Abstract
Background
Wire localization is historically the most common method for guiding excision of non-palpable breast lesions, but there are limitations to the technique. Newer technologies such as magnetic seeds may allow some of these challenges to be overcome. The aim was to compare safety and effectiveness of wire and magnetic seed localization techniques.
Methods
Women undergoing standard wire or magnetic seed localization for non-palpable lesions between August 2018 and August 2020 were recruited prospectively to this IDEAL stage 2a/2b platform cohort study. The primary outcome was effectiveness defined as accurate localization and removal of the index lesion. Secondary endpoints included safety, specimen weight and reoperation rate for positive margins.
Results
Data were accrued from 2300 patients in 35 units; 2116 having unifocal, unilateral breast lesion localization. Identification of the index lesion in magnetic-seed-guided (946 patients) and wire-guided excisions (1170 patients) was 99.8 versus 99.1 per cent (P = 0.048). There was no difference in overall complication rate. For a subset of patients having a single lumpectomy only for lesions less than 50 mm (1746 patients), there was no difference in median closest margin (2 mm versus 2 mm, P = 0.342), re-excision rate (12 versus 13 per cent, P = 0.574) and specimen weight in relation to lesion size (0.15 g/mm2 versus 0.138 g/mm2, P = 0.453).
Conclusion
Magnetic seed localization demonstrated similar safety and effectiveness to those of wire localization. This study has established a robust platform for the comparative evaluation of new localization devices.
Magseed® localization has been shown to demonstrate safety and effectiveness not inferior to wire localization. Furthermore, this study has established a robust platform for the comparative evaluation of new breast localization devices.
Introduction
The expansion of breast screening services worldwide1 has resulted in identification of increasing numbers of non-palpable breast lesions that require preoperative localization. Historically, wire-guided lesion localization has been performed in the majority of units2. Most breast surgeons and breast radiologists have extensive experience using wires, but this technique has several disadvantages. These include migration of the wire, difficult perioperative wire tip localization resulting in excessive excision of normal breast tissue, and logistical challenges. The latter in particular impacts on surgical scheduling as wires need to be placed on the day of surgery. Despite these difficulties, wire localization remains cheap and effective.
Several alternative methods to wire localization have been developed including radio-occult lesion localization (ROLL)3, radioactive seed localization, carbon-track marking4 and intraoperative ultrasonography5. These are limited by radioactivity regulations, difficult intraoperative lesion detection and need for specialist ultrasound training. Recently, additional novel non-radioactive devices have entered the market that use varying methodologies to guide surgeons to the target lesion. These can be placed in the breast or axilla weeks to months prior to surgery. Devices include Savi Scout®6 (Cianna Medical Inc., Aliso Viejo, California, USA), LOCalizer™ radiofrequency identification (RFID) tags7 (Hologic, Santa Carla, California, USA), Magseed®8 (Endomagnetics Inc., Cambridge, UK), Pintuition®9 (Sirius Medical, Eindhoven, Netherlands) and MOLLI™10 (MOLLI surgical, Toronto, Canada). The above devices differ notably in their mode of action, and in the absence of comparative evidence, product selection is largely dependent on surgeon preference.
Magseed® was the first of these newer-generation devices to gain CE marking (2017) following early clinical studies proving early efficacy11. Emerging data from users demonstrate that the device can localize lesions accurately, and may reduce re-excision rates, pain12 and excision specimen weight13,14. These results must be interpreted with caution as the majority of data sets are from single-site case series often reported without a control group and clear prespecified outcome measures. A recent meta-analysis compared wire-guided lesion localization with magnetic seed; however, only one study contained a control group of wire localization and an intervention group of magnetic seed8,13 and RFID7. Although these findings were supportive of the technique, there remains the need for high-quality research to establish the safety and effectiveness of magnetic seed localization, to determine the key outcomes and how it compares with standard wire localization.
It is likely that even more devices will enter the market in the coming years and both surgeons and patients will require data on efficacy and safety15. Although randomized clinical trials are ideal, these are challenging in the context of breast lesion localization. Techniques need to be stable and standardized, and adopted by sufficient number of surgeons. Efficacy data are required so that further studies can be powered adequately. An alternative approach is a well powered multicentre observational study, within an IDEAL (idea, development, exploration, assessment, long-term study) framework16 and the use of shared learning to minimize learning curve effects. IDEAL framework phases 2a (development) and 2b (exploration) focus on studies that examine benefits of a device or technique, indications of use and the ability to be adopted by a wider group of surgeons, and to develop feasibility data for a future trial.
The iBRA-NET (implant Breast Reconstruction evaluation-NETwork) Localisation Study is an IDEAL 2a/2b platform study that aims to compare new localization devices with the standard of wire localization. The results from phase 1 of the study, a national practice questionnaire to understand current practice, were reported previously2. The results from the first two comparative arms of the IDEAL 2a/2b platform study investigating the outcomes of wire-guided and magnetic-seed-guided localization are reported here.
Methods
Study design and participants
The iBRA-NET Localisation Study is a UK-based national, multicentre, prospective, IDEAL stage 2a/2b platform cohort study, with embedded novel shared-learning methodology, that compared safety and effectiveness of magnetic-seed- versus wire-guided breast lesion localisation17. The shared learning methodology and results will be reported elsewhere.
All UK breast and plastic surgical units performing wire or magnetic seed breast localization were invited to participate in the study, through national professional organizations, namely the Association of Breast Surgery, National Trainee Research Collaborative Network, Mammary Fold Research Network and the iBRA-NET network of surgeons.
Women aged 16 and over, who had breast-conserving surgery requiring a preoperative localization procedure were recruited consecutively to the study between August 2018 and August 2020. Due to potential interference with detection of an implanted magnetic seed, patients were excluded from the magnetic seed arm if they had received iron oxide injection in the previous 6 months or had a pacemaker or implantable electronic device in their chest wall. Ethics approval was not required, as this was a service evaluation, as defined by the Health Research Authority decision tool (http://www.hra-decisiontools.org.uk/research/). Each participating centre was required to obtain local audit approvals and register the study before commencing recruitment—consistent with the methods reported previously for multicentre prospective trainee collaborative studies18,19. Patient consent was not required for routine clinical data collection. Study data were collected in an anonymized format and managed using REDCap electronic data capture tools hosted at Kennedy Institute of Rheumatology, University of Oxford20. The study involved the collection of clinical outcome data as routinely recommended by UK guidelines for good practice and outcomes were assessed against their quality standards21,22.
Procedures
Study centres recruited consecutive patients undergoing breast localization procedures, with modality of lesion localization (magnetic seed versus wire) depending on local availability and policy. Units recruited into either one (if only performing wire localization or having switched to magnetic seed) or both arms of the study depending on local localization practice. Centres offering magnetic seed localization were provided with patient information leaflets and a suggested protocol for magnetic seed localization to ensure consistent quality in insertion, localization and surgical excision (Appendix S1), but participating units were able to perform the procedures according to their routine practice. Wire-guided localization was performed on the morning of surgery in all patients, whereas magnetic-seed-guided localization could be performed in advance.
Quality assurance
In wire-guided localization, it was expected that the operating surgeon should have completed a minimum of 10 wire-guided wide local excisions in the last year. For magnetic seed localization surgery, it was expected that the participating unit should have adopted magnetic seed as their method of localization and were not just trialling the device. This was to ensure that there was adequate expertise in both radiological placement and surgical removal of the magnetic seed. Individual surgeons must have completed a minimum of five successful magnetic seed localization cases and have completed their local training requirements before recruiting to the study.
Outcomes
The primary outcome was effectiveness, defined as the identification and successful surgical removal or partial removal of the index lesion in the primary operation (or clip/fibrosis in the event of neoadjuvant therapy), based on final pathology. Secondary outcomes were safety (defined as proportion of patients having a peri- and postoperative complication, and complications related to the device (Appendix S2)); close margins (defined as ductal carcinoma in situ (DCIS)/invasive cancer less than 1 mm from nearest margin); weight of wide local excision specimen (in grams); breast reoperation rate (planned and unplanned); cancellation on day of surgery (proportion of patients cancelled within 24 hours of time of surgery); and the duration and start time of the surgical procedure. In order to account for lesion size in the assessment of excision weight, the authors used size as a dominator over two dimensions, reporting this as weight/size2, in g/mm2.
Comparisons of magnetic seed versus wire
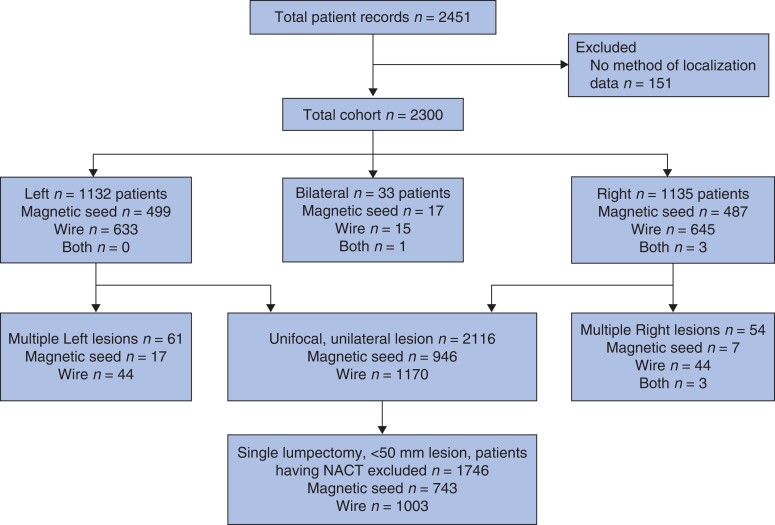
For ease of comparison, only patients having unifocal, unilateral lesion localization were considered. For comparison of surgical parameters, a subset of this group were included, that is patients having a lumpectomy for a single unifocal lesion less than 50 mm in size (cT1 and cT2 only). In this comparison patients operated after neoadjuvant therapy were excluded. If localization was stereotactic-guided, then preoperative size on mammography was used, if localization was ultrasound-guided, then size on ultrasonography was used. Patients having two separate lumpectomies or those having a therapeutic mammoplasty or volume replacement with lipofilling or perforator flap were excluded from this level of analysis (Fig. 1).
Fig 1.
Flow chart of patients included in study
NACT, received neoadjuvant chemotherapy
Statistical analysis
The failure rate of wires in the literature is 0.6 per cent23 giving an identification rate of 99.4 per cent. A clinically significant difference between techniques was considered to be less than 0.9 per cent. Assuming both methods to have an identification rate of 99.4 per cent, the power calculation (upper limit of the observed one-sided 95 per cent confidence interval for the difference between identification rates (magnetic seed versus wire) to be less than 0.9 per cent with 80 per cent power) indicated that the sample size should be 1000 patients per group. Simple summary statistics were calculated for each outcome and data were tested for distribution and differences between groups using unpaired t-tests, Mann–Whitney U tests and chi-squared tests as appropriate. Analyses were conducted using Stata® IC, version 14 (StataCorp, College Station, Texas, USA).
Results
There were 2451 patients recruited from 35 UK breast units, of whom 151 were excluded due to incomplete data. The final cohort therefore consisted of 2300 patients (Fig. 1). A total of 1003 patients had magnetic-seed-guided localization, 1296 patients had wire-guided localization and four patients had both magnetic-seed- and wire-guided localization. Bilateral lesion localization was required for 33 patients. A total of 120 patients had multifocal/multicentric lesion localization (Table S1). Magnetic seed was used infrequently compared with wire for localization of a second lesion, 22.5 versus 77.5 per cent of second lesions (Table S1). Where three lesions were present, magnetic seed was not used and all three had ultrasound-guided wires placed. There were no patients in the database with bilateral multifocal disease.
Primary endpoint
Patients within the two comparator groups were well matched, with no differences in clinicopathological variables (Table 1). For magnetic-seed-guided excisions, the lesion was present in the excision specimen in 99.8 per cent of cases (905 of 907 patients, unknown 39 patients), and for wire-guided excisions, the lesion was present in the excision specimen in 99.1 per cent of cases (1150 of 1161 patients, unknown 9 patients) (Table 2). There was a statistically significant difference between magnetic seed and wire guidance for the primary endpoint of successful index lesion localization (P = 0.048, Fisher’s exact test). Of the 13 cases where the index lesion was not removed in the excision specimen, most (9 patients) were reported to have been removed in the core biopsy, with no residual disease in the excision specimen. Other reasons included localization of incorrect lesion (2 patients), lesion present in shave but not main specimen (1 patient), and pathological complete response after neoadjuvant systemic therapy (1 patient).
Table 1.
Clinicopathological variables in patients with unifocal, unilateral breast lesions
| Magnetic seed (n = 946) | Wire (n = 1170) | P ‡ | |
|---|---|---|---|
| Age (years)* | 59.9(10.4) | 60.4(10.6) | 0.210 |
| BMI (kg/m2 )† | 27.9 (24.4–32.1) | 28 (24–32) | 0.555 |
| Unknown | 93 | 235 | |
| Lesion size (mm)† | n = 764 | n = 931 | |
| Mammogram | 13 (9–20) | 13 (9–20) | 0.598 |
| Unknown | 105 | 75 | |
| Ultrasound | 12 (9–18) | 12 (8–17) | 0.219 |
| Unknown | 182 | 239 | |
| Tumour stage | n = 891 | n = 1147 | |
| Tis | 167 (18.7) | 256 (22.3) | 0.189 |
| T1 | 541 (60.7) | 637 (55.5) | |
| T2 | 126 (14.1) | 172 (15) | |
| T3 | 12 (1.3) | 16 (1.4) | |
| yT0 | 45 (5.1) | 66 (5.8) | |
| Unknown | 55 | 23 | |
| Nodal stage | n = 881 | n = 1147 | |
| N0 | 747 (84.8) | 973 (85.4) | 0.543 |
| N1 | 92 (10.4) | 105 (9.2) | |
| N2 | 7 (0.8) | 11 (1) | |
| N3 | 2 (0.2) | 8 (0.7) | |
| yN0 | 33 (3.7) | 42 (3.7) | |
| Unknown | 65 | 31 | |
| Histological diagnosis | n = 945 | n = 1169 | |
| Invasive ductal and lobular | 592 (62.6) | 715 (61.2) | 0.051 |
| DCIS | 172 (18.2) | 264 (22.6) | |
| Mixed invasive/DCIS | 120 (12.7) | 127 (10.9) | |
| Other | 61 (6.5) | 63 (5.4) | |
| Unknown | 1 | 1 | |
| Neoadjuvant therapy | n = 946 | n = 1170 | |
| Chemotherapy | 100 (10.6) | 117 (10) | 0.679 |
| Endocrine therapy | 34 (3.6) | 50 (4.3) | |
| None | 812 (85.8) | 1003 (85.7) | |
| Surgery | n = 943 | n = 1170 | |
| Lumpectomy for single lesion | 759 (80.5) | 1009 (86.2) | 0.002 |
| Mammoplasty involving skin/volume reduction | 164 (17.4) | 142 (12.1) | |
| Lumpectomy for multiple lesions | 4 (0.4) | 8 (0.7) | |
| Other | 16 (1.7) | 11 (0.9) | |
| Unknown | 3 | - | |
| Concurrent axillary surgery | n = 935 | n = 1168 | |
| No | 225 (24.1) | 328 (28.1) | 0.095 |
| Sentinel node biopsy | 658 (70.4) | 784 (67.1) | |
| Axillary clearance | 52 (5.6) | 56 (4.8) | |
| Unknown | 11 | 2 |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.), †values are median (i.q.r.). DCIS, ductal carcinoma in situ. ‡t–test was used for Age, Mann–Whitney U test was used for BMI, mammogram, ultrasound, and Chi squared was used for all the others namely Tis, N0, Invasive ductal and lobular, chemotherapy, lumpectomy for single lesion, concurrent axillary surgery (No).
Table 2.
Primary endpoint: localization success of wire- and magnetic-seed-guided localization
| Successful localization | Magnetic seed | Wire | P | ||||
|---|---|---|---|---|---|---|---|
| Stereo (n = 282) | US (n = 664) | Total (n = 946) | Stereo (n = 337) | US (n = 833) | Total (n = 1170) | ||
| No | 2 (0.7) | - | 2 (0.2) | 8 (2.4) | 3 (0.4) | 11 (0.9) | 0.048 |
| Yes | 265 (99.3) | 640 (100.0) | 905 (99.8) | 325 (97.6) | 825 (99.6) | 1150 (99.1) | |
| Unknown | 15 | 24 | 39 | 4 | 5 | 9 | |
Values in parentheses are percentages. Stereo, stereotactic localisation; US, ultrasonographic localisation. Fisher's exact test, comparing "Total Magseed" versus "Total Wire". *Fisher's exact test.
Secondary endpoints
For comparison of surgical parameters, only patients having a unilateral lumpectomy for a single unifocal lesion less than 50 mm were included, and those having neoadjuvant therapy were excluded. When magnetic-seed-guided surgery (743 patients) and wire-guided surgery (1003 patients) were compared, there were no significant differences in closest/involved margin, rates of re-excision, rates of routine shaves taken during surgery, duration of procedure, and specimen weight/size2 (Table 3).
Table 3.
Surgical outcomes following lumpectomy for cT1/cT2 unifocal, unilateral lesions guided by magnetic seed and wire. Patients having neoadjuvant chemotherapy were excluded
| Magnetic seed | Wire | P ‡ | |||||
|---|---|---|---|---|---|---|---|
| Stereo | USS | Total | Stereo | USS | Total | ||
| (n = 227) | (n = 516) | (n = 743) | (n = 266) | (n = 737) | (n = 1003) | ||
| Margins | n = 212 | n = 478 | n = 690 | n = 254 | n = 720 | n = 974 | |
| Positive (0 mm at ink) | 26 (12.3) | 66 (13.8) | 92 (13.3) | 43 (16.8) | 103 (14.3) | 146 (15.0) | 0.342 |
| Unknown | 15 | 38 | 53 | 12 | 17 | 29 | |
| Closest margin (mm)* | 2 (0–55) | 2 (0–20) | 2 (0–55) | 2 (0–20) | 2 (0–55) | 2 (0–35) | 0.400 |
| Orientation of closest margin | n = 212 | n = 477 | n = 689 | n = 252 | n = 707 | n = 959 | |
| Anterior | 44 (20.8) | 107 (22.4) | 151 (21.9) | 53 (21) | 192 (27.2) | 245 (25.3) | 0.137 |
| Posterior | 31 (14.6) | 102 (21.4) | 133 (19.3) | 49 (19.4) | 146 (20.7) | 195 (20.5) | |
| Radial | 137 (64.6) | 268 (56.2) | 405 (58.8) | 150 (59.5) | 369 (52.2) | 519 (54.2) | |
| Unknown | 15 | 39 | 54 | 14 | 30 | 44 | |
| Re-excision | n = 216 | n = 491 | n = 707 | n = 260 | n = 730 | n = 990 | |
| Required | 27 (12.5) | 60 (12.2) | 87 (12.3) | 44 (16.9) | 87 (11.9) | 131 (13.2) | 0.574 |
| Unknown | 11 | 25 | 36 | 6 | 7 | 13 | |
| Of which, residual disease present | n = 25 | n = 58 | n = 83 | n = 42 | n = 82 | n = 124 | |
| Yes | 8 (32) | 21 (36.2) | 29 (34.9) | 15 (35.7) | 26 (31.7) | 41 (33.1) | 0.780 |
| Unknown | 2 | 2 | 4 | 2 | 5 | 7 | |
| Routine shaves/margins taken during surgery? | n = 227 | n = 516 | n = 743 | n = 266 | n = 737 | n = 1003 | |
| No | 79 (34.8) | 186 (36.0) | 265 (35.7) | 76 (28.6) | 280 (38) | 356 (35.5) | 0.941 |
| Orientation of shave/margin taken § | n = 148 | n = 330 | n = 478 | n = 190 | n = 457 | n = 647 | |
| Anterior | 7 (4.7) | 17 (5.2) | 24 (5.0) | 13 (6.8) | 28 (6.1) | 42 (6.5) | 0.350 |
| Posterior | 22 (14.9) | 64 (19.4) | 86 (18) | 39 (20.5) | 79 (17.3) | 119 (18.2) | 0.916 |
| Radial | 131 (88.5) | 283 (85.8) | 414 (86.6) | 175 (92.1) | 431 (94.3) | 606 (93.7) | <0.001 |
| Number of radial shaves* | 1 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 2 (1–4) | |
| Duration of procedure | n = 213 | n = 483 | n = 696 | n= 261 | n = 728 | n = 989 | |
| Duration (min)† | 60 (45–75) | 65 (52–84.5) | 60.5 (50–81) | 60 (45–75) | 60 (45–85) | 60 (45–82) | 0.100 |
| Unknown | 14 | 33 | 47 | 5 | 9 | 14 | |
| Specimen weight/size2 | n= 186 | n = 458 | n = 644 | n = 227 | n = 675 | n = 902 | |
| Specimen (g/mm2)† | 0.159 (0.06–0.42) | 0.148 (0.07–0.27) | 0.15 (0.07–0.31) | 0.133 (0.03–0.94) | 0.14 (0.07–0.29) | 0.138 (0.06–0.32) | 0.453 |
| Unknown | 41 | 58 | 99 | 39 | 62 | 101 | |
Values in parentheses are percentages unless indicated otherwise; *values are median (range); †values are median (i.q.r.). §Not mutually exclusive, so for each patient, multiple shaves have been taken. ‡Mann–Whitney U test for continuous variables and chi-squared test for categorical variables.
A failed localization, where a second method of localization was required (for example magnetic seed or wire not placed in index lesion) was a statistically more common occurrence in wire-guided localization (23 of 1162 patients, 1.98 per cent, unknown 8 patients) compared with magnetic-seed-guided localization (15 of 913 patients, 1.64 per cent, unknown 33 patients) (P = 0.032). Magnetic seed was also less likely to be dislodged from the lesion during surgery compared with wire (0.4 versus 1.4 per cent, P = 0.039, Table 4). There were no differences in peri- and postoperative complications related to surgery (Table 4). There were eight patients where the magnetic seed was not detectable transcutaneously, but no procedures were abandoned, hence surgeons were able to complete the procedure despite initial difficulty in transcutaneous detection.
Table 4.
Surgical complications in magnetic-seed- and wire-guided surgery
| Magnetic seed (n = 946) | Wire (n = 1170) | Total (n = 2116) | P* | |
|---|---|---|---|---|
| Perioperative complications/localization | n = 902 | n = 1163 | n = 2065 | |
| None | 891 (98.8) | 1127 (96.9) | 2018 (97.7) | 0.132 |
| Magnetic seed/wire dislodged from lesion | 4 (0.4) | 16 (1.4) | 20 (0.9) | 0.039 |
| Index lesion/clip not visible on specimen Xrays | 4 (0.4) | 9 (0.8) | 13 (0.6) | 0.406 |
| Procedure abandoned, further localization required | - | - | - | - |
| Other | 3 (0.3) | 11 (0.9) | 14 (0.7) | 0.106 |
| Unknown | 44 | 7 | 51 | |
| Postoperative complications/surgery | n = 946 | n = 1170 | n = 2116 | |
| Seroma requiring aspiration | 14 (1.5) | 25 (2.1) | 39 (1.8) | 0.264 |
| Haematoma requiring aspiration in clinic | 3 (0.3) | 8 (0.7) | 11 (0.5) | 0.364 |
| Haematoma requiring surgical evacuation | 3 (0.3) | 4 (0.3) | 7 (0.3) | 1.000 |
| Minor wound infection (oral antibiotics) | 14 (1.5) | 27 (2.3) | 41 (1.9) | 0.170 |
| Major wound infection (IV antibiotics) | 3 (0.3) | 7 (0.6) | 10 (0.5) | 0.527 |
| Major wound infection (drainage/debridement) | 1 (0.1) | 4 (0.3) | 5 (0.2) | 0.388 |
| In-hospital complication including systemic complications such as DVT/PE/MI | 6 (0.7) | 4 (0.3) | 10 (0.5) | 0.349 |
| Unexpected readmission to hospital within 30 days† | 7 (0.8) | 11 (1) | 18 (0.9) | 0.676 |
| Readmission directly related to the localization procedure | ||||
| Reoperation within 30 days‡ | 29 (3.2) | 53 (4.6) | 82 (4) | 0.119 |
| Complications directly related to localization device (magnetic seed or wire)§ | 2 (0.2) | 2 (0.2) | 4 (0.2) | 1.000 |
Values in parentheses are percentages. *All were done using Chi squared test. †Unknown data in 50 magnetic seed and 18 wire. ‡Unknown data in 51 magnetic seed and 19 wire. §Unknown data in 49 magnetic seed and 19 wire. IV, intravenous; DVT, deep venous thrombosis; PE, pulmonary embolism; MI, myocardial infarction.
Multifocal lesion localization using bracketing
Bracketing, the use of two or more localization devices to define the extent of the lesion(s) that require excision, was employed in 49 patients. There were 12 patients who had bracketing with magnetic seed and 37 with wires. Localization was successful in 100 per cent of the index lesions and 97.7 per cent of the second lesion (43 of 44 patients, data missing on 5 patients). When bracketing was used, the median distance between lesions was 40 (i.q.r. 28–49) mm for magnetic seed and 34 (i.q.r. 26–45) mm for wires.
Logistical considerations
There was a low rate of cancellation on the day of surgery for both modalities; 0.9 per cent (9 of 997 patients) for magnetic seed, and 0.5 per cent (6 of 1290 patients) for wire and none for dual modality (wire and magnetic seed). No cancellations were related to the localization procedure.
Magnetic seed lesion localization was performed a median of 6 days before surgery (range 0–167 days, i.q.r. 3–12 days). Most surgery was done as a day-case, with a median length of stay being 0 (range 0–11) days for magnetic-seed-guided surgery, and 0 (range 0–7) days for wire-guided surgery. Magnetic-seed-guided surgeries were started earlier in the day (Fig. S1).
Discussion
This study reports on the first arm of the iBRA-NET Localisation Study, and is the largest study to date comparing wire-guided and magnetic-seed-guided localization in breast surgery. Results show that magnetic seed compared equivalently with wire in terms of localization of non-palpable breast lesions. Additionally, reported complications were low demonstrating safety of this innovative localization technique. This study has contributed substantially to the collective safety data of this novel technique, and aims to set a standard for a robust IDEAL 2a/2b evaluation for future surgical devices.
Magnetic seeds have been subject to multiple published evaluations, albeit largely in single-centre, small cohorts. The results of the present study compare to some but are in contrast to others. Early US data were reassuring for accuracy of placement, with 100 per cent (73 patients) successful placement, defined as positioning within 10 mm of the target, most of which (51 of 73 patients, 70 per cent) were located within 1 mm (either directly contacting the target or immediately adjacent to it)24. Data from the UK were limited to small single-institution cohorts, with contrasting results. A series from London (128 patients) reported smaller specimen weights, but similar rates of positive margins14. A series from Lincoln (137 patients) reported a mean specimen weight of 75.5 g (0.327 g/mm2 for comparison) and a re-excision rate of 14.8 per cent. A series from Manchester reported no significant differences in re-excision rate (104 patients, 16 per cent with magnetic seed and 14 per cent wire, P = 0.692)8. A systemic review and pooled analysis of 1559 procedures from 16 studies concluded that magnetic seed provided an effective, non-inferior alternative to wires, with a successful localization rate of 99.86 per cent (16 studies), and a re-excision rate of 11.19 per cent (12 studies). A re-excision rate was determined based on results from only four studies, comparing a total of 319 magnetic-seed-localized excisions against 507 wires, with a non-significant re-excision rate of 18.5 per cent for magnetic seed and 16.17 per cent for wires13.
A series from Shrewsbury (106 patients with magnetic seed versus 90 with wire) concluded that there was a significant reduction in re-excision rate from 22.4 to 12 per cent, and average specimen weight from 40 to 27 g25. A randomized trial of radioactive seed versus wire from Australia demonstrated an improved re-excision rate of 13.9 per cent for radioactive seed (327 patients) versus 18.9 per cent for wires (332 patients) (P = 0.019)26. For the latter study, it must be noted that for the purposes of power calculation, the re-excision rate was expected to be 30 per cent, resulting in an underpowered study. In comparison, margin positivity is reported in the world literature to be between 16.4 and 20 per cent27,28. These two studies have demonstrated a much higher rate of re-excision in patients undergoing wire-guided localization, resulting in a potentially false ‘improvement’. The results of the iBRA-NET Localisation Study are in keeping with recent published data from the UK, reflecting more current practice29. In a follow-up cost-effectiveness analysis of the use of radioactive seed localization in preventing future re-excisions, data from the ROLLIS study group showed that using seeds is cost-effective. It was concluded that the marginal additional cost when compared with wire localization is less than the cost of reoperations avoided30.
The present study demonstrated that magnetic seed localization did not lead to improvement in the majority of secondary surgical outcomes, including re-excision. Wires and magnetic seeds are both highly effective at localizing lesions, and the majority of complications are related to adjuncts to surgery rather than the localization technique. Several single-unit, smaller studies have demonstrated a reduction in re-excision rate after adopting the new technology, particularly for radioactive seeds31. However, these studies have selection bias and are generally single site. A randomized trial of radioactive seed localizations from Denmark in 201732 concluded that there were major logistical advantages, but with no differences in positive resection margins (11.8 versus 13.3 per cent), duration of the procedure or specimen weight. It is thus plausible that some surgical outcomes are not dependent on the localization device, but rather a reflection of the disease. Re-excision is most commonly required for DCIS at the margins33 and is a consequence occult disease being present rather than poor localization. Re-excision is thus most commonly a mismatch between preoperative clinical expectation and postoperative histology.
The limitations of this study are recognized. There were no data collected on distance of magnetic seed placement from the index tumour or on transcutaneous signal detection. The study was not powered for more complex scenarios of non-palpable breast lesion localization, such as multiple lesions and ‘bracketing’. Being an observational study, there was potential for selection bias because localization modality was dependent on surgeon preference and service convenience. Moreover, not all participating surgeons or services offered both localization modalities. The lack of difference in secondary outcomes could be explained by subtleties that are difficult to capture. For example, during localization of the index lesion, on rare occasions, a second lesion is identified. If this were to be found in advance (as is the case with magnetic seed) this would give time for diagnostic biopsy, and surgery could proceed as planned, whereas with wires the operation would have to be postponed.
Magnetic-seed-guided surgery is a novel technique, and most present data were collected during the ‘early years’ of introduction in the UK. Despite all users having completed their initial learning curve, it is feasible that an on-going learning curve may still have a part to play in the lack of expected superiority in re-excision rates and localization success. Additionally, there may be a lack of standardization of practice amongst individual units performing this technique. It was not the intention of the authors to dictate practice in individual units, and it is likely that this evolved during the implementation of the technology, which cannot be controlled. This, however, could be argued to be beneficial in capturing real-world evidence of current practice in varied healthcare settings. The embedded shared learning allowed for dissemination of surgical technique and challenges, and the technique evolving with experience.
This study does not examine clinician and patient satisfaction, or cost analysis which would help to determine the localization modality that individual breast units choose to adopt. Previous studies have reported favourable clinician satisfaction, but no difference in patient satisfaction, although they did report lower preoperative patient anxiety with magnetic seed, when compared with wire13,14. This study did not report on the utilization of magnetic seed in axillary surgery, and it has been reported previously that magnetic seed localization appears to be a safe, non-radioactive method to localize axillary lymph nodes accurately before surgery34.
The development of magnetic seed and other non-palpable breast lesion localization devices may provide a safe alternative to wires, with logistical benefits. Progressing from the IDEAL 2a/2b design of this present study could involve a randomized study of several localization modalities. This would, however, have to be highly powered, and may not be feasible due to the large range of localization devices available, the difficulties in training and operational standardization of these devices in a study involving multiple surgeons, and the lack of large-scale baseline data for the newer emerging devices. Subsequent arms of the iBRA-NET Localisation Study will, however, report on other localization devices including LOCalizer™ and Savi-Scout®. The study design will enable direct comparison with all other arms of the study giving patients and clinicians robust and reliable data to inform them of the effectiveness and operative outcomes. Further work should also include examining patient and clinician preference using a qualitative approach, a cost analysis to evaluate the technology fully, and assessment of the ability to localize lymph nodes in the axilla accurately to facilitate targeted axillary dissection.
Supplementary Material
Acknowledgements
J.H. and R.V.D. conceived the study. J.H. and R.V.D. designed the pilot study. R.V.D., S.E. and J.H. designed and trialled the pilot data-collection forms. All authors contributed to final study design and finalized data-collection forms. R.V.D., J.M. amd M.C. coordinated collaborator recruitment and provided collaborator support. J.H., N.B., S.E., N.B., T.M., S.D., C.H., S.P., S.S., M.G., S.M. and A.M. provided clinical leadership and promoted unit participation and data collection. E.B. and M.G. provided methodological support. R.V.D., E.B. and J.H. drafted the statistical analysis plan and analysed the data. R.V.D., J.H., C.H., S.P., S.S. and A.M. contributed to data interpretation. R.V.D. led the study and wrote the first draft of the paper, with support from J.H. N.B., S.P., C.H., S.S. and A.M. reviewed and critically revised the manuscript and approved it before submission.
Declaration: R.V.D., E.B., J.M., M.C., S.E., N.B., A.S., T.M., S.D., C.H., S.P., S.S., M.G., S.M., and A.M. have nothing to declare. J.H. has been a Chief Investigator for Endomag®-funded research or CE marking of Magseed®, and has performed consultancy work for Endomag® 2018–2019.
Acknowledgements
Whipps Cross University Hospital, London: F. Teklebrhan, P. Frecker, Z. Ullah; Chesterfield Royal Hospital, Chesterfield: J. Massey, I. Azmy, E. Tokidis; St Helens and Knowsley Teaching Hospital NHS Trust, Prescot: C. Theodorou, B. Riogi, L. Chagla, J. Fong, H. Lennon, J. Atherton, K. Wilce, M. Reeves, S. Bathla, A. Ray; Luton and Dunstable University Hospital, Luton: K. Kirkpatrick, R. James; Kingston Hospital NHS Foundation Trust, Kingston-upon-Thames: Z. Winters, C. Richardson; Nottingham Breast Institute, Nottingham: G. Oni, K. Asgeirsson, L. Whisker, H. Khout, T. Rasheed, E. Gutteridge, D. Macmillan; The Royal Wolverhampton NHS Trust, Wolverhampton: B. Isgar, R. Vidya; North Manchester General Hospital, Manchester: K. Williams, M. Bramley, N. Nasir, M. S. Absar; Brighton and Sussex University Hospitals, Brighton: C. Zammit, S. Shaheed, A. Chouhan; St James University Hospital, Leeds: B. Hogan, B. Kim, K. Horgan, C. Navin, P. Turton, R. Achuthan, S. McKenzie; Manchester University NHS Foundation Trust, Manchester: A. Gandhi, S. Chatterjee, G. Byrne, A. Satpathy, C. C. Kirwan, L. Highton, J. Murphy, R. Johnson, V. Mathen, Z. Saad, N. Dimopoulos; Southmead Hospital, Bristol: J. Cook, S. Govindarajulu, S. Cawthorn, Z. Rayter, A. Sahu; Ninewells Hospital, Dundee: D. Brown, J. Macaskill, V. Pitsinis; Mid Cheshire Hospitals, Crewe: V. Pope, L. Khan, E. Khalifa; Royal Marsden Hospital, London: P. Barry, F. MacNeill, G. Gui, J. Rusby, K. Krupa, N. Roche, W. Allum; Frimley Health NHS Foundation Trust, Frimley: H. Osman, R. Daoud, I. Karat; Countess of Chester Hospital, Chester: J. Ooi, C. Harding-MacKean, E. Redmond, J. Seward; St Barts Hospital, London: J. Kelsall, S. Ledwidge, L. Johnson, J. Hu; Lincoln County Hospital, Lincoln: A. Jibril, L. Hyklova, A. Gvaramadze; Basingstoke and North Hampshire Hospital, Basingstoke: K. Harris, R. Stanton; Ulster Hospital, South Eastern Trust, Belfast: R. Kennedy, S. Kirk; Gartnaval General Hospital, Glasgow: S. Stallard, L. Romics; Hampshire Hospitals Foundation Trust, Winchester: N. Chand; Rotherham Hospital NHS Foundation Trust, Rotherham: L. Caldon, I. Kumar; Glenfield Hospital, Leicester: S. Shokuhi, K. Valassiadou, J. Krupa, S. Pilgrim, W. Sasi, F. Kenny, K. Lambert, M. Kaushik; Blackpool Victoria Hospital, Blackpool: P. Kiruparan, D. Archampong, D. Debnath; Wirral University Teaching Hospital NHS Foundation Trust, Birkenhead: J. Lund, M. Callaghan, R. Vinayagam, S. Poonawala, R. Burrah; Aintree University Hospital, Liverpool: L. Martin, M. Lafi; Lancaster Royal Infirmary, Lancaster: R. Parmeshwar, P. McManus; University Hospital North Tees, Stockton-on-Tees: P. Bhaskar, V. Kurup, C. Hennessy, K. Toe; Nobles Hospital, Douglas, Isle of Man: A. Chrysafi; Southern Health and Social Care Trust, Lurgan: H. Mathers.
Contributor Information
Rajiv V. Dave, The Nightingale Breast Cancer Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK
Emma Barrett, Department of Medical Statistics, Manchester University Hospitals NHS Foundation Trust, Wythenshawe Hospital, Manchester, UK.
Jenna Morgan, Department of Oncology and Metabolism, University of Sheffield Medical School, Sheffield, UK.
Mihir Chandarana, Breast Unit, Lincoln County Hospital, United Lincolnshire Hospitals NHS Trust, Lincoln, UK.
Suzanne Elgammal, Breast Unit, University Hospital Crosshouse, NHS Ayrshire and Arran, Kilmarnock, UK.
Nicola Barnes, The Nightingale Breast Cancer Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK.
Amtul Sami, Breast Unit, Lincoln County Hospital, United Lincolnshire Hospitals NHS Trust, Lincoln, UK.
Tahir Masudi, Breast screening and assessment unit, Rotherham General Hospital, Rotherham NHS Foundation Trust, Rotherham, UK.
Sue Down, Breast Unit, James Paget University Hospital, Great Yarmouth, UK.
Chris Holcombe, Breast Unit, Liverpool University Hospitals Foundation Trust, Liverpool, UK.
Shelley Potter, National Institute for Health Research Bristol Biomedical Research Centre, University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK; Bristol Breast Care Centre, North Bristol NHS Trust, Bristol, UK.
Santosh K. Somasundaram, Breast Screening Unit, Royal Lancaster Infirmary, Lancaster, UK
Matthew Gardiner, Department of Plastic Surgery, Frimley Health NHS Foundation Trust, Slough, UK; Kennedy Institute of Rheumatology, University of Oxford, Oxford, UK.
Senthurun Mylvaganam, Health Education West Midlands, Royal Wolverhampton NHS Trust, Wolverhampton, UK.
Anthony Maxwell, The Nightingale Breast Cancer Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK; Division of Informatics, Imaging & Data Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
James Harvey, The Nightingale Breast Cancer Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK; Division of Cancer Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
iBRA-NET Localisation Study collaborative:
A. Tanska, A. Hurley, A. Leusink, E. St John, I. Giono, K. Shanthakunalan, K. Harborough, K. Shenton, N. Gonen, Q. Ain, R. O’Connell, R. Law, V. Teoh, Z. Yan, A. Gaber Eltatawy, T. Rattay, A. Micha, M. Faheem, A. Tenovici, C. Baban, G. Ahmed, M. Joshi, K. Contractor, M. P. Charalambous, M. Kharashgah, M. Hanief, A. Milica, A. Khan, A. Bell, B. Smith, C. Sproson, C. Hollywood, K. A. Hodgkins, C. L. Rutherford, D. Thekkinkattil, D. Shanthakumar, E. Rahman, N. Amulya Mullapudi, A. Morad, E. Quinn, F. Moura, H. Bromley, J. Chen, L. Walter, M. Preston, N. Neyaz, S. Jafferbhoy, R. Osborne, E. Borg, E. Lumley, K. Wijesinghe, F. A. Ross, T. Davies, S. Tovey, H. Fatayer, I. J. Whitehead, J. Mondani, K. James, L. Darragh, T. Kiernan, U. Sridharan, S. Ashford, S. Laws, N. Robson, M. R. A. Matias, R. L. Wilson, S. H. Ali, M. Salman, M. Buhleigah, R. Rathinaezhil, S. Hignett, T. D. Schrire, and W. Lambert
Funding
This study was funded by the Association of Breast Surgery, London, UK. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or, writing of the report. The corresponding author had full access to the data in the study and had final responsibility for the decision to submit for publication.
Collaborators
Local investigators and members of the iBRA-NET Research Collaborative are recognized as PubMed citable collaborators:
The Royal Marsden Hospital, London: A. Tanska, A. Hurley, A. Leusink, E. St John, I. Giono, K. Shanthakunalan, K. Harborough, K. Shenton, N. Gonen, Q. Ain, R. O’Connell, R. Law, V. Teoh, Z. Yan; Glenfield Hospital, Leicester: A. Gaber Eltatawy; University Hospitals of Leicester NHS Trust, Leicester: T. Rattay; St Bartholomew Hospital, London: A. Micha, M. Faheem; The Parapet, King Edward VII Hospital, Frimley Health NHS Foundation Trust, London: A. Tenovici, C. Baban, G. Ahmed, M. Joshi, K. Contractor, M. P. Charalambous, M. Kharashgah, M. Hanief; Basingstoke and North Hampshire Hospital, Basingstoke: A. Milica; Ninewells Hospital, Dundee: A. Khan; University Hospital North Tees, Stockton-on-Tees: A. Bell; Royal Berkshire Hospital, Reading: B. Smith; Rotherham NHS Foundation Trust, Rotherham: C. Sproson; Chesterfield Royal Hospital, Chesterfield: C. Hollywood, K. A. Hodgkins. Gartnavel General Hospital, Glasgow: C. L. Rutherford; Lincoln County Hospital, Lincoln: D. Thekkinkattil; Whipps Cross University Hospital, London: D. Shanthakumar; The Royal Wolverhampton NHS Trust, Wolverhampton: E. Rahman, N. Amulya Mullapudi; Manchester University NHS Foundation Trust, Manchester: A. Morad, E. Quinn, F. Moura, H. Bromley, J. Chen, L. Walter, M. Preston, N. Neyaz, S. Jafferbhoy, R. Osborne; St James University Hospital, Leeds: E. Borg, E. Lumley, K. Wijesinghe; University Hospital Crosshouse, Kilmarnock: F. A. Ross, T. Davies, S. Tovey; North Manchester General Hospital, Manchester: H. Fatayer; Countess of Chester Hospital, Chester: I. J. Whitehead; Nottingham Breast Institute, Nottingham: J. Mondani; Wirral University Teaching Hospital NHS Foundation trust, Birkenhead: K. James; Ulster Hospital, South Eastern Trust, Belfast: L. Darragh; St Helens and Knowsley Teaching Hospitals NHS Trust, Prescot: T. Kiernan, U. Sridharan, S. Ashford; Hampshire Hospitals Foundation Trust, Winchester: S. Laws, N. Robson; Blackpool Victoria Hospital, Blackpool: M. R. A. Matias, R. L. Wilson; Royal Lancaster Infirmary, Lancaster: S. H. Ali; Nobles Hospital, Douglas, Isle of Man: M. Salman; Brighton and Sussex University Hospitals NHS Trust, Brighton: M. Buhleigah, R. Rathinaezhil; Mid Cheshire Hospitals, Crewe: S. Hignett; Southmead Hospital, Bristol: T. D. Schrire; Kingston Hospital NHS Foundation Trust, Kingston-upon-Thames: W. Lambert.
Supplementary material
Supplementary material is available at BJS online.
References
- 1. Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol 2005;34:405–412 [DOI] [PubMed] [Google Scholar]
- 2. Somasundaram SK, Potter S, Elgammal S, Maxwell AJ, Sami AS, Down SK et al. Impalpable breast lesion localisation, a logistical challenge: results of the UK iBRA-NET national practice questionnaire. Breast Cancer Res Treat 2021;185:13–20 [DOI] [PubMed] [Google Scholar]
- 3. Moreira IC, Ventura SR, Ramos I, Fougo JL, Rodrigues PP. Preoperative localisation techniques in breast conservative surgery: a systematic review and meta-analysis. Surg Oncol 2020;35:351–373 [DOI] [PubMed] [Google Scholar]
- 4. Rose A, Collins JP, Neerhut P, Bishop CV, Mann GB. Carbon localisation of impalpable breast lesions. Breast 2003;12:264–269 [DOI] [PubMed] [Google Scholar]
- 5. Haloua MH, Volders JH, Krekel NMA, Lopes Cardozo AMF, de Roos WK, de Widt-Levert LM et al. Intraoperative ultrasound guidance in breast-conserving surgery improves cosmetic outcomes and patient satisfaction: results of a multicenter randomized controlled trial (COBALT). Ann Surg Oncol 2016;23:30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kasem I, Mokbel K. Savi Scout® radar localisation of non-palpable breast lesions: systematic review and pooled analysis of 842 cases. Anticancer Res 2020;40:3633–3643 [DOI] [PubMed] [Google Scholar]
- 7. Lowes S, Bell A, Milligan R, Amonkar S, Leaver A. Use of Hologic LOCalizerTM radiofrequency identification (RFID) tags to localise impalpable breast lesions and axillary nodes: experience of the first 150 cases in a UK breast unit. Clin Radiol 2020;75:942–949 [DOI] [PubMed] [Google Scholar]
- 8. Zacharioudakis K, Down S, Bholah Z, Lee S, Khan T, Maxwell AJ et al. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur J Surg Oncol 2019;45:2016–2021 [DOI] [PubMed] [Google Scholar]
- 9. Schermers B, van der Hage JA, Loo CE, Vrancken Peeters MTFD, Winter-Warnars HAO, van Duijnhoven F et al. Feasibility of magnetic marker localisation for non-palpable breast cancer. Breast 2017;33:50–56 [DOI] [PubMed] [Google Scholar]
- 10. Nicolae A, Dillon J, Semple M, Hong NL, Ravi A. Evaluation of a ferromagnetic marker technology for intraoperative localization of nonpalpable breast lesions. AJR Am J Roentgenol 2019;212:727–733 [DOI] [PubMed] [Google Scholar]
- 11. Harvey JR, Lim Y, Murphy J, Howe M, Morris J, Goyal A et al. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Cancer Res Treat 2018;169:531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kühn F, Simon CEE, Aliyeva I, Kußmaul J, Groß J, Schweizerhof O et al. A German study comparing standard wire localization with magnetic seed localization of non-palpable breast lesions. In Vivo 2020;34:1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gera R, Tayeh S, Al-Reefy S, Mokbel K. Evolving role of Magseed in wireless localization of breast lesions: systematic review and pooled analysis of 1,559 procedures. Anticancer Res 2020;40:1809–1815 [DOI] [PubMed] [Google Scholar]
- 14. Micha AE, Sinnett V, Downey K, Allen S, Bishop B, Hector LR et al. Patient and clinician satisfaction and clinical outcomes of Magseed compared with wire-guided localisation for impalpable breast lesions. Breast Cancer 2021;28:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haskell H. Cumberlege review exposes stubborn and dangerous flaws in healthcare. BMJ 2020;370:m3099. [DOI] [PubMed] [Google Scholar]
- 16. Hirst A, Philippou Y, Blazeby J, Campbell B, Campbell M, Feinberg J et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg 2019;269:211–220 [DOI] [PubMed] [Google Scholar]
- 17. Bromley HL, Dave R, Holcombe C, Potter S, Maxwell AJ, Kirwan C et al. A novel mixed-methods platform study protocol for investigating new surgical devices, with embedded shared learning: iBRA-NET breast lesion localisation study. Int J Surg Protoc 2021;25:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Courtney A, O’Connell R, Rattay T, Kim B, Cutress RI, Kirwan CC et al. The B-MaP-C study: Breast cancer management pathways during the COVID-19 pandemic. Study protocol. Int J Surg Protoc 2020;24:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dave R, O’Connell R, Rattay T, Tolkien Z, Barnes N, Skillman J et al. The iBRA-2 (immediate breast reconstruction and adjuvant therapy audit) study: protocol for a prospective national multicentre cohort study to evaluate the impact of immediate breast reconstruction on the delivery of adjuvant therapy. BMJ Open 2016;6:e012678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mylvaganam S, Conroy EJ, Williamson PR, Barnes NLP, Cutress RI, Gardiner MD et al. Adherence to best practice consensus guidelines for implant-based breast reconstruction: results from the iBRA national practice questionnaire survey. Eur J Surg Oncol 2018;44:708–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Downey S, Chagla L, Chandran V, Gandhi A, Layer G, Sahu A et al. Association of Breast Surgery. QA Guidelines for Surgeons in Breast Cancer Screening https://associationofbreastsurgery.org.uk/media/64269/screening-guidelines.pdf (accessed 1 August 2018)
- 23. Chan BK, Wiseberg-Firtell JA, Jois RH, Jensen K, Audisio RA. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev 2015; (12)CD009206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price ER, Khoury AL, Esserman LJ, Joe BN, Alvarado MD. Initial clinical experience with an inducible magnetic seed system for preoperative breast lesion localization. AJR Am J Roentgenol 2018;210:913–917 [DOI] [PubMed] [Google Scholar]
- 25. Lake B, Wilson M, Thomas G, Williams S, Usman T. P006: The triple effect of the Magseed for localisation of impalpable breast cancer: significant reduction in re-excision rate, cost saving by reducing further surgery and high patient satisfaction. Eur J Surg Oncol 2020;46:e12 [Google Scholar]
- 26. Taylor DB, Bourke AG, Westcott EJ, Marinovich ML, Chong CYL, Liang R et al. Surgical outcomes after radioactive 125I seed versus hookwire localization of non-palpable breast cancer: a multicentre randomized clinical trial. Br J Surg 2021;108:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haloua MH, Volders JH, Krekel NMA, Barbe E, Sietses C, Jozwiak K et al. A nationwide pathology study on surgical margins and excision volumes after breast-conserving surgery: there is still much to be gained. Breast 2016;25:14–21 [DOI] [PubMed] [Google Scholar]
- 28. Langhans L, Jensen MB, Talman MLM, Vejborg I, Kroman N, Tvedskov TF. Reoperation rates in ductal carcinoma in situ vs invasive breast cancer after wire-guided breast-conserving surgery. JAMA Surg 2017;152:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Public Health England . NHS Breast Screening Programme & Association of Breast Surgery. An Audit of Screen Detected Breast Cancers for the Year of Screening April 2017 to March 2018; 2019. https://associationofbreastsurgery.org.uk/media/65088/nhsbsp-abs-audit-2017-to-2018.pdf (accessed June 2021)
- 30. Wright CM, Moorin RE, Saunders C, Marinovich ML, Taylor DB, the ROLLIS study group. Cost-effectiveness of radioguided occult lesion localization using 125I seeds versus hookwire localization before breast-conserving surgery for non-palpable breast cancer. Br J Surg 2021;108:843–850 [DOI] [PubMed] [Google Scholar]
- 31. Pieri A, Milligan R, Critchley A, O’Donoghue JM, Sibal N, Peace R et al. The introduction of radioactive seed localisation improves the oncological outcome of image guided breast conservation surgery. Breast 2017;36:49–53 [DOI] [PubMed] [Google Scholar]
- 32. Langhans L, Tvedskov TF, Klausen TL, Jensen MB, Talman ML, Vejborg I et al. Radioactive seed localization or wire-guided localization of nonpalpable invasive and in situ breast cancer: a randomized, multicenter, open-label trial. Ann Surg 2017;266:29–35 [DOI] [PubMed] [Google Scholar]
- 33. Boundouki G, Wong Sik Hee JR, Croghan N, Stocking K, Pieri A, Critchley A et al. Comparing long-term local recurrence rates of surgical and non-surgical management of close anterior margins in breast conserving surgery. Breast Cancer Res Treat 2019;176:311–319 [DOI] [PubMed] [Google Scholar]
- 34. Greenwood HI, Wong JM, Mukhtar RA, Alvarado MD, Price ER. Feasibility of magnetic seeds for preoperative localization of axillary lymph nodes in breast cancer treatment. AJR Am J Roentgenol 2019;213:953–957 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.