Abstract
Background
Anastomotic leak is a severe complication after oesophagectomy. Anastomotic leak has diverse clinical manifestations and the optimal treatment strategy is unknown. The aim of this study was to assess the efficacy of treatment strategies for different manifestations of anastomotic leak after oesophagectomy.
Methods
A retrospective cohort study was performed in 71 centres worldwide and included patients with anastomotic leak after oesophagectomy (2011–2019). Different primary treatment strategies were compared for three different anastomotic leak manifestations: interventional versus supportive-only treatment for local manifestations (that is no intrathoracic collections; well perfused conduit); drainage and defect closure versus drainage only for intrathoracic manifestations; and oesophageal diversion versus continuity-preserving treatment for conduit ischaemia/necrosis. The primary outcome was 90-day mortality. Propensity score matching was performed to adjust for confounders.
Results
Of 1508 patients with anastomotic leak, 28.2 per cent (425 patients) had local manifestations, 36.3 per cent (548 patients) had intrathoracic manifestations, 9.6 per cent (145 patients) had conduit ischaemia/necrosis, 17.5 per cent (264 patients) were allocated after multiple imputation, and 8.4 per cent (126 patients) were excluded. After propensity score matching, no statistically significant differences in 90-day mortality were found regarding interventional versus supportive-only treatment for local manifestations (risk difference 3.2 per cent, 95 per cent c.i. −1.8 to 8.2 per cent), drainage and defect closure versus drainage only for intrathoracic manifestations (risk difference 5.8 per cent, 95 per cent c.i. −1.2 to 12.8 per cent), and oesophageal diversion versus continuity-preserving treatment for conduit ischaemia/necrosis (risk difference 0.1 per cent, 95 per cent c.i. −21.4 to 1.6 per cent). In general, less morbidity was found after less extensive primary treatment strategies.
Conclusion
Less extensive primary treatment of anastomotic leak was associated with less morbidity. A less extensive primary treatment approach may potentially be considered for anastomotic leak. Future studies are needed to confirm current findings and guide optimal treatment of anastomotic leak after oesophagectomy.
The aim of this retrospective cohort study was to investigate the efficacy of different treatment strategies for anastomotic leak after oesophagectomy. The study found that less extensive primary treatment resulted in less morbidity. Therefore, less extensive primary treatment could potentially lead to better clinical outcomes, but findings should be confirmed in future studies.
Introduction
Oesophagectomy is a crucial component of multimodal treatment of patients with curable oesophageal cancer1,2. However, oesophagectomy is associated with considerable morbidity and risk of complications3–5. Anastomotic leak (AL) is a severe and potentially life-threatening complication after oesophagectomy. AL occurs in up to 30 per cent of patients and annually 20 000 patients develop AL after oesophagectomy worldwide3–8. AL is a main contributor to postoperative morbidity and mortality after oesophagectomy and is associated with poor oncological survival and decreased quality of life4,9–14. Although various treatment strategies have been described10,15,16, the optimal treatment strategy for patients with AL is unknown5,17–19.
The arsenal for treatment of AL includes an array of supportive, radiological, endoscopic, and surgical interventions. Several studies have reported outcomes of a single modality, such as endoscopic vacuum-assisted closure (EVAC)20–22. However, recent systematic reviews have concluded that evidence to support a specific treatment of AL is currently lacking, as most studies are small, descriptive studies, lacking comparative analyses, and do not consider leak severity or clinical manifestation of AL17,18. The clinical presentation of AL is diverse and, in clinical practice, the treatment strategy is generally determined by the presence of intrathoracic fluid collections and the vitality of the conduit19. Consequently, investigating the optimal treatment should be guided by these findings. Furthermore, instead of evaluating the outcomes of a single intervention, identifying a treatment strategy based on treatment principles may have more clinical relevance. Different principles of AL treatment have been identified: supportive interventions (for example antibiotics and feeding support); drainage of fluid collections; closing the defect to prevent further leakage (for example stent or EVAC); and oesophageal diversion (that is resection of the conduit and diversion using an oesophagostomy)19.
Gaining insight into the efficacy of treatment strategies for AL is crucial, in order to provide effective care and improve clinical outcomes. The aim of this study was to investigate the efficacy of different treatment strategies for different manifestations of AL after oesophagectomy.
Methods
Study design
The TENTACLE—Esophagus study is an international retrospective cohort study in 71 centres across 20 countries. Details of the TENTACLE—Esophagus study have been published previously (NCT03829098)23. Characteristics of the participating centres can be found in Table S1. Data quality validation by independent local validators showed a data accuracy of 96.5 per cent23. The protocol was approved by the institutional review board of Radboud University Medical Centre (review file 2018-4585) and by local ethical committees if needed. The need for individual informed consent was waived by the institutional review board due to the retrospective study design and pseudonymous data collection. This study was conducted in accordance with STROBE guidelines24.
Population
Consecutive patients with AL after oesophagectomy with gastric tube reconstruction for resectable oesophageal or gastro-oesophageal junction (GOJ) carcinoma (cT1–4a N0–3 M0) between January 2011 and June 2019 were included. AL was defined according to the Esophagectomy Complications Consensus Group (ECCG) definition: ‘a full thickness gastrointestinal defect involving oesophagus, anastomosis, staple line, or conduit irrespective of presentation or method of identification’25. Patients were excluded if they underwent extended gastrectomy or emergency resection, or if they died before treatment for AL was started.
Based on a recent mixed-methods study, consisting of an international survey and expert discussions, three different manifestations of AL were distinguished: (1) patients with local manifestations (that is confirmed leak without mediastinal/pleural fluid collections; well perfused conduit); (2) patients with intrathoracic manifestations (that is mediastinal and/or pleural collections; well perfused conduit); and (3) patients with overall ischaemia/necrosis of the gastric conduit19.
Treatment strategies
In line with recommendations of the mixed-methods study mentioned above, the current analysis focused on treatment principles rather than individual modalities, and four main treatment principles were defined: supportive care; drainage; defect closure; and oesophageal diversion19. Supportive care was defined as treatment that aimed to support the patient and that was not directed at the leak itself. This included antibiotic treatment and feeding interventions (for example feeding tube placement or jejunostomy). Drainage included any method to drain infectious fluids: chest tube placement; radiological drain placement; endoscopic drainage (that is nasogastric tube with suction or drain placement through the defect); EVAC; or surgical drainage via reoperation. Defect closure was defined as closure or covering the anastomotic defect using endoscopic techniques (that is EVAC, stent placement, or clipping) or surgical techniques (that is suturing, resection, and re-anastomosis, covering with muscle flap or other tissue). Oesophageal diversion was defined as resection of the conduit and diversion with cervical oesophagostomy.
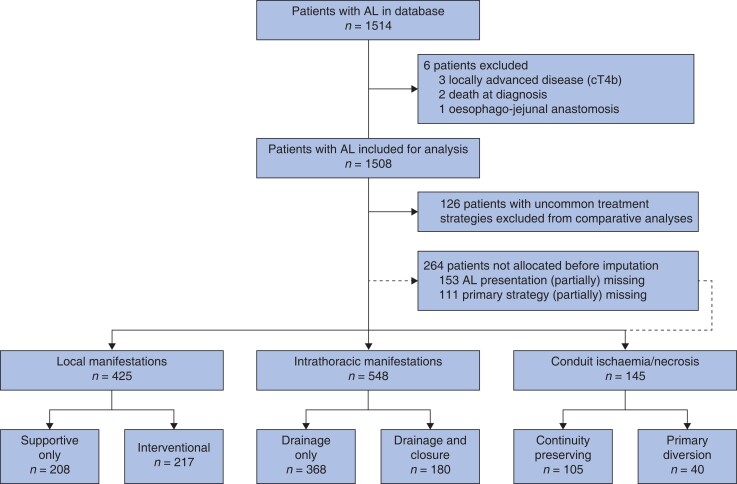
This study focused on the efficacy of primary treatment strategies and primary treatment was defined as treatment within 48 h after diagnosis of AL. Any treatment initiated greater than or equal to 48 h after diagnosis or after failure of primary treatment was considered secondary treatment. For each manifestation of AL, two common primary treatment strategies were identified based on previous literature. In patients with local manifestations, outcomes of a primary interventional strategy were compared with primary supportive-only treatment19. In patients with intrathoracic manifestations, the outcomes of drainage and defect closure (for example EVAC or stent combined with drainage) during primary treatment were compared with drainage only18,19,26,27. In patients with conduit ischaemia/necrosis, outcomes of primary oesophageal diversion were compared with ‘continuity-preserving’ treatment (that is no oesophageal diversion)19,28. An overview of the three manifestations of AL and compared primary treatment strategies is presented in Fig. 1. Patients were included in only one comparative analysis, in line with the defined manifestations and investigated treatment strategies. Patients who underwent different, uncommon primary treatment (for example oesophageal diversion for local manifestations) were not included in comparative analyses.
Fig. 1.
Distribution of patients per manifestation and treatment strategy
Broken lines represent patients who could not be allocated before multiple imputation owing to missing data. These patients have been allocated after multiple imputation and are not included in the number of cases per clinical manifestation presented. AL, anastomotic leak.
Outcomes
The primary outcome was 90-day mortality, defined as overall mortality within 90 days after oesophagectomy. Secondary outcomes included length of stay in hospital and on ICU, leak healing time (assessed by imaging or clinical confirmation by resuming a non-clear liquid diet), and comprehensive complication index (CCI). The CCI expresses overall patient morbidity, ranging from 0 to 100, by combining the severity of all postoperative complications29. No detailed sample size calculation was performed for the evaluation of treatment efficacy within the manifestations of AL, due to the explorative character of the study.
Statistical analysis
Missing data were assumed ‘missing at random’ and multiple imputation using chained equations was used to avoid bias during analysis30. Additional information on the handling of missing data is presented in Methods S1. Patient, leak-related, and treatment characteristics are described as count (percentage) or median (interquartile range (i.q.r.)), as appropriate.
Propensity score matching (PSM) was performed to minimize confounding bias in comparative analyses (Methods S2)31. PSM was performed separately per AL manifestation using multivariable logistic regression including known confounders: age; co-morbidity; performance status; tumour histology; postoperative day (POD) of diagnosis; level of care at diagnosis; diet at diagnosis; organ failure (that is respiratory failure, haemodynamic failure, renal failure, and quick sequential organ failure assessment (qSOFA) score); leucocyte count; intrathoracic fluid collections; defect circumference; hospital volume; and year of surgery. Cases were matched using nearest-neighbour matching, with a caliper of 0.2 and 2 : 1 ratio32,33. Standardized mean difference (SMD) was calculated to assess covariate balance between treatment groups before and after PSM, and an SMD less than 0.1 was considered to indicate sufficient balance34. Differences regarding primary (that is 90-day mortality) and secondary outcomes between treatment strategies were assessed using logistic regression for binary outcomes or linear regression for continuous outcomes and expressed as risk difference (RD) and OR with 95 per cent c.i. and absolute difference with 95 per cent c.i. respectively.
Multiple sensitivity analyses were performed to assess the robustness of the findings. First, in patients with local manifestations and intrathoracic manifestations, treatment efficacy was assessed separately for patients with either cervical or intrathoracic anastomosis after PSM, as previous studies have suggested that treatment may depend on the location of the anastomosis28,35. Second, to assess whether it is safe to start less extensive primary treatment and reserve more extensive interventions for secondary treatment if needed, outcomes of patients undergoing secondary treatment after less extensive primary treatment were compared with patients undergoing more extensive primary treatment (for example secondary treatment after drainage only versus primary drainage and defect closure for intrathoracic manifestations) after PSM. Third, as variation has been found in the treatment of AL related to annual resection volume, treatment efficacy was assessed in patients treated in middle- or high-volume centres (greater than or equal to 20 resections annually) and compared with findings in the entire cohort after PSM36,37. Finally, treatment success and other outcomes of different treatment modalities were assessed. Treatment success was defined as avoidance of 90-day mortality, secondary ICU readmission, and secondary oesophagostomy. No PSM was performed in this analysis due to small groups of patients.
PSM and comparative analyses were performed in each data set and results were pooled subsequently using Rubin’s rule. Statistical analysis was performed in R (version 3.6.2) with packages ‘mice’ and ‘matchit’.
Results
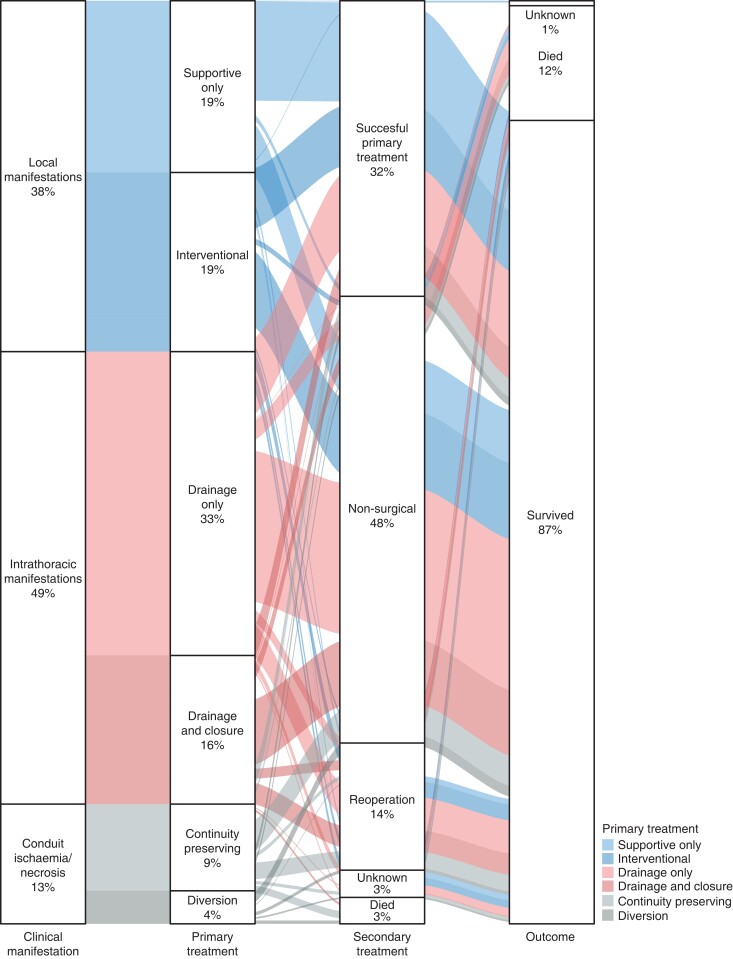
In total, 1514 patients were recorded in the database, of which 1508 patients were included in the current study. Six patients were excluded from the analysis; four patients because they did not meet the inclusion criteria (locally advanced diseases, three patients; and oesophago-jejunal anastomosis, one patient) and two patients because of death before treatment could be started. Figure 1 shows the flow chart of the study. Of 1508 patients, 425 patients (28.2 per cent) had local manifestations, 548 patients (36.3 per cent), had intrathoracic manifestations and 145 patients (9.6 per cent) had overall conduit ischaemia/necrosis (Table S2 and Table S3); 126 patients (8.4 per cent) were excluded from comparative analyses, as these patients were not treated in line with the predefined investigated treatment strategies, and 264 patients (17.5 per cent) could not be allocated before imputation due to missing data and were allocated after multiple imputation. Treatment and outcomes of patients with local manifestations, intrathoracic manifestations, and overall conduit ischaemia/necrosis before PSM are shown in Table 1, Table 2, and Table 3 respectively. In addition, Fig. 2 provides a graphical representation of primary treatment, secondary treatment, and outcome of different leak treatments per manifestation of AL.
Table 1.
Local manifestations before propensity score matching
| Parameter | Supportive only | Intervention |
|---|---|---|
| Patients, n | 208 | 217 |
| Age (years), median (i.q.r.) | 65 (57–71) | 67 (62–72) |
| Co-morbidity* | ||
| ASA I | 28 (13.5) | 14 (6.5) |
| ASA II | 125 (60.1) | 113 (52.1) |
| ASA ≥III | 54 (26.0) | 88 (40.6) |
| Performance status* | ||
| ECOG 0 | 99 (47.6) | 90 (41.5) |
| ECOG 1 | 62 (29.8) | 68 (31.3) |
| ECOG ≥2 | 16 (7.7) | 13 (6.0) |
| Resection type* | ||
| TTO-CA | 79 (38.0) | 73 (33.6) |
| TTO-IA | 57 (27.4) | 110 (50.7) |
| THO-CA | 71 (34.1) | 34 (15.7) |
| POD of diagnosis, median (i.q.r.) | 7 (6–9) | 7 (6–10) |
| Level of care* | ||
| Surgical ward | 153 (73.6) | 132 (60.8) |
| ICU/HC/MC/PACU | 43 (20.7) | 73 (33.6) |
| ED/other | 9 (4.3) | 10 (4.6) |
| Leucocyte count (×109/L), median (i.q.r.) | 10.5 (8.3–13.7) | 11.9 (9.1–15.4) |
| qSOFA score, median (i.q.r.) | 0 (0–0) | 0 (0–1) |
| Respiratory failure | 9 (4.3) | 33 (15.2) |
| Haemodynamic failure | 1 (0.5) | 10 (4.6) |
| Renal failure | 4 (1.9) | 10 (4.6) |
| Defect circumference ≥25% | 9 (4.3) | 22 (10.1) |
| Primary treatment | ||
| Primary strategy | ||
| Supportive only | 208 (100.0) | 0 (0.0) |
| Drainage | 0 (0.0) | 142 (65.4) |
| Defect closure | 0 (0.0) | 19 (8.8) |
| Drainage and defect closure | 0 (0.0) | 56 (25.8) |
| Feeding intervention | 25 (12.0) | 32 (15.7) |
| Endoscopic drainage | 0 (0.0) | 50 (23.0) |
| Stent placement | 0 (0.0) | 47 (21.7) |
| EVAC | 0 (0.0) | 16 (7.4) |
| Reoperation | 3 (1.4) | 51 (23.5) |
| ICU/HC/MC readmission | 11 (5.3) | 61 (28.1) |
| Secondary treatment | ||
| Need for secondary treatment | 78 (37.5) | 123 (56.7) |
| Secondary strategy | ||
| Supportive only | 52 (25.0) | 50 (23.0) |
| Drainage | 7 (3.4) | 23 (10.6) |
| Defect closure | 12 (5.8) | 19 (8.8) |
| Drainage and defect closure | 4 (1.9) | 25 (11.5) |
| Missing | 2 (1.0) | 2 (0.9) |
| Stent placement | 15 (7.2) | 28 (12.9) |
| EVAC | 0 (0.0) | 9 (4.1) |
| Reoperation | 7 (3.4) | 24 (11.1) |
| ICU/HC/MC readmission | 9 (4.3) | 21 (9.7) |
| Outcomes | ||
| 90-day mortality | 9 (4.3) | 23 (10.6) |
| LOS, hospital (days), median (i.q.r.) | 20 (15–28) | 28 (20–42) |
| LOS, ICU (days), median (i.q.r.) | 2 (1–5) | 5 (2–12) |
| Healing time (days), median (i.q.r.) | 19 (10–29) | 21.50 (9–37) |
| CCI, median (i.q.r.) | 31 (21–50) | 43 (32–65) |
Values are n (%) unless otherwise indicated. *Percentages may not add up to 100% due to missing data. i.q.r., interquartile range; ECOG, Eastern Collaborative Oncology Group; TTO, transthoracic oesophagectomy; CA, cervical anastomosis; IA, intrathoracic anastomosis; THO, transhiatal oesophagectomy; POD, postoperative day; HC, high care; MC, medium care; PACU, post-anaesthesia care unit; ED, emergency department; qSOFA, quick sequential organ failure assessment; EVAC, endoscopic vacuum-assisted closure; LOS, length of stay; CCI, comprehensive complication index.
Table 2.
Intrathoracic manifestations before propensity score matching
| Parameter | Drainage only | Drainage and defect closure |
|---|---|---|
| Patients, n | 368 | 180 |
| Age (years), median (i.q.r.) | 65 (58–71) | 65 (59–71) |
| Co-morbidity* | ||
| ASA I | 35 (9.5) | 16 (8.9) |
| ASA II | 213 (57.9) | 97 (53.9) |
| ASA ≥III | 116 (31.5) | 62 (34.4) |
| Performance status* | ||
| ECOG 0 | 172 (46.7) | 74 (41.1) |
| ECOG 1 | 96 (26.1) | 57 (31.7) |
| ECOG ≥2 | 30 (8.2) | 5 (2.8) |
| Resection type* | ||
| TTO-CA | 79 (21.5) | 21 (11.7) |
| TTO-IA | 257 (69.8) | 156 (86.7) |
| THO-CA | 29 (7.9) | 3 (1.7) |
| POD of diagnosis, median (i.q.r.) | 8 (6–11) | 8 (5–11) |
| Level of care* | ||
| Surgical ward | 206 (56.0) | 83 (46.1) |
| ICU/HC/MC/PACU | 140 (38.0) | 90 (50.0) |
| ED/other | 12 (3.3) | 6 (3.3) |
| Leucocyte count (×109/L), median (i.q.r.) | 13.6 (10.3–17.6) | 14.2 (11.0–18.6) |
| qSOFA score, median (i.q.r.) | 0 (0–1) | 1 (0–1) |
| Respiratory failure | 69 (18.8) | 51 (28.3) |
| Haemodynamic failure | 40 (11.7) | 29 (17.8) |
| Renal failure | 40 (10.9) | 29 (16.1) |
| Defect circumference ≥25% | 38 (10.3) | 49 (27.2) |
| Primary treatment | ||
| Primary strategy | ||
| Drainage | 368 (100.0) | 0 (0.0) |
| Drainage and defect closure | 0 (0.0) | 180 (100.0) |
| Radiological drainage | 109 (29.6) | 21 (11.7) |
| Chest tube drainage | 85 (23.1) | 38 (21.1) |
| Endoscopic drainage | 132 (35.9) | 53 (29.4) |
| Stent placement | 0 (0.0) | 104 (57.8) |
| EVAC | 0 (0.0) | 27 (15.0) |
| Reoperation | 99 (26.9) | 80 (44.4) |
| ICU/HC/MC readmission | 153 (41.6) | 110 (61.1) |
| Secondary treatment | ||
| Need for secondary treatment | 263 (71.5) | 138 (76.7) |
| Secondary strategy | ||
| Supportive only | 63 (17.1) | 20 (11.1) |
| Drainage | 110 (29.9) | 33 (18.3) |
| Defect closure | 20 (5.4) | 24 (13.3) |
| Drainage and defect closure | 56 (15.2) | 42 (23.3) |
| Oesophageal diversion | 3 (0.8) | 12 (6.7) |
| Missing | 11 (3.0) | 8 (4.4) |
| Stent placement | 59 (16.0) | 54 (30.0) |
| EVAC | 10 (2.7) | 21 (11.7) |
| Reoperation | 55 (14.9) | 38 (21.1) |
| ICU/HC/MC readmission | 74 (20.1) | 46 (25.6) |
| Outcomes | ||
| 90-day mortality | 42 (11.4) | 34 (18.9) |
| LOS, hospital (days), median (i.q.r.) | 37 (26–56) | 41 (28–65) |
| LOS, ICU (days), median (i.q.r.) | 8 (3, 18) | 11 (5–25) |
| Healing time (days), median (i.q.r.) | 31 (18–50) | 34 (18–58) |
| CCI, median (i.q.r.) | 50 (36–66) | 50 (35–69) |
Values are n (%) unless otherwise indicated. *Percentages may not add up to 100% due to missing data. i.q.r., interquartile range; ECOG, Eastern Collaborative Oncology Group; TTO, transthoracic oesophagectomy; CA, cervical anastomosis; IA, intrathoracic anastomosis; THO, transhiatal oesophagectomy; POD, postoperative day; HC, high care; MC, medium care; PACU, post-anaesthesia care unit; ED, emergency department; qSOFA, quick sequential organ failure assessment; EVAC, endoscopic vacuum-assisted closure; LOS, length of stay; CCI, comprehensive complication index.
Table 3.
Overall conduit ischaemia/necrosis before propensity score matching
| Parameter | Continuity preserving | Oesophageal diversion |
|---|---|---|
| Patients, n | 105 | 40 |
| Age (years), median (i.q.r.) | 66 (60–72) | 67 (61–73) |
| Co-morbidity* | ||
| ASA I | 8 (7.6) | 5 (13) |
| ASA II | 52 (49.5) | 18 (45) |
| ASA ≥III | 38 (36.2) | 17 (42) |
| Performance status* | ||
| ECOG 0 | 32 (30.5) | 19 (48) |
| ECOG 1 | 24 (22.9) | 15 (38) |
| ECOG ≥2 | 10 (9.5) | 5 (13) |
| Resection type* | ||
| TTO-CA | 36 (34.3) | 15 (38) |
| TTO-IA | 49 (46.7) | 21 (53) |
| THO-CA | 20 (19.0) | 1 (3) |
| POD of diagnosis, median (i.q.r.) | 7 (5–11) | 6 (4–9) |
| Level of care* | ||
| Surgical ward | 42 (40.0) | 13 (32.5) |
| ICU/HC/MC/PACU | 57 (54.3) | 25 (62.5) |
| ED/other | 5 (4.8) | 0 (0) |
| Leucocyte count (×109/L), median (i.q.r.) | 11.4 (8.7–18.6) | 12.7 (8.2–19.9) |
| qSOFA score, median (i.q.r.) | 1 (0–2) | 1 (0–2) |
| Respiratory failure | 34 (32.4) | 11 (28) |
| Haemodynamic failure | 23 (21.9) | 8 (20) |
| Renal failure | 5 (4.8) | 5 (13) |
| Defect circumference ≥25% | 24 (22.9) | 22 (55) |
| Primary treatment | ||
| Primary strategy | ||
| Supportive only | 12 (11.4) | 0 (0) |
| Drainage | 32 (30.5) | 0 (0) |
| Defect closure | 12 (11.4) | 0 (0) |
| Drainage and defect closure | 35 (33.3) | 0 (0) |
| Oesophageal diversion | 0 (0.0) | 40 (100) |
| Radiological drainage | 7 (6.7) | 2 (5) |
| Chest tube drainage | 14 (13.3) | 2 (5) |
| Endoscopic drainage | 24 (22.9) | 3 (8) |
| Stent placement | 30 (28.6) | 0 (0) |
| EVAC | 5 (4.8) | 0 (0) |
| Reoperation | 41 (39.0) | 40 (100) |
| ICU/HC/MC readmission | 39 (37.1) | 27 (68) |
| Secondary treatment | ||
| Need for secondary treatment | 69 (65.7) | 24 (60) |
| Secondary strategy | ||
| Supportive only | 14 (13.3) | 8 (20) |
| Drainage | 19 (18.1) | 6 (15) |
| Defect closure | 4 (3.8) | 0 (0) |
| Drainage and defect closure | 20 (19.0) | 1 (3) |
| Oesophageal diversion | 5 (4.8) | 0 (0) |
| Missing | 7 (6.7) | 9 (23) |
| Stent placement | 17 (16.2) | 0 (0) |
| EVAC | 2 (1.9) | 0 (0) |
| Reoperation | 25 (23.8) | 5 (13) |
| ICU/HC/MC readmission | 24 (22.9) | 6 (15) |
| Outcomes | ||
| 90-day mortality | 20 (19.0) | 11 (28) |
| LOS, hospital (days), median (i.q.r.) | 43 (27–61) | 42 (28–72) |
| LOS, ICU (days), median (i.q.r.) | 12 (3–29) | 17 (7–30) |
| Healing time (days), median (i.q.r.) | 32 (13–54) | 24 (24–26) |
| CCI, median (i.q.r.) | 49 (34–63) | 56 (40–69) |
Values are n (%) unless otherwise indicated. *Percentages may not add up to 100 per cent due to missing data. i.q.r., interquartile range; ECOG, Eastern Collaborative Oncology Group; TTO, transthoracic oesophagectomy; CA, cervical anastomosis; IA, intrathoracic anastomosis; THO, transhiatal oesophagectomy; POD, postoperative day; HC, high care; MC, medium care; PACU, post-anaesthesia care unit; ED, emergency department; qSOFA, quick sequential organ failure assessment; EVAC, endoscopic vacuum-assisted closure; LOS, length of stay; CCI, comprehensive complication index.
Fig. 2.
Alluvial diagram on primary treatment, secondary treatment, and outcome of patients with anastomotic leak
Local manifestations
Of 425 patients with local manifestations, 217 patients (51.1 per cent) underwent primary interventional treatment and 208 patients (48.9 per cent) underwent supportive-only treatment (Table 1). After PSM, a good balance was achieved between the interventional and supportive-only treatment groups (Table S4). There was no statistically significant difference in 90-day mortality after interventional versus supportive-only primary treatment (RD 3.2 per cent, 95 per cent c.i. −1.8 to 8.2 per cent). After primary interventional treatment, length of stay in hospital and on ICU were statistically significantly longer (hospital 8 days, 95 per cent c.i. 6 to 10 days; ICU 3 days, 95 per cent c.i. 1 to 4 days) and CCI was higher (9.0, 95 per cent c.i. 6.7 to 11.3) compared with supportive-only treatment (Table 4).
Table 4.
Outcomes of leak treatment after propensity score matching
| Outcome | Local manifestations (interventional versus supportive only (reference)) | Intrathoracic manifestations (drainage and defect closure versus drainage only (reference)) | Conduit ischaemia/necrosis (oesophageal diversion versus continuity-preserving (reference)) |
|---|---|---|---|
| 90-day mortality | |||
| Risk difference (95% c.i.) | 3.2% (−1.8%,8.2%) | 5.8% (−1.2%,12.8%) | 0.1% (−21.4%,21.6%) |
| OR (95% c.i.) | 1.96 (0.72,5.39) | 1.58 (0.92,2.71) | 1.01 (0.29,3.43) |
| LOS in hospital in days (95% c.i.) | 8 (6,10) | 6 (4,8) | −1 (−10,7) |
| LOS on ICU in days (95% c.i.) | 3 (1,4) | 5 (4,7) | −2 (−8,4) |
| Healing time in days (95% c.i.) | 2 (−1,6) | 5 (2,9) | −12 (−30,7)* |
| CCI (95% c.i.) | 9.0 (6.7,11.3) | 6.2 (4.0,8.4) | 6.2 (−2.1,14.5) |
*Not available for most patients who underwent oesophageal diversion. LOS, length of stay; CCI, comprehensive complication index.
Intrathoracic manifestations
Of 548 patients with intrathoracic manifestations, 368 patients (67.2 per cent) underwent primary drainage only and 180 patients (32.8 per cent) underwent primary drainage and defect closure (Table 2). After PSM, covariates were appropriately balanced between the two groups (Table S5). No statistically significant difference in 90-day mortality was found after primary drainage and defect closure versus drainage only (RD 5.8 per cent, 95 per cent c.i. −1.2 to 12.8 per cent). After drainage and defect closure, length of stay in hospital and on ICU (hospital 6 days, 95 per cent c.i. 4 to 8 days; ICU 5 days, 95 per cent c.i. 4 to 7 days) and healing time (5 days, 95 per cent c.i. 2 to 9 days) were statistically significantly longer and CCI was higher (6.2, 95 per cent c.i. 4.0 to 8.4) compared with drainage only (Table 4).
Overall conduit ischaemia/necrosis
Of 145 patients with overall conduit ischaemia/necrosis, 40 patients (27.6 per cent) underwent primary oesophageal diversion and 105 patients (72.4 per cent) underwent continuity-preserving treatment (Table 3). Only five patients (4.8 per cent) underwent secondary oesophageal diversion after primary continuity-preserving treatment. After PSM, covariate balance was achieved (Table S6). No statistically significant difference in 90-day mortality was found between the two treatments (RD 0.1 per cent, 95 per cent c.i. −21.4 to 21.6 per cent). After primary oesophageal diversion, length of stay in hospital and on ICU were shorter (1 day (95 per cent c.i. −10 to 7) and 2 days (95 per cent c.i. −8 to 4) respectively) and CCI was higher (6.2, 95 per cent c.i. −2.1 to 14.5), but not statistically significant (Table 4).
Sensitivity analyses
Adjusted outcomes of either cervical or intrathoracic AL in patients with local manifestations and intrathoracic manifestations are presented in Table S7. In patients with intrathoracic manifestations, differences between drainage only versus drainage and defect closure were larger in cervical leaks than in intrathoracic leaks. For example there was a larger difference in duration of ICU care; 19 days (95 per cent c.i. 13 to 25 days) longer after drainage and defect closure in cervical leaks versus 3 days (95 per cent c.i. 2 to 4 days) longer after drainage and defect closure in intrathoracic leaks. However, only 28 patients with cervical leaks underwent drainage and defect closure.
In all manifestations of AL, patients who underwent secondary treatment after (failure of) less extensive primary treatment did not have poorer outcomes than patients who primarily underwent more extensive treatment (Table S8). Treatment outcomes of patients treated in middle- and high-volume centres are presented in Table S9, and showed no substantial differences compared with treatment outcomes in all centres. Details on the primary treatment modalities per manifestation of AL and consequent outcomes are presented in Table S10.
Discussion
This large, collaborative cohort study investigated the efficacy of primary treatment strategies for different manifestations of AL after oesophagectomy. Across the three manifestations of AL, patients who underwent less extensive primary treatment had better outcomes and reduced morbidity compared with patients who underwent more extensive treatment. These findings suggest that a less extensive approach to primary treatment of AL may potentially lead to better clinical outcomes.
Previous studies were hampered by limited numbers of patients, lack of detailed data, confounding bias, and heterogeneity17. To the best of our knowledge, the current study has been the first to perform robust comparative analyses on the efficacy of treatment strategies in a large, detailed cohort of patients with AL. Furthermore, the manifestations of AL and treatment strategies investigated were based on a recent mixed-methods study, which conducted a survey and expert discussions, and thus represent treatment dilemmas of current clinical practice19.
Some limitations need to be discussed. First, confounding and missing data were potential sources of bias. Confounding bias (for example more severe leaks could be treated more aggressively) was minimized through defining different manifestations of AL and through propensity score matching31. Whereas subtle differences may still have been present, an appropriate balance was achieved in comparative analyses. Although residual confounding and selection cannot be ruled out, all well known patient-related and leak-related confounders were included during propensity score matching. Missing data in different leak-related and treatment parameters were anticipated during study initiation and meticulous data registration and data quality validation was performed to optimize data quality23. In addition, multiple imputation was performed to avoid bias due to missing data during analysis30. Second, the large number of participating centres led to a heterogeneous cohort. Previous studies have found substantial differences between centres, including differences in patient parameters, leak severity at diagnosis, and treatment of AL19,28,36,37. In addition, differences in leak management and outcomes of patients with AL were associated with annual resection volume36–39. In the current study, characteristics of participating centres have been reported transparently. Hospital volume was included in propensity score matching to correct for variation between centres where possible. Furthermore, a sensitivity analysis was performed in patients treated in middle-/high-volume centres to evaluate findings in these centres. As the outcomes of this analysis were largely similar to the overall analysis, findings appear to be robust. Third, current analysis focused on primary leak treatment and did not fully take into account secondary treatment. Although secondary treatment may affect outcomes, it was not possible to further investigate the impact of secondary treatments, as this would have resulted in patient groups that were too small and data regarding the indications and timing of secondary treatment were not available. Moreover, the current focus on primary treatment prevents unjustified selection of patients. Fourth, the comparative analysis did not consider the location of the anastomosis. It has been much debated whether treatment of AL is fundamentally different for cervical and intrathoracic anastomosis19,28,35. A sensitivity analysis in the current study showed differences between cervical and intrathoracic leaks in patients with intrathoracic manifestations, but groups were of a limited size. Finally, data on health-related quality of life (HRQOL) and long-term survival were not available. Next to mortality, HRQOL may be an important outcome measure to evaluate different treatments of AL.
In line with previous studies, the overall 90-day mortality rate was 11.7 per cent and large differences in mortality were observed between different manifestations of AL5. In patients with local manifestations, many surgeons intervene using drain placement and/or defect closure to promote recovery and prevent formation of fluid collections, whereas others rely solely on supportive treatment such as antibiotics and feeding support19. Current findings showed no benefit of intervening in these patients; supportively-only treatment was safe and resulted in less morbidity. Antibiotics may be indicated for any signs of systemic infection and supporting feeding is important to promote recovery5,40,41. In patients with intrathoracic manifestations, no benefit was found for performing defect closure in addition to drainage of fluid collections; overall morbidity was higher and closing the defect did not reduce the leak healing time. These findings contrast with multiple recent studies, which (although often lacking comparative analyses) have propagated the possible benefits of defect closure in addition to drainage, for example using stent and EVAC20,21,42–45. Patients with conduit ischaemia/necrosis had the highest postoperative mortality, in line with a previous study5. Interestingly, most patients with ischaemia/necrosis were treated with a continuity-preserving approach and, in this group, secondary diversion was only needed in 5 per cent of patients. Even though the number of patients with conduit ischaemia/necrosis was limited and no detailed data on the extent of ischaemia/necrosis were available, current findings indicate that a continuity-preserving treatment strategy may be feasible and secondary diversion is rarely needed. More generally, our findings indicate that, in current clinical practice, AL rarely results in oesophageal diversion with oesophagostomy, which aligns with current beliefs regarding oesophageal diversion19,46,47.
This study could provide guidance for treatment of AL in clinical practice. Across the different manifestations, less extensive primary treatment strategies showed at least similar mortality rates and resulted in lower morbidity. Although a substantial number of patients required secondary (invasive) treatment after less extensive primary treatment, these patients did not have poorer outcomes than patients who underwent more extensive treatment directly at diagnosis. Consequently, if confronted with a treatment dilemma in clinical practice, clinicians may potentially choose the less extensive strategy for primary treatment and reserve more extensive treatment for secondary step-up if needed. This less extensive approach to primary treatment of AL also underscores the need for adequate monitoring of patients. Knowing when and how to intervene is of great importance and may contribute to lower failure to rescue36.
To further progress evidence-based treatment of AL, prospective studies and ideally randomized trials are warranted. The benefits of a less extensive approach to primary treatment of AL found in the current study should be confirmed in future studies evaluating treatment strategies for AL. In addition, future studies (both quantitative and qualitative) should investigate the indications, timing, and strategy of secondary treatment, in order to provide further support regarding the treatment of AL. Prospective data provide insight into contemporary management of AL, in which the use of advanced techniques (for example EVAC) may have become more widespread. In addition, prospective registries offer the opportunity to standardize the recording of leak characteristics and treatment. Currently, different prospective initiatives investigate specific modalities for management of AL; the VAC-Stent Registry (NCT03962179) and the Eso-Sponge Registry (NCT02662777) may provide high-quality data on the use of these techniques and may promote standardization45. However, these studies will not perform comparative analyses, which are needed to further identify optimal treatment strategies for AL. Despite the fact that conducting large prospective or randomized studies will be hugely challenging, there are examples of successful clinical trials and innovative study designs in rare surgical conditions48,49. Whilst awaiting further evidence for optimal treatment of AL, clinical guidance for management of AL is much needed19,28. Although an evidence-based guideline may require more support than is provided by this study, the current findings may inform the development of a clinical consensus statement. Developing a consensus statement on the management of AL is one of the future projects of the TENTACLE—Esophagus Study Group and may guide clinical practice, promote standardization, and improve outcomes of patients with anastomotic leak after oesophagectomy.
Conclusion
In conclusion, in all different manifestations of AL, patients who underwent less extensive primary treatment were found to have less morbidity compared with more extensive primary treatment of AL. Potentially, a less extensive primary treatment strategy may lead to better outcomes and more extensive treatment may be reserved for secondary step-up if needed. However, current findings may be affected by selection and thus, future studies are needed to confirm our findings. More scientific evidence is needed to progress treatment of AL and ultimately improve clinical outcomes.
Collaborators
TENTACLE—Esophagus Collaborative Group
Eric Matthée, Cettela A. M. Slootmans, Gijs Ultee (Radboud University Medical Centre, Nijmegen, The Netherlands); Suzanne S. Gisbertz, Wietse J. Eshuis, Marianne C. Kalff, Minke L. Feenstra (Amsterdam UMC, University of Amsterdam, Cancer Centre Amsterdam, Cancer Treatment and Quality of Life, Amsterdam, The Netherlands); Donald L. van der Peet, Wessel T. Stam (Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands); Boudewijn Van Etten, Floris Poelmann, Nienke Vuurberg, Jan Willem van den Berg (University Medical Centre Groningen, University of Groningen, Groningen, The Netherlands); Ingrid S. Martijnse, Robert M. Matthijsen (Elisabeth-TweeSteden Ziekenhuis, Tilburg, The Netherlands); Misha Luyer, Wout Curvers, Tom Nieuwenhuijzen (Catharina Ziekenhuis Eindhoven, Eindhoven, The Netherlands); Annick E. Taselaar (Erasmus Medical Centre, Rotterdam, The Netherlands); Ewout A. Kouwenhoven, Merel Lubbers (Ziekenhuisgroep Twente, Almelo, The Netherlands); Meindert Sosef, Frederik Lecot, Tessa C. M. Geraedts (Zuyderland Medisch Centrum, Heerlen, The Netherlands); Stijn van Esser, Jan Willem T. Dekker (Reinier de Graaf Gasthuis, Delft, The Netherlands); Frits van den Wildenberg (Canisius-Wilhelmina Ziekenhuis, Nijmegen, The Netherlands); Wendy Kelder, Merel Lubbers, Peter C. Baas, Job W. A. de Haas (Martini Ziekenhuis, Groningen, The Netherlands); Henk H. Hartgrink, Renu R. Bahadoer (Leiden University Medical Centre, Leiden, The Netherlands); Johanna W. van Sandick, Koen J. Hartemink, Xander Veenhof (Netherlands Cancer Institute—Antoni van Leeuwenhoek, Amsterdam, The Netherlands); Hein Stockmann, Burak Gorgec, Pepijn Weeder (Spaarne Gasthuis, Haarlem, The Netherlands); Marinus J. Wiezer, Charlotte M. S. Genders (St Antonius Ziekenhuis, Nieuwegein, The Netherlands); Eric Belt, Bjorn Blomberg (Albert Schweitzer, Dordrecht, The Netherlands); Peter van Duijvendijk, Linda Claassen, David Reetz (Gelre, Apeldoorn, The Netherlands); Pascal Steenvoorde, Walter Mastboom, Henk Jan Klein Ganseij (Medisch Spectrum Twente, Enschede, The Netherlands); Annette D. van Dalsen, Annalie Joldersma, Marije Zwakman (Isala, Zwolle, The Netherlands); Richard P. R. Groenendijk, Mahsa Montazeri (IJsselland Ziekenhuis, Cappelle aan de Ijssel, The Netherlands); Stuart Mercer, Benjamin Knight, Gijs van boxel (Portsmouth Hospital University Trust, Portsmouth, UK); Richard J. McGregor, Richard J. E. Skipworth, Cristina Frattini, Alice Bradley (University of Edinburgh, Royal Infirmary of Edinburgh, Edinburgh, UK); Magnus Nilsson, Masaru Hayami, Biying Huang (Karolinska University Hospital, Stockholm, Sweden); James Bundred, Richard Evans (Queen Elizabeth Hospital, Birmingham, UK); Peter P. Grimminger, Pieter C. van der Sluis, Uzun Eren (University Medical Centre Mainz, Mainz, Germany); John Saunders, Elena Theophilidou, Zubair Khanzada (Nottingham University Hospitals NHS Trust, Nottingham, UK); Jessie A. Elliott, Jeroen E. H. Ponten, Sinead King, John V. Reynolds (Trinity S. James’s Cancer Institute, Dublin, Ireland); Bruno Sgromo, Khalid Akbari, Samar Shalaby (Churchill Hospital, Oxford University Hospitals, Oxford, UK); Christian A. Gutschow, Henner Schmidt, Diana Vetter (University Hospital Zurich, Zurich, Switzerland); Krishna Moorthy, Mohamed A. H. Ibrahim, Grigorious Christodoulidis (Imperial, London, UK); Jari V. Räsänen, Juha Kauppi, Henna Söderström (Helsinki University Hospital, Helsinki University, Helsinki, Finland); Renol Koshy (Conventry and Warwickshire, Conventry, UK); Dimitrios K. Manatakis, Dimitrios P. Korkolis, Dimitrios Balalis, Aliki Rompu (Saint Savvas Cancer Hospital, Athens, Greece); Bilal Alkhaffaf, Mohamed Alasmar, Moaad Arebi (Salford Royal NHS Foundation Trust, Division of Cancer Sciences, University of Manchester, Manchester, UK); Guillaume Piessen, Frederiek Nuytens, Sebastien Degisors (University Lille, Claude Huriez University Hospital, CHU de Lille, Lille, France); Ahmed Ahmed, Alex Boddy, Suraj Gandhi, Oluwatomini Fashina (University of Leicester, Leicester, UK); Elke Van Daele, Piet Pattyn (Ghent University Hospital, Ghent, Belgium); William B. Robb, Mayilone Arumugasamy, Mohammed Al Azzawi, Jack Whooley (Beaumont Hospital, Dublin, Ireland); Elif Colak, Engin Aybar, Ahmet C. Sari, Mustafa S. Uyanik, Ahmet B. Ciftci (Samsun Training and Research Hospital, Samsun, Turkey); Raza Sayyed, Bushra Ayub, Ghulam Murtaza, Aniqa Saeed, Priyanka Ramesh (Patel Hospital, Karachi, Pakistan); Alexandros Charalabopoulos, Theodore Liakakos, Dimitrios Schizas, Efstratia Baili, Alkistis Kapelouzou (Laiko General Hospital, Athens, Greece); Michele Valmasoni, Elisa Sefora Pierobon, Giovanni Capovilla, Stefano Merigliano (University Hospital of Padova, Padova, Italy); Silviu Constantinoiu, Rodica Birla, Florin Achim, Cristian Gelu Rosianu, Petre Hoara (Sf. Maria Hospital, Bucharest, Romania); Raúl Guevara Castro, Andrés Felipe Salcedo (Clinica Universitaria Colombia, Bogota, Colombia); Ionut Negoi, Valentina M. Negoita, Cezar Ciubotaru, Bogdan Stoica, Sorin Hostiuc (Carol Davila University of Medicine and Pharmacy Bucharest, Emergency Hospital of Bucharest, Bucharest, Romania); Nicola Colucci, Stefan P. Mönig, Charles-Henri Wassmer, Jeremy Meyer (Geneva University Hospitals, Geneva, Switzerland); Flavio Roberto Takeda, Rubens Antonio Aissar Sallum, Ulysses Ribeiro, Ivan Cecconello (University of Sao Paulo, Sao Paulo, Brazil); Enrique Toledo, Maria Soledad Trugeda, María José Fernández, Carolina Gil, Sonia Castanedo (Valdecilla Hospital, Santander, Spain); Arda Isik, Eray Kurnaz (Erzincan Binali Yildirim University, Erzincan, Turkey); José Flávio Videira, Mariana Peyroteo, Rita Canotilho (Instituto Português de Oncologica, Porto, Portugal); Jacopo Weindelmayer, Simone Giacopuzzi, Carlo Alberto De Pasqual (Verona University Hospital, Verona, Italy); Marcos Bruna, Fernando Mingol, Javier Vaque, Carla Pérez (La Fe Hospital, Valencia, Spain); Alexander W. Phillips, Jakub Chmelo, Joshua Brown, Renol Koshy, Laura E. Han (Royal Victoria Infirmary, Newcastle upon Tyne, UK); James A. Gossage, Andrew R. Davies, Cara R. Baker, Mark Kelly, Mohamed Saad (Guy’s and St Thomas’ Hospitals, London, UK); Daniele Bernardi, Luigi Bonavina, Emanuele Asti, Carlo Riva, Rosa Scaramuzzo (University of Milan, Milan, Italy); Muhammed Elhadi, Hazem Abdelkarem Ahmed, Ahmed Elhadi, Faruk Ali Elnagar, Ahmed A. A. Msherghi (Tripoli University Hospital, Tripoli, Libya); Vanessa Wills, Cassidy Campbell, Marisol Perez Cerdeira, Scott Whiting (John Hunter New England LHD, Newcastle, Australia); Neil Merrett, Amitabha Das, Christos Apostolou, Aldenb Lorenzo (South Western Sydney Local Health District, Sydney, Australia); Fabiana Sousa, José Adelino Barbosa, Vítor Devezas, Elisabete Barbosa, Cristina Fernandes (Centro Hospitalar Universitário São João, Oporto, Portugal); Garett Smith, Edward Y. Li, Nazim Bhimani, Priscilla Chan, Krishna Kotecha (Royal North Shore Hospital, Sydney, Australia); Michael W. Hii, Salena M. Ward, MaryAnn Johnson, Matthew Read, Lynn Chong (St Vincent’s Hospital, Melbourne, Australia); Michael J. Hollands, Matthew Allaway, Arthur Richardson, Emma Johnston, Andy Z. L. Chen (Westmead Hospital Hospital, Sydney, Australia); Harsh Kanhere, Shalvin Prasad, Patrick McQuillan, Tim Surman (Royal Adelaide Hospital, Adelaide, Australia); Markus I. Trochsler, W. A. Schofield, Syeda Khadijah Ahmed, Jessica L. Reid, Mark C. Harris (The Queen Elizabeth Hospital, Adelaide, Australia); Sivakumar Gananadha, Jessica Farrant, Nicole Rodrigues, James Fergusson (Canberra Hospital, Canberra, Australia); Andrew Hindmarsh, Zeeshan Afzal, Peter Safranek, Vijay Sujendran, Siobhan Rooney (Addenbrooke’s Hospital, Cambridge, UK); Carlos Loureiro, Saioa Leturio Fernández, Ismael Díez del Val (University Hospital Basurto, Bilbao, Spain); Shameen Jaunoo, Lauren Kennedy, Ahmed Hussain (Brighton and Sussex University Hospitals, Brighton, UK); Dimitrios Theodorou, Tania Triantafyllou, Charalampos Theodoropoulos, Theodora Palyvou (Hippocration Hospital, Athens, Greece); Muhammed Elhadi, Fatima Abdullah Ben Taher, Mustafa Ekheel, Ahmed A. A. Msherghi (Sabratha National Cancer Institute, Sabratha, Libya).
Supplementary Material
Acknowledgements
The authors thank all of the people involved in the TENTACLE—Esophagus study for their contribution to this collaborative study. The TENTACLE—Esophagus study was registered in the Clinical Trials Registry (NCT03829098) before the start of the study. S.U. and M.H.P.V. share first authorship (both authors contributed equally), and F.v.W. and C.R. share senior authorship (both authors contributed equally).
Contributor Information
Sander Ubels, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Moniek H P Verstegen, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Bastiaan R Klarenbeek, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Stefan Bouwense, Department of Surgery, Maastricht University Medical Centre+, Maastricht, The Netherlands.
Mark I van Berge Henegouwen, Department of Surgery, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Cancer Centre Amsterdam, Cancer Treatment and Quality of Life, Amsterdam, The Netherlands.
Freek Daams, Department of Surgery, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Cancer Centre Amsterdam, Cancer Treatment and Quality of Life, Amsterdam, The Netherlands.
Marc J van Det, Department of Surgery, ZGT Hospital Group, Almelo, The Netherlands.
Ewen A Griffiths, Department of Upper Gastrointestinal Surgery, University Hospitals Birmingham NHS Foundation Trust, Queen Elizabeth Hospital Birmingham, Birmingham, UK; Institute of Cancer and Genomic Sciences, College of Medical and Dental Sciences, University of Birmingham, Birmingham, UK.
Jan Willem Haveman, Department of Surgery, University Medical Centre Groningen, University of Groningen, Groningen, The Netherlands.
Joos Heisterkamp, Department of Surgery, Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands.
Grard Nieuwenhuijzen, Department of Surgery, Catharina Hospital, Eindhoven, The Netherlands.
Fatih Polat, Department of Surgery, Canisius-Wilhelmina Hospital, Nijmegen, The Netherlands.
Jeroen Schouten, Department of Intensive Care, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Peter D Siersema, Department of Gastroenterology and Hepatology, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Pritam Singh, Department of Surgery, Nottingham University Hospitals NHS Trust, Nottingham, UK; Department of Surgery, Regional Oesophago-Gastric Unit, Royal Surrey County Hospital, Guildford, UK.
Bas Wijnhoven, Department of Surgery, Erasmus University Medical Centre, Rotterdam, The Netherlands.
Gerjon Hannink, Department of Operating Rooms, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
Frans van Workum, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands; Department of Surgery, Canisius-Wilhelmina Hospital, Nijmegen, The Netherlands.
Camiel Rosman, Department of Surgery, Radboud Institute for Health Sciences, Radboud University Medical Centre, Nijmegen, The Netherlands.
the TENTACLE—Esophagus Collaborative Group:
Eric Matthée, Cettela A M Slootmans, Gijs Ultee, Suzanne S Gisbertz, Wietse J Eshuis, Marianne C Kalff, Minke L Feenstra, Donald L van der Peet, Wessel T Stam, Boudewijn Van Etten, Floris Poelmann, Nienke Vuurberg, Jan Willem van den Berg, Ingrid S Martijnse, Robert M Matthijsen, Misha Luyer, Wout Curvers, Tom Nieuwenhuijzen, Annick E Taselaar, Ewout A Kouwenhoven, Merel Lubbers, Meindert Sosef, Frederik Lecot, Tessa C M Geraedts, Stijn van Esser, Jan Willem T Dekker, Frits van den Wildenberg, Wendy Kelder, Merel Lubbers, Peter C Baas, Job W A de Haas, Henk H Hartgrink, Renu R Bahadoer, Johanna W van Sandick, Koen J Hartemink, Xander Veenhof, Hein Stockmann, Burak Gorgec, Pepijn Weeder, Marinus J Wiezer, Charlotte M S Genders, Eric Belt, Bjorn Blomberg, Peter van Duijvendijk, Linda Claassen, David Reetz, Pascal Steenvoorde, Walter Mastboom, Henk Jan Klein Ganseij, Annette D van Dalsen, Annalie Joldersma, Marije Zwakman, Richard P R Groenendijk, Mahsa Montazeri, Stuart Mercer, Benjamin Knight, Gijs van boxel, Richard J McGregor, Richard J E Skipworth, Cristina Frattini, Alice Bradley, Magnus Nilsson, Masaru Hayami, Biying Huang, James Bundred, Richard Evans, Peter P Grimminger, Pieter C van der Sluis, Uzun Eren, John Saunders, Elena Theophilidou, Zubair Khanzada, Jessie A Elliott, Jeroen E H Ponten, Sinead King, John V Reynolds, Bruno Sgromo, Khalid Akbari, Samar Shalaby, Christian A Gutschow, Henner Schmidt, Diana Vetter, Krishna Moorthy, Mohamed A H Ibrahim, Grigorious Christodoulidis, Jari V Räsänen, Juha Kauppi, Henna Söderström, Renol Koshy, Dimitrios K Manatakis, Dimitrios P Korkolis, Dimitrios Balalis, Aliki Rompu, Bilal Alkhaffaf, Mohamed Alasmar, Moaad Arebi, Guillaume Piessen, Frederiek Nuytens, Sebastien Degisors, Ahmed Ahmed, Alex Boddy, Suraj Gandhi, Oluwatomini Fashina, Elke Van Daele, Piet Pattyn, William B Robb, Mayilone Arumugasamy, Mohammed Al Azzawi, Jack Whooley, Elif Colak, Engin Aybar, Ahmet C Sari, Mustafa S Uyanik, Ahmet B Ciftci, Raza Sayyed, Bushra Ayub, Ghulam Murtaza, Aniqa Saeed, Priyanka Ramesh, Alexandros Charalabopoulos, Theodore Liakakos, Dimitrios Schizas, Efstratia Baili, Alkistis Kapelouzou, Michele Valmasoni, Elisa Sefora Pierobon, Giovanni Capovilla, Stefano Merigliano, Silviu Constantinoiu, Rodica Birla, Florin Achim, Cristian Gelu Rosianu, Petre Hoara, Raúl Guevara Castro, Andrés Felipe Salcedo, Ionut Negoi, Valentina M Negoita, Cezar Ciubotaru, Bogdan Stoica, Sorin Hostiuc, Nicola Colucci, Stefan P Mönig, Charles-Henri Wassmer, Jeremy Meyer, Flavio Roberto Takeda, Rubens Antonio Aissar Sallum, Ulysses Ribeiro, Ivan Cecconello, Enrique Toledo, Maria Soledad Trugeda, María José Fernández, Carolina Gil, Sonia Castanedo, Arda Isik, Eray Kurnaz, José Flávio Videira, Mariana Peyroteo, Rita Canotilho, Jacopo Weindelmayer, Simone Giacopuzzi, Carlo Alberto De Pasqual, Marcos Bruna, Fernando Mingol, Javier Vaque, Carla Pérez, Alexander W Phillips, Jakub Chmelo, Joshua Brown, Renol Koshy, Laura E Han, James A Gossage, Andrew R Davies, Cara R Baker, Mark Kelly, Mohamed Saad, Daniele Bernardi, Luigi Bonavina, Emanuele Asti, Carlo Riva, Rosa Scaramuzzo, Muhammed Elhadi, Hazem Abdelkarem Ahmed, Ahmed Elhadi, Faruk Ali Elnagar, Ahmed A A Msherghi, Vanessa Wills, Cassidy Campbell, Marisol Perez Cerdeira, Scott Whiting, Neil Merrett, Amitabha Das, Christos Apostolou, Aldenb Lorenzo, Fabiana Sousa, José Adelino Barbosa, Vítor Devezas, Elisabete Barbosa, Cristina Fernandes, Garett Smith, Edward Y Li, Nazim Bhimani, Priscilla Chan, Krishna Kotecha, Michael W Hii, Salena M Ward, MaryAnn Johnson, Matthew Read, Lynn Chong, Michael J Hollands, Matthew Allaway, Arthur Richardson, Emma Johnston, Andy Z L Chen, Harsh Kanhere, Shalvin Prasad, Patrick McQuillan, Tim Surman, Markus I Trochsler, W A Schofield, Syeda Khadijah Ahmed, Jessica L Reid, Mark C Harris, Sivakumar Gananadha, Jessica Farrant, Nicole Rodrigues, James Fergusson, Andrew Hindmarsh, Zeeshan Afzal, Peter Safranek, Vijay Sujendran, Siobhan Rooney, Carlos Loureiro, Saioa Leturio Fernández, Ismael Díez del Val, Shameen Jaunoo, Lauren Kennedy, Ahmed Hussain, Dimitrios Theodorou, Tania Triantafyllou, Charalampos Theodoropoulos, Theodora Palyvou, Muhammed Elhadi, Fatima Abdullah Ben Taher, Mustafa Ekheel, and Ahmed A A Msherghi
Funding
The TENTACLE—Esophagus study was funded by the company Medtronic, with an unrestricted research grant. The study was performed independently, and Medtronic had no role in the: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript.
Disclosure
All authors declare funding from Medtronic for the submitted work. P.D.S. reports grants from The Enose Company, grants and other from Motus GI, grants from Pentax, grants from Micro-Tech, and other from Boston Scientific, outside the submitted work. B.R.K. reports grants from Medtronic and ZonMw, outside the submitted work. M.I.v.B.H. reports other from Mylan, other from Alesi Surgical, other from Johnson and Johnson, other from BBraun, other from Medtronic, grants from Olympus, and grants from Stryker, outside the submitted work. All fees unrelated to submitted work, paid to institution. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Study data are not openly available. The authors are willing to share data upon reasonable request to the corresponding author.
References
- 1. van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, Henegouwen M, Wijnhoven BPLet al. . Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084 [DOI] [PubMed] [Google Scholar]
- 2. Al-Batran S-E, Homann N, Schmalenberg H, Kopp H-G, Haag GM, Luley KBet al. . Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. J Clin Oncol 2017;35:4004–4004 [Google Scholar]
- 3. Biere SSAY, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JRet al. . Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887–1892 [DOI] [PubMed] [Google Scholar]
- 4. van Workum F, Verstegen MHP, Klarenbeek BR, Bouwense SAW, van Berge Henegouwen MI, Daams Fet al. . Intrathoracic vs cervical anastomosis after totally or hybrid minimally invasive esophagectomy for esophageal cancer: a randomized clinical trial. JAMA Surg 2021;156:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oesophago-Gastric Anastomosis Study Group on behalf of the West Midlands Research Collaborative. Rates of anastomotic complications and their management following esophagectomy: results of the Oesophago-Gastric Anastomosis Audit (OGAA). Ann Surg 2022;275:e382–e391 [DOI] [PubMed] [Google Scholar]
- 6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424 [DOI] [PubMed] [Google Scholar]
- 7. Dutch Upper Gastrointestinal Cancer Audit (DUCA) . Core Figures 2015–2019 (Basis Tabel 2015–2019). Leiden: Dutch Institute for Cancer Auditing (DICA), 2020 [Google Scholar]
- 8. Netherlands Comprehensive Cancer Organization (IKNL) . Netherlands Cancer Registry (NCR). NCR Figures (NKR Cijfers). 2019. https://iknl.nl/nkr-cijfers (accessed 09-05-2022)
- 9. Goense L, Meziani J, Ruurda JP, van Hillegersberg R. Impact of postoperative complications on outcomes after oesophagectomy for cancer. Br J Surg 2019;106:111–119 [DOI] [PubMed] [Google Scholar]
- 10. Turkyilmaz A, Eroglu A, Aydin Y, Tekinbas C, Muharrem Erol M, Karaoglanoglu N. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Dis Esophagus 2009;22:119–126 [DOI] [PubMed] [Google Scholar]
- 11. Lubbers M, Workum F, Berkelmans G, Rosman C, Luyer M, Nieuwenhuijzen Get al. . Variations in treatment of an anastomotic leakage after Ivor Lewis esophagectomy. J Clin Images Med Case Rep 2021;2:1417 [Google Scholar]
- 12. Scarpa M, Saadeh LM, Fasolo A, Alfieri R, Cagol M, Cavallin Fet al. . Health-related quality of life in patients with oesophageal cancer: analysis at different steps of the treatment pathway. J Gastrointest Surg 2013;17:421–433 [DOI] [PubMed] [Google Scholar]
- 13. Jezerskyte E, van Berge Henegouwen MI, van Laarhoven HWM, van Kleef JJ, Eshuis WJ, Heisterkamp Jet al. . Postoperative complications and long-term quality of life after multimodality treatment for esophageal cancer: an analysis of the Prospective Observational Cohort Study of Esophageal-Gastric Cancer Patients (POCOP). Ann Surg Oncol 2021;28:7259–7276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Derogar M, Orsini N, Sadr-Azodi O, Lagergren P. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 2012;30:1615–1619 [DOI] [PubMed] [Google Scholar]
- 15. Page RD, Shackcloth MJ, Russell GN, Pennefather SH. Surgical treatment of anastomotic leaks after oesophagectomy. Eur J Cardiothorac Surg 2005;27:337–343 [DOI] [PubMed] [Google Scholar]
- 16. Ye H-Y, Huang W-Z, Wu Y-M, Liang Y, Zheng J-M, Jiang H-M. Personalized management of anastomotic leak after surgery for esophageal carcinoma. Chin Med Sci J 2012;27:35–40 [DOI] [PubMed] [Google Scholar]
- 17. Verstegen MHP, Bouwense SAW, van Workum F, Ten Broek R, Siersema PD, Rovers Met al. . Management of intrathoracic and cervical anastomotic leakage after esophagectomy for esophageal cancer: a systematic review. World J Emerg Surg 2019;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scognamiglio P, Reeh M, Karstens K, Bellon E, Kantowski M, Schön Get al. . Endoscopic vacuum therapy versus stenting for postoperative esophago-enteric anastomotic leakage: systematic review and meta-analysis. Endoscopy 2020;52:632–642 [DOI] [PubMed] [Google Scholar]
- 19. Ubels S, Lubbers M, Verstegen MHP, Bouwense SAW, van Daele E, Ferri Let al. . Treatment of anastomotic leak after esophagectomy: insights of an international case vignette survey and expert discussions. Dis Esophagus 2022;35:doac020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rausa E, Asti E, Aiolfi A, Bianco F, Bonitta G, Bonavina L. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus 2018;31:doy020 [DOI] [PubMed] [Google Scholar]
- 21. Brangewitz M, Voigtländer T, Helfritz FA, Lankisch TO, Winkler M, Klempnauer Jet al. . Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013;45:433–438 [DOI] [PubMed] [Google Scholar]
- 22. van Rossum PSN, Haverkamp L, Carvello M, Ruurda JP, van Hillegersberg R. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus 2017;30:1–8 [DOI] [PubMed] [Google Scholar]
- 23. Ubels S, Verstegen M, Klarenbeek B, Bouwense S, van Berge Henegouwen M, Daams Fet al. . Severity of oEsophageal Anastomotic Leak in patients after oesophagectomy: the SEAL score. Br J Surg 2022;109:864–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 2007;85:867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, D’Journo XBet al. . International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286–294 [DOI] [PubMed] [Google Scholar]
- 26. Hayami M, Klevebro F, Tsekrekos A, Samola Winnberg J, Kamiya S, Rouvelas Iet al. . Endoscopic vacuum therapy for anastomotic leak after esophagectomy: a single-center’s early experience. Dis Esophagus 2020;34:doaa122 [DOI] [PubMed] [Google Scholar]
- 27. Tavares G, Tustumi F, Tristão LS, Bernardo WM. Endoscopic vacuum therapy for anastomotic leak in esophagectomy and total gastrectomy: a systematic review and meta-analysis. Dis Esophagus 2021;34:doaa132 [DOI] [PubMed] [Google Scholar]
- 28. Hagens ERC, Anderegg MCJ, van Berge Henegouwen MI, Gisbertz SS. International survey on the management of anastomotic leakage after esophageal resection. Ann Thorac Surg 2018;106:1702–1708 [DOI] [PubMed] [Google Scholar]
- 29. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1–7 [DOI] [PubMed] [Google Scholar]
- 30. Van Buuren S. Flexible Imputation of Missing Data. Boca Raton: CRC Press, 2018 [Google Scholar]
- 31. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 2012;21:69–80 [DOI] [PubMed] [Google Scholar]
- 33. Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol 2010;172:1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mardin WA, Palmes D, Bruewer M. Current concepts in the management of leakages after esophagectomy. Thorac Cancer 2012;3:117–124 [DOI] [PubMed] [Google Scholar]
- 36. Ubels S, Matthée E, Verstegen M, Klarenbeek B, Bouwense S, van Berge Henegouwen MIet al. . Practice variation in anastomotic leak after esophagectomy: unravelling differences in failure to rescue. Eur J Surg Oncol 2023; DOI: 10.1016/j.ejso.2023.01.010 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37. Oesophago-Gastric Anastomosis Study Group on behalf of the West Midlands Research Collaborative . International variation in surgical practices in units performing oesophagectomy for oesophageal cancer: a unit survey from the Oesophago-Gastric Anastomosis Audit (OGAA). World J Surg 2019;43:2874–2884 [DOI] [PubMed] [Google Scholar]
- 38. Markar SR, Karthikesalingam A, Thrumurthy S, Low DE. Volume-outcome relationship in surgery for esophageal malignancy: systematic review and meta-analysis 2000–2011. J Gastrointest Surg 2012;16:1055–1063 [DOI] [PubMed] [Google Scholar]
- 39. van Lanschot JJ, Hulscher JB, Buskens CJ, Tilanus HW, ten Kate FJ, Obertop H. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–1578 [DOI] [PubMed] [Google Scholar]
- 40. Dent B, Griffin SM, Jones R, Wahed S, Immanuel A, Hayes N. Management and outcomes of anastomotic leaks after oesophagectomy. Br J Surg 2016;103:1033–1038 [DOI] [PubMed] [Google Scholar]
- 41. Crestanello JA, Deschamps C, Cassivi SD, Nichols FC, Allen MS, Schleck Cet al. . Selective management of intrathoracic anastomotic leak after esophagectomy. J Thorac Cardiovasc Surg 2005;129:254–260 [DOI] [PubMed] [Google Scholar]
- 42. Hwang JJ, Jeong YS, Park YS, Yoon H, Shin CM, Kim Net al. . Comparison of endoscopic vacuum therapy and endoscopic stent implantation with self-expandable metal stent in treating postsurgical gastroesophageal leakage. Medicine (Baltimore) 2016;95:e3416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoeppner J, Kulemann B, Seifert G, Marjanovic G, Fischer A, Hopt UTet al. . Covered self-expanding stent treatment for anastomotic leakage: outcomes in esophagogastric and esophagojejunal anastomoses. Surg Endosc 2014;28:1703–1711 [DOI] [PubMed] [Google Scholar]
- 44. Mennigen R, Harting C, Lindner K, Vowinkel T, Rijcken E, Palmes Det al. . Comparison of endoscopic vacuum therapy versus stent for anastomotic leak after esophagectomy. J Gastrointest Surg 2015;19:1229–1235 [DOI] [PubMed] [Google Scholar]
- 45. Richter F, Hendricks A, Schniewind B, Hampe J, Heits N, von Schönfels Wet al. . Eso-Sponge® for anastomotic leakage after oesophageal resection or perforation: outcomes from a national, prospective multicentre registry. BJS Open 2022;6:zrac030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manghelli JL, Ceppa DP, Greenberg JW, Blitzer D, Hicks A, Rieger KMet al. . Management of anastomotic leaks following esophagectomy: when to intervene? J Thorac Dis 2019;11:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang H, Zhang Y, Zhang Y, Liu W, Wang J, Liu Get al. . Practice of cervical end-esophageal exteriorization in patients with severe intrathoracic anastomotic leakage after esophagectomy. J Int Med Res 2018;46:5090–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CHet al. . A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491–1502 [DOI] [PubMed] [Google Scholar]
- 49. Gagne JJ, Thompson L, O’Keefe K, Kesselheim AS. Innovative research methods for studying treatments for rare diseases: methodological review. BMJ 2014;349:g6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study data are not openly available. The authors are willing to share data upon reasonable request to the corresponding author.