Abstract
Background
The aim of this study was to investigate whether the PAM-50-based 46-gene assay carries prognostic value for risk of local recurrence of breast cancer.
Methods
The Austrian Breast and Colorectal Cancer Study Group (ABCSG) 8 RCT compared 5 years of tamoxifen with tamoxifen for 2 years followed by anastrozole for 3 years in postmenopausal women with endocrine receptor-positive breast cancer. This study included patients from the trial who had breast-conserving surgery for whom tumour blocks were available for PAM-50 analysis.
Results
Tumour blocks from 1204 patients who had breast-conserving surgery were available for the PAM-50 analysis, and 1034 of these received radiotherapy. After a median follow-up of 10.8 years, 23 local events had been observed, corresponding to an overall local recurrence risk of 2.2 per cent. Univariable competing-risk analysis demonstrated that patients at low risk according to PAM-50 analysis (risk-of-recurrence (ROR) score less than 57) had a significantly lower incidence of local recurrence than those in the high-risk group at 5 years (0.1 (95 per cent c.i. 0 to 0.7) versus 2.2 (0.9 to 4.6) per cent respectively; subhazard ratio (SHR) 17.18, 95 per cent c.i. 2.06 to 142.88; P = 0.009) and 10 years (0.9 (0.4 to 2.0) versus 3.8 (1.9 to 6.6) per cent; SHR 4.76, 1.72 to 13.17; P = 0.003). Multivariable analyses that included ROR score, age, tumour size, nodal status, type of surgery, tumor grade, and trial-specific endocrine therapy confirmed that ROR score was an independent prognostic factor for risk of local recurrence. Analysis of the women randomized to radiotherapy or control after breast conservation showed that PAM-50 was not predictive of radiotherapy effect.
Conclusion
PAM-50 can be used as a prognostic tool for local recurrence risk in postmenopausal women with hormone receptor-positive breast cancer treated with endocrine therapy. The test was not predictive for the benefit of radiotherapy.
Predicting local recurrence risk after early breast cancer treatment has important implications for decision-making regarding local therapy. The aim of this study was to investigate whether a PAM-50-based risk-of-recurrence score adds additional prognostic value to known clinical risk factors for local recurrence risk in early-stage breast cancer, and to evaluate its predictive role regarding radiotherapy benefit. It was demonstrated that a PAM-50-based assay can be used as a prognostic tool for local recurrence risk in postmenopausal women with hormone receptor-positive breast cancer treated with endocrine therapy and who had received standard-of-care local therapy. Predictive value for the benefit of radiotherapy was not shown.
Predicting local recurrence (LR)-risk after early breast cancer treatment has important implications for local therapy decision making.
The aim of this study was to investigate whether a PAM-50-based assay (Prosigna, NanoString Technologies) risk of recurrence (ROR) score adds additional prognostic value to known clinical risk-factors for LR risk in early stage breast cancer, and to evaluate its predictive role regarding radiotherapy benefit.
We accomplished demonstrating that a PAM-50-based assay can be used as a prognostic tool for LR risk in postmenopausal hormone-receptor positive endocrine-treated breast cancer patients who have received standard of care local therapy. A predictive value for radiotherapy benefit was not shown.
Finds ultra low-risk patients
Resumen
Antecedentes
El objetivo de este estudio fue investigar si el análisis de 46 genes de la plataforma de expresión génica PAM-50 tenía valor pronóstico para determinar el riesgo de recidiva local en el cáncer de mama.
Métodos
ABCSG-8 es un ensayo prospectivo aleatorizado y controlado efectuado en pacientes postmenopáusicas con cáncer de mama y receptores endocrinos positivos, que comparaba la administración de tamoxifeno durante 5 años con tamoxifeno 2 años seguido de anastrozol durante 3 años. De este ensayo, se disponía de bloques de tumor de 1.204 pacientes sometidas a cirugía conservadora de mama para el análisis de PAM-50 y, de estas pacientes, 1.034 recibieron radioterapia.
Resultados
Después de una mediana de seguimiento de 10,8 años, se observaron 23 recidivas locales, lo que correspondía a un riesgo global de recidiva local del 2,2%. El análisis univariado de riesgos competitivos demostró que pacientes con PAM-50 de bajo riesgo (puntuación de riesgo de recidiva, risk-of-recurrence score, ROR < 57) tenían una incidencia significativamente menor de recidiva local (0,1%, i.c. del 95% (0,0-0.7) y 0,9%, (0,4-2,0)) a 5 y 10 años, respectivamente, cuando se comparaban con pacientes con PAM-50 de elevado riesgo (2,2%, (0,9-4,6) a los 5 años, cociente de riesgos instantáneos, hazard ratio, HR 17,18, (2,06-142,9), P = 0,0085 y 3,8% (1,9-6,6) a los 10 años, HR = 4,76, (1,72-13,17), P = 0,0026)). Los análisis multivariables que incluían la puntuación ROR, edad, tamaño tumoral, estadio ganglionar, tipo de cirugía y el tratamiento endocrino específico del ensayo clínico confirmaron que la puntuación ROR era un factor pronóstico independiente de riesgo de recidiva local. En el análisis de las mujeres aleatorizadas a grupo de radioterapia o al grupo control tras la preservación de la mama, PAM-50 no era un factor predictor del efecto de la radioterapia.
Conclusión
PAM-50 puede ser utilizada como herramienta pronóstica de riesgo de recidiva en pacientes postmenopáusicas con cáncer de mama positivo para receptores hormonales tratadas con terapia endocrina. La prueba no tuvo valor predictivo del beneficio de la radioterapia.
Introduction
Predicting local recurrence risk after early breast cancer treatment has important implications for decision-making. Factors such as grade, tumour size, age, and lymph node positivity correlate independently with local recurrence-free survival. However, efforts to de-escalate local therapy using these factors, such as omitting radiotherapy for patients perceived as low risk after breast-conserving surgery (BCS), have so far failed in prospective clinical trials1–5. Similarly, age and categorization as low risk based on clinical factors were not able to distinguish between patients who may or may not benefit from mastectomy compared with BCS6,7. With respect to radiotherapy after BCS, even patients with small low-grade breast cancers benefited from adjuvant radiotherapy in terms of risk of local recurrence in clinical trials8 .
There is an urgent need to develop diagnostic tools to better estimate the local recurrence risk in order to predict the benefit of local therapy, guide optimal and tailored locoregional treatment strategies, and thus further reduce avoidable side-effects and costs of breast cancer treatment. The development of multigene expression assays has improved ability to distinguish between intrinsic molecular subtypes of breast cancer (such as luminal A and B), and to differentiate between patients at low and high risk of recurrence, independently of classical clinical prognostic factors such as nodal status or age9–11. Perhaps more controversially, these tests have also been accepted by some to predict benefit from systemic chemotherapy and extended endocrine therapy. Although there seems to be a good prognostic correlation with the risk of local recurrence determined by Endopredict®12 (Myriad Genetics, Salt Lake City, USA), Oncotype DX®13 (Genomic Health, USA) or Mammaprint®14 (Agendia, Amsterdam NL) multigene tests, it is unknown whether these also predict the efficacy of local therapy15.
The aim of this study was to investigate whether a risk-of-recurrence (ROR) score derived from the PAM-50-based 46-gene assay (Prosigna®) adds additional prognostic value to known clinical risk factors for local recurrence in early-stage breast cancer, and to evaluate its predictive value for the benefit of radiotherapy.
Methods
The ABCSG initiated a prospective randomized trial (ABCSG-8) comparing 5 years of adjuvant tamoxifen with sequential therapy consisting of tamoxifen for 2 years followed by anastrozole for 3 years in postmenopausal women with endocrine receptor-positive early-stage breast cancer16. Between January 1996 and June 2004, 3901 patients were enrolled (NCT00291759). A CONSORT diagram is available in the original publication16. Notably, 869 patients in ABCSG-8 were also included in a second randomization built into that trial, comparing radiotherapy with no radiotherapy after breast conservation (ABCSG-8A)8.
All patients provided written informed consent and the trial was approved by the relevant ethics committees, and done in accordance with the Declaration of Helsinki. Formalin-fixed, paraffin-embedded tumour blocks from 1620 patients from the ABCSG-8 trial were available for the PAM-50 multigene assay, with 1478 samples passing quality control standards17. Local therapy and relapse data for these patients were analysed in the present study.
Local therapy
In this multicentre trial, local therapy strategies followed established clinical practice guidelines, except the additional randomization to radiotherapy or no radiotherapy in selected patients with a favourable prognosis5. In short, all patients underwent BCS or mastectomy with resection-free margins (R0 definition according to centres’ preference). Sentinel node biopsy was performed in patients with clinically lymph node-negative disease, and axillary lymph node dissection in all with macrometastases or micrometastases. Patients who underwent mastectomy were excluded from the present analysis. All patients, except those randomized to no adjuvant radiotherapy within the ABCSG-8A subtrial, received radiotherapy after BCS. For consistency, patients who were randomized to no radiotherapy after BCS within ABCSG-8A were not included in the present analysis except for the interaction test of ROR and radiotherapy (Fig. S1).
Follow-up
All patients were routinely followed up every 3 months throughout the first year, at 6-month intervals through the second and third years, and yearly thereafter. Physical examination by breast palpation, gynaecological examination, thoracic X-ray, abdominal ultrasonography or CT of the chest and abdomen, and mammography were used as appropriate to screen for local and distant recurrence. Overall survival was recorded by documented deaths. Local relapses documented as events for this study were usually confirmed histologically.
Definition of local recurrence as endpoint
Local recurrence was defined as in-breast or chest wall recurrence. Regional recurrence, distant recurrence, and deaths from breast cancer or second primary carcinoma, and deaths without breast cancer or with unknown breast cancer status, were considered competing-risk events.
PAM-50-based Prosigna® test
A detailed description of the PAM-50-based 46-gene Prosigna® assay, and the conversion of gene expression measurements into ROR scores and intrinsic molecular subtypes has been reported elsewhere11,18. In short, RNA was extracted from formalin-fixed, paraffin-embedded blocks from breast cancer excision specimens from the ABCSG-8 trial. The PAM-50 assay simultaneously measures the expression levels of 50 target genes plus eight housekeeping genes in a single hybridization reaction18.
Statistical analysis
The primary objective of the study was to investigate whether the ROR score can provide prognostic information on local recurrence. Secondary objectives were to evaluate whether such prognostic information can be gained from intrinsic subtypes, and whether the ROR score is predictive of radiotherapy benefit. The data were analysed in a competing-risk framework with local recurrences as the events of interest, and regional recurrences, distant metastases, secondary malignancies, and death as competing events. Fine–Gray subdistribution hazard models were used to assess the effects of the ROR score, ROR score-derived risk groups, intrinsic subtypes, and individual prognostic factors, such as age (continuous), tumour size (T1 versus T2 or T3), nodal status (N0 versus N1–N3), treatment (tamoxifen or tamoxifen followed by anastrozole), and progesterone receptor status (positive versus negative), as well as a possible interaction between ROR and radiotherapy. Summary statistics from Fine–Gray models19 include subhazard ratios (SHRs) with 95 per cent confidence intervals. A two-sided α value of 0.05 was used for all tests. Additionally, 5- and 10-year cumulative rates of local recurrence were estimated (with 95 per cent confidence intervals). In all analyses, data from the full investigational period were considered, except for the univariable comparisons of risk groups, which were censored at 5 and 10 years. Censoring at 5 and 10 years was done to ensure that estimated SHRs and P values were based on the data up to these time points.
Patients were initially assigned to one of three ROR score-derived risk groups using the original cut-off values defined for distant recurrence. They were divided into three groups with low, intermediate, and high risk, and were also dichotomized by combinations of high–intermediate and intermediate–low risk. To assess whether classification into risk groups based on local relapses was possible, the patients were recategorized into a low- and a high-risk group using a receiver operating characteristic (ROC) curve for local recurrence data (time-dependent ROC curves) and the Youden index20. In contrast to distant recurrence, no difference in 10-year risk was observed between patients with node-negative and node-positive disease with respect to local recurrence. Owing to the low number of events, a division into more than two risk groups was not deemed appropriate.
SAS® version 9.4 (SAS Institute, Cary, North Carolina, USA) was used for all analyses.
Results
Table 1 shows demographic data for 1034 patients undergoing BCS and radiotherapy. All patients were assigned randomly to either five years of tamoxifen (525) or tamoxifen followed by anastrozole (509) after surgery. There were no patients with high-grade (G3) breast cancer in this analysis because such women were not included in the ABCSG-8 feeder trial. Although nearly all of the patients had oestrogen receptor-positive disease as confirmed by immunohistochemistry, 36 (3.5 per cent) had either human epidermal growth factor receptor 2-enriched or basal-like breast cancer as evaluated by the PAM-50 multigene test.
Table 1.
Demographic data for patients with PAM-50 analyses randomized within the ABCSG 8 trial and who underwent breast-conserving surgery with subsequent radiotherapy
| Low risk (ROR score < 57) (n = 765) |
High risk (ROR score ≥ 57) (n = 269) |
Total (n = 1034) |
|
|---|---|---|---|
| Age (years) | |||
| < 65 | 478 (62.5) | 153 (56.9) | 631 (61.0) |
| ≥ 65 | 287 (37.5) | 116 (43.1) | 403 (39.0) |
| Tumour category | |||
| T1 | 612 (80.0) | 156 (58.0) | 768 (74.3) |
| T2 | 152 (19.9) | 112 (41.6) | 264 (25.5) |
| T3 | 1 (0.1) | 1 (0.4) | 2 (0.2) |
| Node category | |||
| N0 | 550 (71.9) | 175 (65.1) | 725 (70.1) |
| N1 | 195 (25.5) | 84 (31.2) | 279 (27.0) |
| N2/N3 | 20 (2.6) | 10 (3.7) | 30 (2.9) |
| Tumour grade | |||
| G1 | 173 (22.6) | 21 (7.8) | 194 (18.8) |
| G2 | 569 (74.4) | 239 (88.8) | 808 (78.1) |
| Unknown | 23 (3.0) | 9 (3.3) | 32 (3.1) |
| ER status | |||
| – | 7 (0.9) | 0 (0) | 7 (0.7) |
| + | 92 (12.0) | 29 (10.8) | 121 (11.7) |
| ++/+++ | 666 (87.1) | 240 (89.2) | 906 (87.6) |
| PgR status | |||
| – | 118 (15.4) | 61 (22.7) | 179 (17.3) |
| + | 140 (18.3) | 57 (21.2) | 197 (19.1) |
| ++/+++ | 507 (66.3) | 151 (56.1) | 658 (63.6) |
| Treatment | |||
| Tamoxifen* | 383 (50.1) | 142 (52.8) | 525 (50.8) |
| Tamoxifen/anastozole† | 382 (49.9) | 127 (47.2) | 509 (49.2) |
| Subtype | |||
| Luminal A | 694 (90.7) | 15 (5.6) | 709 (68.6) |
| Luminal B | 64 (8.4) | 225 (83.6) | 289 (27.9) |
| HER2-enriched | 2 (0.3) | 26 (9.7) | 28 (2.7) |
| Basal like | 5 (0.7) | 3 (1.1) | 8 (0.8) |
Values in parentheses are percentages. *Tamoxifen for 5 years; †tamoxifen for 2–3 years followed by anastrozole for 2–3 years. ROR, risk of recurrence; ER, oestrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 1.
There were 765 patients (74.0 per cent) with a low ROR score (below 57). A total of 709 patients (68.6 per cent) had luminal A tumours according to the multigene assay. Patients with high-risk cancer, as defined by the ROR score, tended to be older (aged at least 65 years), and had significantly larger and more intermediate-grade tumours.
Local recurrences
After a median follow-up of 10.8 years, a total of 23 local recurrence events were observed (Table S1), corresponding to an overall risk of local recurrence of 2.2 per cent. Distant recurrence was observed in 11.6 per cent of patients and 6.8 per cent died from breast cancer during follow-up. A secondary malignancy was diagnosed in 14.0 per cent, and 11.6 per cent died without breast cancer recurrence. In 1.1 per cent of patients with local recurrence, distant recurrences developed synchronously (1) or metachronously (14).
Risk-of-recurrence score, intrinsic subtype, and local recurrence risk
Univariable analyses of the continuous ROR score showed the prognostic value of PAM-50 for local recurrence; SHRs for a 10-point increase were 1.72 (95 per cent c.i. 1.38 to 2.14; P < 0.001) and 1.41 (1.11 to 1.77; P = 0.004) at 5 and 10 years respectively. This remained significant in multivariable analyses for the full period (SHR 1.40, 1.15 to 1.71; P = 0.001).
No significant differences between the three original ROR risk groups based on distant recurrence were observed at 10 years (high versus low risk: SHR 3.11, 0.84 to 11.49, P = 0.089; intermediate versus low risk: SHR 1.36, 0.30 to 6.07, P = 0.688). SHRs were not computable at 5 years owing to few events.
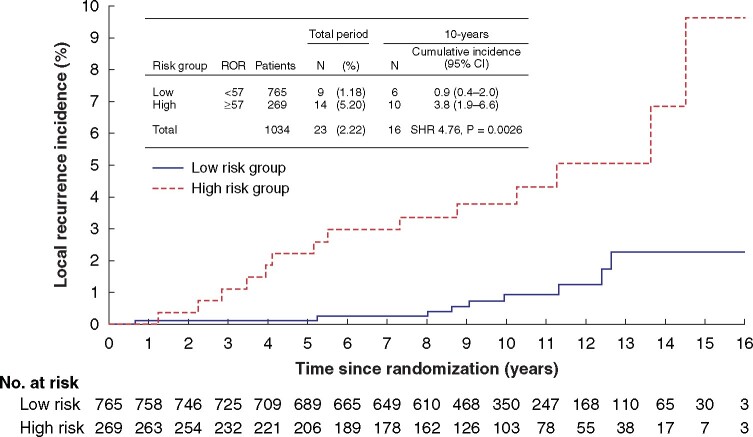
As these cut-offs based on distant metastases were not applicable, a cut-off based on local recurrences was calculated from the data using a time-dependent ROC curve (Fig. S2). According to these results, patients with ROR scores below 57 were considered to be at genomic low risk of local recurrence. Univariable analysis demonstrated that low-risk patients had a significantly lower incidence of local recurrence than those in the high-risk group at 5 years (0.1 (95 per cent c.i. 0 to 0.7) versus 2.2 (0.9 to 4.6) per cent respectively; subhazard ratio (SHR) 17.18, 95 per cent c.i. 2.06 to 142.88; P = 0.009) and 10 years (0.9 (0.4 to 2.0) versus 3.8 (1.9 to 6.6) per cent; SHR 4.76, 1.72 to 13.17; P = 0.003) (Fig. 1). Multivariable analyses including risk group, age, tumour size, nodal status, progesterone receptor status, tumor grade, and adjuvant treatment, confirmed that PAM-50 ROR score was an independent predictor for local recurrence (Table 2).
Fig. 1.
Cumulative incidence of local recurrence according to risk-of-recurrence score-derived risk group
Table 2.
Results of multivariable Fine–Gray subdistribution analysis including risk-of-recurrence score-derived risk groups for predicting local recurrence-free survival (full period)
| Subhazard ratio | P | |
|---|---|---|
| PgR status (positive versus negative) | 0.46 (0.19, 1.10) | 0.082 |
| Age (per year) | 0.98 (0.94, 1.03) | 0.505 |
| Grade (G2 or Gx versus G1) | 0.73 (0.23, 2.32) | 0.592 |
| Node status (N1–N3 versus N0) | 0.97 (0.39, 2.44) | 0.951 |
| PAM-50 risk group (high versus low)* | 4.60 (1.99, 10.63) | < 0.001 |
| Treatment (tamoxifen/anastozole† versus tamoxifen alone‡) | 1.00 (0.43, 2.31) | 0.997 |
| Tumour category (T2 or T3 versus T1) | 0.72 (0.30, 1.74) | 0.472 |
Values in parentheses are 95 per cent confidence intervals. The analysis included 1034 patients.
High risk, PAM-50 risk-of-recurrence score 57 or more.
Tamoxifen for 2–3 years followed by anastrozole for 2–3 years;
tamoxifen for 5 years.
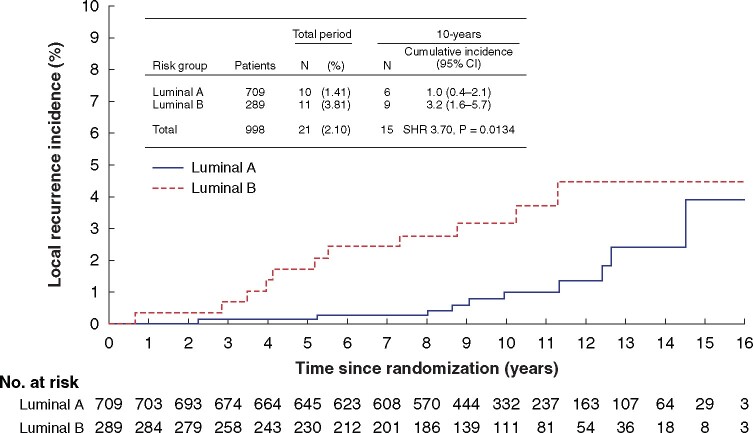
In the PAM-50 subtype assessment, univariable analysis showed an increased risk of local recurrence in patients with luminal B tumours compared with luminal A disease, at 5 years (1.7 (95 per cent c.i. 0.7 to 3.8) versus 0.1 (0 to 0.8) per cent respectively; SHR 12.33, 95 per cent c.i. 1.44 to 105.59; P = 0.022) and 10 years (3.2 (1.6 to 5.7) versus 1.0 (0.4 to 2.1) per cent; SHR 3.70, 1.31 to 10.41; P = 0.013) (Fig. 2). In the multivariable Fine–Gray subhazard model including clinical and pathological variables, definition of breast cancer subtypes by PAM-50 also reached statistical significance for the complete investigational period (Table 3).
Fig. 2.
Cumulative incidence of local recurrence according to luminal subtype
Table 3.
Results of multivariable Fine–Gray subdistribution analysis including luminal subtypes for predicting local recurrence-free survival (full period)
| Subhazard ratio | P | |
|---|---|---|
| PgR status (positive versus negative) | 0.41 (0.17, 1.03) | 0.058 |
| Age (per year) | 0.98 (0.94, 1.03) | 0.485 |
| Grade (G2 or Gx versus G1) | 0.78 (0.25, 2.47) | 0.675 |
| Node status (N1–N3 versus N0) | 1.06 (0.40, 2.79) | 0.909 |
| Luminal type B versus A) | 2.83 (1.11, 7.17) | 0.029 |
| Treatment (tamoxifen/anastozole* versus tamoxifen alone†) | 0.99 (0.41, 2.38) | 0.975 |
| Tumour category (T2 or T3 versus T1) | 1.04 (0.41, 2.60) | 0.937 |
Values in parentheses are 95 per cent confidence intervals. The analysis included 998 patients.
Tamoxifen for 2–3 years followed by anastrozole for 2–3 years;
tamoxifen for 5 years.
Interaction between risk-of-recurrence score and radiotherapy
The prognostic relevance of the PAM-50 assay for local recurrence after breast conservation was also seen in 170 women who did not received radiotherapy. The 5- and 10-year local recurrence rates were 14.6 and 20.8 per cent respectively among the 48 women without radiotherapy assigned to the high-risk group, compared with 1.6 and 5.0 per cent among 122 women in the low-risk group.
A Fine–Gray subhazard interaction model including ROR score, radiotherapy, and the respective interaction showed similar SHRs for a 10-point increase in ROR score among patients who received radiotherapy (SHR 1.37, 95 per cent 1.11 to 1.70) as well as for those who did not have radiotherapy (SHR 1.23, 0.98 to 1.54) (P = 0.479 for interaction), suggesting no predictive effect of PAM-50 for efficacy of radiotherapy (Table 4).
Table 4.
Fine–Gray subhazard interaction models including risk-of-recurrence score (continuous), risk group, and interaction with radiotherapy
| SHR | P | |
|---|---|---|
| Interaction between ROR score (continuous) and radiotherapy (SHR per 10-point risk increase) | 0.479 | |
| No radiation | 1.23 (0.98, 1.54) | |
| Radiation | 1.37 (1.11, 1.70) | |
| Interaction between ROR risk group and radiotherapy (SHR for high versus low risk) | 0.383 | |
| No radiation | 2.50 (1.04, 5.99) | |
| Radiation | 4.28 (1.85, 9.90) | |
| Interaction between ROR risk group and radiotherapy (SHR for radiotherapy versus no radiotherapy) | 0.383 | |
| ROR high risk | 0.23 (0.10, 0.53) | |
| ROR low risk | 0.13 (0.06, 0.32) |
Values in parentheses are 95 per cent confidence intervals. SHR, subhazard ratio; ROR, risk of recurrence.
Discussion
The present data show that the PAM-50 ROR score and intrinsic molecular subtypes can identify a genomic low-risk population within the group of patients with a clinically low risk of local recurrence (superlow risk of local recurrence). It has been shown that the PAM-50 ROR score is continuously related to the probability of disease recurrence. Consequently, cut-off points defining low- and high-risk disease strongly depend on the clinical setting and the clinical decision being made. Here an unbiased model-free approach was used to define a cut-off point for local recurrence risk based on the Youden’s index for a time-dependent ROC curve. This cut off-point (ROR score less than 57) is different from the commercial cut-off point for low risk of distant recurrence in the Prosigna® assay (ROR score less than 40 for patients with node-negative disease) and therefore requires further validation. Women with breast cancer who had a low-risk PAM-50 ROR score (below 57) after BCS and radiotherapy had a 0.9 per cent probability of local relapse within 10 years, whereas patients with an intrinsic luminal A subtype breast cancer had a 1.0 per cent risk of local recurrence. Multivariable analysis in the present study demonstrated that PAM-50 is prognostic for local recurrence risk, independently of clinical risk factors such as nodal status, age or tumour size. Some 74.0 per cent of patients had a low-risk ROR score, whereas 68.6 per cent of tumours were classified as luminal A. Both the PAM-50 ROR score and intrinsic subtypes were able to identify a subgroup of patients with a very good prognosis regarding local recurrence risk in whom de-escalation of therapy may be an option. Exploratory analysis of the ability of PAM-50 to predict the efficacy of adjuvant radiotherapy did not, however, show any such benefit.
Mammaprint® was the first multigene test evaluated for local recurrence prognosis in a retrospective data set14. Although this test had no significant prognostic efficacy for clinically high-risk patients, it was found to be an independent prognostic risk factor for clinically low-risk patients and identified a subgroup with superlow local recurrence risk14. In the Mammaprint® trial14, the local recurrence risk after 10 years in the low-risk category was 6.1 per cent, whereas in the present study the PAM-50 superlow-risk group had a 10-year local recurrence risk of 0.9 per cent. Similarly, the authors’ group12 previously reported a 10-year local recurrence risk of 1.4 per cent for the Endopredict® low-risk group. Thus, the latter two tests seem to perform better in this respect; alternatively, the difference may reflect the superiority of more recent local therapy and differences in clinical risk parameters between studies.
Oncotype DX® has been shown to be an independent prognostic tool for identification of a low-risk population for local recurrence within a clinically low-risk group of patients aged less than 50 years13. This low-risk group had a 10-year local recurrence risk of 1.6 per cent with adjuvant chemotherapy and tamoxifen, and 4.3 per cent with tamoxifen alone. The latter group had adjuvant treatment similar to the patients in the present study. As the 10-year local recurrence risk was 0.9 per cent with endocrine therapy alone, a slightly better performance for PAM-50 regarding local recurrence risk prognosis can be assumed. Moreover, Oncotype DX® did not provide statistically significant prognostic information for patients older than 50 years (10-year local recurrence risk 3.6 per cent in low-risk group, 3.7 per cent in intermediate-risk group, and 4.8 per cent in high-risk group)13, whereas the present study was based on a trial of postmenopausal women, the vast majority of whom were aged over 50 years. Notably, of all data reported, the Oncotype DX® intermediate-risk group receiving placebo in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial13 had the highest local recurrence rate (20 per cent versus 10 per cent in low-risk and 18 per cent in high-risk groups) suggesting that intermediate groups are of no clear value.
Indirect comparisons between studies should be interpreted with great caution. Inclusion criteria for the trials analysed were different regarding age and tumour grade, with more G3 tumours and younger patients in the Mammaprint® and Oncotype DX® trials. The present results suggest that PAM-50 and Endopredict® are two possible multigene tests that clearly define a very low-risk group for local recurrence among postmenopausal women with a clinically low risk of breast cancer.
To investigate whether the PAM-50 test may predict the efficacy of radiotherapy after breast conservation, a subset of 170 women in the ABCSG-8 trial who did not receive adjuvant radiotherapy was analysed. These women were not included in the general risk score assessment in the present study. The 10-year local recurrence risk of only 5.0 per cent in the group of 122 patients with a low risk based on PAM-50 and clinical features, and who did not receive adjuvant radiotherapy, indicates that the option of no further radiotherapy can be discussed for this specific patient group. However the reduction of local recurrence rate in the low-risk group showed a similar hazard ratio compared with the high-risk group SHRs were compared between women who did or not receive radiotherapy in the groups with low- and high-risk scores. There was no significant difference between SHRs, and radiotherapy improved local recurrence-free survival in both groups. Owing to the limited number of patients and events, however, no final conclusions can be drawn from this exploratory analysis.
Strengths of this study are the use of a prospectively controlled data set with a subset randomized to omission of radiotherapy, the large number of tumour blocks analysed, and the long follow-up of more than 10 years. Limitations include the retrospective nature of this analysis, and the small number of events (although the latter may be indicative of an excellent local treatment modality and may be a good quality indicator). The results have shown the possibility of classification into risk groups based on local recurrences. However, the validity of separation into risk groups needs to be determined and confirmed in larger cohorts with more events. The same is true regarding the potential predictive role of PAM-50 in terms of radiotherapy benefit.
The present study has demonstrated that a PAM-50-based assay can be used as a prognostic tool for local recurrence risk in postmenopausal women with hormone receptor- positive breast cancer treated with endocrine therapy. PAM-50 has the ability to accurately and reliably define a superlow-risk population within this low-risk group of women with breast cancer. This justifies a prospective trial comparing patients with and without adjuvant radiotherapy in the low-risk group.
Supplementary Material
Acknowledgements
The study was funded by AstraZeneca, the ABCSG, and Nanostring Technologies (Seattle, Washington, USA).
Supplementary material
Supplementary material is available at BJS online.
Disclosure. The authors declare no conflicts of interest.
References
- 1. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266–273 [DOI] [PubMed] [Google Scholar]
- 2. Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA,, McCormick B et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol 2013;31:2382–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med 2004;351:971–977 [DOI] [PubMed] [Google Scholar]
- 4. Blamey RW, Bates T, Chetty U, Duffy SW, Ellis IO, George D et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 2013;49:2294–2302 [DOI] [PubMed] [Google Scholar]
- 5. Potter R, Gnant M, Kwasny W, Tausch C, Handl-Zeller L, Pakisch B et al. Lumpectomy plus tamoxifen or anastrozole with or without whole breast irradiation in women with favorable early breast cancer. Int J Radiat Oncol Biol Phys 2007;68:334–340 [DOI] [PubMed] [Google Scholar]
- 6. Hind D, Wyld L, Reed MW. Surgery, with or without tamoxifen, vs tamoxifen alone for older women with operable breast cancer: Cochrane review. Br J Cancer 2007;96:1025–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chakrabarti J, Kenny FS, Syed BM, Robertson JF, Blamey RW, Cheung KL. A randomised trial of mastectomy only versus tamoxifen for treating elderly patients with operable primary breast cancer-final results at 20-year follow-up. Crit Rev Oncol Hematol 2011;78:260–264 [DOI] [PubMed] [Google Scholar]
- 8. Fastner G, Sedlmayer F, Widder J, Metz M, Geinitz H, Kapp K. et al. Endocrine therapy with or without whole breast irradiation in low-risk breast cancer patients after breast-conserving surgery: 10-year results of the Austrian Breast and Colorectal Cancer Study Group 8A trial. Eur J Cancer 2020;127:12–20 [DOI] [PubMed] [Google Scholar]
- 9. Sestak I, Cuzick J, Dowsett M, Lopez-Knowles E, Filipits M, Dubsky P. et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian Breast and Colorectal Cancer Study Group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015;33:916–922 [DOI] [PubMed] [Google Scholar]
- 10. Dowsett M, Sestak I, Lopez-Knowles E, Sidhu K, Dunbier AK, Cowens JW. et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol 2013;31:2783–2790 [DOI] [PubMed] [Google Scholar]
- 11. Gnant M, Filipits M, Greil R, Stoeger H, Rudas M, Bago-Horvath Z et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014;25:339–345 [DOI] [PubMed] [Google Scholar]
- 12. Fitzal F, Filipits M, Rudas M, Greil R, Dietze O, Samonigg H et al. The genomic expression test EndoPredict is a prognostic tool for identifying risk of local recurrence in postmenopausal endocrine receptor-positive, her2neu-negative breast cancer patients randomised within the prospective ABCSG 8 trial. Br J Cancer 2015;112:1405–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol 2010;28:1677–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drukker CA, Elias SG, Nijenhuis MV, Wesseling J, Bartelink H, Elkhuizen P et al. Gene expression profiling to predict the risk of locoregional recurrence in breast cancer: a pooled analysis. Breast Cancer Res Treat 2014;148:599–613 [DOI] [PubMed] [Google Scholar]
- 15. Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P et al. Members of the St. Gallen International Consensus Panel on the Primary Therapy of Early Breast Cancer 2019. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 2019;30:1541–1557 [DOI] [PubMed] [Google Scholar]
- 16. Dubsky PC, Jakesz R, Mlineritsch B, Postlberger S, Samonigg H, Kwasny W et al. Tamoxifen and anastrozole as a sequencing strategy: a randomized controlled trial in postmenopausal patients with endocrine-responsive early breast cancer from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 2012;30:722–728 [DOI] [PubMed] [Google Scholar]
- 17. Gnant M, Sestak I, Filipits M, Dowsett M, Balic M, Lopez-Knowles E et al. Identifying clinically relevant prognostic subgroups of postmenopausal women with node-positive hormone receptor-positive early-stage breast cancer treated with endocrine therapy: a combined analysis of ABCSG-8 and ATAC using the PAM50 risk of recurrence score and intrinsic subtype. Ann Oncol 2015;26:1685–1691 [DOI] [PubMed] [Google Scholar]
- 18. Wallden B, Storhoff J, Nielsen T, Dowidar N, Schaper C, Ferree S et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med Genomics 2015;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 20. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.