Abstract
Background
In rectal cancer, watch and wait for patients with a cCR after neoadjuvant treatment has an established evidence base. However, there is a lack of consensus on the definition and management of a near-cCR. This study aimed to compare outcomes in patients who achieved a cCR at first reassessment versus later reassessment.
Methods
This registry study included patients from the International Watch & Wait Database. Patients were categorized as having a cCR at first reassessment or at later reassessment (that is near-cCR at first reassessment) based on MRI and endoscopy. Organ preservation, distant metastasis-free survival, and overall survival rates were calculated. Subgroup analyses were done for near-cCR groups based on the response evaluation according to modality.
Results
A total of 1010 patients were identified. At first reassessment, 608 patients had a cCR; 402 had a cCR at later reassessment. Median follow-up was 2.6 years for patients with a cCR at first reassessment and 2.9 years for those with a cCR at later reassessment. The 2-year organ preservation rate was 77.8 (95 per cent c.i. 74.2 to 81.5) and 79.3 (75.1 to 83.7) per cent respectively (P = 0.499). Similarly, no differences were found between groups in distant metastasis-free survival or overall survival rate. Subgroup analyses showed a higher organ preservation rate in the group with a near-cCR categorized exclusively by MRI.
Conclusion
Oncological outcomes for patients with a cCR at later reassessment are no worse than those of patients with a cCR at first reassessment.
Patients from the International Watch & Wait Database with a cCR were analysed. The oncological outcomes for patients achieving a cCR at later reassessment, who had a near-cCR at first reassessment, were similar to those of patients with a cCR at first reassessment.
Introduction
The oncological safety of a watch-and-wait (W&W) approach, with deferral of surgery aiming at organ preservation (OP) with a cCR after neoadjuvant treatment in patients with rectal cancer, has a growing evidence base1–5. For patients with no sign of remaining tumour at first reassessment, this approach may be considered uncontroversial. However, there is a considerable proportion of patients who are shown to have a good, albeit not complete, response to treatment at first reassessment. These good responders are often referred to as having a near-cCR, although this clinical entity is poorly defined. Most often, a near-cCR is attributed to patients with a good chance of reaching a cCR, but who lack the typical features on endoscopy and/or MRI. For such patients, extension of the observation period and repeated reassessments are gradually becoming more common.
Despite the lack of an exact definition of near-cCR, the term is commonly used by clinicians and also in recent publications5–7. The principal tools used for clinical response assessment are MRI, digital rectal examination (DRE), and flexible endoscopy. For MRI, guidelines for evaluation of tumour regression grade (mrTRG) are available8,9 and are presumably used at W&W centres. Although interpretation of images could be considered subjective, DRE and endoscopy may contain an even greater element of subjectivity. With inclusion of modern endoscopy techniques, criteria for endoscopic reassessment after neoadjuvant (chemo)radiotherapy ((C)RT) are being developed10–12. However, there is no clear framework to decide whether a patient has near-cCR and, when one eventually reaches a cCR, if outcomes are similar6,13.
Several different neoadjuvant regimens that could lead to a cCR (and near-cCR) have been reported1. This leads to variability in the time interval between the conclusion of (C)RT and first reassessment in studies to date. For example, one patient could be treated with short-course radiotherapy (SCRT) over 1 week and have first reassessment 6 weeks later, whereas another could receive 5 weeks of CRT followed by additional chemotherapy before the first reassessment. Although this renders comparison difficult between patients with a near-cCR who have undergone different neoadjuvant treatments, the clinical situation of a patient displaying an excellent, but not complete, response, is very much the same, irrespective of neoadjuvant therapy delivered. To date, few data on long-term oncological outcome for patients with a near-cCR have been reported, and only small cohorts of patients analysing cCR at different reassessment times have been published13,14.
The aim of this study was to compare outcomes in patients treated using a W&W strategy who had achieved a cCR at first reassessment versus later reassessment.
Methods
The International Watch and Wait Database (IWWD) is a retrospective and prospective registry with participation from 60 centres across 15 countries15. The registry was started in 2014, and contains information on preoperative staging, type of treatment, reassessment modalities, follow-up, and outcome. All patients with rectal cancer, in whom surgery was deferred after neoadjuvant therapy, and instead followed a W&W approach, were included. Participating centres routinely entered data on individual patients for whom an OP strategy was decided during a multidisciplinary therapy meeting. Data from patients managed with a W&W approach before 2014 could also be collected retrospectively. Entered data were subjected to quality checks in case of irregularities or discrepancies in follow-up time.
For this report, a data set (data lock 1 April 2022) was retrieved from the IWWD. All patients whose information was were entered into the IWWD from 1991 until 1 April 2022 were included in the data set. Data were coded and patient anonymity assured. The IWWD was approved by a certified Medical Ethics Committee (Leiden Den Haag Delft) and this study was approved by the Swedish Ethical Review Authority (2022-02044-01).
Definitions
For the purpose of this study, cCR at first reassessment was defined by register data reporting the combination of mrTRG 1 (absence of any tumour signal) and an endoscopic finding of normal, white scar and/or teleangiectasia at the first reassessment. All patients who had an mrTRG of 2 or higher and/or an endoscopic finding of ulcer, polypoid tissue or stenosis were classified as having a near-cCR at first reassessment and thus a cCR at later reassessment. Therefore, both an mrTRG classification and an endoscopy report at first reassessment after neoadjuvant therapy were mandatory for inclusion in the study.
Population
This study included only patients who, at some point during follow-up, reached a cCR. All patients labelled by their centre at first data entry as having a complete response were thus included. For the group of patients who did not have this label, IWWD follow-up data were scrutinized to exclude those who never obtained a cCR. This was done by combining the results from MRI, endoscopy, and/or transanal microscopic microsurgery (TEM) during follow-up.
Patients with distant metastasis at baseline were excluded. As OP was the outcome, patients with other reasons for non-operative management, such as severe co-morbidity making the patient inoperable or patient refusal for surgery, were also excluded from further analyses.
Outcomes
The outcome of primary interest was OP, defined as absence of transabdominal resectional surgery (anterior resection, Hartmann’s procedure or abdominoperineal resection), and absence of locoregional regrowth unless salvaged by transanal R0 excision. Other outcomes included distant metastasis-free survival (DMFS) and overall survival (OS).
Additional analyses for these outcome measures were done for subgroups of cCR at later reassessment based on results from the first reassessment: near-cCR on MRI (favourable endoscopy but mrTRG 2 or more); near-cCR on endoscopy (mrTRG 1 but ulcer, polypoid tissue or stenosis endoscopically), and near-cCR on both modalities.
Statistical analysis
Baseline and reassessment characteristics are presented as numbers with percentages for categorical variables and as median (i.q.r.) for continuous variables. χ2 tests were used to describe differences in binomial and categorical variables. The Mann–Whitney U test and Kruskal–Wallis test were used for continuous variables. Reassessment time intervals were calculated from the last day of radiotherapy to the first reassessment in all patients, and described as median (i.q.r.). Median follow-up time was calculated from the first reassessment using the reversed Kaplan–Meier method, a method used in cohort studies whereby the event and censor of the normal Kaplan–Meier are reversed16.
Survival curves were constructed using the Kaplan–Meier method. The log rank test was used to describe differences and 95 per cent confidence intervals were calculated. OP was calculated from the first reassessment to the date of (first) transabdominal surgery or, in some patients, regrowth. Two- and 5-year DMFS and OS rates were calculated from the first reassessment to the first date of distant metastasis or death. Cumulative rates for incidence of first events were calculated and plotted with the Aalen–Johansen estimator, taking competing risks into account. Gray’s test was used to compare the subdistribution for each cause across groups. For all statistical tests, P < 0.050 was considered significant.
Statistical analyses were undertaken using SPSS® version 27 (IBM, Armonk, NY, USA) and R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
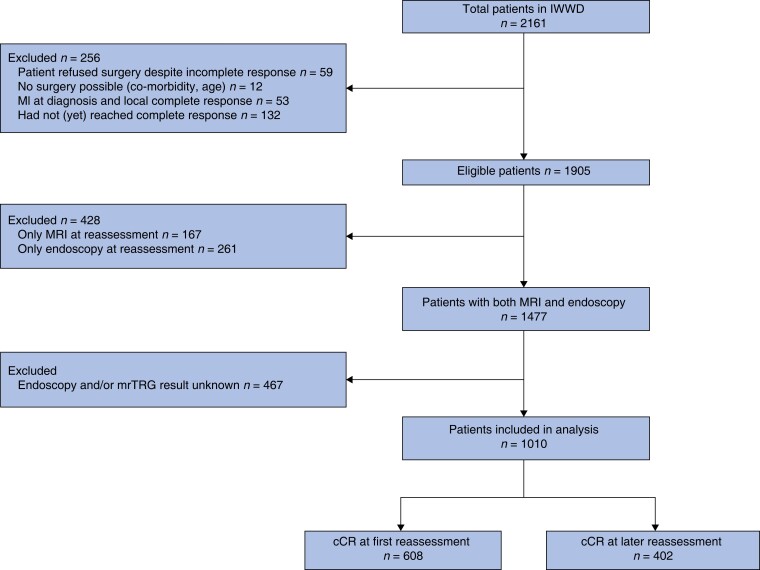
At time of data lock, information on 2161 patients had been entered into the IWWD registry. Figure 1 shows exclusions, leading to a final study population of 1010 patients. Among the 1010 patients in whom the results of both endoscopy and mrTRG were recorded in the IWWD, 608 were classified as having a cCR at first reassessment and 402 as having a cCR at later reassessment. Baseline data for both groups are shown in Table 1. Patients with a cCR at later reassessment were more often women, had more co-morbidity, more advanced T category, and were more commonly included in the IWWD after 2015.
Fig. 1.
Study flow diagram
IWWD, International Watch & Wait Database; mrTRG, magnetic resonance tumour regression grade.
Table 1.
Baseline patient and tumour characteristics for patients with a cCR at first reassessment or later reassessment
| cCR at first reassessment (n = 608) | cCR at later reassessment (n = 402) | P* | |
|---|---|---|---|
| Sex ratio (M : F) | 413 : 195 | 245 : 157 | 0.023 |
| Age at diagnosis (years), median (i.q.r.) | 65 (56–72) | 63 (54–72) | 0.224† |
| BMI (kg/m2), median (i.q.r.) | 26.0 (23.6–29.1) | 26.4 (23.0–29.1) | 0.685† |
| Co-morbidity present | 165 (27.1) | 165 (41.0) | <0.001 |
| Tumour distance from anorectal junction on MRI, median (i.q.r.) | 4.0 (2.0–6.0) | 3.0 (0–5.4) | <0.001† |
| Year of decision for W&W | <0.001 | ||
| ȃBefore 2015 | 197 (32.4) | 73 (18.2) | |
| ȃAfter 2015 | 411 (67.6) | 329 (81.8) | |
| Baseline clinical T category | <0.001 | ||
| ȃcT1 | 6 (1.0) | 3 (0.7) | |
| ȃcT2 | 173 (28.5) | 67 (16.7) | |
| ȃcT3 | 387 (63.7) | 287 (71.4) | |
| ȃcT4 | 35 (5.8) | 42 (10.4) | |
| ȃMissing | 7 (1.2) | 3 (0.7) | |
| Baseline N category | 0.630 | ||
| ȃN0 | 198 (32.6) | 131 (32.6) | |
| ȃN1 | 248 (40.8) | 155 (38.6) | |
| ȃN2 | 154 (25.3) | 113 (28.1) | |
| ȃMissing | 8 (1.3) | 3 (0.7) | |
| Induction therapy | 0.991 | ||
| ȃCRT | 496 (81.6) | 328 (81.6) | |
| ȃCRT + CT | 21 (3.5) | 13 (3.2) | |
| ȃSCRT | 54 (8.9) | 35 (8.7) | |
| ȃSCRT + CT | 33 (5.4) | 24 (6.0) | |
| ȃCT | 4 (0.7) | 2 (0.5) |
Values are n (%) unless otherwise indicated. W&W, watch and wait; CRT, chemoradiotherapy; CT, chemotherapy; SCRT, short-course radiotherapy. *χ2 test, except †Mann–Whitney U test.
Table 2 summarizes MRI and endoscopy findings at first reassessment for the two groups. Among patients with a cCR at later reassessment, 149 (37.1 per cent) had both an mrTRG of 2 or higher and unfavourable endoscopy findings, 130 (32.3 per cent) had an mrTRG of 2 or higher but favourable endoscopic findings, and 123 (30.5 per cent) had unfavourable endoscopy characteristics combined with mrTRG1.
Table 2.
Findings at endoscopy and MRI recorded at first reassessment for patients with a cCR at first reassessment or later reassessment
| cCR at first reassessment (n = 608) | cCR at later reassessment (n = 402) | |
|---|---|---|
| Endoscopy | ||
| ȃNormal/white scar | 585 (96.2) | 193 (48.0) |
| ȃTelangiectasia | 181 (29.7) | 80 (19.9) |
| ȃUlcer | – | 211 (52.5) |
| ȃPolypoid tissue | – | 64 (15.9) |
| ȃStenosis | – | 13 (3.2) |
| MRI | ||
| ȃmrTRG 1 | 608 (100) | 123 (30.6) |
| ȃmrTRG 2 | – | 196 (48.7) |
| ȃmrTRG 3 | – | 75 (18.7) |
| ȃmrTRG 4 | – | 7 (1.7) |
| ȃmrTRG 5 | – | 1 (0.2) |
Values are n (%). mrTRG, magnetic resonance tumour regression grade.
Median follow-up from the first reassessment for patients who had a cCR at first reassessment and those with a cCR at later reassessment was 2.6 and 2.9 years respectively. The median interval from the end of radiotherapy to date of the first MRI was 8.9 (i.q.r. 6.7–11.7) weeks for patients with a cCR at first reassessment and 7.9 (5.9–10.0) weeks for those with a cCR at later reassessment (P < 0.001). The first endoscopy was carried out 9.9 (7.7–12.9) and 8.8 (6.9–11.4) weeks after the end of radiotherapy respectively (P < 0.001). The median interval between first reassessment and decision to W&W was 1.1 (0–3.1) and 1.9 (0.4–4.7) weeks respectively (P < 0.001). Twenty-three patients (3.8 per cent) in the group with a cCR at first reassessment received chemotherapy within 6 months after the first reassessment, compared with 52 (12.9 per cent) of those with a cCR at later reassessment (P < 0.001). In group with a cCR at first reassessment, 9 patients (1.5 per cent) had a transanal local excision within 6 months after first reassessment, compared with 14 (3.5 per cent) with a cCR at later reassessment (P = 0.037). Seven patients in group with a cCR at first reassessment had an R0 resection after local excision, one an R1 resection, and in one patient the resection margin was unknown. Among patients with cCR at later reassessment, 11 patients had an R0 resection and 3 had an unknown resection margin.
Outcomes
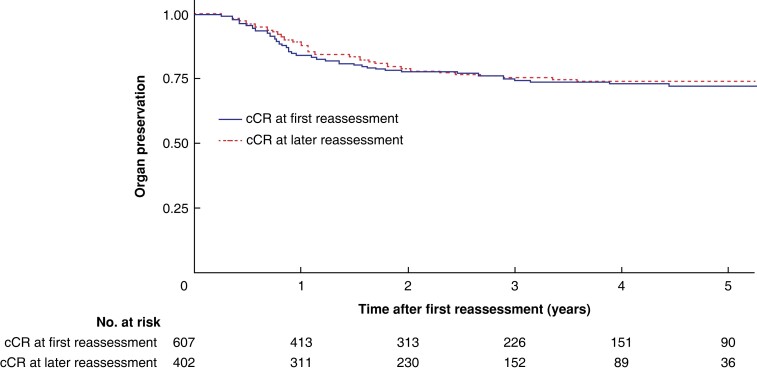
Overall, OP was achieved in 478 patients (78.6 per cent) with a cCR at first reassessment; the corresponding figure for patients with a cCR at later reassessment was 318 (79.1 per cent). The 2- and 5-year OP rates were 77.8 (95 per cent c.i. 74.2 to 81.5) and 72.0 (67.8 to 76.6) per cent respectively for group with a cCR at first reassessment, and 79.3 (75.1 to 83.7) and 74.0 (69.2 to 79.2) per cent for those with a cCR at later reassessment (P = 0.499) (Fig. 2).
Fig. 2.
Five-year organ preservation rate since first reassessment for patients with a cCR at first reassessment or later reassessment
P = 0.499 (log rank test).
The 2- and 5-year DMFS rates were 95.2 (95 per cent c.i. 93.3 to 97.1) and 89.2 (85.6 to 92.9) per cent respectively for patients with a cCR at first reassessment; corresponding figures for patients with a cCR at later reassessment were 93.7 (91.2 to 96.4) and 89.6 (86.1 to 93.4) per cent (P = 0.676).
Five-year OS rates were 92.2 (95 per cent c.i. 88.8 to 95.7) per cent for patients with a cCR at first reassessment and 86.0 (79.8 to 92.7) per cent for those with a cCR at later reassessment (P = 0.497).
Subgroup analyses
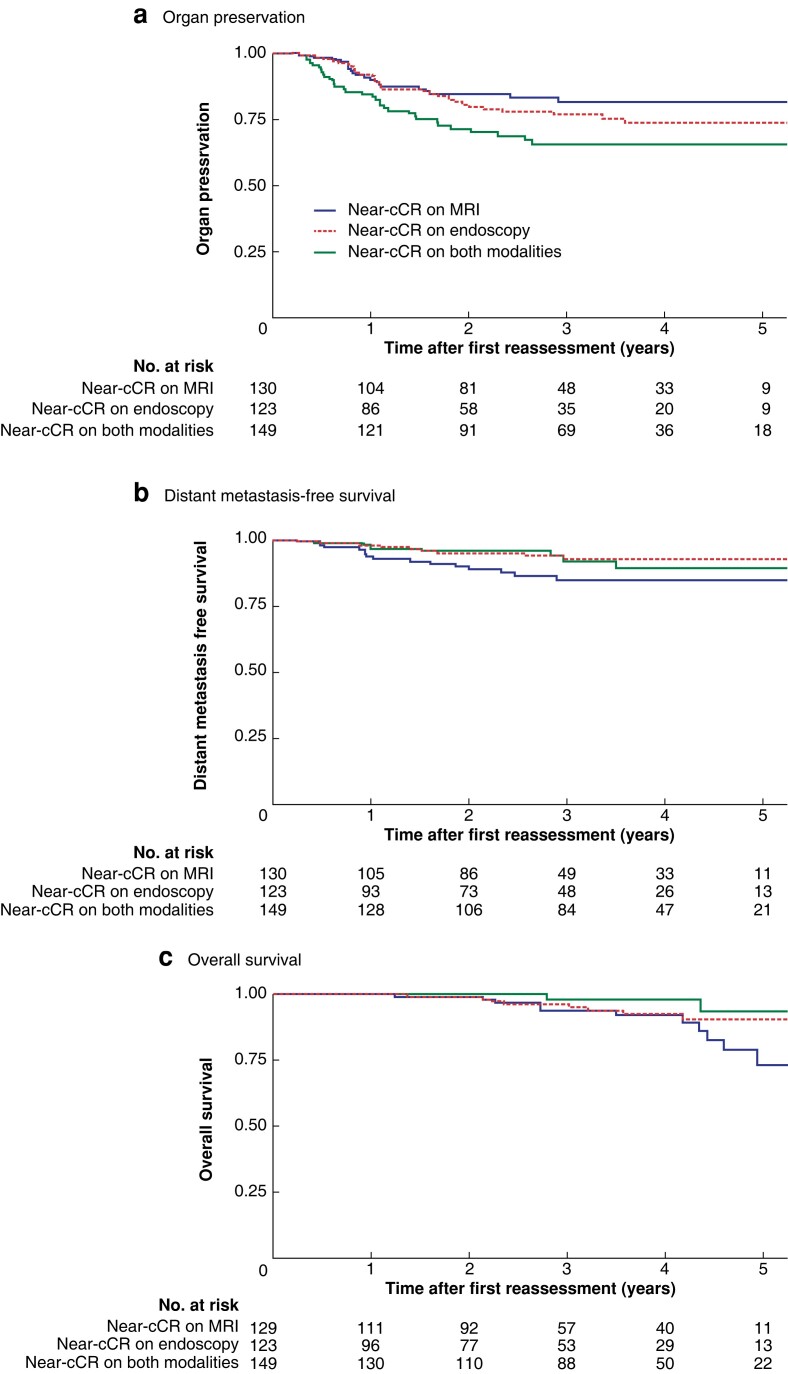
Comparing baseline characteristics in the three subgroups of patients with a cCR at later reassessment, those with a near-cCR on endoscopy at the first reassessment were more often men, but there were no other statistically significant differences (Table S1). OP, DMFS, and OS rates in the subgroups with a cCR at later reassessment are shown in Fig. 3. Patients who were classified as having a near-cCR only on the basis of a MRI finding at the first assessment had a significantly increased OP rate (P = 0.020).
Fig. 3.
Five-year organ preservation, distant metastasis-free survival, and overall survival since first reassessment for near-cCR subgroups based on modality
a Organ preservation, b distant metastasis-free survival, and c overall survival. aP = 0.020, bP = 0.095, cP = 0.073 (log rank test).
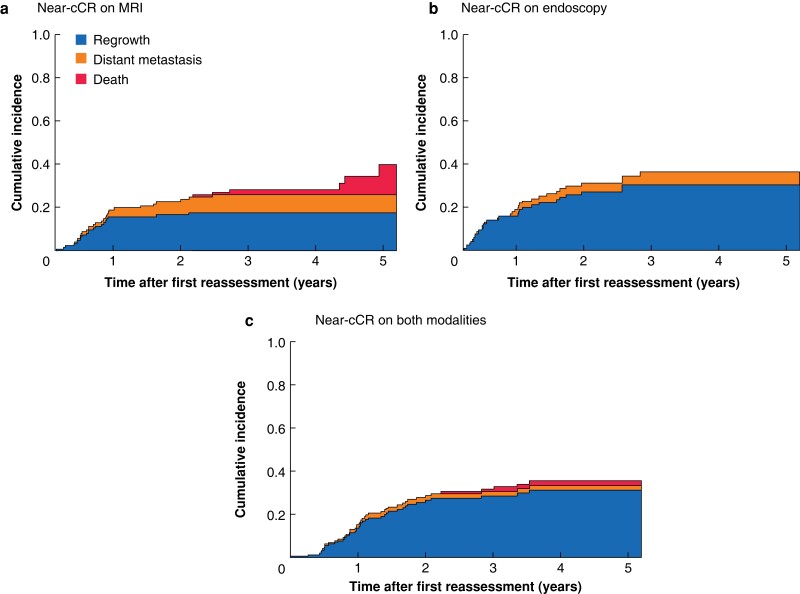
Regrowth was the predominant first event during follow-up for all three subgroups (Fig. 4). The 3-year total cumulative incidence of any first event was 28.2 per cent for the group with a near-cCR on MRI (17.6 per cent local regrowth, 8.2 per cent distant metastasis, and 2.4 per cent death), 36.3 per cent for those with a near-cCR on endoscopy (30.4 per cent local regrowth, 5.9 per cent distant metastasis, and 0.0 per cent death), and 31.7 per cent for patients with a near-cCR on both modalities (28.6 per cent local regrowth, 2.2 per cent distant metastasis, and 0.9 per cent death). The likelihood of any first event happening was no different between the groups (P = 0.674). Across the three groups, there was no difference in the subdistribution of regrowth (P = 0.100), distant metastasis (P = 0.131), or death (P = 0.065).
Fig. 4.
Cumulative incidence rates for competing first events since first reassessment for near-cCR subgroups on MRI, endoscopy, and both modalities
a MRI, b endoscopy, and c both modalities.
Discussion
This large study, reporting on oncological outcomes of patients with a cCR after an initial near-cCR, has demonstrated that first reassessment after neoadjuvant therapy for rectal cancer can be a clinical challenge. For patients with an unquestionable remnant tumour, the recommendation is generally to proceed to surgery; for those with mrTRG 1 and favourable endoscopy and DRE findings, deferral of surgery aiming at OP is a valid option. However, the optimal management of patients with an excellent, albeit not complete, clinical response remains controversial. A substantial proportion of these patients has been shown to progress to a cCR with extension of the observation period and repeated reassessments. This study, based on 1010 patients from the IWWD, showed that OP, OS, and DMFS rates in patients with a cCR at later reassessment were similar to those of patients with a cCR at first reassessment.
Data from the IWWD register on both MRI and endoscopic findings were used to define cCR and near-cCR. This reflects current clinical practice at W&W centres, where the combined results of these modalities are generally used to assess the response to neoadjuvant therapy. Although the term near-cCR is frequently used, no universally accepted definition exists. Using mrTRG and/or the presence of an ulcer or polypoid tissue on endoscopy is in line with the general definition of near-cCR commonly used by investigators13,17.
In this study, the OP rate for patients with a cCR at later reassessment was no worse than that for patients with a cCR at first reassessment. Accordingly, the DMFS and OS rates were comparable between the two groups. The overall OP rate was consistent with other reported patient cohorts within a W&W strategy18,19. Although all patients in the present study eventually achieved a cCR, the results include a group with an initial near-cCR for whom, in general, there is a paucity of data in the literature. One Dutch study17 from 2018, with a smaller cohort, reported similar non-significant differences in regrowth rate and OS between cCR at first reassessment and cCR at the second reassessment.
Oncological outcomes were similar in the two groups, even though the group with a cCR at later reassessment included a significantly larger proportion of patients with T3 and T4 tumours. This finding is in accordance with the observation of Habr-Gama et al.14 that patients with more advanced T categories may take longer, but can eventually achieve and sustain a cCR. This should be considered when a decision to defer surgery has to be made for patients with an advanced T category and a near-cCR at first reassessment.
It is notable that a significantly larger proportion of patients with a cCR at later reassessment received chemotherapy within 6 months after the first reassessment. A pCR rate of 10 per cent in the Stockholm III trial versus 28 per cent in the RAPIDO trial supports the notion that adding chemotherapy after SCRT can improve the response rate20,21. The strategy including consolidation chemotherapy was also applied in the OPRA trial7, where more than half of patients receiving chemotherapy after CRT achieved and sustained a cCR. Furthermore, 3.5 per cent of patients with a cCR at later reassessment underwent local excision during the first 6 months after first reassessment compared with only 1.5 per cent of those with a cCR at first reassessment. This reflects recent studies22–24 in which TEM has proven to be a safe and a feasible alternative to abdominal surgery for limited residual disease after neoadjuvant treatment.
Patients with a cCR at later reassessment, and thus a near-cCR at first reassessment, were further subdivided depending on modality indicating the near-cCR. Those with favourable MRI findings but a near-cCR on endoscopy had a lower OP rate than patients with a near-cCR on MRI (mrTRG 2 or higher) and favourable endoscopy. The proportion of patients with a near-cCR referred for surgery and who never achieved a cCR is not included in this report but, speculatively, it is possible that clinicians might react more intuitively to endoluminal abnormalities and opt for surgery earlier, compared with when abnormalities are reported on MRI. Although mrTRG 2 at first reassessment has been shown to be a predictor of surgery later in follow-up25, it is also known that MRI as a single modality provides incomplete information26. Moreover, it is conceivable that a tumour which is not detected endoscopically, although suspected on MRI, may lead to a prolonged persistence of viable (submucosal) tumour cells that eventually result in disseminated disease and a fatal outcome. However, although the discrete numerical differences in DMFS and OS rates between the near-cCR subgroups reported here support this interpretation, it should be noted that these differences were not statistically significant.
In this study, the proportion of patients with co-morbidity was significantly higher among those with a cCR at later reassessment. One possible explanation is that the threshold for opting for surgery when observing a near-cCR may have been lower among patients fit for surgery than in co-morbid, high-risk patients. The data presented here, showing similar outcomes for patients with a cCR at first and later reassessment, may provide additional guidance for clinicians at reassessment of patients, irrespective of performance status, after neoadjuvant therapy for rectal cancer.
This study has several limitations. The major one is that patients with a near-cCR who never achieved a cCR could not be included owing to the structure of the IWWD, making it impossible to compare outcomes of cCR and near-cCR. Many patients with a near-cCR who were referred for surgery and did not achieve a cCR are not registered in the IWWD because data collection is retrospective. Inclusion in the IWWD begins when a decision to defer surgery is taken at a multidisciplinary tumour board meeting but, owing to heterogeneity in clinical practice, patients inevitably have been included at different time points. To minimize inclusion bias and make comparable groups, the IWWD was scrutinized in order to include patients only who eventually achieved a cCR. However, it should be recognized that the exact definitions of cCR may vary between centres.
Second, important parameters for response assessment, such as elaborate descriptions of endoscopic findings (and endoscopic images) and results of diffusion-weighted imaging, are lacking in the IWWD. In addition, DRE findings were insufficiently reported. Although this report may thus include an oversimplified near-cCR grouping, the data nevertheless reflect a realistic view of actual clinical practice. The median interval between the end of radiotherapy and first response evaluation was between 8 and 10 weeks for both groups. This is representative of clinical practice and consistent with the recommended time interval8,14. Even though this study does not provide exact clinical or radiological criteria for the identification of a near-cCR, patients who underwent subsequent reassessments leading to a cCR did no worse than those who a achieved a cCR at first reassessment. Therefore, to arrange a second reassessment following a good, but not complete, response at first reassessment may the improve chances of OP, with OS and DMFS rates maintained if a cCR is reached. These results emphasize the importance of defining optimal intervals between reassessments and the time point at which a definitive decision should be made. This report also underlines the importance of using all available modalities for response evaluation and not discarding MRI findings, even in the presence of a favourable endoscopy result. It is recognized that further research and a universal definition of a near-cCR is necessary for the safe identification and inclusion of patients in W&W programmes and future interpretation of research results.
Collaborators
Aghili M., Keshvari A., Nouritaromlou M.K. (Imam Khomeini Hospital Complex, Tehran, Iran); Ahlberg M., Kordnejad S. (Karolinska Universitetssjukhuset, Stockholm, Sweden); Aleinikov A., Dulskas A. (National Cancer Institute, Vilnius, Lithuania); Asoğlu O., Tokmak H. (Boğaziçi Klinik Bilimler Akademisi, Istanbul, Turkey); Barroca R.G., Caiado A.F., Rosa I.A.L. (IPO Francisco Gentil, Lisbon, Portugal); Breukink S.O. (Department of general surgery MUMC+, Maastricht, the Netherlands, GROW - School for Oncology and Developmental, the Netherlands, NUTRIM School of Nutrition and Translational Research in Metabolism the Netherlands); Coraglio M.F., Iseas S. (Hospital Udaondo, Buenos Aires, Argentina); Creaven B., Winter D.C., Zaborowski A. (St. Vincent's University Hospital, Dublin, Ireland); Cunningham C., Gregory E. (Oxford University Hospitals, Oxford, United Kingdom); Custers P.A., Geubels B.M. (Nederlands Kanker Instituut, Amsterdam, The Netherlands); DeBrun L., D’Hoore A. (UZ Gasthuisberg, Leuven, Belgium); Dimofte G. (Regional Institute of Oncology Iasi, Universitatea de Medicina si Farmacie “Grigore T. Popa”, Iași, Romania); Fechner K., Matzel K. (Universitätsklinikum Erlangen, Erlangen, Germany); Fernandez L., Herrando A.I., Vieira P. (Centro Clínico Champalimaud, Lisbon, Portugal); Gaertner W.B., Madoff R.D. (University of Minnesota, Minneapolis, USA); Gerard J.P., Jacquinot F., Schiappa R. (Centre Antoine-Lacassagne, Nice, France); Gollins S. (Glan Clwyd Hospital, Rhyl, United Kingdom); Gonzalez M.,); Vaccaro C.A. (Hospital Italiano de Buenos Aires, Buenos Aires, Argentina); Habr-Gama A., São Julião G.P. (Instituto A&J Gama, São Paulo, Brasil); Holman F.A. (Leids Universitair Medisch Centrum, Leiden, the Netherlands); Hompes R., Lameris W. (Amsterdum UMC, Amsterdam, the Netherlands); Ketelaers S.H.J., Rutten H.J.T. (Catharina Ziekenhuis, Eindhoven, the Netherlands); Leitner K., Mazzarisi C. (Ospedale di Bressanone, Bressanone, Italy); Malcomson L., O’Dwyer S.T., Saunders M. (The Christie NHS Foundation Trust, Manchester, United Kingdom); Maroli A. (IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy); Mitchell P. (Royal Preston Hospital, Preston, United Kingdom); Murad-Regadas S. (Universidade Federal do Ceará, Fortaleza, Brasil); Pairola A., Pedraza Salazar I., Sanchez Loria F. (Instituto Alexander Fleming, Buenos Aires, Argentina); Pennings A.J. (Maastricht Universitair medisch Centrum+, Maastricht, the Netherlands+/GROW school for oncology and developmental biology); Spinelli A. (Department of Biomedical Sciences, Humanitas University, Milan, Italy; IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy); Sun Myint A. (The Clatterbridge Cancer Centre, Bebbington, United Kingdom)
Supplementary Material
Acknowledgements
The development of the IWWD was partly funded by the European Registration of Cancer Care, financed by the European Society of Surgical Oncology, the Champalimaud Foundation in Lisbon, and the Netherlands Cancer Institute.
Contributor Information
Sofieke J D Temmink, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
Koen C M J Peeters, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Renu R Bahadoer, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Elma Meershoek-Klein Kranenbarg, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Annet G H Roodvoets, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Jarno Melenhorst, Department of Surgery, Maastricht Universitair Medisch Centrum+, Maastricht, the Netherlands.
Jacobus W A Burger, Department of Surgery, Catharina Ziekenhuis, Eindhoven, the Netherlands.
Albert Wolthuis, Department of Abdominal Surgery, University Hospitals Leuven, Leuven, Belgium.
Andrew G Renehan, Manchester Cancer Research Centre, National Institute for Health Research Manchester Biomedical Research Centre, Division of Cancer Sciences, School of Medical Sciences, Faculty of Biology, Medicine, and Health, University of Manchester, Manchester, UK; Colorectal and Peritoneal Oncology Centre, Christie National Health Service Foundation Trust, Manchester, UK.
Nuno L Figueiredo, Colorectal Surgery, Hospital Lusíadas, Lisbon, Portugal.
Oriol Pares, Department of Radiation Oncology, Champalimaud Foundation, Lisbon, Portugal.
Anna Martling, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
Rodrigo O Perez, Department of Colorectal Surgery, Angelita and Joaquim Gama Institute, São Paulo, Brazil; Department of Surgical Oncology, Hospital Beneficencia Portuguesa, São Paulo, Brazil; Colorectal Surgery Division, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil; Ludwig Institute for Cancer Research, São Paulo Branch, São Paulo, Brazil.
Geerard L Beets, Department of Surgery, Netherlands Cancer Institute—Antoni van Leeuwenhoek, Amsterdam, the Netherlands; GROW School for Oncology and Developmental Biology, Maastricht University, Maastricht, the Netherlands.
Cornelis J H van de Velde, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Per J Nilsson, Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
the International Watch & Wait Database (IWWD) Consortium:
M Aghili, A Keshvari, M K Nouritaromlou, M Ahlberg, S Kordnejad, A Aleinikov, A Dulskas, O Asoğlu, H Tokmak, R G Barroca, A F Caiado, I A L Rosa, S O Breukink, M F Coraglio, S Iseas, B Creaven, D C Winter, A Zaborowski, C Cunningham, E Gregory, P A Custers, B M Geubels, L DeBrun, A D’Hoore, G Dimofte, K Fechner, K Matzel, L Fernandez, A I Herrando, P Vieira, W B Gaertner, R D Madoff, J P Gerard, F Jacquinot, R Schiappa, S Gollins, M Gonzalez, C A Vaccaro, A Habr-Gama, Julião G P São, F A Holman, R Hompes, W Lameris, S H J Ketelaers, H J T Rutten, K Leitner, C Mazzarisi, L Malcomson, S T O’Dwyer, M Saunders, A Maroli, P Mitchell, S Murad-Regadas, A Pairola, Salazar I Pedraza, Loria F Sanchez, A J Pennings, A Spinelli, and Myint A Sun
Funding
This project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement number 857894—CAST. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication.
Author contributions
Sofieke Temmink (Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing—original draft, Writing—review & editing), Koen Peeters (Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—review & editing), Renu Bahadoer (Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing—review & editing), W.M. Meershoek-Klein Kranenbarg (Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Writing—review & editing), Annet Roodvoets (Data curation, Investigation, Project administration, Resources, Software, Writing—review & editing), J Melenhorst (Data curation, Supervision, Writing—review & editing), Jacobus Burger (Data curation, Supervision, Writing—review & editing), Albert Wolthuis (Data curation, Supervision, Writing—review & editing), Andrew Renehan (Data curation, Methodology, Supervision, Writing—review & editing), Nuno Figueiredo (Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing—review & editing), Oriol Pares (Conceptualization, Data curation, Investigation, Resources, Supervision, Writing—review & editing), Anna Martling (Conceptualization, Data curation, Methodology, Resources, Supervision, Writing—review & editing), R Perez (Conceptualization, Data curation, Methodology, Supervision, Writing—review & editing), Geerard Beets (Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing—review & editing), C.J.H. van de Velde (Conceptualization, Data curation, Project administration, Resources, Supervision, Writing—review & editing), and Per Nilsson (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Visualization, Writing—original draft, Writing—review & editing).
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The data that support the findings of this study are stored at the Clinical Research Centre of the Leiden University Medical Centre. Data are available from the corresponding author or IWWD steering committee upon reasonable request.
References
- 1. van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NLet al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018;391:2537–2545 [DOI] [PubMed] [Google Scholar]
- 2. Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Silva e Sousa AHet al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711–717; discussion 17–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint ASet al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016;17:174–183 [DOI] [PubMed] [Google Scholar]
- 4. Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MRet al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 2012;256:965–972 [DOI] [PubMed] [Google Scholar]
- 5. Nilsson PJ, Ahlberg M, Kordnejad S, Holm T, Martling A. Organ preservation following short-course radiotherapy for rectal cancer. BJS Open 2021;5:zrab093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fokas E, Appelt A, Glynne-Jones R, Beets G, Perez R, Garcia-Aguilar Jet al. International consensus recommendations on key outcome measures for organ preservation after (chemo)radiotherapy in patients with rectal cancer. Nat Rev Clin Oncol 2021;18:805–816 [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HMet al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol 2022;40:2546–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo Let al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol 2018;28:1465–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santiago I, Rodrigues B, Barata M, Figueiredo N, Fernandez L, Galzerano Aet al. Re-staging and follow-up of rectal cancer patients with MR imaging when ‘watch-and-wait’ is an option: a practical guide. Insights Imaging 2021;12:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tjalma JJJ, Koller M, Linssen MD, Hartmans E, de Jongh S, Jorritsma-Smit Aet al. Quantitative fluorescence endoscopy: an innovative endoscopy approach to evaluate neoadjuvant treatment response in locally advanced rectal cancer. Gut 2020;69:406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chino A, Konishi T, Ogura A, Kawachi H, Osumi H, Yoshio Tet al. Endoscopic criteria to evaluate tumor response of rectal cancer to neoadjuvant chemoradiotherapy using magnifying chromoendoscopy. Eur J Surg Oncol 2018;44:1247–1253 [DOI] [PubMed] [Google Scholar]
- 12. van der Sande ME, Maas M, Melenhorst J, Breukink SO, van Leerdam ME, Beets GL. Predictive value of endoscopic features for a complete response after chemoradiotherapy for rectal cancer. Ann Surg 2021;274:e541–ee47 [DOI] [PubMed] [Google Scholar]
- 13. Martens MH, Maas M, Heijnen LA, Lambregts DMJ, Leijtens JWA, Stassen LPSet al. Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst 2016;108:djw171 [DOI] [PubMed] [Google Scholar]
- 14. Habr-Gama A, Sao Juliao GP, Fernandez LM, Vailati BB, Andrade A, Araújo SEAet al. Achieving a complete clinical response after neoadjuvant chemoradiation that does not require surgical resection: it may take longer than you think!. Dis Colon Rectum 2019;62:802–808 [DOI] [PubMed] [Google Scholar]
- 15. Beets GL, Figueiredo NL, Habr-Gama A, van de Velde CJH. A new paradigm for rectal cancer: organ preservation: introducing the International Watch & Wait Database (IWWD). Eur J Surg Oncol 2015;41:1562–1564 [DOI] [PubMed] [Google Scholar]
- 16. Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343–346 [DOI] [PubMed] [Google Scholar]
- 17. Hupkens BJP, Maas M, Martens MH, van der Sande ME, Lambregts DMJ, Breukink SO. Organ preservation in rectal cancer after chemoradiation: should we extend the observation period in patients with a clinical near-complete response? Ann Surg Oncol 2018;25:197–203 [DOI] [PubMed] [Google Scholar]
- 18. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501–513 [DOI] [PubMed] [Google Scholar]
- 19. Custers PA, Hupkens BJP, Grotenhuis BA, Kuhlmann KFD, Breukink SO, Beets GLet al. Selected stage IV rectal cancer patients managed by the watch-and-wait approach after pelvic radiotherapy: a good alternative to total mesorectal excision surgery? Colorectal Dis 2022;24:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EMKet al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29–42 [DOI] [PubMed] [Google Scholar]
- 21. Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu Cet al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336–346 [DOI] [PubMed] [Google Scholar]
- 22. Bach SP, Gilbert A, Brock K, Korsgen S, Geh I, Hill Jet al. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): a randomised, open-label feasibility study. Lancet Gastroenterol Hepatol 2021;6:92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rullier E, Vendrely V, Asselineau J, Rouanet P, Tuech JJ, Valverde Aet al. Organ preservation with chemoradiotherapy plus local excision for rectal cancer: 5-year results of the GRECCAR 2 randomised trial. Lancet Gastroenterol Hepatol 2020;5:465–474 [DOI] [PubMed] [Google Scholar]
- 24. Bach SP; Collaborative STAR-TREC. Can we Save the rectum by watchful waiting or TransAnal surgery following (chemo)Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC)? Protocol for the international, multicentre, rolling phase II/III partially randomized patient preference trial evaluating long-course concurrent chemoradiotherapy versus short-course radiotherapy organ preservation approaches. Colorectal Dis 2022;24:639–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki C, Halperin SK, Nilsson PJ, Martling A, Holm T. Initial magnetic resonance imaging tumour regression grade (mrTRG) as response evaluation after neoadjuvant treatment predicts sustained complete response in patients with rectal cancer. Eur J Surg Oncol 2022;48:1643–1649 [DOI] [PubMed] [Google Scholar]
- 26. Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JWAet al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol 2015;22:3873–3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are stored at the Clinical Research Centre of the Leiden University Medical Centre. Data are available from the corresponding author or IWWD steering committee upon reasonable request.