Introduction
Neoadjuvant chemotherapy (NACT) is increasingly being used to treat early breast cancer, and offers several advantages, including reducing the extent of breast and axillary surgery, and providing an in vivo assessment of tumour sensitivity to treatment1–4. Clinical trials have identified tumour subgroups with high rates of pCR. A pCR can be achieved in 45–90 per cent of human epidermal growth factor receptor 2-positive (HER2+) tumours and triple-negative breast cancer (TNBC), but the rate in oestrogen receptor-positive (ER+)/HER2-negative (HER2–) breast cancer remains below 10 per cent3,5. Historically, increasing pCR rates following NACT have not translated into more breast-conserving surgery (BCS), but more recent data suggest that NACT can result in surgical downstaging6.
NACT use in the UK appears inconsistent, as highlighted by a recent prospective audit7. Although UK guidelines suggest considering NACT in patients with HER2+ cancers and TNBC, detailed guidance does not exist8. Furthermore, it is unclear whether pCR rates in routine clinical practice reflect those observed in trials, and whether tumour downstaging influences surgical decision-making beyond the trial setting. Moreover, there remains a lack of consensus on whether definitive surgery should aim to excise the original or post-treatment tumour footprint, as highlighted in a recent UK survey9, in which 24 per cent of centres stated that their routine practice was to excise the original tumour footprint rather than carry out response-adapted surgery.
This prospective study aimed to determine surgical decision-making in the breast and axilla and pCR rates in routine clinical practice following NACT for early breast cancer.
Methods
The NeST study protocol has been published previously10. Briefly, this was a prospective multicentre cohort study, including consecutive patients in participating units undergoing NACT as primary treatment for breast cancer, between 1 December 2017 and 30 November 2018. Demographics, multidisciplinary team (MDT) recommendations for NACT and preoperative planning, operative outcomes, and oncological data were collected for each participant. MDTs were asked to record prospectively whether patients were eligible for breast conservation at diagnosis. A pCR was defined by the absence of residual invasive disease with or without the presence of residual in situ disease (ypT0/ypTis) with negative axillary nodes (ypN0)11. Further details are provided in the Supplementary material.
Results
A total of 1283 patients were entered into the NeST study database from 39 UK units; complete histopathological and surgical outcomes data were available for 900 patients (916 tumours). Patient demographics and baseline tumour characteristics are summarized in Table S1.
Pathological response
A pCR in the breast (ypT0/ypTis) was reported in 379 tumours (41.4 per cent), and 330 of 448 (36.0 per cent) node-positive tumours (ypT0/is, ypN0). The pCR rates by tumour subtype are summarized in Table 1 and pCR rates in patients with node-positive disease by subtype in Table S2.
Table 1.
Overall pathological response rates by tumour subtype in both the breast and breast/axilla
| Final pathological response | ||||||
|---|---|---|---|---|---|---|
| pCR | pPR | No response | Total | |||
| Pretreatment pathology | ypT0 | ypT0/ypTis | ypT0/yTis, yN0 | |||
| HER2+ | 166 (38.9) | 236* (55.1) | 205* (48.1) | 180* (42.3) | 10 (2.3) | 426 (46.5) |
| HER2+/ER+ | 87 (31.9) | 129 (47.3) | 115 (42.1) | 137 (50.2) | 7 (2.7) | 273 (29.8) |
| HR2+/ER– | 79 (52.3) | 106 (70.2) | 85 (56.3) | 42 (27.8) | 3 (2.0) | 151 (16.5) |
| TNBC | 89 (33.9) | 110 (41.9) | 101 (38.5) | 129 (49.2) | 23 (8.8) | 262 (28.6) |
| ER+/HER2– | 27 (11.8) | 34 (14.9) | 24 (10.5) | 175 (76.8) | 19 (8.3) | 228 (24.9) |
| Total | 282 (30.8) | 379 (41.4) | 330 (36.1) | 484 (52.9) | 52 (6.1) | 916 |
Values in parentheses are percentages. A pCR was defined by the absence of residual invasive disease (ypT0) but in situ disease could be present (ypTis); absence of residual disease in the breast or axilla was classified as ypT0/ypTis, ypN0. Patients with unknown oestrogen receptor (ER) status on core biopsy were excluded. pPR, pathological partial response; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Surgical management
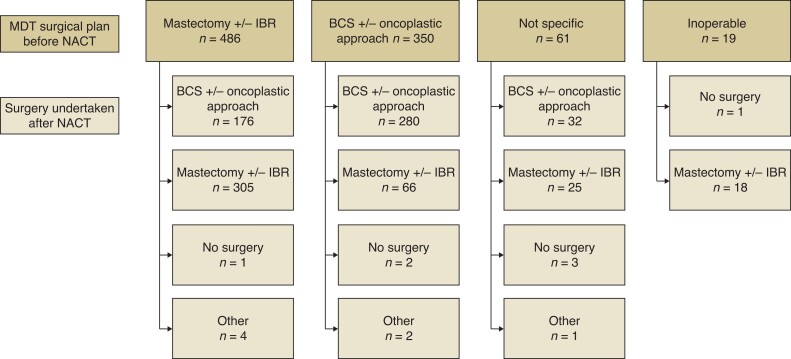
Figure 1 shows the initial surgical management plan and actual treatment received. At diagnosis, 486 tumours were ineligible for BCS, and were 350 deemed suitable for BCS. Following NACT, operation for 176 of 486 tumours (36.2 per cent) was converted from mastectomy to BCS. Downstaging rates varied according to subtype; 48.8 per cent of TNBC, 32 per cent of HER2+, and 30 per cent of ER+/HER2– procedures were converted to BCS (P = 0.004; χ2 test). Among 350 patients suitable for BCS at diagnosis, 280 underwent BCS and 66 had a mastectomy. A breast pCR was reported in 143 patients (34.5 per cent) who had a mastectomy and 231 (47.3 per cent) who underwent BCS (P < 0.001; Fisher’s exact test). Of 176 tumours downstaged from mastectomy, 45 per cent had a pCR and 50 per cent a partial response to NACT.
Fig. 1.
Surgical management, showing surgical plan at diagnosis and actual surgical management after systemic therapy
MDT, multidisciplinary team; NACT, neoadjuvant chemotherapy; IBR, immediate breast reconstruction; BCS, breast-conserving surgery.
The intention to resect either the original breast tumour or post-treatment tumour footprint was stated for 855 patients: resection of the original tumour footprint in 383 (44.8 per cent) and of the post-treatment tumour footprint in 472 (55.2 per cent). After BCS, 54 patients (11.4 per cent) had involved margins. Of these, surgery in 22 (40.7 per cent) was downstaged to BCS from an original plan for mastectomy, and 28 (51.9 per cent) were eligible for BCS at baseline. Further surgery was recommended for 40 patients, BCS in 34 (85 per cent) and completion mastectomy in 6 (15 per cent). The final overall mastectomy rate was 45.9 per cent when re-excision was considered.
Axillary surgery
Nodal status at diagnosis is shown in Table S1. Sentinel lymph node biopsy (SLNB) was planned before NACT in 42 patients (9.4 per cent) with cN0 disease, and after NACT in 372 (82.9 per cent). Among cN1+ axillae, radiological reassessment after NACT was planned in 149 (32.3 per cent) and carried out in 112, with disease in 70 patients (62.5 per cent) downstaged to cN0. The axillary surgery performed was recorded for 908 of 916 tumours (99.1 per cent) (Table S3). SLNB was undertaken after NACT in 405 (44.6 per cent), with pN+ reported on final histology in 30 patients (7.4 per cent). Targeted axillary dissection was carried out in 55 (6 per cent), and the majority had pN0 disease (85.5 per cent). In total, 385 patients had axillary lymph node dissection after NACT and, of these, 186 (48.3 per cent) had pathologically negative nodes.
Discussion
This prospective multicentre cohort study reports real-world pCR rates after NACT, which are broadly comparable to those in clinical trials, with pCR rates of 41.9 per cent (TNBC), 47.3 per cent (HER2+/ER+), and 70.3 per cent (HER2+/ER-negative), in keeping with published data. With respect to ER+/HER2– breast cancer, it is known that pCR rates in such tumours are low, the rate of 14.9 per cent in the present series being in line with reported rates of 7.5–15.2 per cent. Despite this, NACT remains commonly used in ER+/HER2– disease, as indicated by this group making up 24.9 per cent of patients in the present study. This finding highlights the need for careful consideration of neoadjuvant treatment options for patient with ER+/HER2– disease.
Variation in surgical practice following NACT was seen, with 44 per cent of centres excising the pretreatment tumour footprint following treatment, and 55 per cent undertaking risk-adapted surgery. Despite this, for 36.2 per cent of tumours requiring mastectomy at diagnosis, the procedure was converted to BCS, in keeping with published data suggesting that modern chemotherapeutic regimens can downstage disease12. Patients undergoing BCS were significantly more likely to have a pCR than those undergoing mastectomy, suggesting that, as pCR rates increase with improved patient selection and treatment, it is likely that BCS rates will also rise. This study confirms that a proportion of patients with tumours suitable for BCS will elect to undergo mastectomy. This might be because of multifocal/bilateral disease, patient preference or a mutation in a risk predisposition gene (data on this were not available for the cohort). Following BCS, rates of margin involvement were low and comparable with those reported after BCS without NACT, and this did not appear to be related to whether or not treatment of the tumour was downstaged from a planned mastectomy at baseline13.
Downstaging of axillary surgery was less commonly seen after NACT, with greater variation in treatment. SLNB before treatment continues to be performed in patients with cN0 disease, despite the known low false-negative rate after NACT. Not all patients with node-positive tumours underwent axillary reassessment after NACT, and 64.9 per cent of patients with node-positive disease at baseline proceeded to axillary dissection after NACT, with almost half (184 patients, 49.5 per cent) having no evidence of axillary disease. Taken together, these data imply that some patients could undergo less extensive axillary surgery following NACT, but are not currently considered for this. More patients could be offered pretreatment nodal marking and targeted axillary dissection, with the options of either being treated in the UK’s ongoing trial of axillary surgery after neoadjuvant therapy (ATNEC, https://clinicaltrials.gov/ct2/show/NCT04109079), or receiving axillary radiotherapy.
There are limitations to this study. Data were collected from only a proportion of around 150 UK breast units; participating units may be those with a high use of NACT, and so these results may not necessarily be more widely generalizable. Additionally, an observational study introduces the possibility of bias, although measures were taken to minimize this. There are some missing data and, to minimize the impact of this, such patients were excluded from the analysis of pCR and surgical decision-making. Finally, data were collected during 2017–2018, and practice may have changed in the intervening period, in particularly with increasing use of platinums in TNBC further increasing pCR rates in this subtype. Furthermore, since inception of the study, guidelines on surgical management of the axilla have been published in the UK, and this may have changed clinical practice14.
The NeST study has demonstrated variation in use of and decision-making around NACT across the UK, with surgical downstaging more apparent in the breast than the axilla, and variation according to disease subtype. These findings highlight the need for clear guidelines for decision-making in terms of patient selection and both breast and axillary surgery, to address treatment variation and optimize patient outcomes.
Supplementary Material
Contributor Information
Hiba Fatayer, Liverpool Breast Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Rachel L O’Connell, Department of Breast Surgery, Royal Marsden NHS Foundation Trust, Sutton, UK.
Finian Bannon, Centre for Public Health, Queen’s University Belfast, Institute of Clinical Science, Belfast, UK.
Charlotte E Coles, Department of Oncology, University of Cambridge, Cambridge, UK.
Ellen Copson, Cancer Sciences Academic Unit, Faculty of Medicine, University of Southampton and University Hospital Southampton, Southampton, UK.
Ramsey I Cutress, Cancer Sciences Academic Unit, Faculty of Medicine, University of Southampton and University Hospital Southampton, Southampton, UK.
Rajiv V Dave, Nightingale Breast Cancer Centre, Wythenshawe Hospital, Manchester University NHS Foundation Trust, Manchester, UK.
Matthew D Gardiner, Department of Plastic Surgery, Wexham Park Hospital, Frimley Health NHS Foundation Trust, Slough, UK; Kennedy Institute of Rheumatology, Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK.
Margaret Grayson, Northern Ireland Cancer Research Consumer Forum, Northern Ireland Cancer Trials Network, Belfast City Hospital, Belfast, UK.
Christopher Holcombe, Liverpool Breast Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Sheeba Irshad, Guy’s Cancer Centre, Guy’s and St Thomas’ NHS Trust, London, UK; School of Cancer and Pharmaceutical Sciences, King’s College London, London, UK.
Gareth W Irwin, Breast Surgery Department, Belfast City Hospital, Belfast Health and Social Care Trust, Belfast, UK.
Ciara O’Brien, Department of Medical Oncology, Christie Hospital NHS Foundation Trust, Manchester, UK; School of Medical Sciences Faculty of Biology, Medicine and Health University of Manchester, Manchester, UK.
Carlo Palmieri, University of Liverpool, Institute of Systems, Molecular and Integrative Biology, Department of Molecular and Clinical Cancer Medicine, Liverpool, UK; Clatterbridge Cancer Centre NHS Foundation Trust, Liverpool, UK.
Abeer M Shaaban, Department of Pathology, Queen Elizabeth Hospital Birmingham and University of Birmingham, Birmingham, UK.
Nisha Sharma, Breast Unit, St James’s Hospital, Leeds, UK.
Jagdeep K Singh, Surrey and Sussex Healthcare NHS Trust, East Surrey Hospital, Redhill, UK.
Ian Whitehead, Liverpool Breast Unit, Liverpool University Hospitals NHS Foundation Trust, Liverpool, UK.
Shelley Potter, Bristol Centre for Surgical Research, Population Health Sciences, Bristol Medical School, Bristol, UK; Bristol Breast Care Centre, North Bristol NHS Trust, Southmead Hospital, Bristol, UK.
Stuart A McIntosh, Patrick G Johnston Centre for Cancer Research, Queen’s University Belfast, Belfast, UK.
NeST Study Research Collaborative:
H Curry, E Iddles, M Mahmood, Y Masannat, J Schneider, L Simpson, M Sidapra, L Baker, H Capitelli-McMahon, M Hughes, A Isaac, B Skelly, C Sirianni, N Hirst, R Linforth, A Botes, T Robinson, T Schrire, J Alfred, H Lennon, D Dumitru, E Kleidi, F Hoar, E MacInnes, K Sharma, T Alaguthurai, N Chand, C A Farulla, A Hayward, B Pearce, M Tatterton, S Laws, J Iqbal, M S Mirza, K V Sainarayanan, L Humphreys, S Tayeh, S Jones, A Ansari, R Bate, B C J Wei, B Gurung, F M T Leone, C Mitchell, G Mondani, S Pilgrim, T Sun, G Boundouki, R Broadbent, A Khan, F Morgans-Slader, J Rai, R Soulsby, H Cain, R Thomas, B Elsberger, G Walls, S Cadwell-Sneath, J Couch, M D’Auria, C Grundy, S Hitchin, H Khout, F Latief, J Mondani, A Nessa, G Oni, L Sawers, S S Rajan, Q Tan, L Whisker, A Ghoneima, M Rezacova, N Marikakis, L Ballance, U Andaleeb, N Basu, T Hubbard, A Maxwell, M Roland, C Weerasinghe, Q Ain, G Bitsakou, C Chamberlain, N Chopra, A Micha, C Norman, P Padmanabhan, N Patani, K Shanthakunalan, E St John, S Jafferbhoy, C Bransgrove, A Hussein, J Livingstone, O Waker, J Hack, S Hadad, J Newell, A Heetun, A Hargreaves, E Rahman, and R Vidya
Collaborators
NeST Study Research Collaborative: H. Curry, E. Iddles, M. Mahmood, Y. Masannat, J. Schneider, L. Simpson, M. Sidapra (Aberdeen Royal Infirmary, Aberdeen, UK), L. Baker, H. Capitelli-McMahon, M. Hughes (Airedale Hospital, Keighley, UK), A. Isaac, B. Skelly (Belfast City Hospital, Belfast, UK), C. Sirianni (Betsi Cadwaladr University Health Board, Bangor, UK), N. Hirst, R. Linforth (Bradford Teaching Hospitals NHS Foundation Trust, Bradford, UK), A. Botes, T. Robinson, T. Schrire (Southmead Hospital, Bristol, UK), J. Alfred, H. Lennon (St Helens Hospital, St Helens, UK), D. Dumitru, E. Kleidi (Addenbrookes Hospitasl, Cambridge, UK), F. Hoar (City Hospital, Birmingham, UK), E. MacInnes (Doncaster Royal Infirmary, Doncaster, UK), K. Sharma (Glasgow Royal Infirmary, Glasgow, UK), T. Alaguthurai (Guy's Hospital, London, UK), N. Chand, C. A. Farulla, A. Hayward, B. Pearce, M. Tatterton, S. Laws (Royal Hampshire County Hospital, Winchester, UK), J. Iqbal, M. S. Mirza, K. V. Sainarayanan (Heart of England NHS Trust, Birmingham, UK), L. Humphreys, S. Tayeh (Homerton Hospital, London, UK), S. Jones (St James's Hospital, Leeds, UK), A. Ansari, R. Bate, B. C. J. Wei, B. Gurung, F. M. T. Leone, C. Mitchell, G. Mondani, S. Pilgrim, T. Sun (Glenfield Hospital, Leicester, UK), G. Boundouki (Wythenshawe Hospital, Manchester, UK), R. Broadbent, A. Khan, F. Morgans-Slader (Christie Hospital, Manchester, UK), J. Rai, R. Soulsby (Milton Keynes University Hospital, Milton Keynes, UK), H. Cain, R. Thomas (Royal Victoria Infirmary, Newcastle, UK), B. Elsberger (Ninewells Hospital, Dundee, UK), G. Walls (Altnagelvin Hospital, Londonderry, UK), S. Cadwell-Sneath, J. Couch, M. D’Auria, C. Grundy, S. Hitchin, H. Khout, F. Latief, J. Mondani, A. Nessa, G. Oni, L. Sawers, S. S. Rajan, Q. Tan, L. Whisker (Notthingham University Hospitals, Nottingham, UK), A. Ghoneima, M. Rezacova, N. Marikakis (Queen Alexandra Hospital, Portsmouth, UK), L. Ballance (Royal Preston Hospital, Preston, UK), U. Andaleeb, N. Basu (Queen Elizabeth Hospitals, Birmingham, UK), T. Hubbard, A. Maxwell, M. Roland (Royal Devon & Exeter Hospital, Exeter, UK), C. Weerasinghe (Royal Liverpool Hospitals, Liverpool, UK), Q. Ain, G. Bitsakou, C. Chamberlain, N. Chopra, A. Micha, C. Norman, P. Padmanabhan, N. Patani, K. Shanthakunalan, E. St John (Royal Marsden Hospital, Sutton, UK), S. Jafferbhoy (University Hospital North Midlands, Stoke-on-Trent, UK), C. Bransgrove, A. Hussein, J. Livingstone, O. Waker (Royal Surrey County Hospital, Guildford, UK), J. Hack (Salisbury District Hospital, Salisbury, UK), S. Hadad (Royal Halamshire Hospital, Sheffield, UK), J. Newell (Ulster Hospital, Dundonald, UK), A. Heetun (University Hospital Southampton, Southampton, UK), A. Hargreaves (Warrington and Halton NHS Trust, Warrington, UK), E. Rahman, R. Vidya (Royal Wolverhampton Hospital, Wolverhampton, UK).
Funding
This work was funded by a grant from the Association of Breast Surgery
Disclosure. S.M. declares advisory board fees and travel and conference support from Roche and Lilly, and institutional research funding from Novartis, Sanofi and Almac Diagnostic Services. The other authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
References
- 1. Makris A, Powles T, Ashley S, Chang J, Hickish T, Tidy V et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol 1998;9:1179. [DOI] [PubMed] [Google Scholar]
- 2. Kuerer HM, Hunt KK, Newman LA, Ross MI, Ames FC, Singletary SE. Neoadjuvant chemotherapy in women with invasive breast carcinoma: conceptual basis and fundamental surgical issues. J Am Coll Surg 2000;190:350–363 [DOI] [PubMed] [Google Scholar]
- 3. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 2017;35:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loibl S, O’Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 2018;19:497–509 [DOI] [PubMed] [Google Scholar]
- 6. Criscitiello C, Curigliano G, Burstein HJ, Wong S, Esposito A, Viale G et al. Breast conservation following neoadjuvant therapy for breast cancer in the modern era: are we losing the opportunity? Eur J Surg Oncol 2016;42:1780–1786 [DOI] [PubMed] [Google Scholar]
- 7.Mastectomy Decisions Audit Collaborative on behalf of the West Midlands Research Collaborative; Singh JK, McEvoy K, Marla S, Rea D, Hallissey M et al. Multicentre prospective observational study evaluating recommendations for mastectomy by multidisciplinary teams. Br J Surg 2020;107:227–237 [DOI] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence . Early and Locally Advanced Breast Cancer: Diagnosis and Management. https://www.nice.org.uk/guidance/ng101/chapter/Recommendations#primary-systemic-therapy (accessed 11 February 2022) [PubMed]
- 9. Whitehead I, Irwin GW, Bannon F, Coles CE, Copson E, Cutress RI et al. The NeST (Neoadjuvant systemic therapy in breast cancer) study: National Practice Questionnaire of United Kingdom multi-disciplinary decision making. BMC Cancer 2021;21:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irwin GW, Bannon F, Coles CE, Copson E, Cutress RI, Dave RV et al. The NeST (neoadjuvant systemic therapy in breast cancer) study—protocol for a prospective multi-centre cohort study to assess the current utilization and short-term outcomes of neoadjuvant systemic therapies in breast cancer. Int J Surg Protoc 2019;18:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amin MB, Byrd DR, Edge SB, Greene FL. AJCC Cancer Staging Manual. Cham: Springer International Publishing, 2016 [Google Scholar]
- 12. Petruolo O, Sevilimedu V, Montagna G, Le T, Morrow M, Barrio AV. How often does modern neoadjuvant chemotherapy downstage patients to breast-conserving surgery? Ann Surg Oncol 2021;28:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang SS, Kaptanis S, Haddow JB, Mondani G, Elsberger B, Tasoulis MK et al. Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: a national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur J Cancer 2017;84:315–324 [DOI] [PubMed] [Google Scholar]
- 14. Gandhi A, Coles C, Makris A, Provenzano E, Goyal A, Maxwell AJ et al. Axillary surgery following neoadjuvant chemotherapy—multidisciplinary guidance from the Association of Breast Surgery, Faculty of Clinical Oncology of the Royal College of Radiologists, UK Breast Cancer Group, National Coordinating Committee for Breast Pathology and British Society of Breast Radiology. Clin Oncol (R Coll Radiol) 2019;31:664–668 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.