Introduction
Small intestinal neuroendocrine tumours (SI-NETs) are rare lesions with a prevalence of 1 per 100 000 patients per year1. They typically grow slowly and without initial symptoms, resulting in the majority of patients presenting with advanced local disease, multiple primary tumours, and distant metastases2–4. Staging of SI-NETs is of paramount importance, as curative surgery is indicated only for patients with resectable disease. In general, tumour resection is not commonly undertaken in asymptomatic patients with unresectable metastatic disease5.
Small peritoneal or lymph node metastases, or multiple primaries can be too small to be detected with preoperative imaging and can therefore be missed. Therefore, the European and North American guidelines5,6 state that curative surgery should be carried out via a laparotomy in order to allow palpation of the whole abdominal cavity before proceeding with curative resection. Despite this extensive procedure to optimally select patients for curative surgery, recurrence is common. Recurrence rates of 9 per cent after 1 year and over 50 per cent after 10 years have been reported7. Most disease recurrence develops in the liver or peritoneum, with a small proportion of patients developing recurrence in the small bowel8. This may indicate that, with the current staging protocol, small tumour deposits are still being missed.
Near-infrared (NIR) fluorescence imaging can be used to improve visualization of tumours and vital structures during surgery9. Methylene blue (MB) is a blue dye that has been used off-label for fluorescence imaging purposes. The aim of this study was to investigate whether SI-NETs can be detected with intraoperative fluorescence imaging using MB as a fluorescent contrast agent.
Methods
This study was reviewed and approved by the medical ethical committee of the Erasmus Medical Centre (MEC-2021–0021) and was conducted according to the declaration of Helsinki (10th version, Fortaleza, 2013). The trial was registered in the International Clinical Trials Registry Platform (ICTRP registration code NL9305; https://trialsearch.who.int). Seventeen patients undergoing open surgery for SI-NETs were included. MB was administered intravenously during surgery, after which NIR fluorescence imaging was performed for 10–15 min. MB doses of 0.5 and 1.0 mg/kg were studied. The primary endpoint of this study was the in vivo tumour-to-background ratio (TBR) of the primary tumour. A TBR of 1.5 or higher was defined as fluorescence-positive10. Secondary endpoints were the TBR of occult multiple primaries and metastatic lesions, and the optimal dose of MB. Fluorescence imaging was performed with a Quest Spectrum V2 fluorescence camera (Quest Medical Imaging®, Middenmeer, the Netherlands). Full methods (including intraoperative set-up) are available in the supplementary material.
Results
The study comprised 12 men and 5 women with a median age of 60 (i.q.r. 50–72) years. Patient, surgical, and pathological characteristics are shown in Table S1.
Primary small intestinal neuroendocrine tumours
Seventeen primary tumours were detected in 16 patients by preoperative imaging and subsequently confirmed histopathologically as SI-NETs. Median TBR of the primary tumours was 1.10 (i.q.r. 1.00–1.13) (Fig. S1). None of the primary tumours met the predetermined criterion (TBR 1.5 or higher) for fluorescence-positive.
Multiple primaries
Six additional primary lesions were identified during surgery in four patients after inspection and palpation of the abdominal cavity. Median TBR was 1.16 (1.04–1.18) and none had a TBR of 1.5 or higher. No occult additional primaries were detected by fluorescence imaging. After histopathological assessment of the resected bowel, 37 occult primaries (median 4, range 2–20) were detected in five patients. One of these five patients also had additional primary lesions detected during surgery.
Metastatic lesions
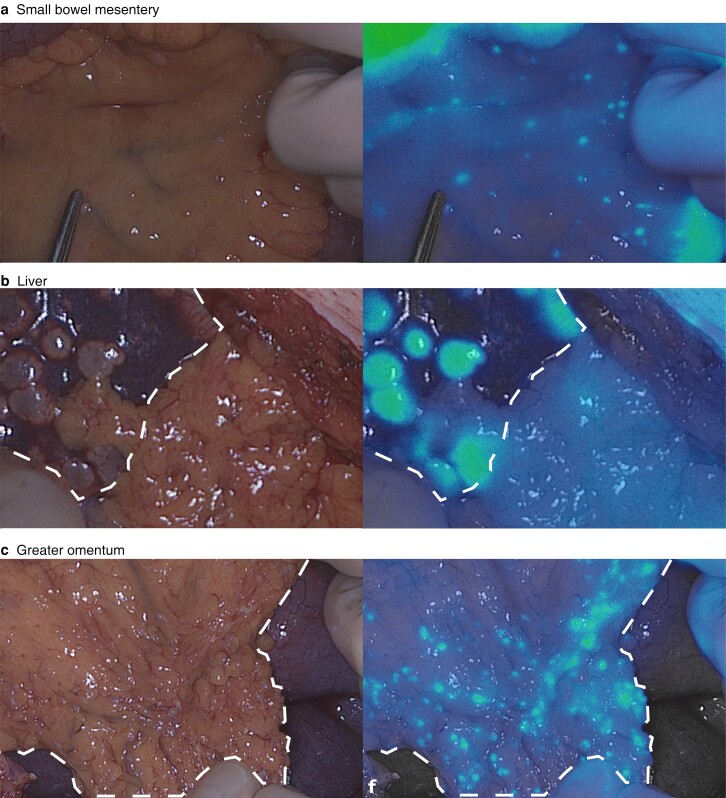
One hundred and fifteen lesions suspected to be metastases were resected or biopsied from 15 patients, of which 109 lesions were histopathologically proven SI-NETs. Of the 109 confirmed SI-NET metastases, 101 (92.6 per cent) were detected by fluorescence imaging with a TBR over 1.5 (true-positives) (Fig. 1). Median TBR for all metastatic SI-NET deposits was 1.90 (1.71–2.01) (Figs S2 and S3). For the eight metastatic lesions with a TBR of less than 1.5 (false-negatives), the median TBR was 1.36 (1.31–1.47). Fourteen liver metastases were detected in two patients, of which 13 (93 per cent) were fluorescence-positive. Four patients had a total of 82 peritoneal metastases, of which 76 (94 per cent) were fluorescence-positive. In three of the patients with peritoneal metastases, fluorescence imaging showed additional lesions that did not affect the surgical strategy (Fig. 1). One benign peritoneal lesion was suspected clinically to be a peritoneal metastasis, but was fluorescence-negative (true-negative) (Fig. S4). Table 1 provides an overview of all lesions and their characteristics, stratified by lesion type.
Fig. 1.
Representative images of metastases
Images of metastases in a small bowel, b liver, and c greater omentum shown in white light (left panels) and gradient fluorescence overlay (right panels).
Table 1.
Fluorescence characteristics of all tumour sites stratified by lesion type
| No. of patients* | No. of lesions | TP | FN | FP | TBR†‡ | Occult lesions identified with FLI* | Figure | |
|---|---|---|---|---|---|---|---|---|
| Primary tumours | 16 (94) | 17 | 0 (0) | 17 (100) | 0 (0) | 1.10 (1.00–1.13) | 0 (0) | S1 |
| Multiple primaries | 8 (47) | 43 | 0 (0) | 43 (100) | 0 (0) | 1.16 (1.04–1.18) | 0 (0) | – |
| Peritoneal metastases | 4 (24) | 82 | 76 (94) | 5 (6) | 0 (0) | 1.88 (1.70–2.05) | 3 (18) | 1 |
| Liver metastases | 2 (12) | 14 | 13 (93) | 1 (7) | 0 (0) | 2.13 (1.89–2.46) | 0 (0) | 1 |
| Mesenteric masses | 13 (76) | 13 | 11 (85) | 2 (15) | 0 (0) | 1.91 (1.60–2.12) | 0 (0) | S3 |
| Lymph nodes | 4 (24) | 6 | 1 (17) | 0 (0) | 5 (83) | 1.78 | 1 (6) | S5 |
Values are n (%) with respect to lesions unless indicated otherwise; *values in parentheses are percentage of patients; †values are median (i.q.r.). ‡Benign lesions (false-positives) were excluded from tumour-to-background ratio (TBR) analysis. Note: As clinically unsuspected, fluorescence-negative tissue was not biopsied, true-negatives could not be assessed. TP, true-positives; FP, false-positives; FN, false-negatives; FLI, fluorescence imaging.
Methylene blue dose
There was no difference in median TBR of the primary tumours at either 0.5 or 1.0 mg/kg MB (1.00 versus 1.11; P = 0.554). For metastatic lesions, median TBR was higher in patients who received 1.0 mg/kg MB (1.54 versus 1.91; P = 0.016) (Fig. S5).
Discussion
In this study, primary SI-NETs and multiple primaries could not be detected with NIR fluorescence imaging using MB as a contrast agent. Conversely, peritoneal and liver metastases had an excellent fluorescence signal. Of all metastatic lesions, 93 per cent had a positive fluorescence signal (TBR 1.5 or more) and fluorescence allowed the identification of additional (occult) metastases in three patients. One clinically suspected peritoneal lesion that was fluorescence-negative was proven benign (true-negative). Although this was only one lesion, it suggests that MB selectively stains malignant lesions and not benign peritoneal lesions. It appears, therefore, that there is a role for NIR fluorescence imaging with MB for improved staging of SI-NET peritoneal and liver metastases, especially considering that the majority of disease recurrences present in the liver or peritoneum8. Using this technique, small occult metastases could potentially be detected during operation to guide the surgeon’s operative strategy. These results also confirm that multiple primaries are common and, even after extensive preoperative imaging, inspection and palpation of the complete small bowel, these lesions are challenging to identify. Eight patients (47 per cent) had multiple primaries, in five of whom these lesions were not detected before or during the operation. Two of these five patients had surgery with curative intent.
As the MB compound could not be traced in the ex vivo specimens, the mechanism behind tumour-specific imaging of MB in SI-NET metastases remains unclear. The authors hypothesize that the enhanced permeability and retention (EPR) effect could be responsible for the targeting mechanism. Because of a lack of specific binding, a common issue with the EPR effect is that no residual tracer can be detected after ex vivo tissue processing.
For a laparoscopic approach to be feasible, identification of primary tumours is vital and needs to be improved. Promising results were recently published for a somatostatin receptor 2-specific tracer that might improve intraoperative identification of SI-NETs11. As this tracer is labelled to an 800-nm fluorophore, dual-wavelength imaging with MB (approximately 700 nm) is feasible, adding complementary value. In contrast to other fluorescent contrast agents (for example indocyanine green), MB is not excreted through the liver, potentially allowing detection of hepatic metastases after intraoperative administration.
In conclusion, NIR fluorescence imaging with MB improves intraoperative detection of metastatic lesions from SI-NETs. Detection of the primary tumour and occult multiple primaries is not possible with this contrast agent. There is a need for development and clinical validation of tumour-targeted tracers to improve the intraoperative identification of SI-NETs.
Supplementary Material
Acknowledgements
D.E.H. and S.K. are joint senior authors.
Contributor Information
Hidde A Galema, Department of Surgical Oncology and Gastrointestinal Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands; Department of Otorhinolaryngology, Head and Neck Surgery, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Tessa M van Ginhoven, Department of Surgical Oncology and Gastrointestinal Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Gaston J H Franssen, Department of Surgical Oncology and Gastrointestinal Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Johannes Hofland, Department of Internal Medicine, Division of Endocrinology, ENETS Centre of Excellence, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Claire G O T Bouman, Department of Anaesthesiology, Erasmus Medical Centre, Rotterdam, the Netherlands.
Cornelis Verhoef, Department of Surgical Oncology and Gastrointestinal Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Alexander L Vahrmeijer, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Merlijn Hutteman, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands; Department of Cardiothoracic Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Denise E Hilling, Department of Surgical Oncology and Gastrointestinal Surgery, Erasmus MC Cancer Institute, Rotterdam, the Netherlands; Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Stijn Keereweer, Department of Otorhinolaryngology, Head and Neck Surgery, Erasmus MC Cancer Institute, Rotterdam, The Netherlands.
Funding
The authors have no funding to declare.
Author contributions
Hidde A. Galema (Methodology, Formal analysis, Investigation, Data Curation, Writing - Original Draft, Visualization), Tessa M. van Ginhoven (Methodology, Investigation, Resources, Writing - Review & Editing, Supervision), Gaston J.H. Franssen (Methodology, Investigation, Resources, Writing - Review & Editing), Johannes Hofland (Supervision, Writing - Review & Editing), Claire G.O.T. Bouman (Methodology, Writing - Review & Editing, Supervision), Cornelis Verhoef (Resources, Writing - Review & Editing, Supervision), Merlijn Hutteman (Conceptualization, Methodology, ResourcesWriting - Review & Editing, Supervision), Denise E. Hilling (Methodology, Investigation, Resources, Writing - Review & Editing, Supervision, Project administration), and Stijn Keereweer (Methodology, Investigation, Resources, Writing - Review & Editing, Supervision, Project administration).
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Data from this study are available upon reasonable request.
References
- 1. Wu L, Fu J, Wan L, Pan J, Lai S, Zhong Jet al. Survival outcomes and surgical intervention of small intestinal neuroendocrine tumors: a population based retrospective study. Oncotarget 2017;8:4935–4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keck KJ, Maxwell JE, Utria AF, Bellizzi AM, Dillon JS, O’Dorisio TMet al. The distal predilection of small bowel neuroendocrine tumors. Ann Surg Oncol 2018;25:3207–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Snorradottir S, Asgeirsdottir A, Rögnvaldsson S, Jonasson JG, Björnsson ES. Incidence and prognosis of patients with small intestinal neuroendocrine tumors in a population based nationwide study. Cancer Epidemiol 2022;79:102197 [DOI] [PubMed] [Google Scholar]
- 4. Gangi A, Siegel E, Barmparas G, Lo S, Jamil LH, Hendifar Aet al. Multifocality in small bowel neuroendocrine tumors. J Gastrointest Surg 2018;22:303–309 [DOI] [PubMed] [Google Scholar]
- 5. Howe JR, Cardona K, Fraker DL, Kebebew E, Untch BR, Wang YZet al. The surgical management of small bowel neuroendocrine tumors: consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas 2017;46:715–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niederle B, Pape UF, Costa F, Gross D, Kelestimur F, Knigge Uet al. ENETS consensus guidelines update for neuroendocrine neoplasms of the jejunum and ileum. Neuroendocrinology 2016;103:125–138 [DOI] [PubMed] [Google Scholar]
- 7. Blažević A, Zandee WT, Franssen GJH, Hofland J, van Velthuysen MF, Hofland LJet al. Mesenteric fibrosis and palliative surgery in small intestinal neuroendocrine tumours. Endocr Relat Cancer 2018;25:245–254 [DOI] [PubMed] [Google Scholar]
- 8. Folkestad O, Wasmuth HH, Mjønes P, Fougner R, Hauso Ø, Fossmark R. Survival and disease recurrence in patients operated for small intestinal neuroendocrine tumors at a referral hospital. Surg Oncol 2020;35:336–343 [DOI] [PubMed] [Google Scholar]
- 9. Mieog JSD, Achterberg FB, Zlitni A, Hutteman M, Burggraaf J, Swijnenburg RJet al. Fundamentals and developments in fluorescence-guided cancer surgery. Nat Rev Clin Oncol 2022;19:9–22 [DOI] [PubMed] [Google Scholar]
- 10. Azargoshasb S, Boekestijn I, Roestenberg M, KleinJan GH, van der Hage JA, van der Poel HGet al. Quantifying the impact of signal-to-background ratios on surgical discrimination of fluorescent lesions. Mol Imaging Biol 2022. 10.1007/s11307-022-01736-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dijkstra BM, de Jong M, Stroet MCM, Andreae F, Dulfer S, Everts M et al. Evaluation of Ac-Lys(0)(IRDye800CW)Tyr(3)-octreotate as a novel tracer for SSTR(2)-targeted molecular fluorescence guided surgery in meningioma. J Neurooncol 2021;153:211–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are available upon reasonable request.