Abstract
Background
The timing at which venous thromboembolism (VTE) occurs after major surgery has major implications for the optimal duration of thromboprophylaxis. The aim of this study was to perform a systematic review and meta-analysis of the timing of postoperative VTE up to 4 weeks after surgery.
Methods
A systematic search of MEDLINE, Scopus, and CINAHL databases was performed between 1 January 2009 and 1 April 2022. Prospective studies that recruited patients who underwent a surgical procedure and reported at least 20 symptomatic, postoperative VTE events by time were included. Two reviewers independently selected studies according to the eligibility criteria, extracted data, and evaluated risk of bias. Data were analysed with a Poisson regression model, and the GRADE approach was used to rate the certainty of evidence.
Results
Some 6258 studies were evaluated, of which 22 (11 general, 5 urological, 4 mixed, and 2 orthopaedic postoperative surgical populations; total 1 864 875 patients and 24 927 VTE events) were eligible. Pooled evidence of moderate certainty showed that 47.1 per cent of the VTE events occurred during the first, 26.9 per cent during the second, 15.8 per cent during the third, and 10.1 per cent during the fourth week after surgery. The timing of VTE was consistent between individual studies.
Conclusion
Although nearly half of symptomatic VTE events in first 4 weeks occur during the first postoperative week, a substantial number of events occur several weeks after surgery. These data will inform clinicians and guideline developers about the duration of postoperative thromboprophylaxis.
The timing of venous thromboembolism after surgery was modelled based on a new systematic review and meta-analysis of 22 prospective studies that included thousands of VTE events from various surgical fields. For symptomatic VTEs occurring within 4 weeks after surgery, 47 per cent occurred by the first, 74 per cent by the second, and 90 per cent by the third week after surgery. This model offers evidence of moderate certainty that, although half of symptomatic VTE events occur during the first postoperative week, a substantial number occur up to 3 weeks after surgery.
Introduction
The annual number of surgical procedures performed worldwide exceeds 300 million1. Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), represents a serious, and on occasion fatal, complication of surgery. Pharmacological prophylaxis decreases the risk of VTE in surgical patients but also increases the risk of bleeding. The decision to use pharmacological prophylaxis therefore presents a trade-off between a reduction in VTE and an increase in major bleeding.
Crucial issues when considering decisions regarding VTE prevention include the starting time and duration of pharmacological thromboprophylaxis. Understanding the timing of postoperative events is therefore necessary. Owing to limitations in the available data, prominent guidelines2–7 on thromboprophylaxis have been unable to provide consistent and actionable guidance on the timing of initiation and duration of thromboprophylaxis. The absence of clear guidance contributes to substantial practice variation within and between centres and countries8–10.
A recent systematic review and meta-analysis11 reported a statistically non-significant decrease in the rate of any (mostly asymptomatic) VTE when thromboprophylaxis was initiated before surgery (risk ratio 0.77, 95 per cent c.i. 0.55 to 1.08). However, there were only 10 symptomatic VTEs (6 symptomatic VTEs among 938 patients (0.6 per cent) in the preoperative and 4 symptomatic VTEs among 941 patients (0.4 per cent) in the postoperative initiation group), highlighting the fragility of current estimates. A Cochrane review12 compared the impact of extended thromboprophylaxis with low molecular weight heparin for at least 14 days with in-hospital-only prophylaxis for abdominal or pelvic surgery procedures. The seven randomized trials the authors included reported a total of only eight symptomatic VTE events. Meta-analysis suggested better VTE reduction with extended prophylaxis (1.0 per cent in the in-hospital-only group versus 0.1 per cent in patients receiving extended thromboprophylaxis; OR 0.30, 95 per cent c.i. 0.08 to 1.11). The aim of the present study was to systematically review the incidence of symptomatic venous thromboembolism by postoperative day after surgery.
Methods
The review protocol was registered before starting work on the systematic review (PROSPERO CRD42021241159), and followed PRISMA13,14 and MOOSE15 guidance.
Data sources and searches
With the aid of an information specialist, comprehensive searches were performed for studies in general/gastrointestinal, urological, and gynaecological (not obstetric) surgery, without language restrictions, in the MEDLINE, Scopus, and CINAHL databases to search for potentially eligible articles published between 1 January 2009 and 1 April 2022 (AppendicesS1-3). The review team manually searched reference lists of the included articles and systematic reviews.
Eligibility criteria
Prospective studies were included if they recruited all patients from the year 2000 or later, in which at least 95 per cent of patients underwent a surgical procedure (in general/gastrointestinal, urological and gynaecological (not obstetric), orthopaedic, thoracic, plastic, hand, breast, endocrine and/or transplant surgery) and reported the timing of at least 20 symptomatic, postoperative VTE (or PE or DVT) events within 90 days after surgery.
Study selection and data extraction
Standardized forms with detailed instructions were developed for screening of abstracts and full texts, risk of bias, assessment of evidence certainty, and data extraction. Pairs of methodologically trained reviewers independently applied the forms to screen study reports for eligibility and extracted data using online-based DistillerSR™ software (Evidence Partners® Inc., Ottawa, Ontario, Canada). The lead author and/or clinician-methodologist adjudicator resolved potential disagreements.
The following data were extracted from all eligible studies: first author; year of publication; country/countries; surgical field/specialties; number of patients; age; sex; proportion of patients with malignant disease; duration of hospital stay; patient recruitment years, and DVT, PE, and VTE events. Data were retrieved from text, tables or figures. When data were only available in figures, they were retrieved by digitalizing from screenshots of figures.
Analysis
Outcomes
The primary outcome was the proportion of cumulative occurrence of VTE up to 28 days (4 weeks) after surgery. Secondary outcomes included: proportion of cumulative occurrence of PE up to 28 days (4 weeks) after surgery; proportion of cumulative occurrence of DVT up to 28 days (4 weeks) after surgery; and proportion of cumulative occurrence of VTE up to 90 days (3 months) after surgery.
If a study reported the timing of DVT or PE events (but not VTE events), DVT or PE events were converted to VTE events using a previously published method16. In that study, data were reviewed from 50 studies that reported DVT, PE, and VTE totals. The overlap was estimated from these studies, and then the degree of overlap was applied to estimate the actual numbers of VTEs in studies that provided only separate reports of DVT and/or PE.
Risk of bias
As methods to evaluate the risk of bias in studies of prognosis are less developed than the methods for RCTs, through discussion and consensus building, and considering previous literature17–20, an instrument was developed to categorize individual studies as being at low or high risk of bias (Table S1). This instrument includes issues of sampling and representativeness of the population, study type, loss to follow-up, and thromboprophylaxis documentation.
Estimation of thromboprophylaxis use
The reported incidence of VTE was adjusted for the use of pharmacological and mechanical thromboprophylaxis separately for each study. For patients who received prophylaxis, the reported incidence was multiplied by the relative risk (RR) of thromboprophylaxis for the duration of prophylaxis use21. The updated meta-analyses22 informed the RR estimates of thromboprophylaxis as follows: for pharmacological prophylaxis (heparin), RR 0.46 for VTE; for any mechanical prophylaxis, RR 0.43 for VTE; and for combination therapy of pharmacological plus mechanical (versus pharmacological alone), RR 0.59 for VTE. Finally, it was inferred that preoperative thromboprophylaxis provided no extra benefit for VTE prevention11.
For studies that did not report on use of thromboprophylaxis, thromboprophylaxis use was estimated as follows: web-based survey on thromboprophylaxis informed the authors’ decisions (AppendixS4); and, if the survey did not include the procedure(s) undertaken in the study, a study that reported thromboprophylaxis for the procedure(s) from the same time interval and procedure was identified (Tables S2 and 3).
Statistical and sensitivity analyses
A Poisson regression model was fitted using number of VTE (and PE/DVT) events as the dependent variable and population size as the offset variable. Splines were used for days (knots on 2, 6, 10, 14, 18, and 22 days) and categorical variables (study) as predictors. The interaction between time and study proved significant (P < 0.001) and was included in the model. Cumulative incidence was predicted for each study separately and predictions were pooled using the inverse of variance of predictions as weights. All analyses were carried out using R language and package Epi23,24, and figures were plotted with package ggplot225.
Sensitivity analyses were undertaken. First, the pooled analysis included only studies that reported VTE. Second, sensitivity analyses explored what would have happened under various conditions of thromboprophylaxis, in particular assuming: no pharmacological thromboprophylaxis for all patients (with or without 2 days of mechanical prophylaxis); 1 week of pharmacological thromboprophylaxis for all patients (with or without 2 days of mechanical prophylaxis); 2 weeks of pharmacological thromboprophylaxis for all patients (with or without 2 days of mechanical prophylaxis); and 3 weeks of pharmacological thromboprophylaxis for all patients (with or without 2 days of mechanical prophylaxis).
Quality of evidence
In the GRADE (Grading of Recommendations Assessment, Development and Evaluation) framework for assessing prognosis, a body of observational studies begins as high-certainty evidence (synonymously, evidence certainty or quality of evidence)26,27. Several categories of limitations may, however, reduce the certainty of evidence, including risk of bias, imprecision, inconsistency, and indirectness.
Results
Literature search and study characteristics
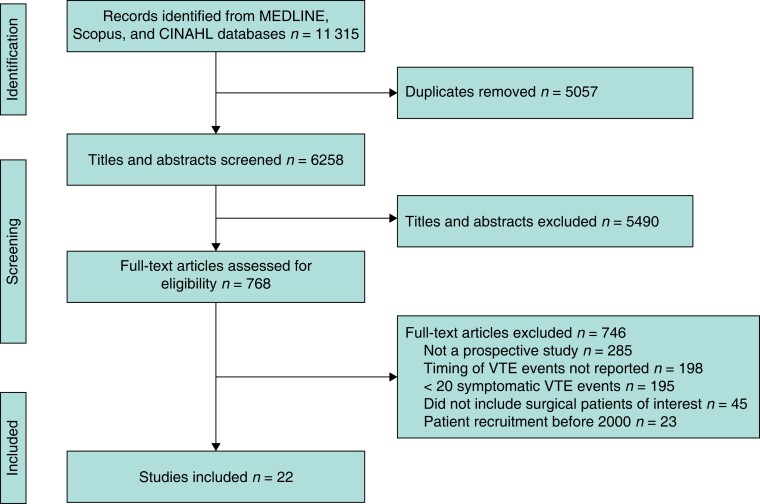
Some 6258 titles and abstracts were screened and 768 potentially eligible full-text reports were retrieved, of which 22 studies28–49 (with 1 864 875 patients and 24 927 VTE events) proved eligible (Fig. 1). Of these 22 studies, 11 included general, 5 urological, 4 mixed, and 2 orthopaedic postoperative populations. The median size of the study population across the studies was 30 468 patients, the median proportion of female patients 49 per cent, the median proportion of patients with malignant disease 85 per cent, and the median duration of hospital stay was 6.5 days (Table 1). In 20 of 22 studies28–30,32–42,44–49, DVT and PE diagnoses were confirmed by definitive imaging, such as duplex ultrasound examination or CT. In individual studies, the estimated duration of pharmacological thromboprophylaxis varied from 0 to 27 (median 7, i.q.r. 5–11) days, and that of mechanical thromboprophylaxis from 0 to 9 (median 2, i.q.r. 1–2) days (Table S3). Table 1 summarizes the characteristics of the included studies; more details are available in Tables S2 and S4.
Fig. 1.
PRISMA flow chart showing selection of articles for review
VTE, venous thromboembolism.
Table 1.
Characteristics of included studies
| Reference | Surgical category* | No. of patients | Age (years)† |
Female (%) |
Malignancy (%) |
Duration of hospital stay (days)† | No. of VTE events | Recruitment years |
|---|---|---|---|---|---|---|---|---|
| Agnelli et al.43 | Mixed | 2373 | 64 | 46 | 100 | 10 | 92 | n.r. |
| Kwon et al.31 | General | 4195 | 61 | 54 | 39 | 8 | 47 | 2005–2009 |
| Merkow et al.42 | Mixed | 44 656 | n.r. | 65 | 100 | 4‡ | 719 | 2006–2008 |
| Davenport et al.28 | General | 21 943 | 66 | 49 | 100 | 7‡ | 446 | 2005–2009 |
| Shah et al.36 | Mixed | 471 867 | 54 | 41 | 20 | 4‡ | 7078 | 2005–2010 |
| Tzeng et al.38 | General | 7621 | 60‡ | 52 | 85 | 6‡ | 210 | 2005–2010 |
| Tzeng et al.39 | General | 13 771 | 64‡ | 52 | 82 | 8‡ | 427 | 2005–2010 |
| Lavallée et al.32 | Urological | 2303 | 68 | 21 | 100 | 8‡ | 123 | 2006–2012 |
| VanDlac et al.40 | Urological | 1307 | 69‡ | 24 | 100 | 8‡ | 78 | 2005–2011 |
| Gross et al.41 | General | 37 076 | 66 | 48 | 100 | 10 | 1018 | 2005–2010 |
| Moghadamyeghaneh et al.44 | General | 116 029 | 62 | 52 | n.r. | 6 | 4556 | 2005–2011 |
| Kester et al.45 | Orthopaedic | 23 620 | NR | 61 | 0 | 3‡ | 366 | 2008–2010 |
| Martin et al.33 | General | 3208 | 64‡ | n.r. | 100 | 11‡ | 161 | 2005–2012 |
| Moghadamyeghaneh et al.35 | General | 219 477 | 61 | 52 | 61 | 6 | 2278 | 2005–2013 |
| Spaniolas et al.37 | General | 71 694 | 45‡ | 79 | 0 | n.r. | 283 | 2006–2011 |
| Jordan et al.30 | Urological | 13 208 | 61 | 42 | n.r. | 4‡ | 160 | 2006–2012 |
| McAlpine et al.34 | Urological | 65 100 | n.r. | n.r. | 85 | n.r. | 956 | 2006–2014 |
| Benlice et al.48 | General | 24 182 | 43 | 49 | 0 | 8 | 614 | 2005–2016 |
| Herforth et al.29 | Mixed | 503 602 | n.r. | 51 | n.r. | n.r. | 3912 | 2016 |
| Sager et al.46 | Orthopaedic | 39 825 | 59 | 42 | 0 | n.r. | 102 | 2005–2017 |
| Merhe et al.49 | Urological | 36 753 | 62 | 0 | 100 | 2‡ | 423 | 2008–2015 |
| Kumar et al.47 | General | 141 065 | 57 | 57 | n.r. | 1‡ | 878 | 2011–2017 |
| Total | 1 864 875 | 24 927 |
Details available in supplementary material (Tables S2 and S4). †Mean values are shown, except ‡median. VTE, venous thromboembolism; n.r., not reported.
Risk of bias and evidence certainty
All studies involved multiple centres and 18 of the 22 studies used consecutive patient recruitment (Table 2). In one study it was certain that loss to follow-up was less than 10 per cent. None of the studies accurately reported the proportion of patients receiving thromboprophylaxis, including type and duration of prophylaxis. Overall, 1 study was judged as having a low and 21 studies a high risk of bias (Table 2), and so the certainty of evidence was rated down owing to risk of bias. Evidence review raised no concerns regarding imprecision, inconsistency, or indirectness, and therefore a quality rating (evidence certainty) of moderate was warranted.
Table 2.
Risk of bias
| Sampling and representativeness of population | Documentation of thromboprophylaxis | Follow-up of patients | Study type | Risk of bias | |
|---|---|---|---|---|---|
| Agnelli et al.43 | + | – | + | + | Low |
| Kwon et al.31 | + | – | – | + | High |
| Merkow et al.42 | + | – | – | + | High |
| Davenport et al.28 | – | – | – | + | High |
| Shah et al.36 | – | – | – | + | High |
| Tzeng et al.38 | + | – | – | + | High |
| Tzeng et al.39 | – | – | – | + | High |
| Lavallée et al.32 | + | – | – | + | High |
| VanDlac et al.40 | – | – | – | + | High |
| Gross et al.41 | + | – | – | + | High |
| Moghadamyeghaneh et al.44 | + | – | – | + | High |
| Kester et al.45 | + | – | – | + | High |
| Martin et al.33 | + | – | – | + | High |
| Moghadamyeghaneh et al.35 | + | – | – | + | High |
| Spaniolas et al.37 | + | – | – | + | High |
| Jordan et al.30 | + | – | – | + | High |
| McAlpine et al.34 | + | – | – | + | High |
| Benlice et al.48 | + | – | – | + | High |
| Herforth et al.29 | + | – | – | + | High |
| Sager et al.46 | + | – | – | + | High |
| Merhe et al.49 | + | – | – | + | High |
| Kumar et al.47 | + | – | – | + | High |
+, Low risk; –, high risk.
Timing of events
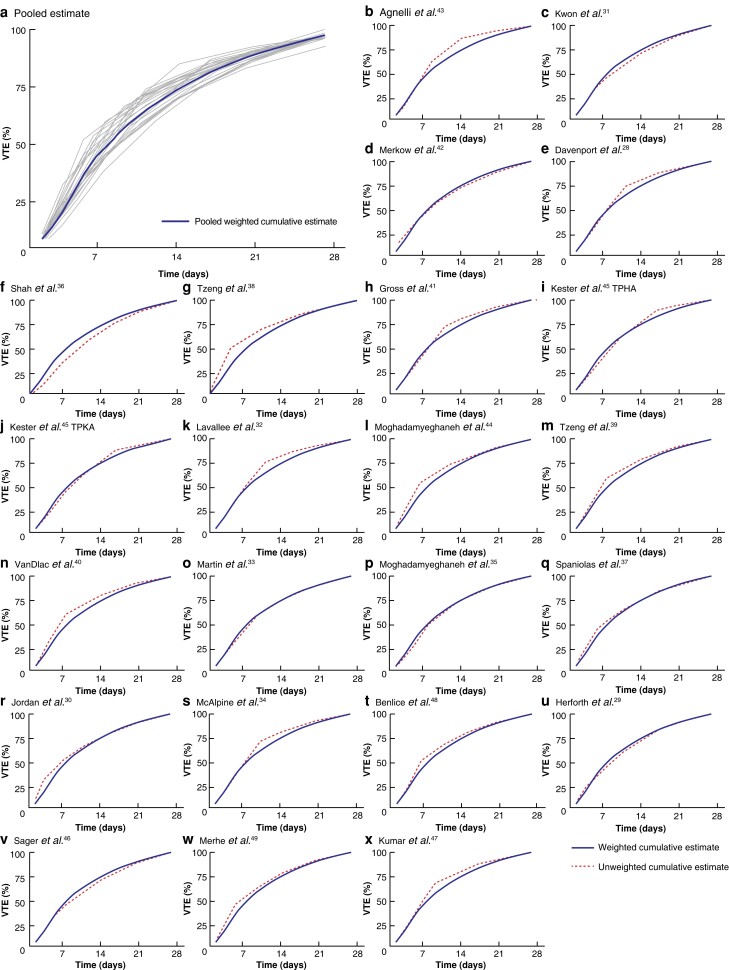
Regarding the cumulative VTE risk during the first 4 weeks after surgery, 47.1 per cent of the VTE events occurred during the first week, 26.9 per cent during the second week, 15.8 per cent during the third week, and 10.1 per cent during the fourth week after operation (Fig. 2 and Table S5).
Fig. 2.
Proportion of cumulative occurrence of venous thromboembolism by time during the first 28 days (4 weeks) after surgery: all included studies pooled
VTE, venous thromboembolism.
The timing of VTE was consistent between individual studies (Fig. 3). For instance, from the cumulative VTE risk of 4 weeks, the median estimate of the proportion of VTEs that occurred by 2 weeks was 77.7 (i.q.r. 74.8–80.2) per cent, with highest and lowest estimates of 86.0 and 68.2 per cent (Fig. 3 and Fig. S1).
Fig. 3.
Proportion of cumulative occurrence of venous thromboembolism by time during the first 28 days (4 weeks) after surgery in individual studies.
a Weighted cumulative estimates (pooled in blue line and individual studies in grey lines) of venous thromboembolism (VTE) occurrence and b–x cumulative weighted and unweighted estimates for individual studies. VTE, venous thromboembolism; TPHA, total or partial hip arthroplasty; TPKA, total or partial knee arthroplasty.
Data on the timing of PE, DVT, and VTE events separately are provided in Figs S2–S4. The sensitivity analyses did not change the results materially (Figs S5–S7). No eligible studies reported on VTE up to 90 days, so pooled estimates did not extend beyond 28 days after surgery.
Discussion
This systematic review and meta-analysis, pooling 22 studies, represents the first available summary of the postoperative timing of symptomatic VTE. The pooled results provide evidence of moderate quality that, of the cumulative VTE risk during the first 28 days (4 weeks) after surgery, 47.1 per cent of the VTE events occur during the first, 26.9 per cent during the second, 15.8 per cent during the third, and 10.1 per cent during the fourth week after operation.
Strengths of this study include a comprehensive search (studies published 2009 or later; patients recruited after 2000). The search was limited to contemporary studies, because the baseline risks of VTE and bleeding have likely changed over time50–52. To mitigate the effect of publication bias, studies with at least 20 symptomatic VTE events were included. Teams of two reviewers assessed eligibility and risk of bias, and undertook data extraction with a clinician-methodologist adjudicating discrepancies. A total of 22 prospective studies (each directly providing information regarding timing of VTE in dozens of surgical procedures in various fields of surgery) that included thousands of VTE events (high statistical power leading to high precision for the pooled results) were identified. Considering the use of thromboprophylaxis, the timing of postoperative VTE events was pooled up to 28 days (4 weeks) after surgery, a duration of extended prophylaxis frequently used by clinicians4,7,53–58. Studies proved consistent regarding timing of VTE (as well as PE and DVT) and sensitivity analyses yielded results similar to the primary analyses. Applying the GRADE approach to certainty of evidence, the results were judged to provide evidence of moderate certainty.
This study has limitations, reflecting limitations in the available evidence. Because observational studies have less established indexing than randomized trials, some relevant studies may have been missed. Second, most of the included studies used the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which does not collect data on perioperative thromboprophylaxis. Owing to lack of data regarding thromboprophylaxis, thromboprophylaxis practice was estimated based on the published literature and results of a clinician survey (Supplementary methods S4). The finding that sensitivity analyses assuming different thromboprophylaxis regimens did not materially change the results suggests that they are trustworthy. Third, as most studies used the ACS-NSQIP database, some studies included the same procedures, and sometimes even data from the same patient recruitment years, resulting in some double-counting of patients and events. It is unlikely, however, that this double-counting would have seriously biased the results, although it likely led to some degree of false precision. The reason is the striking consistency of results across studies; thus, any double-counting would be of patients with results similar to those of patients counted only once59. Finally, owing to the lack of studies reporting this information, it was not possible to pool timing of VTE events beyond the initial postoperative 4 weeks, or report the proportion of patients with more proximal venous thrombosis or embolism.
Little previous work has attempted to summarize the literature informing the timing of postoperative VTE60–62. Globally, the first procedure-specific guideline in any field of surgery (urological surgery)4 was based on a series of systematic reviews and meta-analyses on procedure-specific risks of VTE and bleeding after urological surgery16,63. To be able to account for the timing of VTE62, the authors pooled the results from two large studies60,61: a prospective study that included British women, of whom almost 900 had VTE within 12 weeks after surgery; and a retrospective study that included US surgical patients with 305 VTE events (172 among patients who had abdominal surgery) within 6 months after operation. VTE events occurred later when the results of these two studies were pooled60–62 than in the present work: 27 versus 47 per cent respectively of the VTE events occurred by the first week, and 54 versus 74 per cent by the second week. One important reason for the earlier occurrence of VTEs in the present study is that the previously published work did not take the use of thromboprophylaxis into account. As thromboprophylaxis is often used only during the first week after surgery10, not taking it into account overestimates the proportion of late VTEs as VTEs that would have occurred early and were prevented by prophylaxis are missed, whereas those that occur later when prophylaxis is given less frequently are not missed.
Both the present and previous work62 benefited from focusing on symptomatic VTE events. This is especially important because scanning for asymptomatic events (typically at fixed time points such as a week or two post-surgery) would bias the timing (treatment of asymptomatic events would prevent the occurrence of symptomatic events at a later time point) and focus on an outcome that is not important to patients. The present systematic review also benefits from including only prospective studies (not the case for the US study61), as retrospective studies often miss VTEs that occur after discharge, and studies with contemporary patient recruitment years, and therefore more up-to-date surgical and perioperative practices (not the case for the British study60). These new results therefore represent more accurate and up-to-date estimates of the timing of VTE within the first 28 days after surgery.
The results of this systematic review have important implications for the surgical practice globally. Surgical thromboprophylaxis practice, especially after discharge, varies widely both within and between countries8–10,64. The timing and duration of postoperative VTE prophylaxis is a key question in daily clinical practice. Although the evidence establishes that almost half of VTE events in the first month after surgery occur during the first postoperative week, it also demonstrates that a substantial number of VTE events arise during the third, or even fourth, week after surgery. These results suggest the possible importance of extended prophylaxis, especially in patients with high risk of VTE. Although meta-analyses of the randomized trials have failed, owing to insufficient statistical power11,12, to establish the optimal starting time and duration of thromboprophylaxis, clinicians and guideline developers can use the results of the present systematic review, together with knowledge of baseline risks of VTE and bleeding, to guide the starting time and duration of thromboprophylaxis.
These results will also prove useful for the planning and conduct of future clinical research, which should benefit from the present identification of limitations in past studies. There were limitations regarding reporting of use, starting time, and duration of thromboprophylaxis, and so data from a contemporary survey of surgeons’ practices on thromboprophylaxis had to be relied on. Because of lack of direct evidence on this issue, most studies were judged as having a high risk of bias, and the certainty of evidence was therefore lowered from high to moderate. Future prospective studies, including use of representative patient populations, clear documentation of VTE, DVT, PE, and their follow-up times, and documentation of thromboprophylaxis used, would improve the evidence base, and consequently further rationalize the global practice of thromboprophylaxis in surgery.
Supplementary Material
Acknowledgements
The authors thank T. M. Heino for help with the literature search; Y. Aoki, P. Bastani, S. Hajebrahimi, T. Kurokawa and E. Sunami for assistance with the survey of thromboprophylaxis practice; and I. Ilonen regarding selection of eligible surgical procedures.
Contributor Information
Tino Singh, Faculty of Medicine, University of Helsinki, Helsinki, Finland; Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland.
Lauri I Lavikainen, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Alex L E Halme, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Riikka Aaltonen, Department of Obstetrics and Gynaecology, Turku University Hospital and University of Turku, Turku, Finland.
Arnav Agarwal, Division of General Internal Medicine, Department of Medicine, McMaster University, Hamilton, Ontario, Canada; Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada.
Marco H Blanker, Department of General Practice and Elderly Care Medicine, University Medical Centre Groningen, University of Groningen, Groningen, the Netherlands.
Kostiantyn Bolsunovskyi, Faculty of Medicine, University of Helsinki, Helsinki, Finland; Raseborg Health Centre, City of Raseborg, Raseborg, Finland.
Rufus Cartwright, Departments of Gynaecology and Gender Affirmation Surgery, Chelsea and Westminster NHS Foundation Trust, London, UK; Department of Epidemiology and Biostatistics, Imperial College London, London, UK.
Herney García-Perdomo, Division of Urology/Uro-oncology, Department of Surgery, School of Medicine, Universidad del Valle, Cali, Colombia.
Rachel Gutschon, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada; Department of Surgery, Woodstock Hospital, Woodstock, Ontario, Canada.
Yung Lee, Department of Surgery, McMaster University, Hamilton, Ontario, Canada.
Negar Pourjamal, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Robin W M Vernooij, Julius Centre for Health Sciences and Primary Care, University Medical Centre Utrecht, Utrecht University, Utrecht, the Netherlands; Department of Nephrology and Hypertension, University Medical Centre Utrecht, Utrecht, the Netherlands.
Philippe D Violette, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada; Department of Surgery, Woodstock Hospital, Woodstock, Ontario, Canada.
Jari Haukka, Faculty of Medicine, University of Helsinki, Helsinki, Finland.
Gordon H Guyatt, Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Ontario, Canada; Department of Medicine, McMaster University, Hamilton, Ontario, Canada.
Kari A O Tikkinen, Faculty of Medicine, University of Helsinki, Helsinki, Finland; Department of Urology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; Department of Surgery, South Karelian Central Hospital, Lappeenranta, Finland.
Funding
Supported by the Academy of Finland (309387, 340957), Sigrid Jusélius Foundation, Helsinki and Uusimaa Hospital District (TYH2019321, TYH2020248, TYH2022330), and Turku University Hospital.
Author contributions
Tino Singh (Conceptualization, Data curation, Formal analysis, Project administration, Visualization, Writing—original draft, Writing—review & editing), Lauri Lavikainen (Conceptualization, Data curation, Formal analysis, Supervision, Writing E28093 review & editing), Alex Halme (Data curation, Formal analysis, Visualization, Writing—original draft, Writing—review & editing), Riikka Aaltonen (Data curation, Funding acquisition, Writing—review & editing), Arnav Agarwal (Data curation, Writing—review & editing), Marco Blanker (Data curation, Writing—review & editing), Kostiantyn Bolsunovskyi (Data curation, Writing—review & editing), Rufus Cartwright (Data curation, Writing—review & editing), Herney GarcC3ADa-Perdomo (Data curation, Writing—review & editing), Rachel Gutschon (Data curation, Writing—review & editing), Yung Lee (Data curation, Writing—review & editing), Negar Pourjamal (Data curation, Writing—review & editing), Robin Vernooij (Data curation, Writing—review & editing), Philippe Violette (Data curation, Writing—review & editing), Jari Haukka (Conceptualization, Formal analysis, Visualization, Writing—original draft, Writing—review & editing), Gordon Guyatt (Conceptualization, Formal analysis, Methodology, Supervision, Writing E28093 review & editing), and Kari Tikkinen (Conceptualization, Formal analysis, Funding acquisition, Supervision, Visualization, Writing—original draft, Writing—review & editing).
Disclosure
R.C., P.D.V. and K.A.O.T. are panel members of the European Society of Anaesthesiology and Intensive Care Task Force for the European Guidelines on VTE. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
The corresponding author is the custodian of the data and will provide access to data on request.
References
- 1. Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz Tet al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385:S11. [DOI] [PubMed] [Google Scholar]
- 2. Afshari A, Ageno W, Ahmed A, Duranteau J, Faraoni D, Kozek-Langenecker Set al. European guidelines on perioperative venous thromboembolism prophylaxis: executive summary. Eur J Anaesthesiol 2018;35:77–83 [DOI] [PubMed] [Google Scholar]
- 3. National Guideline Centre (UK) . Venous Thromboembolism in over 16s: Reducing the Risk of Hospital-Acquired Deep Vein Thrombosis or Pulmonary Embolism. NICE Guideline No. 89. http://www.ncbi.nlm.nih.gov/books/NBK493720/ (accessed 15 August 2022) [PubMed]
- 4. Tikkinen KAO, Cartwright R, Gould MK, Naspro R, Novara G, Sandset PMet al. 2017. EAU Guidelines on Thromboprophylaxis in Urological Surgery. European Association of Urology. https://uroweb.org/guidelines/thromboprophylaxis (accessed 4 January 2023)
- 5. Anderson DR, Morgano GP, Bennett C, Dentali F, Francis CW, Garcia DAet al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv 2019;3:3898–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farge D, Frere C, Connors JM, Khorana AA, Kakkar A, Ay Cet al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol 2022;23:e334–e347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology . Prevention of venous thromboembolism in gynecologic surgery: ACOG practice bulletin, number 232. Obstet Gynecol 2021;138:e1–e15 [DOI] [PubMed] [Google Scholar]
- 8. Krell RW, Scally CP, Wong SL, Abdelsattar ZM, Birkmeyer NJO, Fegan Ket al. Variation in hospital thromboprophylaxis practices for abdominal cancer surgery. Ann Surg Oncol 2016;23:1431–1439 [DOI] [PubMed] [Google Scholar]
- 9. Liu DS, Stevens S, Wong E, Fong J, Mori K, Fleming Net al. Variations in practice of thromboprophylaxis across general surgical subspecialties: a multicentre (PROTECTinG) study of elective major surgeries. ANZ J Surg 2020;90:2441–2448 [DOI] [PubMed] [Google Scholar]
- 10. Pourjamal N, Lavikainen LI, Halme ALE, Cartwright R, Ahopelto K, Guyatt GHet al. Global practice variation in pharmacologic thromboprophylaxis for general and gynecologic surgery. BJS Open 2022;6:zrac129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAlpine K, Breau RH, Werlang P, Carrier M, Le Gal G, Fergusson DAet al. Timing of perioperative pharmacologic thromboprophylaxis initiation and its effect on venous thromboembolism and bleeding outcomes: a systematic review and meta-analysis. J Am Coll Surg 2021;233:619–631.e14 [DOI] [PubMed] [Google Scholar]
- 12. Felder S, Rasmussen MS, King R, Sklow B, Kwaan M, Madoff Ret al. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev 2019; (3)CD004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269 [DOI] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie Det al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 16. Tikkinen KAO, Craigie S, Agarwal A, Violette PD, Novara G, Cartwright Ret al. Procedure-specific risks of thrombosis and bleeding in urological cancer surgery: systematic review and meta-analysis. Eur Urol 2018;73:242–251 [DOI] [PubMed] [Google Scholar]
- 17. Tähtinen RM, Cartwright R, Tsui JF, Aaltonen RL, Aoki Y, Cárdenas JLet al. Long-term impact of mode of delivery on stress urinary incontinence and urgency urinary incontinence: a systematic review and meta-analysis. Eur Urol 2016;70:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C.. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–286 [DOI] [PubMed] [Google Scholar]
- 19. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn Set al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013;66:408–414 [DOI] [PubMed] [Google Scholar]
- 20. Pesonen JS, Cartwright R, Vernooij RWM, Aoki Y, Agarwal A, Mangera Aet al. The impact of nocturia on mortality: a systematic review and meta-analysis. J Urol 2020;203:486–495 [DOI] [PubMed] [Google Scholar]
- 21. Lavikainen LI, Guyatt GH, Lee Y, Couban RJ, Luomaranta AL, Sallinen VJet al. Systematic reviews of observational studies of Risk of Thrombosis and Bleeding in General and Gynecologic Surgery (ROTBIGGS): introduction and methodology. Syst Rev 2021;10:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavikainen LI, Guyatt G, Luomaranta ALet al. Risk of venous thromboembolism and major bleeding in gynaecological cancer surgery: series of systematic reviews and meta-analyses. Int J Gynecol Cancer 2022;32:A196–A197 [Google Scholar]
- 23. R Core Team . R: a Language and Environment for Statistical Computing.https://www.R-project.org/ (accessed 4 January 2023)
- 24. Carstensen B, Plummer M, Laara E, Hills M. Epi: a Package For Statistical Analysis In Epidemiology.https://CRAN.R-project.org/package=Epi (accessed 4 January 2023)
- 25. Wickham H. ggplot2: Elegant graphics for Data Analysis.https://ggplot2.tidyverse.org (accessed 4 January 2023)
- 26. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJet al. What is ‘quality of evidence’ and why is it important to clinicians? BMJ 2008;336:995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand Bet al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870. [DOI] [PubMed] [Google Scholar]
- 28. Davenport DL, Vargas HD, Kasten MW, Xenos ES. Timing and perioperative risk factors for in-hospital and post-discharge venous thromboembolism after colorectal cancer resection. Clin Appl Thromb Hemost 2012;18:569–575 [DOI] [PubMed] [Google Scholar]
- 29. Herforth C, Rocco N, Christman M. The ‘rule of W’ in urology: testing surgical dictum. Urology 2019;130:29–35 [DOI] [PubMed] [Google Scholar]
- 30. Jordan BJ, Matulewicz RS, Trihn B, Kundu S. Venous thromboembolism after nephrectomy: incidence, timing and associated risk factors from a national multi-institutional database. World J Urol 2017;35:1713–1719 [DOI] [PubMed] [Google Scholar]
- 31. Kwon S, Meissner M, Symons R, Steele S, Thirlby R, Billingham Ret al. Perioperative pharmacologic prophylaxis for venous thromboembolism in colorectal surgery. J Am Coll Surg 2011;213:596–603.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lavallée LT, Schramm D, Witiuk K, Mallick R, Fergusson D, Morash Cet al. Peri-operative morbidity associated with radical cystectomy in a multicenter database of community and academic hospitals. PLoS One 2014;9:e111281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin JT, Mahan AL, Ferraris VA, Saha SP, Mullett TW, Zwischenberger JBet al. Identifying esophagectomy patients at risk for predischarge versus postdischarge venous thromboembolism. Ann Thorac Surg 2015;100:932–938; discussion 938 [DOI] [PubMed] [Google Scholar]
- 34. McAlpine K, Breau RH, Mallick R, Cnossen S, Cagiannos I, Morash Cet al. Current guidelines do not sufficiently discriminate venous thromboembolism risk in urology. Urol Oncol 2017;35:457.e1–457.e8 [DOI] [PubMed] [Google Scholar]
- 35. Moghadamyeghaneh Z, Alizadeh RF, Hanna MH, Hwang G, Carmichael JC, Mills Set al. Post-hospital discharge venous thromboembolism in colorectal surgery. World J Surg 2016;40:1255–1263 [DOI] [PubMed] [Google Scholar]
- 36. Shah DR, Wang H, Bold RJ, Yang X, Martinez SR, Yang ADet al. Nomograms to predict risk of in-hospital and post-discharge venous thromboembolism after abdominal and thoracic surgery: an American College of Surgeons National Surgical Quality Improvement Program analysis. J Surg Res 2013;183:462–471 [DOI] [PubMed] [Google Scholar]
- 37. Spaniolas K, Kasten KR, Sippey ME, Pender JR, Chapman WH, Pories WJ. Pulmonary embolism and gastrointestinal leak following bariatric surgery: when do major complications occur? Surg Obes Relat Dis 2016;12:379–383 [DOI] [PubMed] [Google Scholar]
- 38. Tzeng CWD, Curley SA, Vauthey JN, Aloia TA. Distinct predictors of pre- versus post-discharge venous thromboembolism after hepatectomy: analysis of 7621 NSQIP patients. HPB (Oxford) 2013;15:773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tzeng CWD, Katz MHG, Lee JE, Fleming JB, Pisters PWT, Vauthey JNet al. Predicting the risks of venous thromboembolism versus post-pancreatectomy haemorrhage: analysis of 13 771 NSQIP patients. HPB (Oxford) 2014;16:373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. VanDlac AA, Cowan NG, Chen Y, Anderson RE, Conlin MJ, La Rochelle JCet al. Timing, incidence and risk factors for venous thromboembolism in patients undergoing radical cystectomy for malignancy: a case for extended duration pharmacological prophylaxis. J Urol 2014;191:943–947 [DOI] [PubMed] [Google Scholar]
- 41. Gross ME, Vogler SA, Mone MC, Sheng X, Sklow B. The importance of extended postoperative venous thromboembolism prophylaxis in IBD: a National Surgical Quality Improvement Program analysis. Dis Colon Rectum 2014;57:482–489 [DOI] [PubMed] [Google Scholar]
- 42. Merkow RP, Bilimoria KY, McCarter MD, Cohen ME, Barnett CC, Raval MVet al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg 2011;254:131–137 [DOI] [PubMed] [Google Scholar]
- 43. Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni Eet al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006;243:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moghadamyeghaneh Z, Hanna MH, Carmichael JC, Nguyen NT, Stamos MJ. A nationwide analysis of postoperative deep vein thrombosis and pulmonary embolism in colon and rectal surgery. J Gastrointest Surg 2014;18:2169–2177 [DOI] [PubMed] [Google Scholar]
- 45. Kester BS, Merkow RP, Ju MH, Peabody TD, Bentrem DJ, Ko CYet al. Effect of post-discharge venous thromboembolism on hospital quality comparisons following hip and knee arthroplasty. J Bone Joint Surg 2014;96:1476–1484 [DOI] [PubMed] [Google Scholar]
- 46. Sager B, Ahn J, Tran J, Khazzam M. Timing and risk factors for venous thromboembolism after rotator cuff repair in the 30-day perioperative period. Arthroscopy 2019;35:3011–3018 [DOI] [PubMed] [Google Scholar]
- 47. Kumar SB, Mettupalli D, Carter JT. Extended-duration thromboprophylaxis after ventral hernia repair: a risk model to predict venous thrombotic events after hospital discharge. Hernia 2022;26:919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benlice C, Holubar SD, Gorgun E, Stocchi L, Lipman JM, Kalady MFet al. Extended venous thromboembolism prophylaxis after elective surgery for IBD patients: nomogram-based risk assessment and prediction from nationwide cohort. Dis Colon Rectum 2018;61:1170–1179 [DOI] [PubMed] [Google Scholar]
- 49. Merhe A, Abou Heidar N, Hout M, Bustros G, Mailhac A, Tamim Het al. An evaluation of the timing of surgical complications following radical prostatectomy: data from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). Arab J Urol 2020;18:136–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carrier M, Altman AD, Blais N, Diamantouros A, McLeod D, Moodley Uet al. Extended thromboprophylaxis with low-molecular weight heparin (LMWH) following abdominopelvic cancer surgery. Am J Surg 2019;218:537–550 [DOI] [PubMed] [Google Scholar]
- 51. Heit JA, Ashrani A, Crusan DJ, McBane RD, Petterson TM, Bailey KR. Reasons for the persistent incidence of venous thromboembolism. Thromb Haemost 2017;117:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vendler MMI, Haidari TA, Waage JE, Kleif J, Kristensen B, Gögenur Iet al. Incidence of venous thromboembolic events in enhanced recovery after surgery for colon cancer: a retrospective, population-based cohort study. Colorectal Dis 2017;19:O393–O401 [DOI] [PubMed] [Google Scholar]
- 53. Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JAet al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e227S–e277S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner Met al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clin Nutr 2013;32:879–887 [DOI] [PubMed] [Google Scholar]
- 55. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis Net al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg 2019;43:659–695 [DOI] [PubMed] [Google Scholar]
- 56. Lassen K, Coolsen MME, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer Met al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:240–258 [DOI] [PubMed] [Google Scholar]
- 57. Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CHCet al. Guidelines for perioperative care for liver surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 2016;40:2425–2440 [DOI] [PubMed] [Google Scholar]
- 58. Nygren J, Thacker J, Carli F, Fearon KCH, Norderval S, Lobo DNet al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2013;37:285–305 [DOI] [PubMed] [Google Scholar]
- 59. Ko CY, Hall BL, Hart AJ, Cohen ME, Hoyt DB. The American College of Surgeons National Surgical Quality Improvement Program: achieving better and safer surgery. Jt Comm J Qual Patient Saf 2015;41:199–204 [DOI] [PubMed] [Google Scholar]
- 60. Sweetland S, Green J, Liu B, Berrington de González A, Canonico M, Reeves Get al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 2009;339:b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Amin AN, Lenhart G, Princic N, Lin J, Thompson S, Johnston S. Retrospective administrative database study of the time period of venous thromboembolism risk during and following hospitalization for major orthopedic or abdominal surgery in real-world US patients. Hosp Pract (1995) 2011;39:7–17 [DOI] [PubMed] [Google Scholar]
- 62. Tikkinen KA, Agarwal A, Craigie S, Cartwright R, Gould MK, Haukka Jet al. Systematic reviews of observational studies of risk of thrombosis and bleeding in urological surgery (ROTBUS): introduction and methodology. Syst Rev 2014;3:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tikkinen KAO, Craigie S, Agarwal A, Siemieniuk RAC, Cartwright R, Violette PDet al. Procedure-specific risks of thrombosis and bleeding in urological non-cancer surgery: systematic review and meta-analysis. Eur Urol 2018;73:236–241 [DOI] [PubMed] [Google Scholar]
- 64. Violette PD, Vernooij RWM, Aoki Y, Agarwal A, Cartwright R, Arai Yet al. An international survey on the use of thromboprophylaxis in urological surgery. Eur Urol Focus 2021;7:653–658 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The corresponding author is the custodian of the data and will provide access to data on request.