Abstract
Background
To evaluate the risk factors and prognosis of patients with acute cholangitis recurrence.
Methods
A total of 503 patients with acute cholangitis admitted to the First Affiliated Hospital of Chongqing Medical University between July 2013 and January 2022 were included in this retrospective observational study, who were followed up for 360 days and divided into relapse group and non-recurrence group according to the recurrence of acute cholangitis. Risk factors and prognosis of patients with acute cholangitis recurrence were analyzed by univariate, multivariate analyses and proportional hazards model.
Results
A total of 161 patients with recurrent acute cholangitis were identified. Recurrent acute cholangitis usually occurred within 125 days; Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus faecalis, and Enterococcus faecium was the most common positive record both in blood and bile culture. In the multivariate analysis, abdominal pain (OR = 2.448, 95% CI = 1.196–5.010, P = 0.014), bile stones (OR = 2.429, 95% CI = 1.024–5.762, P = 0.044), diabetes (OR = 1.790, 95% CI = 1.007–3.182, P = 0.047), pathogen (OR = 3.305, 95% CI = 1.932–5.654, P<0.001), and chronic kidney disease (OR = 2.500, 95% CI = 1.197–5.221, P = 0.015) may be ascertained as the risk factors of acute cholangitis recurrence. The recurrence of acute cholangitis was identified as an independent risk factor for patient death (HR = 4.524, 95% CI = 1.426–14.357, P = 0.010) by Cox proportional-hazards regression.
Conclusion
Abdominal pain, bile stones, diabetes and chronic kidney disease may be risk factors of acute cholangitis recurrence. Patients with recurrent acute cholangitis have poor prognosis and high mortality. Early control of recurrent risk factors and active intervention are beneficial to high-risk patients.
Keywords: recurrence, acute cholangitis, risk factors, prognosis
Introduction
Acute cholangitis is a clinical syndrome characterized by fever, jaundice, and abdominal pain, with symptoms ranging from mild recurrent illness to severe sepsis.1 Patients die of cholangitis due to delayed diagnosis, systemic disease, or failure to respond to conservative treatment, which poses a huge challenge to clinical resource consumption.2
In recent years, with the further understanding of the pathogenesis and pathophysiological evolution of acute cholangitis, as well as the improvement of examination and surgical techniques, the localization diagnosis, treatment and therapeutic effect of the disease have made great progress.3,4 There are various treatment methods for acute cholangitis caused by complex and extensive biliary tract and liver lesions of hepatic duct stones.5,6 Currently, individualized treatment is advocated for the specific conditions of patients.7 However, many patients still relapse after receiving what is considered to be a relatively ideal treatment.
Recurrent acute cholangitis is often accompanied by repeated infection of the biliary system, forming calculi and stenosis, which brings challenges to the diagnosis and treatment of patients. A study suggested that common bile duct corrosion after endoscopic cholecystectomy leads to recurrent choledocholithiasis and acute cholangitis.8 A prospective study of 210 patients with acute cholangitis due to choledocholithiasis suggests that cholecystectomy does not prevent recurrence of acute cholangitis in Asian patients, and that early endoscopic papillectomy reduces the frequency of recurrence in these patients.9 Ling et al investigated that biliary duodenal anastomosis is not an ideal method to reduce intrahepatic cholangitis nor is it the best choice to curb the recurrence of cholangitis.10 The incidence of recurrent primary sclerosing cholangitis was found to be higher than before and was associated with increased morbidity and mortality. Patients with recurrent primary sclerosing cholangitis appear to have a more aggressive, immunoreactive phenotype.11 It has been found that acute cholangitis, liver abscess, cirrhosis complications and CCA (cholangiocarcinoma) frequently occur in patients with recurrent pyogenic cholangitis and may be risk factors.12 There are some researches to analyse the risk factors for the recurrence of acute cholangitis, but more efforts should be focused. In view of this, we retrospectively analyzed the risk factors and prognosis of recurrent acute cholangitis.
Patients and Methods
Study Design
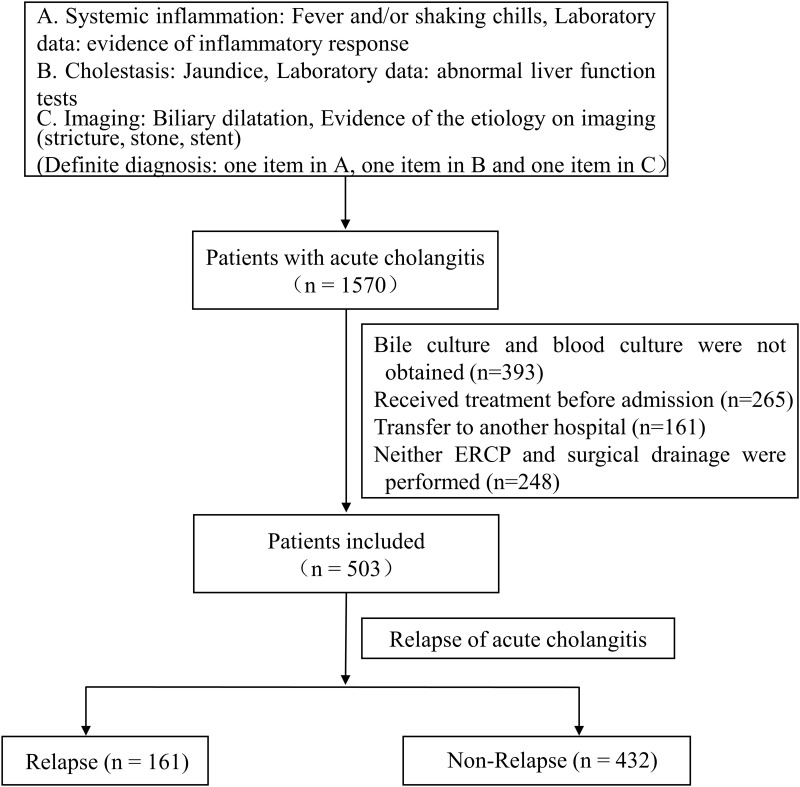
All 1570 inpatients with acute cholangitis admitted to the First Affiliated Hospital of Chongqing Medical University from July 2013 to January 2022 were considered for being preliminary included, the inclusion and exclusion criteria and case identification refer to our previous study,13 as shown in Figure 1. Taking into account some irresistible factors, as detailed in our previous study,14 503 patients were eventually included. All patients were followed closely, 29 of whom died during hospitalization, and 474 patients who were discharged were followed up by telephone every month after discharge. For 161 patients with recurrent acute cholangitis, the time interval between the recurrence and the last onset was strictly recorded, which was recorded as Re Time. Patients without recurrence were followed up for one year. Our median duration of follow-up was 365. Based on the one-year follow-up of patients with recurrent acute cholangitis, a total of 161 cases were included in the relapse group, while 432 cases were arranged into the non-relapse group just as described in Figure 1. This research was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Figure 1.
503 patients with acute cholangitis were divided into relapse group and non-relapse group via one-year follow-up.
Basic Information
Information of all 503 patients were collected in the light of the form uniformly formulated by the team in the preliminary study, including patient’s gender, age, hospital stays, cause of biliary obstruction, history of any biliary procedures, baseline diseases, clinical manifestation, laboratory inspection, 14 days antibiotic use, relapse, severity of acute cholangitis, prognosis, the specific content of the patient’s basic information has been shown in the previous study.14
Statistical Analysis
Statistical analysis was performed via SPSS 27.0 (IBM, Armonk, NY, USA). Descriptive statistics were performed on the frequency (percentage) for categorical variables and mean ± standard deviation (x ± s) and median (interquartile range, IQR) for continuous variables. Univariate analysis was used to compare variables between relapse group and non-relapse group, and Mann-Whitney U-test, Chi-square or Fisher’s exact test were applied to evaluate whether the variables were statistically significant. Subsequently, factors identified by single-factor analysis or worthy of discussion based on clinical experience were further verified by multiple logistic regression. Cox proportional-hazards regression model was used to assess the risk of death. P < 0.05 indicated that the difference was statistically significant.
Results
Characteristics of All 503 Patients
According to acute cholangitis recurred, 161 patients were defined as relapse group, and 432 patients without relapse were defined as non-relapse group. The summary description of baseline characteristics of patients is shown in Table 1. Gender, bile stones, biliary stent, surgery, ERCP, cholecystectomy, biliary stent, chronic kidney disease, fever, abdominal pain, PLT < 100 × 109, INR, PLT, ALB, MDR, ESBL, APACHE II, and SOFA have been described in Table 1 with P < 0.05 and could be related to the recurrence of acute cholangitis.
Table 1.
Demographic Characteristics of 503 Patients with Acute Cholangitis
| Total | Relapse | Non-Relapse | P-value | |
|---|---|---|---|---|
| Number of patients | 503 | 161 | 342 | — |
| Gender (male) | 90 (17.89%) | 159 (98.76%) | 212 (61.99%) | 0.049 |
| Age | 64±16 | 64.25±16.238 | 66.34±14.715 | 0.091 |
| Cause of biliary obstruction | ||||
| Tumor | 26 (5.17%) | 12 (7.45%) | 14 (4.09%) | 0.131 |
| Bile stones | 475 (94.43%) | 147 (91.30%) | 328 (95.91%) | 0.036 |
| History of any biliary procedures | ||||
| Surgery | 75 (14.91%) | 75 (46.58%) | — | < 0.001 |
| ERCP | 75 (14.91%) | 75 (46.58%) | — | < 0.001 |
| Biliary anastomosis | 8 (1.59%) | 4 (2.48%) | 4 (1.17%) | 0.275 |
| Cholecystectomy | 129 (25.65%) | 62 (38.51%) | 67 (19.59%) | < 0.001 |
| Biliary stent | 42 (8.35%) | 42 (26.09%) | — | < 0.001 |
| Baseline Diseases | ||||
| Cardiovascular | 157 (31.21%) | 42 (26.09%) | 115 (33.63%) | 0.078 |
| Chronic pulmonary disease | 32 (6.36%) | 6 (3.73%) | 26 (7.60%) | 0.082 |
| Malignancies | 38 (7.55%) | 15 (9.32%) | 23 (6.73%) | 0.305 |
| Diabetes, mellitus | 78 (15.51%) | 18 (11.18%) | 60 (17.54%) | 0.059 |
| Chronic kidney disease | 10 (1.99%) | 7 (4.35%) | 3 (0.88%) | 0.015 |
| Chronic liver disease | 30 (5.96%) | 11 (6.83%) | 19 (5.56%) | 0.552 |
| Neurological disease | 24 (4.77%) | 10 (6.21%) | 14 (4.09%) | 0.299 |
| Clinical manifestation | ||||
| Shock | 93 (18.49%) | 34 (21.12%) | 59 (17.25%) | 0.325 |
| Fever | 253 (50.30%) | 97 (60.25%) | 156 (45.61%) | 0.002 |
| Abdominal pain | 462 (91.85%) | 141 (87.58%) | 321 (93.86%) | 0.022 |
| Jaundice | 357 (70.97%) | 112 (69.57%) | 245 (71.64%) | 0.633 |
| Mental status change | 78 (15.51%) | 30 (18.63%) | 48 (14.04%) | 0.189 |
| Laboratory inspection | ||||
| PLT < 100 × 109 | 121 (24.06%) | 51 (31.68%) | 70 (20.47%) | 0.007 |
| INR | 1.1 (1.0–1.3) | 1.15 (1.02–1.33) | 1.10 (1.01–1.22) | 0.029 |
| WBC | 11.5 (9.1–15.2) | 11.21 (8.42–15.27) | 11.22 (7.93–15.27) | 0.344 |
| N | 90.7 (84–92.9) | 90.6 (83.4–92.7) | 89.1 (82.7–93.1) | 0.265 |
| PLT | 119.5 (70–193.5) | 124 (72–194) | 164 (90–236) | 0.002 |
| TB | 79.7 (34.4–129.7) | 82.9 (36.9–142.4) | 75.6 (31.7–137.2) | 0.412 |
| DB | 57.6 (18.4–91.7) | 60.5 (22.0–111.4) | 55.6 (17.2–108) | 0.306 |
| ALT | 103.5 (51.8–224) | 106 (50–218) | 130 (61–241) | 0.149 |
| AST | 110 (49–219) | 109 (50–220) | 117 (50–226) | 0.305 |
| ALB | 32.5±6.8 | 32.53±6.886 | 34.32±6.384 | 0.010 |
| BUN | 5.7 (4.4–7.4) | 5.7 (4.4–7.2) | 5.5 (3.8–7.8) | 0.997 |
| Cr | 68 (55.3–85) | 67 (56–84) | 68 (55–89) | 0.803 |
| 14 days antibiotic use | 194 (38.57%) | 64 (39.75%) | 130 (38.01%) | 0.768 |
| CRE | 35 (7.00%) | 15 (9.31%) | 20 (5.85%) | 0.154 |
| MDR | 187 (37.18%) | 93 (57.76%) | 94 (27.49%) | < 0.001 |
| ESBL | 116 (23.06%) | 57 (35.40%) | 59 (17.25%) | < 0.001 |
| Pathogen | 374 (74.35%) | 142 (88.20%) | 232 (67.84%) | < 0.001 |
| Severity of acute cholangitis | ||||
| Grade I | 116 (23.06%) | 34 (21.11%) | 82 (23.98%) | 0.498 |
| Grade II | 212 (42.15%) | 62 (38.51%) | 150 (43.86%) | 0.287 |
| Grade III | 175 (34.79%) | 65 (40.37%) | 110 (32.16%) | 0.071 |
| APACHE II | 11 (8.25–15) | 11 (9–15) | 11 (8–14) | 0.01 |
| SOFA | 4 (2–9) | 4 (2–9) | 3 (2–6) | 0.004 |
| Prognosis | ||||
| ICU admission | 171 (34.00%) | 52 (32.30%) | 119 (34.80%) | 0.580 |
| Hospital stays | 15.5 (12–22) | 15 (11–21) | 16 (11–21) | 0.924 |
| Death | 27 (5.68%) | 10 (6.21%) | 17 (4.97%) | 0.565 |
Abbreviations: ERCP, endoscopic retrograde cholangio-pancreatography; PLT, platelet; INR, international normalized ratio; WBC, white blood cell; N, neutrophil; TB, total bilirubin; DB, direct bilirubin; ALT, alanine transaminase; AST, aspartate transaminase; ALB, albumin; BUN, blood urea nitrogen; Cr, creatinine; APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment.
The Days Since the Last Occurrence of Acute Cholangitis
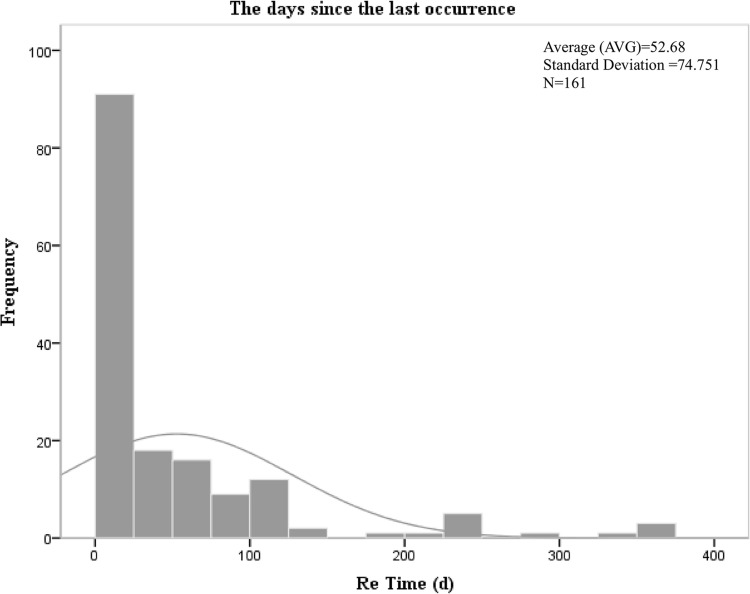
For 161 patients with acute cholangitis recurred, the number of days since recurrence of acute cholangitis is analyzed in Figure 2. The overall number of days presents a skewed distribution, with the mean value of 52.68 and the standard deviation of 74.751, and IQR was 24 (6, 72). The results indicated that recurrence occurred within 125 days after the occurrence of acute cholangitis, and the highest recurrence rate was 92 within 25 days, accounting for about 57.14%. Detailed information is presented in Table S1 of our Supplementary Materials.
Figure 2.
Recurrence time profile of 161 patients with recurrent acute cholangitis.
Microbiological Characteristics in Blood Culture and Bile Culture
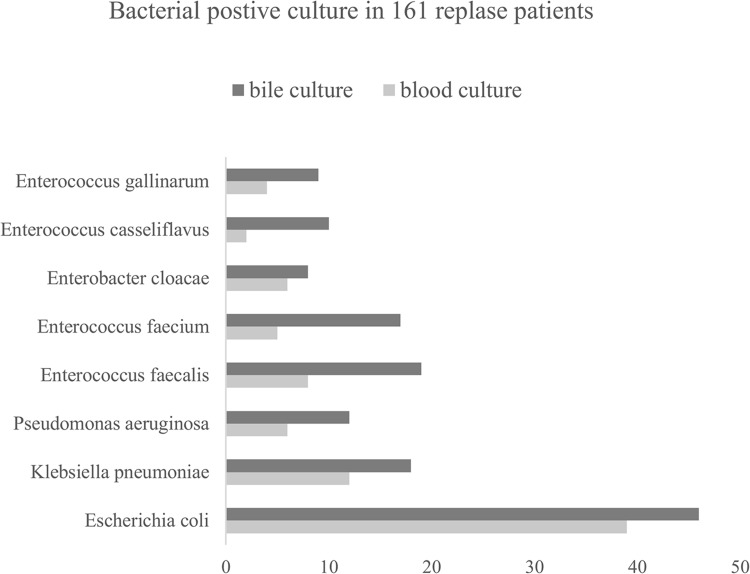
Among 503 patients with acute cholangitis, 313 (62.2%) positive blood culture records and 365 (72.6%) positive bile culture records were demonstrated in our previous study.14 For this research, as shown in Table 2, Aeromonas hydrophila, Proteus vulgaris, Enterococcus avium, Enterobacter areogenes, other Enterococcus spp. and Enterococcus raffinosus were not detected in the blood culture but bile culture, while Klebsiella oxytoca was only found in the relapsed group. In addition, for Gram-negative bacilli, Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae and Pseudomonas aeruginosa occupy higher proportion in relapse group (24.2%/28.6%, 7.5%/11.2%, 3.7%/5.0%, 3.7%/7.5%). For Gram-positive cocci, Enterococcus faecalis, Enterococcus faecium, Enterococcus gallinarum and Streptococcus spp. accounted for higher proportion in relapse group (5.0%/11.8%, 3.1%/10.6%, 2.5%/5.6%, 1.2%/3.1%). But Enterococcus casseliflavus accounted for a higher blood culture proportion in relapse group (1.2%/0.9%) but a lower positive bile culture proportion in relapse group (0.6%/2.3%). The distribution of pathogenic bacteria in patients with recurrent acute cholangitis is shown in Figure 3.
Table 2.
Results of Blood and Bile Cultures in Patients with Acute Cholangitis
| Pathogens | Blood Culture | Bile Culture | ||
|---|---|---|---|---|
| Relapse (n = 161) | No-Relapse (n = 342) | Relapse (n = 161) | No-Relapse (n = 342) | |
| Gram-negative bacilli | ||||
| Escherichia coli | 39(24.22%) | 55(16.08%) | 46(28.57%) | 76(22.22%) |
| Klebsiella pneumoniae | 12(7.45%) | 22(6.43%) | 18(11.18%) | 30(8.77%) |
| Enterobacter cloacae | 6(3.73%) | 5(1.46%) | 8(4.97%) | 16(4.68%) |
| Pseudomonas aeruginosa | 6(3.73%) | 5(1.46%) | 12(7.45%) | 16(4.68%) |
| Acinetobacter baumannii | 1(0.62%) | — | 3(1.86%) | 2(0.58%) |
| Other Enterobacter spp. | 2(1.24%) | 1(0.29%) | 3(1.86%) | 1(0.29%) |
| Klebsiella oxytoca | 2(1.24%) | — | 10(0.62%) | — |
| Citrobacter spp. | 1(0.62%) | — | 5(3.11%) | 7(2.05%) |
| Aeromonas hydrophila | — | — | 1(0.62%) | 3(0.88%) |
| Proteus vulgaris | — | — | 2(1.24%) | 1(0.29%) |
| Gram-positive cocci | ||||
| Enterococcus faecalis | 8(4.97%) | 4(1.17%) | 19(11.80%) | 43(12.57%) |
| Enterococcus faecium | 5(3.11%) | 7(2.05%) | 17(10.56%) | 31(9.06%) |
| Enterococcus casseliflavus | 2(1.24%) | 3(0.87%) | 10(6.21%) | 8(2.34%) |
| Enterococcus gallinarum | 4(2.48%) | 2(0.58%) | 9(2.63%) | 5(1.46%) |
| Streptococcus spp. | 2(1.24%) | 3(0.88%) | 5(3.11%) | 7(2.05%) |
| Staphylococcus hominis | 1(0.62%) | 2(0.58%) | — | 2(0.58%) |
| Enterococcus avium | — | — | 3(1.86%) | 3(0.88%) |
| Enterobacter aerogenes | — | — | — | 1(0.29%) |
| Other Enterococcus spp. | — | — | — | 3(0.88%) |
| Enterococcus raffinosus | — | — | 2(1.24%) | 1(0.29%) |
| Other Gram-positive cocci | 3(1.86%) | 1(0.29%) | 3(1.86%) | 1(0.29%) |
| Fungus | 1(0.62%) | 1(0.29%) | 8(4.97%) | 10(2.92%) |
Notes: “—” indicates that the value is zero, Gram-negative bacilli: Bacteria with a red gram staining reaction, Gram-positive cocci: Bacteria dyed dark blue or purple by Gram stain.
Figure 3.
Both blood culture and bile culture results of 161 recurrent acute cholangitis.
The Cox Proportional-Hazards Regression Model and Organ Function Between Relapse and Non-Relapse
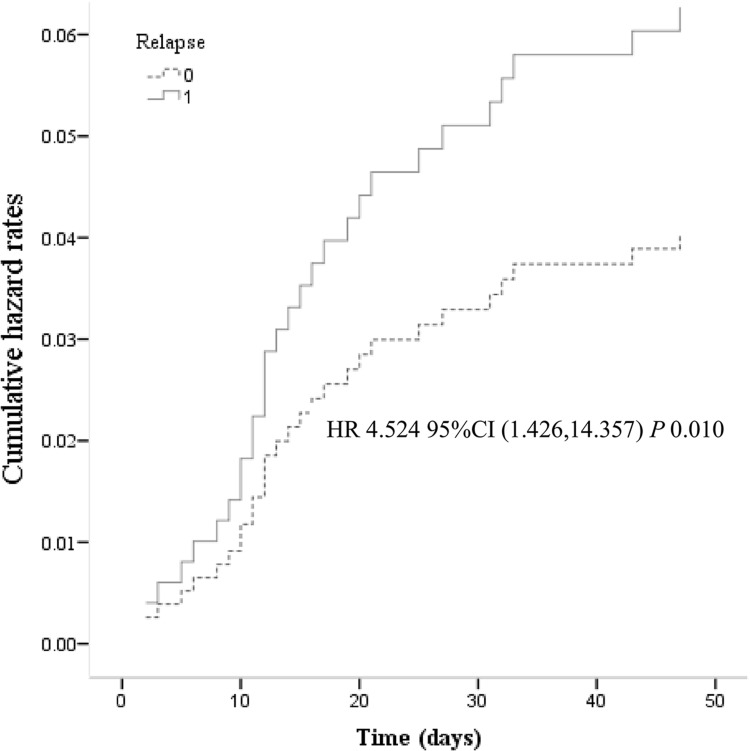
Relapse of acute cholangitis was identified as an independent risk factor for patient death (HR = 4.524, 95% CI = 1.426–14.357, P = 0.010) by Cox proportional-hazards regression just as shown in Figure 4. Cox proportional-hazards regression model was adjusted for age, gender, abdominal pain, fever, bile stones, tumor, malignancies, chronic liver disease, chronic kidney disease, chronic pulmonary disease, diabetes, CRO, surgery, PLT < 100 × 109, WBC, TB, relapse.
Figure 4.
The results of Cumulative hazard rates (Cox) proportional-hazards regression. Cox proportional-hazards regression model were adjusted for age, gender, abdominal pain, fever, bile stones, tumor, malignancies, chronic liver disease, chronic kidney disease, chronic pulmonary disease, diabetes, CRO, surgery, PLT < 100 × 109, WBC, TB, relapse.
The results of risk ratio of organ dysfunction expounded that the renal dysfunction was more severe in relapse group than non-relapse group (OR = 2.17, 95% CI = 1.08–4.36, P = 0.029). Correspondingly, there was no significant difference in septic shock, cardiovascular, neurological dysfunction, hepatic dysfunction, respiratory dysfunction between relapse group and non-relapse group as described in Table 3.
Table 3.
Risk Ratio of Organ Dysfunction in Relapse Group by Logistic-Regression Model
| Variables | No-Relapse (n = 342) | Relapse (n = 161) | |
|---|---|---|---|
| Adjusted OR (95% CI) | P-value | ||
| Septic shock | 1.0 (reference) | 0.80 (0.47–1.36) | 0.408 |
| Cardiovascular | 1.0 (reference) | 1.54 (0.99–2.37) | 0.043 |
| Neurological dysfunction | 1.0 (reference) | 0.57 (0.24–1.35) | 0.201 |
| Hepatic dysfunction | 1.0 (reference) | 0.32 (0.04–2.86) | 0.309 |
| Renal dysfunction | 1.0 (reference) | 2.17 (1.08–4.36) | 0.029 |
| Respiratory dysfunction | 1.0 (reference) | 2.47 (0.96–6.37) | 0.044 |
Risk Factors for Recurrence of Acute Cholangitis
Our results of univariate analysis in Table 4 shows that abdominal pain (OR = 2.168, 95% CI = 1.139–4.127, P = 0.018), bile stones (OR = 2.231, 95% CI = 1.037–4.799, P = 0.040), diabetes mellitus (OR = 1.815, 95% CI = 1.012–3.254, P = 0.045), pathogen (OR = 3.544, 95% CI = 2.086–6.019, P<0.001), and chronic kidney disease (OR = 7.509, 95% CI = 1.533–36.782, P = 0.013) may be the risk factors of the relapse of acute cholangitis. In addition, gender, male (OR = 0.685, 95% CI = 0.470–0.999, P = 0.049), cholecystectomy (OR = 0.389, 95% CI = 0.257–0.589, P = 0.000), 14 days antibiotic use (OR = 0.516, 95% CI = 0.306–0.872, P = 0.013) were identified by univariate analysis.
Table 4.
Univariate and Multivariable Analysis of Risk Factors for the Recurrent Acute Cholangitis
| Characteristics | Univariate Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age, year, median (range) | 0.990 | 0.978–1.002 | 0.098 | — | — | — |
| Gender, male, n (%) | 0.685 | 0.470–0.999 | 0.049 | — | — | — |
| Tumor, n (%) | 0.530 | 0.239–1.174 | 0.118 | — | — | — |
| Abdominal pain, n (%) | 2.168 | 1.139–4.127 | 0.018 | 2.448 | 1.196–5.010 | 0.014 |
| Bile stones, n (%) | 2.231 | 1.037–4.799 | 0.040 | 2.429 | 1.024–5.762 | 0.044 |
| Cholecystectomy, n (%) | 0.389 | 0.257–0.589 | 0.000 | — | — | — |
| Cardiovascular, n (%) | 1.454 | 0.958–2.207 | 0.078 | — | — | — |
| Chronic pulmonary, n (%) | 2.126 | 0.857–5.271 | 0.104 | — | — | — |
| Malignancies, n (%) | 0.702 | 0.356–1.384 | 0.307 | — | — | — |
| Diabetes mellitus, n (%) | 1.815 | 1.012–3.254 | 0.045 | 1.790 | 1.007–3.182 | 0.047 |
| Liver cirrhosis, n (%) | 0.797 | 0.370–1.717 | 0.562 | — | — | — |
| Pathogen, n (%) | 3.544 | 2.086–6.019 | 0.000 | 3.305 | 1.932–5.654 | 0.000 |
| 14 Antibiotic use, n (%) | 0.516 | 0.306–0.872 | 0.013 | — | — | — |
| Chronic kidney disease, n (%) | 7.509 | 1.533–36.782 | 0.013 | 2.500 | 1.197–5.221 | 0.015 |
Our results of multivariable analysis rely on gender, tumor, abdominal pain, bile stones, cardiovascular and chronic pulmonary, malignancies, diabetes, and chronic kidney disease in Table 4 and suggest that abdominal pain (OR = 2.448, 95% CI = 1.196–5.010, P = 0.014), bile stones (OR = 2.429, 95% CI = 1.024–5.762, P = 0.044), diabetes mellitus (OR = 1.790, 95% CI = 1.007–3.182, P = 0.047), pathogen (OR = 3.305, 95% CI = 1.932–5.654, P<0.001), and chronic kidney disease (OR = 2.500, 95% CI = 1.197–5.221, P = 0.015) may be regarded as the risk factors of the occurrence of recurrent acute cholangitis.
Discussion
Acute cholangitis remains a serious problem due to its complex pathophysiological features.15 Acute cholangitis is often caused by bacterial infection in the bile ducts and can result in a range of complications, such as sepsis, biliary sepsis, and organ failure.16 The pathophysiology of acute cholangitis is complex, involving multiple factors, including obstruction of the bile ducts, bacterial infection, and immune system dysfunction.17 In addition, the diagnosis and treatment of acute cholangitis can be challenging due to its various clinical presentations and potential complications.18 Early diagnosis and appropriate management of the underlying biliary tract disease are crucial for the prevention and treatment of acute cholangitis.19 Treatment options for acute cholangitis include antibiotics, endoscopic interventions, and surgical interventions, and the choice of treatment depends on the severity of the disease and the individual patient’s clinical condition.20 Despite advances in the understanding of the pathophysiology and treatment of acute cholangitis, the disease remains a significant clinical challenge. Ongoing research efforts are aimed at further understanding the complex pathophysiology of acute cholangitis, developing more effective diagnostic and treatment strategies, and improving the overall prognosis for patients with this condition.
The incidence of recurrence of acute cholangitis varies depending on the underlying cause and the patient’s individual risk factors. However, studies have shown that the recurrence rate of acute cholangitis ranges from 10% to 30%.21 Several risk factors have been identified that can increase the likelihood of recurrence of acute cholangitis. Biliary tract obstruction, any obstruction in the bile ducts, such as biliary stones, strictures, or tumors, can increase the risk of recurrence of acute cholangitis.22–25 Patients with other underlying medical conditions, such as diabetes, chronic liver disease, or inflammatory bowel disease, are at an increased risk of recurrent acute cholangitis.26–28 Management of these risk factors, such as removal of biliary stones, treating underlying medical conditions, and lifestyle modifications, can help reduce the risk of recurrence of acute cholangitis.29,30
Our results suggested that abdominal pain may be the risk factors of the relapse of acute cholangitis. A case report showing that recurrent bouts of cholangitis are often accompanied by acute abdominal pain, which is consistent with our study.31 Alzerwi et al reported a case of recurrent cholangitis in a patient with multiple episodes of abdominal pain, jaundice, anorexia, fever, and apparent unintentional weight loss and noted that surgeons should be aware of these rare symptoms to avoid misdiagnosis and delay or inappropriate management.32 Studies have pointed out that bile duct stenosis caused by recurrent cholangitis is a common complication in patients with advanced chronic pancreatitis. Its clinical manifestations range from incidental findings to obvious jaundice and cholangitis. However, diagnosis is mostly found in the investigation of abdominal pain, and jaundice may only be the initial clinical manifestation.33,34
Acute cholangitis is often caused by cholelithiasis, biliary obstruction, and cholecystitis. Some studies have pointed out that predicting the tumor risk of patients with gallbladder polyps based on nomogram model is helpful to reduce the occurrence of tumor-related biliary obstruction, which may reduce the recurrence of acute cholangitis to some extent.35,36 Treatment usually includes the use of antibiotics and the removal of biliary obstruction. In severe cases, surgical intervention may be required. If acute cholangitis is not treated promptly, it may cause serious complications, such as biliary peritonitis and sepsis. Our results also confirm that bile stones were one of the major risk factors for recurrence of acute cholangitis in patients. A study reported that a 34-year-old man presented to the emergency department with a chief complaint of upper abdominal pain. Acute cholangitis from choledocholithiasis occurred again 3 months after removal of black stones and debris. Intraductal ultrasound revealed hyperechoic lesions with acoustic shadows in the diverticulum, indicating the presence of stones or fragments accompanied by diverticular stones, and the patient was considered to have recurrent acute cholangitis.37 A retrospective cohort study illustrated that biliary obstruction may be associated with 30-day readmission rates in patients with acute cholangitis.29
Diabetes mellitus was a risk factor for recurrence of acute cholangitis. An analysis from the National Readmission Database suggests that diabetes is one of the factors contributing to the increased risk of readmission in patients with acute cholangitis.29 Cozma et al indicated that patients with T2DM have a higher risk of acute cholangitis compared with non-diabetic patients. Diabetics with acute cholangitis are more likely than non-diabetics to have longer hospital stays and sepsis, as well as a higher risk of death and more serious consequences, such as repeated bouts of cholangitis.26 People with diabetes have poor resistance and are prone to infections, including biliary tract infections. Acute cholangitis is an infection of the biliary tract, usually caused by bile duct stones or cholestasis. If people with diabetes develop acute cholangitis, their immune system may not be able to fight the infection effectively, leading to a recurrence and persistence of the infection. In addition, patients with diabetes may also have impaired bile production due to inadequate pancreatic function, which may increase the risk of biliary tract infections. Therefore, diabetic patients should avoid the formation of biliary stones and cholestasis as much as possible to keep the biliary tract unobstructed. If patients with diabetes develop symptoms of acute cholangitis, such as upper abdominal pain, fever, nausea, vomiting, etc., they should seek medical attention and receive treatment promptly. Treatment usually includes medications such as antibiotics, pain relievers, and surgery such as biliary drainage if necessary. At the same time, diabetes patients should also pay attention to control blood sugar levels, maintain a healthy diet, avoid excessive alcohol consumption, etc., in order to prevent the recurrence of biliary tract infection.
The relationship between chronic kidney disease and recurrence of acute cholangitis is rarely reported. A patient with autosomal dominant polycystic kidney disease (ADPKD) developed 6 episodes of acute pancreatitis and 2 episodes of cholangitis within a period of 12 months. Various imaging studies revealed multiple cysts of the kidney, liver, and pancreas, mild dilatation of the pancreatic duct, dilatation of the biliary system, and no gallstones. A single-center retrospective study of 1438 patients with acute cholangitis demonstrated that the Kaplan–Meier analysis showed significantly higher mortality in the AKI group than in the non-AKI group. AKI (HR = 1.853, 95% CI = 1.115–3.079) was an independent predictor of all-cause acute cholangitis mortality.38 Our results confirmed that chronic kidney disease was also identified as possible independent risk factors for recurrence of acute cholangitis. The effect of acute kidney injury and chronic kidney injury on recurrence of acute cholangitis remains to be further explored.
The results of pathogenic bacteria culture indicated that the proportions of E. coli, E. pneumoniae, E. faecalis and E. faecium were all high in blood culture and bile culture, which may play a certain guiding role in the selection of antimicrobial agents in clinical practice. The pathogenic bacteria spectra of 161 patients with recurrence also provided theoretical basis for further clinical prevention of recurrence of acute cholangitis.
Compared with patients without recurrent acute cholangitis, 161 patients with recurrent acute cholangitis had higher mortality, poor prognosis, and renal dysfunction. Chronic kidney disease is a risk factor for recurrence in patients with acute cholangitis, and the results of organ function analysis suggest that renal dysfunction gradually worsens over time, which may suggest that patients with acute cholangitis with poor renal function should pay special attention to renal protection treatment. In addition, gender, cholecystectomy, 14 days antibiotic use may be negative regulatory factors for recurrence of acute cholangitis.
There are some limitations in our study. Our study did not carefully classify the tumors and further analyze whether biliary obstruction affected recurrence of acute cholangitis. Despite these limitations, it is helpful to elucidate some risk factors of cholangitis recurrence for timely intervention and precise treatment. Clinical diagnosis model construction is an important research field in clinical medicine. By collecting the patient’s medical history, signs, laboratory tests and other relevant information, an accurate and practical clinical diagnosis model can be established to help doctors diagnose diseases more accurately and make appropriate treatment plans. Our study failed to construct an acute cholangitis recurrence model, which is also a reflection of the limitations of the study. In the following study, we will expand the sample size and work on the construction of the disease model.
Conclusion
Abdominal pain, bile stones, diabetes, pathogen, and chronic kidney disease may be risk factors for relapse of acute cholangitis. Recurrent acute cholangitis increases the severity and mortality of patients, and renal protection therapy may benefit patients.
Funding Statement
This study was supported by the Science and Technology Commission of Chongqing (project number X1-2703 and 0202czzx2106(CQYC202004).
Abbreviations
CRO, Carbapenem-resistant organism; TG18, Tokyo Guidelines 2018; MDR, Multiple drug resistance; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; ERCP, Endoscopic Retrograde Cholangio-Pancreatography; PLT, Platelet; INR, International Normalized Ratio; WBC, White Blood Cell; N, Neutrophil; TB, Total Bilirubin; DB, Direct Bilirubin; ALT, Alanine Transaminase; AST, Aspartate transaminase; ALB, Albumin; BUN, Blood urea nitrogen; Cr, Creatinine; ESBL, Extended-spectrum β-lactamases; ICU, Intensive care unit.
Ethics Approval and Consent to Participate
Our study was conducted in accordance with the Helsinki Declaration, and all methods were carried out in accordance with relevant guidelines and regulations of the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
The experimental protocols and reference number (K2023-068) of our research was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Informed consent of our research was obtained from all subjects or their legal guardian.
The data that support the findings of this study are available from the Department of Medical Records, the First Affiliated Hospital of Chongqing Medical University, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6(9):533–541. doi: 10.1038/nrgastro.2009.126 [DOI] [PubMed] [Google Scholar]
- 2.Taha R, Khamaysi I. ERCP for severe acute cholangitis: critical timing. Gastrointest Endosc. 2020;92(4):984. doi: 10.1016/j.gie.2020.05.022 [DOI] [PubMed] [Google Scholar]
- 3.Buxbaum JL, Buitrago C, Lee A, et al. ASGE guideline on the management of cholangitis. Gastrointest Endosc. 2021;94(2):207–221.e214. doi: 10.1016/j.gie.2020.12.032 [DOI] [PubMed] [Google Scholar]
- 4.An Z, Braseth AL, Sahar N. Acute cholangitis: causes, diagnosis, and management. Gastroenterol Clin North Am. 2021;50(2):403–414. doi: 10.1016/j.gtc.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 5.Sulzer JK, Ocuin LM. Cholangitis: causes, diagnosis, and management. Surg Clin North Am. 2019;99(2):175–184. doi: 10.1016/j.suc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Hirschfield GM, Beuers U, Corpechot C, et al. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67(1):145–172. doi: 10.1016/j.jhep.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 7.Doi A, Morimoto T, Iwata K. Shorter duration of antibiotic treatment for acute bacteraemic cholangitis with successful biliary drainage: a retrospective cohort study. Clin Microbiol Infect. 2018;24(11):1184–1189. doi: 10.1016/j.cmi.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 8.Chaudhry H, Sohal A, Gulati A, Chintanaboina J. Endoscopic removal of postcholecystectomy clip eroding in the common bile duct causing recurrent choledocholithiasis and acute cholangitis. ACG Case Rep J. 2022;9(10):e00875. doi: 10.14309/crj.0000000000000875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui CK, Lai KC, Yuen MF, Ng MM, Lam SK, Lai CL. Role of cholecystectomy in preventing recurrent cholangitis. Gastrointest Endosc. 2002;56(1):55–60. doi: 10.1067/mge.2002.125545 [DOI] [PubMed] [Google Scholar]
- 10.Ling XF, Xu Z, Wang LX, et al. Long-term outcomes of choledochoduodenostomy for hepatolithiasis. Chin Med J. 2010;123(2):137–141. [PubMed] [Google Scholar]
- 11.Faust TW. Recurrent primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis after transplantation. Liver Transpl. 2001;7(11 Suppl 1):S99–108. doi: 10.1053/jlts.2001.28514 [DOI] [PubMed] [Google Scholar]
- 12.You MS, Lee SH, Kang J, et al. Natural course and risk of cholangiocarcinoma in patients with recurrent pyogenic cholangitis: a retrospective cohort study. Gut Liver. 2019;13(3):373–379. doi: 10.5009/gnl18339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian S, Li K, Tang H, et al. Clinical characteristics of Gram-negative and Gram-positive bacterial infection in acute cholangitis: a retrospective observational study. BMC Infect Dis. 2022;22(1):269. doi: 10.1186/s12879-021-06964-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li K, Jiang S, Fu H, Hao Y, Tian S, Zhou F. Risk factors and prognosis of carbapenem-resistant organism colonization and infection in acute cholangitis. Infect Drug Resist. 2022;15:7777–7787. doi: 10.2147/IDR.S398581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadakia SC. Biliary tract emergencies. Acute cholecystitis, acute cholangitis, and acute pancreatitis. Med Clin North Am. 1993;77(5):1015–1036. doi: 10.1016/S0025-7125(16)30208-5 [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya T, Sofuni A, Tsuji S, et al. Endoscopic management of acute cholangitis according to the TG13. Dig Endosc. 2017;29 Suppl 2:94–99. doi: 10.1111/den.12799 [DOI] [PubMed] [Google Scholar]
- 17.Buckman SA, Mazuski JE. Review of the Tokyo guidelines 2018: antimicrobial therapy for acute cholangitis and cholecystitis. JAMA Surg. 2019;154(9):873–874. doi: 10.1001/jamasurg.2019.2169 [DOI] [PubMed] [Google Scholar]
- 18.Ramchandani M, Pal P, Reddy DN. Endoscopic management of acute cholangitis as a result of common bile duct stones. Dig Endosc. 2017;29 Suppl 2:78–87. doi: 10.1111/den.12848 [DOI] [PubMed] [Google Scholar]
- 19.Sharma BC, Kumar R, Agarwal N, Sarin SK. Endoscopic biliary drainage by nasobiliary drain or by stent placement in patients with acute cholangitis. Endoscopy. 2005;37(5):439–443. doi: 10.1055/s-2005-861054 [DOI] [PubMed] [Google Scholar]
- 20.Lavillegrand JR, Mercier-Des-Rochettes E, Baron E, et al. Acute cholangitis in intensive care units: clinical, biological, microbiological spectrum and risk factors for mortality: a multicenter study. Crit Care. 2021;25(1):49. doi: 10.1186/s13054-021-03480-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Simo K. Recurrent pyogenic cholangitis. In: StatPearls. Treasure Island (FL): StatPearls Publishing, Copyright © 2023, StatPearls Publishing LLC; 2023. [PubMed] [Google Scholar]
- 22.Hara T, Taniguchi M, Hattori C, et al. Microbiological analysis of patients with first and recurrent episodes of acute cholangitis in a middle-sized hospital: a single-center retrospective study in rural North Kyoto, Japan. J Infect Chemother. 2022;28(3):413–419. doi: 10.1016/j.jiac.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 23.Kihara Y, Yokomizo H. The clinical features of late postoperative cholangitis following pancreaticoduodenectomy brought on by conditions other than cancer recurrence: a single-center retrospective study. BMC Surg. 2022;22(1):301. doi: 10.1186/s12893-022-01752-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez M, Perito ER, Valentino P, et al. Recurrence of primary sclerosing cholangitis after liver transplant in children: an international observational study. Hepatology. 2021;74(4):2047–2057. doi: 10.1002/hep.31911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duclos-Vallée JC. Recurrence of autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis after liver transplantation. Acta Gastroenterol Belg. 2005;68(3):331–336. [PubMed] [Google Scholar]
- 26.Cozma MA, Dobrică EC, Shah P, Shellah D, Găman MA, Diaconu CC. Implications of type 2 diabetes mellitus in patients with acute cholangitis: a systematic review of current literature. Healthcare. 2022;10(11). doi: 10.3390/healthcare10112196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiménez Castillo RA, Jáquez-Quintana JO, Reyna-Aréchiga AI, et al. Risk factors for mortality in patients with acute bacterial cholangitis - Type-2 diabetes is a significant clinical predictor. Rev Esp Enferm Dig. 2022;114(1):59–61. doi: 10.17235/reed.2021.8251/2021 [DOI] [PubMed] [Google Scholar]
- 28.Khoury T, Sbeit W. Diabetes mellitus is associated with a higher rate of acute cholangitis among patients with common bile duct stones: a retrospective study. Medicine. 2022;101(4):e28687. doi: 10.1097/MD.0000000000028687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parikh MP, Garg R, Chittajallu V, et al. Trends and risk factors for 30-day readmissions in patients with acute cholangitis: analysis from the national readmission database. Surg Endosc. 2021;35(1):223–231. doi: 10.1007/s00464-020-07384-z [DOI] [PubMed] [Google Scholar]
- 30.Navaneethan U, Gutierrez NG, Jegadeesan R, et al. Delay in performing ERCP and adverse events increase the 30-day readmission risk in patients with acute cholangitis. Gastrointest Endosc. 2013;78(1):81–90. doi: 10.1016/j.gie.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 31.Martín-Lagos.Maldonado A, Alcázar Jaén LM, Martínez Tirado Mdel P, Salmerón Escobar J, Mundi Sánchez-Ramade JL. An Asian man with recurrent abdominal pain. Gastroenterol Hepatol. 2012;35(8):572–576. doi: 10.1016/j.gastrohep.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 32.Alzerwi NAN. Recurrent ascending cholangitis with acute pancreatitis and pancreatic atrophy caused by a juxtapapillary duodenal diverticulum: a case report and literature review. Medicine. 2020;99(27):e21111. doi: 10.1097/MD.0000000000021111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdallah AA, Krige JE, Bornman PC. Biliary tract obstruction in chronic pancreatitis. HPB. 2007;9(6):421–428. doi: 10.1080/13651820701774883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catalano MF, Linder JD, George S, Alcocer E, Geenen JE. Treatment of symptomatic distal common bile duct stenosis secondary to chronic pancreatitis: comparison of single vs. multiple simultaneous stents. Gastrointest Endosc. 2004;60(6):945–952. doi: 10.1016/S0016-5107(04)02275-8 [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Wang J, Wu B, et al. A nomogram-based model to predict neoplastic risk for patients with gallbladder polyps. J Clin Transl Hepatol. 2022;10(2):263–272. doi: 10.14218/JCTH.2021.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Wang J, Wu B, et al. A nomogram-based model and ultrasonic radiomic features for gallbladder polyp classification. J Gastroenterol Hepatol. 2022;37(7):1380–1388. doi: 10.1111/jgh.15841 [DOI] [PubMed] [Google Scholar]
- 37.Azami T, Takano Y, Kobayashi T, et al. A case of bile duct diverticulum with repeated acute cholangitis due to diverticular stone fall. Clin J Gastroenterol. 2021;14(5):1555–1560. doi: 10.1007/s12328-021-01462-y [DOI] [PubMed] [Google Scholar]
- 38.Lee TW, Bae W, Kim S, et al. Incidence, risk factors, and prognosis of acute kidney injury in hospitalized patients with acute cholangitis. PLoS One. 2022;17(4):e0267023. doi: 10.1371/journal.pone.0267023 [DOI] [PMC free article] [PubMed] [Google Scholar]