Introduction
For patients with hepatic, pancreatic or biliary tumours, surgical resection offers the highest chance of cure. Although postoperative survival rates have improved, morbidity owing to postoperative complications remains significant1. Low aerobic fitness has been shown to increase the risk of postoperative complications in hepatopancreatobiliary surgery2–4. Preoperative optimization of aerobic fitness (prehabilitation) in high-risk (low aerobically fit) patients might reduce the incidence and impact of postoperative complications5,6. However, both attrition and adherence, as well as gaining an adequate response, remain a challenge7.
Ferreira et al.8 reported that patients’ preferred method of delivery of preoperative exercise programmes is home-based, with at least one supervised exercise session per week. To improve aerobic fitness in a short time period, high-intensity interval training seems to be most effective9. Furthermore, adequate protein intake is necessary to increase muscle protein synthesis10. Insights into the ability to improve a high-risk patient’s aerobic fitness and feasibility of a high-intensity supervised bimodal home-based exercise programme would be of great interest, as this might be the most preferred and effective method for exercise prehabilitation.
The primary aim of this study was to evaluate the effects of a 4-week home-based high-intensity interval training programme with nutritional support on preoperative improvement of aerobic fitness of high-risk patients scheduled for elective liver or pancreatic resection. Secondary aims were to evaluate the feasibility of this bimodal prehabilitation programme, and its (preliminary) effect on other performance indicators of aerobic fitness and preoperative perceived quality of life.
Methods
Medical ethical approval was granted by the Medical Ethics Committee Twente, Enschede, the Netherlands (P17-08, NL59702.044.16, April 2017) and the study was registered in the Netherlands Trial Registry (NL6151). Written informed consent was received from all patients before enrolment.
A complete description of the methodology for this multicentre study is available in Appendix S1 and in the previously published study protocol11. In brief, using a pretest–post-test design, high-risk patients (preoperative oxygen uptake (VO2) at the ventilatory anaerobic threshold (VAT) 11 ml per kg per min or less) scheduled for elective liver or pancreatic resection, and who provided informed consent, participated in a 4-week semisupervised home-based exercise programme (12 sessions in total). The programme consisted of individualized goal setting followed by titration of high-intensity interval training and moderate-intensity endurance interval training on an advanced cycle ergometer (Lode Corival; Lode, Groningen, the Netherlands), combined with functional task exercises and protein and vitamin/mineral supplementation. The primary endpoint of this study was the change in VO2 at the VAT and oxygen uptake at peak exercise (VO2peak) after the 4-week prehabilitation programme. Secondary endpoints were: programme feasibility (recruitment rate, adherence, completion rate, drop-out rate, attrition rate, and adverse events); the (preliminary) effect of the programme on other cardiopulmonary exercise testing (CPET) values; and the effect of the programme on perceived health-related quality of life.
Results
In total, 112 patients were assessed for eligibility. Of the 52 initially eligible patients (VO2 at the VAT 11 ml per kg per min or less), 8 were eventually not scheduled for surgery, whereas surgeons preferred short-term surgery because of borderline resectability in 6 patients. Of the 38 eligible patients, 26 were included, corresponding to a recruitment rate of 68 per cent (Fig. S1). The other 12 eligible patients were not included in the study for various reasons (Appendix S2). Baseline characteristics of the study participants are summarized in Table 1.
Table 1.
Preoperative characteristics of patients in the study cohort
| Study cohort (n = 26) | |
|---|---|
| Age (years) | |
| 18−64 | 6 |
| 65–74 | 9 |
| ≥ 75 | 11 |
| Mean (s.d.) | 71.6 (8.7) |
| Sex | |
| M | 18 |
| F | 8 |
| BMI (kg/m2) | |
| < 18.5 | 0 |
| 18.5–25.0 | 6 |
| 25.1–29.9 | 4 |
| ≥ 30.0 | 16 |
| Median (i.q.r.) | 31.3 (27.0-34.9) |
| Smoker | 8 |
| Charlson Co-morbidity Index score | |
| < 5 | 3 |
| 5–9 | 17 |
| ≥ 10 | 6 |
| Mean (s.d.) | 7.6 (2.4) |
| ASA fitness grade | |
| I−II | 10 |
| ≥ III | 16 |
| Aerobic fitness | |
| VO2 at VAT (ml per kg per min), mean (s.d.)* | 9.5 (0.9) |
| VO2peak (ml per kg per min), mean (s.d.)† | 14.5 (2.2) |
| Haemoglobin (mmol/l), median (i.q.r.) | 8.3 (7.2-9.2) |
| Indication for referral | |
| Colorectal liver metastases | 3 |
| Liver tumour | 6 |
| Gallbladder and biliary tract tumour | 5 |
| Pancreatic head tumour | 8 |
| Pancreatic body/tail tumour | 3 |
| Other‡ | 1 |
| Eventually underwent surgery | |
| Yes | 20 |
| No, (partly) owing to patient’s condition | 1 |
| No, other reason§ | 5 |
| Interval between baseline CPET and surgery (days), median (i.q.r.)¶ | 53 (50-72) |
| Interval between second CPET and surgery (days), median (i.q.r.)# | 12 (3-18) |
*Based on 25 patients, as oxygen uptake (VO2) at the ventilatory anaerobic threshold (VAT) during pre-prehabilitation cardiopulmonary exercise testing (CPET) could not be determined for 1 patient; the patient was nonetheless included in the study, as the oxygen uptake at peak exercise (VO2peak) at pre-prehabilitation CPET was below 11 ml per kg per min. †Based on 19 patients as 7 patients did not meet the criteria for a valid maximal effort during pre-prehabilitation CPET. ‡Colonic tumour with invasion of pancreas.§Tumour found to be unresectable when the patient was already listed for surgery. ¶Based on 20 patients as 6 did not have surgery. #Based on 15 patients as 7 did not undergo second CPET and 4 did not have surgery.
Aerobic fitness
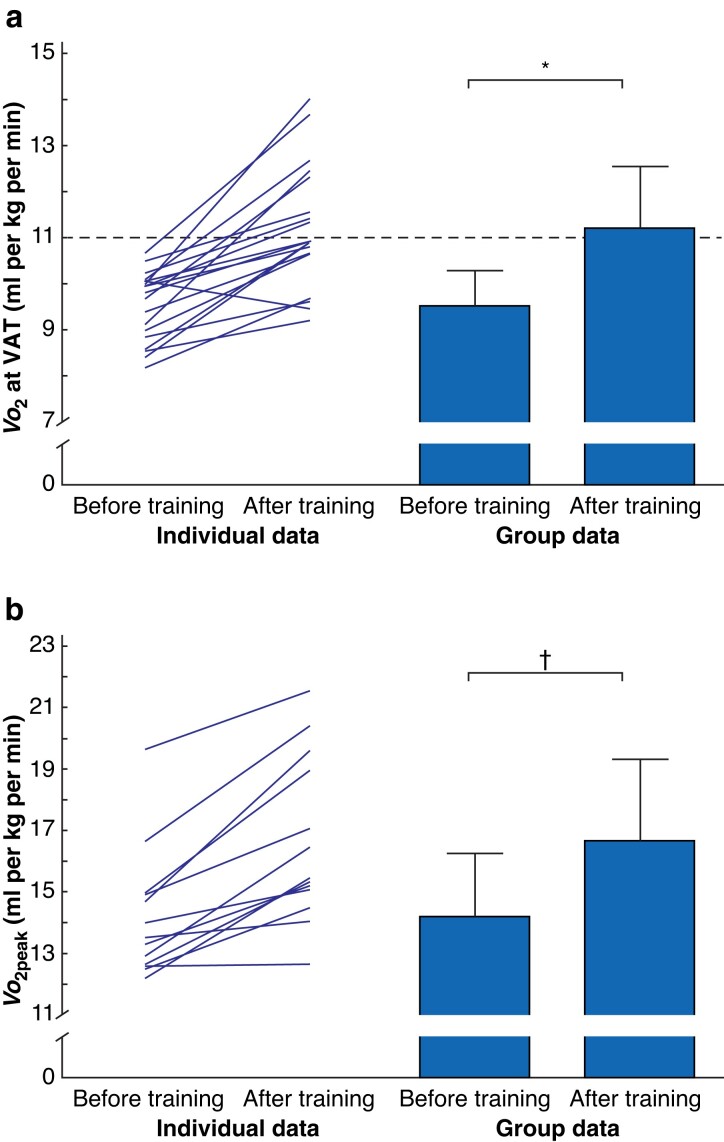
Progress on preoperative aerobic fitness during the 4-week prehabilitation programme is shown in Fig. 1 and Table S1. Prehabilitation led to a mean(s.d.) improvement in VO2 at the VAT of 1.7(1.1) (range −0.6 to 4.1) ml per kg per min (19 patients) indicating a 17.8 (95 per cent c.i. −2.23 to −1.15) per cent improvement (P < 0.001). VO2peak improved by 2.4(1.4) (range 0.1–4.9) ml per kg per min, representing a 17.2 per cent increase (P = 0.001; no 95 per cent c.i. as data were not distributed normally). The oxygen pulse and work rate at peak exercise improved significantly, whereas the oxygen uptake efficiency slope demonstrated no change. Eight patients had a preoperative VO2 at the VAT higher than 11 ml per kg per min after the prehabilitation programme. Five showed an improvement of less than 1 ml per kg per min in VO2 at the VAT in the post-prehabilitation CPET, and 2 had an increase in VO2peak of less than 1 ml per kg per min.
Fig. 1.
Pre- and post-prehabilitation aerobic fitness measurements
a Oxygen uptake (VO2) at the ventilatory anaerobic threshold (VAT) and b oxygen uptake at peak exercise (VO2peak). Group data are presented as mean (s.d.). The dashed line represents the cut-off for high risk based on VO2 at the VAT; patients who score below this cut-off have an increased risk of postoperative complications, which was an inclusion criterion for the present study. *P < 0.001 (paired samples t-test), †P = 0.001 (Wilcoxon signed-rank test).
Feasibility
Next to a recruitment rate of 68 per cent, patients attended a mean (s.d.) of 9.9 (3.2) of the 12 training sessions, resulting in an adherence rate of 83 per cent. Fifteen of the 26 patients attended all of the training sessions, giving a completion rate of 58 per cent. The drop-out rate was 3 of 26 (12 per cent), which led to an attrition rate of 61 per cent. Reasons for drop-out or missing training sessions are listed in Appendix S3. No serious adverse events were registered. Patients were satisfied with the training programme. Patients scored a median value of 4 (range of median values 4–5) on 8 statements. The median scores for each statement separately are presented in Fig. S2.
Perceived health-related quality of life
There was no significant difference in health-related quality of life pre- and post-prehabilitation, as measured with the Short Form 36 (SF-36) questionnaire. Scores for each item in the SF-36 are presented in Fig. S3. Mean(s.d.) scores were 7.2(1.8) before training and 7.7(1.3) after prehabilitation (P = 0.106). Furthermore, an improving trend was observed in the EQ-5D™ (EuroQol Group, Rotterdam, the Netherlands) questionnaire scores after prehabilitation; however, this improvement was not statistically significant (P = 0.262).
Discussion
The results of the present study showed that this prehabilitation programme led to a mean improvement in VO2 at the VAT of 17.8 per cent, whereas VO2peak improved by 17.2 per cent. In addition, patients completed 83 per cent of the training sessions, and on average they were satisfied with the programme. These results indicate that semisupervised home-based training is a highly efficient in improving preoperative aerobic fitness in these high-risk patients.
Following a 3-week supervised community-based exercise prehabilitation programme in high-risk patients with colorectal cancer (VO2 at the VAT 11 ml per kg per min or less), VO2 at the VAT increased by 10.1 per cent (P = 0.006) and VO2peak by 8.8 per cent (P = 0.05)6. Patients attended a mean(s.d.) of 8.1(2.4) of the 9 supervised exercise sessions (90 per cent)6. The present study showed an improvement of 17.8 per cent in VO2 at the VAT and 17.2 per cent in VO2peak, with an adherence rate of 83 per cent. A similar improvement was observed in a 4-week prehabilitation programme before liver resection12. As reported adherence rates to training sessions in prehabilitation programmes vary from 70 per cent on average in unsupervised programmes to 98 per cent on average in supervised programmes13, an adherence rate of 83 per cent seems acceptable for the present semisupervised programme.
The moderate recruitment rate (68 per cent) might have led to selection bias, as the results of the programme are unknown for the patients who were not recruited. However, the main reason for non-participation was lack of a physiotherapist specialized in oncology available in the living context of the patient for the supervised home-based training sessions. Most patients were included in the study from a tertiary referral centre with a large catchment area, which made it difficult to use the same physiotherapist for multiple patients. As well as compromising the recruitment rate, this logistical challenge resulted in each physiotherapist training only one or two patients, which limited the ability of physiotherapists to gain experience with the training protocol. Community-based perioperative care networks should be established, in which trained and competent physiotherapists, along with the patient and their informal support system, aim to make a patient fit for surgery, either in a home- or community-based context14.
Strengths of this study were that the training sessions were personalized based on the steep ramp test, partly supervised by a specialised physiotherapist, supplemented with nutritional support, and that the effect of the programme was evaluated with CPET. An important limitation is the fact that adherence to the nutritional intervention was not monitored. Furthermore, not only does a patient’s aerobic fitness need to be optimized before treatment, but their nutritional status, presence of anaemia, frailty, use/abuse of intoxicants, and low psychological resilience should also be addressed in a multimodal prehabilitation programme15.
In the context of all recent evidence in favour of prehabilitation, the authors urge profound but swift dialogue on the next experimental step(s) in the context of remedies like multimodal prehabilitation to further improve the recruitment rate, adherence, attrition, and effectiveness. This might translate into improved postoperative outcomes and a reduced demand on hospital resources.
Supplementary Material
Acknowledgements
The authors thank Lode Holding for the use of cycle ergometers and Nutricia for the provision of nutritional supplements; H. Kotte (physiotherapist, Fysio Twente, Enschede, the Netherlands), P. Weltevreden (physiotherapist, FITclinic, Enschede, the Netherlands), and E. van Zutphen (physiotherapist, Paramedics, Assen, the Netherlands) for their contribution in delivering the training programme; and all the physiotherapists who contributed to this study by supervising the patients. This article is original work, and has not been published before, and is not being considered for publication elsewhere in its final form, in either printed or electronic media. No preregistration exists for the study reported in this article.
Disclosure. The authors declare no conflict of interest.
Contributor Information
Laura van Wijk, Department of Hepatobiliary Surgery and Liver Transplantation, University Medical Centre Groningen, Groningen, the Netherlands.
Bart C Bongers, Department of Nutrition and Movement Sciences, School of Nutrition and Translational Research in Metabolism (NUTRIM), Maastricht University, Maastricht, the Netherlands; Department of Epidemiology, Care and Public Health Research Institute (CAPHRI), Maastricht University, Maastricht, the Netherlands.
Annefleur E M Berkel, Department of Surgery, Medical Spectrum Twente, Enschede, the Netherlands.
Carlijn I Buis, Department of Hepatobiliary Surgery and Liver Transplantation, University Medical Centre Groningen, Groningen, the Netherlands.
Muriël Reudink, Department of Surgery, Máxima Medical Centre, Veldhoven, the Netherlands.
Mike S L Liem, Department of Surgery, Medical Spectrum Twente, Enschede, the Netherlands.
Gerrit D Slooter, Department of Surgery, Máxima Medical Centre, Veldhoven, the Netherlands.
Nico L U van Meeteren, Department of Anaesthesiology, Erasmus MC, Rotterdam, the Netherlands; Top Sector Life Sciences & Health (Health∼Holland), The Hague, the Netherlands.
Joost M Klaase, Department of Hepatobiliary Surgery and Liver Transplantation, University Medical Centre Groningen, Groningen, the Netherlands.
Funding
The authors have no funding to declare.
Supplementary material
Supplementary material is available at BJS online.
Data Availability
Data, analytical methods, and study materials will be available from the corresponding author (l.van.wijk@umcg.nl) upon reasonable request.
References
- 1. Straatman J, Cuesta MA, de Lange-de Klerk ES, van der Peet DL. Hospital cost-analysis of complications after major abdominal surgery. Dig Surg 2015;32:150–156 [DOI] [PubMed] [Google Scholar]
- 2. Kumar R, Garcea G. Cardiopulmonary exercise testing in hepato-biliary & pancreas cancer surgery—a systematic review: are we any further than walking up a flight of stairs? Int J Surg 2018;52:201–207 [DOI] [PubMed] [Google Scholar]
- 3. Junejo MA, Mason JM, Sheen AJ, Moore J, Foster P, Atkinson D et al. Cardiopulmonary exercise testing for preoperative risk assessment before hepatic resection. Br J Surg 2012;99:1097–1104 [DOI] [PubMed] [Google Scholar]
- 4. Junejo MA, Mason JM, Sheen AJ, Bryan A, Moore J, Foster P et al. Cardiopulmonary exercise testing for preoperative risk assessment before pancreaticoduodenectomy for cancer. Ann Surg Oncol 2014;21:1929–1936 [DOI] [PubMed] [Google Scholar]
- 5. Barberan-Garcia A, Ubre M, Roca J, Lacy AM, Burgos F, Risco R et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50–56 [DOI] [PubMed] [Google Scholar]
- 6. Berkel AEM, Bongers BC, Kotte H, Weltevreden P, de Jongh FHC, Eijsvogel MMM et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg 2022;275:e299–e306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dewulf M, Verrips M, Coolsen MME, Olde Damink SWM, Den Dulk M, Bongers BC et al. The effect of prehabilitation on postoperative complications and postoperative hospital stay in hepatopancreatobiliary surgery a systematic review. HPB (Oxford) 2021;23:1299–1310 [DOI] [PubMed] [Google Scholar]
- 8. Ferreira V, Agnihotram RV, Bergdahl A, van Rooijen SJ, Awasthi R, Carli F et al. Maximizing patient adherence to prehabilitation: what do the patients say? Support Care Cancer 2018;26:2717–2723 [DOI] [PubMed] [Google Scholar]
- 9. Franssen RFW, Janssen-Heijnen MLG, Barberan-Garcia A, Vogelaar FJ, Van Meeteren NLU, Bongers BC. Moderate-intensity exercise training or high-intensity interval training to improve aerobic fitness during exercise prehabilitation in patients planned for elective abdominal cancer surgery? Eur J Surg Oncol 2022;48:3–13 [DOI] [PubMed] [Google Scholar]
- 10. Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:713–719 [DOI] [PubMed] [Google Scholar]
- 11. Berkel AEM, Van Wijk L, Bongers BC, Van Der Palen J, Buis CI, Reudink M et al. Study protocol of a single-arm pre–post study to assess the preliminary effectiveness and feasibility of a home-based bimodal prehabilitation program on preoperative aerobic fitness in high-risk patients scheduled for liver or pancreatic resection. Int J Clin Trials 2020;7:103–111 [Google Scholar]
- 12. Dunne DF, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 2016;103:504–512 [DOI] [PubMed] [Google Scholar]
- 13. Thomas G, Tahir MR, Bongers BC, Kallen VL, Slooter GD, van Meeteren NL. A systematic review of randomised controlled trials investigating prehabilitation before major intra-abdominal cancer surgery: an analysis of prehabilitation content and outcome measures. Eur J Anaesthesiol 2019;36:933–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bongers BC, Dejong CHC, den Dulk M. Enhanced recovery after surgery programmes in older patients undergoing hepatopancreatobiliary surgery: what benefits might prehabilitation have? Eur J Surg Oncol 2021;47:551–559 [DOI] [PubMed] [Google Scholar]
- 15. van Wijk L, van der Snee L, Buis CI, Hentzen JEKR, Haveman ME, Klaase JM. A prospective cohort study evaluating screening and assessment of six modifiable risk factors in HPB cancer patients and compliance to recommended prehabilitation interventions. Perioper Med (Lond) 2021;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, analytical methods, and study materials will be available from the corresponding author (l.van.wijk@umcg.nl) upon reasonable request.