Abstract
Graft-versus-host disease (GvHD) is a common, morbid complication after intestinal transplantation (ITx) with poorly-understood pathophysiology. Resident memory T cells (TRM) are a recently described CD69+ memory T cell subset localizing to peripheral tissue. We observed that T effector memory cells (TEM) in the blood increase during GvHD and hypothesized that they derive from donor graft CD69+TRM migrating into host blood and tissue. To probe this hypothesis, graft and blood lymphocytes from 10 ITx patients with overt GvHD and 34 without were longitudinally analyzed using flow cytometry. As hypothesized, CD4+ and CD8+CD69+TRM were significantly increased in blood and grafts of GvHD patients, alongside higher cytokine and activation marker expression. The majority of CD69+TRM were donor derived as determined by multiplex immunostaining. Notably, CD8/PD-1 was significantly elevated in blood prior to transplantation in patients who later had GvHD, and percentages of HLA-DR, CD57, PD-1, and naïve T cells differed significantly between GvHD patients who died vs. those who survived. Overall, we demonstrate that (1) there were significant increases in TEM, at the time of GvHD, possibly of donor derivation; (2) donor TRM in the graft are a possible source; and (3) potential biomarkers for the development and prognosis of GvHD exist.
1. Introduction
Intestinal transplantation (ITx) is the only therapeutic option for patients with intestinal failure once parenteral nutrition is no longer an option.(1) Unfortunately, two profound and related drawbacks limit its use. First, high rates of rejection necessitate increased levels of immunosuppression. Second, this predisposes patients to infections, malignancy, and graft-versus-host disease (GvHD).(2, 3) Better understanding of these limitations, and ability to ameliorate them, could make ITx a more practical therapeutic option.
GvHD, in which host-reactive donor cells attack the recipient, classically causes injury to the skin, liver, and GI tract.(4–6) It is difficult to treat and often results in poor outcomes.(7, 8) While it has been extensively studied in the context of bone marrow transplantation (BMT), little is known about its immunology after solid organ transplantation, particularly after ITx.
T resident memory cells (TRM) are a recently described subset of tissue resident T cells that have developed memory phenotype, defined as T effector memory cells (TEM) (CCR7−, CD27+/−, CD28+/−, CD45RA−) or TEMRA (CCR7−, CD27−, CD28−, CD45RA+), through antigen-experience and that express receptors and integrins that specifically home to peripheral tissues. The most reliable of these markers is CD69, which is expressed by most TRM, whereas CD49a and CD103 are more specific for TRM homing to epithelial tissue.(9–11)
Since it has been speculated that TRM can downregulate retention markers such as S1PR1 and upregulate CCR7 and CD62L markers promoting both tissue egress and recirculation to lymphatic tissue, respectively,(9, 10, 12) we hypothesized that donor derived TRM cells transplanted with the graft enter the recipient circulation after ITx and play a potential role in the underlying pathophysiology of GvHD.
There has also been recent significant interest in the role of programmed death-1 (PD-1) in the context of GvHD as well as graft rejection and cancer. PD-1 is an inhibitory marker expressed by activated lymphocytes to regulate the immune response. When interacting with its ligands, PD-L1 and PD-L2, expressed by antigen presenting cells, cells in immune privileged sites, and other activated T cells, this serves to maintain peripheral self-tolerance and downregulate exuberant immune responses.(13, 14) In rejection, its absence on antigen-experienced cells is associated with treatment resistance.(15, 16) In certain types of cancer, one method by which tumor cells evade the host immune system is by expressing PD-L1 and PD-L2 to inhibit tumor-reactive host T cells, and, conversely, blocking the PD-1/PD-L1 pathway activates the anti-tumor response.(13)
In GvHD after bone marrow transplantation, increased PD-1 expression within target host tissue at the time of GvHD is interpreted as increased graft T cells that have shifted from naïve to effector phenotype.(17) Blocking the PD-1/PD-L1 interaction has been shown to exacerbate GvHD, just as increased expression of PD-L1 on recipient cells has been shown to inhibit it.(14, 18) Conversely, increased expression of PD-L1 on donor cells correlates with increased incidence of GvHD, implying that the GvH-HvG axis can be altered by downregulating alloreactive host cells that express PD-1.(18)
It has also been shown after liver transplantation that donor cells expressing high levels of PD-1 are increased at the time of GvHD, and blocking the PD-1/PD-L1 axis increased the alloreactivity of the host-reactive cells.(19) Thus it is clear that PD-1 plays an important role in GvHD, but its importance has not been well-delineated, nor has PD-1 expression been quantified longitudinally, in the context of GvHD after solid organ transplantation. We sought to characterize the role of PD-1 in GvHD, and since it is known to be expressed by TRM,(20, 21) we hypothesized that PD-1 would be elevated in the setting of GvHD.
To study this, we utilized longitudinal blood and graft analysis to characterize the changes in lymphocyte phenotype after ITx. As hypothesized, we found potential relationships between TRM in both graft and blood, as well as PD-1 and other markers of antigen experience, activation, and maturity in the pathophysiology of GvHD. Our major findings were (1) that there were significant increases in TEM, at the time of GvHD, possibly of donor derivation; (2) that donor TRM in the graft are a possible source, indicating the potential role these cells may play in the pathogenesis of GvHD; and (3) the potential existence of several biomarkers, including PD-1, for the development and prognosis of GvHD.
2. Materials and Methods
2.1. Study populations
This study received IRB approval (IRB studies #2004–008 and #2017–0365). Patients studied received transplants between 2004 and 2018. Inclusion criteria for control patients were absence of clinical or pathological signs of rejection or GvHD. GvHD patients were diagnosed based on clinical and pathological signs.
Surgical technique, immunosuppression, and medical management were standardized.(22–24) Patients with GvHD received initial treatment with methylprednisolone (1g x 2 then tapered) and thymoglobulin (1.5mg/kg/day) for 10–14 doses if severe. If refractory, photopheresis (2 patients) and ruxolitinib (1 patient) were used.
2.2. Blood and tissue sample collection and flow cytometric analysis
Blood samples were obtained in ethylenediaminetetraacetic acid tubes. Post-transplantation tissue samples were obtained from routine endoscopic biopsies. Peripheral blood and biopsy samples from graft and/or native intestines were taken pre-transplant, day 0, weeks 1, 2, and 4, then months 2, 3, 6, 9, and 12, and/or when clinically indicated. Blood leukocytes were profiled via the staining panel described in Supporting Materials and Methods and via the DuraClone® panel for T cell subsets (Beckman-Coulter, Indianapolis, IN) following the manufacturer’s protocol.(23, 25) Isolation of lamina propria leukocytes from bowel biopsy samples and flow cytometric analysis were performed as described in Supporting Materials and Methods.
2.3. Histology
Tissue samples were fixed in 4% paraformaldehyde, were paraffin embedded, and sectioned at 4μm per our pathology department’s protocol. Presence or absence of GvHD as well as grade of the disease were determined by hematoxylin-eosin staining and clinical signs. When histology was not definitive, GvHD was diagnosed by clinical criteria (e.g., rash, fever, etc.) as is standard.
2.4. Multiplex immunostaining
Sample preparation, tissue staining, imaging and analysis for multiplex immunostaining were performed as described in Supporting Materials and Methods.
2.5. Chimerism testing
Chimerism testing was performed by BloodCenter of Wisconsin, Inc. (Milwaukee, WI) using amplification of genetic loci containing tandemly repeated sequences. Specifically, post-transplant blood samples were sorted for CD3+, CD33+, CD19+, and CD56+ cell subsets, and each was tested at eight STR/VNTR loci (Tho1, SE33, FGA, PentaE, TPOX, D18S51, PentaD, and D1S80). The sensitivity of the assays was 2–5%.
2.6. Statistical analysis
Demographic features were compared using either chi-square or Fisher’s exact test where appropriate. Distribution of data in each group was checked for normality by Shapiro-Wilk test. Unpaired/paired t test was used for normally distributed groups; otherwise the non-parametric Mann-Whitney/Wilcoxon test was used. For more than two independent groups, the Kruskal-Wallis test was performed followed with Dunn’s multiple comparisons. All analyses were done in Prism 8 (Graphpad) software; the p-value of 0.05 was used as a threshold for significance of variance.
3. Results
ITx patients with GvHD have higher rates of mortality
From 2003 to 2018, we performed 268 bowel transplants. 153 (57.1%) were isolated intestinal grafts, 59 (22.0%) were liver/pancreas/intestine, 7 (2.6%) were modified multivisceral, and 49 (18.3%) were full multivisceral grafts (MvTx). Twenty-one (7.8%) of these patients were subsequently diagnosed with GvHD with a mortality rate of 57.1%, of whom ten have had their immune responses extensively assessed longitudinally.
34 intestinal and multivisceral recipients, who had no prior GvHD or rejection and whose immune responses had been characterized at similar time points as the GvHD cohort were used as controls.
Table 1 compares GvHD and control group characteristics. Relative to control patients, there were significantly higher percentages of GvHD patients who received MvTx grafts (p<0.001) and were transplanted for malignancy (p=0.007). More GvHD patients died (p<0.001) with substantially fewer alive at 1-year post-transplant (p=0.017). The re-transplant rate in the GvHD group was 2/10 (20%) vs. 1/34 (2.9%) in the control group (p=0.06).
Table 1:
Patient characteristics and demographic data
| GvHD (n, %) 10 patients | Control (n, %) 34 patients | P-value | ||
|---|---|---|---|---|
| Induction | Thymoglobulin | 5, 50% | 8, 23.5% | 0.13 |
| Simulect | 5, 50% | 26, 76.5% | ||
| Graft type | SB+C | 3, 30% | 19, 55.9% | 0.28 |
| L/SB/P | 0, 0% | 8, 23.5% | 0.16 | |
| MvTx | 7, 70% | 5, 14.7% | 0.002 | |
| MMvTx | 0, 0% | 2, 5.9% | 0.99 | |
| Age at transplantation | Mean (range) | 32 (0.17–53) | 21 (0.75–56) | 0.22 |
| Adults | 7, 70% | 17, 50% | 0.3 | |
| Pediatric | 3, 30% | 17, 50% | ||
| Gender | Male | 8, 80% | 20, 58.8% | 0.28 |
| Female | 2, 20% | 14, 41.2% | ||
| Race | Caucasian | 9, 90% | 24, 70.6% | 0.4 |
| African American | 1, 10% | 7, 20.6% | 0.66 | |
| Hispanic | 0, 0% | 3, 8.8% | 0.99 | |
| Indication | Short gut | 5, 50% | 20, 58.8% | 0.72 |
| Enteropathy | 2, 20% | 12, 35.3% | 0.46 | |
| Malignancy | 2, 20% | 0, 0% | 0.048 | |
| Other | 1, 10% | 2, 5.9% | 0.55 | |
| Alive | At 1 year | 7, 70% | 33, 97.1% | 0.032 |
| More than 1 year | 5, 50% | 32, 94.1% | 0.004 | |
| Retransplant | 2, 20% | 1, 2.9% | 0.125 |
C, colon; L/SB/P, liver/small bowel/pancreas; MMvTx, modified multivisceral transplant (multivisceral without liver); MvTx, multivisceral transplant; SB, small bowel.
Figure 1 shows representative images of GvHD in various tissues compared to controls, as well as the gross appearance of characteristic skin and native colon changes. It also summarizes the specific effects of GvHD experienced by each of the 10 patients. Where possible, the stage of each finding is recorded as well as the percentage of donor chimerism in the blood, days to first GvHD diagnosis, and the overall GvHD grade. Of the 10 patients, 8 had skin findings, the most severe of which correlated with higher GvHD grade and increased mortality. Mortality was also higher in patients with multiple manifestations or more severe manifestations (higher grade) of GvHD.
Figure 1. Manifestations of GvHD in our patient cohort.
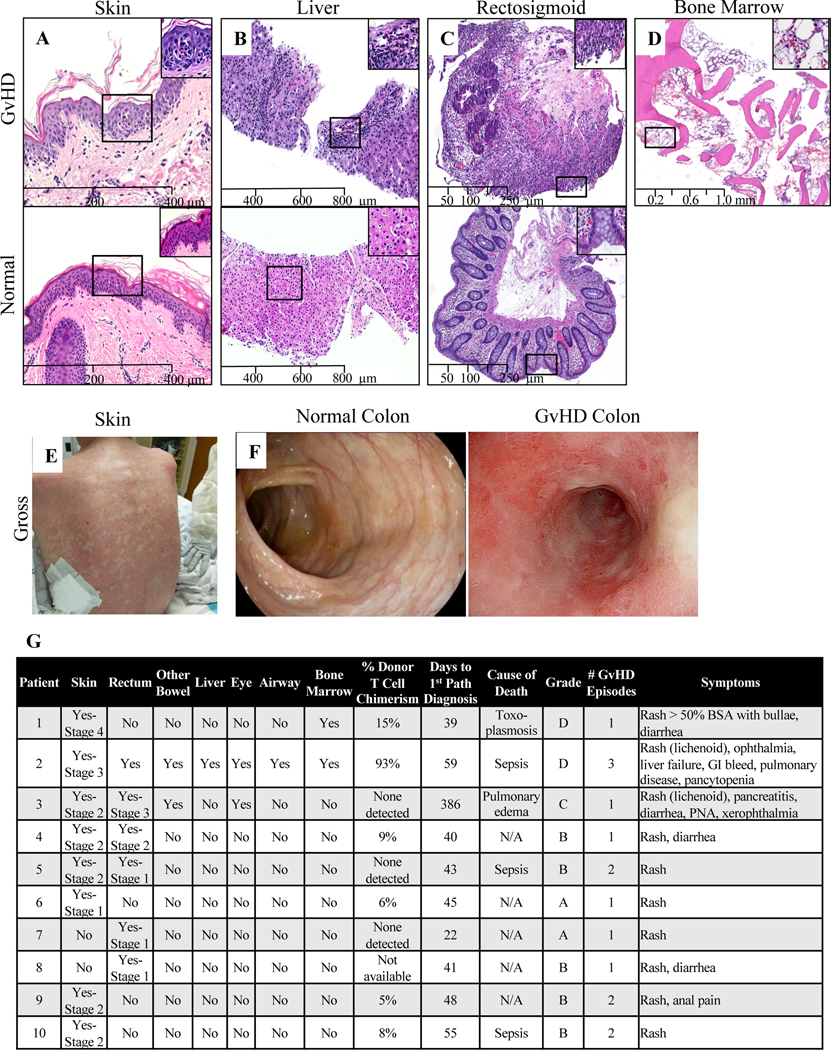
For all micrographs, images were captured from scanned slides using nanozoomer 2.0RS digital slight scanner (Hamamatsu, Iawata city, Japan) at original magnifications of 100 X for A-C and 25X for D. These images were viewed and analyzed using NDP.view2 software (Hamamatsu, Iawata city, Japan). (A) Representative microscopic findings in GvHD vs. normal skin. Skin punch biopsy shows vacuolar degeneration with necrotic keratinocytes, features consistent with grade 2 GVHD (top) in contrast to an unremarkable skin biopsy (bottom). (B) Representative microscopic findings in GvHD vs. normal liver. Liver biopsy shows increased mixed inflammatory infiltrate including eosinophils in the portal triad (top) in contrast to an unremarkable liver biopsy. (C) Representative microscopic findings in GvHD vs. normal colon. Rectosigmoid biopsy shows mucosal ulceration with marked lymphocytic infiltrates, crypt epithelial damage and apoptosis, consistent with grade 3 GvHD (top) in contrast to an unremarkable rectosigmoid biopsy (bottom). (D) Representative microscopic findings in GvHD bone marrow. Markedly aplastic bone marrow for the stated age. This histologic diagnosis of GvHD was supported by chimerism studies in the patient’s peripheral blood showing increased donor’s cells in the circulation. (E) Gross appearance of characteristic erythematous macular and popular GvHD rash. (F) Gross appearance of characteristic erythematous, inflamed appearance of native colon in GvHD compared to normal colon. (G) Summary of the above manifestations of GvHD for each affected patient.
Chimerism in blood associates with severity of GvHD
T cell chimerism in peripheral blood at time of GvHD is shown in Figure 1. Data are available for 9 of the 10 patients, and chimerism was detected in 6 of them, with a test sensitivity of 2–5%. Greater chimerism correlated with more severe GvHD to some extent. Of the 6 patients who developed T cell chimerism detectable in the blood, the 3 who died had the first, second, and fourth highest chimerism percentages. Moreover, the patient with by far the highest chimerism percentage (93%) had the most severe and chronic case of GvHD with lichenoid skin changes and involvement of host rectum, stomach, duodenum, liver, eye, lung, and bone marrow.
Patients with GvHD had increased percentages of circulating TEM, specifically TRM, and higher expression of HLA-DR, CD57, and PD-1 by T cells
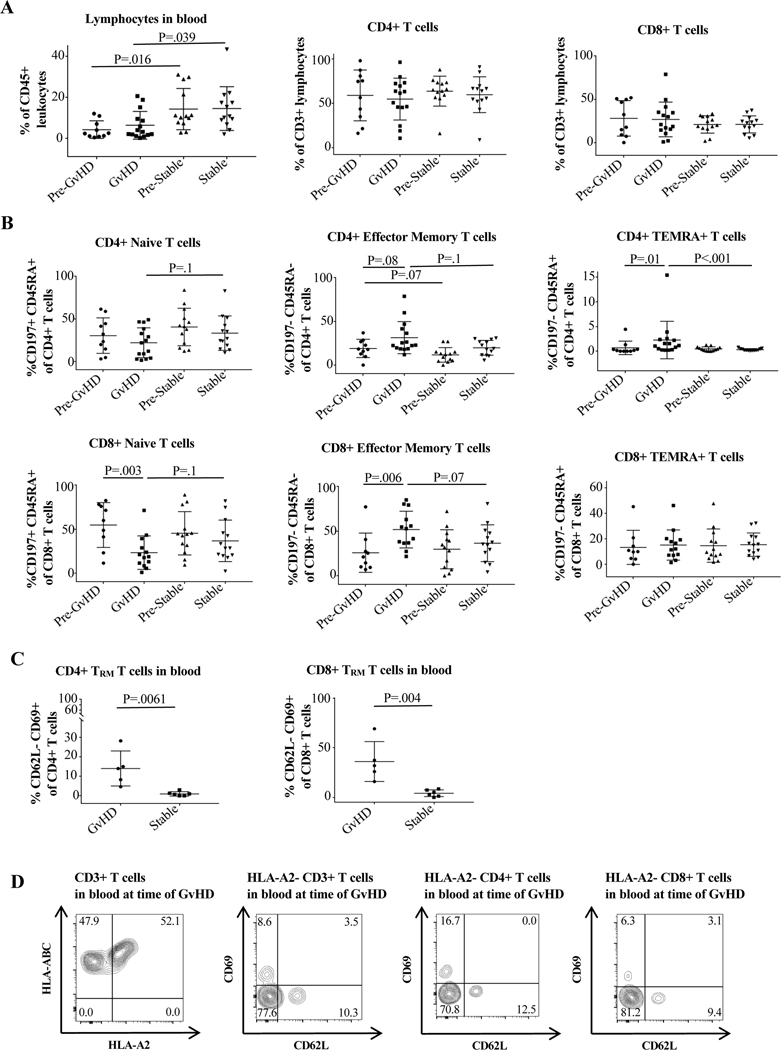
Overall lymphocyte population in the blood was decreased in GvHD vs. control patients, even prior to GvHD onset (Figure 2A). For CD8+, the difference in TEM in patients with GvHD compared to their pre-GvHD state was 51.7% vs. 25.7% (p=0.006) and for CD4+ was 31.3% vs. 18.9% (p=0.08). Compared to control patients, the difference in TEM percentage in the blood of GvHD patients approached significance: 31.3% vs. 19.7% (p=0.1) for CD4+ and 51.7% vs. 36.5% (0.07) for CD8+. The percentage of naïve CD8+ cells also significantly decreased at the time of GvHD (54.5% vs. 23.2%, p=0.003) (Figure 2B).
Figure 2. Cell surface marker expression in peripheral blood of GvHD vs. control patients.
Cells were stained and analyzed by flow cytometry as described in Materials and Methods and Supporting Materials and Methods. The GvHD timepoint was defined as the sample taken closest to the time of GvHD clinical manifestation but not after the initiation of treatment. Pre-GvHD was the immediately preceding timepoint. The stable time point was defined as the highest percentage of CD4+ and CD8+ effector memory T cells (TEM) during the post-transplant course in control patients, and the pre-stable timepoint as the timepoint directly preceding this. (A) Differences in overall lymphocyte, CD4+, and CD8+ expression at the different timepoints, with lymphocyte percentage differing significantly between the GvHD and control groups. (B) Differences in CD4+ and CD8+ naïve, TEM, and TEMRA percentages between the groups. CD8+ naïve cell percentage decreased at the time of GvHD, while CD4+TEMRA and CD8+TEM increased. The average percentage of CD4+ and CD8+TEM was large between the groups but only approached statistical significance (p=0.1 for CD4 and p=0.07 for CD8). (C) Statistically significant difference in CD4+ and CD8+CD62L-CD69+TRM in peripheral blood of GvHD compared to control patients. Unpaired/paired t test was used for normally distributed groups; otherwise the non-parametric Mann Whitney/Wilcoxon test was used. For more than two independent groups, the Kruskal-Wallis test was performed followed with Dunn’s multiple comparisons. (D) Flow cytometry plots showing presence of donor derived HLA-A2-CD3+T cells as well as HLA-A2-CD62L-CD69+CD4+ and CD8+TRM in peripheral blood of an HLA-A2+ recipient of an HLA-A2- graft at the time of active GvHD.
Both CD4+ and CD8+CD69+TRM also significantly increased in the blood at the time of GvHD. Compared to control patients, there was a 24-fold difference in CD4+TRM percentage (13.9% vs. 0.58%, p=0.006) and a 9.3-fold difference in CD8+TRM percentage (34.6% vs. 3.7%, p=0.004) (Figure 2C). Representative flow cytometry plots for Figure 2 are shown in Supplemental Figure 1. Further analysis of available blood samples obtained during an active GvHD episode from an HLA-A2+ ITx patient who received an HLA-A2- allograft demonstrated that 47.9% CD3+T cells in peripheral blood were HLA-A2- and of donor origin (Figure 2D). Notably, 16.7% of donor derived CD4+HLA-A2-T cells and 6.25% of CD8+HLA-A2-T cells had a CD62L-CD69+TRM phenotype (Figure 2D).
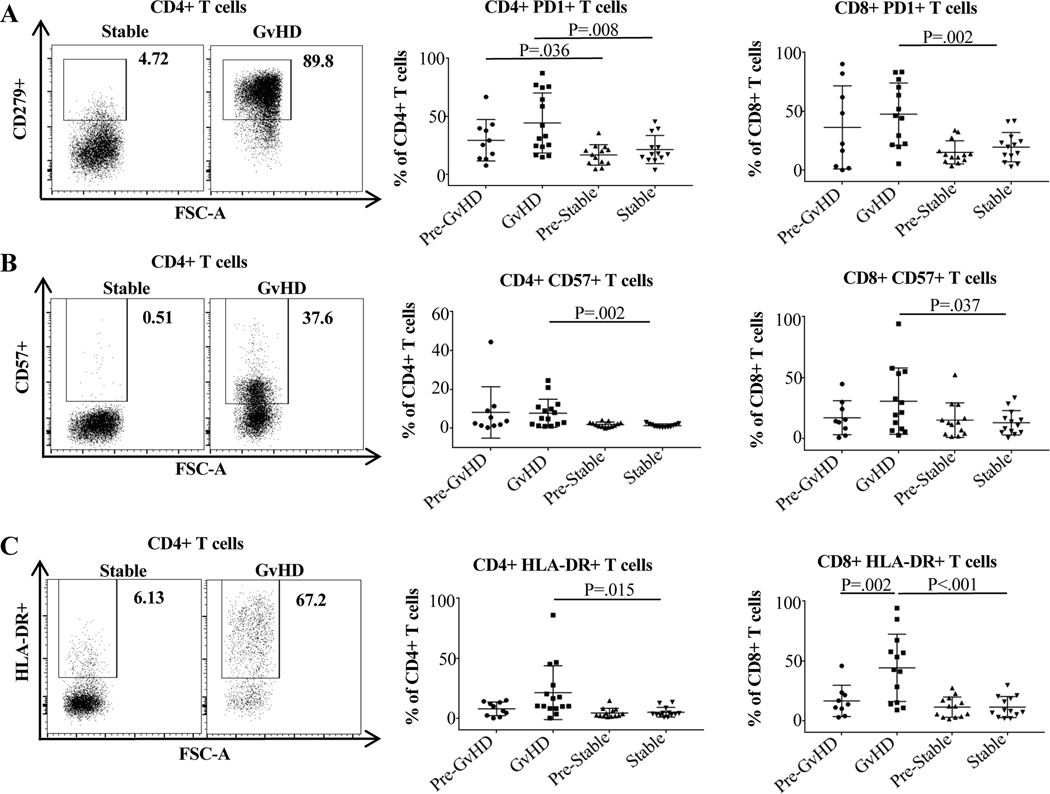
Corresponding to the increased percentage of TEM and CD69+TRM at the time of GvHD, we also found increases in T cells expressing activation, memory, antigen-experience, and exhaustion markers. CD4+ and CD8+ expression of PD-1 was significantly higher in the blood of GvHD vs. control patients. The difference between mean PD-1 expression by CD4+ cells was 44.3% vs. 21.3% (p=0.008) and by CD8+ cells was 47.5% vs. 19.4% (p=0.002). Moreover, there was significantly higher levels of expression of PD-1 on CD4+ cells even prior to GvHD episodes between those who later experienced GvHD and those who did not (29.3% vs. 16.6%, p=0.036, Figure 3A). Expression of HLA-DR and CD57 also differed significantly. In patients with GvHD vs. controls, percentage of HLA-DR expression in CD4+ and CD8+ cells was 21.2% vs. 4.9% (p=0.015) and 44.2% vs. 11.3% (p<0.001) (Figure 3B), and the percentage of CD8+HLA-DR+ expression at the time of GvHD vs. the last normal timepoint increased from 7.7% to 21.2% (p=0.002) (Figure 3C). Similarly, in patients with GvHD vs. controls, percentage of CD57 expression in CD4+ and CD8+ cells was 7.7% vs. 1.4% (p=0.002) and 30.5% vs. 12.8% (p=0.037).
Figure 3. Expression of the maturity, exhaustion, antigen-experience, and activation markers PD-1, CD57, and HLA-DR on CD4 and CD8 cells in the peripheral blood of GvHD vs. control patients after transplantation.
Cell staining and group definition is described in Figure 2. All three markers were significantly elevated on CD4+ and CD8+ cells in patients with GvHD compared to control patients. Representative flow cytometry plots are displayed. Unpaired/paired t test was used for normally distributed groups; otherwise the non-parametric Mann-Whitney/Wilcoxon test was used. For more than two independent groups, the Kruskal-Wallis test was performed followed with Dunn’s multiple comparisons.
Grafts of patients with GvHD have increased percentage of CD69+TRM
Because of these finding in the blood, particularly those involving TRM, we investigated whether similar changes in memory cells would also be seen in the grafts of patients with and without GvHD. Moreover, we investigated whether the memory cells within the graft could potentially be associated with the increased memory cells in the blood at time of GvHD.
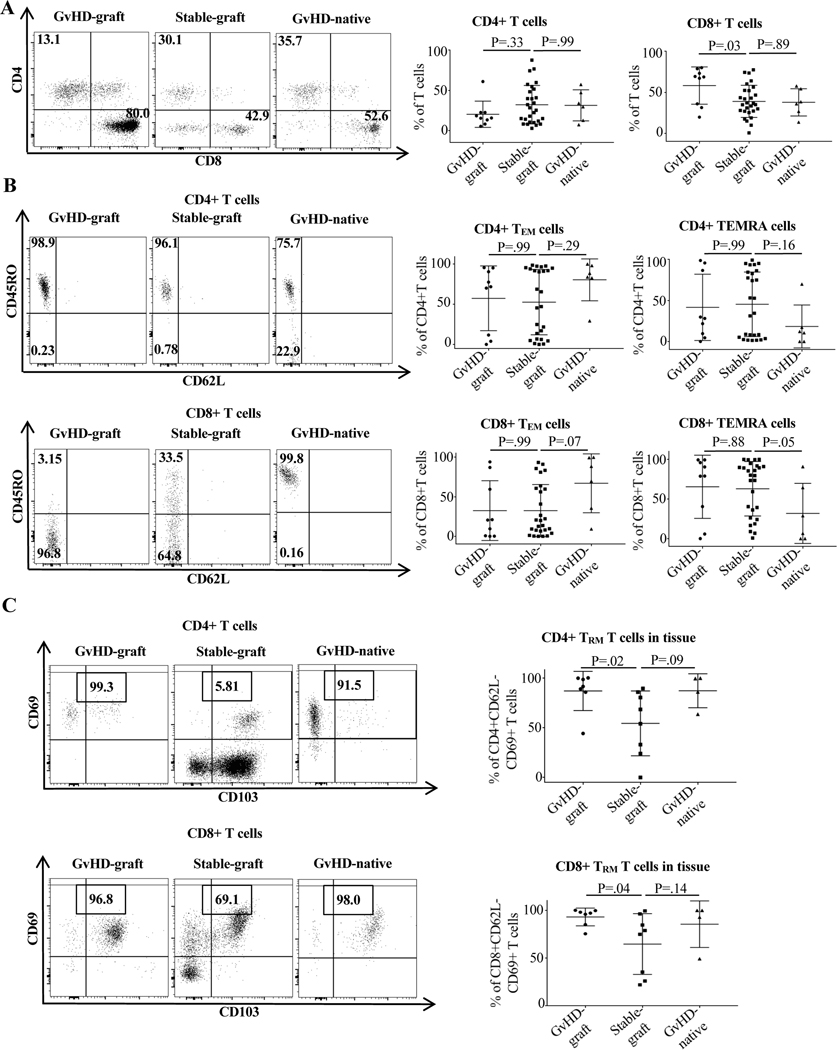
The percentages of CD8+ and CD69+TRM increased significantly in grafts at the time of GvHD compared to grafts in control patients (Figure 4). For CD4+TRM, there was a 32.5% difference between grafts in GvHD and control patients (86.9% vs. 54.4%, p=0.02), and the difference for CD8+TRM was 28.4% (93.0% vs. 64.6%, p=.04) (Figure 4C). These data indicate that, within the graft, the percentage of memory T cells that may have the capacity to potentially home to host tissue in the periphery increases at the time of GvHD.
Figure 4. T cell phenotype in graft and native intestine in GvHD and control patients.
All samples were collected and analyzed as described in Materials and Methods and Supporting Materials and Methods. GvHD graft samples were taken from biopsies at the time of GvHD episodes. Control graft samples were taken at protocol biopsy timepoints in patients who never experienced GvHD or rejection. GvHD native bowel samples were taken from biopsies at the time of GvHD episodes. (A) Overall differences in CD4 and CD8 expression showing increased CD8 expression in graft bowel at the time of GvHD. (B) Comparison of CD4+ and CD8+TEM and TEMRA expression showing numerically large significantly decreased+ CD8 TEMRA percentage and nearly significantly increased CD8+TEM percentage in native gut (the tissue target in in GvHD) at the time of GvHD. (C) Comparison of TRM expression between groups showing numerically large and significantly increased percentage of CD4+ and CD8+TRM in graft bowel and numerically large and nearly significant increased percentage of CD4+ and CD8+TRM in native bowel at the time of GvHD. Non-parametric Mann-Whitney test was used. For more than two independent groups, the Kruskal-Wallis test was performed followed with Dunn’s multiple comparisons.
Notably, it is specifically the percentage of CD69+TRM that is elevated in the grafts of patients with GvHD and not the percentage of TEM or TEMRA (Figure 4B). Since TRM make up a subset of TEM and TEMRA, both being CD62L-negative, the difference appears to lie in the expression of the tissue-resident markers CD69 and CD103 (Figure 4).
T cell phenotypes are similar in the host and graft bowel at the time of GvHD when compared to graft bowel in control patients
Analyses of T cell subsets in host bowel and graft bowel with and without GvHD demonstrates that the TRM immune phenotype in native host bowel in patients with GvHD was similar to that in graft bowel at the same time. These differed from the TRM phenotypes of graft bowel from control patients (Figure 4C).
CD69+TRM in both the host and graft bowel of GvHD patients with host-donor HLA-A2 mismatches are of donor origin
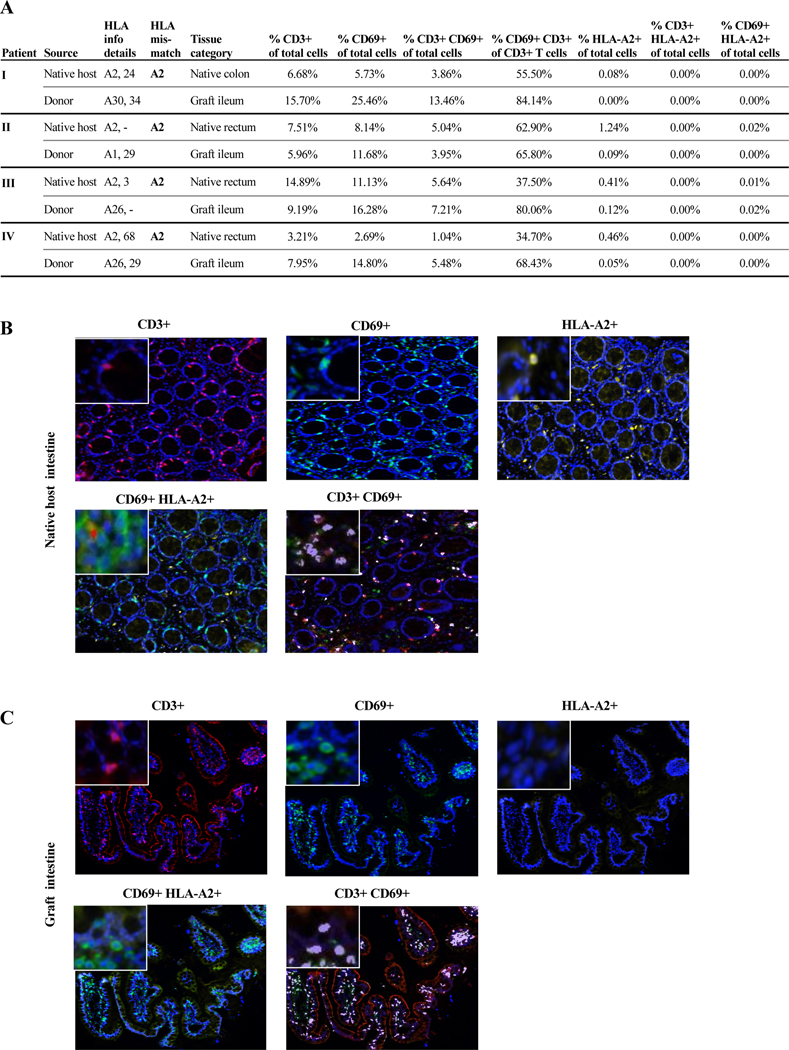
Further analyses utilizing host-donor mismatches for the HLA-haplotype-A2 were performed to determine whether CD69+TRM in host and graft bowel were, as speculated, of donor origin. Specifically, we studied 4 GvHD recipients with HLA-A2+ haplotypes, who received allografts from HLA-A2- donors (Figure 5A), from which sufficient tissue samples from both host and graft bowel biopsies obtained at the time of GvHD episodes were available for analysis. Multiplex immunostainings for CD3, CD69, and HLA-A2 were performed for all 4 active GvHD cases and demonstrated that up to 84.1% of CD3+T cells in graft bowel and up to 62.9% of CD3+T cells in host bowel had a CD69+TRM phenotype (Figure 5A). Moreover, almost all CD3+T cells in both graft and host bowel were HLA-A2- and of donor origin at the time of GvHD (Figure 5A–C), in line with our hypothesis.
Figure 5. CD69+ TRM phenotype in the host and graft bowel of GvHD patients with host-donor HLA-A2 mismatches.
All tissue samples were collected, processed and analyzed via multiplex immunostaining as described in Supporting Materials and Methods. Both graft and native host bowel samples were taken from biopsies at the time of active GvHD episodes from 4 patients with host-donor HLA-A2 mismatches as shown in (A). (A) Relevant host and donor HLA information, HLA-A2 mismatch data, tissue categories, and CD3+, CD69+, and HLA-A2+ cells as a percentage of total cells analyzed and CD69+CD3+ cells as a percentage of total CD3+ cells analyzed are shown. (B and C) Vectra 3.0 Automated Quantitative Pathology Imaging System (PerkinElmer/Akoya) was used to captured high-powered images at 20X (resolution of 0.5mm per pixel) for multispectral image capture. Representative images of different markers CD3 (red), CD69 (green), HLA-A2 (yellow; host marker in all 4 cases) in the intestinal tissues are shown. Insert in the left upper corner shows 40X magnification of the respective markers. (B) Co-localization of CD3+CD69+ (purple) and CD69+HLA-A2+ (red) is evident in the native intestinal tissue at the time of GvHD. (C). Co-localization of CD3+CD69+ (purple) is evident in the allograft intestinal tissue of patients with GvHD and there is absence of HLA-A2+ cells.
Select inflammatory cytokines are increased in graft and native bowel at the time of GvHD
Expression patterns of CD69+TRM are mirrored by cytokine expression in immune cells. Analysis of cytokines released by CD4+ and CD8+T cell subsets in host bowel with GvHD, graft bowel with and without GvHD, and blood with and without GvHD shows two distinct patterns. First, the highest producers of TNF-α and IFN-γ were host and graft bowel at the time of GvHD. Second, the amount of cytokines released by immune cells in host bowel at the time of GvHD was more similar – albeit not reaching statistical significance – to that released by graft bowel at the time of GvHD than graft bowel without GvHD, pointing to a possible link between activity in the graft and native bowel.
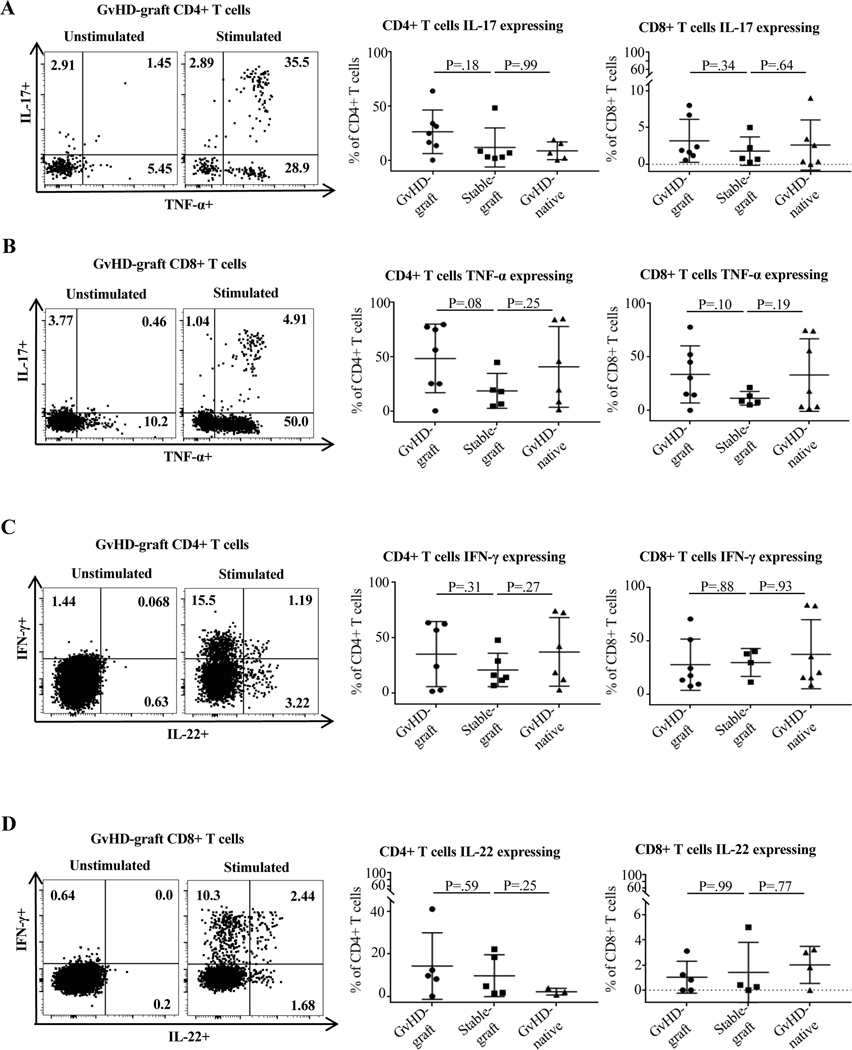
The percentage of CD4+ cells staining positive for TNF-α was 40.9% for native bowel at the time of GvHD vs. 48.6% for graft bowel and 17.4% for graft bowel without GvHD (Figure 6B); the percent staining positive for IFN-γ was 37.3% vs. 41.8% and 21.1% (Figure 6C). For CD8+, the TNF-α percentages were 33.1% vs. 36.6% and 11.3%, and the IFN-γ percentages were 37.6% vs. 30.2% and 29.9% (Figure 6C). IL-17 and IL-22 expression was also assessed, but there was no specific pattern of expression or significant difference in percentage of expression among the three groups (Figure 6A and D).
Figure 6. Cytokine expression in graft and native intestine in GvHD and control patients.
All samples were collected and analyzed as described in Materials and Methods and Supporting Materials and Methods. Groups are defined as described in Figure 4. (A-B) Representative flow cytometry plots and comparison of IL-17 and TNF-α expression on CD4+ and CD8+ cells between groups showing numerically large but not statistically significant increases in TNF-α expression by both CD4+ and CD8+ cells in graft and native bowel at the time of GvHD compared to graft bowel in control patients. (C-D) Representative flow cytometry plots and comparison of IFN-γ and IL-22 expression on CD4+ and CD8+ cells between groups showing numerically large but not statistically significant increases in IFN-γ expression by both CD4+ cells in graft and native bowel at the time of GvHD compared to graft bowel in control patients. Non-parametric Mann-Whitney test was used. For more than two independent groups, the Kruskal-Wallis test was performed followed with Dunn’s multiple comparisons.
We found possible biomarkers for the development and severity of GvHD
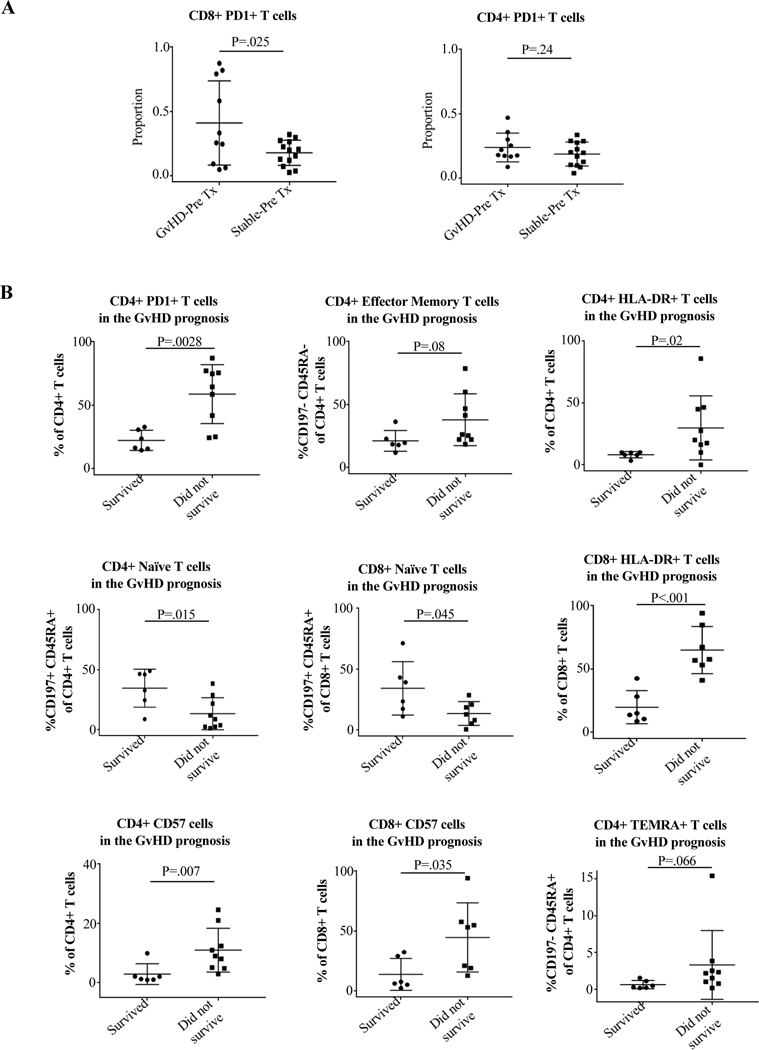
Remarkably, there was a significant difference in CD8 expression of PD-1 in blood prior to transplantation in patients who eventually experienced GvHD vs those who did not (41.0% vs. 17.8%, p=0.025) (Figure 7). Since this is a pre-transplant value, these cells are entirely recipient derived, indicating an increased expression of this activation/exhaustion marker prior to transplantation in those who later experience GvHD. Moreover, while all patients who had >45% of CD8+ cells expressing PD-1 prior to transplantation later developed GvHD, none of the non-GvHD patients had >33% expression prior to transplant, meaning that in our cohort, a pre-transplant CD8+PD-1+ level >45% was a predictor of later GvHD 100% of the time.
Figure 7. Use of the maturity, exhaustion, antigen-experience, and activation markers PD-1, CD57, and HLA-DR on CD4 and CD8 cells as biomarkers for the risk, diagnosis, and prognosis of GvHD.
Blood samples were collected and analyzed as described in Materials and Methods and Supporting Materials and Methods. Tx indicates transplant. (A) Comparison of CD4+ and CD8+ expression of PD-1 expression in peripheral blood prior to implantation into the recipient in patients who later experienced GvHD vs. those who did not. Remarkably, there was a significantly higher expression of PD-1 by CD8+ cells in blood of patients who later had GvHD compared to those who did not, indicating that pre-transplant PD-1 expression by CD8+ cells in blood could be a biomarker for the risk of later developing GvHD. (B) Comparison of CD4+ and CD8+ phenotype and expression of PD-1, HLA-DR, and CD57 in GvHD patients who survived vs. those who did not. Blood samples were taken at the time of diagnosis of each GvHD episode. Patients who succumbed to GvHD compared to those who recovered had significantly higher percentages of PD-1, HLA-DR, and CD57, and lower percentages of naïve phenotype for CD4+ and significantly higher percentages of HLA-DR and CD57 and lower percentages of naïve phenotype for CD8+. Increases in CD4+TEM and TEMRA approached statistical significance. Non-parametric Mann-Whitney test was used.
Additionally, our data show that elevated percentages of T cell activation and maturity markers at the time of GvHD are associated with higher mortality. When we compare GvHD episodes from patients with GvHD who died vs. those who survived, we find significantly higher percentages of CD4+HLA-DR+ (29.9% vs. 8.2%, p=0.037), CD8+HLA-DR+ (65.0% vs. 19.9%, p<0.001), CD4+CD57+ (11.0% vs. 2.9%, p=0.015), CD8+CD57+ (44.7% vs. 14.0%, p=0.033), CD4+PD-1+ (58.9% vs. 22.4%, p=0.001), and CD4+TEM (38.0% vs. 19.6%, p=0.08), with decreased CD4+ and CD8+ naïve phenotype (12.7% vs. 38.5%, p=0.015; 14.6% vs. 41.2%, p=0.045). Differences in CD4+TEM and TEMRA approached significance (Figure 7, non-significant differences in markers shown in Supplemental Figure 2). Values that 100% correlated with mortality were CD8+CD57+ >35%, CD4+HLA-DR+ >15%, CD8+HLA-DR+ >45%, and CD4+PD-1+ >35%.
Finally, overall grade of GvHD correlated with prognosis. Of the 5 patients who died following GvHD diagnosis, 100% were grade B or higher (D, D, C, B, B); of the 5 patients who survived, 100% were grade B or lower (B, B, B, A, A). No patient with grade A GvHD died, and no patient with grades C or D survived (Figure 1).
4. Discussion
This study represents one of the largest longitudinal characterization of the immune response in patients with and without GvHD after any type of solid organ transplant. Because of protocol analyses of blood and graft at set timepoints in all recipients, we were able to assess immunologic changes not only at the time of GvHD, but also before and after, and even pre-transplant in both donors and recipients, leading to three major findings.
First, building on prior studies in BMT and animal models that demonstrate that the percentage of T effector memory cells in the blood increases at the time of GvHD compared to control patients, we are able to report this finding in a large cohort of bowel recipients, further corroborating that they play a potentially important role in the pathophysiology of GvHD after ITx.(26–28) Our data suggest that these increased TEM may be potentially donor derived. While we acknowledge that our chimerism data are heterogenous and not necessarily conclusive, we see increased donor T cell chimerism in 6 of 10 patients at the time of GvHD, in one case up to 93%. Of the others, 1 was not tested for chimerism, and 3 did not show detectable levels of donor T cell chimerism, which could be explained by low cell yields and poor purities of the T cell sorts in these cases, as well as the relatively low donor sensitivity of 2–5% of the performed chimerism assay. Of note, the patient with the highest chimerism percentage had the most severe case of GvHD with chronic manifestations. This high level of T cell chimerism indicates that the recipient’s immune system has potentially been replaced by the donor’s. The increased CD4+ and CD8+ TRM cells were potentially donor derived, possibly linking GvHD to increased donor TEM in the blood. Of note, this patient also had significantly elevated percentages of PD-1, TEM, HLA-DR, and CD57 by CD4+ cells (including the highest PD-1 and CD57 expression) and PD-1, TEM, HLA-DR, and CD57 by CD8+ cells (including the highest HLA-DR and CD57 expression). While we cannot definitely say that these correlations between elevated chimerism and T cell activation, memory, exhaustion, and antigen experience markers in our patient set can be generalized, we hope they are a starting point for further ITx GvHD research by our and other centers.
Second, our data points to potentially donor derived CD69+ TRM as the likely source of the increased TEM. If TEM appear in the blood, the natural question is, what is their source? Since these could be donor derived, we looked to the graft, where we saw a parallel increase in CD69+ TRM in the setting of GvHD. Multiplex immunostaining confirmed that CD69+ TRM are of donor origin, at least in the subset of GvHD patients with HLA-A2 host-donor mismatch. The significant increase of these cells in the bowel mirrored the increase in TEM in the blood. The concurrent increase in TEM indicates that, as Beura et al. suggest, TRM may be induced by engraftment, inflammation, or immunosuppression to lose the tissue resident phenotype and reenter circulation as alloreactive memory cells.(9) This is further supported by the increased expression of the inflammatory cytokines IFN-γ and TNF-α in the graft and host bowel of patients with GvHD since these cytokines are released by CD8+ cells in GvHD.(29–31)
We also saw increased percentages of CD69+ TRM in the blood and native bowel of patients with GvHD, which were shown to be donor derived in the cases with HLA-A2 host-donor mismatch. These CD69+ TRM are known to be the source of GvH T cell clones after bowel transplant.(32) This supports our hypothesis that the pathophysiology of GvHD may depend on donor derived TRM in the graft migrating to recipient blood as host-reactive effector memory cells with resident memory phenotype and then homing to native tissue.
This finding, once corroborated with deeper studies across centers, could have both prognostic and therapeutic implications. In donor tissue sampled before any exposure to the recipient, there are suggestive trends towards altered numbers of CD8+ TEM, CD4+ TRM, and CD8+ TRM in grafts of patients who will develop GvHD compared to those who will not (data not shown). These data are in keeping with the preliminary hypothesis that grafts with higher percentages of TRM, by carrying more effector T cells capable of reacting to the host, may be more prone to inducing GvHD after transplantation. Whether this is a potential target of therapeutics, for example by more aggressively depleting the percentage of TRM in the graft prior to transplantation, merits further study with a larger patient set.
Our third finding is the identification of significant increases in antigen-experience and maturity markers in patients with GvHD. These included CD57, HLA-DR, and PD-1. This further supports our hypothesis about the importance of effector memory cells during GvHD, since these cells would be expected to express such markers.(15, 16, 33, 34) It also informs two important diagnostic and prognostic purposes. From a diagnostic perspective, the higher percentages of these markers could potentially serve as additional data points for diagnosing GvHD, a clinical diagnosis that can be difficult to make.(4, 7) From a prognostic perspective, we show that higher percentages of these markers correlate with severity of disease. Specifically, as mentioned above, values that 100% correlated with mortality in our study were CD8+ CD57+ >35%, CD4+ HLA-DR+ >15%, CD8+ HLA-DR+ >45%, and CD4+ PD-1+ >35%.
PD-1 is especially interesting since it not only increases significantly at the time of GvHD and correlates with more severe disease, it is also the single marker we identified that might indicate increased GvHD risk prior to transplantation. There was a statistically significant average of 23% higher levels of CD8+ PD-1 prior to transplantation in the blood of patients who later developed GvHD. There was a cutoff of 33% CD8+ PD-1 below which no patient developed GvHD, and a cutoff of 45% CD8+ PD-1 above which all patients went on to have GvHD. As PD-1 can be measured prior to transplantation, it could be used to help with risk stratification and to potentially inform directed therapies in patients who may be predisposed to GvHD.
We further help elucidate the role of PD-1 in GvHD. As discussed above, it has previously been shown that, after BMT, there is an increase in graft PD-1 expression at the time of GvHD;(17) however, it has also been shown that the GvH-HvG axis can be altered by downregulating alloreactive host cells that express PD-1 by increased graft expression of PD-1L.(18) Taken together, this seems to indicate that the increase in PD-1 observed in the blood at the time of GvHD was possibly due to the increased, potentially donor derived TEM and TRM that we observed, while higher expression of CD8+ PD-1 prior to transplantation represented alloreactive cells that were possibly more prone to being downregulated by PD-1L expressing cells in the graft and correlated with future GvHD. This may also help explain why patients with multivisceral grafts were more likely to experience GvHD than those with isolated intestinal grafts since, besides carrying a higher load of donor cells, these grafts specifically carry more PD-1L expressing myeloid cells in the liver component of the graft.(32) Tracking the percentage of PD-1 and PD-1L in grafts is the subject of our future work.
Overall, we provide data showing the role of potentially donor derived TRM in the pathophysiology of GvHD in ITx, as well as the identification of several potential biomarkers for the prediction, diagnosis, and prognosis of GvHD, even prior to transplantation. These observations can inform further studies that can lay the tracks for future targeted diagnostics and therapeutics.
Supplementary Material
Acknowledgments
AK, SCR, MZ, and TMF acknowledge funding support from the National Institute of Allergy and Infectious Diseases (R01AI132389). The authors thank Valerie Bockstette for critical review of the manuscript. The authors thank all coordinators, research associates, assistants, and students who were involved in this project for their technical contributions.
Abbreviations
- BMT
Bone marrow transplantation
- GvHD
Graft-versus-host disease
- GvH-HvG
Graft-versus-host-host-versus-graft
- ITx
Intestinal transplantation
- MvTx
Multivisceral transplantation
- PD-1
Programmed death-1
- TEM
Effector memory T cells
- TRM
Resident memory T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest pertinent to this article as described by the American Journal of Transplantation.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Fishbein TM. Intestinal transplantation. The New England journal of medicine 2009;361(10):998–1008. [DOI] [PubMed] [Google Scholar]
- 2.Ganoza AJ, Farmer DG, Marquez MA, Mazariegos GV. Intestinal Transplantation: International Outcomes. Clinical transplants 2014:49–54. [PubMed] [Google Scholar]
- 3.Reyes JD. Intestinal transplantation: an unexpected journey. Robert E. Gross Lecture. Journal of pediatric surgery 2014;49(1):13–18. [DOI] [PubMed] [Google Scholar]
- 4.Zeiser R, Blazar BR. Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. The New England journal of medicine 2017;377(22):2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 2009;373(9674):1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf D, von Lilienfeld-Toal M, Wolf AM, Schleuning M, von Bergwelt-Baildon M, Held SA et al. Novel treatment concepts for graft-versus-host disease. Blood 2012;119(1):16–25. [DOI] [PubMed] [Google Scholar]
- 7.Jaglowski SM, Devine SM. Graft-versus-host disease: why have we not made more progress? Current opinion in hematology 2014;21(2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2012;18(8):1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beura LK, Rosato PC, Masopust D. Implications of Resident Memory T Cells for Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2017;17(5):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prosser AC, Kallies A, Lucas M. Tissue-Resident Lymphocytes in Solid Organ Transplantation: Innocent Passengers or the Key to Organ Transplant Survival? Transplantation 2018;102(3):378–386. [DOI] [PubMed] [Google Scholar]
- 11.Turner DL, Gordon CL, Farber DL. Tissue-resident T cells, in situ immunity and transplantation. Immunol Rev 2014;258(1):150–166. [DOI] [PubMed] [Google Scholar]
- 12.Fan X, Rudensky AY. Hallmarks of Tissue-Resident Lymphocytes. Cell 2016;164(6):1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. The New England journal of medicine 2016;375(18):1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briones J, Novelli S, Sierra J. T-cell costimulatory molecules in acute-graft-versus host disease: therapeutic implications. Bone marrow research 2011;2011:976793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinosa J, Herr F, Tharp G, Bosinger S, Song M, Farris AB 3rd et al. CD57(+) CD4 T Cells Underlie Belatacept-Resistant Allograft Rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2016;16(4):1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Bendersky VA, Brennan TV, Espinosa JR, Kirk AD. IL-7 receptor heterogeneity as a mechanism for repertoire change during postdepletional homeostatic proliferation and its relation to costimulation blockade-resistant rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2018;18(3):720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stikvoort A, Gaballa A, Solders M, Nederlof I, Onfelt B, Sundberg B et al. Risk Factors for Severe Acute Graft-versus-Host Disease in Donor Graft Composition. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018;24(3):467–477. [DOI] [PubMed] [Google Scholar]
- 18.Saha A, O’Connor RS, Thangavelu G, Lovitch SB, Dandamudi DB, Wilson CB et al. Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality. The Journal of clinical investigation 2016;126(7):2642–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuchmann M, Meyer RG, Distler E, von Stebut E, Kuball J, Schnurer E et al. The programmed death (PD)-1/PD-ligand 1 pathway regulates graft-versus-host-reactive CD8 T cells after liver transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2008;8(11):2434–2444. [DOI] [PubMed] [Google Scholar]
- 20.Petrelli A, Mijnheer G, Hoytema van Konijnenburg DP, van der Wal MM, Giovannone B, Mocholi E et al. PD-1+CD8+ T cells are clonally expanding effectors in human chronic inflammation. The Journal of clinical investigation 2018;128(10):4669–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Wang S, Goplen NP, Li C, Cheon IS, Dai Q et al. PD-1(hi) CD8(+) resident memory T cells balance immunity and fibrotic sequelae. Science immunology 2019;4(36). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishbein T, Novitskiy G, Mishra L, Matsumoto C, Kaufman S, Goyal S et al. NOD2-expressing bone marrow-derived cells appear to regulate epithelial innate immunity of the transplanted human small intestine. Gut 2008;57(3):323–330. [DOI] [PubMed] [Google Scholar]
- 23.Elsabbagh AM, Hawksworth J, Khan KM, Kaufman SS, Yazigi NA, Kroemer A et al. Long-term survival in visceral transplant recipients in the new era: A single-center experience. Am J Transplant 2019;19(7):2077–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroemer A, Belyayev L, Khan K, Loh K, Kang J, Duttargi A et al. Rejection of intestinal allotransplants is driven by memory T helper type 17 immunity and responds to infliximab. Am J Transplant 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streitz M, Miloud T, Kapinsky M, Reed MR, Magari R, Geissler EK et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transplantation research 2013;2(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khandelwal P, Lane A, Chaturvedi V, Owsley E, Davies SM, Marmer D et al. Peripheral Blood CD38 Bright CD8+ Effector Memory T Cells Predict Acute Graft-versus-Host Disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015;21(7):1215–1222. [DOI] [PubMed] [Google Scholar]
- 27.Loschi M, Porcher R, Peffault de Latour R, Vanneaux V, Robin M, Xhaard A et al. High number of memory t cells is associated with higher risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015;21(3):569–574. [DOI] [PubMed] [Google Scholar]
- 28.Ali N, Flutter B, Sanchez Rodriguez R, Sharif-Paghaleh E, Barber LD, Lombardi G et al. Xenogeneic graft-versus-host-disease in NOD-scid IL-2Rgammanull mice display a T-effector memory phenotype. PloS one 2012;7(8):e44219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henden AS, Hill GR. Cytokines in Graft-versus-Host Disease. Journal of immunology 2015;194(10):4604–4612. [DOI] [PubMed] [Google Scholar]
- 30.Piper C, Drobyski WR. Inflammatory Cytokine Networks in Gastrointestinal Tract Graft vs. Host Disease. Frontiers in immunology 2019;10:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Chu J, Yu J, Wei W. Cellular and molecular mechanisms in graft-versus-host disease. Journal of leukocyte biology 2016;99(2):279–287. [DOI] [PubMed] [Google Scholar]
- 32.Zuber J, Shonts B, Lau SP, Obradovic A, Fu J, Yang S et al. Bidirectional intragraft alloreactivity drives the repopulation of human intestinal allografts and correlates with clinical outcome. Science immunology 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed A, Adiga V, Nayak S, Uday Kumar JAJ, Dhar C, Sahoo PN et al. Circulating HLA-DR+CD4+ effector memory T cells resistant to CCR5 and PD-L1 mediated suppression compromise regulatory T cell function in tuberculosis. PLoS pathogens 2018;14(9):e1007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ammirati E, Cianflone D, Vecchio V, Banfi M, Vermi AC, De Metrio M et al. Effector Memory T cells Are Associated With Atherosclerosis in Humans and Animal Models. Journal of the American Heart Association 2012;1(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.