Abstract
Background
Endoscopic vacuum therapy (EVT) with or without early surgical closure (ESC) is considered an effective option in the management of pelvic anastomotic leakage. This meta-analysis evaluated the effectiveness of EVT in terms of stoma reversal rate and the added value of ESC.
Methods
A systematic search of PubMed, MEDLINE, and the Cochrane Library was conducted in November 2021 to identify articles on EVT in adult patients with pelvic anastomotic leakage. The primary outcome was restored continuity rate. Following PRISMA guidelines, a meta-analysis was undertaken using a random-effects model.
Results
Twenty-nine studies were included, accounting for 827 patients with leakage who underwent EVT. There was large heterogeneity between studies in design and reported outcomes, and a high risk of bias. The overall weighted mean restored continuity rate was 66.8 (95 per cent c.i. 58.8 to 73.9) per cent. In patients undergoing EVT with ESC, the calculated restored continuity rate was 82 per cent (95 per cent c.i. 50.1 to 95.4) as compared to 64.7 per cent (95 per cent c.i. 55.7 to 72.7) after EVT without ESC. The mean number of sponge exchanges was 4 (95 per cent c.i. 2.7 to 4.6) and 9.8 (95 per cent c.i. 7.3 to 12.3), respectively. Sensitivity analysis showed a restored continuity rate of 81 per cent (95 per cent c.i. 55.8 to 99.5) for benign disease, 69.0 per cent (95 per cent c.i. 57.3 to 78.7) for colorectal cancer, and 65 per cent (95 per cent c.i. 48.8 to 79.1) if neoadjuvant radiotherapy was given.
Conclusion
EVT is associated with satisfactory stoma reversal rates that may be improved if it is combined with ESC.
Available literature suggests that endoscopic vacuum therapy is associated with a satisfactory stoma reversal rate, especially if combined with early surgical closure, but there is substantial heterogeneity and high risk of bias.
Introduction
Anastomotic leakage is the most feared complication in colorectal surgery. This adverse event increases morbidity, mortality, and healthcare costs, and decreases health-related quality of life, and may increase the risk of locoregional recurrence1–4. Despite surgical advances and newly developed preventive strategies5–10, low anterior resection is still associated with anastomotic leak rates of about 10–15 per cent1,11.
A significant number of pelvic leaks do not heal or may develop into a chronic sinus12,13. This late complication has a substantial impact on quality of life, with symptoms such as pelvic pain, purulent discharge, or even septicaemia14,15. Borstlap and colleagues16 reported absence of long-term healing after 48 per cent of leaks13, and the stoma is never closed in half of all patients who develop an anastomotic leak. These data emphasize the need for more effective treatment strategies.
In 2008, a new treatment comprising endoscopic placement of a vacuum sponge into the abscess cavity was introduced, referred to as endoscopic vacuum therapy (EVT)17. The effectiveness of EVT has been explored in several cohort studies18–20, with increasing interest in this technique in most recent years. Early surgical closure (ESC) by transanal suturing of the defect after a few sponge exchanges may improve outcomes further, if technically feasible21,22. However, complete anastomotic healing might still be difficult to achieve, with a risk of recurrent sinus after an apparent healing.
The reported incidence of anastomotic healing after EVT varies from 56 to 100 per cent; this in part reflects lack of consensus on the definition of anastomotic healing18,23. Several studies have considered both complete and partial anastomotic healing as a primary outcome for therapeutic success owing to this heterogeneity20. A more objective endpoint that better reflects the success of therapy from a patient perspective is the rate of living with a functional anastomosis. Therefore, this systematic review and meta-analysis was designed to evaluate the effectiveness of EVT in treating patients with pelvic anastomotic leak based on stoma closure rate, and to assess whether the outcomes improve with ESC.
Methods
Study design and registration
This study was conducted in accordance with PRISMA guidelines24. The protocol was registered in PROSPERO, the International Prospective Register of Systematic Reviews (CRD42019118088).
Search strategy and study selection
An expert librarian assisted with a systematic search conducted in PubMed, MEDLINE, and the Cochrane Library for relevant articles between inception and February 2019, with an update in November 2021. The search strategy and information resources are detailed in Appendix S1. RCTs and observational studies of patients with pelvic intestinal anastomotic leakage treated with EVT were included. Only manuscripts written in English, and for which the full text was available, were included. Case reports and case series with fewer than five patients were excluded, as were animal studies. If the same group published different articles in the same interval, only the largest study was included.
The literature search was performed independently by two authors in March 2019 and two authors in November 2021. Disagreements were settled by discussion between the two reviewers, and reasons for exclusion were recorded during the screening processes. References in relevant publications were searched manually for additional potentially eligible studies.
Procedures and definitions
Treatment with EVT consisted of endoscopic placement of an open-pored polyurethane sponge into the abscess cavity. The procedure was performed as described in previous articles17,21,25. Sponges were replaced every 3–4 days, allowing continuous monitoring of the development of granulation tissue and preventing ingrowth of the sponge. The sponge was connected to a low-vacuum suction bottle to generate a negative pressure and continuous evacuation of pus. Although EVT without faecal diversion has been described, the anastomosis was generally defunctioned.
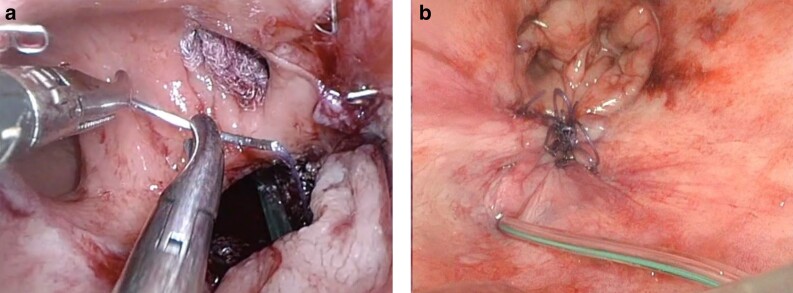
ESC is a transanal surgical procedure, carried out under general anaesthesia, in which the anastomotic defect is closed. This can be considered when the abscess cavity is covered with granulation tissue and the rectal cuff can be reapproximated21,22,26. ESC is performed in the Lloyd-Davies position. Depending on the height of the anastomosis, an anal retractor (for example, Lonestar®; Cooper Surgical, Trumbull, CT, USA) or an endoscopic transanal platform, such as the flexible Gelpoint Path (Applied Medical, Rancho Santa Margarita, CA, USA), are used. A suction drain is placed in the cavity behind the reconstructed anastomosis, which results in obliteration of the cavity, after which the neorectum will stick to the sacrum (Fig. 1).
Fig. 1.
Early surgical closure a Anastomotic endoluminal view (closing of the anastomotic defect). b Final closure (reconstructed anastomosis with suction drain in the cavity).
Outcome measures and data collection
The primary outcome was restored gastrointestinal continuity at the end of follow-up. Secondary outcomes included time from index surgery to start of EVT, number of sponge exchanges, time to restored continuity, and short- and long-term complication rates.
The following data were extracted for each selected study: title, first author, year of publication, country, journal name, study design, strength of evidence, inclusion and exclusion criteria, sample size, patient characteristics (mean age, sex, BMI, neoadjuvant radiotherapy, ASA fitness grade, indication for index surgery), primary operative and postoperative outcomes (type of surgery, primary diverting stoma, time to diagnosis of anastomotic leakage), and EVT outcomes (technical details, time to initiation of EVT, number of sponge exchanges, need for secondary stoma, drain placement and removal, adjunct treatments, procedure-related events, and late complications).
Quality assessment
Two authors independently assessed methodological quality using the Newcastle–Ottawa Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). A maximum of four points can be awarded for selection, two points for comparability, and three for outcome.
Statistical analysis
Study and baseline characteristics are reported using descriptive statistics. A meta-analysis was performed for single proportions (restored continuity rate, and procedure-related and late complication rates) using a pooled random-effects analysis with inverse-variance weighting. The I2 value was calculated to assess statistical heterogeneity. A meta-analysis was undertaken for single means (interval from surgery to diagnosis of anastomotic leak, interval from surgery to start of EVT, number of sponge exchanges, and time to stoma reversal) from mean(s.d.) values reported in the studies. When data were missing, these were calculated from other data if possible (such as median or i.q.r.), using methods described by Wan and co-workers27. Both fixed-effect and random-effects analysis were performed using an inverse-variance method, and statistical heterogeneity was assessed by calculating the I2 value. Sensitivity analyses for restored continuity rates were conducted for EVT with or without ESC, benign disease (or more than 90 per cent benign disease among included patients) versus colorectal cancer (or over 90 per cent colorectal cancer among included patients), colorectal cancer with radiotherapy versus any type of disease without radiotherapy, and primary diverting stoma (or more than 80 per cent of included patients) versus no primary diverting stoma (or less 20 per cent of included patients). Publication bias was investigated by visual inspection of the funnel plot of restored continuity, and using the Peters’ test to assess linear regression of funnel plot asymmetry (based on sample size)28.
No comparative meta-analysis between EVT with or without ESC was undertaken because only single cohort studies were found; results are presented separately for the two subgroups. A meta-analysis of healed anastomosis rate was not done because of the high level of heterogeneity in definition of a healed anastomosis. Meta-analysis was performed using RStudio version 1.2.1335 (RStudio: Integrated Development for R; RStudio, PBC, Boston, MA, USA).
Results
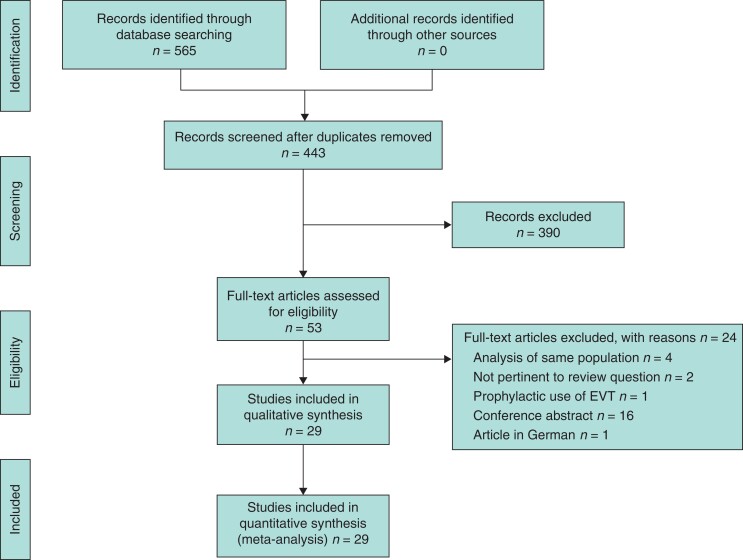
The literature search yielded 442 records. After screening titles and abstracts, 53 articles were eligible for full-text review. Of these, 29 studies18,19,21–23,25,29–51 were finally included. Reasons for exclusion are shown in Fig. 2. No RCT was found. Six studies22,29,31,45,47,50 were cohort studies, including one that used matching to handle allocation bias. The remaining studies18,19,21,23,25,30,32–44,46,48,49,51 were case series from institutional databases. Four studies21,22,25,45 used ESC as an adjunct to EVT. However, the study by Huisman and colleagues45 was excluded from the subgroup analysis as it was not possible to extract specific information for the ESC cohort (3 patients, 15 per cent of the whole group).
Fig. 2.
PRISMA flow diagram showing selection of articles for review
EVT, endoscopic vacuum therapy.
Quality assessment of the included studies is reported in Table S2. The funnel plot appeared potentially asymmetrical, but Peters’ linear regression indicated no asymmetry in the funnel plot, indicating a low likelihood of publication bias (P = 0.356) (Fig. S1).
Table 1 summarizes the characteristics of the included studies, accounting for a total of 827 patients. Surgery for colorectal cancer was the primary indication for surgery (613 of 817 patients, 75.0 per cent)18,19,21–23,25,29–39,41–51. Sixty-six patients (8 per cent) were treated for inflammatory bowel disease and 134 patients (16.4 per cent) had various underlying diseases as an indication for initial surgery18,19,21–23,25,29–39,41–51.
Table 1.
Characteristics of included studies of endoscopic vacuum therapy for pelvic anastomotic leakage
| Reference | Study design | Inclusion criteria | Indication | n | Male sex | Age (years) |
Primary stoma | NART | Adjunct treatment (%) |
|---|---|---|---|---|---|---|---|---|---|
| Mees et al.29 | Prospective matched cohort | Symptomatic leak after AR or IPAA | Rectal cancer and UC | 5 | 4 of 5 | 47 | n.a. | 0 of 5 | None |
| Glitsch et al.30 | Prospective case series | Symptomatic leak after AR or colectomy with extraperitoneal anastomosis | Rectal cancer | 17 | 14 of 17 | 61 | 8 of 17 | 9 of 17 | 15 Fibrin glue |
| van Koperen et al.18 | Prospective case series | Symptomatic leak after AR or IPAA | Rectal cancer and UC | 16 | 9 of 16 | 64 | 8 of 16 | 11 of 16 | None |
| von Bernstorff et al.19 | Prospective case series | Symptomatic leak after AR | Rectal cancer | 26 | 21 of 26 | 58 | 14 of 26 | 14 of 26 | None |
| Chopra et al.31 | Retrospective cohort | Symptomatic leak after AR | Rectal cancer | 5 | n.a. | n.a. | n.a. | 5 of 5 | Fibrin glue: 2 |
| Riss et al.32 | Retrospective case series | Symptomatic leak after AR or Hartmann insufficiency | Rectal cancer | 9 | 5 of 9 | 64 | 4 of 9 | 4 of 9 | None |
| Verlaan et al.25 | Prospective case series | Symptomatic leak after AR or IPAA | Rectal cancer and UC or FAP | 6 | 5 of 6 | 50 | 0 | 1 of 6 | ESC: 4 Clip: 1 |
| Srinivasamurthy et al.33 | Retrospective case series | Extraperitoneal anastomosis and symptomatic leak after AR or IPAA | Rectal cancer and UC | 8 | 7 of 8 | 67 | n.a. | 7 of 8 | None |
| Nerup et al.23 | Retrospective case series | Symptomatic leak after AR | Rectal cancer | 13 | 11 of 13 | 64 | 13 of 13 | 6 of 13 | None |
| Keskin et al.34 | Retrospective case series | Symptomatic leak after AR, IPAA or IRA | Rectal cancer, FAP and diverticular disease | 15 | 7 of 15 | 55 | 14 of 15 | 6 of 15 | None |
| Arezzo et al.35 | Retrospective case series | Symptomatic leak after AR, TEM or STARR | Rectal cancer, rectal adenoma, RV fistula | 14 | 7 of 14 | 68 | 8 of 14 | 7 of 14 | Glue and clip |
| Strangio et al.36 | Prospective case series | Symptomatic leak after AR, IPAA or left colectomy | Rectal cancer, endometriotic nodule, UC, colonic cancer, diverticulitis | 25 | 18 of 25 | 67 | 13 of 25 | 8 of 25 | None |
| Kuehn et al.37 | Retrospective case series | Symptomatic leak after AR, Hartmann insufficiency, IPAA, TEM or STARR | Rectal cancer, diverticulitis, UC, rectal perforation, UC, fistula | 41 | 31 of 41 | 70 | 19 of 19 | 12 of 41 | None |
| Mussetto et al.38 | Retrospective case series | Symptomatic leak after AR | Rectal cancer | 11 | 6 of 11 | 71 | n.a. | 5 of 11 | None |
| Milito et al.39 | Prospective case series | AL of low rectal anastomosis | Rectal cancer | 14 | 10 of 14 | 65 | 14 of 14 | 14 of 14 | None |
| Mencio et al.40 | Retrospective case series | Patients with different GI leaks | n.a. | 10 | 5 of 10 | 55 | 7 of 10 | n.a. | None |
| Jimenez-Rodriguez et al.41 | Prospective case series | Symptomatic leak after AR or Hartmann insufficiency | Rectal cancer | 22 | 18 of 22 | 65 | 13 of 22 | 17 of 22 | Fibrin glue: 10 |
| Borstlap et al.21 | Prospective case series | Symptomatic leak after AR | Rectal cancer | 30 | 19 of 30 | 66 | 23 of 30 | 22 of 30 | ESC: 30 |
| Rottoli et al.42 | Prospective case series | Symptomatic leak after IPAA | UC and FAP | 8 | n.a. | 37 | 8 of 8 | 0 of 8 | None |
| Katz et al.43 | Retrospective case series | Symptomatic leak after AR, IPAA | Rectal cancer, Hirschprung, FAP, ovarian cancer with rectal involvement | 6 | 5 of 6 | 54 | 3 of 6 | n.a. | None |
| Wasmann et al.22 | Retrospective cohort | Symptomatic leak after IPAA | UC | 18 | 12 of 18 | 41 | 1 of 18 | 0 of 18 | ESC: 18 |
| Boschetti et al.44 | Retrospective case series | Symptomatic leakage | Colonic cancer, rectal cancer, sigmoiditis | 29 | 22 of 29 | 68 | 12 of 29 (41.4) | 19 of 29 (65.5) | None |
| Huisman et al.45 | Retrospective cohort | Symptomatic leakage after rectal surgery | Rectal cancer, IBD | 20 | 14 of 20 | 64 | 14 of 20 | 14 of 20 | ESC: 3 |
| Kantowski46 | Retrospective case series | AL after colorectal resection | Rectal cancer, diverticular disease, IBD, ischaemia | 89 | 68 of 89 | 58 | 87 of 89 | 27 of 89 | Transanal rinsing therapy after EVT: 58 |
| Abdalla et al.47 | Prospective case series | Leakage after elective proctectomy | Rectal cancer, IBD | 47 | 36 of 47 | 65 | 40 of 47 | 27 of 47 | None |
| Weréen et al.48 | Retrospective cohort study | Symptomatic leakage after AR | Rectal cancer | 14 | 9 of 14 | 64 | 12 of 14 | 13 of 14 | None |
| Kühn et al.49 | Prospective case series | Colorectal defects | Rectal cancer, IBD, diverticular disease, other malignancies, perforation | 281 | 186 of 281 | 65 | 224 of 281 | 95 of 281 | None |
| Jagielski et al.50 | Prospective cohort study | AL after rectal cancer surgery | Rectal cancer | 18 | 18 of 18 | 61 | 8 of 18 | 16 of 18 | None |
| Keshvari et al.51 | Prospective case series | AL after LAR | Rectal cancer | 10 | 6 of 10 | 56 | 10 of 10 | 10 of 10 | None |
Values in parentheses are percentages. NART, neoadjuvant radiotherapy; (L)AR, (low) anterior resection; IPAA, ileal pouch–anal anastomosis; UC, ulcerative colitis; n.a., not available; FAP, familial adenomatous polyposis; ESC, early surgical closure; IRA, ileorectal anastomosis; TEM, transanal endoscopic microsurgery; STARR, stapled transanal rectal resection; RV, rectovaginal; GI, gastrointestinal; IBD, inflammatory bowel disease; AL, anastomotic leakage; EVT, endoscopic vacuum therapy.
Baseline characteristics
The pooled mean age for all patients was 62.9 years, and the overall male to female ratio, calculated on the basis of the studies reporting sex, was 2.5 : 1. Weighted mean BMI was 25.4 kg/m2 (Table 2). The weighted mean time interval between index surgery and diagnosis of leakage was 20.2 (95 per cent c.i. 15.9 to 24.6) days.
Table 2.
Baseline characteristics of included studies
| No. of studies | Total | No ESC | ESC | ||||
|---|---|---|---|---|---|---|---|
| n | Pooled value (%)* | n | Pooled value (%)* |
n | Pooled value (%)* |
||
| Patient characteristics | |||||||
| Men | 27 | 573 of 814 | 70.4 | 537 of 760 | 70.7 | 36 of 54 | 67 |
| Age (years) | 27 | 804 | 62.9† | 750 | 63.4† | 54 | 56† |
| BMI (kg/m2) | 10 | 197 | 25.4† | 149 | 25.5† | 48 | 25† |
| Neoadjuvant radiotherapy | 27 | 369 of 811 | 45.5 | 346 of 757 | 45.7 | 23 of 54 | 43 |
| Indication for primary surgery | |||||||
| Colorectal cancer | 28 | 613 of 817 | 75.0 | 582 of 763 | 76.3 | 31 of 54 | 57 |
| IBD | 28 | 66 of 817 | 8.1 | 43 of 763 | 5 | 23 of 54 | 43 |
| Other | 28 | 134 of 817 | 16.4 | 134 of 763 | 17.6 | 0 of 54 | 0 |
| Primary stoma (created during index surgery) | 24 | 577 of 776 | 74.4 | 553 of 722 | 73.6 | 24 of 54 | 44 |
| Secondary stoma (created after index surgery) | 23 | 119 of 687 | 17.3 | 86 of 613 | 14.0 | 30 of 54 | 56 |
| EVT in outpatient setting | 9 | 216 of 423 | 51.1 | 216 of 423 | 51.1 | 0 | 0 |
| Duration of follow-up (months) | 13 | 246 | 19.4† | 170 | 17.5† | 54 | 30† |
*Unless indicated otherwise; †mean value. ESC, early surgical closure; n, number of patients; IBD, inflammatory bowel disease; EVT, endoscopic vacuum therapy.
Of 776 patients, 577 (74.4 per cent) had a diverting stoma after primary surgery, and 119 of 687 (17.3 per cent) received a secondary stoma following anastomotic leakage after the primary resection (Table 2). The pooled mean follow-up for all patients was 19.4 months. Among patients undergoing EVT without ESC, 553 of 722 (73.6 per cent) had faecal diversion with a primary stoma, 86 of 613 (14.0 per cent) had a secondary stoma, and mean follow-up was 17.5 months. In patients undergoing EVT with ESC, 24 of 54 patients had faecal diversion (44 per cent) with primary stoma, 30 of 54 had faecal diversion with a secondary stoma (55 per cent) and the mean follow-up was 29.8 months.
Outcomes of endoscopic vacuum therapy
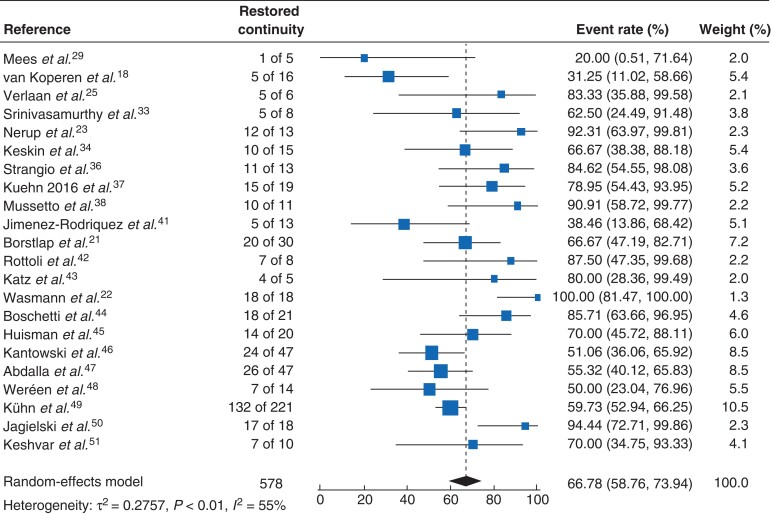
Table 3 shows the general outcomes of EVT, including all studies independent of adjunct ESC. Random-effects meta-analysis showed that the weighted mean rate of restored continuity after stoma formation (either primary or secondary) was 66.8 (95 per cent c.i. 58.8 to 73.9) per cent (I2 = 55 per cent) (Fig. 3)18,21–23,25,29,33,34,36–38,41–51. The calculated mean rate of procedure-related complications was 6.7 (4.7 to 9.6) per cent18,19,21–23,29–39,41–47,50,51. Healed anastomosis rates and definitions are presented separately for the included studies in Table S1. From the available information, EVT could be continued in an outpatient setting in 216 patients (representing 51.1 per cent of the total of 423 patients from studies reporting this information)19,29,30,34,35,41,44,48,49. The documented late complication rate was 10.8 (6.8 to 16.7) per cent among 21 studies comprising 440 patients18,19,21–23,29,30,32–39,41,42,44,45,47,51.
Table 3.
Pooled outcomes after endoscopic vacuum therapy in patients with pelvic anastomotic leakage
| Total | No ESC | ESC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | n | Pooled value (%)* | No. of studies | n | Pooled value (%)* | No. of studies | n | Pooled value (%)* | |
| Interval from surgery to AL diagnosis (days) | 16 | 272 | 20.2 (15.9, 24.6)† | 12 | 198 | 23.5 (17.2, 29.9)† | 3 | 54 | 15 (8.3, 22.5)† |
| Interval from surgery to EVT (days) | 15 | 265 | 35.9 (27.8, 44.0)† | 11 | 191 | 38.3 (28.8, 47.8)† | 3 | 54 | 23 (9.1, 37.0)† |
| No. of sponges used | 26 | 710 | 9.1 (7.0, 11.3)† | 22 | 636 | 9.8 (7.3, 12.3)† | 3 | 54 | 4 (2.7, 4.6)† |
| Anastomotic function | |||||||||
| Restored continuity (%) | 22 | 578 | 66.8 (58.8, 73.9) | 18 | 505 | 64.7 (55.7, 72.7) | 3 | 54 | 82.0 (50.1, 95.4) |
| Time to restored continuity (months)‡ | 7 | 114 | 5.1 (3.3, 6.9)† | 3 | 51 | 4 (2.5, 4.9)† | 3 | 43 | 2 (0.9, 4.0)† |
| Complications | |||||||||
| Procedure-related | 25 | 516 | 6.7 (4.7, 9.6) | 22 | 461 | 10.2 (6.7, 15.1) | 2 | 48 | 2 (0, 0.1) |
| Late (during follow-up) | 21 | 440 | 10.8 (6.8, 16.7) | 18 | 372 | 9.7 (6.0, 15.3) | 2 | 48 | 14 (1.0, 72.3) |
Values in parentheses are 95 per cent confidence intervals. *Unless indicated otherwise; †mean. ‡After diagnosis of anastomotic leakage (AL). ESC, early surgical closure; n, number of patients; EVT, endoscopic vacuum therapy.
Fig. 3.
Forest plot showing restored continuity rates after endoscopic vacuum therapy
Event rates are shown with 95 per cent confidence intervals.
Time to start of endoscopic vacuum therapy
Several authors have suggested that the timing of EVT may influence treatment outcomes. However, these analyses usually focused on anastomotic healing, and only three reported data on stoma reversal rate at the end of follow-up. Borstlap and colleagues21 found that starting EVT within the first 21 days was associated with a non-significant increase in stoma reversal rate (73 versus 60 per cent; median follow-up 14 months). With a median follow-up of 10 months, Huisman et al.45 reported a cumulative probability of stoma removal of 77 (95 per cent c.i. 22 to 93) per cent when EVT was started within the first 21 days, compared with 70 (23 to 88) per cent in the late-initiation group (P = 0.31). Abdalla and co-workers47 documented a higher stoma reversal rate when EVT was started 15 days after diagnosis of anastomotic leakage than when it was initiated later (72.4 versus 27.8 per cent; P = 0.003).
Endoscopic vacuum therapy with or without early surgical closure
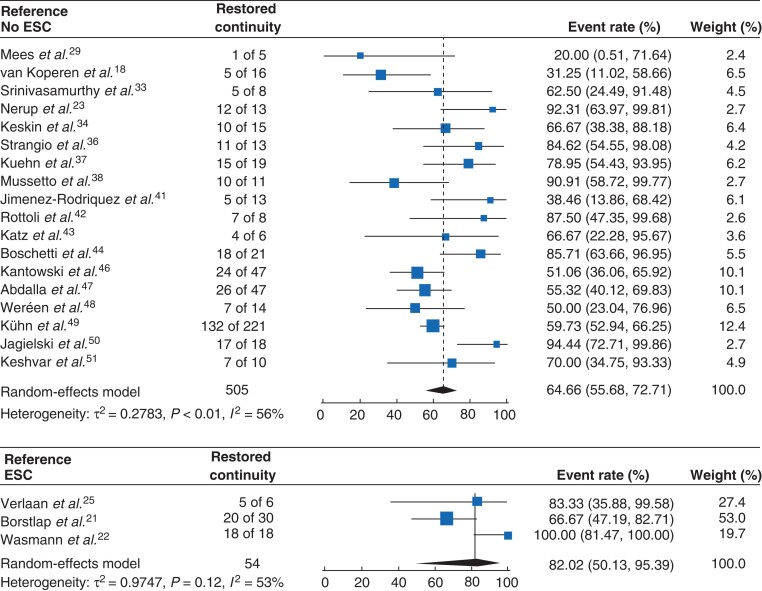
Fifty-four patients had EVT with ESC, of whom 23 underwent ileal pouch–anal anastomosis (IPAA). Regarding baseline characteristics, primary resection for colorectal cancer was performed in 31 of 54 patients who underwent EVT with ESC (57 per cent) and in 582 of 763 (76.3 per cent) without ESC. Corresponding proportions neoadjuvant radiotherapy were 23 of 54 (43 per cent) and 346 of 757 (45.7 per cent), respectively. Random-effects meta-analysis showed that the weighted mean rate of restoration of continuity in the ESC group was 82 per cent (95 per cent c.i. 50.1 to 95.4)21,22,25, which was 64.7 per cent (95 per cent c.i. 55.7 to 72.7) in the group without ESC (Table 3 and Fig. 4). The mean number of sponge exchanges was 4 (95 per cent c.i. 2.7 to 4.6) in the EVT with ESC group, compared to a mean of 9.8 (95 per cent c.i. 7.3 to 12.3) in the EVT-only group.
Fig. 4.
Forest plot showing restored continuity rates after endoscopic vacuum therapy with or without early surgical closure
Event rates are shown with 95 per cent confidence intervals. ESC, early surgical closure.
Sensitivity analysis
Sensitivity analysis showed a restored continuity rate of 81.0 (95 per cent c.i. 55.8 to 99.5) per cent for patients with benign disease, 69.0 (57.3 to 78.7) per cent for those with colorectal cancer, and 65.5 (48.8 to 79.1) per cent if neoadjuvant radiotherapy was administered (Table 4). The restored continuity rate was 61.9 (53.4 to 69.7) per cent in patients who received a primary diverting stoma, and 83.1 (66.2 to 92.5) per cent among those without a primary stoma.
Table 4.
Sensitivity analysis for restored continuity in different subgroups of patients undergoing endoscopic vacuum therapy for pelvic anastomotic leakage
| No. of studies | n | Restored continuity rate (%) | |
|---|---|---|---|
| Benign disease (or > 90%) | 5 | 39 | 81.0 (55.8, 99.5) |
| Colorectal cancer (or ≥ 90%) | 11 | 201 | 69.0 (57.3, 78.7) |
| Colorectal cancer with radiotherapy | 5 | 76 | 65.5 (48.8, 79.1) |
| Any type of disease, no radiotherapy | 6 | 57 | 70 (38.8, 89.7) |
| Primary diverting stoma (or ≥ 80%) | 11 | 420 | 61.9 (53.4, 69.7) |
| No primary stoma (or ≤ 20%) | 3 | 81 | 83 (66.2, 92.5) |
Values in parentheses are 95 per cent confidence intervals. n, number of patients.
Discussion
In this systematic review including 29 studies, EVT was associated with successful restoration of continuity, with a functional anastomosis in two-thirds of patients. The stoma reversal rate at the end of follow-up seemed to be higher for patients treated with combined EVT plus ESC compared with EVT alone. Most studies were retrospective cohort studies, with a large difference in cohort size ranging from 5 to 281 patients, and a wide variety of underlying diseases as well as primary treatment modalities (colonic anastomosis or IPAA, with or without neoadjuvant radiotherapy). This resulted in a high risk of bias. Therefore, the present findings should be interpreted carefully for the different subgroups and indications. Nevertheless, these results justify further investigation in larger prospective series and international registries with extended follow-up, given the ethical and other practical and methodological issues related to controlled randomized conditions in this specific population.
EVT aims to control pelvic sepsis and gradually reduce the size of the sinus. In the original publication, Weidenhagen and colleagues17 reported definitive anastomotic healing in more than 96 per cent of patients. Since then, a number of observational studies17–19,23,29–31,35–39,41 have been published, with variable success rates in heterogeneous patient populations. Meta-analyses20,52–54 have been undertaken in this area. The present review is an update, with a substantially larger number of studies and patients, which also enabled sensitivity analyses of clinically relevant subgroups. Furthermore, the additional value of ESC was not analysed in the previous reviews.
There is a lack of consensus on how to classify anastomotic healing after leakage. Across the included studies, there was a wide range of definitions. Imaging and/or endoscopic confirmation was included in some of these, whereas others did not describe any specific criteria at all. This hinders the ability to compare results and, more importantly, underlines the need for consensus on an objective and reproducible universal definition. For future research, objective measures for anastomotic healing should be used, such as the absence of any extraluminal air or fluid on CT with rectal contrast, and absence of symptoms indicative of reactivation of leakage following stoma closure.
Among the currently used definitions, a healed anastomosis may refer to true healing but also pelvic symptom containment. However, restored continuity (without the need for any major salvage surgery) is a hard endpoint that reflects the rate of functional anastomoses. Several studies have reported permanent stoma rates after conventional management of anastomotic leakage. Maggiori and co-workers55, with a median follow-up of 3 years, reported a 36 per cent rate in patients with symptomatic anastomotic leak treated with a secondary stoma. In the 2011 Dutch Surgical Colorectal Audit, Borstlap et al.13 analysed 998 patients who underwent low anterior resection, and reported an early anastomotic leak rate of 13.4 per cent. The rate of unintentional permanent stoma after anastomotic leak was 46 per cent after a median of 43 months, which is similar to the 51 per cent rate in the Dutch TME trial16 with 7 years of follow-up. The findings of the present meta-analysis showed that, with a median follow-up of less than 2 years, EVT was associated with a long-term stoma rate of 33 per cent, which is somewhere between the permanent stoma rates ranging from 24 to 49 per cent in previously published meta-analyses20,52,53. This 33 per cent stoma rate seems acceptable, but at the same time does not convincingly show better stoma-free survival than that achieved with conventional leakage management. This might represent selection bias, with more severe leaks treated using EVT, and more asymptomatic radiological leaks managed in a conventional passive way.
The addition of ESC was associated with better outcomes, with a long-term stoma rate of 18 per cent. However, it should be noted that the proportion of IPAAs was relatively high in the ESC group compared with that among patients who received EVT alone, and these results cannot be extrapolated to rectal cancer populations undergoing neoadjuvant radiotherapy. Anastomotic leakage severity scores need to be developed for the purpose of better comparison between treatment strategies56.
Establishing the cost-effectiveness of a new therapy is important before its use becomes widespread in reimbursed healthcare systems. The financial impact of treating a patient with anastomotic leakage is already high, with additional costs of approximately €18 000 compared with those for patients with no leak57. It has been reported previously that five patients must be treated with EVT and ESC in order to save one extra anastomosis, compared with standard passive anastomotic leak management21. The present study found that EVT with ESC required six fewer endoscopies for sponge replacement than EVT alone. This implies a direct reduction in resources, but also in time to completion of treatment. Moreover, the suggested improved clinical outcomes observed with the addition of ESC indicate potential cost-effectiveness, but this has to be confirmed in properly designed studies.
The development of a pelvic anastomotic leak may lead to significant postoperative bowel dysfunction. For this reason, in addition to studying how these leaks are treated using hard endpoints such as stoma closure, it is important to include functional and quality-of-life outcomes. The ability to control pelvic sepsis and close a defect earlier by means of EVT and ESC, with fewer sponge replacements, may also improve function. This was shown recently in a cohort study22 of patients undergoing IPAA, which found that EVT with ESC was associated with preservation of pouch function and preclusion of pouch failure, in contrast to conventional leak management. Unfortunately, very few studies have reported on function after EVT with or without ESC; this represents an important knowledge gap that should also be addressed in future studies.
Of all the factors that may increase the effectiveness of EVT, it seems that early diagnosis and initiation of treatment are crucial52. Late initiation of EVT might be ineffective owing to the retraction of the anastomotic edges and reduced pliability of the neorectum. An especially susceptibility group of patients are those with primary diversion and an asymptomatic anastomotic leak, in whom dehiscence may be diagnosed only after stoma reversal. Therefore, to detect occult leaks, and with the aim of initiating EVT as soon as possible, highly selective diversion with early C-reactive protein measurement in all patients receiving a pelvic anastomosis, followed by CT or endoscopy when necessary, is recommended58. The sensitivity analysis also hints in a similar direction, with a higher rate of restored continuity in patients without a primary stoma (83.1 versus 61.9 per cent).
This study has several limitations. The sample sizes of the included studies were mostly small and there was considerable heterogeneity among the inclusion criteria. Moreover, the studies had methodological limitations, mostly based on imperfect designs and reporting. The primary outcome—stoma reversal rate—was considered to be the rate at the end of the follow-up; nevertheless, additional stomas might have been created after manuscript publication, for example for a small persistent sinus or faecal incontinence. The majority of articles included patients with anastomotic leakage, but a few also included patients with rectal stump insufficiency following a low Hartmann’s procedure. These data could not be analysed separately and may be a source of bias.
Supplementary Material
Acknowledgements
F.B.d.L. and K.T. are joint first authors of this review. The authors thank F. S. van Etten-Jamaludin (AMC Medical Library), who was in charge of the full systematic research process, and S. van Dieren (statistician), who was involved in the data analysis.
Disclosure. P.J.T. and R.H. received a grant from B. Braun for a prospective trial (IMARI), but no grants, equipment or drugs have been received for this work. Dr W.A.B. reports grants from VIFOR, grants from Medtronic, and grants from B. Braun, outside the submitted work. The authors declare no other conflict of interest.
Contributor Information
F Borja de Lacy, Gastrointestinal Surgery Department, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Kevin Talboom, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Cancer Centre Amsterdam, Amsterdam, the Netherlands.
Sapho X Roodbeen, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Cancer Centre Amsterdam, Amsterdam, the Netherlands.
Robin Blok, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Cancer Centre Amsterdam, Amsterdam, the Netherlands.
Anna Curell, Gastrointestinal Surgery Department, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Pieter J Tanis, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Cancer Centre Amsterdam, Amsterdam, the Netherlands; Department of Oncological and Gastrointestinal Surgery, Erasmus MC, Rotterdam, the Netherlands.
Wilhelmus A Bemelman, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Cancer Centre Amsterdam, Amsterdam, the Netherlands.
Roel Hompes, Department of Surgery, Amsterdam University Medical Centres, University of Amsterdam, Cancer Centre Amsterdam, Amsterdam, the Netherlands.
Supplementary material
Supplementary material is available at BJS online.
References
- 1. Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH et al. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg 2016;20:2035–2051 [DOI] [PubMed] [Google Scholar]
- 2. McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg 2005;92:1150–1154 [DOI] [PubMed] [Google Scholar]
- 3. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011;253:890–899 [DOI] [PubMed] [Google Scholar]
- 4. Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser D et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 2011;254:907–913 [DOI] [PubMed] [Google Scholar]
- 5. Beard JD, Nicholson ML, Sayers RD, Lloyd D, Everson NW. Intraoperative air testing of colorectal anastomoses: a prospective, randomized trial. Br J Surg 1990;77:1095–1097 [DOI] [PubMed] [Google Scholar]
- 6. Hirst NA, Tiernan JP, Millner PA, Jayne DG. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis 2014;16:95–109 [DOI] [PubMed] [Google Scholar]
- 7. Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 2013;27:3003–3008 [DOI] [PubMed] [Google Scholar]
- 8. Karliczek A, Benaron DA, Baas PC, Zeebregts CJ, Wiggers T, van Dam GM. Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis 2010;12:1018–1025 [DOI] [PubMed] [Google Scholar]
- 9. Li VK, Wexner SD, Pulido N, Wang H, Jin HY, Weiss EG et al. Use of routine intraoperative endoscopy in elective laparoscopic colorectal surgery: can it further avoid anastomotic failure? Surg Endosc 2009;23:2459–2465 [DOI] [PubMed] [Google Scholar]
- 10. Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA et al. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg 2015;220:82–92.e1 [DOI] [PubMed] [Google Scholar]
- 11. Vallance A, Wexner S, Berho M, Cahill R, Coleman M, Haboubi N et al. A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis 2017;19:O1–O12 [DOI] [PubMed] [Google Scholar]
- 12. Blumetti J, Chaudhry V, Cintron JR, Park JJ, Marecik S, Harrison JL et al. Management of anastomotic leak: lessons learned from a large colon and rectal surgery training program. World J Surg 2014;38:985–991 [DOI] [PubMed] [Google Scholar]
- 13. Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ. Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross-sectional study. Ann Surg 2017;266:870–877 [DOI] [PubMed] [Google Scholar]
- 14. Musters GD, Borstlap WA, Bemelman WA, Buskens CJ, Tanis PJ. Intersphincteric completion proctectomy with omentoplasty for chronic presacral sinus after low anterior resection for rectal cancer. Colorectal Dis 2016;18:147–154 [DOI] [PubMed] [Google Scholar]
- 15. Sloothaak DA, Buskens CJ, Bemelman WA, Tanis PJ. Treatment of chronic presacral sinus after low anterior resection. Colorectal Dis 2013;15:727–732 [DOI] [PubMed] [Google Scholar]
- 16. den Dulk M, Smit M, Peeters KC, Kranenbarg EM, Rutten HJ, Wiggers T et al. A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol 2007;8:297–303 [DOI] [PubMed] [Google Scholar]
- 17. Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 2008;22:1818–1825 [DOI] [PubMed] [Google Scholar]
- 18. van Koperen PJ, van Berge Henegouwen MI, Rosman C, Bakker CM, Heres P, Slors JF et al. The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc 2009;23:1379–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. von Bernstorff W, Glitsch A, Schreiber A, Partecke LI, Heidecke CD. ETVARD (endoscopic transanal vacuum-assisted rectal drainage) leads to complete but delayed closure of extraperitoneal rectal anastomotic leakage cavities following neoadjuvant radiochemotherapy. Int J Colorectal Dis 2009;24:819–825 [DOI] [PubMed] [Google Scholar]
- 20. Shalaby M, Emile S, Elfeki H, Sakr A, Wexner SD, Sileri P. Systematic review of endoluminal vacuum-assisted therapy as salvage treatment for rectal anastomotic leakage. BJS Open 2019;3:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borstlap WAA, Musters GD, Stassen LPS, van Westreenen HL, Hess D, van Dieren S et al. Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc 2018;32:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wasmann KA, Reijntjes MA, Stellingwerf ME, Ponsioen CY, Buskens CJ, Hompes R et al. Endo-sponge assisted early surgical closure of ileal pouch–anal anastomotic leakage preserves long-term function: a cohort study. J Crohns Colitis 2019;13:1537–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nerup N, Johansen JL, Alkhefagie GA, Maina P, Jensen KH. Promising results after endoscopic vacuum treatment of anastomotic leakage following resection of rectal cancer with ileostomy. Dan Med J 2013;60:A4604. [PubMed] [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verlaan T, Bartels SA, van Berge Henegouwen MI, Tanis PJ, Fockens P, Bemelman WA. Early, minimally invasive closure of anastomotic leaks: a new concept. Colorectal Dis 2011;13:18–22 [DOI] [PubMed] [Google Scholar]
- 26. Talboom K, van Kesteren J, Sonneveld DJA, Tanis PJ, Bemelman WA, Hompes R. Early transanal closure after vacuum-assisted drainage for anastomotic leakage in rectal cancer surgery—a video vignette. Colorectal Dis 2020;22:973–974 [DOI] [PubMed] [Google Scholar]
- 27. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676–680 [DOI] [PubMed] [Google Scholar]
- 29. Mees ST, Palmes D, Mennigen R, Senninger N, Haier J, Bruewer M. Endo-vacuum assisted closure treatment for rectal anastomotic insufficiency. Dis Colon Rectum 2008;51:404–410 [DOI] [PubMed] [Google Scholar]
- 30. Glitsch A, von Bernstorff W, Seltrecht U, Partecke I, Paul H, Heidecke CD. Endoscopic transanal vacuum-assisted rectal drainage (ETVARD): an optimized therapy for major leaks from extraperitoneal rectal anastomoses. Endoscopy 2008;40:192–199 [DOI] [PubMed] [Google Scholar]
- 31. Chopra SS, Mrak K, Hünerbein M. The effect of endoscopic treatment on healing of anastomotic leaks after anterior resection of rectal cancer. Surgery 2009;145:182–188 [DOI] [PubMed] [Google Scholar]
- 32. Riss S, Stift A, Meier M, Haiden E, Grunberger T, Bergmann M. Endo-sponge assisted treatment of anastomotic leakage following colorectal surgery. Colorectal Dis 2010;12:e104–e108 [DOI] [PubMed] [Google Scholar]
- 33. Srinivasamurthy D, Wood C, Slater R, Garner J. An initial experience using transanal vacuum therapy in pelvic anastomotic leakage. Tech Coloproctol 2013;17:275–281 [DOI] [PubMed] [Google Scholar]
- 34. Keskin M, Bayram O, Bulut T, Balik E. Effectiveness of endoluminal vacuum-assisted closure therapy (Endosponge) for the treatment of pelvic anastomotic leakage after colorectal surgery. Surg Laparosc Endosc Percutan Tech 2015;25:505–508 [DOI] [PubMed] [Google Scholar]
- 35. Arezzo A, Verra M, Passera R, Bullano A, Rapetti L, Morino M. Long-term efficacy of endoscopic vacuum therapy for the treatment of colorectal anastomotic leaks. Dig Liver Dis 2015;47:342–345 [DOI] [PubMed] [Google Scholar]
- 36. Strangio G, Zullo A, Ferrara EC, Anderloni A, Carlino A, Jovani M et al. Endo-sponge therapy for management of anastomotic leakages after colorectal surgery: a case series and review of literature. Dig Liver Dis 2015;47:465–469 [DOI] [PubMed] [Google Scholar]
- 37. Kuehn F, Janisch F, Schwandner F, Alsfasser G, Schiffmann L, Gock M et al. Endoscopic vacuum therapy in colorectal surgery. J Gastrointest Surg 2016;20:328–334 [DOI] [PubMed] [Google Scholar]
- 38. Mussetto A, Arena R, Buzzi A, Fuccio L, Dari S, Brancaccio ML et al. Long-term efficacy of vacuum-assisted therapy (Endo-SPONGE®) in large anastomotic leakages following anterior rectal resection. Ann Gastroenterol 2017;30:649–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Milito G, Lisi G, Venditti D, Campanelli M, Aronadio E, Grande S et al. endoluminal vacuum therapy as treatment for anastomotic colorectal leakage. Surg Technol Int 2017;30:125–130 [PubMed] [Google Scholar]
- 40. Mencio MA, Ontiveros E, Burdick JS, Leeds SG. Use of a novel technique to manage gastrointestinal leaks with endoluminal negative pressure: a single institution experience. Surg Endosc 2018;32:3349–3356 [DOI] [PubMed] [Google Scholar]
- 41. Jimenez-Rodriguez RM, Araujo-Miguez A, Sobrino-Rodriguez S, Heller F, Diaz-Pavon JM, Bozada Garcia JM et al. A new perspective on vacuum-assisted closure for the treatment of anastomotic leak following low anterior resection for rectal cancer, is it worthy? Surg Innov 2018;25:350–356 [DOI] [PubMed] [Google Scholar]
- 42. Rottoli M, Di Simone MP, Vallicelli C, Vittori L, Liguori G, Boschi L et al. Endoluminal vacuum-assisted therapy as treatment for anastomotic leak after ileal pouch–anal anastomosis: a pilot study. Tech Coloproctol 2018;22:223–229 [DOI] [PubMed] [Google Scholar]
- 43. Katz E, White I, Shpitz B, Ghinea R, Avital S. Different approaches for Endo-SPONGE® insertion to treat rectal anastomotic leaks. Tech Coloproctol 2018;22:231–233 [DOI] [PubMed] [Google Scholar]
- 44. Boschetti G, Moussata D, Lahlou W, Passot G, Belkhodia H, Chauvenet M. Endo-sponge treatment of anastomotic leakage after colorectal surgery: a report of 29 cases compared to the main studies in the literature. J Hepato Gastroenterol 2018;1
- 45. Huisman JF, van Westreenen HL, van der Wouden EJ, Vasen HFA, de Graaf EJR, Doornebosch PG et al. Effectiveness of endosponge therapy for the management of presacral abscesses following rectal surgery. Tech Coloproctol 2019;23:551–557 [DOI] [PubMed] [Google Scholar]
- 46. Kantowski M, Kunze A, Bellon E, Rösch T, Settmacher U, Tachezy M. Improved colorectal anastomotic leakage healing by transanal rinsing treatment after endoscopic vacuum therapy using a novel patient-applied rinsing catheter. Int J Colorectal Dis 2020;35:109–117 [DOI] [PubMed] [Google Scholar]
- 47. Abdalla S, Cotte E, Epin A, Karoui M, Lefevre JH, Berger A et al. Short-term and long-term outcome of endoluminal vacuum therapy for colorectal or coloanal anastomotic leakage: results of a nationwide multicenter cohort study from the French GRECCAR group. Dis Colon Rectum 2020;63:371–380 [DOI] [PubMed] [Google Scholar]
- 48. Weréen A, Dahlberg M, Heinius G, Pieniowski E, Saraste D, Eklöv K et al. Long-term results after anastomotic leakage following rectal cancer surgery: a comparison of treatment with endo-sponge and transanal irrigation. Dig Surg 2020;37:456–462 [DOI] [PubMed] [Google Scholar]
- 49. Kühn F, Wirth U, Zimmermann J, Beger N, Hasenhütl SM, Drefs M et al. Endoscopic vacuum therapy for in- and outpatient treatment of colorectal defects. Surg Endosc 2021;35:6687–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jagielski M, Piątkowski J, Jarczyk G, Jackowski M. Transrectal endoscopic drainage with vacuum-assisted therapy in patients with anastomotic leaks following rectal cancer resection. Surg Endosc 2021;36:959–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keshvari A, Badripour A, Keramati MR, Kazemeini A, Behboudi B, Fazeli MS et al. Introduction of a handmade vacuum-assisted sponge drain for the treatment of anastomotic leakage after low anterior rectal resection. Ann Coloproctol 2021; DOI: 10.3393/ac.2021.00059.0008 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mahendran B, Rossi B, Coleman M, Smolarek S. The use of Endo-SPONGE® in rectal anastomotic leaks: a systematic review. Tech Coloproctol 2020;24:685–694 [DOI] [PubMed] [Google Scholar]
- 53. Popivanov GI, Mutafchiyski VM, Cirocchi R, Chipeva SD, Vasilev VV, Kjossev KT et al. Endoluminal negative pressure therapy in colorectal anastomotic leaks. Colorectal Dis 2020;22:243–253 [DOI] [PubMed] [Google Scholar]
- 54. Sharp G, Steffens D, Koh CE. Evidence of negative pressure therapy for anastomotic leak: a systematic review. ANZ J Surg 2021;91:537–545 [DOI] [PubMed] [Google Scholar]
- 55. Maggiori L, Bretagnol F, Lefèvre JH, Ferron M, Vicaut E, Panis Y. Conservative management is associated with a decreased risk of definitive stoma after anastomotic leakage complicating sphincter-saving resection for rectal cancer. Colorectal Dis 2011;13:632–637 [DOI] [PubMed] [Google Scholar]
- 56. van Workum F, Talboom K, Hannink G, Wolthuis A, de Lacy BF, Lefevre JH et al. Treatment of anastomotic leakage after rectal cancer resection: the TENTACLE-rectum study. Colorectal Dis 2021;23:982–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ashraf SQ, Burns EM, Jani A, Altman S, Young JD, Cunningham C et al. The economic impact of anastomotic leakage after anterior resections in English NHS hospitals: are we adequately remunerating them? Colorectal Dis 2013;15:e190–e198 [DOI] [PubMed] [Google Scholar]
- 58. Talboom K, Vogel I, Blok RD, Roodbeen SX, Ponsioen CY, Bemelman WA et al. Highly selective diversion with proactive leakage management after low anterior resection for rectal cancer. Br J Surg 2021;108:609–612 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.