Abstract
Background
The National Oesophago-Gastric Cancer Audit (NOGCA) captures patient data from diagnosis to end of primary treatment for all patients with oesophagogastric (OG) cancer in England and Wales. This study assessed changes in patient characteristics, treatments received, and outcomes for OG cancer surgery for the period 2012–2020, and examined which factors may have led to changes in clinical outcomes over this time.
Methods
Patients diagnosed with OG cancer between April 2012 and March 2020 were included. Descriptive statistics were used to summarize patient demographics, disease site, type, and stage, patterns of care, and outcomes over time. The treatment variables of unit case volume, surgical approach, and neoadjuvant therapy were included. Regression models were used to examine associations between surgical outcomes (duration of stay and mortality), and patient and treatment variables.
Results
In total, 83 393 patients diagnosed with OG cancer during the study period were included. Patient demographics and cancer stage at diagnosis showed little change over time. Altogether, 17 650 patients underwent surgery as part of radical treatment. These patients had increasingly more advanced cancers, and a greater likelihood of pre-existing comorbidity in more recent years. Significant decreases in mortality rates and duration of stay were noted, along with improvements in oncological outcomes (nodal yields and margin positivity rates). Following adjustment for patient and treatment variables, increasing audit year and trust volume were associated, respectively, with improved postoperative outcomes: lower 30-day mortality (odds ratio (OR) 0.93 (95 per cent c.i. 0.88 to 0.98) and OR 0.99 (95 per cent c.i. 0.99–0.99)) and lower 90-day mortality (OR 0.94 (95 per cent c.i. 0.91 to 0.98) and OR 0.99 (95 per cent c.i. 0.99–0.99)), and a reduction in duration of postoperative stay (incidence rate ratio (IRR) 0.98 (95 per cent c.i. 0.97 to 0.98) and IRR 0.99 (95 per cent c.i. 0.99 to 0.99)).
Conclusion
Outcomes of OG cancer surgery have improved over time, despite little evidence of improvements in early diagnosis. The underlying drivers for improvements in outcome are multifactorial.
National population data for patients with oesophagogastric cancer in England and Wales were analysed for patients diagnosed between 2012 and 2020. Improvements in surgical and oncological outcomes were noted, despite failures to improve early diagnosis and increasingly comorbid patients. Year of audit (more recent care) and unit volume were significantly associated with surgical outcomes on multivariable regression analysis.
Introduction
As part of a continuing process to improve care and patient outcomes, multimodal treatment for oesophagogastric (OG) cancer has changed significantly over the past decade. Within surgery, there has been the increasing adoption of minimal-access or robotic approaches1,2, while developments in oncological treatments have increased the efficacy of systemic and local tumour response3,4. However, radical surgery for OG cancer continues to be associated with major morbidity, with curative multimodal treatment resulting in relatively poor overall survival compared to other tumour types4–7.
In England and Wales, the National Oesophago-Gastric Cancer Audit (NOGCA) was established in its current form in 2012 to evaluate the quality of care in National Health Service (NHS) services and promote improvement in outcomes for patients with oesophageal and gastric cancer in England and Wales8. NOGCA is commissioned by an independent national body, the UK Healthcare Quality Improvement Partnership, and delivered by a multiorganizational partnership, including national specialty societies representing surgeons, gastroenterologists, and radiologists. While data entry is not mandated, participation in NOGCA is recommended as a priority by NHS England in its Quality Accounts List; units’ audit participation and case ascertainment are included as quality metrics in NOGCA reports. The audit covers the care pathway from diagnosis to the end of primary treatment, and includes therapies given with curative or palliative intent, covering surgery, antisystemic cancer therapies, and radiotherapy. Information about individual NHS trusts (and, in previous iterations, individual surgeons) is publicly available to view online, with the aim of increasing transparency and driving quality improvement (QI) by enabling NHS organizations to benchmark themselves on different aspects of patient management. The audit website (www.nogca.org.uk) also directs organizations to various QI tools.
National clinical audit programmes have highlighted their roles in successfully improving care for OG cancer in the Netherlands9, and for other cancer types in England9–12. Past improvements in outcomes for patients undergoing OG cancer surgery in England have been linked to the centralization of services that took place during the 2000s13. However, more recent trends in OG cancer presentation, treatment, and outcomes, as well as the impact of more recent developments in OG cancer care have not been described.
This study aimed to investigate changes in characteristics of patients (demographics and tumour characteristics), treatments received, and clinical outcomes for OG cancer surgery (duration of postoperative stay, lymph node yield, and 30-day mortality) in England and Wales since 2012, and to examine which factors may have led to changes in clinical outcomes in this time.
Methods
All patients
The study used an extract of NOGCA data that included information about patients diagnosed with OG cancer between 1 April 2012 and 31 March 2020. Patients were eligible for inclusion if they were aged 18 years and older, and had a histological diagnosis of epithelial OG cancer; hospital staff complete a data set that covers the care pathway from diagnosis to the end of initial treatment. Patient records are then linked with information from other national healthcare data sets that cover hospital admissions, chemotherapy, and radiotherapy (see Park et al.8 for more details). Case ascertainment has progressively improved during the lifetime of NOGCA, from approximately 80 per cent to 90 per cent14. Survival data are obtained by linking to national mortality data (Office for National Statistics (ONS)). Data collection is permitted under section 251 of the NHS Act 2006, with study analysis permitted as this entailed analysis of pre-existing anonymized data.
Study variables
The following patient and tumour variables from the NOGCA data set were included: age at diagnosis (in years); date of diagnosis; sex; quintile of socio-economic deprivation (based on the Index of Multiple Deprivation ranking for small geographical areas in England and Wales15,16, derived from the patient’s postcode of residence and assigned to national quintiles); number of documented comorbidities (from list: ischaemic heart disease, chronic obstructive pulmonary disease/asthma, chronic renal impairment, liver failure/cirrhosis, diabetes, peripheral vascular disease, cerebrovascular disease, mental illness, or other significant condition); disease site and histology (categorized as oesophageal squamous cell carcinoma, upper or mid-oesophageal adenocarcinoma, lower oesophageal adenocarcinoma, stomach); clinical tumour (T) stage and clinical node (N) stage; performance status; and intended treatment. For those patients who had curative surgery, the following surgical variables were included: receipt of neoadjuvant therapy (yes/no); main procedure; and surgical approach (minimally invasive or open).
The NOGCA data set was linked to ONS death registration data for information on mortality outcomes.
Outcomes after surgery included duration of postoperative stay in days (defined as from operation date to discharge date), 30- and 90-day postoperative mortality, the proportion of specimens with 15 or more lymph nodes examined, and the proportion of specimens with positive margins (longitudinal resection margins for oesophagectomy and gastrectomy; circumferential margins for oesophagectomy).
Surgical case volumes were calculated for each individual NHS organization (trusts in England and local health boards in Wales) where curative surgery was undertaken, and reported as the number of surgical resection procedures performed with curative intent per organization per year.
Analysis
Descriptive statistics were used to summarize patient characteristics, patterns of care, and outcomes over time (analysed as individual audit years, from 1 April to 31 March, but grouped into 2-year periods for presentation), and by tumour site (oesophagus or stomach), using χ2 tests to evaluate associations between audit year and categorical variables. Linear regression analysis was used to assess the associations between audit year and continuous variables.
Multivariable logistic regression models were used to examine the associations between 30- and 90-day postoperative mortality and audit year (odds ratio (OR) for annual change in mortality, relative to 2012–13). An initial model adjusted for patient variables (age at diagnosis, sex, deprivation quintile, number of comorbidities, tumour group, clinical T stage, clinical N stage, and performance status). In a second stage, the model was additionally adjusted for variables related to the patterns of care (neoadjuvant therapy, surgical procedure, and NHS trust volume). Robust standard errors were used to account for clustering within NHS trusts.
Poisson regression models (with robust standard errors) were used to assess the associations between duration of postoperative stay and audit year. As above, two models were used with adjustment in two stages to examine the degree to which the changes in duration of stay over time were associated with practice variables. Outcomes were expressed as incidence rate ratios (IRRs), indicating the relative risk of incurring an additional day in hospital.
Missing values for patient characteristics were imputed with multiple imputation using chained equations creating 10 data sets with the assumption that missing values were only related to the observed data (that is missing at random). The imputation model included all of the variables in the analysis models. Rubin’s rules were used to pool the estimated regression model coefficients and intercept. All statistical tests were two sided and the threshold of statistical significance was defined as a P value of 0.05. Statistical analysis was performed using STATA version 17 (StataCorp, College Station, Texas, USA).
Ethics approval
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research. Patient consent for publication was not required. The study was exempt from UK National Research Ethics Committee approval as it involved secondary analysis of an existing data set of anonymized data. The National Oesophago-Gastric (OG) Cancer Audit has approval for processing healthcare information under Section 251 (reference number: ECC 1-06 (c)/2011) for all NHS patients diagnosed with OG cancer in England and Wales. Data for this study were based on patient-level information collected by the NHS, as part of the care and support of patients with cancer.
Results
NOGCA records were available for a total of 83 393 patients diagnosed during the study period. The characteristics of this cohort are summarized in Table 1.
Table 1.
Characteristics of patients diagnosed with oesophagogastric cancer by time period
| 2012–2014 (n = 21 004) | 2014–2016 (n = 21 356) | 2016–2018 (n = 20 714) | 2018–2020 (n = 20 319) | |
|---|---|---|---|---|
| Age group (years) | ||||
| ȃ<60 | 3362 (16.0) | 3456 (16.2) | 3322 (16.0) | 3245 (16.0) |
| ȃ60–69 | 5362 (25.5) | 5380 (25.2) | 5155 (24.9) | 4878 (24.0) |
| ȃ70–79 | 6624 (31.5) | 6921 (32.4) | 6904 (33.3) | 6974 (34.3) |
| ȃ≥80 | 5656 (26.9) | 5599 (26.2) | 5333 (25.8) | 5222 (25.7) |
| Male sex | 14 273 (68.1) | 14 577 (68.4) | 14 395 (69.6) | 14 256 (70.3) |
| ȃMissing | 49 | 31 | 37 | 40 |
| Deprivation quintiles | ||||
| ȃ1 (least deprived) | 3692 (17.7) | 3685 (17.3) | 3750 (18.2) | 3730 (18.5) |
| ȃ2 | 4129 (19.8) | 4310 (20.3) | 4170 (20.2) | 4086 (20.2) |
| ȃ3 | 4262 (20.4) | 4391 (20.7) | 4325 (21.0) | 4241 (21.0) |
| ȃ4 | 4330 (20.7) | 4393 (20.7) | 4119 (20.0) | 4082 (20.2) |
| ȃ5 (most deprived) | 4487 (21.5) | 4476 (21.1) | 4259 (20.6) | 4075 (20.2) |
| ȃMissing | 104 | 101 | 91 | 105 |
| Comorbidities | ||||
| ȃNone | 13 106 (62.4) | 12 749 (59.7) | 10 368 (50.1) | 9031 (44.4) |
| ȃ1 | 4454 (21.2) | 4978 (23.3) | 6161 (29.7) | 7371 (36.3) |
| ȃ2 | 2328 (11.1) | 2492 (11.7) | 2900 (14.0) | 2939 (14.5) |
| ȃ3 or more | 1116 (5.3) | 1137 (5.3) | 1285 (6.2) | 978 (4.8) |
| Tumour group | ||||
| ȃOesophageal SCC | 3844 (18.3) | 3810 (17.8) | 3857 (18.6) | 3878 (19.1) |
| ȃOesophageal ACA upper/mid | 1415 (6.7) | 1611 (7.5) | 1487 (7.2) | 1599 (7.9) |
| ȃOesophageal ACA lower (including SI–SII) | 8742 (41.6) | 9248 (43.3) | 9195 (44.4) | 9231 (45.4) |
| ȃStomach (including SIII) | 7003 (33.3) | 6687 (31.3) | 6175 (29.8) | 5611 (27.6) |
| Clinical T category | ||||
| ȃ0/1 | 1049 (5.4) | 1099 (5.6) | 995 (5.3) | 1008 (5.5) |
| ȃ2 | 2525 (13.0) | 2557 (12.9) | 2323 (12.3) | 2352 (12.8) |
| ȃ3 | 9612 (49.6) | 10 001 (50.6) | 9486 (50.4) | 9087 (49.3) |
| ȃ4 | 3618 (18.7) | 3855 (19.5) | 3907 (20.7) | 3828 (20.8) |
| ȃX | 2579 (13.3) | 2259 (11.4) | 2118 (11.2) | 2140 (11.6) |
| ȃMissing | 1621 | 1585 | 1885 | 1904 |
| Clinical N category | ||||
| ȃ0 | 5500 (28.4) | 5502 (27.8) | 5529 (29.4) | 5540 (30.1) |
| ȃ1 | 6920 (35.7) | 7104 (35.9) | 6210 (33.0) | 6033 (32.8) |
| ȃ2 | 3551 (18.3) | 3883 (19.6) | 3887 (20.6) | 3736 (20.3) |
| ȃ3 | 1572 (8.1) | 1747 (8.8) | 1813 (9.6) | 1622 (8.8) |
| ȃX | 1840 (9.5) | 1535 (7.8) | 1390 (7.4) | 1484 (8.0) |
| ȃMissing | 1621 | 1585 | 1885 | 1904 |
| Clinical M category | ||||
| ȃ0 | 12 857 (67.2) | 13 015 (66.7) | 12 436 (66.6) | 12 604 (69.0) |
| ȃ1 | 6268 (32.8) | 6506 (33.3) | 6226 (33.4) | 5660 (31.0) |
| ȃMissing | 1879 | 1835 | 2052 | 2049 |
| Performance status | ||||
| ȃ0 | 6196 (29.5) | 7117 (33.3) | 7031 (33.9) | 7749 (38.1) |
| ȃ1 | 7197 (34.3) | 7112 (33.3) | 6981 (33.7) | 6738 (33.2) |
| ȃ≥2 | 7611 (36.2) | 7127 (33.4) | 6702 (32.4) | 5832 (28.7) |
Values are n (%) unless otherwise stated. SCC, squamous cell carcinoma; ACA, adenocarcinoma; SI–III, Siewert type I–III.
Over the 8-year study period, there was little change in the distribution of patient demographics (age, sex, or socio-economic deprivation index). The pattern of clinical stage was also stable, with approximately one-third of patients presenting with metastatic disease at diagnosis. In more recent audit years, a greater proportion of patients had one or more documented comorbidities (55.6 per cent in 2018–2020 versus 37.6 per cent in 2012–2014; P < 0.001), but a greater proportion also had a WHO performance status of 0 (38.1 per cent versus 29.5 per cent; P < 0.001).
Patients undergoing radical surgery
Of all patients diagnosed with OG cancer between 2012 and 2020, 17 650 underwent radical surgery as part of treatment with curative intent; the percentage of all diagnosed patients who had a record of radical surgical resection decreased from 23.2 per cent to 18.0 per cent (P < 0.001) between 2012 and 2020 (Table 2).
Table 2.
Characteristics of patients having curative surgery for oesophagogastric cancer by time period
| Patients having curative surgery | 2012–2014 (n = 4806; 22.9%) | 2014–2016 (n = 4684; 21.9%) | 2016–2018 (n = 4246; 20.5%) | 2018–2020 (n = 3914; 19.3%) |
|---|---|---|---|---|
| Age group (years) | ||||
| ȃ<60 | 1136 (23.6) | 1137 (24.3) | 1000 (23.6) | 971 (24.8) |
| ȃ60–69 | 1690 (35.2) | 1590 (33.9) | 1433 (33.7) | 1277 (32.6) |
| ȃ70–79 | 1636 (34.0) | 1571 (33.5) | 1501 (35.4) | 1385 (35.4) |
| ȃ≥80 | 344 (7.2) | 386 (8.2) | 312 (7.3) | 281 (7.2) |
| Male sex | ||||
| 3569 (74.4) | 3505 (74.9) | 3177 (75.1) | 3001 (76.8) | |
| ȃMissing | 11 | 6 | 17 | 7 |
| Deprivation quintiles | ||||
| ȃ1 (least deprived) | 936 (19.6) | 890 (19.1) | 892 (21.1) | 800 (20.5) |
| ȃ2 | 1003 (21.0) | 1039 (22.3) | 869 (20.6) | 833 (21.4) |
| ȃ3 | 969 (20.3) | 964 (20.7) | 881 (20.9) | 805 (20.7) |
| ȃ4 | 928 (19.5) | 930 (20.0) | 818 (19.4) | 782 (20.1) |
| ȃ5 (most deprived) | 931 (19.5) | 830 (17.8) | 758 (18.0) | 677 (17.4) |
| ȃMissing | 39 | 31 | 28 | 17 |
| Comorbidities | ||||
| ȃNone | 2510 (52.2) | 2524 (53.9) | 2069 (48.7) | 1861 (47.5) |
| ȃ1 | 1262 (26.3) | 1158 (24.7) | 1280 (30.1) | 1312 (33.5) |
| ȃ2 | 691 (14.4) | 678 (14.5) | 610 (14.4) | 574 (14.7) |
| ȃ3 or more | 343 (7.1) | 324 (6.9) | 287 (6.8) | 167 (4.3) |
| Tumour group | ||||
| ȃOesophageal SCC | 414 (8.6) | 418 (8.9) | 359 (8.5) | 314 (8.0) |
| ȃOesophageal ACA upper/mid | 211 (4.4) | 239 (5.1) | 198 (4.7) | 198 (5.1) |
| ȃOesophageal ACA lower (w SI, SII) | 2456 (51.1) | 2448 (52.3) | 2229 (52.5) | 2131 (54.4) |
| ȃStomach (w SIII) | 1725 (35.9) | 1579 (33.7) | 1460 (34.4) | 1271 (32.5) |
| Clinical T category | ||||
| ȃ0/1 | 433 (9.4) | 469 (10.4) | 349 (8.6) | 293 (7.6) |
| ȃ2 | 981 (21.3) | 931 (20.6) | 861 (21.2) | 779 (20.3) |
| ȃ3 | 2561 (55.7) | 2519 (55.6) | 2256 (55.6) | 2134 (55.6) |
| ȃ4 | 361 (7.9) | 400 (8.8) | 371 (9.1) | 406 (10.6) |
| ȃX | 259 (5.6) | 209 (4.6) | 223 (5.5) | 223 (5.8) |
| ȃMissing | 211 | 156 | 186 | 79 |
| Clinical N category | ||||
| ȃ0 | 1967 (42.8) | 1953 (43.1) | 1864 (45.9) | 1727 (45.0) |
| ȃ1 | 1770 (38.5) | 1733 (38.3) | 1412 (34.8) | 1365 (35.6) |
| ȃ2 | 608 (13.2) | 605 (13.4) | 614 (15.1) | 571 (14.9) |
| ȃ3 | 140 (3.0) | 148 (3.3) | 110 (2.7) | 100 (2.6) |
| ȃX | 110 (2.4) | 89 (2.0) | 60 (1.5) | 72 (1.9) |
| ȃMissing | 211 | 156 | 186 | 79 |
| Performance status | ||||
| ȃ0 | 2532 (52.7) | 2678 (57.2) | 2479 (58.4) | 2509 (64.1) |
| ȃ1 | 1748 (36.4) | 1677 (35.8) | 1466 (34.5) | 1230 (31.4) |
| ȃ≥2 | 526 (10.9) | 329 (7.0) | 301 (7.1) | 175 (4.5) |
Values are n (%) unless otherwise stated. SCC, squamous cell carcinoma; ACA, adenocarcinoma; w SI–III, with Siewert type I–III.
Among patients undergoing curative surgery for OG cancer, patient age, sex, and socio-economic deprivation did not change over the study period. The percentage of patients with any comorbidities recorded increased from 48.5 per cent among those diagnosed in 2012–13 to 51.9 per cent diagnosed in 2019–20 (P < 0.001). There was a statistically insignificant increase in the proportion of lower oesophageal and Siewert I–II adenocarcinomas (from 50.4 per cent to 55.5 per cent), and a decline in Siewert III/stomach cancers (from 37.0 per cent to 32.4 per cent; P = 0.055). The percentage of patients with more locally advanced cancers increased; for instance, the percentage of patients with cT4 disease increased from 7.2 per cent to 10.5 per cent (P < 0.001). Patients undergoing surgery in more recent years had a better performance status than those in the earlier period, with the percentage with performance status 0 (fully active) increasing from 53.3 per cent to 65.2 per cent (P < 0.001).
Multimodal treatment and surgical practice
The use of neoadjuvant treatment increased over the study period in patients undergoing oesophagectomy (from 70.0 per cent to 74.8 per cent; P < 0.001) and gastrectomy (from 6.4 per cent to 56.3 per cent; P < 0.001) (Table 3).
Table 3.
Patterns of care for patients having curative surgery for oesophagogastric cancer by time period and tumour site
| 2012–2014 | 2014–2016 | 2016–2018 | 2018–2020 | |
|---|---|---|---|---|
| Oesophagectomy, n | 3071 | 3078 | 2765 | 2557 |
| Neoadjuvant therapy, n (%) | 2151 (70.0) | 2090 (67.9) | 1904 (68.9) | 1878 (73.5) |
| Main procedure | ||||
| ȃThoracoabdominal | 309 (10.1) | 283 (9.2) | 181 (6.6) | 161 (6.3) |
| ȃTwo-phase | 2517 (82.0) | 2473 (80.3) | 2369 (85.7) | 2221 (86.9) |
| ȃThree-phase | 144 (4.7) | 197 (6.4) | 153 (5.5) | 136 (5.3) |
| ȃTranshiatal | 101 (3.3) | 125 (4.1) | 62 (2.2) | 39 (2.5) |
| Surgical access | ||||
| ȃOpen | 1675 (58.5) | 1639 (57.7) | 1367 (51.7) | 1150 (47.0) |
| ȃHybrid | 771 (26.9) | 867 (30.5) | 789 (29.9) | 745 (30.4) |
| ȃLaparoscopic completed | 290 (10.1) | 256 (9.0) | 412 (15.6) | 450 (18.4) |
| ȃLaparoscopic converted | 129 (4.5) | 81 (2.9) | 75 (2.8) | 65 (2.7) |
| ȃRobotic | 0 | 0 | <5 | 37 (1.6) |
| ȃMissing | 206 | 235 | 120* | 110 |
| Gastrectomy | 1735 | 1606 | 1481 | 1357 |
| Neoadjuvant therapy | 801 (46.2) | 673 (41.9) | 618 (41.7) | 695 (51.2) |
| Main procedure | ||||
| ȃTotal gastrectomy | 794 (45.8) | 673 (41.9) | 700 (42.3) | 640 (47.2) |
| ȃExtended gastrectomy | 122 (7.0) | 143 (8.9) | 127 (8.6) | 125 (9.2) |
| ȃProximal | 28 (1.6) | 37 (2.3) | 24 (1.6) | 16 (1.2) |
| ȃDistal gastrectomy | 733 (42.3) | 714 (44.5) | 587 (39.6) | 556 (41.0) |
| ȃOther | 58 (3.4) | 39 (2.5) | 43 (2.9) | 20 (1.5) |
| Surgical access | ||||
| ȃOpen | 1481 (85.4) | 1346 (83.8) | 1213 (82.0) | 1079 (80.1) |
| ȃLaparoscopic completed | 207 (11.9) | 219 (13.6) | 237 (16.0) | 248 (18.4) |
| ȃLaparoscopic converted | 47 (2.7) | 41 (2.6) | 29 (2.0) | 20 (1.5) |
| ȃRobotic | 0 | 0 | 0 | <5 |
| ȃMissing | 0 | 0 | 2 | 10* |
| Surgical volumes | ||||
| ȃNHS organizations performing surgery | 56 | 66 | 51 | 57 |
| ȃMedian number of patients per organization over 2 years (i.q.r.) | 84.5 (28–129.5) | 72 (1–113) | 87 (36–127) | 64 (2–105) |
Values are n (%) unless otherwise stated. Data suppression applied to small numbers (<5) and percentages calculated treating suppressed values as 0. *Rounded to the nearest 5. NHS, National Health Service; i.q.r., interquartile range.
Among patients undergoing oesophagectomy, use of the two-phase (Ivor Lewis) procedure increased from 80.3 per cent to 86.8 per cent, while the use of thoracoabdominal (from 11.5 per cent to 7.0 per cent) and transhiatal (from 3.1 per cent to 1.7 per cent) procedures decreased (P < 0.001). For both oesophagectomy and gastrectomy, the use of minimally invasive approaches increased over the study period. Open approaches accounted for 57.3 per cent of oesophagectomies in 2012–14 versus 47.1 per cent in 2018–20 (P < 0.001), and 84.7 per cent of gastrectomies in 2012–14 versus 80.3 per cent in 2018–20. Few procedures involved robotic surgery during the study period. There was substantial hospital-level variation in the use of minimally invasive approaches (including hybrid and converted procedures) over the study period, for both oesophagectomy (interquartile range (i.q.r.) 12.5 per cent to 81.0 per cent) and gastrectomy (i.q.r. 0.5 per cent to 41.1 per cent).
The number of NHS organizations performing curative surgery was relatively stable over the study period, ranging from 45 to 59 per audit year. Surgical volumes were also largely consistent, with median annual volumes ranging from 38 to 49 per organization.
Outcomes after radical surgery
Thirty- and 90-day postoperative mortality rates decreased over the study period. The overall 30-day mortality rate was 2.6 per cent in 2012–13 versus 1.3 per cent in 2019–20 (P = 0.062), while 90-day mortality decreased from 4.7 per cent to 2.7 per cent (P = 0.005). After adjusting for patient factors, audit year was associated with both 30-day mortality (adjusted OR 0.93, 95 per cent c.i. 0.89 to 0.98) and 90-day mortality (adjusted OR 0.95, 95 per cent c.i. 0.91 to 0.98). Further adjustment for treatment factors had little impact on the observed associations, and audit year remained significantly associated with 30-day (OR 0.93, 95 per cent c.i. 0.88 to 0.98) and 90-day mortality (OR 0.94, 95 per cent c.i. 0.91 to 0.98) (Table 4).
Table 4.
Multivariable logistic regression models for 30- and 90-day postoperative mortality and Poisson regression model for duration of postoperative stay in patients diagnosed from 2012 to 2020 who had curative surgery for oesophagogastric cancer
| 30-day mortality | 90-day mortality | Duration of stay | ||||
|---|---|---|---|---|---|---|
| aOR (95% c.i.) (n = 17 650) | P value | aOR (95% c.i.) (n = 17 650) | P value | aIRR (95% c.i.) (n = 16 392) | P value | |
| Audit year | 0.93 (0.88–0.98) | 0.004 | 0.94 (0.91–0.98) | 0.003 | 0.98 (0.97–0.98) | <0.001 |
| Age (years) | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.02–1.04) | <0.001 | 1.00 (1.00–1.01) | <0.001 |
| Male sex | 1.15 (0.87–1.53) | 0.318 | 1.15 (0.94–1.42) | 0.184 | 0.98 (0.95–1.00) | 0.103 |
| Deprivation quintiles | ||||||
| ȃ1 (least deprived) | 1 | 0.662 | 1 | 0.300 | 1 | <0.001 |
| ȃ2 | 1.09 (0.76–1.55) | 1.24 (0.95–1.61) | 1.02 (0.98–1.06) | |||
| ȃ3 | 1.10 (0.77–1.58) | 1.25 (0.96–1.64) | 1.03 (0.99–1.07) | |||
| ȃ4 | 1.29 (0.91–1.83) | 1.30 (0.99–1.70) | 1.02 (0.98–1.06) | |||
| ȃ5 (most deprived) | 1.22 (0.84–1.76) | 1.31 (0.99–1.73) | 1.09 (1.05–1.13) | |||
| Comorbidities | ||||||
| ȃNone | 1 | <0.001 | 1 | <0.001 | 1 | 0.001 |
| ȃ1 | 1.80 (1.37–2.37) | 1.56 (1.28–1.92) | 1.04 (1.01–1.07) | |||
| ȃ2 | 2.09 (1.51–2.88) | 1.84 (1.45–2.33) | 1.05 (1.01–1.09) | |||
| ȃ3 or more | 2.78 (1.89–4.08) | 2.58 (1.94–3.42) | 1.10 (1.04–1.16) | |||
| Tumour group | ||||||
| ȃOesophageal SCC | 1 | 0.146 | 1 | 0.195 | 1 | 0.007 |
| ȃOes ACA upper/mid | 1.02 (0.58–1.82) | 1.06 (0.69–1.64) | 0.93 (0.87–0.99) | |||
| ȃOes ACA lower (SI, SII) | 0.84 (0.56–1.27) | 0.84 (0.62–1.15) | 0.90 (0.86–0.94) | |||
| ȃStomach (w SIII) | 0.52 (0.28–0.96) | 0.64 (0.40–1.04) | 0.85 (0.79–0.90) | |||
| Clinical T category | ||||||
| ȃ0/1 | 1 | 0.350 | 1 | 0.152 | 1 | 0.008 |
| ȃ2 | 0.68 (0.43–1.06) | 1.07 (0.74–1.54) | 1.03 (0.98–1.08) | |||
| ȃ3 | 0.90 (0.59–1.37) | 1.37 (0.96–1.94) | 1.00 (0.95–1.05) | |||
| ȃ4 | 0.81 (0.44–1.47) | 1.19 (0.76–1.89) | 1.07 (1.00–1.14) | |||
| ȃX | 0.99 (0.53–1.87) | 1.44 (0.89–2.31) | 0.95 (0.89–1.02) | |||
| Clinical N category | ||||||
| ȃ0 | 1 | 0.517 | 1 | 0.162 | 1 | 0.124 |
| ȃ1 | 1.22 (0.92–1.62) | 1.27 (1.04–1.56) | 1.00 (0.97–1.03) | |||
| ȃ2 | 1.18 (0.80–1.74) | 1.28 (0.97–1.69) | 1.02 (0.98–1.07) | |||
| ȃ3 | 0.87 (0.37–2.05) | 0.97 (0.55–1.73) | 1.07 (0.99–1.15) | |||
| ȃX | 1.59 (0.72–3.50) | 1.32 (0.72–2.42) | 1.09 (0.99–1.19) | |||
| Performance status | ||||||
| ȃ0 | 1 | 0.041 | 1 | <0.001 | 1 | <0.001 |
| ȃ1 | 1.05 (0.82–1.35) | 1.05 (0.88–1.26) | 1.06 (1.03–1.09) | |||
| ȃ≥2 | 1.57 (1.09–2.25) | 1.74 (1.33–2.26) | 1.16 (1.11–1.22) | |||
| ȃNeoadjuvant therapy | 0.78 (0.60–1.02) | 0.068 | 0.73 (0.60–0.89) | 0.002 | 0.96 (0.93–0.99) | 0.008 |
| Main procedure | ||||||
| ȃOesophagectomy | 1 | 0.790 | 1 | 0.425 | 1 | <0.001 |
| ȃGastrectomy | 0.94 (0.57–1.53) | 0.85 (0.58–1.26) | 0.78 (0.74–0.82) | |||
| Trust volume | 0.99 (0.99–0.99) | <0.001 | 0.99 (0.99–0.99) | <0.001 | 0.99 (0.99–0.99) | <0.001 |
aOR, adjusted odds ratio; aIRR, adjusted incidence rate ratio; SCC, squamous cell carcinoma; Oes ACA, oesophageal adenocarcinoma; w SI–III, with Siewert type I–III. Bold values indicate statistically significant at p < 0.05.
Median duration of postoperative stay declined from 12 days (i.q.r. 9 to 18) in 2012–13 to 10 days (i.q.r. 8 to 15) in 2019–20 (P < 0.001, from non-parametric equality of medians test). This reduction remained statistically significant after adjustment for patient characteristics (IRR 0.98, 95 per cent c.i. 0.97 to 0.98). Additional adjustment for practice variables produced similar results (IRR 0.98, 95 per cent c.i. 0.97 to 0.98). Table 4 describes the models in detail.
Increasing age, comorbidity, worse performance status, not receiving neoadjuvant therapy, and lower annual hospital case volume were associated with an increased risk of mortality and longer duration of stay (Table 4). Increased deprivation was associated with a longer length of stay (IRR for patients in the most deprived versus those in the least deprived quintile 1.09, 95 per cent c.i. 1.05 to 1.13) but not mortality.
Other outcomes
The median number of lymph nodes examined after surgery increased from 23 (i.q.r. 16 to 32) in 2012–13 to 28 (i.q.r. 20 to 37) in 2019–20. The percentage of procedures in which 15 or more lymph nodes were examined increased from 82.0 per cent to 89.1 per cent (P < 0.001; Fig. 1).
Fig. 1.
Number of lymph nodes examined, by audit year
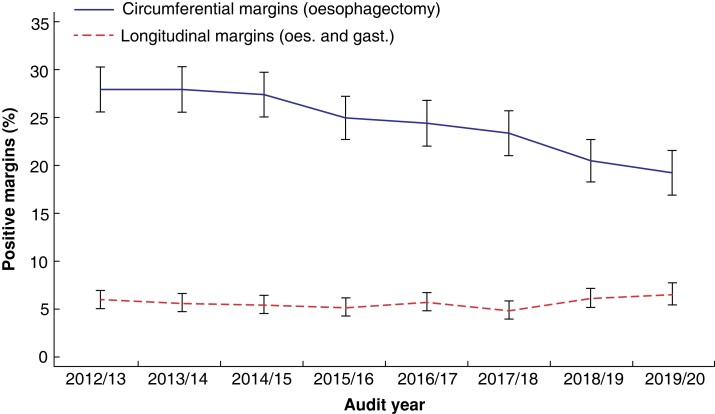
Positive circumferential margin rates for oesophagectomy decreased from 27.8 per cent in 2012–13 to 19.2 per cent in 2019–20 (P < 0.001). Rates of positive longitudinal margins (oesophagectomy and gastrectomy combined) did not change over the study period (P = 0.370; Fig. 2).
Fig. 2.
Unadjusted rates of positive margins for oesophagectomy (oes.) and gastrectomy (gast.), 2012–2020
Discussion
This study describes the changes in patient characteristics, management, and outcomes of radical surgery for OG cancer in England and Wales over an 8-year period since the national audit of OG cancer care began. Over the course of the 8 years, there have been significant reductions in postoperative mortality rates and durations of stay, even after adjusting for patient and treatment factors, as well as improvement in histological outcome metrics, with reduced positive margin rates and improved nodal yields.
The publication of hospital- and surgeon-specific audit figures by NOGCA has, in the past, resulted in fears that this might result in selection bias against more comorbid patients, to improve published outcomes. This study suggests that improvements in outcomes have occurred despite a shift in patient and disease demographics, with more comorbid patients and patients presenting with more advanced disease being treated with radical surgery, suggesting that these fears are unfounded. However, there has also been an overall decline in the proportion of patients being offered radical multimodal therapy, with those undergoing surgery having a better performance status. Further research into how changing presentations of disease, patient comorbidity, and oncological treatment options may affect patient selection and treatment recommendations is warranted. The use of neoadjuvant therapy has increased over the audit’s lifetime, and the increasing efficacy of neoadjuvant chemotherapy and chemoradiation regimens may have contributed to the increasing proportion of patients with late-stage disease progressing to surgery. Ongoing trials of new chemotherapeutic agents, and of existing agents in expanded contexts, including oligometastatic disease, may, in future, broaden the scope of patients considered for surgery even further17,18.
The centralization of major OG cancer services, since the last major reorganization of UK cancer services in the 1990s, has resulted in a stable number of centres and centre volumes. However, some centres still report low case volumes. Annual centre volume was the only organizational factor considered and, in adjusted analysis, demonstrated a statistically significant association with surgical outcomes; the well-established relationship between higher surgical volume and improved outcomes across various surgical disciplines suggests that low-volume centres may be at greater risk of fluctuations in care quality19.
NOGCA data suggest that minimally invasive approaches continue to be adopted, with steady increases in laparoscopy and thoracoscopy, in keeping with current evidence1,2. Robotically assisted procedures, for which the evidence remains less clear, has remained novel in the UK, although they have been adopted at pace by a number of centres in the time since the period of audit covered in this study (Personal communication, Intuitive Surgical (Sunnyvale, CA, USA)). An increased standardization of surgical approach means that increasingly fewer thoracoabdominal or transhiatal oesophagectomies are being performed, while proximal gastrectomies are almost completely absent in UK practice.
The data described herein also raise important broader issues to be considered at policy and organizational levels. Despite efforts to increase the awareness and earlier diagnosis of oesophageal cancer such as straight-to-test referrals to endoscopy from primary care and statutory 2-week targets from primary care referral to initial diagnostic test (that is endoscopy)20,21, data presented in this study demonstrate there has been effectively no change in disease stage at presentation over the past decade. Concerns remain that the diagnostic delays induced by the COVID-19 pandemic may yet further exacerbate the problem of late diagnosis of cancers in the near future. In the absence of screening endoscopy programmes in Western countries, less invasive screening tests such as swallowable cell collection devices (Cytosponge)22 or breath testing for cancer-associated volatile organic compounds23 have not yet been broadly adopted, but may help increase rates of early diagnosis in future. Variations in care and outcomes across socio-economic deprivation levels have been well described in many other developed health systems, and are partially reflected here with increased durations of stay for more deprived patients24,25.
The use of outcome metrics and how to adjust for case mix continues to be debated. The use of oncological outcomes such as nodal count and positive resection margin (R1) rates are subject to significant variations in histopathological practice26,27. Duration of stay, as demonstrated here, is subject to multiple factors including socio-economic and social care considerations, which will vary by geographical area. With short-term postoperative mortality an increasingly rare occurrence, other outcomes such as longer-term mortality may provide more information about quality of care. A multifactorial composite metric that considers all areas of the patient pathway at a unit level is mostly likely to best represent hospital care quality, and is currently being introduced by NOGCA8.
We contrast our findings to those from other recently published national data sets. A recently published analysis of the Dutch Upper Gastrointestinal Cancer Audit (DUCA) most closely approximates the timespan (2011–2018) of the data reported in this study9. DUCA is a mandatory national audit with data completeness reported at >99 per cent; data from DUCA show similar trends in the characteristics of patients undergoing surgery for OG cancer as in England and Wales, with parallels in changes in histological subtype, more advanced stage at presentation, and increased comorbidity. Dutch centres were much more likely to employ hybrid or fully minimally invasive techniques (90 per cent hybrid/minimally invasive oesophagectomy rate in 2017–2018 versus 53 per cent in England and Wales in 2018–2020) but reported slightly higher 30-day mortality rates (as high as 4.4 per cent in 2013–2014 versus 2.5 per cent in UK). The publication of surgeon-level data by NOGCA versus hospital-level data only in DUCA does not appear to have impacted on these positive outcome trends. A particular strength of this publication and of NOGCA data is the inclusion of information about all patients diagnosed with histologically confirmed OG cancer, as opposed to DUCA, which captures surgical patient data only and therefore lacks a population denominator of patients not suitable for surgical treatment. Analyses of the Swedish (2007–2016) and Danish (2004–2013) national OG cancer registries found comparable improvements in clinical and histological outcomes; the Swedish registry also reported roughly similar rates of case ascertainment (89.2 per cent)28,29.
The limitations of this data must be considered. Outcomes for non-surgically based treatment with curative intent, such as definitive chemoradiation or endoscopic therapy, have been historically less well captured by NOGCA, but consist of < 5 per cent of the total patient cohort and are thought to have a minimal impact on the overall results presented here. Surgical case ascertainment in NOGCA has been shown to be very high, but incomplete, when compared to national hospital data sets (Hospital Episode Statistics, HES) at over 90 per cent. No conclusions can be drawn over any potential bias incurred by these missing patients; efforts to further improve data capture in other areas are ongoing30.
The results of this analysis of population-level audit data demonstrate both a failure to improve early diagnostic rates, as well as successful improvements in OG cancer care over time. The underlying drivers for improvements in outcome are multifactorial and cannot be easily isolated. However, audit and public accountability for outcomes are undoubtedly a critical factor to measure and improve any process, and, as such, national registries such as NOGCA remain vital to monitoring and improving OG cancer care.
Acknowledgements
This study was undertaken as part of the work by the National Oesophago-Gastric (OG) Cancer Audit. The Audit is commissioned by the Healthcare Quality Improvement Partnership (HQIP) as part of the National Clinical Audit and Patient Outcomes Programme and funded by NHS England and the Welsh Government (www.hqip.org.uk/national-programmes). The authors had full independence from the Healthcare Quality Improvement Partnership. The aim of National Oesophago-Gastric Cancer Audit is to evaluate the care of patients with OG cancer in England and Wales, and support NHS providers to improve the quality of hospital care for these patients. More information can be found at: www.nogca.org.uk. Neither HQIP nor the funders had any involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Contributor Information
Philip H Pucher, Department of Surgery, Portsmouth Hospitals University NHS Trust, Portsmouth, UK; School of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth, UK.
Min Hae Park, Clinical Effectiveness Unit, Royal College of Surgeons of England, London, UK; Department of Health Services Research and Policy, London School of Hygiene & Tropical Medicine, London, UK.
David A Cromwell, Clinical Effectiveness Unit, Royal College of Surgeons of England, London, UK; Department of Health Services Research and Policy, London School of Hygiene & Tropical Medicine, London, UK.
Tom C Crosby, Department of Clinical Oncology, Velindre Cancer Centre, Velindre University NHS Trust, Cardiff, UK.
Betsan Thomas, Department of Clinical Oncology, Velindre Cancer Centre, Velindre University NHS Trust, Cardiff, UK.
Nigel Trudgill, Department of Gastroenterology, Sandwell and West Birmingham Hospitals NHS Trust, Birmingham, UK.
Muhammad Wahedally, Clinical Effectiveness Unit, Royal College of Surgeons of England, London, UK.
Nick Maynard, Department of Surgery, Oxford University Hospitals NHS Trust, Oxford, UK.
James A Gossage, Department of Surgery, Guy’s and St Thomas’ Hospital NHS Foundation Trust, London, UK.
Author contributions
Philip Pucher (Conceptualization, Formal analysis, Methodology, Project administration, Visualization, Writing—original draft, Writing—review & editing), Min Hae Park (Conceptualization, Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review & editing), David Cromwell (Conceptualization, Data curation, Formal analysis, Resources, Supervision, Writing—original draft, Writing—review & editing), Tom Crosby (Conceptualization, Methodology, Writing—review & editing), Betsan Thomas (Data curation, Writing—review & editing), Nigel Trudgill (Conceptualization, Data curation, Methodology, Writing—review & editing), Muhammad Wahedally (Data curation, Formal analysis, Resources, Software, Supervision, Validation), Nick Maynard (Conceptualization, Data curation, Methodology, Supervision, Writing—review & editing), and James Gossage (Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing—review & editing).
Funding
The authors have no funding to declare.
Disclosure
The authors declare no conflict of interest.
Data availability
Researchers may apply to the Healthcare Quality Improvement Partnership to access National Oesophago-Gastric Cancer Audit data for a research study. The approvals process for such requests is outlined here: https://www.hqip.org.uk/national-programmes/accessing-ncapop-data/
References
- 1. van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCAet al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg 2019;269:621–630 [DOI] [PubMed] [Google Scholar]
- 2. Biere SSAY, Van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JRet al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887–1892 [DOI] [PubMed] [Google Scholar]
- 3. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper Set al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948–1957 [DOI] [PubMed] [Google Scholar]
- 4. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPLet al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–1098 [DOI] [PubMed] [Google Scholar]
- 5. Warps AK, Saraste D, Westerterp M, Detering R, Sjövall A, Martling Aet al. National differences in implementation of minimally invasive surgery for colorectal cancer and the influence on short-term outcomes. Surg Endosc 2022;36:5986–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling Get al. Benchmarking complications associated with esophagectomy. Ann Surg 2019;269:291–298 [DOI] [PubMed] [Google Scholar]
- 7. Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold Det al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979–989 [DOI] [PubMed] [Google Scholar]
- 8.Park MH, Wahedally H, Cromwell DA, Maynard N, Crosby T, Trudgill N, et al. [National Oesophago-Gastric Cancer Audit 2020. London. 2020 (accessed 28 November 2022)].
- 9. Voeten DM, Busweiler LAD, van der Werf LR, Wijnhoven BPL, Verhoeven RHA, van Sandick JWet al. Outcomes of esophagogastric cancer surgery during eight years of surgical auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann Surg 2021;274:866–873 [DOI] [PubMed] [Google Scholar]
- 10. Almoudaris AM, Burns EM, Bottle A, Aylin P, Darzi A, Faiz O. A colorectal perspective on voluntary submission of outcome data to clinical registries. Br J Surg 2011;98:132–139 [DOI] [PubMed] [Google Scholar]
- 11. Vallance AE, Fearnhead NS, Kuryba A, Hill J, Maxwell-Armstrong C, Braun Met al. Effect of public reporting of surgeons’ outcomes on patient selection, “gaming,” and mortality in colorectal cancer surgery in England: population based cohort study. BMJ 2018;361:1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeevan R, Cromwell DA, Browne JP, Caddy CM, Pereira J, Sheppard Cet al. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. J Plast Reconstr Aesthetic Surg 2014;67:1333–1344 [DOI] [PubMed] [Google Scholar]
- 13. Varagunam M, Hardwick R, Riley S, Chadwick G, Cromwell DA, Groene O. Changes in volume, clinical practice and outcome after reorganisation of oesophago-gastric cancer care in England: a longitudinal observational study. Eur J Surg Oncol 2018;44:524–531 [DOI] [PubMed] [Google Scholar]
- 14. Healthcare Quality Improvement Partnership . National OesophagoGastric Cancer Audit Short Report: Comparison of Patients Captured by NOGCA and the National Cancer Registration and Analysis Service.https://www.nogca.org.uk/content/uploads/2020/06/NOGCA_Short-report-2020_Final_June2020.pdf (accessed 28 November 2022)
- 15. Welsh Index of Multiple Deprivation (full Index update with ranks): 2019. https://gov.wales/welsh-index-multiple-deprivation-full-index-update-ranks-2019 (accessed 28 November 2022)
- 16. English Indices of Deprivation 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (accessed 28 November 2022)
- 17. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen Let al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al-Batran SE, Goetze TO, Mueller DW, Vogel A, Winkler M, Lorenzen Set al. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction—a phase III trial. BMC Cancer 2017;17:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Jan B Lanschot J, Hulscher JBF, Buskens CJ, Tilanus HW, Ten Kate FJW, Obertop H. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–1578 [DOI] [PubMed] [Google Scholar]
- 20. Patel J, McNair A. Identification of upper gastrointestinal malignancy in patients with uncomplicated dyspepsia referred under the two-week-wait cancer pathway: a single-centre, 10-year experience. Eur J Gastroenterol Hepatol 2020;32:22–25 [DOI] [PubMed] [Google Scholar]
- 21. Spahos T, Hindmarsh A, Cameron E, Tighe MR, Igali L, Pearson Det al. Endoscopy waiting times and impact of the two week wait scheme on diagnosis and outcome of upper gastrointestinal cancer. Postgrad Med J 2005;81:728–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fitzgerald RC, di Pietro M, O’Donovan M, Maroni R, Muldrew B, Debiram-Beecham Iet al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet 2020;396:333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Markar SR, Wiggins T, Antonowicz S, Chin ST, Romano A, Nikolic Ket al. Assessment of a noninvasive exhaled breath test for the diagnosis of oesophagogastric cancer. JAMA Oncol 2018;4:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evans N III, Grenda T, Alvarez NH, Okusanya OT. Narrative review of socioeconomic and racial disparities in the treatment of early stage lung cancer. J Thorac Dis 2021;13:3758–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang SE, Kendall BJ, Hodge AM, Dixon-Suen SC, Dashti SG, Makalic Eet al. Demographic and lifestyle risk factors for gastroesophageal reflux disease and Barrett’s esophagus in Australia. Dis Esophagus 2022;35:doab058. [DOI] [PubMed] [Google Scholar]
- 26. Pucher PH, Green M, Bateman AC, Underwood TJ, Maynard N, Allum WHet al. Variation in histopathological assessment and association with surgical quality indicators following oesophagectomy. Br J Surg 2021;108:74–79 [DOI] [PubMed] [Google Scholar]
- 27. Pucher PH, Allum WH, Bateman AC, Green M, Maynard N, Novelli Met al. Consensus recommendations for the standardized histopathological evaluation and reporting after radical oesophago-gastrectomy (HERO consensus). Dis Esophagus 2021;34:doab033. [DOI] [PubMed] [Google Scholar]
- 28. Jeremiasen M, Linder G, Hedberg J, Lundell L, Björ O, Lindblad Met al. Improvements in esophageal and gastric cancer care in Sweden-population-based results 2007-2016 from a national quality register. Dis Esophagus 2020;33:doz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kjaer DW, Larsson H, Svendsen LB, Jensen LS. Changes in treatment and outcome of oesophageal cancer in Denmark between 2004 and 2013. Br J Surg 2017;104:1338–1345 [DOI] [PubMed] [Google Scholar]
- 30.Cromwell DA, Wahedally H, Park MH, Maynard N, Crosby T, Trudgill N, et al. [National Oesophago-Gastric Cancer Audit 2019. London; 2019 (accessed 28 November 2022)].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Researchers may apply to the Healthcare Quality Improvement Partnership to access National Oesophago-Gastric Cancer Audit data for a research study. The approvals process for such requests is outlined here: https://www.hqip.org.uk/national-programmes/accessing-ncapop-data/
References
- 1. van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCAet al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg 2019;269:621–630 [DOI] [PubMed] [Google Scholar]
- 2. Biere SSAY, Van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JRet al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887–1892 [DOI] [PubMed] [Google Scholar]
- 3. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper Set al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948–1957 [DOI] [PubMed] [Google Scholar]
- 4. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPLet al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–1098 [DOI] [PubMed] [Google Scholar]
- 5. Warps AK, Saraste D, Westerterp M, Detering R, Sjövall A, Martling Aet al. National differences in implementation of minimally invasive surgery for colorectal cancer and the influence on short-term outcomes. Surg Endosc 2022;36:5986–6001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling Get al. Benchmarking complications associated with esophagectomy. Ann Surg 2019;269:291–298 [DOI] [PubMed] [Google Scholar]
- 7. Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold Det al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979–989 [DOI] [PubMed] [Google Scholar]
- 8.Park MH, Wahedally H, Cromwell DA, Maynard N, Crosby T, Trudgill N, et al. [National Oesophago-Gastric Cancer Audit 2020. London. 2020 (accessed 28 November 2022)].
- 9. Voeten DM, Busweiler LAD, van der Werf LR, Wijnhoven BPL, Verhoeven RHA, van Sandick JWet al. Outcomes of esophagogastric cancer surgery during eight years of surgical auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann Surg 2021;274:866–873 [DOI] [PubMed] [Google Scholar]
- 10. Almoudaris AM, Burns EM, Bottle A, Aylin P, Darzi A, Faiz O. A colorectal perspective on voluntary submission of outcome data to clinical registries. Br J Surg 2011;98:132–139 [DOI] [PubMed] [Google Scholar]
- 11. Vallance AE, Fearnhead NS, Kuryba A, Hill J, Maxwell-Armstrong C, Braun Met al. Effect of public reporting of surgeons’ outcomes on patient selection, “gaming,” and mortality in colorectal cancer surgery in England: population based cohort study. BMJ 2018;361:1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeevan R, Cromwell DA, Browne JP, Caddy CM, Pereira J, Sheppard Cet al. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. J Plast Reconstr Aesthetic Surg 2014;67:1333–1344 [DOI] [PubMed] [Google Scholar]
- 13. Varagunam M, Hardwick R, Riley S, Chadwick G, Cromwell DA, Groene O. Changes in volume, clinical practice and outcome after reorganisation of oesophago-gastric cancer care in England: a longitudinal observational study. Eur J Surg Oncol 2018;44:524–531 [DOI] [PubMed] [Google Scholar]
- 14. Healthcare Quality Improvement Partnership . National OesophagoGastric Cancer Audit Short Report: Comparison of Patients Captured by NOGCA and the National Cancer Registration and Analysis Service.https://www.nogca.org.uk/content/uploads/2020/06/NOGCA_Short-report-2020_Final_June2020.pdf (accessed 28 November 2022)
- 15. Welsh Index of Multiple Deprivation (full Index update with ranks): 2019. https://gov.wales/welsh-index-multiple-deprivation-full-index-update-ranks-2019 (accessed 28 November 2022)
- 16. English Indices of Deprivation 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019 (accessed 28 November 2022)
- 17. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen Let al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al-Batran SE, Goetze TO, Mueller DW, Vogel A, Winkler M, Lorenzen Set al. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction—a phase III trial. BMC Cancer 2017;17:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Jan B Lanschot J, Hulscher JBF, Buskens CJ, Tilanus HW, Ten Kate FJW, Obertop H. Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–1578 [DOI] [PubMed] [Google Scholar]
- 20. Patel J, McNair A. Identification of upper gastrointestinal malignancy in patients with uncomplicated dyspepsia referred under the two-week-wait cancer pathway: a single-centre, 10-year experience. Eur J Gastroenterol Hepatol 2020;32:22–25 [DOI] [PubMed] [Google Scholar]
- 21. Spahos T, Hindmarsh A, Cameron E, Tighe MR, Igali L, Pearson Det al. Endoscopy waiting times and impact of the two week wait scheme on diagnosis and outcome of upper gastrointestinal cancer. Postgrad Med J 2005;81:728–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fitzgerald RC, di Pietro M, O’Donovan M, Maroni R, Muldrew B, Debiram-Beecham Iet al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet 2020;396:333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Markar SR, Wiggins T, Antonowicz S, Chin ST, Romano A, Nikolic Ket al. Assessment of a noninvasive exhaled breath test for the diagnosis of oesophagogastric cancer. JAMA Oncol 2018;4:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Evans N III, Grenda T, Alvarez NH, Okusanya OT. Narrative review of socioeconomic and racial disparities in the treatment of early stage lung cancer. J Thorac Dis 2021;13:3758–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang SE, Kendall BJ, Hodge AM, Dixon-Suen SC, Dashti SG, Makalic Eet al. Demographic and lifestyle risk factors for gastroesophageal reflux disease and Barrett’s esophagus in Australia. Dis Esophagus 2022;35:doab058. [DOI] [PubMed] [Google Scholar]
- 26. Pucher PH, Green M, Bateman AC, Underwood TJ, Maynard N, Allum WHet al. Variation in histopathological assessment and association with surgical quality indicators following oesophagectomy. Br J Surg 2021;108:74–79 [DOI] [PubMed] [Google Scholar]
- 27. Pucher PH, Allum WH, Bateman AC, Green M, Maynard N, Novelli Met al. Consensus recommendations for the standardized histopathological evaluation and reporting after radical oesophago-gastrectomy (HERO consensus). Dis Esophagus 2021;34:doab033. [DOI] [PubMed] [Google Scholar]
- 28. Jeremiasen M, Linder G, Hedberg J, Lundell L, Björ O, Lindblad Met al. Improvements in esophageal and gastric cancer care in Sweden-population-based results 2007-2016 from a national quality register. Dis Esophagus 2020;33:doz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kjaer DW, Larsson H, Svendsen LB, Jensen LS. Changes in treatment and outcome of oesophageal cancer in Denmark between 2004 and 2013. Br J Surg 2017;104:1338–1345 [DOI] [PubMed] [Google Scholar]
- 30.Cromwell DA, Wahedally H, Park MH, Maynard N, Crosby T, Trudgill N, et al. [National Oesophago-Gastric Cancer Audit 2019. London; 2019 (accessed 28 November 2022)].