Abstract
Background
Venous resection of the superior mesenteric or portal vein is increasingly performed in pancreatic cancer surgery, whereas results of studies on short- and long-term outcomes are contradictory. The aim of this study was to evaluate the impact of the type of venous resection in pancreatoduodenectomy for pancreatic cancer on postoperative morbidity and overall survival.
Methods
This nationwide retrospective cohort study included all patients who underwent pancreatoduodenectomy for pancreatic cancer in 18 centres (2013–2017).
Results
A total of 1311 patients were included, of whom 17 per cent underwent wedge resection and 10 per cent segmental resection. Patients with segmental resection had higher rates of major morbidity (39 versus 20 versus 23 per cent, respectively; P < 0.001) and portal or superior mesenteric vein thrombosis (18 versus 5 versus 1 per cent, respectively; P < 0.001) and worse overall survival (median 12 versus 16 versus 20 months, respectively; P < 0.001), compared to patients with wedge resection and those without venous resection. Multivariable analysis showed patients with segmental resection, but not those who had wedge resection, had higher rates of major morbidity (odds ratio = 1.93, 95 per cent c.i. 1.20 to 3.11) and worse overall survival (hazard ratio = 1.40, 95 per cent c.i. 1.10 to 1.78), compared to patients without venous resection. Among patients who received neoadjuvant therapy, there was no difference in overall survival among patients with segmental and wedge resection and those without venous resection (median 32 versus 25 versus 33 months, respectively; P = 0.470), although there was a difference in major morbidity rates (52 versus 19 versus 21 per cent, respectively; P = 0.012).
Conclusion
In pancreatic surgery, the short- and long-term outcomes are worse in patients with venous segmental resection, compared to patients with wedge resection and those without venous resection.
Of 1311 patients who underwent pancreatoduodenectomy, 17 per cent underwent venous wedge resection and 10 per cent underwent venous segmental resection. Venous segmental, but not venous wedge, resection was associated with higher major morbidity rates (odds ratio = 1.93, 95 per cent c.i. 1.20 to 3.11) and worse overall survival (hazard ratio = 1.40, 95 per cent c.i. 1.10 to 1.78), compared to no venous resection. This nationwide study found worse short- and long-term outcomes in patients who had venous segmental resection. The results of this study urge the need for improving outcomes in patients who require venous segmental resection.
Introduction
Pancreatic cancer is one of the few types of cancer for which the survival rate has barely improved in the last decades1. Radical tumour resection preceded or followed by chemo(radio)therapy is the current standard treatment for patients with pancreatic cancer2,3. The International Study Group of Pancreatic Surgery (ISGPS) suggests that partial resection of the portal vein or superior mesenteric vein (PV-SMV) should be performed in case of their suspected involvement in order to achieve radical resection4. Use of venous resection during pancreatoduodenectomy is increasing and is expected to increase further with the use of neoadjuvant therapy5–8.
An international survey reported that most pancreatic surgeons prefer venous segment resection with primary anastomosis over partial venous wedge resection, because of a lower perceived risk of complications9. Literature regarding complications after different types of venous resection is contradictory8,10–12. A recent meta-analysis of mostly single-centre observational studies showed that venous resection is associated with increased mortality and worse survival13. Data on the type of venous resection are not available. Nationwide studies with contemporary data representing current clinical practice are lacking.
The aim of this nationwide study was to evaluate the impact of venous resection type during pancreatoduodenectomy for pancreatic cancer on postoperative morbidity, mortality and overall survival.
Methods
Study design and patient selection
This nationwide retrospective cohort study included all 18 centres (18 patients) that are part of the multidisciplinary Dutch Pancreatic Cancer Group (DPCG)14. All patients registered in the mandatory prospective nationwide Dutch Pancreatic Cancer Audit (DPCA)15 who underwent pancreatoduodenectomy for pancreatic adenocarcinoma (postoperative pathological diagnosis) from 2013 to 2017 were included. Due to the retrospective nature of the study, the Medical Ethics Committee of the Leiden University Medical Centre waived the need for obtaining informed consent (G18.103). This study was performed in accordance with the Declaration of Helsinki and is reported in accordance with the STROBE criteria16.
Data collection
Data were requested from the DPCA, including baseline, intraoperative, postoperative, and histopathological characteristics. Additional data were manually extracted from patients’ medical records (for example, type of venous resection, blood loss, duration of surgery, PV-SMV thrombosis, tumour invasion in resected vein, lymphangio invasion, perineural invasion, follow-up characteristics).
Definitions
The type of venous resection was scored according to ISGPS classification as follows: type 1, partial venous excision with direct suture closure (venorrhaphy); type 2, partial venous excision using a patch; type 3, venous segment resection with primary venovenous anastomosis; and type 4, venous segment resection with interposed venous conduit and at least two anastomoses4. For the present analysis, type 1 and 2 resections were categorized as ‘wedge resection’, and type 3 and 4 resections as ‘segmental resection’.
Venous involvement on preoperative imaging was defined as absence or presence of a fat plane between the tumour and PV-SMV. Resectability was defined according to the DPCG criteria: resectable (tumour without arterial involvement and with venous involvement < 90°); borderline resectable (tumour with arterial involvement < 90° and/or venous involvement 90–269° without occlusion); and locally advanced (tumour with arterial involvement ≥ 90° and/or venous involvement ≥ 270° or occlusion). Neoadjuvant preoperative therapy was categorized as no/yes, regardless of type, duration, and dose of chemo(radio)therapy. Neoadjuvant therapy was mainly administered according to the protocol of the PREOPANC trial17 in which patients with resectable and borderline resectable disease were included (preoperative chemoradiotherapy, which consisted of three courses of gemcitabine, with the second course combined with 15 × 2.4 Gy radiotherapy) and occasionally outside this trial setting at the discretion of the treating physician. Additional organ resection was defined as any additional organ resection not including standard pancreatoduodenectomy18. Pancreatic surgery-specific complications were classified in accordance with ISGPS criteria. Only grade B and C complications were reported, as these complications were considered clinically relevant19–24. Postoperative PV-SMV thrombosis within 30 days following surgery was scored, based on imaging studies which were performed at the discretion of the attending physician. The Clavien–Dindo classification was used for scoring within 30 days following surgery, with grade ≥ III considered as major morbidity25. Postoperative mortality was defined as death within 90 days following surgery, unless the cause of death was clearly disease-related (for example, early recurrence or metastasis), and not surgery-related26. Textbook outcome was defined as absence of postoperative pancreatic fistula, bile leak, postpancreatectomy haemorrhage (all ISGPS grades B and C), major morbidity, readmission, and postoperative mortality27. The eighth edition of the TNM classification was used for histological classification28. An R1 resection margin was defined as the presence of tumour cells within 1 mm of the resection margin29. Due to inclusion of patients with neoadjuvant therapy, overall survival was calculated as time length in months between the start of treatment (day of surgery or start of neoadjuvant therapy) and the date of death (or last follow-up visit) and was truncated at 48 months.
Outcomes and comparisons
Primary outcomes of this study were major morbidity (Clavien–Dindo grade ≥ III) and overall survival (since start of treatment). Secondary outcomes were postoperative characteristics: postoperative mortality; PV-SMV thrombosis; postoperative pancreatic fistula; postpancreatectomy haemorrhage; bile leakage; delayed gastric emptying; chyle leak; pneumonia; wound infection; relaparotomy; radiological intervention; (duration of) intensive care unit admission; (duration of) hospital stay; readmission; textbook outcome and adjuvant therapy; and histopathological characteristics (resection margin status, tumour invasion in the resected vein, tumour size on pathology, pN-stage, pM-stage, tumour differentiation grade, lymphangio invasion, and perineural invasion).
Patients were analysed by category of venous resection: without venous resection, wedge and segmental resection. Subgroup analysis was performed on patients who received neoadjuvant therapy.
Statistical analysis
Statistical analyses were performed using SPSS Statistics for Windows, Version 23.0 (IBM, Armonk, New York, USA). Continuous variables are presented as the mean with s.d. or the median with interquartile range (i.q.r.), depending on the distribution. Categorical variables are presented as frequencies with percentages. Continuous variables were compared using the Mann–Whitney U test or Kruskal–Wallis test. Categorical variables were compared using the chi-square test or Fisher’s exact test.
Missing data for multivariable analysis (BMI, Eastern Cooperative Oncology Group, aspect of the pancreatic remnant, diameter of the pancreatic duct, blood loss, duration of surgery, tumour size on pathology, pN-stage, tumour differentiation grade, lymphangio invasion, perineural invasion) were imputed 25 times based on relevant prognostic factors (venous resection, sex, age, biliary drainage, neoadjuvant therapy, ASA score, minimally invasive procedure, arterial resection, additional organ resection, resection margin status, pM-stage) and outcome variables (major morbidity and overall survival).
Log-transformation was performed for not normally distributed variables30. Multivariable binary logistic regression analysis was performed to assess the impact of the category of venous resection on major morbidity and to adjust for potential confounders. Overall survival was reported as the median with 95 per cent confidence intervals, and Kaplan–Meier curves and log rank tests were used to compare groups. A multivariable Cox proportional hazards model was used to assess the impact of the type of venous resection on overall survival and adjust for potential confounders. A sensitivity analysis was performed for the impact of category of venous resection on major morbidity and overall survival with complete cases, without multiple imputation, to show robustness of the results. A two-sided P-value < 0.050 was considered statistically significant.
Results
In total, 1311 patients who underwent pancreatoduodenectomy for pancreatic cancer were included, of whom 351 (27 per cent) underwent venous resection. Characteristics are shown in Table 1. Of the patients who had venous resection, 227 (65 per cent) underwent wedge resection (196 patients with type 1, 31 patients with type 2) and 124 (35 per cent) underwent segmental resection (97 patients with type 3, 27 patients with type 4). Several baseline characteristics differed significantly across the categories of venous resection (Table 1). Patients with segmental resection more often had venous involvement on preoperative imaging, compared to patients with wedge resection and those without venous resection (93 (75 per cent) versus 134 (59 per cent) versus 252 (26 per cent) patients, respectively; P < 0.001). Patients with segmental resection more often received neoadjuvant therapy, compared to patients with wedge resection and those without venous resection (23 (19 per cent) versus 21 (9 per cent) versus 57 (6 per cent) patients, respectively; P = 0.012).
Table 1.
Population characteristics by category of venous resection
| Without venous resection | Wedge resection | Segmental resection | P-value | ||
|---|---|---|---|---|---|
| Total | 960 (73.2) | 227 (17.3) | 124 (9.5) | – | |
| Sex | M | 554 (57.7) | 115 (50.7) | 65 (52.4) | 0.11 |
| F | 406 (42.3) | 112 (49.3) | 59 (47.6) | ||
| Age (years), median (i.q.r.) | 68 (61–74) | 68 (61–73) | 69 (62–74) | 0.73 | |
| BMI (kg/m2), mean (s.d.) | 25.1 (4.2) | 24.5 (3.9) | 23.8 (3.4) | 0.002 | |
| ECOG | 0–1 | 862 (89.8) | 196 (86.3) | 112 (90.3) | 0.31 |
| 2–4 | 98 (10.2) | 31 (13.7) | 12 (9.7) | ||
| Preoperative biliary drainage | 542 (56.5) | 135 (59.5) | 68 (54.8) | 0.64 | |
| Venous involvement on preoperative imaging | 252 (26.3 | 134 (59.0) | 93 (75.0) | < 0.001 | |
| Preoperative resectability status* | Resectable | 780 (83.3) | 126 (56.8) | 46 (38.3) | < 0.001 |
| Borderline resectable | 113 (12.1) | 76 (34.2) | 62 (51.7) | ||
| Locally advanced | 43 (4.6) | 20 (9.0) | 12 (10.0) | ||
| Neoadjuvant therapy | 57 (5.9) | 21 (9.3) | 23 (18.5) | < 0.001 | |
| Type of neoadjuvant therapy | Chemoradiotherapy | 33 (57.9†) | 12 (57.1†) | 13 (56.5†) | 0.99 |
| Chemotherapy | 24 (42.1†) | 9 (42.9†) | 10 (43.5†) | ||
| ASA score | I–II | 742 (77.3) | 176 (77.5) | 97 (78.2) | 0.97 |
| III–IV | 218 (22.7) | 51 (22.5) | 27 (21.8) | ||
| Minimally invasive procedure | 109 (11.4) | 10 (4.4) | 4 (3.2) | < 0.001 | |
| Type of surgery | Classic Whipple | 347 (36.1) | 75 (33.0) | 53 (42.7) | 0.45 |
| PPPD | 591 (61.6) | 145 (63.9) | 68 (54.8) | ||
| PRPD | 22 (2.3) | 7 (3.1) | 3 (2.4) | ||
| Texture of pancreatic remnant | Normal/soft | 451 (47.0) | 79 (34.8) | 38 (30.6) | < 0.001 |
| Fibrotic/hard | 509 (53.0) | 148 (65.1) | 86 (69.4) | ||
| Pancreatic duct diameter (mm), median (i.q.r.) | 5 (3–8) | 6 (4–9) | 6 (4–9) | < 0.001 | |
| Arterial resection | 9 (0.9) | 5 (2.2) | 3 (2.4) | 0.16 | |
| Additional resection | 51 (5.3) | 9 (4.0) | 13 (10.5) | 0.031 | |
| Duration of surgery (min), median (i.q.r.) | 295 (239–377) | 344 (278–423) | 388 (321–458) | < 0.001 | |
| Blood loss during surgery (ml), median (i.q.r.) | 600 (350–1000) | 700 (450–1100) | 1200 (600–2000) | < 0.001 | |
*According to the Dutch Pancreatic Cancer Group criteria.
Percentage is based on the number of patients who received neoadjuvant therapy. Values are frequencies (per cent) unless indicated otherwise. Missing data imputed: BMI (n = 8); ECOG (n = 6); texture of pancreatic remnant (n = 103); pancreatic duct diameter (n = 256); duration of surgery (n = 136); blood loss (n = 148). Missing data not imputed: preoperative resectability status (n = 33). i.q.r., interquartile range; ECOG, Eastern Cooperative Oncology Group; PPPD, pylorus-preserving pancreatoduodenectomy; PRPD, pyloric ring pancreatoduodenectomy.
Over the study period, the annual rate of venous resection increased from 20 to 32 per cent (P = 0.001; Figure S1). Variation was observed in the number of pancreatoduodenectomies (range 38–129), the percentage of venous resection (range 10–53 per cent), and the segmental-to-wedge resection ratio (range 0–6) per centre during the study period (Figure S2).
Major morbidity
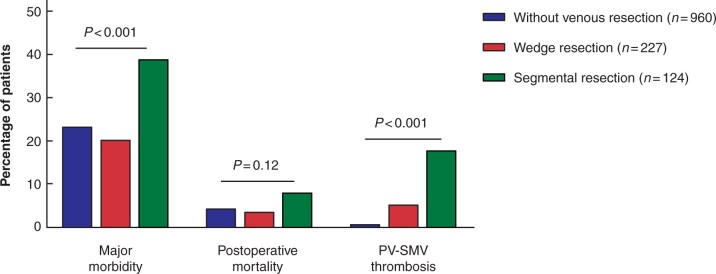
Patients with segmental resection had a higher rate of major morbidity, compared to patients with wedge resection and those without venous resection (Fig. 1). Results of multivariable analysis for major morbidity is shown in Table 2. Segmental resection was an independent predictor for major morbidity, whereas wedge resection was not. A sensitivity analysis with complete cases showed similar results (Table S1). Major morbidity rates were not different between patients with and those without venous involvement on preoperative imaging for wedge (30 (22 per cent) versus 16 (17 per cent) patients, respectively; P = 0.34) and segmental resection (13 (42 per cent) versus 35 (38 per cent) patients, respectively; P = 0.67).
Fig. 1.
Major morbidity (Clavien–Dindo grade ≥ III), postoperative mortality, and portal vein–superior mesenteric vein thrombosis after pancreatoduodenectomy for pancreatic cancer by category of venous resection
Table 2.
Multivariable analysis of major morbidity (Clavien–Dindo grade ≥ III) and overall survival by category of venous resection
| Major morbidity |
Overall survival |
||||||
|---|---|---|---|---|---|---|---|
| Odds ratio | 95% c.i. | P-value | Hazard ratio | 95% c.i. | P-value | ||
| Category of venous resection* | Wedge resection | 0.95 | 0.64–1.40 | 0.79 | 1.04 | 0.86–1.27 | 0.68 |
| Segmental resection | 1.93 | 1.20–3.11 | 0.007 | 1.40 | 1.10–1.78 | 0.007 | |
| Sex † | F | 1.06 | 0.81–1.39 | 0.68 | 1.01 | 0.87–1.17 | 0.95 |
| Age (years) ‡ | 1.00 | 0.99–1.02 | 0.95 | 1.02 | 1.01–1.02 | 0.001 | |
| BMI (kg/m2)‡ | 1.01 | 0.98–1.05 | 0.41 | 0.99 | 0.97–1.01 | 0.25 | |
| ECOG § | 2–4 | 0.80 | 0.51–1.28 | 0.36 | 0.87 | 0.68–1.11 | 0.25 |
| Preoperative biliary drainage* | 0.90 | 0.69–1.18 | 0.44 | – | – | – | |
| Preoperative resectability status¶ | Borderline resectable | 0.89 | 0.62–1.28 | 0.54 | – | – | – |
| Locally advanced | 0.46 | 0.23–0.91 | 0.024 | – | – | – | |
| Neoadjuvant therapy* | 1.46 | 0.88–2.43 | 0.15 | 0.90 | 0.66–1.22 | 0.51 | |
| ASA score# | III–IV | 1.68 | 1.23–2.31 | 0.001 | 1.45 | 1.22–1.73 | < 0.001 |
| Minimally invasive procedure* | 1.49 | 0.94–2.36 | 0.09 | – | – | – | |
| Arterial resection* | 1.59 | 0.55–4.55 | 0.39 | – | – | – | |
| Additional resection* | 1.59 | 0.92–2.73 | 0.10 | – | – | – | |
| Texture pancreatic remnant** | Fibrotic/hard | 0.79 | 0.60–1.05 | 0.11 | – | – | – |
| Pancreatic duct diameter (mm) ‡ | 0.94 | 0.90–0.98 | 0.005 | – | – | – | |
| Duration of surgery (min) ‡ | 1.00 | 1.00–1.00 | 0.55 | – | – | – | |
| Blood loss (ml) ‡ | 1.00 | 1.00–1.00 | < 0.001 | – | – | – | |
| Resection margin status †† | R1 | – | – | – | 1.26 | 1.08–1.48 | 0.004 |
| Tumour size on pathology (mm) ‡ | – | – | – | 1.01 | 1.00–1.02 | 0.008 | |
| pN-stage ‡‡ | N1 | – | – | – | 1.11 | 0.92–1.36 | 0.29 |
| N2 | – | – | – | 1.45 | 1.17–1.80 | 0.001 | |
| pM-stage §§ | M1 | – | – | – | 1.22 | 0.79–1.89 | 0.36 |
| Tumour differentiation grade ¶¶ | Moderate | – | – | – | 1.55 | 1.17–2.04 | 0.002 |
| Poor/undifferentiated | – | – | – | 2.26 | 1.69–3.02 | < 0.001 | |
| Lymphangio invasion* | – | – | – | 1.10 | 0.92–1.31 | 0.30 | |
| Perineural invasion* | – | – | – | 1.21 | 0.94–1.36 | 0.29 | |
*Reference category: ‘without/no’. †Reference category: ‘male’.
‡Continuous variable.
§Reference category: ‘0–1’.
¶Reference category: ‘resectable’. #Reference category: ‘I–II’.
**Reference category: ‘normal/soft’.
††Reference category: ‘R0’.
‡‡Reference category: ‘N0’
§§Reference category: ‘M0’.
Reference category: ‘good’. ECOG, Eastern Cooperative Oncology Group.
Overall survival
Patients with segmental resection had worse overall survival (median 12 months, 95 per cent c.i. 9 to 15 months), compared to patients with wedge resection (median 16 months, 95 per cent c.i. 12 to 20 months) and without venous resection (median 20 months, 95 per cent c.i. 18 to 22 months; P < 0.001; Fig. 2). Results of multivariable analysis for overall survival is shown in Table 2. Segmental resection was an independent predictor for worse overall survival, whereas this could not be demonstrated for wedge resection (Table 2). A sensitivity analysis with complete cases showed similar results (segmental resection: hazard ratio (HR) 1.35, 95 per cent c.i. 1.02 to 1.77; wedge resection: HR 0.97, 95 per cent c.i. 0.77 to 1.23; Table S1). A post-hoc analysis, which also adjusted for use of adjuvant therapy in patients without postoperative mortality, showed similar results (segmental resection: HR 1.34, 95 per cent c.i. 1.04 to 1.72; wedge resection: HR 1.11, 95 per cent c.i. 0.91 to 1.36; Table S2).
Fig. 2.
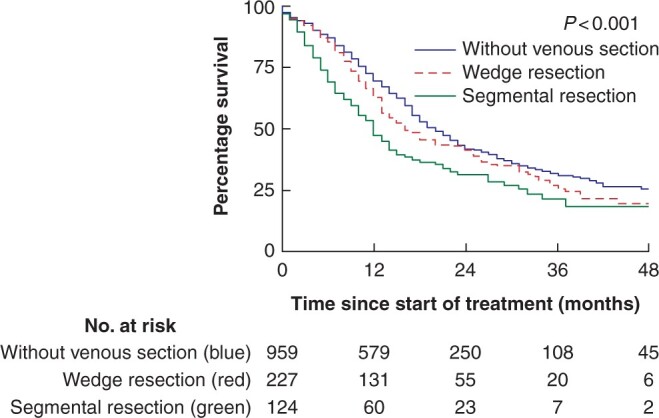
Kaplan–Meier curves of overall survival after pancreatoduodenectomy for pancreatic cancer by category of venous resection
Postoperative characteristics
Postoperative mortality did not differ significantly among patients with segmental resection, those with wedge resection, and those without venous resection (Fig. 1). Patients with segmental resection had a higher rate of PV-SMV thrombosis, compared to patients with wedge resection and those without venous resection (22 (18 per cent) versus 12 (5 per cent) versus 9 (1 per cent) patients, respectively; P < 0.001). Patients with segmental resection had a higher rate of relaparotomy, chyle leak, radiological intervention, intensive care unit admission and readmission, longer hospital stay, and a lower rate of textbook outcome, compared to patients with wedge resection and those without venous resection (Table 3). Vascular complications (PV-SMV thrombosis or haemorrhage) were the indication for relaparotomy in 18 out of 23 (78 per cent) patients with segmental resection (Table S3).
Table 3.
Postoperative and histopathological characteristics by category of venous resection
| Without venous resection | Wedge resection | Segmental resection | P-value | ||
|---|---|---|---|---|---|
| Postoperative characteristics | |||||
| Postoperative pancreatic fistula | 87 (9.1) | 11 (4.8) | 7 (5.6) | 0.07 | |
| Postpancreatectomy haemorrhage | 72 (7.5) | 9 (4.0) | 12 (9.7) | 0.09 | |
| Bile leakage | 29 (3.0) | 5 (2.2) | 4 (3.2) | 0.78 | |
| Delayed gastric emptying | 160 (16.7) | 31 (13.7) | 25 (20.3) | 0.26 | |
| Missing | 1 | 0 | 1 | ||
| Chyle leak | 25 (2.6) | 12 (5.3) | 18 (14.5) | < 0.001 | |
| Pneumonia | 58 (6.0) | 10 (4.4) | 9 (7.3) | 0.51 | |
| Wound infection | 100 (10.4) | 19 (8.4) | 11 (8.9) | 0.60 | |
| Relaparotomy | 69 (7.2) | 13 (5.7) | 23 (18.5) | < 0.001 | |
| Radiological intervention | 135 (14.1) | 21 (9.3) | 23 (18.5) | 0.041 | |
| ICU admission | 92 (9.6) | 23 (10.1) | 27 (21.8) | < 0.001 | |
| Duration of ICU admission in days*, median (i.q.r.) | 4 (2–12) | 6 (3–13) | 5 (2–13) | 0.77 | |
| Missing | 5 | 2 | 1 | ||
| Duration of hospital stay in days † , median (i.q.r.) | 11 (8–16) | 10 (8–14) | 15 (11–23) | < 0.001 | |
| Missing | 2 | 1 | 0 | ||
| Readmission † | 134 (14.6) | 32 (14.6) | 35 (30.7) | < 0.001 | |
| Textbook outcome | 638 (66.5) | 159 (70.0) | 60 (48.4) | < 0.001 | |
| Adjuvant therapy † | 646 (71.2) | 169 (77.5) | 66 (58.4) | 0.001 | |
| Missing | 12 | 1 | 1 | ||
| Histopathological characteristics | |||||
| Resection margin status | R0 | 519 (54.1) | 80 (35.2) | 44 (35.5) | < 0.001 |
| R1 | 441 (45.9) | 147 (64.8) | 80 (64.5) | ||
| Tumour invasion in resected vein | – | 69 (57.5) | 58 (66.7) | 0.18 | |
| Missing | 107 | 37 | |||
| Tumour size on pathology in mm, median (i.q.r.) | 30 (22–38) | 31 (25–40) | 35 (27–41) | < 0.001 | |
| pT-stage | T1 | 135 (14.1) | 19 (8.4) | 11 (8.9) | < 0.001 |
| T2 | 590 (61.8) | 141 (62.4) | 62 (50.4) | ||
| T3 | 214 (22.4) | 55 (24.3) | 45 (36.6) | ||
| T4 | 16 (1.7) | 11 (4.9) | 5 (4.1) | ||
| pN-stage | N0 | 255 (26.6) | 59 (26.0) | 34 (27.4) | 0.97 |
| N1 | 381 (39.7) | 86 (37.9) | 49 (39.5) | ||
| N2 | 324 (33.8) | 82 (36.1) | 41 (33.1) | ||
| pM-stage | M0 | 936 (97.5) | 222 (97.8) | 120 (96.8) | 0.84 |
| M1 | 24 (2.5) | 5 (2.2) | 4 (3.2) | ||
| Tumour differentiation grade | Good | 135 (14.0) | 27 (11.9) | 14 (11.3) | 0.78 |
| Moderate | 543 (56.6) | 123 (54.2) | 70 (56.5) | ||
| Poor/undifferentiated | 282 (29.4) | 77 (33.9) | 40 (32.3) | ||
| Lymphangio invasion | 518 (54.0) | 144 (63.4) | 73 (58.9) | 0.49 | |
| Perineural invasion | 792 (82.5) | 208 (91.6) | 104 (83.9) | 0.95 | |
*Patients admitted to the ICU.
†Patients without postoperative mortality. Values are frequencies (per cent) unless indicated otherwise. Missing data imputed: pN-stage (n = 1); pT-stage and tumour size on pathology (n = 7); tumour differentiation grade (n = 125); lymphangio invasion (n = 225); perineural invasion (n = 147). ICU, intensive care unit; i.q.r., interquartile range.
The rate of adjuvant therapy was lower in patients with segmental resection, compared to patients with wedge resection and those without venous resection (66 (58 per cent) versus 169 (78 per cent) versus 646 (71 per cent) patients, respectively; P < 0.001). Similar results regarding the rate of adjuvant therapy were obtained in the subgroup of patients without neoadjuvant chemotherapy and no postoperative mortality (51 (54 per cent) versus 149 (76 per cent) versus 607 (71 per cent) patients, respectively; P < 0.001).
Histopathological characteristics
Patients with segmental and wedge resection had a higher rate of R1 resections, compared to patients without venous resection (Table 3). Data on tumour invasion in the resected vein were available for 207 patients (59 per cent). Tumour invasion did not differ between patients with wedge resection and those with segmental resection. Patients with segmental resection had larger tumours, compared to patients with wedge resection and those without venous resection.
Patients who received neoadjuvant therapy
In total, 101 (8 per cent) patients received neoadjuvant therapy. Baseline characteristics and histopathological characteristics were largely comparable among the categories of venous resection (Table S4). Patients with segmental resection had higher rates of major morbidity, postoperative mortality, and PV-SMV thrombosis, compared to patients with wedge resection and those without venous resection. Multivariable analysis showed that segmental resection was an independent predictor for major morbidity, whereas this could not be demonstrated for wedge resection (Table S5).
There was no difference in overall survival among patients with segmental resection, those with wedge resection, and those without venous resection (Figure S3). Multivariable analysis showed both segmental and wedge resection did not predict overall survival (Table S5).
Discussion
This nationwide study of patients who underwent pancreatoduodenectomy for pancreatic cancer demonstrated that patients with venous segmental resection had a twofold increase in major morbidity rate and a 17 per cent increased risk of PV-SMV thrombosis, compared to patients without venous resection. The segmental resection group had worse overall survival, compared to patients with wedge resection and those without venous resection (median 12 versus 16 versus 20 months), even after correction for clinical and pathological factors. Among patients who received neoadjuvant therapy, overall survival showed no difference between patients with segmental resection, those who had wedge resection, and those without venous resection, whereas major morbidity and postoperative mortality rates were higher after venous segmental resection.
In contrast to the preference observed for segmental resection in the international survey, two-thirds of patients who had venous resection underwent wedge resection, with only one-third who had segmental resection. The reasons for choosing to perform venous resection and reconstruction type are multifactorial and based on the surgeon’s preference and skills, as well as on the perceived circumference and length of vein involvement31. Little is known about what exactly drives the surgeon’s preference with regard to choice of type of venous reconstruction9.
Large studies focusing on outcome and type of venous resection are sparse. The largest study (977 venous resections) used the National Surgical Quality Improvement Program (NSQIP) database and showed that, compared to no venous resection, direct repair (72 per cent) was associated with higher morbidity and graft repair (28 per cent) was associated with higher morbidity and mortality8. Unfortunately, comparison with the present study is difficult since the study did not use the ISGPS venous resection definition and Clavien–Dindo classification. Another large study (229 venous resections) showed no difference in morbidity, mortality, and survival among the types of venous resection11. In contrast to a single-centre study of 249 patients (period 2000–2010)32, patients with and those without venous involvement on preoperative imaging and venous resection had comparable major morbidity rates. Based on the available data, it can only be speculated what the exact reasons were for the higher major morbidity rates after segmental resection. Previously, vascular complications were shown to be the main causes of postoperative mortality33 and were the main indication for relaparotomy in these patients. There are no studies available investigating the association between outcome and the number or proportion of venous resections performed at an institution. This was not investigated here since only patients who underwent pancreatoduodenectomy for pancreatic cancer were included and there was no clear association between the volume of pancreatoduodenectomies and the proportion of venous resection or category of venous resection. Future research should focus on identifying optimal venous reconstruction techniques and protocols (for example, clamping time, length of vein resected, type of conduit, preservation or ligation of the splenic vein, heparinization, etc.).
The rate of PV-SMV thrombosis after segmental resection (18 per cent) was higher, compared to other studies (approximately 8 per cent)11,34,35. The present study had no patient-level data on thromboprophylaxis to study the effect on PV-SMV thrombosis. However, it was reported that only 29 per cent of Dutch surgeons adjusted thromboprophylaxis following venous resection (some start a platelet aggregation inhibitor or increase the dose of low-molecular-weight heparin)9. A previous meta-analysis found no differences in PV-SMV thrombosis in patients with and those without thromboprophylaxis34. Moreover, intensified thromboprophylaxis might result in more haemorrhages36, reflecting the fragile balance between thromboprophylaxis, postoperative thrombosis, and haemorrhage in pancreatic surgery.
Segmental resection, but not wedge resection, was a predictor for worse overall survival in this study. This is most likely explained by the fact that patients who require segmental resection have more advanced disease, despite the fact that multivariable analysis adjusted for several patient and histopathological characteristics. The question of whether wedge, rather than segmental, resection produces improved outcome in otherwise identical patients is a topic for further research.
Tumour invasion in the resected vein was observed in 61 per cent of patients with venous resection, which is within the range reported in the literature (32–82 per cent)37. It is difficult for a surgeon to distinguish a tumour from peritumoural inflammation and fibrosis on a scale of millimetres. Several studies have shown varying results regarding the significance of circumference and length of vein involvement on preoperative imaging38,39. The added value of intraoperative ultrasound for this assessment is being investigated within the DPCG. A previous study showed that radical venous resection can rarely be achieved due to the microanatomy at the venous margin and the broadly invasive growth pattern of pancreatic cancer40. More research is needed to identify patients who would truly benefit from venous resection, so that patients are not put at unnecessary risk of surgical complications.
In this cohort, only 8 per cent of patients received neoadjuvant therapy. This is comparable with recently published results from Germany (5 per cent) and Sweden (3 per cent), though lower than results from the United States (28 per cent)41. This is probably due to the fact that neoadjuvant therapy was mainly administered in a trial setting during the study period in most European countries (including the Netherlands). The comparable overall survival across the categories of venous resection after neoadjuvant therapy may be explained by the effect of neoadjuvant therapy, as well as by patient selection, as patients with advanced, aggressive, or therapy-resistant tumours are no longer considered good candidates for resection. Patients who received neoadjuvant therapy with segmental resection had a very high rate of major morbidity and postoperative mortality. There is little evidence on outcomes of venous resection after neoadjuvant therapy. A previous study showed major morbidity in 7 out of 15 (47 per cent) patients who underwent venous resection after neoadjuvant therapy for locally advanced pancreatic cancer. It should be noted that these resections were performed in a high-volume centre42.
This study has several limitations. First, because this was a retrospective study, collecting and interpreting data from medical records have the risk of information and classification bias. However, a previous study by the DPCA showed that data registration is complete, with high accuracy15. Multiple imputations were used to solve the problem of missing data. Sensitivity analysis with complete cases showed similar outcomes, indicating robustness of the results. Second, given the observational design of this study, confounding by indication should be considered as the surgeon’s decision (for example, selection for neoadjuvant therapy and venous resection) was made in the clinical and surgical context of the patient. Although multivariable analysis adjusted for potential confounders, inherent differences among the categories of venous resection may partly explain the observed results and residual confounding cannot be ruled out. Furthermore, no definitive conclusions can be drawn regarding neoadjuvant therapy since the sample size was relatively small and details of neoadjuvant therapy (type, cycles, doses, fractions, etc.) were not available for analysis. Lastly, there were missing data in the pathology reports on tumour invasion in the resected vein (41 per cent). Unclear or absent marking of the specimen and pathology request forms can make it difficult for pathologists to recognize the resected vein, especially in the case of wedge resection43. Within the DPCG, several initiatives have been set up to standardize pathology requests and reports. The strengths of the present study are, unlike previous studies, its nationwide design, including all Dutch centres performing pancreatic surgery, and the resulting large cohort of patients spanning a relatively short study period (2013–2017).
Data availability
Data sets used in this study are available upon reasonable request from the corresponding author after completion of the MULTI-VERS PROJECT (www.trialregister.nl – TC 7644).
Funding
This study was supported by a Bas Mulder Award (UL2015-7665) from the Alpe d’HuZes Foundation/Dutch Cancer Society (J.S.D.M.).
Supplementary Material
Acknowledgements
The authors would like to acknowledge M. Suker (Department of Surgery, Erasmus Medical Centre, Rotterdam, the Netherlands), L. Damen (Department of Surgery, University Medical Centre Utrecht, Utrecht, the Netherlands), B. Pranger (Department of Surgery, University of Groningen and University Medical Centre Groningen, Groningen), M. Ligthart (Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands), and L. Boyd (Department of Surgery, Cancer Centre Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands) for providing administrative support. Concept and design of the manuscript: J.V.G., M.G.B., C.H.J.vE., I.Q.M., J.dV., M.N.W., B.A.B., M.W.J.S., J.S.D.M. Data acquisition: J.V.G., N.M., S.vR., K.B., O.R.B., R.vD., C.H.J.vE., B.G.K., E.vd.H., I.H.dH., T.M.K., D.J.L., V.E.dM., I.Q.M., V.B.N., D.R., H.C.vS., J.H.W., F.W., B.M.Z., B.A.B, M.W.J.S., J.S.D.M. Data analysis and interpretation: J.V.G., N.M., S.vR., M.G.B., C.H.J.vE., B.G.K., I.Q.M., J.dV., M.N.W., B.A.B., M.W.J.S., J.S.D.M. Drafting of the manuscript: J.V.G., N.M., S.vR., M.G.B., C.H.J.vE., B.G.K., I.Q.M., J.dV., M.N.W., B.A.B., M.W.J.S., J.S.D.M. Critical revision of the manuscript: all authors. Final approval of the manuscript: all authors.
Disclosure. I.H.dH. received unrestricted research funding paid to the institute from QP&S, RanD Biotech, and Roche for research that is not related to the current manuscript. All other authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Contributor Information
Jesse V Groen, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Nynke Michiels, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Stijn van Roessel, Department of Surgery, Cancer Centre Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Marc G Besselink, Department of Surgery, Cancer Centre Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Koop Bosscha, Department of Surgery, Jeroen Bosch Hospital, Den Bosch, the Netherlands.
Olivier R Busch, Department of Surgery, Cancer Centre Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands.
Ronald van Dam, Department of Surgery, Maastricht University Medical Centre, Maastricht, the Netherlands.
Casper H J van Eijck, Department of Surgery, Erasmus Medical Centre, Rotterdam, the Netherlands.
Bas Groot Koerkamp, Department of Surgery, Erasmus Medical Centre, Rotterdam, the Netherlands.
Erwin van der Harst, Department of Surgery, Maasstad Hospital, Rotterdam, the Netherlands.
Ignace H de Hingh, Department of Surgery, Catharina Hospital, Eindhoven, the Netherlands; Department of Epidemiology, GROW—School for Oncology and Developmental Biology, Maastricht UMC+, Maastricht, the Netherlands.
Tom M Karsten, Department of Surgery, Onze Lieve Vrouwe Gasthuis (loc. Oost), Amsterdam, the Netherlands.
Daan J Lips, Department of Surgery, Medisch Spectrum Twente, Enschede, the Netherlands.
Vincent E de Meijer, Department of Surgery, University of Groningen and University Medical Centre Groningen, Groningen, the Netherlands.
Isaac Q Molenaar, Department of Surgery, UMC Utrecht Cancer Centre, St Antonius Hospital Nieuwegein; Regional Academic Cancer Centre Utrecht, Utrecht, the Netherlands.
Vincent B Nieuwenhuijs, Department of Surgery, Isala, Zwolle, the Netherlands.
Daphne Roos, Department of Surgery, Reinier de Graaf Gasthuis, Delft, the Netherlands.
Hjalmar C van Santvoort, Department of Surgery, UMC Utrecht Cancer Centre, St Antonius Hospital Nieuwegein; Regional Academic Cancer Centre Utrecht, Utrecht, the Netherlands.
Jan H Wijsman, Department of Surgery, Amphia Hospital, Breda, the Netherlands.
Fennie Wit, Department of Surgery, Tjongerschans Hospital, Heerenveen, the Netherlands.
Babs M Zonderhuis, Department of Surgery, Cancer Centre Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, the Netherlands.
Judith de Vos-Geelen, Department of Internal Medicine, Division of Medical Oncology, GROW—School for Oncology and Developmental Biology, Maastricht UMC+, Maastricht, the Netherlands.
Martin N Wasser, Department of Radiology, Leiden University Medical Centre, Leiden, the Netherlands.
Bert A Bonsing, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
Martijn W J Stommel, Department of Surgery, Radboud University Medical Centre, Nijmegen, the Netherlands.
J Sven D Mieog, Department of Surgery, Leiden University Medical Centre, Leiden, the Netherlands.
References
- 1. Malvezzi M, Carioli G, Bertuccio P, Boffetta P, Levi F, La Vecchia C et al. European cancer mortality predictions for the year 2019 with focus on breast cancer. Ann Oncol 2019;30:781–787. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed 20 March 2020).
- 3. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goere D et al. ; ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26(Suppl 5):v56–68. [DOI] [PubMed] [Google Scholar]
- 4. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA et al. ; International Study Group of Pancreatic Surgery. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977–988. [DOI] [PubMed] [Google Scholar]
- 5. Kleive D, Sahakyan MA, Berstad AE, Verbeke CS, Gladhaug IP, Edwin B et al. Trends in indications, complications and outcomes for venous resection during pancreatoduodenectomy. Br J Surg 2017;104:1558–1567. [DOI] [PubMed] [Google Scholar]
- 6. van Roessel S, Mackay TM, Tol J, van Delden OM, van Lienden KP, Nio CY et al. Impact of expanding indications on surgical and oncological outcome in 1434 consecutive pancreatoduodenectomies. HPB (Oxford) 2019;21:865–875. [DOI] [PubMed] [Google Scholar]
- 7. Worni M, Castleberry AW, Clary BM, Gloor B, Carvalho E, Jacobs DO et al. Concomitant vascular reconstruction during pancreatectomy for malignant disease: a propensity score-adjusted, population-based trend analysis involving 10,206 patients. JAMA Surg 2013;148:331–338. [DOI] [PubMed] [Google Scholar]
- 8. Kantor O, Talamonti MS, Wang CH, Roggin KK, Bentrem DJ, Winchester DJ et al. The extent of vascular resection is associated with perioperative outcome in patients undergoing pancreaticoduodenectomy. HPB (Oxford) 2018;20:140–146. [DOI] [PubMed] [Google Scholar]
- 9. Groen JV, Stommel MWJ, Sarasqueta AF, Besselink MG, Brosens LAA, van Eijck CHJ et al. ; Dutch Pancreatic Cancer Group. Surgical management and pathological assessment of pancreatoduodenectomy with venous resection: an international survey among surgeons and pathologists. HPB (Oxford) 2021;23:80–89. [DOI] [PubMed] [Google Scholar]
- 10. Kleive D, Berstad AE, Verbeke CS, Haugvik SP, Gladhaug IP, Line PD et al. Cold-stored cadaveric venous allograft for superior mesenteric/portal vein reconstruction during pancreatic surgery. HPB (Oxford) 2016;18:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ravikumar R, Sabin C, Abu Hilal M, Al-Hilli A, Aroori S, Bond-Smith G et al. Impact of portal vein infiltration and type of venous reconstruction in surgery for borderline resectable pancreatic cancer. Br J Surg 2017;104:1539–1548. [DOI] [PubMed] [Google Scholar]
- 12. Glebova NO, Hicks CW, Piazza KM, Abularrage CJ, Cameron AM, Schulick RD 3rd et al. Technical risk factors for portal vein reconstruction thrombosis in pancreatic resection. J Vasc Surg 2015;62:424–433. [DOI] [PubMed] [Google Scholar]
- 13. Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg 2016;103:179–191. [DOI] [PubMed] [Google Scholar]
- 14. Strijker M, Mackay TM, Bonsing BA, Bruno MJ, van Eijck CHJ, de Hingh I et al. ; Dutch Pancreatic Cancer Group. Establishing and coordinating a Nationwide Multidisciplinary Study Group: lessons learned by the Dutch Pancreatic Cancer Group. Ann Surg 2020;271:e102–e104. [DOI] [PubMed] [Google Scholar]
- 15. van Rijssen LB, Koerkamp BG, Zwart MJ, Bonsing BA, Bosscha K, van Dam RM et al. ; Dutch Pancreatic Cancer Group. Nationwide prospective audit of pancreatic surgery: design, accuracy, and outcomes of the Dutch Pancreatic Cancer Audit. HPB (Oxford) 2017;19:919–926. [DOI] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP et al. ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499. [DOI] [PubMed] [Google Scholar]
- 17. Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA et al. ; for the Dutch Pancreatic Cancer Group. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol 2020;38:1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartwig W, Vollmer CM, Fingerhut A, Yeo CJ, Neoptolemos JP, Adham M et al. ; International Study Group on Pancreatic Surgery. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery 2014;156:1–14. [DOI] [PubMed] [Google Scholar]
- 19. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J et al. ; International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an International Study Group (ISGPF) definition. Surgery 2005;138:8–13. [DOI] [PubMed] [Google Scholar]
- 20. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M et al. ; International Study Group on Pancreatic Surgery. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584–591. [DOI] [PubMed] [Google Scholar]
- 21. Besselink MG, van Rijssen LB, Bassi C, Dervenis C, Montorsi M, Adham M et al. ; International Study Group on Pancreatic Surgery. Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery 2017;161:365–372. [DOI] [PubMed] [Google Scholar]
- 22. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–688. [DOI] [PubMed] [Google Scholar]
- 23. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–768. [DOI] [PubMed] [Google Scholar]
- 24. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20–25. [DOI] [PubMed] [Google Scholar]
- 25. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mise Y, Vauthey JN, Zimmitti G, Parker NH, Conrad C, Aloia TA et al. Ninety-day postoperative mortality is a legitimate measure of hepatopancreatobiliary surgical quality. Ann Surg 2015;262:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Roessel S, Mackay TM, van Dieren S, van der Schelling GP, Nieuwenhuijs VB, Bosscha K et al. ; Dutch Pancreatic Cancer Group. Textbook outcome: nationwide analysis of a novel quality measure in pancreatic surgery. Ann Surg 2020;271:155–162. [DOI] [PubMed] [Google Scholar]
- 28. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours ( 7th edn). Geneva: International Union Against Cancer, 2009. [Google Scholar]
- 29. Campbell F, Cairns A, Duthie F, Feakins R. Dataset for the Histopathological Reporting of Carcinomas of the Pancreas, Ampulla of Vater and Common Bile Duct. London: Royal College of Pathologists, 2010. [Google Scholar]
- 30. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dua MM, Tran TB, Klausner J, Hwa KJ, Poultsides GA, Norton JA et al. Pancreatectomy with vein reconstruction: technique matters. HPB (Oxford) 2015;17:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim PT, Wei AC, Atenafu EG, Cavallucci D, Cleary SP, Moulton CA et al. Planned versus unplanned portal vein resections during pancreaticoduodenectomy for adenocarcinoma. Br J Surg 2013;100:1349–1356. [DOI] [PubMed] [Google Scholar]
- 33. Clark W, Silva M, Donn N, Luberice K, Humphries LA, Paul H et al. Targeting early deaths following pancreaticoduodenectomy to improve survival. J Gastrointest Surg 2012;16:1869–1874. [DOI] [PubMed] [Google Scholar]
- 34. Chandrasegaram MD, Eslick GD, Lee W, Brooke-Smith ME, Padbury R, Worthley CS et al. Anticoagulation policy after venous resection with a pancreatectomy: a systematic review. HPB (Oxford) 2014;16:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kleive D, Berstad AE, Sahakyan MA, Verbeke CS, Naper C, Haugvik SP et al. Portal vein reconstruction using primary anastomosis or venous interposition allograft in pancreatic surgery. J Vasc Surg Venous Lymphat Disord 2018;6:66–74. [DOI] [PubMed] [Google Scholar]
- 36. Hanna-Sawires RG, Groen JV, Klok FA, Tollenaar R, Mesker WE, Swijnenburg RJ et al. Outcomes following pancreatic surgery using three different thromboprophylaxis regimens. Br J Surg 2019;106:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song A, Liu F, Wu L, Si X, Zhou Y. Histopathologic tumor invasion of superior mesenteric vein/portal vein is a poor prognostic indicator in patients with pancreatic ductal adenocarcinoma: results from a systematic review and meta-analysis. Oncotarget 2017;8:32600–32607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kishi Y, Nara S, Esaki M, Hiraoka N, Shimada K. Feasibility of resecting the portal vein only when necessary during pancreatoduodenectomy for pancreatic cancer. BJS Open 2019;3:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Imamura T, Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R et al. Prognostic role of the length of tumour-vein contact at the portal-superior mesenteric vein in patients having surgery for pancreatic cancer. Br J Surg 2019;106:1649–1656. [DOI] [PubMed] [Google Scholar]
- 40. Kleive D, Labori KJ, Line PD, Gladhaug IP, Verbeke CS. Pancreatoduodenectomy with venous resection for ductal adenocarcinoma rarely achieves complete (R0) resection. HPB (Oxford) 2020;22:50–57. [DOI] [PubMed] [Google Scholar]
- 41. Mackay TM, Gleeson EM, Wellner UF, Williamsson C, Busch OR, Groot Koerkamp B et al. Transatlantic registries of pancreatic surgery in the United States of America, Germany, the Netherlands, and Sweden: comparing design, variables, patients, treatment strategies, and outcomes. Surgery 2021;169:396–402. [DOI] [PubMed] [Google Scholar]
- 42. Hackert T, Strobel O, Michalski CW, Mihaljevic AL, Mehrabi A, Muller-Stich B et al. The TRIANGLE operation – radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford) 2017;19:1001–1007. [DOI] [PubMed] [Google Scholar]
- 43. Roch AM, House MG, Cioffi J, Ceppa EP, Zyromski NJ, Nakeeb A et al. Significance of portal vein invasion and extent of invasion in patients undergoing pancreatoduodenectomy for pancreatic adenocarcinoma. J Gastrointest Surg 2016;20:479–487; discussion 487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sets used in this study are available upon reasonable request from the corresponding author after completion of the MULTI-VERS PROJECT (www.trialregister.nl – TC 7644).