Abstract
Background
The Scandinavian Diverticulitis (SCANDIV) trial and the LOLA arm of the LADIES trial randomized patients with Hinchey III perforated diverticulitis to laparoscopic peritoneal lavage or sigmoid resection. The aim of this analysis was to identify risk factors for treatment failure in patients with Hinchey III perforated diverticulitis.
Methods
This was a post hoc analysis of the SCANDIV trial and LOLA arm. Treatment failure was defined as morbidity requiring general anaesthesia (Clavien–Dindo grade IIIb or higher) within 90 days. Age, sex, BMI, ASA fitness grade, smoking status, previous episodes of diverticulitis, previous abdominal surgery, time to surgery, and surgical competence were all tested in univariable and multivariable logistic regression analyses using an interaction variable.
Results
The pooled analysis included 222 patients randomized to laparoscopic lavage and primary resection (116 and 106 patients respectively). Univariable analysis found ASA grade to be associated with advanced morbidity in both groups, and the following factors in the laparoscopic lavage group: smoking, corticosteroid use, and BMI. Significant factors for laparoscopic lavage morbidity in multivariable analysis were smoking (OR 7.05, 95 per cent c.i. 2.07 to 23.98; P = 0.002) and corticosteroid use (OR 6.02, 1.54 to 23.51; P = 0.010).
Conclusion
Active smoking status and corticosteroid use were risk factors for laparoscopic lavage treatment failure (advanced morbidity) in patients with perforated diverticulitis.
This study analysed a prospectively collected cohort of patients with Hinchey III diverticulitis from two large randomized trials (SCANDIV and LOLA), aiming to find risk factors for treatment failure. The results showed that only active smoking status and corticosteroid use in the laparoscopic lavage group were risk factors for treatment failure.
Introduction
Complicated diverticulitis is characterized by perforation, stenosis, fistulas, and obstruction1. The extension of abdominal contamination owing to perforation is classified according to Hinchey2. Initially, the classification was based on surgical findings, but the improvement in radiological diagnostics, especially CT, has resulted in localized abscesses (Hinchey grade I and II) often being treated with antibiotics with or without percutaneous drainage. Surgery is indicated in patients with perforation causing abdominal contamination and purulent or faecal peritonitis (Hinchey grade III or IV respectively), particularly if the patient develops sepsis3,4.
The role of laparoscopic lavage as means of treating Hinchey III disease has been debated and investigated. Recently, the results of three RCTS5–7 comparing laparoscopic lavage with primary resection for Hinchey grade III perforated diverticulitis have been published. Meta-analyses8 of these studies showed that laparoscopic lavage in patients with Hinchey III diverticulitis is associated with the need for repeated interventions because of ongoing sepsis, but no difference in mortality rates were found and fewer patients undergoing laparoscopic lavage had a stoma at 1-year or longer follow-up5,9–12. Moreover, laparoscopic lavage is more easily performed than primary resection, is cheaper, and long-term follow-up has shown no differences in terms of morbidity or mortality11,13. However, laparoscopic lavage fails in about one in five patients, so identifying preoperative risk factors for treatment failure would be helpful. Until now, risk factors for treatment failure have been explored only in a few heterogeneous studies14–16.
The SCANDIV trial6 and the LOLA arm of the LADIES trial5 randomized a total of 222 patients with Hinchey III diverticulitis to either laparoscopic lavage or primary resection (116 and 106 respectively). The aim of this study was to identify potential risk factors for treatment failure in patients with Hinchey III perforated diverticulitis in this large, prospectively collected, randomized study population.
Methods
Both studies were approved by national ethics boards initially, and a supplement to the primary ethics approval was sent in and approved (2021-06755-02) in Sweden and Norway. In the Netherlands, additional approval was not considered necessary by the Medical Ethics Review Committee of the Academic Medical Centre.
Individualized data were retrieved from SCANDIV and the LOLA arm of the LADIES trial. All data were collected prospectively. The SCANDIV trial was designed as a pragmatic, two-armed, open-labelled, multicentre, superiority randomized trial. Patients were enrolled in 21 surgical units (9 in Sweden and 12 in Norway)6. Primary enrolment was conducted between 5 February 2010 and 28 June 2014. Included patients were older than 18 years, and had a clinical suspicion of perforated diverticulitis and a need for emergency surgery. The LOLA study was a parallel-group, multicentre, randomized, open-label trial conducted in 30 hospitals in Belgium (1), Italy (1), and the Netherlands (28)5. Primary enrolment was undertaken between 1 July 2010 and 22 February 2013. The inclusion criterion was perforated purulent diverticulitis at laparoscopy. Patients with faecal peritonitis, age over 85 years, high-dose corticosteroid use (at least 20 mg daily), and haemodynamic instability were excluded.
Patients were evaluated on an intention-to-treat basis. All factors that may predict treatment failure, defined as complications of at least Clavien–Dindo grade IIIb (requiring general anaesthesia), were analysed17. These included patient characteristics, and perioperative and postoperative variables such as age, sex, BMI, ASA fitness grade, smoking (yes or no), and immunosuppression (defined as use of immunosuppressive medication in SCANDIV and use of corticosteroids (less than 20 mg daily) in LOLA). Corticosteroid use referred to oral intake. Furthermore, duration of operation, time to surgery, and duration of surgical ICU stay were assessed. Only factors that were registered in both studies were analysed.
Statistical analysis
Logistic regression in a two-step approach was used to investigate risk factors for failure and compare potential risk factors between operation types. ORs with 95 per cent confidence intervals and P values were calculated. In the first step, potential risk factors and interaction terms between potential risk factors and operation type were analysed in separate models for each risk factor. Risk factors with P < 0.100 for at least one operation type were included in a multiple logistic regression model. In both steps, all analyses were adjusted for study (SCANDIV or LOLA). All analyses were performed using the statistical software SAS® version 9.4 (SAS Institute, Cary, NC, USA).
Results
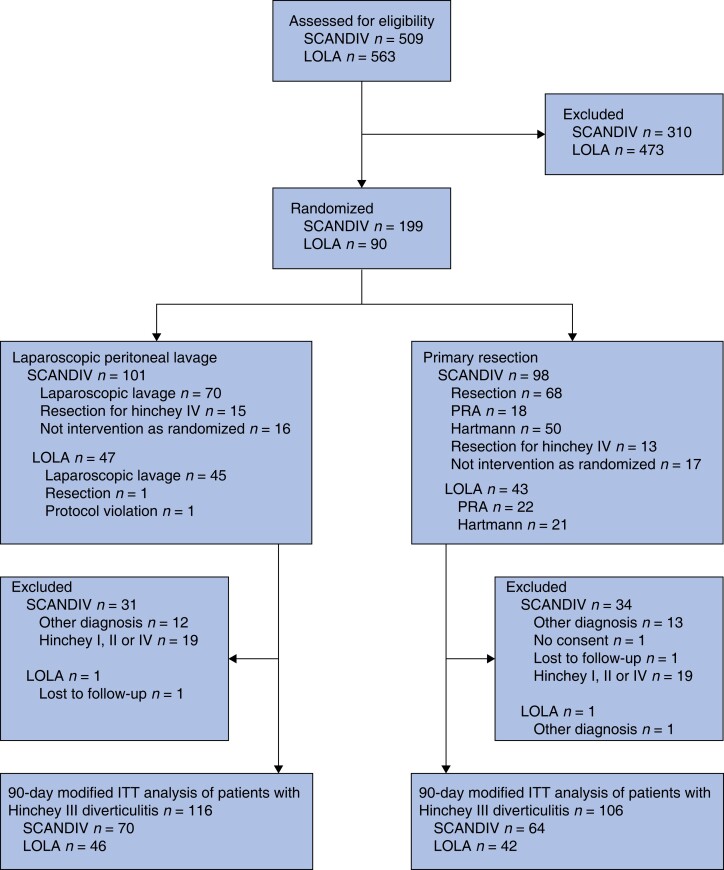
A total of 222 patients with Hinchey III perforated diverticulitis were included in the pooled analysis, of whom 116 were randomized to the laparoscopic lavage group and 106 to the primary resection group (Fig. 1). Baseline characteristics for all patients, as well as those for each separate study population and procedure, are shown in Tables 1–3. There were no significant differences between patients in the two studies, except greater cortisone use in SCANDIV and more smokers in the LOLA study.
Fig. 1.
Study flow chart
PRA, primary resection with anastomosis; ITT, intention to treat.
Table 1.
Baseline characteristics of all patients with Hinchey III diverticulitis
| All patients (n = 222) |
SCANDIV (n = 134) |
LOLA (n = 88) |
|
|---|---|---|---|
| Age (years), mean(s.d.) | 64.4(13.7) | 65.2(14.5) | 63.1(12.5) |
| Sex ratio (M : F) | 125 : 97 | 74 : 60 | 51 : 37 |
| BMI (kg/m2), mean(s.d.) | 26.7(5.0) | 26.3(4.7) | 27.3(5.3) |
| ASA fitness grade | |||
| I | 39 | 21 | 18 |
| II | 93 | 59 | 34 |
| III | 69 | 50 | 19 |
| IV | 9 | 4 | 5 |
| Missing | 12 | 0 | 12 |
| History of diverticulitis | 54 | 32 | 22 |
| History of laparotomy | 19 | 12 | 7 |
| Corticosteroid use | 33 | 29 | 4 |
| Insulin use | 5 | 5 | 0 |
| Smoking | 40 | 19 | 21 |
Table 2.
Baseline characteristics for all patients who had laparoscopic lavage
| Laparoscopic lavage (n = 116) |
SCANDIV (n = 70) |
LOLA (n = 46) |
|
|---|---|---|---|
| Age (years), mean(s.d.) | 65.0(13.3) | 66.8(13.5) | 62.4(12.7) |
| Sex ratio (M : F) | 64 : 52 | 38 : 32 | 26 : 20 |
| BMI (kg/m2), mean(s.d.) | 26.8(5.)5 | 26.5(5.0) | 27.5(6.2) |
| ASA fitness grade | |||
| I | 21 | 11 | 10 |
| II | 57 | 36 | 21 |
| III | 25 | 20 | 5 |
| IV | 6 | 3 | 3 |
| Missing | 7 | 0 | 7 |
| History of diverticulitis | 28 | 16 | 12 |
| History of laparotomy | 8 | 4 | 4 |
| Corticosteroid use | 16 | 15 | 1 |
| Insulin use | 3 | 3 | 0 |
| Smoking | 21 | 8 | 13 |
Table 3.
Baseline characteristics of all patients who underwent resection
| Resection (n = 106) |
SCANDIV (n = 64) |
LOLA (n = 42) |
|
|---|---|---|---|
| Age (years), mean(s.d.) | 63.7(14.2) | 63.6(15.3 | 64.0(12.3 |
| Sex ratio (M : F) | 61 : 45 | 36 : 28 | 25 : 17 |
| BMI (kg/m2) | 26.5(4.4) | 26.1(4.)5 | 27.1(4.4) |
| ASA fitness grade | |||
| I | 18 | 10 | 8 |
| II | 36 | 23 | 13 |
| III | 44 | 30 | 14 |
| IV | 3 | 1 | 2 |
| Missing | 5 | 0 | 5 |
| History of diverticulitis | 26 | 16 | 10 |
| History of laparotomy | 11 | 8 | 3 |
| Corticosteroid use | 17 | 14 | 3 |
| Insulin use | 2 | 2 | 0 |
| Smoking | 19 | 11 | 8 |
In the resection group, patients had either Hartmann's operation (67), or sigmoid resection with (15) or without (18) a stoma. Two patients had an anastomosis that was later converted into Hartmann's operation, and four patients in the resection group underwent lavages. The median age of patients in the Hartmann's group was 69 (i.q.r 58–76) years and that of patients undergoing primary anastomosis was 58 (i.q.r 47–69) years.
The treatment failure rate was 32.8 per cent (38 patients) in the laparoscopic lavage group and 18.9 per cent (20 patients) in the resection group (P = 0.019).
In univariable analysis, smoking was a risk factor for treatment failure in the laparoscopic lavage group (OR 3.24, 95 per cent c.i. 1.19 to 8.81; P = 0.024), but not in the resection group (OR 0.46, 0.10 to 2.18; P = 0.326). The same was true for corticosteroid use in the laparoscopic lavage (OR 4.83, 1.55 to 15.08; P = 0.022) and resection (OR 1.88, 0.52 to 6.81; P = 0.335) groups. Higher ASA grade was a risk factor for both laparoscopic lavage (OR 3.21, 1.31 to 7.90; P = 0.011) and resection (OR 3.30, 1.12 to 9.71; P = 0.030) (Table 4).
Table 4.
Univariable logistic regression analysis of risk factors for treatment failure with interaction model for adjustment for study
| Resection | Lavage | |||
|---|---|---|---|---|
| OR | P | OR | P | |
| Age (years) | 1.04 (0.10, 1.08) | 0.086 | 1.01 (0.98, 1.05) | 0.389 |
| Age ≥ 65 years | 1.28 (0.48, 3.45) | 0.622 | 2.09 (0.92, 4.75) | 0.077 |
| Sex | 0.90 (0.33, 2.45) | 0.839 | 1.40 (0.63, 3.08) | 0.407 |
| BMI (kg/m2) | 0.99 (0.88, 1.11) | 0.848 | 0.91 (0.83, 0.10) | 0.049 |
| BMI ≥ 25 kg/m2 | 1.04 (0.37, 2.95) | 0.935 | 0.65 (0.28, 1.52) | 0.321 |
| ASA fitness grade | 3.30 (1.12, 9.71) | 0.030 | 3.21 (1.31, 7.90) | 0.011 |
| History of diverticulitis | 1.04 (0.33, 3.28) | 0.941 | 0.77 (0.30, 2.01) | 0.593 |
| History of laparotomy | 3.18 (0.81, 12.49) | 0.097 | 1.13 (0.25, 5.15) | 0.871 |
| Corticosteroid use | 1.88 (0.52, 6.81) | 0.335 | 4.83 (1.55, 15.08) | 0.007 |
| Smoker | 0.46 (0.10, 2.18) | 0.326 | 3.24 (1.19, 8.81) | 0.022 |
| GI surgeon present during surgical procedure | 0.82 (0.24, 2.82) | 0.749 | 1.174 (0.50, 2.73) | 0.710 |
| Interval between admission and surgery (h) | 1.01 (1.00, 1.02) | 0.184 | 1.00 (0.97, 1.01) | 0.197 |
Values in parentheses are 95% confidence intervals. GI, gastrointestinal.
In multivariable analysis, the only statistically significant risk factors for treatment failure were smoking (OR 7.05, 2.07 to 23.98; P = 0.002) and corticosteroid use (OR 6.02, 1.54 to 23.51; P = 0.010) in the laparoscopic lavage group (Table 5).
Table 5.
Multivariable logistic regression analysis of risk factors for treatment failure with interaction model for adjustment of study
| Resection | Lavage | |||
|---|---|---|---|---|
| OR | P | OR | P | |
| ASA fitness grade | 3.11 (0.98, 9.90) | 0.055 | 2.13 (0.69, 6.59) | 0.188 |
| BMI (kg/m2) | 0.99 (0.86, 1.14) | 0.895 | 0.94 (0.85, 1.04) | 0.245 |
| Corticosteroid use | 0.99 (0.25, 3.97) | 0.991 | 6.02 (1.54, 23.51) | 0.010 |
| Smoker | 0.42 (0.08, 2.11) | 0.288 | 7.05 (2.07, 23.98) | 0.002 |
Values in parentheses are 95% confidence intervals.
Discussion
By combining two of the largest randomized trials of treatment of perforated diverticulitis, a large prospectively collected data set within a trial setting of patients with Hinchey III disease could be assessed. Main risk factors for treatment failure in the laparoscopic lavage group were smoking and corticosteroid use. No other factors analysed were associated with treatment failure. Smoking is known to impair wound healing and is a well acknowledged risk factor for complications18. Therefore, in elective surgery, smoking cessation is advised possibly in conjunction with increased awareness in postoperative care for these patients as they are at higher risk of complications both in the elective and emergency settings.
Concerns have been raised regarding patients who were diagnosed with cancers and misdiagnosed as having Hinchey III perforated diverticulitis initially. In the SCANDIV study, five patients were assessed as having Hinchey III perforated diverticulitis and three of these were randomized to the laparoscopic lavage arm. Two of these patients had failure of lavage, and both were diagnosed with sigmoid cancer and had surgery within 90 days of laparoscopic lavage (1 sigmoid resection and 1 transversostomy before neoadjuvant treatment for sigmoid cancer with liver metastasis). Of the nine patients who experienced treatment failure after lavage in the LADIES trial, one was diagnosed with an underlying carcinoma during pathological assessment. In both studies, these patients were included in the analysis, as this best reflects clinical practice with the inherent difficulty in differentiating correctly between perforated diverticulitis and perforated carcinoma.
In recent years, trials19,20 have compared laparoscopic peritoneal lavage with resectional surgery. Primary anastomosis appears the preferred option over Hartmann's procedure in haemodynamically stable patients as there are fewer stomas and fewer additional procedures, with comparable morbidity and mortality19,21. Primary anastomosis, however, is not always easily performed, particularly in the emergency setting or in the absence of a surgeon experienced in emergency colorectal procedures. A recent study22 comparing colorectal with non-colorectal surgeons performing primary anastomosis in acute diverticulitis surgery revealed a 1.4 times higher mortality rate if the anastomosis was created by a non-colorectal surgeon. Laparoscopic lavage may potentially serve as a bridge to convert an acute situation to an elective situation, ensuring better treatment outcomes both in the short and long term. Therefore, to minimize the risk of treatment failure in patients undergoing lavage, patient selection has become essential, necessitating risk assessment. In this analysis, the authors chose not to subdivide the resection group into anastomosis with or without stoma and Hartmann's procedure because the groups were heterogeneous. The choice of primary anastomosis or Hartmann's procedure was at the surgeon's discretion in the SCANDIV study, and it is known from clinical practice that younger and more stable patients are usually chosen for primary anastomosis. Such selection bias was demonstrated by the 11-year difference in median age between the groups in this analysis.
Other studies have examined risk factors for failure of laparoscopic lavage. In a study by Radé et al.14, risk factors for treatment failure following laparoscopic peritoneal lavage were age over 80 years, immunosuppression, and ASA grade above III. In multivariable analysis, only ASA grade over III remained associated with treatment failure. Greilsamer et al.15 investigated potential risk factors in a cohort of 71 patients with Hinchey III disease, and found immunosuppression to be the only independent predictor of treatment failure from a broad range of factors. Finally, the larger LLO study16, which included 231 patients with Hinchey III diverticulitis, reported a short-term failure rate of 25 per cent (within 60 days) after laparoscopic lavage. A Mannheim Peritonitis Index score of at least 24 and ASA grade above III were identified as risk factors for treatment failure that could be assessed before surgery. Furthermore, an increased BMI or higher Mannheim Peritonitis Index score was independently associated with an increased risk of reoperation within the first 60 days after the procedure. The present study adds new information to these potential risk factors and might further aid the decision-making process.
The strength of this study lies in the inclusion of the two largest randomized cohorts studying Hinchey III diverticulitis in a prospective manner. Nevertheless, there are several methodological differences between the SCANDIV and LOLA studies which limit interpretation of the results. For example, the set of baseline characteristics collected and secondary outcomes studied were different. Hence, not all baseline characteristics could be considered in the risk factor analyses. To correct for study heterogeneity in the statistical analyses, care was taken to adjust for study population. As exclusion criteria were different, many patients with high corticosteroid use were excluded from the LOLA study, but were included in the SCANDIV study, making the baseline populations different. Furthermore, both trials did not register Mannheim Peritonitis Index and it was therefore not possible to assess this as a risk factor in the present analysis. Although this is the largest cohort of patients with Hinchey III diverticulitis who underwent laparoscopic lavage in a trial setting, the total number of included patients and variables still limited the extent of multivariable analyses.
In the largest assessment of risk factors for treatment failure of laparoscopic peritoneal lavage in a prospectively collected Hinchey III cohort, active smoking and corticosteroid use at index surgery were found to be statistically significant. Smoking and corticosteroid use are, therefore, factors that should be considered when assessing surgical options for patients with perforated Hinchey III diverticulitis.
Collaborators
A Papp, U Ersson, T Zittel, N Fagerström, D Gustafsson, G Dafnis,: M Cornelius, M Egenvall, P O Nyström, I Syk, D Vilhjalmsson, C Wallon, G Arbman, J Folkesson, A Chabok, M Helgeland, J Bondi, A Husby, R Helander, A Kjos, H Gregussen, A J Talabani, G Tranø, I H Nygaard, G Wiedswang, O Helmer, K F Desserud, S Norderval, M Vikhammer Gran, T Pettersen, A Sæther, H Kørner; L Blesic, H M Forsmo, S Vennix, GD Musters, IM Mulder, HA Swank, EC Consten, EH Belgers, AA van Geloven, MF Gerhards, MJ Govaert, WM van Grevenstein, A Hoofwijk, PM Kruyt, SW Nienhuijs, MA Boermeester, J Vermeulen, S van Dieren, WC Hop, BC Opmeer, JB Reitsma, RA Scholte, EWH Waltmann, DA Legemate, JF Bartelsman, DW Meijer, M de Brouwer, J van Dalen, M Durbridge, M Geerdink, GJ Ilbrink, S Mehmedovic, P Middelhoek, M J Boom, ECJ Consten, JDW van der Bilt, GDJ van Olden, MAW Stam, MS Verweij, ORC Busch, CJ Buskens, Y El-Massoudi, AB Kluit, C van Rossem, MP Schijven, PJ Tanis, C Unlu, MF Gerhards, TM Karsten, LC de Nes, H Rijna B, A van Wagensveld, GI Koffeman, EP Steller, JB Tuynman, SC Bruin, DL van der Peet, CFJ M Blanken-Peeters, HA Cense, E Jutte, RMPH Crolla, GP van der Schelling, M van Zeeland, EJR de Graaf, RPR Groenendijk, TM Karsten, M Vermaas, O Schouten, MR de Vries, HA Prins, DJ Lips, RJI Bosker, JAB van der Hoeven, J Diks, PW Plaisier, PM Kruyt, C Sietses, MWJ Stommel, SW Nienhuijs, IHJT de Hingh, MDP Luyer, G van Montfort, EH Ponten, JF Smulders, EB van Duyn, JM Klaase, DJ Swank, RT Ottow, HBAC Stockmann, J Vermeulen, RJCLM Vuylsteke, HJ Belgers, S Fransen, EM von Meijenfeldt, MN Sosef, AA W van Geloven, ER Hendriks, B ter Horst, MMN Leeuwenburgh, O van Ruler, JM Vogten, EJ C Vriens, M Westerterp, QAJ Eijsbouts, A Bentohami, TS Bijlsma, N de Korte, D Nio, JJA Joosten, LPS Stassen, MJ Wiezer, EJ Hazebroek, AB Smits, HL van Westreenen, A Brandt, WN Nijboer, BR Toorenvliet, WF Weidema, PPL O Coene, GHH Mannaerts, D den Hartog, RJ de Vos, JF Zengerink, AGM Hoofwijk, KW E Hulsewé, J Melenhorst, JHMB Stoot, WH Steup, PJ Huijstee, JWS Merkus, JJ Wever, JK Maring, J Heisterkamp, WMU van Grevenstein, MR Vriens, MGH Besselink, IHM Borel Rinkes, AJ Witkamp, GD Slooter, JLM Konsten, AF Engel, EGJM Pierik, TG Frakking, D van Geldere, GA Patijn, AJL D’Hoore, A de Buck van Overstraeten, M Miserez, I Terrasson, A Wolthuis, S Di Saverio, MG De Blasiis.
Acknowledgements
The authors thank T. Schyman and A. Åkesson at Forum South, Clinical Studies Sweden, for help with the statistical analysis.
Contributor Information
Najia Azhar, Department of Surgery, Skåne University Hospital, Malmö, Sweden; Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden.
Daniël Lambrichts, Department of Surgery, Erasmus University Medical Centre, Rotterdam, The Netherlands; Department of Surgery, University Medical Centre Amsterdam, AMC, Amsterdam, The Netherlands.
Johan Lange, Department of Surgery, Erasmus University Medical Centre, Rotterdam, The Netherlands.
Sheraz Yaqub, Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Gastrointestinal Surgery, Oslo University Hospital, Oslo, Norway.
Tom Øresland, Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Johannes Schultz, Faculty of Medicine, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of Digestive Surgery, Akershus University Hospital, Lørenskog, Norway.
Willem Bemelman, Department of Surgery, University Medical Centre Amsterdam, AMC, Amsterdam, The Netherlands.
Pamela Buchwald, Department of Surgery, Skåne University Hospital, Malmö, Sweden; Department of Clinical Sciences Malmö, Lund University, Malmö, Sweden.
Funding
The investigator-initiated LADIES trial was supported by a grant from the Netherlands Organization for Health Research and Development (ZonMw). The SCANDIV trial was funded by the South-Eastern Norway Regional Health Authority (PNR 2719011).
Author contributions
Najia Azhar (Conceptualization, Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review & editing), Daniël Lambrichts (CRediT contribution not specified), Sheraz Yaqub (Conceptualization, Methodology, Supervision, Writing—review & editing), Johan Lange (CRediT contribution not specified), Tom Oresland (Conceptualization, Methodology, Writing—review & editing), Willem Bemelman (Conceptualization, Data curation, Methodology, Supervision, Writing—review & editing), Johannes Schultz (Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing—original draft, Writing—review & editing), and P. Buchwald (Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Writing—original draft, Writing—review & editing).
Disclosure
The authors declare no conflict of interest.
Data availability
Original data will be available upon request.
References
- 1. Bharucha AE, Parthasarathy G, Ditah I, Fletcher JG, Ewelukwa O, Pendlimari Ret al. . Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol 2015;110:1589–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hinchey EJ, Schaal PG, Richards GK. Treatment of perforated diverticular disease of the colon. Adv Surg 1978;12:85–109 [PubMed] [Google Scholar]
- 3. Schultz JK, Azhar N, Binda GA, Barbara G, Biondo S, Boermeester MAet al. . European Society of Coloproctology: guidelines for the management of diverticular disease of the colon. Colorectal Dis 2020;22:5–28 [DOI] [PubMed] [Google Scholar]
- 4. Hall J, Hardiman K, Lee S, Lightner A, Stocchi L, Paquette IMet al. . The American Society of Colon and Rectal Surgeons clinical practice guidelines for the treatment of left-sided colonic diverticulitis. Dis Colon Rectum 2020;63:728–747 [DOI] [PubMed] [Google Scholar]
- 5. Vennix S, Musters GD, Mulder IM, Swank HA, Consten EC, Belgers EHet al. . Laparoscopic peritoneal lavage or sigmoidectomy for perforated diverticulitis with purulent peritonitis: a multicentre, parallel-group, randomised, open-label trial. Lancet 2015;386:1269–1277 [DOI] [PubMed] [Google Scholar]
- 6. Schultz JK, Yaqub S, Wallon C, Blecic L, Forsmo HM, Folkesson Jet al. . Laparoscopic lavage vs primary resection for acute perforated diverticulitis: the SCANDIV randomized clinical trial. JAMA 2015;314:1364–1375 [DOI] [PubMed] [Google Scholar]
- 7. Angenete E, Thornell A, Burcharth J, Pommergaard HC, Skullman S, Bisgaard Tet al. . Laparoscopic lavage is feasible and safe for the treatment of perforated diverticulitis with purulent peritonitis: the first results from the randomized controlled trial DILALA. Ann Surg 2016;263:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marshall JR, Buchwald PL, Gandhi J, Schultz JK, Hider PN, Frizelle FAet al. . Laparoscopic lavage in the management of Hinchey grade III diverticulitis: a systematic review. Ann Surg 2017;265:670–676 [DOI] [PubMed] [Google Scholar]
- 9. Schultz JK, Wallon C, Blecic L, Forsmo HM, Folkesson J, Buchwald Pet al. . One-year results of the SCANDIV randomized clinical trial of laparoscopic lavage versus primary resection for acute perforated diverticulitis. Br J Surg 2017;104:1382–1392 [DOI] [PubMed] [Google Scholar]
- 10. Thornell A, Angenete E, Bisgaard T, Bock D, Burcharth J, Heath Jet al. . Laparoscopic lavage for perforated diverticulitis with purulent peritonitis: a randomized trial. Ann Intern Med 2016;164:137–145 [DOI] [PubMed] [Google Scholar]
- 11. Azhar N, Johanssen A, Sundström T, Folkesson J, Wallon C, Kørner Het al. . Laparoscopic lavage vs primary resection for acute perforated diverticulitis: long-term outcomes from the Scandinavian Diverticulitis (SCANDIV) randomized clinical trial. JAMA Surg 2020;156:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoek VT, Edomskis PP, Stark PW, Lambrichts DPV, Draaisma WA, Consten ECJet al. . Laparoscopic peritoneal lavage versus sigmoidectomy for perforated diverticulitis with purulent peritonitis: three-year follow-up of the randomised LOLA trial. Surg Endosc 2022;36:7764–7774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gehrman J, Angenete E, Bjorholt I, Bock D, Rosenberg J, Haglind E. Health economic analysis of laparoscopic lavage versus Hartmann’s procedure for diverticulitis in the randomized DILALA trial. Br J Surg 2016;103:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Radé F, Bretagnol F, Auguste M, Di Guisto C, Huten N, de Calan L. Determinants of outcome following laparoscopic peritoneal lavage for perforated diverticulitis. Br J Surg 2014;101:1602–1606 [DOI] [PubMed] [Google Scholar]
- 15. Greilsamer T, Abet E, Meurette G, Comy M, Hamy A, Lehur PAet al. . Is the failure of laparoscopic peritoneal lavage predictable in Hinchey III diverticulitis management? Dis Colon Rectum 2017;60:965–970 [DOI] [PubMed] [Google Scholar]
- 16. Binda GA, Bonino MA, Siri G, Di Saverio S, Rossi G, Nascimbeni Ret al. . Multicentre international trial of laparoscopic lavage for Hinchey III acute diverticulitis (LLO study). Br J Surg 2018;105:1835–1843 [DOI] [PubMed] [Google Scholar]
- 17. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sorensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy: a systematic review. Ann Surg 2012;255:1069–1079 [DOI] [PubMed] [Google Scholar]
- 19. Lambrichts DPV, Vennix S, Musters GD, Mulder IM, Swank HA, Hoofwijk AGMet al. . Hartmann’s procedure versus sigmoidectomy with primary anastomosis for perforated diverticulitis with purulent or faecal peritonitis (LADIES): a multicentre, parallel-group, randomised, open-label, superiority trial. Lancet Gastroenterol Hepatol 2019;4:599–610 [DOI] [PubMed] [Google Scholar]
- 20. Tartaglia D, Di Saverio S, Stupalkowska W, Giannessi S, Robustelli V, Coccolini Fet al. . Laparoscopic peritoneal lavage versus laparoscopic sigmoidectomy in complicated acute diverticulitis: a multicenter prospective observational study. Int J Colorectal Dis 2019;34:2111–2120 [DOI] [PubMed] [Google Scholar]
- 21. Bridoux V, Regimbeau JM, Ouaissi M, Mathonnet M, Mauvais F, Houivet Eet al. . Hartmann’s procedure or primary anastomosis for generalized peritonitis due to perforated diverticulitis: a prospective multicenter randomized trial (DIVERTI). J Am Coll Surg 2017;225:798–805 [DOI] [PubMed] [Google Scholar]
- 22. Goldstone RN, Cauley CE, Chang DC, Kunitake H, Ricciardi R, Bordeianou L. The effect of surgical training and operative approach on outcomes in acute diverticulitis: should guidelines be revised? Dis Colon Rectum 2019;62:71–78 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Original data will be available upon request.