Abstract
Background
Incisional hernias cause morbidity and may require further surgery. HART (Hughes Abdominal Repair Trial) assessed the effect of an alternative suture method on the incidence of incisional hernia following colorectal cancer surgery.
Methods
A pragmatic multicentre single-blind RCT allocated patients undergoing midline incision for colorectal cancer to either Hughes closure (double far–near–near–far sutures of 1 nylon suture at 2-cm intervals along the fascia combined with conventional mass closure) or the surgeon’s standard closure. The primary outcome was the incidence of incisional hernia at 1 year assessed by clinical examination. An intention-to-treat analysis was performed.
Results
Between August 2014 and February 2018, 802 patients were randomized to either Hughes closure (401) or the standard mass closure group (401). At 1 year after surgery, 672 patients (83.7 per cent) were included in the primary outcome analysis; 50 of 339 patients (14.8 per cent) in the Hughes group and 57 of 333 (17.1 per cent) in the standard closure group had incisional hernia (OR 0.84, 95 per cent c.i. 0.55 to 1.27; P = 0.402). At 2 years, 78 patients (28.7 per cent) in the Hughes repair group and 84 (31.8 per cent) in the standard closure group had incisional hernia (OR 0.86, 0.59 to 1.25; P = 0.429). Adverse events were similar in the two groups, apart from the rate of surgical-site infection, which was higher in the Hughes group (13.2 versus 7.7 per cent; OR 1.82, 1.14 to 2.91; P = 0.011).
Conclusion
The incidence of incisional hernia after colorectal cancer surgery is high. There was no statistical difference in incidence between Hughes closure and mass closure at 1 or 2 years.
Registration number
ISRCTN25616490 (http://www.controlled-trials.com).
In this single-blind multicentre RCT, 802 patients were assigned randomly to either Hughes closure (double far–near–near–far sutures combined with mass closure) compared with surgeon choice of mass closure to assess the incidence of incisional hernia at 1-year follow-up. There was no significant difference between the Hughes closure technique and standard mass closure in the rate of incisional hernia at 1-year follow-up. This was the first randomized trial to include a combination of interrupted and mass closure in the intervention arm.
Introduction
Incisional hernias are defined as ‘abdominal wall gaps around postoperative scars, perceptible or palpable by clinical examination or imaging’1,2. They are a common complication after abdominal surgery and can result in a reduction in quality of life3.
A meta-regression4 of 13 400 patients reported a weighted incidence of incisional hernia of 12.8 per cent at 2 years after midline incisional surgery, and suggested that approximately one-third of these patients end up having further surgery. This indicates that patients with a midline abdominal incision have a 5 per cent chance of undergoing further surgery for incisional hernia. Incisional hernia can cause morbidity ranging from discomfort and embarrassment through to obstruction and strangulation. Repairing incisional hernias is also associated with significant morbidity and mortality, with reported recurrence rates of up to 45 per cent5 and considerable healthcare resource use6–10. Therefore, surgeons are beholden to adopt strategies that reduce the risk of incisional hernias, and the subsequent burden on both the patient and the healthcare system.
Incisional hernia prevention has begun to achieve more attention in recent years. The European Hernia Society11 set out its recommendations in 2015 for abdominal wall closure. These included avoiding midline incisions, and using a slowly absorbable continuous suture and a small-stitch closure technique in elective procedures. A Cochrane review12 in 2017 concluded that there was no evidence for one specific closure technique over another. However, the review excluded the small-stitch technique as it had not compared suture material or technique as the authors classified it.
The small-stitch technique (5-mm bites, 5 mm apart on the anterior sheath using a 2/0 needle) was examined in the STITCH RCT13. The trial recruited 560 patients and found a statistically significant difference in the incidence of incisional hernia at 1-year follow-up between the small-bite and traditional large-bite groups (13 versus 21 per cent; P = 0.02). The incidence in the small-bite group is thus consistent with that in the meta-regression analysis. Current published evidence suggests that small stitch is the strongest evidenced suture technique for abdominal wall closure but, worldwide, its adoption has been slow even though it is relatively straightforward to learn. Explanations offered for this include increased time for closure and the lack of evidence to support its use in an emergency setting or in obese patients.
The eponymously titled Hughes closure was widely promoted as a technique for repairing incisional hernias14. It is also known as the Cardiff repair or far-and-near technique15. HART (Hughes Abdominal Repair Trial), a pragmatic RCT, aimed to assess use of the Hughes closure in the prevention of incisional hernia versus standard mass closure for closure of midline abdominal wall incisions in patients undergoing colorectal cancer surgery.
Methods
Study design
This prospective pragmatic single-blind RCT was performed in 28 UK hospitals. The full protocol has been published previously16. Patients with colorectal cancer were studied owing to their perceived high incidence of incisional hernia, high compliance with follow-up, and the knowledge that CT would be performed routinely at 1 year and often 2 years as part of the normal follow-up process. Inclusion criteria required patients to be aged 18 years or over and able to give informed consent. Both patients scheduled for elective colorectal cancer surgery following full staging investigations including CT of the abdomen and pelvis, and those presenting as an emergency with a strong suspicion of colorectal cancer on CT and a midline incision of 5 cm or more, including laparoscopic extraction sites or conversions, were included. Exclusion criteria were: insertion of mesh as part of the abdominal closure and requirement for myocutaneous flap closure of the perineal defect in abdominoperineal excision of the rectum.
The study was registered with the ISRCTN registry (25616490) and was approved by the Multi-centre Research Ethics Committee for Wales (12/WA/0374).
Randomization and blinding
The trial design required that randomization took place in the operating theatre towards the end of the procedure when all inclusion criteria had been confirmed. Randomization was by telephone using a 1 : 1 computerized adaptive system administered by Sealed EnvelopeTM, London, UK. The patients were blinded to the group allocation. The surgeon carrying out the closure was asked not to record the closure technique in the operative record, and clinicians undertaking assessments at 1 and 2 years were asked to complete the case report form before accessing patient notes in order to maximize blinding. The HART Data Monitoring Committee was able to access unblinded data at 3–6-month intervals to monitor safety, and to recommend full trial stoppage.
Procedures
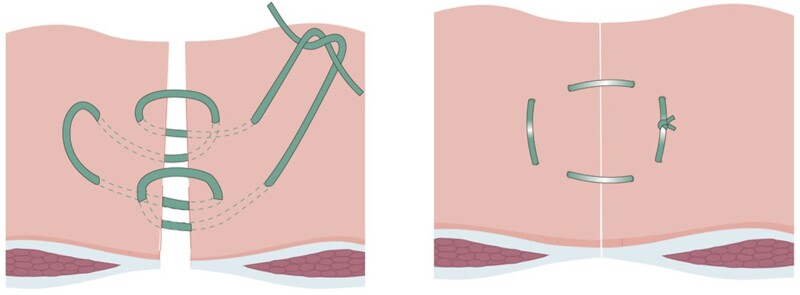
The Hughes closure technique involved placement of double far–near–near–far sutures of 1 nylon suture at 2-cm intervals along the fascia, followed by conventional mass closure using one-loop polydioxanone suture (Fig. 1). In this pragmatic trial, standard mass closure was the individual surgeon’s normal technique of mass closure, and this choice was recorded. A feasibility study was performed with 30 patients to assess recruitment, technique, and trial documentation. This was published17 and the data were not included in the main trial.
Fig. 1.
Hughes closure interrupted suture Reproduced with permission from Cornish et al.16
Training in the technique was provided at each site before the start of recruitment. Patients were asked to complete a 30-day wound diary. They were also asked to complete quality-of-life questionnaires at baseline, 30 days, 6 months, and 12 months. The primary outcome measure was assessed in an outpatient setting at 12 months after surgery (+/− 2-month window). This comprised a clinical examination by a trained assessor blinded to the closure method, with the patient both standing and lying, to assess the wound for evidence of incisional hernia.
Quality-of-life data were collected using recognized and validated patient-reported outcome measurement tools: the generic quality-of-life Short Form 12 (SF-12®)18 and the condition-specific Functional Analysis of Cancer Therapy—Colorectal (FACT-C)19.
Outcomes
The primary outcome was the incidence of incisional hernia over 1 year as assessed by clinical examination of the abdomen. Secondary outcomes included: quality of life over 1 year following colorectal cancer surgery between the Hughes and standard mass closure arms (principal secondary aim); incidence of incisional hernia at 2 years as assessed by clinical examination of the abdomen; incidence of incisional hernia over 1 year as assessed by CT of the abdomen and pelvis, assessed by two independent blinded consultant radiologists; incidence of postoperative ‘burst abdomen’ (complete abdominal wound dehiscence) by day 30; identification of patient and surgical factors that increase the risk of developing incisional hernias; and comparison of quality of life between patients with incisional hernias and those without incisional hernias in both arms of the study over 1 year. Adverse events, including surgical-site infections (SSIs), were also recorded for data monitoring purposes; specific adverse events are presented according to the Clavien–Dindo classification20, and SSIs were defined according to Centers for Disease Control and Prevention recommendations21. Secondary and tertiary outcomes of cost-effectiveness, and comparison of sensitivity and specificity of CT identification of incisional hernia compared with clinical examination are not reported in this paper and will be reported elsewhere.
Statistical analysis
An opportunistic retrospective clinical review at the lead site suggested that the difference in incisional hernia rates between Hughes closure and mass closure could be as high as 18 per cent. Based on data from that and the authors’ systematic literature review, a reduction in incisional hernia rates from 30 per cent for mass closure to 20 per cent for the Hughes closure was postulated. To give 80 per cent power to detect this difference with a 5 per cent significance level required follow-up of 640 patients at 1 year. Anticipating loss to follow-up of 20 per cent at 1 year, the aim was to recruit 800 patients in total.
Statistical analyses, documented and agreed in advance of data release, used the treatment-allocated (intention-to-treat) principle, reflecting group allocations at randomization.
Continuous variables with an approximately normal distribution were summarized using non-missing sample size and mean(s.d.). Non-parametric continuous variables were summarized using median (i.q.r.). Categorical variables were summarized using frequencies and percentages. All hypothesis testing was two-tailed with a 5 per cent significance level and no adjustment for multiple testing.
Binary logistic regression analysis was undertaken for the outcome variable incisional hernia adjusted for all important baseline co-variates and factors. Confounders were selected by stepwise backward selection, starting from the full model with all co-variates/factors, and then iteratively removing the least significant, until only statistically significant confounders and the group indicator remained. In addition to group indicator, the full model included: age; sex; BMI; diabetes; chemotherapy, radiotherapy; history of high level of alcohol use; history of chronic obstructive pulmonary disease; any incisional hernia present clinically; ASA fitness grade; site recruiter status (high, at least 50 enrolled participants; low, less than 50 enrolled participants); and baseline quality-of-life measures, comprising the SF-12® Physical and Mental Component Summary (PCS and MCS) scores, FACT-C score, and POSSUM value. Data processing and analyses were carried out using Stata® version 16 (StataCorp, College Station, TX, USA).
Results
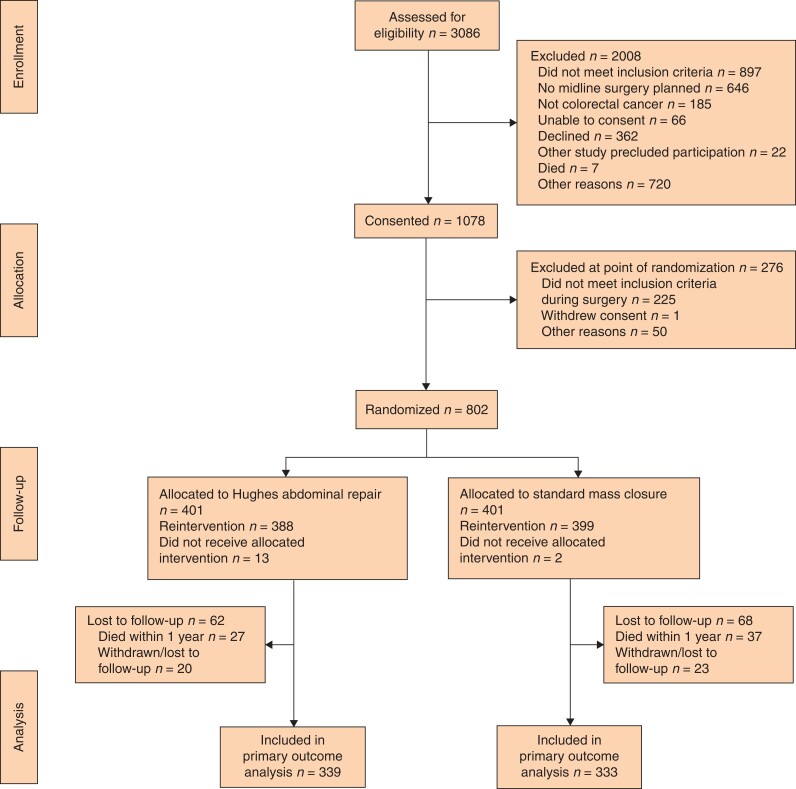
Between August 2014 and February 2018, a total of 802 patients were randomized to either Hughes closure (401) or standard mass closure (401) groups (Fig. 2). The median age was 70 (i.q.r. 60–77) years and there were 509 men. Baseline characteristics were similar in the two groups (Table 1). Two-year follow-up was complete in February 2020. At 1-year follow-up, 672 patients (83.7 per cent) were examined for the primary endpoint, 339 in the Hughes closure and 333 the standard closure group.
Fig. 2.
CONSORT diagram for the trial
*Logistical reasons (376 patients), private patient (35), reason not stated (309). †Logistical reasons (18), surgeon decision (17), patient eligibility 14, reason not stated (1). ‡A small number of patients (Hughes repair, n = 15; standard mass closure, n = 8) reported no year 1 data and could not be included in year 1 analysis, as no study discontinuation form had been completed.
Table 1.
Baseline patient characteristics
| Hughes closure (n = 401) | Standard mass closure (n = 401) | |
|---|---|---|
| Age (years), median (i.q.r.) | 69 (61–77) | 70 (60–78) |
| Sex | ||
| M | 262 (65.3) | 247 (61.6) |
| F | 139 (34.7) | 154 (38.4) |
| BMI (kg/m2), median (i.q.r.) | 27.3 (24.7–30.8) | 27.0 (24–30.6) |
| Missing | 4 (1) | 5 (1.3) |
| Risk factors | ||
| Diabetes mellitus | 65 (16.2) | 68 (17.0) |
| COPD | 50 (12.5) | 62 (15.5) |
| Abdominal aortic aneurysm | 4 (1.0) | 2 (0.5) |
| Current smoker | 31 (7.7) | 37 (9.2) |
| Missing | 2 (0.5) | |
| Excessive alcohol intake* | 25 (6.2) | 35 (8.7) |
| Missing | 1 (0.2) | |
| Neoadjuvant chemotherapy | 43 (10.7) | 34 (8.5) |
| Neoadjuvant radiotherapy | 38 (9.5) | 32 (8.0) |
| Previous abdominal surgery | 167 (41.67) | 169 (42.1) |
| Incisional hernia | 9 (2.2) | 3 (0.7) |
| Missing | 2 (0.5) | |
| Non-incisional hernia† | 35 (8.7) | 28 (7.0) |
| Missing | 2 (0.5) | |
| ASA fitness grade | ||
| I | 52 (13.0) | 51 (12.7) |
| II | 223 (55.6) | 233 (58.1) |
| III | 121 (30.2) | 110 (27.4) |
| IV | 5 (1.2) | 5 (1.2) |
| Missing | 2 (0.5) | |
| Quality-of-life measures, mean (s.d.) | ||
| SF-12®: PCS score | 43.6 (5.5) | 43.7 (5.5) |
| Missing | 78 (19.4) | 82 (20.4) |
| SF-12®: MCS score | 52.5 (11.9) | 52.9 (12.1) |
| Missing | 82 (20.4) | 78 (19.5) |
| FACT-C score | 70.5 (9.9) | 71.7 (10.0) |
| POSSUM, mean(s.d.) | 8.3 (7.9) | 7.8 (7.8) |
| Missing | 88 (21.9) | 90 (22.4) |
Values are n (%) unless indicated otherwise. Missing values are stated only when more than 0.
*More than 21 units/week for women and more than 28 units/week for men.
†Includes inguinal, umbilical, and epigastric hernias. COPD, chronic obstructive pulmonary disease; SF, Short Form; PCS, Physical Component Summary; MCS, Mental Component Summary; FACT-C, Functional Assessment of Cancer Therapy—Colorectal.
At 1 year after surgery, 50 patients (14.8 per cent) in the Hughes closure group and 57 (17.1 per cent) in the standard mass closure group had an incisional hernia on clinical examination (OR 0.84, 95 per cent c.i. 0.55 to 1.27; P = 0.402) (Table 2). Respective numbers at 2 years were 78 (28.7 per cent) and 84 (31.8 per cent) (OR 0.86, 0.59 to 1.25; P = 0.429). Among the 107 patients with an incisional hernia at 1 year, 20 hernias (18.7 per cent) were repaired by year 2 (9 in the Hughes closure group and 11 in the standard mass closure group). A total of 681 CT scans were performed at 1-year follow-up; 158 patients (47.0 per cent) in the Hughes closure group had an incisional hernia diagnosed on CT compared with 165 (47.8 per cent) in the standard mass closure group (OR 0.97, 0.72 to 1.31; P = 0.834).
Table 2.
Incisional hernia incidence by clinical examination in each group
| Hughes closure | Standard mass closure | |
|---|---|---|
| 1 year | 50 of 339 (14.8) | 57 of 333 (17.1) |
| 2 years | 78 of 271 (28.7) | 84 of 264 (31.8) |
Values are n (%).
At 1 year after surgery, 426 (69.3 per cent), 427 (69.5 per cent), and 415 (68.0 per cent) of 672 patients completed the SF-12® PCS, SF-12® MCS, and FACT-C scores respectively. The mean SF-12® PCS, SF-12® MCS, and FACT-C scores at any time point (baseline, 30 days, 6 months, and 1 year) were similar in the two arms (Table S1). Patients with an incisional hernia during follow-up (regardless of allocated arm) had significantly lower mean PCS scores (indicating poorer physical health) at baseline compared with patients without an incisional hernia (P = 0.003), but this difference was not observed at subsequent time points (30 days, 6 months, and 1 year) (Table S2). No significant differences were observed at any time point in mean SF-12® MCS or FACT-C scores between patients with or without incisional hernia at 1 year.
The median time taken for fascial closure was significantly longer for Hughes closure than for standard mass closure (20 versus 11 min; P < 0.001) (Table 3). Three patients had full-thickness wound dehiscence at 30 days after surgery (0.8 per cent), one in the Hughes closure group (0.3 per cent) and two in the standard mass closure group (0.5 per cent). There was no difference between groups in general complications (Table 4). There was a significantly higher SSI rate in the Hughes closure group compared with the standard mass closure group (13.2 versus 7.7 per cent; OR 1.82, 1.14 to 2.91; P = 0.010). There was no difference between SSI types (superficial, deep or organ/space occupying) and closure group. Logistic regression analysis adjusting for the baseline characteristics suggested that increased age, male sex, increased BMI, higher POSSUM value, preoperative radiotherapy, and emergency admission increased the odds of incisional hernia formation; age, BMI, and preoperative radiotherapy were the only variables significant at both 1 and 2 years (Table 5).
Table 3.
Intraoperative characteristics
| Hughes closure (n = 401) | Standard mass closure (n = 401) | |
|---|---|---|
| Grade of surgeon performing closure | ||
| Surgical trainee | 136 (33.9) | 208 (51.9) |
| Consultant/attending | 262 (65.3) | 187 (46.6) |
| Missing | 3 (0.7) | 6 (1.5) |
| Surgical urgency | ||
| Elective | 371 (92.5) | 372 (92.8) |
| Emergency | 30 (7.5) | 29 (7.2) |
| Operative procedure | ||
| Abdominoperineal resection | 19 (4.7) | 13 (3.2) |
| Anterior resection | 130 (32.4) | 122 (30.4) |
| Hartmann’s procedure | 22 (5.5) | 26 (6.5) |
| Left hemicolectomy | 19 (4.7) | 18 (4.5) |
| Right hemicolectomy | 131 (32.7) | 147 (36.7) |
| Extended right hemicolectomy | 24 (6.0) | 34 (8.5) |
| Panproctocolectomy | 3 (0.7) | 1 (0.2) |
| Subtotal colectomy | 9 (2.2) | 11 (2.7) |
| Sigmoid colectomy | 17 (4.2) | 10 (2.5) |
| Other | 27 (6.7) | 19 (4.7) |
| Stoma formed | ||
| End ileostomy | 8 (2.0) | 8 (2.0) |
| Loop ileostomy | 71 (17.7) | 64 (16.0) |
| End colostomy | 49 (12.2) | 49 (12.2) |
| Loop colostomy | 3 (0.7) | 3 (0.7) |
| Other | 11 (2.7) | 0 (0) |
| Surgical approach | ||
| Open | 171 (42.6) | 151 (37.7) |
| Laparoscopic | 125 (31.2) | 127 (31.7) |
| Laparoscopically assisted | 35 (8.7) | 64 (16.0) |
| Laparoscopic converted to open | 70 (17.5) | 59 (14.7) |
| Colorectal cancer resected | 399 (99.5) | 400 (99.8) |
| Intraoperative transfusion requirement | 21 (5.2) | 14 (3.5) |
| No. of units transfused, mean | 2.0 | 1.6 |
| Intraoperative complications | 20 (5.0) | 10 (2.5) |
| Missing | 1 (0.2) | |
| Intraoperative use of antiadhesive agent | 7 (1.7) | 6 (1.5) |
| Missing | 3 (0.7) | 5 (1.2) |
| Final length of midline incision (cm), median (i.q.r.) | 15 (7–21) | 15 (7–20) |
| Missing | 9 (2.2) | 20 (5.0) |
| Skin closure method | ||
| Surgical clips | 162 (40·.4) | 144 (35.9) |
| Subcuticular absorbable suture(s) | 238 (59.4) | 250 (62.3) |
| Interrupted sutures | 1 (0.2) | 2 (0.5) |
| Other | 0 (0) | 4 (1.0) |
| Missing | 1 (0.2) | |
| Total procedure duration (min), median (i.q.r.) | 180 (148–240) | 180 (130–220) |
| Missing | 4 (1.0) | 8 (2.0) |
| Time taken for fascial closure (min), median (i.q.r.) | 20 (15–28) | 11 (9–16) |
| Missing | 8 (2.0) | 19 (4.7) |
| Duration of postoperative hospital stay (days), median (i.q.r.) | 7 (5–12) | 7 (5–11) |
| Level of postoperative care | ||
| ICU (ITU) | 19 (4.7) | 9 (2.2) |
| High-dependency unit | 96 (23.9) | 100 (24.9) |
| Ward-level care | 286 (71.3) | 292 (72.8) |
Values are n (%) unless indicated otherwise. Missing values are stated only when more than 0. ITU, intensive therapy unit.
Table 4.
Surgical complications according to Clavien–Dindo classification
| Complication grade | Hughes closure (n = 139) | Standard mass closure (n = 142) |
|---|---|---|
| I | 21 (15.1) | 24 (16.9) |
| II | 38 (27.3) | 29 (20.4) |
| III | 17 (12.2) | 16 (11.3) |
| IV | 10 (7.2) | 10 (7.0) |
| V | 53 (38.1) | 63 (44.4) |
Values are number of events (%).
Table 5.
Logistic regression model of factors influencing incisional hernia formation at 1 and 2 years
| Year 1 | Year 2 | |||
|---|---|---|---|---|
| OR | P | OR | P | |
| Hughes closure | 0.73 (0.48, 1.12) | 0.165 | 0.79 (0.54, 1.17) | 0.235 |
| Age | 1.03 (1.01, 1.05) | 0.009 | 1.02 (1.00, 1.04) | 0.023 |
| Male sex | 1.72 (1.07, 2.77) | 0.027 | 1.48 (0.98, 2.27) | 0.070 |
| BMI | 1.05 (1.01, 1.10) | 0.053 | 1.07 (1.02, 1.10) | 0.002 |
| Radiotherapy use | 3.80 (1.37, 9.45) | 0.010 | 3.30 (1.20, 9.02) | 0.020 |
| POSSUM | 0.99 (0.95, 1.03) | 0.692 | 1.03 (1.00, 1.08) | 0.034 |
| SF-12®: PCS (baseline) | 0.96 (0.92, 1.00) | 0.054 | 0.97 (0.93,1.01) | 0.096 |
| Emergency admission | 2.46 (1.07, 5.68) | 0.034 | 2.16 (0.90, 5.19) | 0.084 |
Values in parentheses are 95 per cent confidence intervals. SF, Short Form; PCS, Physical Component Summary.
Discussion
This study found no significant difference in the clinically detected incidence of incisional hernia at either 1 or 2 years between the Hughes closure and standard mass closure groups. The 1-year incidence rate of incisional hernia in the intervention arm of the present trial (14.8 per cent) was similar to that in the intervention arm of the STITCH trial (13 per cent)13. The STITCH trial included only patients who had elective procedures with a median BMI of 24 kg/m2, but with ultrasound-detected hernias alongside clinical examination as the primary outcome. Despite the inclusion of emergency procedures and an overall median BMI of 27 kg/m2 in the present trial, there remains a similarity between the rates. Neither study has been able to reduce the incisional hernia rate below that reported in the meta-regression4 by suture technique alone.
At 2 years, the rate of incisional hernia had approximately doubled in both groups (28.7 per cent in the Hughes closure group and 31.8 per cent in the standard mass closure group). This is consistent with other studies22,23 reporting that incidence of incisional hernia increases over time. Many trials do not report outcomes beyond 1–2 years. The present authors have secured funding to obtain follow-up data beyond 2 years, which will be reported in due course.
Since the trial commenced, it is now generally accepted that radiological diagnosis of incisional hernia is crucial in prospective trials, and that primary outcomes should include medical imaging as recommended by the European Hernia Society11. Reporting guidelines24 for interventional trials of incisional ventral hernias have determined CT to be the optimal detection method. Patients with colorectal cancer were the population of choice in the present study because CT is the standard for follow-up surveillance; there was no additional radiation exposure, and it was possible to report CT-detected incisional hernia at 1 year. There was no difference between the two closure groups in radiological detection of incisional hernias at 1 year; however, the hernia detection rate was more than double that by clinical examination, highlighting that clinical examination can underestimate the incidence of incisional hernia.
Patients who underwent Hughes closure had a significantly higher SSI rate than those in the standard mass closure group (13.2 versus 7.7 per cent; OR 1.82, 95 per cent c.i. 1.14 to 2.91; P = 0.010). This would be considered by most commentators as a low rate of SSI for colorectal surgery, and may reflect the higher standard that patients who are effectively in a wound trial are subjected to25. It is hypothesized that two factors may have contribute to significant difference seen. The first is that the far–near suture was performed using nylon, a non-absorbable suture. This is consistent with recent meta-analyses26–28 showing that, although non-absorbable suture reduced incisional hernia rates compared with slowly absorbable sutures, it increased the rate of suture sinuses. The second is that there was more suture, and in particular knot, material present in the closure, thus creating a higher surface area of foreign material for potential SSI. Despite an increase rate of SSI in the Hughes closure group, there was no difference between the two groups across quality-of-life scores at any time during follow-up.
Interestingly, patients who were found to have an incisional hernia during follow-up (regardless of allocated arm) had a significantly lower mean PCS score at baseline than those without an incisional hernia (P = 0.003). This difference was not observed at any other time point during follow-up. This finding suggests the importance of patient fitness before surgery and adds to an ever-growing body of evidence supporting a role for prehabilitation before colorectal cancer surgery29–32. No studies to date have shown the significance of this in relation to subsequent incisional hernia formation. This implies that, as well as focusing on surgical closure technique to prevent incisional hernia, efforts should be made to address risk factors before operation. The logistic regression analysis confirmed risk factors for incisional hernia formation that are widely accepted. However, interestingly, it showed that neoadjuvant radiotherapy increased the rate of incisional hernia formation at both 1 and 2 years. The authors hypothesize that this could be a result of the field of radiation affecting small vessels in the lower aspect of the midline incision. Alternatively, it could be due to a technically more challenging operation with a radiated field resulting in a longer operation, or because the patient was more likely to be deconditioned after preoperative radiotherapy. It is important to note that this finding applied to a relatively small subset of patients in the trial.
The HART study has demonstrated beyond doubt that patients with colorectal cancer are at particular risk of incisional hernia. The study team was conscious that a study looking to prevent incisional hernia may well lead to a reduced rate of the event being studied because of heightened awareness of the condition of interest to the study. The incidence of incisional hernia at 1 and 2 years and based on CT findings illustrates that this is a significant problem in this patient group that colorectal surgeons need to be acutely aware of. This is supported by the retrospective findings from the recently published French national database that colorectal surgery was responsible for 72 per cent of patients requiring repair of incisional hernia after laparotomy33.
This study has some limitations. It was a pragmatic trial, and, although this was chosen to maximize engagement and recruitment, there was wider variation in the surgeon choice of closure technique in the mass closure arm than was anticipated and this may have affected the results. There was potentially a change in practice after publication of the STITCH trial13, shortly after the present study commenced. This is likely to have introduced two issues influencing the present findings. First, surgeons were learning a second new technique (small stitch) in a non-controlled fashion which potentially confounded the control arm. Second, it led to some surgeons to lose equipoise as they may have considered incisional hernia prevention resolved and there was no role for the Hughes closure.
Many incisional hernia prevention trials have excluded emergency surgery. Patients undergoing emergency procedures were included in the present trial in order to reflect real-world practice; however, only 59 such patients were included, representing just 7.3 per cent of the study population. In the UK, up to 20 per cent of patients with colorectal cancer present in the emergency setting, suggesting that a number of potentially eligible patients will not have been identified34. This may be because of concerns about giving eligible patients enough time to decide to take part in the study. There are currently two ongoing randomized trials investigating continuous versus interrupted sutures35, and small-bite versus large-bite sutures36 in the closure of emergency midline laparotomies. The results of these will add to the paucity of evidence for such procedures.
Intraoperative randomization also highlighted the issue of equipoise. The Hughes closure took longer to perform with a median time for fascial closure of 20 min, compared with 11 min in the standard closure arm (P < 0.001). It is possible that, at the end of difficult procedures, surgeons chose not to randomize and so this important subgroup of patients were lost to the study. This conjecture is supported by the observation that not all patients received the allocated treatment. There were 15 such patients in total, notably 13 in the Hughes closure group. In addition, for patients who had consented but were subsequently not randomized, reported reasons for non-randomization included ‘ran out of time’. These arguments around equipoise may also explain the large numbers of patients screened but not entering the trial. In addition, screening logs do not report the route (emergency or elective) through which patients considered eligible, but not consented, were identified.
Despite attempts to prevent incisional hernia by use of different surgical suture techniques, the incidence still remains too high. The use of prophylactic mesh augmentation is likely to be the next area of focus in incisional hernia prevention research. The HULC trial37 is currently recruiting and investigating the role of small-bite closure combined with mesh insertion in elective midline laparotomies. Future research should also include the role of prehabilitation in incisional hernia prevention.
Collaborators
HART Collaborative: J. Torkington (University Hospital of Wales, Cardiff, UK); R. Harries (Swansea Bay University Health Board, Swansea, UK); S. O'Connell, L. Knight (Cedar Healthcare Technology Research Centre, Cardiff, UK); S. Islam, N. Bashir, A. Watkins, G. Fegan (Swansea Trials Unit, Swansea, UK); J. Cornish (Cardiff and Vale University Health Board, Cardiff, UK); B. Rees, H. Cole, H. Jarvis, S. Jones, I. Russell, D. Bosanquet, A. Cleves, B. Sewell, A. Farr, N. Zbrzyzna, N. Fiera, R. Ellis- Owen, Z. Hilton, C. Parry; A. Bradbury (Chair), T. Rockall, A. Windsor, V. Allgar, W. Hollingworth, C. Lovell-Smith, J. Hepburn, M. Gudgeon, R. Gabe, C. Thomson; P. Wall, J. Hill, D. Winter, K. Cocks (University Hospital of Wales, Cardiff, UK); D. Harris (Singleton Hospital, Swansea, UK); J. Hilton (Princess of Wales Hospital, Bridgend, UK); S. Vakis (Queens Hospital, Burton on Trent, UK); D. Hanratty (Royal Glamorgan Hospital, Llantrisant, UK); R. Rajagopal, F. Akbar (Glan Clwd Hospital, Rhyl, UK); A. Ben-Sassi (Wrexham Maelor Hospital, Wrexham, UK); N. Francis (Yeovil District Hospital, Yeovil, UK); L. Jones (Royal Blackburn Hospital, Blackburn, UK); M. Williamson (Royal United Hospital, Bath, UK); I. Lindsey (Churchill Hospital, Oxford, UK); R. West (Weston General Hospital, Weston Super Mare, UK); C. Smart (Macclesfield Hospital, UK); P. Ziprin (St Mary's Hospital, London, UK); T. Agarwal Ealing Hospital, London, UK); G. Faulkner (Royal Bolton Hospital, Bolton, UK); T. Pinkney (Queen Elizabeth II Hospital, Birmingham, UK); D. Vimalachandran (Countess of Chester Hospital, Chester, UK); D. Lawes (Maidstone District Hospital, Maidstone, UK); O. Faiz (St Marks Hospital, London, UK); P. Nisar (St Peter's Hospital, Chertsey, UK); N. Smart (Royal Devon and Exeter Hospital, Exeter, UK); T. Wilson (Doncaster Royal Infirmary, Doncaster, UK); A. Myers (Hillingdon Hospital, Uxbridge, UK); J. Lund (Royal Derby Hospital , Derby, UK); S. Smolarek (Derriford Hospital, Plymouth, UK); A. Acheson (Queens Medical Centre Hospital, Nottingham, UK); J. Horwood, J. Ansell, S. Phillips, M. Davies, L. Davies, S. Bird, N. Palmer, M. Williams, G. Galanopoulos, P. Dhruva Rao, D. Jones, R. Barnett, S. Tate, J. Wheat, N. Patel, S. Rahmani, E. Toynton, L. Smith, N. Reeves, E. Kealaher, G. Williams (University Hospital of Wales, Cardiff, UK); C. Sekaran, M. Evans, J. Beynon, R. Egan, E. Qasem, U. Khot, S. Ather, P. Mummigati, G. Taylor, J. Williamson, J. Lim, A. Powell, H. Nageswaran, A. Williams, J. Padmanabhan, K. Phillips, T. Ford, J. Edwards, N. Varney, L. Hicks, C. Greenway, K. Chesters, H. Jones, P. Blake, C. Brown (Singleton Hospital, Swansea, UK); L. Roche, D. Jones, M. Feeney (Princess of Wales Hospital, Bridgend, UK); P. Shah (Royal Glamorgan Hospital, Llantrisant, UK); C. Rutter (Glan Clwyd Hospital, Rhyl, UK); C. McGrath (Wrexham Maelor Hospital, Wrexham, UK); N. Curtis, L. Pippard, J. Perry, J. Allison, J. Ockrim, R. Dalton, A. Allison, J. Rendell, L. Howard, K. Beesley, G. Dennison, J. Burton (Yeovil District Hospital, Yeovil, UK); G. Bowen, S. Duberley, L Richards, J. Giles (Royal Blackburn Hospital, Blackburn, UK); J. Katebe, S. Dalton, J. Wood, E. Courtney (Royal United Hospital, Bath, UK); R. Hompes, A. Poole (Churchill Hospital, Oxford, UK); S. Ward, L. Wilkinson, L. Hardstaff, M. Bogden, M. Al-Rashedy, C. Fensom, N. Lunt, M. McCurrie, R. Peacock, K. Malik, H. Burns, B. Townley, P. Hill, M. Sadat, U. Khan (Macclesfield Hospital, Macclesfield, UK); C. Wignall (St Mary's Hospital, London, UK); D. Murati, M. Dhanaratne, S. Quaid, S. Gurram (Ealing Hospital, London, UK); D. Smith, P. Harris, J. Pollard, G. DiBenedetto, J. Chadwick, R. Hull (Royal Bolton Hospital, Bolton, UK); S. Bach, D. Morton, K. Hollier, V. Hardy, M. Ghods, D. Tyrrell, S. Ashraf, J. Glasbey, M. Ashraf, S. Garner, A. Whitehouse, D. Yeung, S. Noor Mohamed, R. Wilkin, N. Suggett, C. Lee, A. Bagul, C. McNeill (Queen Elizabeth II Hospital, Birmingham, UK); N. Eardley, R. Mahapatra, C. Gabriel (Countess of Chester Hospital, Chester, UK); P. Datt, S. Mahmud (St Mark's Hospital, London, UK); I. Daniels, F. McDermott, M. Nodolsk, L. Park (Royal Devon and Exeter Hospital, Exeter, UK); H. Scott, J. Trickett, P. Bearn, P. Trivedi, V. Frost, C. Gray, M. Croft (St Peter's Hospital, Chertsey, UK); D. Beral, J. Osborne, R. Pugh, G. Herdman, R. George (Doncaster Royal Infirmary, Doncaster, UK); A.-M. Howell, S. Al-Shahaby, B. Narendrakumar, Y. Mohsen, S. Ijaz, M. Nasseri (Hillingdon Hospital, Uxbridge, UK); P. Herrod, T. Brear, J.-J. Reilly, A. Sohal, C. Otieno (Royal Derby Hospital, Derby, UK); W. Lai, M. Coleman, E. Platt, A. Patrick, C. Pitman, S. Balasubramanya (Derriford Hospital, Plymouth, UK); E. Dickson, R. Warman, C. Newton, S. Tani, J. Simpson, A. Banerjee, A. Siddika, D. Campion, D. Humes, N. Randhawa, J. Saunders, B. Bharathan, O. Hay (Queen's Medical Centre, Nottingham, UK).
Supplementary Material
Acknowledgements
The authors thank all the patients for participating in this study; the principal investigators and their teams at all participating sites for their valuable contribution and support; I. Russell former Director of WWORTH (the forerunner to STU), A. Bradbury (Trial Steering Committee Chair), P. Wall (Chair Data Monitoring and Ethics Commitee [DMEC]), J. Hill (Chair DMEC), and K. Cocks (DMEC statistician) for their contributions; the trial steering committee (T. Rockall, A. Windsor, V. Allgar, W. Hollingworth, C. Lovell-Smith, J. Hepburn, M. Gudgeon, R. Gabe, C. Thomson); and the research administration team (N. Zbrzyzna, N. Fiera) and R. Jeremy (radiology) for coordinating data collection from individual sites.
Disclosure. A.W. is a current member of the NIHR Health and Social Care Delivery Research Funding Committee. The authors declare no other conflict of interest.
Contributor Information
HART Collaborative:
J Torkington, R Harries, S O'Connell, L Knight, S Islam, N Bashir, A Watkins, G Fegan, J Cornish, B Rees, H Cole, H Jarvis, S Jones, I Russell, D Bosanquet, A Cleves, B Sewell, A Farr, N Zbrzyzna, N Fiera, R Ellis-Owen, Z Hilton, C Parry, A Bradbury, P Wall, J Hill, D Winter, K Cocks, D Harris, J Hilton, S Vakis, D Hanratty, R Rajagopal, F Akbar, A Ben-Sassi, N Francis, L Jones, M Williamson, I Lindsey, R West, C Smart, P Ziprin, T Agarwal, G Faulkner, T Pinkney, D Vimalachandran, D Lawes, O Faiz, P Nisar, N Smart, T Wilson, A Myers, J Lund, S Smolarek, A Acheson, J Horwood, J Ansell, S Phillips, M Davies, L Davies, S Bird, N Palmer, M Williams, G Galanopoulos, P Dhruva Rao, D Jones, R Barnett, S Tate, J Wheat, N Patel, S Rahmani, E Toynton, L Smith, N Reeves, E Kealaher, G Williams, C Sekaran, M Evans, J Beynon, R Egan, E Qasem, U Khot, S Ather, P Mummigati, G Taylor, J Williamson, J Lim, A Powell, H Nageswaran, A Williams, J Padmanabhan, K Phillips, T Ford, J Edwards, N Varney, L Hicks, C Greenway, K Chesters, H Jones, P Blake, C Brown, L Roche, D Jones, M Feeney, P Shah, C Rutter, C McGrath, N Curtis, L Pippard, J Perry, J Allison, J Ockrim, R Dalton, A Allison, J Rendell, L Howard, K Beesley, G Dennison, J Burton, G Bowen, S Duberley, L Richards, J Giles, J Katebe, S Dalton, J Wood, E Courtney, R Hompes, A Poole, S Ward, L Wilkinson, L Hardstaff, M Bogden, M Al-Rashedy, C Fensom, N Lunt, M McCurrie, R Peacock, K Malik, H Burns, B Townley, P Hill, M Sadat, U Khan, C Wignall, D Murati, M Dhanaratne, S Quaid, S Gurram, D Smith, P Harris, J Pollard, G DiBenedetto, J Chadwick, R Hull, S Bach, D Morton, K Hollier, V Hardy, M Ghods, D Tyrrell, S Ashraf, J Glasbey, M Ashraf, S Garner, A Whitehouse, D Yeung, S Noor Mohamed, R Wilkin, N Suggett, C Lee, A Bagul, C McNeill, N Eardley, R Mahapatra, C Gabriel, P Datt, S Mahmud, I Daniels, F McDermott, M Nodolsk, L Park, H Scott, J Trickett, P Bearn, P Trivedi, V Frost, C Gray, M Croft, D Beral, J Osborne, R Pugh, G Herdman, R George, A-M Howell, S Al-Shahaby, B Narendrakumar, Y Mohsen, S Ijaz, M Nasseri, P Herrod, T Brear, J-J Reilly, A Sohal, C Otieno, W Lai, M Coleman, E Platt, A Patrick, C Pitman, S Balasubramanya, E Dickson, R Warman, C Newton, S Tani, J Simpson, A Banerjee, A Siddika, D Campion, D Humes, N Randhawa, J Saunders, B Bharathan, and O Hay
Funding
The study received Health Technology Assessment funding from the National Institute for Health Research (NIHR).
Supplementary material
Supplementary material is available at BJS online.
Health Technology Assessment have published the full trial report, which includes extensive results:
Susan O'Connell, Saiful Islam, Bernadette Sewell, Angela Farr, Laura Knight, Nadim Bashir et al. Hughes abdominal closure versus standard mass closure to reduce incisional hernias following surgery for colorectal cancer: the HART RCT. Health Technol Assess 2022;26:34. DOI: https://doi.org/10.3310/CMWC8368
References
- 1. Korenkov M, Paul A, Sauerland S, Neugebauer E, Arndt M, Chevrel JP et al. Classification and surgical treatment of incisional hernia. Results of an experts’ meeting. Langenbecks Arch Surg 2001;386:65–73 [DOI] [PubMed] [Google Scholar]
- 2. Muysoms FE, Miserez M, Berrevoet F, Campanelli G, Champault GG, Chelala E et al. Classification of primary and incisional abdominal wall hernias. Hernia 2009;13:407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 2004;240:578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosanquet D, Ansell J, Abdelrahman T, Cornish J, Harries R, Stimpson A et al. Systematic review and meta-regression of factors affecting midline incisional hernia rates: analysis of 14618 patients. PLoS One 2015;10:e0138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geçim I E, Koçak S, Ersoz S, Bumin C, Aribal D. Recurrence after incisional hernia repair: results and risk factors. Surg Today 1996;26:607–609 [DOI] [PubMed] [Google Scholar]
- 6. Gillion JF, Sanders D, Miserez M, Muysoms F. The economic burden of incisional ventral hernia repair: a multicentric cost analysis. Hernia 2016;20:819–830 [DOI] [PubMed] [Google Scholar]
- 7. Soliani G, De Troia A, Portinari M, Targa S, Carcoforo P, Vasquez G et al. Laparoscopic versus open incisional hernia repair: a retrospective cohort study with costs analysis on 269 patients. Hernia 2017;21:609–618 [DOI] [PubMed] [Google Scholar]
- 8. Earle D, Seymour N, Fellinger E, Perez A. Laparoscopic versus open incisional hernia repair: a single-institution analysis of hospital resource utilization for 884 consecutive cases. Surg Endosc 2006;20:71–75 [DOI] [PubMed] [Google Scholar]
- 9. Finan KR, Kilgore ML, Hawn MT. Open suture versus mesh repair of primary incisional hernias: a cost–utility analysis. Hernia 2009;13:173–182 [DOI] [PubMed] [Google Scholar]
- 10. Plymale MA, Ragulojan R, Davenport DL, Roth SJ. Ventral and incisional hernia: the cost of comorbidities and complications. Surg Endosc 2017;31:341–351 [DOI] [PubMed] [Google Scholar]
- 11. Muysoms FE, Antoniou SA, Bury K, Campanelli G, Conze J, Cuccurullo D et al. European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia 2015;19:1–24 [DOI] [PubMed] [Google Scholar]
- 12. Patel SV, Paskar DD, Nelson RL, Vedula SS, Steele SR. Closure methods for laparotomy incisions for preventing incisional hernias and other wound complications. Cochrane Database Syst Rev 2017; CD005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deerenberg EB, Harlaar JJ, Steyerberg EW, Lont HE, van Doorn HC, Heisterkamp J et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet 2015;386:1254–1260 [DOI] [PubMed] [Google Scholar]
- 14. Mudge M, Harding KG, Hughes LE. Incisional hernia. Br J Surg 1986;73:82. [DOI] [PubMed] [Google Scholar]
- 15. Shukla VK, Gupta A, Singh H, Pandey M, Gautam A. Cardiff repair of incisional hernia: a university hospital experience. Eur J Surg 1998;164:271–274 [DOI] [PubMed] [Google Scholar]
- 16. Cornish J, Harries RL, Bosanquet D, Rees B, Ansell J, Frewer N et al. Hughes Abdominal Repair Trial (HART)—abdominal wall closure techniques to reduce the incidence of incisional hernias: study protocol for a randomised controlled trial. Trials 2016;17:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harries R, Cornish J, Bosanquet D, Rees B, Horwood J, Islam S et al. Hughes Abdominal Repair Trial (HART)—abdominal wall closure techniques to reduce the incidence of incisional hernias: feasibility trial for a multicentre, pragmatic, randomised controlled trial. BMJ Open 2017;7:e017235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33 [DOI] [PubMed] [Google Scholar]
- 19. Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res 1999;8:181–95 [DOI] [PubMed] [Google Scholar]
- 20. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 21. Berrios-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection. JAMA Surg 2017;152:784–791 [DOI] [PubMed] [Google Scholar]
- 22. Bloemen A, van Dooren P, Huizinga BF, Hoofwijk AG. Randomized clinical trial comparing polypropylene or polydioxanone for midline abdominal wall closure. Br J Surg 2011;98:633–639 [DOI] [PubMed] [Google Scholar]
- 23. Fink C, Baumann P, Wente MN, Knebel P, Bruckner T, Ulrich A et al. Incisional hernia rate 3 years after midline laparotomy. Br J Surg 2014;101:51–54 [DOI] [PubMed] [Google Scholar]
- 24. Parker S, Halligan S, Berrevoet F, de Beaux AC, East B, Eker HH et al. Reporting guidelines for interventional trials of primary and incisional ventral hernia repair. Br J Surg 2021;108:1050–1055 [DOI] [PubMed] [Google Scholar]
- 25. Reeves N, Cuff S, Boyce K, Harries R, Roberts C, Harrison W et al. Welsh Barbers SSI Research Collaborative. Diagnosis of colorectal and emergency surgical site infections in the era of enhanced recovery: an all-Wales prospective study. Colorectal Dis 2021;23:1239–1247 [DOI] [PubMed] [Google Scholar]
- 26. Diener MK, Voss S, Jensen K, Büchler MW, Seiler CM. Elective midline laparotomy closure. Ann Surg 2010;251:843–856 [DOI] [PubMed] [Google Scholar]
- 27. van’t Riet M, Steyerberg EW, Nellensteyn J, Bonjer HJ, Jeekel J. Meta-analysis of techniques for closure of midline abdominal incisions. Br J Surg 2002;89:1350–1356 [DOI] [PubMed] [Google Scholar]
- 28. Hodgson NC, Malthaner RA, Ostbye T. The search for an ideal method of abdominal fascial closure: a meta-analysis. Ann Surg 2000;231:436–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 2013;27:1072–1082 [DOI] [PubMed] [Google Scholar]
- 30. Carli F, Charlebois P, Stein B, Feldman L, Zavorsky G, Kim DJ et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg 2010;97:1187–1197 [DOI] [PubMed] [Google Scholar]
- 31. Mayo NE, Feldman L, Scott S, Zavorsky G, Kim DJ, Charlebois P et al. Impact of preoperative change in physical function on postoperative recovery, argument supporting prehabilitation for colorectal surgery. Surgery 2011;150:505–514 [DOI] [PubMed] [Google Scholar]
- 32. Gillis C, Li C, Lee L, Awasthi R, Awasthi R, Augustin B, Gamsa A et al. Prehabilitation versus rehabilitation. Anesthesiology 2014;121:937–947 [DOI] [PubMed] [Google Scholar]
- 33. Gignoux B, Bayon Y, Martin D, Phan R, Augusto V, Darnis B et al. Incidence and risk factors for incisional hernia and recurrence: retrospective analysis of the French national database. Colorectal Dis 2021;23:1515–1523 [DOI] [PubMed] [Google Scholar]
- 34.National Bowel Cancer Audit. National BOwel Cancer Audit 2019 . An Audit of the Care Received by People with Bowel Cancer in England and Wales v2.0. https://www.nboca.org.uk/reports/annual-report-2019/ (accessed 1 June 2020)
- 35. Rahbari NN, Knebel P, Keiser M, Bruckner T, Bartsch DK, Friess H et al. Design and current status of CONTINT: continuous versus interrupted abdominal wall closure after emergency midline laparotomy—a randomized controlled multicenter trial [NCT00544583]. Trials 2012;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ClinicalTrials.gov. Small Versus Large Bite Closure of Emergency Midline Laparotomy (E-STITCH). https://clinicaltrials.gov/ct2/show/NCT04098380 (accessed 1 August 2021)
- 37. Heger P, Feißt M, Krisam J, Klose C, Dörr-Harim C, Tenckhoff S et al. Hernia reduction following laparotomy using small stitch abdominal wall closure with and without mesh augmentation (the HULC trial): study protocol for a randomized controlled trial. Trials 2019;20:738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.