.
Funding
This study was funded by the participating centres.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Data are available for further analysis on request to the first author.
References
- 1. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis 2009;11:364–365. [DOI] [PubMed] [Google Scholar]
- 2. Bertelsen CA, Neuenschwander AU, Jansen JE, Tenma JR, Wilhelmsen M, Kirkegaard-Klitbo Aet al. 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol 2019;20:1556–1565 [DOI] [PubMed] [Google Scholar]
- 3. Bertelsen CA, Neuenschwander AU, Jansen JE, Kirkegaard-Klitbo A, Tenma JR, Wilhelmsen Met al. ; Copenhagen Complete Mesocolic Excision Study (COMES), Danish Colorectal Cancer Group (DCCG). Short-term outcomes after complete mesocolic excision compared with ‘conventional’ colonic cancer surgery. Br J Surg 2016;103:581–589 [DOI] [PubMed] [Google Scholar]
- 4. Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JRet al. ; Danish Colorectal Cancer Group. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 2015;16:161–168 [DOI] [PubMed] [Google Scholar]
- 5. Bernhoff R, Sjovall A, Granath F, Holm T, Martling A, Buchli C. Oncological outcomes after complete mesocolic excision in right-sided colon cancer: a population-based study. Colorectal Dis 2021;23:1404–1413 [DOI] [PubMed] [Google Scholar]
- 6. Gao Z, Wang C, Cui Y, Shen Z, Jiang K, Shen Det al. Efficacy and safety of complete mesocolic excision in patients with colon cancer: three-year results from a prospective, nonrandomized, double-blind, controlled trial. Ann Surg 2020;271:519–526 [DOI] [PubMed] [Google Scholar]
- 7. Merkel S, Weber K, Matzel KE, Agaimy A, Gohl J, Hohenberger W. Prognosis of patients with colonic carcinoma before, during and after implementation of complete mesocolic excision. Br J Surg 2016;103:1220–1229 [DOI] [PubMed] [Google Scholar]
- 8. Ow ZGW, Sim W, Nistala KRY, Ng CH, Koh FH, Wong NWet al. Comparing complete mesocolic excision versus conventional colectomy for colon cancer: a systematic review and meta-analysis. Eur J Surg Oncol 2021;47:732–737 [DOI] [PubMed] [Google Scholar]
- 9. De Simoni O, Barina A, Sommariva A, Tonello M, Gruppo M, Mattara Get al. Complete mesocolic excision versus conventional hemicolectomy in patients with right colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2021;36:881–892 [DOI] [PubMed] [Google Scholar]
- 10. Anania G, Davies RJ, Bagolini F, Vettoretto N, Randolph J, Cirocchi Ret al. Right hemicolectomy with complete mesocolic excision is safe, leads to an increased lymph node yield and to increased survival: results of a systematic review and meta-analysis. Tech Coloproctol 2021;25:1099–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferri V, Vicente E, Quijano Y, Duran H, Diaz E, Fabra Iet al. Right-side colectomy with complete mesocolic excision vs conventional right-side colectomy in the treatment of colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2021;36:1885–1904 [DOI] [PubMed] [Google Scholar]
- 12. Di Buono G, Buscemi S, Cocorullo G, Sorce V, Amato G, Bonventre Get al. Feasibility and safety of laparoscopic complete mesocolic excision (CME) for right-sided colon cancer: short-term outcomes. a randomized clinical study. Ann Surg 2020;274:57-62 [DOI] [PubMed] [Google Scholar]
- 13. Karachun A, Panaiotti L, Chernikovskiy I, Achkasov S, Gevorkyan Y, Savanovich Net al. Short-term outcomes of a multicentre randomized clinical trial comparing D2 versus D3 lymph node dissection for colonic cancer (COLD trial). Br J Surg 2020;107:499–508 [DOI] [PubMed] [Google Scholar]
- 14. Xu L, Su X, He Z, Zhang C, Lu J, Zhang Get al. Short-term outcomes of complete mesocolic excision versus D2 dissection in patients undergoing laparoscopic colectomy for right colon cancer (RELARC): a randomised, controlled, phase 3, superiority trial. Lancet Oncol 2021;22:391-401 [DOI] [PubMed] [Google Scholar]
- 15. Kobayashi H, West NP. CME versus D3 dissection for colon cancer. Clin Colon Rectal Surg 2020;33:344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Venkataramani AS, Bor J, Jena AB. Regression discontinuity designs in healthcare research. BMJ 2016;352:i1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benz S, Stricker I, Tam Y, Tannapfel A. CME or traditional surgery for right-sided colon cancer? Coloproctology 2017;39:184–189 [Google Scholar]
- 18. Deutsche Krebsgesellschaft, Erhebungsbogen für Darmkrebszentren 2019 . https://www.onkozert.de/wordpress/wp-content/uploads/2019/09/eb_dz-J1_190920.pdf
- 19. West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol 2008;9:857–865 [DOI] [PubMed] [Google Scholar]
- 20. Benedix F, Schmidt U, Mroczkowski P, Gastinger I, Lippert H, Kube R. Colon carcinoma – classification into right and left sided cancer or according to colonic subsite?—analysis of 29 568 patients. Eur J Surg Oncol 2011;37:134–139 [DOI] [PubMed] [Google Scholar]
- 21. Kube R, Mroczkowski P, Granowski D, Benedix F, Sahm M, Schmidt Uet al. Anastomotic leakage after colon cancer surgery: a predictor of significant morbidity and hospital mortality, and diminished tumour-free survival. Eur J Surg Oncol 2010;36:120–124 [DOI] [PubMed] [Google Scholar]
- 22. Pearl J. Causality: models, reasoning and inference. Cambridge University press, New York, 2009 [Google Scholar]
- 23. McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods 2004;9:403–425 [DOI] [PubMed] [Google Scholar]
- 24. Hu L, Gu C, Lopez M, Ji J, Wisnivesky J. Estimation of causal effects of multiple treatments in observational studies with a binary outcome. Stat Methods Med Res 2020;29:3218–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ozenne BMH, Sheike TH, Stærk L, Gerds L. On the estimation of average treatment effects with right-censored time to event outcome and competing risks. Biom J 2020;62:751–763 [DOI] [PubMed] [Google Scholar]
- 26. van Buuren S. Flexible imputation of missing data (2nd edn). Chapman and Hall/CRC, Boca Raton, 2018 [Google Scholar]
- 27. Benz S Deutsches Klinische Studienregister, Multizentrische Prospektive Evaluation der Resektatqualität von rechtsseitigen Kolonkarzinomen 2017 . https://www.drks.de/drks_web/navigate.do?navigationId = trial.HTML&TRIAL_ID = DRKS00012400
- 28. West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 2010;28:272–278 [DOI] [PubMed] [Google Scholar]
- 29. Benz S, Tam Y, Tannapfel A, Stricker I. The uncinate process first approach: a novel technique for laparoscopic right hemicolectomy with complete mesocolic excision. Surg Endosc 2016;30:1930–1937 [DOI] [PubMed] [Google Scholar]
- 30. Pearl J. Comment: graphical models, causality and intervention. Stat Sci 1993;8:266–269 [Google Scholar]
- 31. Strey CW, Wullstein C, Adamina M, Agha A, Aselmann H, Becker Tet al. Laparoscopic right hemicolectomy with CME: standardization using the ‘critical view’concept. Surg Endosc 2018;32:5021–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fletcher J, Ilangovan R, Hanna G, Miskovic D, Lung P. The impact of three-dimensional reconstruction and standardised CT interpretation (AMIGO) on the anatomical understanding of mesenteric vascular anatomy for planning complete mesocolic excision surgery: a randomised crossover study. Colorectal Dis 2022;24:388–400 [DOI] [PubMed] [Google Scholar]
- 33. Benz S, Barlag H, Gerken M, Furst A, Klinkhammer-Schalke M. Laparoscopic surgery in patients with colon cancer: a population-based analysis. Surg Endosc 2017;31:2586–2595 [DOI] [PubMed] [Google Scholar]
- 34. Olofsson F, Buchwald P, Elmstahl S, Syk I. No benefit of extended mesenteric resection with central vascular ligation in right-sided colon cancer. Colorectal Dis 2016;18:773–778 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available for further analysis on request to the first author.