Abstract
Background
Perioperative chemotherapy is widely used in the treatment of oesophagogastric adenocarcinoma (OGAC) with a substantial survival benefit over surgery alone. However, the postoperative part of these regimens is given in less than half of patients, reflecting uncertainty among clinicians about its benefit and poor postoperative patient fitness. This study estimated the effect of postoperative chemotherapy after surgery for OGAC using a large population-based data set.
Methods
Patients with adenocarcinoma of the oesophagus, gastro-oesophageal junction or stomach diagnosed between 2012 and 2018, who underwent preoperative chemotherapy followed by surgery, were identified from a national-level audit in England and Wales. Postoperative therapy was defined as the receipt of systemic chemotherapy within 90 days of surgery. The effectiveness of postoperative chemotherapy compared with observation was estimated using inverse propensity treatment weighting.
Results
Postoperative chemotherapy was given to 1593 of 4139 patients (38.5 per cent) included in the study. Almost all patients received platinum-based triplet regimens (4004 patients, 96.7 per cent), with FLOT used in 3.3 per cent. Patients who received postoperative chemotherapy were younger, with a lower ASA grade, and were less likely to have surgical complications, with similar tumour characteristics. After weighting, the median survival time after postoperative chemotherapy was 62.7 months compared with 50.4 months without chemotherapy (hazard ratio 0.84, 95 per cent c.i. 0.77 to 0.94; P = 0.001).
Conclusion
This study has shown that postoperative chemotherapy improves overall survival in patients with OGAC treated with preoperative chemotherapy and surgery.
Less than half of patients undergoing perioperative chemotherapy for gastro-oesophageal adenocarcinoma receive postoperative cycles of treatment. This study aimed to quantify the benefit of postoperative chemotherapy using a large population-based data set and inverse propensity score weighting. A substantial benefit of postoperative chemotherapy was demonstrated (hazard ratio 0.84; median survival 62.7 versus 50.4 months; P < 0.001).
Introduction
The majority of patients suitable for curative treatment of oesophagogastric adenocarcinoma (OGAC) undergo either perioperative chemotherapy or neoadjuvant chemoradiotherapy (NACRT) followed by surgery. There is clear evidence of benefit for both approaches compared with surgery alone in patients with locally advanced disease1–4. Despite this, less than half of these patients will survive for more than 5 years after surgery5. The benefit of perioperative/neoadjuvant treatment outside patients who exhibit substantial cancer regression (primary tumour and/or lymph nodes), who probably represent less than 20 per cent of those receiving perioperative chemotherapy6 and less than 30 per cent having NACRT3, appears to be small in retrospective analyses. Furthermore, the importance of different components of perioperative chemotherapy, particularly treatment given in the postoperative phase, is unclear. This is of particular relevance in OGAC because, even in trial settings, the planned postoperative component of perioperative chemotherapy is started in as few as 50–60 per cent of patients and completed in less than 50 per cent1,7. This leads many treating clinicians to believe that the benefit of perioperative chemotherapy is all derived from the preoperative doses. In the majority of patients who do not receive postoperative chemotherapy, the reason for this is poor fitness after surgery8,9. In this context, quantifying the additional benefit of postoperative treatment and establishing in whom this occurs would allow more informed decision-making about relative risks and benefits.
To date, there are no published RCTs of postoperative chemotherapy following preoperative chemotherapy and surgery. In reality, such a trial would be challenging to conduct. Assuming modest efficacy and 50 per cent of patients undergoing treatment per protocol, several thousand patients would be required for an adequately powered primary analysis (even more for meaningful subgroup analyses), a likely infeasible number to achieve in a changing treatment field. The strongest evidence to guide practice has been generated from observational studies based on large population-based data sets. Research into the benefit of postoperative chemotherapy after NACRT and surgery for oesophageal adenocarcinoma using the US National Cancer Database (NCDB) suggested a survival benefit, particularly when there was residual disease (hazard ratio (HR) 0.69–0.79)10–12. In contrast, evidence for the benefit of postoperative chemotherapy after preoperative chemotherapy and surgery is limited; one large propensity-matched NCDB study13 showed no benefit of postoperative chemotherapy in patients with gastric cancer, and small studies14–16 including cancers of the gastro-oesophageal junction (GOJ) have yielded inconsistent results. The aim of this study was to evaluate the survival benefit of postoperative chemotherapy compared with no chemotherapy in patients with OGAC treated with preoperative chemotherapy and surgery in a planned perioperative regimen.
Methods
Patients and treatment
Patients aged over of 18 years and diagnosed between 1 April 2012 and 31 March 2018 were identified from the National Oesophago-Gastric Cancer Audit (NOGCA). The audit is funded by National Health Service (NHS) England and the Welsh government, and commissioned by the Healthcare Quality Improvement Partnership (HQIP), with data collection approved by the Confidentiality Advisory Group under section 251 of the NHS Act 2006. The NOGCA includes patient demographics, tumour data, details of surgery, surgical pathology results, and treatment. Details of chemotherapeutics were cross-referenced and supplemented with linked data from the Systemic Anti-Cancer Therapy (SACT) data set, to which data submission is mandatory for all NHS chemotherapy providers in England17.
This non-randomized, retrospective study was designed to emulate a hypothetical comparable RCT, that is a target trial18,19. Table S1 summarizes the target trial protocol. Patients were eligible for inclusion if they had a histologically confirmed diagnosis of adenocarcinoma arising from the oesophagus, GOJ (Siewert I–III) or stomach, and underwent chemotherapy followed by planned curative surgery. As response to radiotherapy/chemoradiotherapy is biologically distinct from that to chemotherapy20–22, patients who received preoperative chemoradiotherapy or radiotherapy were excluded. Patients with overt metastatic disease at resection (ypM1) were excluded, as were those whose physical fitness before surgery was likely to preclude a full course of treatment (ASA fitness grade IV or more, WHO performance status 3 or higher). Chemotherapy regimens were ascertained from the SACT data set, and the study cohort was limited to patients who received platinum-based triplet therapy (ECF, epirubicin, cisplatin, 5-fluorouracil (5-FU); ECX, epirubicin, cisplatin, capecitabine; EOX, epirubicin, oxaliplatin, capecitabine; EOF, epirubicin, oxaliplatin, 5-FU) or FLOT (5-FU, leucovorin, oxaliplatin, docetaxel) in both the preoperative and postoperative phases. Treatments other than platinum-based triplet or FLOT regimens were excluded because these regimens are primarily given in the neoadjuvant (as opposed to perioperative) setting.
The treatment intention for platinum-based triplet therapy was three preoperative and three postoperative cycles, where each cycle consisted of epirubicin (50 mg/m2 intravenously (i.v.)) and cisplatin (60 mg/m2 i.v.) or oxaliplatin (130 mg/m2 i.v.) on day 1 in conjunction with daily 5-FU (200 mg/m2 i.v.) or capecitabine (1250 mg/m2 orally) for 21 days. For FLOT, four preoperative and four postoperative 2-week cycles were intended, with docetaxel (50 mg/m2), oxaliplatin (85 mg/m2), leucovorin (200 mg/m2) and 5-FU (2600 mg/m2) administered on cycle day 1.
Postoperative treatment was defined as the receipt of at least one cycle of postoperative chemotherapy started within 90 days of surgery, given with non-palliative intent. Patients who did not receive any postoperative chemotherapy were referred to as receiving observation only.
To be eligible for postoperative treatment, patients had to survive the immediate postoperative period. In retrospective analyses such as the present one, this introduces immortal time bias23, with an apparently higher mortality risk in the untreated group. One method of addressing this is to remove patients who die before a landmark time24 (all in the untreated group) from the analysis. As postoperative treatment was defined as beginning within 90 days of surgery, 90 days after surgery was chosen as landmark time and all patients who died before this were excluded. The primary outcome was overall survival from date of diagnosis.
Ethics approval
The study was exempt from UK National Research Ethics Committee approval as it involved secondary analysis of an existing data set of anonymized data. The NOGCA has approval for processing healthcare information under Section 251 (reference number: ECC 1-06 (c)/2011) for all NHS patients diagnosed with oesophagogastric cancer in England and Wales. Data for this study were based on patient-level information collected by the NHS, as part of the care and support of patients with cancer.
Statistical analysis
Treatment allocation in observational studies is non-random. In routine clinical practice, allocation to postoperative treatment is influenced by a number of factors that are associated with survival16, which will confound the results of an observational study. To reduce selection bias and confounding in this study, a propensity score weighting approach was used. The propensity score25 estimates the probability of treatment given the patient characteristics, and is produced from a logistic regression model. The score is then used to balance the patient characteristics in the different treatment groups during the process of estimating the relative benefit of one treatment compared with another. This propensity score analysis used the inverse probability of treatment weighting (IPTW) method, which has been shown to achieve better co-variable balance in comparison with matching approaches26,27. Robust standard errors were calculated using bootstrapping with 100 replications28. The propensity score analysis was conducted separately for each imputed data set and the relative treatment effect was derived by pooling the 30 treatment effects estimated with IPTW on each imputed data set29.
For this study, 25 variables were used for balancing the treatment groups, and incorporated a combination of relevant patient, disease and treatment factors (Table 1). The co-variable balance was assessed using the standardized mean difference (SMD)31,32, with a value of greater than 0.100 taken to indicate significant imbalance33.
Table 1.
Characteristics used for inverse probability of treatment weighting
| Patient characteristics | Disease characteristics | Treatment characteristics |
|---|---|---|
| Sex | Tumour site | Completion of preoperative chemotherapy |
| Age | ypT category | Time from diagnosis to surgery |
| ASA fitness grade | ypN category | Surgical approach |
| Performance status | CRM involvement | Hospital volume |
| Ischaemic heart disease | Differentiation grade | Complications (any) |
| COPD/asthma | Anastomotic leak | |
| Chronic kidney disease | Respiratory complications | |
| Diabetes mellitus | Duration of hospital stay | |
| Total no. of co-morbidities | Surgical procedure | |
| Social deprivation* | Preoperative chemotherapy regimen (platinum-based triplet or FLOT) |
*English Index of Multiple Deprivation. CRM, circumferential resection margin, defined according to the Royal College of Pathologists guidelines (positive if tumour found at or within 1 mm of cut edge)30; COPD, chronic obstructive pulmonary disease; FLOT, fluorouracil, leucovorin, oxaliplatin, docetaxel.
Missing values were assumed to be missing at random and multiple imputation by chained equations34 was used to generate 30 imputed data sets for the analysis35. Comparisons of patient characteristics across the treatment groups were conducted using weighted Mann–Whitney U and χ2 tests. Survival analysis was undertaken using weighted log rank tests and Cox proportional hazard models. The proportional hazards assumption was assessed by inspection of scaled Schoenfeld residuals36. The restricted mean survival time37–42 and its derivative, life expectancy difference (LED), were also calculated at 36 and 60 months. As IPTW adjusts only for known confounders, a sensitivity analysis was carried out to assess the magnitude of confounding from unmeasured factors required to eliminate the measured effect, using the E-value43. In a second sensitivity analysis, the landmark time was varied up to 6 months after surgery to ensure that immortal time bias had been controlled for adequately in the analysis. A series of subgroup analyses were also conducted to assess the stability of the estimated treatment effect. The effects of tumour site (oesophagus, GOJ, stomach) and lymph node involvement (N0, N+) were assessed separately. Inverse probability of treatment weights were recalculated for each analysis. All analyses were undertaken in R (R Foundation for Statistical Computing, Vienna, Austria)44.
Results
Patients
The study flow chart is shown in Fig. 1. In total, 4139 patients treated in 58 centres were included. Data on patient characteristics were missing for 1124 patients (27.1 per cent); the most frequent missing variables were circumferential resection margin (CRM) (16.0 per cent), cT category (5.3 per cent), cN category (2.8 per cent), ypN category (4.4 per cent), ypT category (4.3 per cent), and duration of hospital stay (4.1 per cent). All other variables had less than 1 per cent missing data. A full course of preoperative chemotherapy was completed in 3025 patients (73.1 per cent). The tumour site was oesophagus in 47.9 per cent, GOJ in 29.2 per cent, and stomach in 22.9 per cent. Median survival for the full study cohort was 57.1 (95 per cent c.i. 51.9 to 63.6) months, with 60.8 and 49.3 per cent of patients surviving at 3 and 5 years respectively. Median follow-up was 37.5 (i.q.r. 21.5 to 53.1) months. In the preoperative setting, ECX was most frequently used (3116 patients, 75.3 per cent) followed by EOX (717, 17.3 per cent), FLOT (135, 3.3 per cent), ECF (134, 3.2 per cent), and EOF (37, 0.9 per cent). The most common procedures were Ivor–Lewis oesophagectomy (2439, 58.9 per cent), total gastrectomy (703, 16.9 per cent), and distal gastrectomy (414, 10.0 per cent).
Fig. 1.
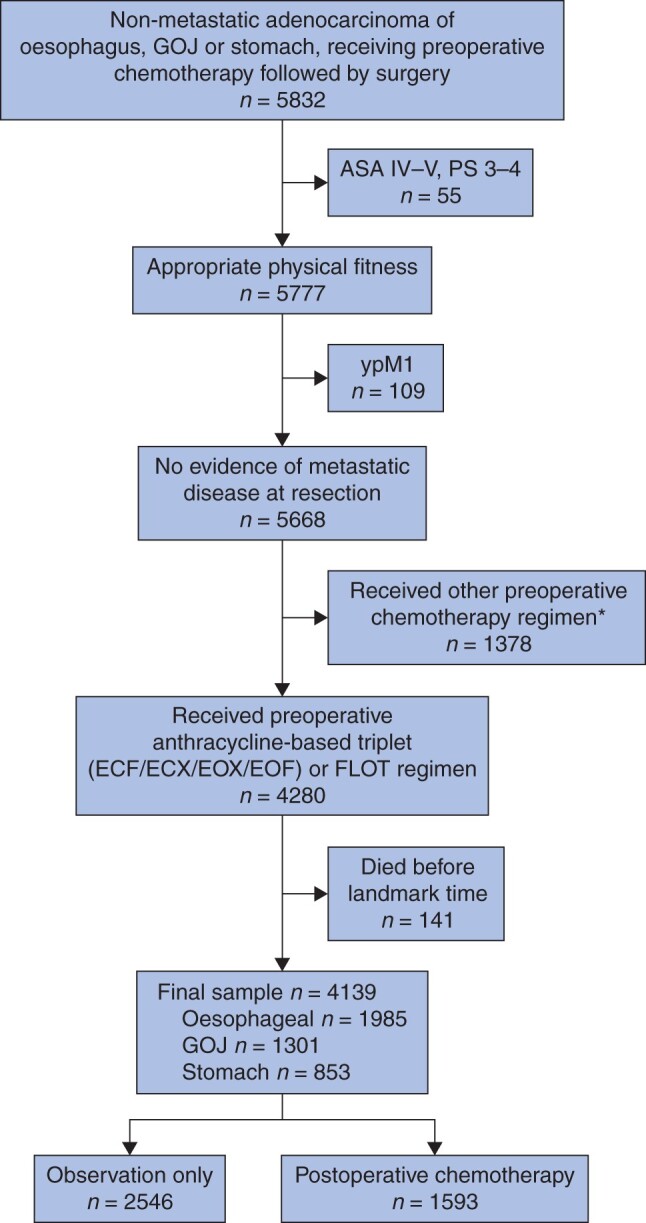
Study flow diagram
The initial cohort comprised patients diagnosed between 1 April 2012 and 30 March 2018. *Patients were excluded if they received non-perioperative (neoadjuvant only) or non-standard regimens. The majority of these received either a platinum and fluoropyrimidine doublet regimen (780, 45.5 per cent) or paclitaxel with carboplatin (206, 15.4 per cent). GOJ, gastro-oesophageal junction; PS, WHO performance status; ECF, epirubicin, cisplatin, 5-fluorouracil (5-FU); ECX, epirubicin, cisplatin, capecitabine; EOX, epirubicin, oxaliplatin, capecitabine; EOF, epirubicin, oxaliplatin, 5-FU; FLOT, 5-FU, leucovorin, oxaliplatin, docetaxel.
Postoperative chemotherapy was started within 90 days of surgery in 1593 patients (38.5 per cent) (treatment group), and 2546 (61.5 per cent) received observation only. In the treatment group, 953 patients (61.9 per cent) were recorded as receiving three or more postoperative cycles of chemotherapy, 384 (25.0 per cent) two cycles, and 256 (16.6 per cent) one cycle. Fewer patients who underwent FLOT received postoperative treatment than those who underwent platinum-based triplet chemotherapy (12.6 versus 39.4 per cent; P < 0.001), although the number of patients receiving FLOT was small (135, 3.3 per cent).
There were substantial differences in characteristics between patients who received postoperative therapy and those who were observed (Table 2). Patients who underwent postoperative treatment were younger, with a more favourable WHO performance status and ASA grade. They also had fewer surgical complications and a shorter hospital stay. The differences in postoperative pathology were less marked, suggesting that tumour biology was less influential in treatment allocation than patient fitness.
Table 2.
Preweighting cohort characteristics stratified by receipt of postoperative treatment
| Overall (n = 4139) | Observation (n = 2546) | Treatment (n = 1593) | P‡ | ||
|---|---|---|---|---|---|
| Women | 810 (19.6) | 506 (19.9) | 304 (19.1) | 0.559 | |
| Age (years) * | 66 (58–71) | 67 (60–72) | 64 (56–70) | < 0.001§ | |
| Tumour site | Oesophagus upper 1/3 | 28 (0.7) | 18 (0.7) | 10 (0.6) | 0.019 |
| Oesophagus middle 1/3 | 176 (4.3) | 108 (4.2) | 68 (4.3) | ||
| Oesophagus lower 1/3 | 1781 (43.0) | 1109 (43.6) | 672 (42.2) | ||
| GOJ Siewert I | 413 (10.0) | 271 (10.6) | 142 (8.9) | ||
| GOJ Siewert II | 439 (10.6) | 268 (10.5) | 171 (10.7) | ||
| GOJ Siewert III | 356 (8.6) | 232 (9.1) | 124 (7.8) | ||
| Gastric fundus | 93 (2.2) | 58 (2.3) | 35 (2.2) | ||
| Gastric body | 515 (12.4) | 305 (12.0) | 210 (13.2) | ||
| Gastric antrum | 232 (5.6) | 117 (4.6) | 115 (7.2) | ||
| Pylorus | 106 (2.6) | 60 (2.4) | 46 (2.9) | ||
| Surgical procedure | Left thoracoabdominal oesophagectomy | 249 (6.0) | 158 (6.2) | 91 (5.7) | 0.115 |
| Two-phase oesophagectomy | 2439 (58.9) | 1520 (59.7) | 919 (57.7) | ||
| Three-phase oesophagectomy | 88 (2.1) | 64 (2.5) | 24 (1.5) | ||
| Transhiatal oesophagectomy | 61 (1.5) | 37 (1.5) | 24 (1.5) | ||
| Total gastrectomy | 703 (17.0) | 426 (16.7) | 277 (17.4) | ||
| Extended total gastrectomy | 171 (4.1) | 100 (3.9) | 71 (4.5) | ||
| Proximal gastrectomy | 14 (0.3) | 8 (0.3) | 6 (0.4) | ||
| Distal gastrectomy | 414 (10.0) | 233 (9.2) | 181 (11.4) | ||
| Performance status | 0 | 2587 (62.5) | 1509 (59.3) | 1078 (67.7) | < 0.001 |
| 1 | 1369 (33.1) | 910 (35.7) | 459 (28.8) | ||
| 2 | 183 (4.4) | 127 (5.0) | 56 (3.5) | ||
| ASA fitness grade | I | 534 (12.9) | 293 (11.5) | 241 (15.1) | 0.002 |
| II | 2533 (61.2) | 1568 (61.6) | 965 (60.6) | ||
| III | 1072 (25.9) | 685 (26.9) | 387 (24.3) | ||
| Social deprivation index† | Least deprived | 847 (20.5) | 493 (19.4) | 354 (22.3) | 0.003 |
| 2nd quintile | 838 (20.3) | 502 (19.8) | 336 (21.2) | ||
| 3rd quintile | 829 (20.1) | 554 (21.8) | 275 (17.3) | ||
| 4th quintile | 825 (20.0) | 519 (20.4) | 306 (19.3) | ||
| Most deprived | 787 (19.1) | 472 (18.6) | 315 (19.9) | ||
| History of specific co-morbidity | IHD | 878 (21.2) | 551 (21.6) | 327 (20.5) | 0.415 |
| COPD | 399 (9.6) | 257 (10.1) | 142 (8.9) | 0.231 | |
| DM | 385 (9.3) | 249 (9.8) | 136 (8.5) | 0.199 | |
| No. of recorded co-morbidities * | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.008§ | |
| Annual hospital volume | 1 to 30 | 308 (7.4) | 190 (7.5) | 118 (7.4) | < 0.001 |
| 31 to 60 | 2117 (51.1) | 1392 (54.7) | 725 (45.5) | ||
| > 60 | 1714 (41.4) | 964 (37.9) | 750 (47.1) | ||
| ypT category | ypT0 | 279 (7.0) | 181 (7.4) | 98 (6.4) | 0.064 |
| ypT1 | 515 (13.0) | 323 (13.3) | 192 (12.5) | ||
| ypT2 | 531 (13.4) | 318 (13.1) | 213 (13.9) | ||
| ypT3 | 2203 (55.6) | 1321 (54.3) | 882 (57.6) | ||
| ypT4 | 435 (11.0) | 289 (11.9) | 146 (9.5) | ||
| ypN category | ypN0 | 1708 (43.2) | 1063 (43.8) | 645 (42.2) | 0.416 |
| ypN1 | 851 (21.5) | 526 (21.7) | 325 (21.3) | ||
| ypN2 | 776 (19.6) | 456 (18.8) | 320 (20.9) | ||
| ypN3 | 622 (15.7) | 383 (15.8) | 239 (15.6) | ||
| CRM-positive | 791 (22.8) | 512 (24.0) | 279 (20.8) | 0.036 | |
| Grade | G1 | 116 (2.8) | 71 (2.8) | 45 (2.8) | 0.852 |
| G2 | 1409 (34.4) | 872 (34.7) | 537 (33.9) | ||
| G3/4 | 2124 (51.8) | 1289 (51.3) | 835 (52.6) | ||
| GX | 450 (11.0) | 281 (11.2) | 169 (10.7) | ||
| Preoperative regimen | FLOT | 135 (3.3) | 118 (4.6) | 17 (1.1) | < 0.001 |
| Platinum-based triplet | 4004 (96.7) | 2428 (95.4) | 1576 (98.9) | ||
| Preoperative treatment not completed | 1114 (26.9) | 854 (33.5) | 260 (16.3) | < 0.001 | |
| Any complication | 1331 (32.4) | 935 (37.0) | 396 (24.9) | < 0.001 | |
| Respiratory complication | 614 (14.8) | 434 (17.0) | 180 (11.3) | < 0.001 | |
| Anastomotic leak | 219 (5.3) | 186 (7.4) | 33 (2.1) | < 0.001 | |
| Duration of hospital stay (days) * | 11 (9–15) | 12 (9–19) | 10 (8–13) | < 0.001§ | |
| Interval from diagnosis to resection (days) * | 153 (139–171) | 153 (138–173) | 153 (140–168) | 0.296§ |
Values in parentheses are percentages unless indicated otherwise;
*values are median (i.q.r.).
†Index of Multiple Deprivation. GOJ, gastro-oesophageal junction; IHD, ischaemic heart disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; CRM, circumferential resection margin; FLOT, fluorouracil, leucovorin, oxaliplatin, docetaxel.
‡χ2 test, except §Mann–Whitney U test.
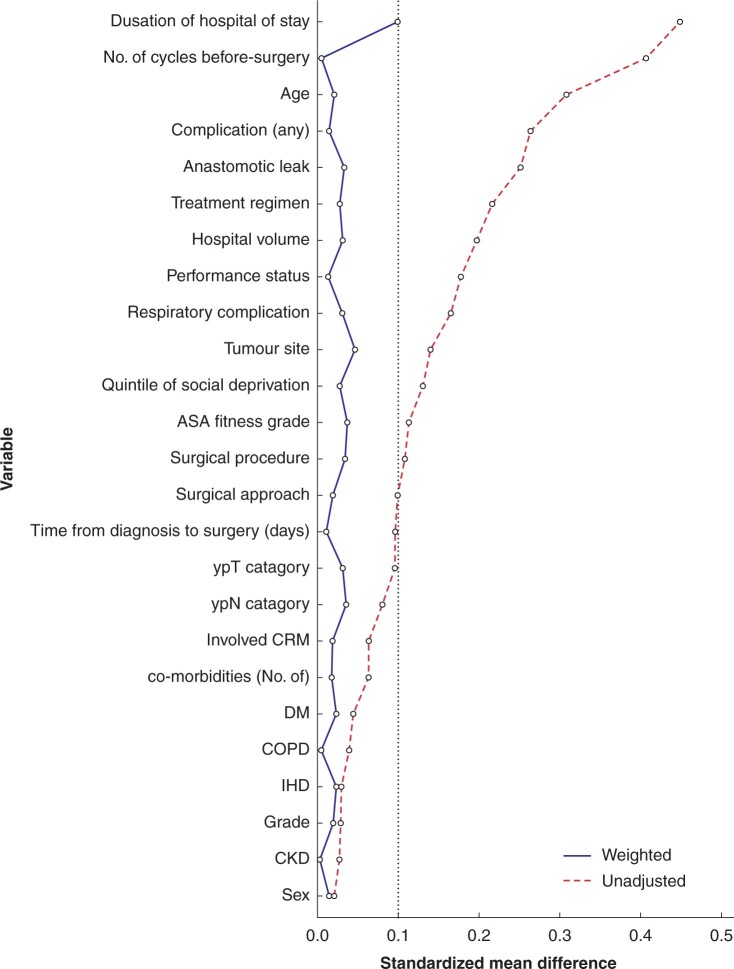
Of the 25 variables used to produce the propensity score, 13 exhibited substantial imbalance (SMD over 0.100) before weighting. Considerable overlap in propensity score distribution between groups was noted (Fig. S1). Following IPTW, the intragroup differences were substantially reduced, with the mean SMD across the variables reduced from 0.145 to 0.024. An SMD of less than 0.100 was achieved for all variables (Fig. 2), indicating similar distribution of measured characteristics between the two groups33.
Fig. 2.
Standardized mean difference of weighting co-variables before and after weighting ordered by preweighting standardized mean difference
After weighting, the standardized mean difference was less than 0.100 for all variables, indicating no substantial imbalance between analysis groups. CRM, circumferential resection margin; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; CKD, chronic kidney disease.
Outcomes
Before weighting, a substantial survival benefit of postoperative chemotherapy was seen, with a HR of 0.78 (95 per cent c.i. 0.70 to 0.86; P < 0.001) and an increase of median survival from 49.4 to 67.2 months (P < 0.001).
After IPTW, postoperative chemotherapy remained associated with significantly longer median overall survival compared with observation (62.7 versus 50.4 months; P = 0.004). Three- and 5-year survival rates were 62.7 and 50.4 per cent respectively in the treatment group, compared with 58.8 and 47.5 per cent in the observation group, corresponding to an absolute increase in survival of 2.9 per cent at 5 years (Fig. 3), and an increase in the restricted mean survival time of 1.5 (95 per cent c.i. 0.8 to 2.1) months (P < 0.001) and 2.2 (0.8 to 3.5) months (P = 0.002) at 3 and 5 years respectively. Weighted Cox analysis yielded a HR for postoperative treatment of 0.84 (95 per cent c.i. 0.77 to 0.94; P = 0.001). Weighted Schoenfeld residuals are shown in Fig. S2.
Fig. 3.
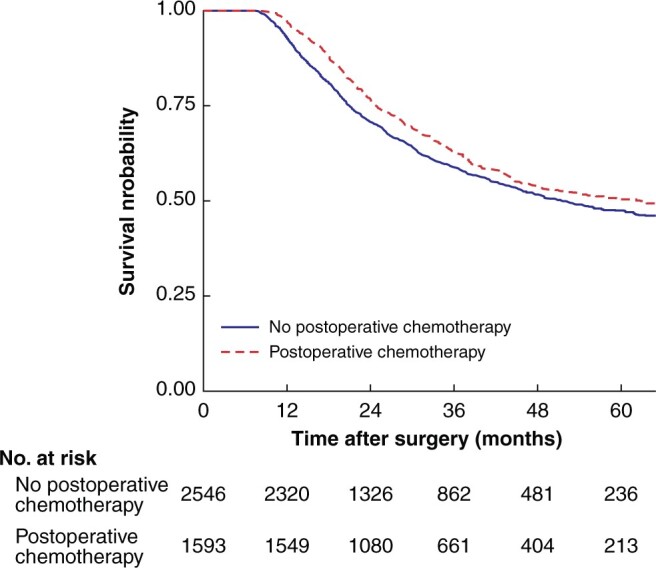
Weighted overall survival for patients receiving postoperative chemotherapy or observation only
P = 0.004 (log rank test).
In assessment of the risk of unmeasured confounding, the E-value was 1.51 (95 per cent c.i. 1.26 to 1.69), meaning that to eliminate the survival benefit described above, residual confounding would have to be expressed 1.51 times more frequently in patients treated with postoperative chemotherapy than in the observed group and exhibit a risk ratio for mortality of 1.51 (HR approximately 1.80). Varying the landmark analysis time from 3 to 6 months (therefore excluding patients who died before this) had minimal influence on the results (Table S2 and Fig. S3), which were consistent with those of the primary analysis.
Subgroup analysis
The 3-year LED and HR for each subgroup analysis are summarized in Table 3. The weighting did not produce as balanced treatment and observation groups in the subgroup analyses, with a SMD exceeding 0.100 in duration of hospital stay for the N+ and gastric subgroups, and in anastomotic leak for the gastric and GOJ subgroups. There were, however, substantial reductions in the mean SMD and for all variables in all subgroups.
Table 3.
Benefit of receiving postoperative chemotherapy for different patient subgroups, estimated using inverse probability of treatment weighting propensity analysis
| No. of patients* |
3-year survival (%) |
3-year LED (months) | P | Hazard ratio | P | |||
|---|---|---|---|---|---|---|---|---|
| Observed | Treated | Observed | Treated | |||||
| ypN0 | 1119 | 671 | 82.4 | 89.9 | 0.95 (0.26, 1.63) | 0.007 | 0.85 (0.61, 1.17) | 0.322 |
| ypN+ | 1427 | 922 | 39.8 | 51.2 | 2.11 (1.20, 3.02) | 0.000 | 0.80 (0.70, 0.92) | 0.001 |
| Oesophagus | 1235 | 750 | 55.4 | 65.8 | 1.82 (0.89, 2.76) | 0.000 | 0.79 (0.68, 0.90) | 0.001 |
| Gastro-oesophageal junction (Siewert I–III) | 771 | 437 | 57.4 | 73.8 | 1.69 (0.42, 2.96) | 0.009 | 0.86 (0.70, 1.06) | 0.156 |
| Gastric | 540 | 406 | 66.3 | 76.3 | 0.72 (−0.51, 1.95) | 0.251 | 0.95 (0.75, 1.20) | 0.686 |
Values in parentheses are 95 per cent confidence intervals.
*Mean number across imputed data sets. LED, life expectancy difference (difference in restricted mean survival times from 3 years of follow-up).
In analyses of effect by tumour site, a survival benefit was most marked for oesophageal tumours, with a LED at 3 years of 1.82 (95 per cent c.i. 0.89 to 2.76) months (P < 0.001), median survival 63.6 versus 43.2 months (P < 0.001), and a HR of 0.79 (95 per cent c.i. 0.68 to 0.90; P = 0.001). For GOJ tumours, the 3-year LED was 1.69 (0.42 to 2.96) months (P = 0.009); however, the HR was not statistically significant (0.86, 0.70 to 1.06; P = 0.156) and violated the proportional hazards assumption (and is therefore less appropriate for group comparisons). For tumours of the stomach, postoperative chemotherapy provided no benefit in terms of 3-year LED (0.72 (−0.51 to 1.95) months; P = 0.251) or HR (0.95, 0.75 to 1.20; P = 0.686).
Patients with tumours of the stomach were more likely to receive postoperative chemotherapy (42.9 per cent versus 36.2 per cent GOJ and 37.8 per cent oesophagus; P < 0.001), possibly reflecting the lower complication burden among these patients (all complications: 22.5 per cent stomach, 33.9 per cent GOJ, and 36.2 per cent oesophagus; P < 0.001) (Table S3). ypN category was similar for all tumour locations (P = 0.111), but patients with gastric tumours were more likely to have a higher ypT category (ypT4: 22.7 per cent for stomach, 11.3 per cent for GOJ, and 5.2 per cent for oesophagus; P < 0.001).
Patients found to have no involved lymph nodes at resection (ypN0) had globally excellent survival outcomes, with no further benefit of postoperative chemotherapy, whereas in the ypN+ subgroup, survival was superior among patients who received postoperative chemotherapy (HR 0.80, 0.70 to 0.92, P < 0.001; 3-year LED 2.11 (1.20 to 3.02) months, P < 0.001). In analyses according to both nodal status and tumour site, survival was longer in patients with ypN+ disease receiving postoperative chemotherapy if the tumour was located in the oesophagus (HR 0.81, 0.69 to 0.95; P = 0.009) or at the GOJ (HR 0.76, 0.61 to 0.95; P = 0.016), but not the stomach (HR 0.94, 0.69 to 1.27; P = 0.686) or for any tumour site with ypN0 status (Table S4).
Discussion
Although drawing definitive conclusions from retrospective analyses is challenging, the results of this study suggest that the postoperative component of perioperative chemotherapy has a clinically meaningful benefit in patients with surgically treated OGAC. Patients undergoing postoperative chemotherapy had a median survival of 62.7 months in comparison with 50.4 months for observation alone, a magnitude of effect comparable to that seen for the benefit of FLOT-type perioperative treatment over ECF/ECX4, which has led to a change in practice. In patients with oesophageal tumours, the effect of postoperative chemotherapy was even more marked, with median survival of 63.6 and 43.2 months, and a HR of 0.79.
Following publication of the Medical Research Council (MRC) MAGIC trial1, perioperative chemotherapy became the standard of care in many settings, especially for gastric/GOJ cancers. Subsequent trials4,45–47 provided further evidence of benefit for multimodal therapy over surgery alone and aimed to optimize regimens. However, fewer than 50 per cent of patients completed treatment according to the protocol in all of these studies. This has left a clear evidence gap, with uncertainty over which patients among a comparatively unfit patient cohort, who have already undergone debilitating treatment, should be targeted for postoperative treatment. The present study provides evidence in support of postoperative chemotherapy in these patients.
The strengths of this study include the large, multicentre national data sets on which it was based. A large number of variables plausibly related to treatment allocation and/or survival were used to derive the propensity score, and it was possible to include details of chemotherapy regimens and surgical complications, which are lacking in studies conducted using the NCDB10–12,16. The main limitation of this study is its observational design, but this must be weighed against the importance of the findings made from real-world settings outside very tightly controlled clinical trials. Robust methods to deal with potential sources of bias were used, and the IPTW produced well balanced groups in the main analysis. A landmark time method was adopted to minimize immortal time bias, and the effect persisted even when the landmark time was significantly extended. There was one unmeasured confounding factor, tumour regression grade (TRG), which has previously been shown to influence postoperative treatment allocation6,15. The propensity score model included several variables with which TRG is strongly correlated (ypT, ypN, CRM), which are likely to reduce or eliminate its independent multivariable effect48. However, its absence is a limitation of this analysis. Further study with direct measurement of tumour and nodal regression49 would be beneficial. It is likely that other unmeasured confounders exist that may reduce the strength of association seen. The potential effect of unmeasured confounding was assessed. To eliminate the demonstrated benefit, it was estimated that a set of confounders would need to be roughly 50 per cent more prevalent in the observation group and would have to be associated with a 1.51 increase in the risk of death to explain the observed risk ratio, which is unlikely. The NOGCA does not record recurrence or cause of death, so it was not possible to calculate disease-free or cancer-specific survival, which may be less susceptible to residual selection bias.
The subgroup analyses were limited by the increased residual confounding in comparison with the main analysis, with duration of stay and the occurrence of anastomotic leak, both factors that strongly correlate with administration of postoperative chemotherapy, unable to be balanced in some subgroups. This reduces the validity of the relationships demonstrated. However, valuable further insights may still be gained. First, no survival benefit was seen with postoperative chemotherapy in patients with ypN0 disease. These patients had very good survival outcomes overall, and they may have either innately favourable biology and/or a substantial response to preoperative treatment that obviate the need for postoperative chemotherapy. A prognostic model for oesophageal cancer was developed in a similar cohort from the NOGCA50, which highlights subpopulations within staging groups with differing survival. Prospective validation of this tool or others should consider whether they can identify either subgroups or individual patients who would benefit from postoperative/adjuvant therapy. The integration of biomarker analysis into routine practice may also help guide treatment decisions in future, particularly for addition of monoclonal antibodies into treatment pathways (for example programmed death ligand-1 status for value of nivolumab), although no such marker of response to traditional preoperative or postoperative chemotherapy has been identified so far. Second, the treatment effect varied with tumour site; a larger effect was noted for oesophageal tumours, a lesser effect for lesions of the GOJ, and no clear benefit for tumours of the stomach, even those in the ypN+ subgroup, similar to previous findings13. These results should be interpreted in the context that the prespecified balance criteria (SMD less than 0.100) were not met for some variables in the GOJ and gastric subgroups, making these analyses largely exploratory. Nonetheless, these results reinforce that these tumours, although often considered together, vary in terms of aetiology, genetics, and response to treatment, at least in certain settings, which should be taken into consideration when analysing outcomes data51.
There were insufficient numbers to analyse patients treated with FLOT separately, as this regimen has only recently entered widespread clinical practice, but, given the similarities in postoperative treatment uptake between the MRC-MAGIC and FLOT4 trials, similar results would be expected. The magnitude of effect is, however, unclear, and the findings in subgroup analysis (such as the lack of benefit in ypN0 disease, which is seen more frequently after FLOT therapy) may not translate to this population. Further research is required, and a further 5–10 years of experience with the regimen is required to answer these questions. Despite the small number of patients who received FLOT in this study, the considerably reduced proportion going on to have postoperative chemotherapy should be noted. Considering the effects of postoperative therapy demonstrated in this study, the more pronounced toxicity of FLOT, perceived or otherwise, could influence survival outcomes in the real-world setting.
The present results highlight the potential impact of increasing the uptake of postoperative chemotherapy in terms of improving survival among patients with OGAC. It was found that postoperative chemotherapy was used less frequently overall than in published trials (38.5 per cent of patients). Treatment allocation appeared to be governed predominantly by patient fitness and postoperative course, rather than tumour biology, with the most discriminatory variables including duration of hospital stay after surgery, surgical complications, and age. Patients who did not complete all preoperative treatment were also less likely receive treatment after surgery, presumably because of their physical condition. Patients who were treated in higher-volume centres (over 60 resections per year) were more likely to be treated with postoperative chemotherapy (Table 2).
In this context, increasing the use of postoperative chemotherapy involves targeting potentially modifiable factors influencing its receipt, predominantly surgical complications. Although the NOGCA reports complications less frequently than international benchmarks52,53, a strong relationship was observed between complications and receipt of postoperative chemotherapy. This may partly explain the association of anastomotic leak and pulmonary complications with decreased overall survival after oesophagectomy54. Strategies to minimize surgical complications and their impact, including centralization55 and minimally invasive techniques56, proceed at pace and may in future yield significant survival benefits. Furthermore, better risk stratification might allow different treatment strategies to be used in higher-risk patients, including both the accepted (chemoradiation3,57) and the experimental (such as immunotherapy58,59), administration of all treatment cycles before operation, or omission of futile treatment for patients who will not benefit.
Funding
S.A.R. is supported by a Royal College of Surgeons of England Research Fellowship and a British Association of Surgical Oncologists Research Project Grant. T.J.U. is supported by a Cancer Research UK and Royal College of Surgeons of England Advanced Clinician Scientist Fellowship, (ID: A23924). This project has been supported by the Association of Upper Gastrointestinal Surgery, Heartburn Cancer UK, and the Royal College of Surgeons of England Surgical Specialty Lead Programme. This study was undertaken as part of the work of the NOGCA. This audit is commissioned by the HQIP as part of the National Clinical Audit and Patient Outcomes Programme, and funded by NHS England and the Welsh Government (www.hqip.org.uk/national-programmes). The authors had full independence from the HQIP. The aim of the NOGCA is to evaluate the care of patients with oesophagogastric cancer in England and Wales, and support NHS providers to improve the quality of hospital care for these patients. More information can be found at www.nogca.org.uk. Neither the HQIP nor the funders had any involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Supplementary Material
Acknowledgements
D.A.C. and T.J.U. contributed equally to this work.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Contributor Information
Saqib Rahman, School of Cancer Sciences, Faculty of Medicine, University of Southampton, Southampton, UK; Clinical Effectiveness Unit, Royal College of Surgeons of England, London, UK.
Betsan Thomas, Department of Oncology, Velindre University NHS Trust, Cardiff, UK.
Nick Maynard, Department of Upper Gastrointestinal Surgery, Oxford University Hospitals NHS Trust, Oxford, UK.
Min Hae Park, Clinical Effectiveness Unit, Royal College of Surgeons of England, London, UK.
Muhammad Wahedally, Clinical Effectiveness Unit, Royal College of Surgeons of England, London, UK.
Nigel Trudgill, Department of Gastroenterology, Sandwell and West Birmingham Hospitals NHS Trust, Birmingham, UK.
Tom Crosby, Department of Oncology, Velindre University NHS Trust, Cardiff, UK.
David A. Cromwell, Clinical Effectiveness Unit, Royal College of Surgeons of England, London, UK
Tim J. Underwood, School of Cancer Sciences, Faculty of Medicine, University of Southampton, Southampton, UK
References
- 1. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJH, Nicolson M et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062–5067. [DOI] [PubMed] [Google Scholar]
- 3. van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, van Berge Henegouwen MIB, Wijnhoven BPL et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 4. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948–1957. [DOI] [PubMed] [Google Scholar]
- 5. Cromwell D, Wahedally H, Park MH, Maynard N, Crosby T, Trudgill N et al. National Oesophago-Gastric Cancer Audit: 2019 Annual Report. London: Healthcare Quality Improvement Partnership (HQIP), 2019. [Google Scholar]
- 6. Noble F, Lloyd MA, Turkington R, Griffiths E, O'Donovan M, O'Neill JR et al. ; OCCAMS consortium. Multicentre cohort study to define and validate pathological assessment of response to neoadjuvant therapy in oesophagogastric adenocarcinoma. Br J Surg 2017;104:1816–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moorcraft SY, Smyth EC, Cunningham D. Adjuvant or neoadjuvant therapy for operable esophagogastric cancer? Gastric Cancer 2015;18:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Derogar M, Lagergren P. Health-related quality of life among 5-year survivors of esophageal cancer surgery: a prospective population-based study. J Clin Oncol 2012;30:413–418. [DOI] [PubMed] [Google Scholar]
- 9. Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens JJME, van Meerten E et al. ; CARTS Study Group. Long-term oncological and functional outcomes of chemoradiotherapy followed by organ-sparing transanal endoscopic microsurgery for distal rectal cancer. JAMA Surg 2019;154:47–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burt BM, Groth SS, Sada YH, Farjah F, Cornwell L, Sugarbaker DJ et al. Utility of adjuvant chemotherapy after neoadjuvant chemoradiation and esophagectomy for esophageal cancer. Ann Surg 2017;266:297–304. [DOI] [PubMed] [Google Scholar]
- 11. Mokdad AA, Yopp AC, Polanco PM, Mansour JC, Reznik SI, Heitjan DF et al. Adjuvant chemotherapy vs postoperative observation following preoperative chemoradiotherapy and resection in gastroesophageal cancer a propensity score-matched analysis. JAMA Oncol 2018;4:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nevala-Plagemann C, Francis S, Cavalieri C, Tao R, Whisenant J, Glasgow R et al. Benefit of adjuvant chemotherapy based on lymph node involvement for oesophageal cancer following trimodality therapy. ESMO Open 2018;3:e000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drake JA, Stiles ZE, Tsao MW, Deneve JL, Glazer ES, Yakoub D et al. Analysis of the survival impact of postoperative chemotherapy after preoperative chemotherapy and resection for gastric cancer. Ann Surg Oncol 2021;28:1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glatz T, Bronsert P, Schäfer M, Kulemann B, Marjanovic G, Sick O et al. Perioperative platin-based chemotherapy for locally advanced esophagogastric adenocarcinoma: postoperative chemotherapy has a substantial impact on outcome. Eur J Surg Oncol 2015;41:1300–1307. [DOI] [PubMed] [Google Scholar]
- 15. Sisic L, Blank S, Nienhüser H, Haag GM, Jäger D, Bruckner T et al. The postoperative part of perioperative chemotherapy fails to provide a survival benefit in completely resected esophagogastric adenocarcinoma. Surg Oncol 2020;33:177–188. [DOI] [PubMed] [Google Scholar]
- 16. Papaxoinis G, Kamposioras K, Weaver JMJ, Kordatou Z, Stamatopoulou S, Germetaki T et al. The role of continuing perioperative chemotherapy post surgery in patients with esophageal or gastroesophageal junction adenocarcinoma: a multicenter cohort study. J Gastrointest Surg 2019;23:1729–1741. [DOI] [PubMed] [Google Scholar]
- 17. Bright CJ, Lawton S, Benson S, Bomb M, Dodwell D, Henson KE et al. Data resource profile: The Systemic Anti-Cancer Therapy (SACT) data set. Int J Epidemiol 2020;49:15L–15L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016;183:758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer 2017;81:183–190. [DOI] [PubMed] [Google Scholar]
- 21. Petrelli F, Ghidini M, Barni S, Sgroi G, Passalacqua R, Tomasello G. Neoadjuvant chemoradiotherapy or chemotherapy for gastroesophageal junction adenocarcinoma: a systematic review and meta-analysis. Gastric Cancer 2019;22:245–254. [DOI] [PubMed] [Google Scholar]
- 22. Pucher PH, Rahman SA, Walker RC, Grace BL, Bateman A, Iveson T et al. Outcomes and survival following neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus: inverse propensity score weighted analysis. Eur J Surg Oncol 2020;46:2248–2256. [DOI] [PubMed] [Google Scholar]
- 23. Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol 2008;167:492–499. [DOI] [PubMed] [Google Scholar]
- 24. Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes 2011;4:363–371. [DOI] [PubMed] [Google Scholar]
- 25. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 26. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Med Res Methodol 2012;12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016;35:5642–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leyrat C, Seaman SR, White IR, Douglas I, Smeeth L, Kim J et al. Propensity score analysis with partially observed covariates: how should multiple imputation be used? Stat Methods Med Res 2019;28:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grabsch HI, Mapstone NP, Novelli M. Standards and Datasets for Reporting Cancers Dataset for the Histopathological Reporting of Oesophageal Carcinoma (2nd edn). London, UK: Royal College of Pathologists, 2019.
- 31. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26:734–753. [DOI] [PubMed] [Google Scholar]
- 32. Austin PC, Mamdani MM, Stukel TA, Anderson GM, Tu JV. The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med 2005;24:1563–1578. [DOI] [PubMed] [Google Scholar]
- 33. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 35. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 36. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241. [Google Scholar]
- 37. Royston P, Parmar MKB. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med 2011;30:2409–2421. [DOI] [PubMed] [Google Scholar]
- 38. Dehbi HM, Royston P, Hackshaw A. Life expectancy difference and life expectancy ratio: two measures of treatment effects in randomised trials with non-proportional hazards. BMJ 2017;357:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trinquart L, Jacot J, Conner SC, Porcher R. Comparison of treatment effects measured by the hazard ratio and by the ratio of restricted mean survival times in oncology randomized controlled trials. J Clin Oncol 2016;34:1813–1819. [DOI] [PubMed] [Google Scholar]
- 40. Zhao L, Claggett B, Tian L, Uno H, Pfeffer MA, Solomon SD et al. On the restricted mean survival time curve in survival analysis. Biometrics 2016;72:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. A’Hern RP. Restricted mean survival time: an obligatory end point for time-to-event analysis in cancer trials? J Clin Oncol 2016;34:3474–3476. [DOI] [PubMed] [Google Scholar]
- 42. A’Hern RP. Cancer biology and survival analysis in cancer trials: restricted mean survival time analysis versus hazard ratios. Clin Oncol 2018;30:e75–e80. [DOI] [PubMed] [Google Scholar]
- 43. Van Der Weele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 44. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2019. [Google Scholar]
- 45. Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–1721. [DOI] [PubMed] [Google Scholar]
- 46. Alderson D, Cunningham D, Nankivell M, Blazeby JM, Griffin SM, Crellin A et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol 2017;18:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol 2017;18:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rahman SA, Walker RC, Lloyd MA, Grace BL, van Boxel GI, Kingma BF et al. ; OCCAMS Consortium. Machine learning to predict early recurrence after oesophageal cancer surgery. Br J Surg 2020;107:1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Davies AR, Myoteri D, Zylstra J, Baker CR, Wulaningsih W, Van Hemelrijck M et al. ; Guy's and St Thomas' Oesophago-Gastric Research Group and PROGRESS Study Group. Lymph node regression and survival following neoadjuvant chemotherapy in oesophageal adenocarcinoma. Br J Surg 2018;105:1639–1649. [DOI] [PubMed] [Google Scholar]
- 50. Rahman SA, Walker RC, Maynard N, Trudgill N, Crosby T, Cromwell DA, et al. The AUGIS survival predictor. Ann Surg 2021; doi: 10.1097/SLA.0000000000004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Curtis NJ, Noble F, Bailey IS, Kelly JJ, Byrne JP, Underwood TJ. The relevance of the Siewert classification in the era of multimodal therapy for adenocarcinoma of the gastro-oesophageal junction. J Surg Oncol 2014;109:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van der Werf LR, Busweiler LAD, van Sandick JW, van Berge Henegouwen MI, Wijnhoven BPL. Reporting national outcomes after esophagectomy and gastrectomy according to the Esophageal Complications Consensus Group (ECCG). Ann Surg 2020;271:1095–1101. [DOI] [PubMed] [Google Scholar]
- 53. Low DE, Kuppusamy MK, Alderson D, Cecconello I, Chang AC, Darling G et al. Benchmarking complications associated with esophagectomy. Ann Surg 2019;269:291–298. [DOI] [PubMed] [Google Scholar]
- 54. Booka E, Takeuchi H, Suda K, Fukuda K, Nakamura R, Wada N et al. Meta-analysis of the impact of postoperative complications on survival after oesophagectomy for cancer. BJS Open 2018;2:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Markar SR, Mackenzie H, Wiggins T, Askari A, Karthikesalingam A, Faiz O et al. Influence of national centralization of oesophagogastric cancer on management and clinical outcome from emergency upper gastrointestinal conditions. Br J Surg 2018;105:113–120. [DOI] [PubMed] [Google Scholar]
- 56. Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med 2019;380:152–162. [DOI] [PubMed] [Google Scholar]
- 57. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL et al. ; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–1098. [DOI] [PubMed] [Google Scholar]
- 58. Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol 2018;36:61–67. [DOI] [PubMed] [Google Scholar]
- 59. Kelly RJ. The emerging role of immunotherapy for esophageal cancer. Curr Opin Gastroenterol 2019;35:337–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.