Abstract
Simultaneous pancreas and kidney transplantation is the optimum treatment for patients with type 1 diabetes and renal failure, providing survival benefit over deceased donor kidney transplant alone. Here the authors demonstrate that utilization of donation after circulatory death pancreases is a safe approach to expanding the donor pool with equivalent results to donation after brainstem death transplantation. They also demonstrate that pancreas transplantation after normothermic regional perfusion is feasible, but it will require ongoing prospective study to ensure that the benefits seen for liver transplantation do not come at the expense of pancreas transplant outcomes.
Introduction
Simultaneous pancreas and kidney transplantation is the optimal treatment for patients with type 1 diabetes and renal failure, providing survival benefit over deceased kidney transplant alone, and improved quality of life1,2. Waiting list mortality is compounded by a shortage of donor organs and high discard rates3,4. To address this, donation after circulatory death (DCD) donors have been increasingly used and now account for about 30 per cent of all simultaneous pancreas and kidney transplantations in the UK. Marked variation in the utilization of DCD pancreases exists3,4, which may reflect a perception that DCD grafts are ‘high risk’ compared to organs procured from brainstem dead donors due to additional warm ischaemia. Other factors include differences in withdrawal of life support and variations in the legality of antemortem interventions5. The authors’ early experience was similar to that of others6,7, in that there was no difference in short-term survival between those receiving grafts from donation after brainstem death (DBD) or conventional DCD (sDCD) donors.
Normothermic regional perfusion is a promising technique to reduce the additional ischaemic insult associated with DCD by placing the donor on a modified extracorporeal membrane oxygenator circuit in order to restore circulation of oxygenated blood to the organs following cardiorespiratory arrest. In liver transplantation, normothermic regional perfusion leads to superior outcomes compared with sDCD8–10. It is unclear if the benefits of normothermic regional perfusion extend to DCD pancreas transplantation.
The aim of this study was to evaluate a decade of a DCD pancreas transplant programme and a cohort of DCD pancreas transplants performed with or without normothermic regional perfusion.
Methods
All consecutive simultaneous pancreas and kidney transplantations performed at Addenbrooke’s Hospital, Cambridge, UK from 1 August 2008 to 31 July 2018 were included in this study. Full methodology is provided in detail in the supplementary material.
Results
A total of 211 patients (139 DBD and 72 DCD, of which 59 were sDCD and 13 normothermic regional perfusion) were included. The donor, recipient and transplant characteristics are summarized in Table S1.
Patient and allograft survival
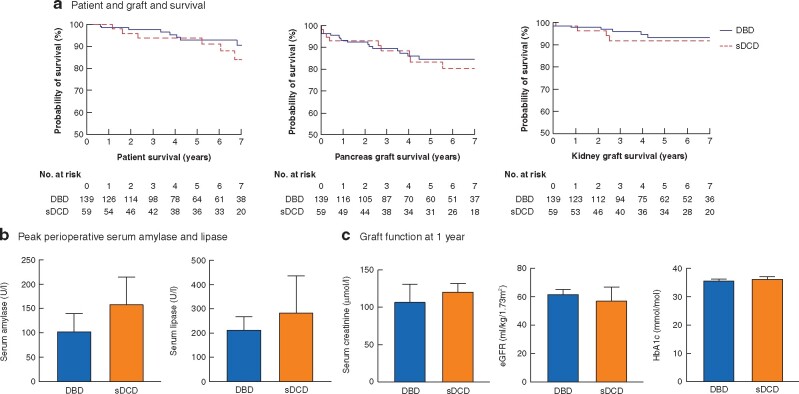
Patient survival at 1, 3, 5 and 10 years was 99.0, 96.6, 93.4 and 84.3 per cent respectively, with no significant difference between those receiving DBD or sDCD grafts (Fig. 1a). Death-censored pancreas and kidney graft survival at 5 years was 83.9 and 93.2 per cent respectively, with no significant difference between sDCD and DBD cohorts (Fig. 1a).
Fig. 1.
Outcomes of simultaneous pancreas and kidney transplantation from conventional donation after circulatory death compared with donation after brainstem death.
a Kaplan–Meier plots of unadjusted patient and death censored graft survival. There was no significant difference in patient (P = 0.754), pancreas (P = 0.876) or kidney graft survival (P = 0.628) between those recipients receiving grafts from donation after brainstem death (DBD), conventional donation after circulatory death (sDCD) or normothermic regional perfusion donors (Mantel–Cox tests). b Graph of median (with 95 per cent confidence intervals) peak serum amylase and lipase levels measured in days 0–3; levels of amylase and lipase were significantly lower in DBD compared with sDCD (P = 0.050 and P = 0.040 respectively; Mann–Whitney U tests). c Graphs of median (with 95 per cent confidence intervals) serum creatinine, estimated glomerular filtration rate (eGFR) and glycated haemoglobin (HbA1c). There was no significant difference between DBD and sDCD cohorts in terms of serum creatinine (P = 0.085), eGFR (P = 0.252) or HbA1c (P = 0.585) at 1 year (Mann–Whitney U tests).
Delayed graft function (DGF) occurred in 33.3 per cent of renal grafts and 3.5 per cent of pancreatic grafts (Table S2). The rate of renal, but not pancreatic, DGF was significantly higher in the sDCD compared with the DBD cohort (Table S3). Serum levels of pancreatic enzymes were significantly lower in days 0–3 in the DBD compared with the DCD cohort (Fig. 1b). There was no significant difference in the serum creatinine, estimated glomerular filtration rate (eGFR) or glycated haemoglobin (HbA1c) at 1 year between groups (Fig. 1c). Rates of graft losses, thrombosis, length of stay, reoperation and episodes of rejection are included in the supplementary results (Fig. S1, Tables S3 and S4).
Outcomes of the normothermic regional perfusion cohort
There was no significant difference in patient or graft survival between sDCD or normothermic regional perfusion donors (Fig. S2a), nor in the rates of primary non-function, DGF, thrombosis, episodes of acute rejection, reoperation or readmission between sDCD or normothermic regional perfusion cohorts (Table S4). Peak serum lipase, but not amylase, levels were significantly lower in patients receiving normothermic regional perfusion organs compared with sDCD (Fig. S2b). There was no significant difference between sDCD and normothermic regional perfusion cohorts in terms of serum creatinine, eGFR or HbA1c at 1 year (Fig. S2c).
Discussion
In this series of DCD simultaneous pancreas and kidney transplantation, long-term follow-up data demonstrate that patient and graft survival are equivalent for sDCD and DBD organs with no difference in graft function at 1 year. Utilization of DCD pancreases is a safe approach to expanding the donor pool with equivalent results to DBD transplantation. Also, pancreas transplantation after normothermic regional perfusion is feasible, but requires on-going prospective study to ensure that the benefits seen for liver transplantation do not come at the expense of pancreas transplant outcomes.
All outcome data for sDCD and DBD simultaneous pancreas and kidney transplantation were similar in the current series, other than the incidence of kidney DGF, which was higher for patients receiving an sDCD simultaneous pancreas and kidney transplantation (26.6 per cent versus 49.2 per cent; Table S3). This mirrors the UK rate of 49 per cent seen with isolated DCD renal transplantation11. sDCD transplantation was not associated with increased graft loss, major ureteric complications, rejection episodes or poorer kidney graft function at 1 year (Table S3).
While appropriate selection of donors and minimizing cold ischaemia time underpins successful DCD outcomes12, the authors think it unlikely that the comparable results achieved for DBD and DCD organs is attributable to stringent selection criteria for DCD organs – Cambridge has the lowest rate of declining DCD pancreases of any UK centre3 and the median Pancreas Donor Risk Index is representative of previous UK13, Eurotransplant14 and US4 data. Of the 45 normothermic regional perfusion donors under 50 years of age, almost half resulted in a pancreas transplant. Furthermore, although it is standard practice to abandon DCD pancreas retrieval if the donor has not reached asystole within an hour from withdrawal of life-supporting treatment, in the current series 12.2 per cent of sDCD pancreases were retrieved from donors with withdrawal of life-supporting treatment of more than 100 minutes, with the longest more than 400 minutes. Patient and graft outcomes were not different in this cohort (data not shown), in accord with previous findings for isolated kidney transplants with a prolonged agonal phase15.
Others have noted higher rates of graft thrombosis in DCD pancreas transplantation16, but this was not observed in the present series. Most episodes were incidental findings on CT (86.8 per cent) and treated non-operatively with systemic anticoagulation alone (73.7 per cent). Only 4.5 per cent of patients required operative intervention and this did not differ significantly between DBD and sDCD cohorts (Supplementary information). This fits with previous work demonstrating that most thrombi can be managed successfully with systemic anticoagulation17.
The present study represents a large experience of pancreas transplantations following normothermic regional perfusion. Although a small cohort, this experience nevertheless accounts for about 70 per cent of the current UK experience. The findings indicate that pancreas transplantation following normothermic regional perfusion is both feasible and offers comparable outcomes. Others have previously reported improved renal outcomes in recipients of normothermic regional perfusion compared with sDCD grafts9,18, but whether this is also seen in the setting of simultaneous pancreas and kidney transplantation will only become evident as experience accrues.
Lower levels of both amylase and lipase were seen in recipients of grafts from DBD compared with sDCD donors (Fig. 1b). Serum lipase, but not amylase, levels were also significantly lower in the normothermic regional perfusion cohort compared with sDCD (Fig. S2b), which may suggest less severe graft pancreatitis19. This warrants further study to confirm or refute this observation.
Given the waiting list mortality and known survival benefits of simultaneous pancreas and kidney transplantation compared with renal transplant alone for diabetic patients1,12, it is difficult to justify the large discrepancies in utilization of DCD pancreases3,4. As with other organs, this may have resulted from a cognitive bias, whereby a single poor outcome has disproportionately influenced the perception of the risks associated with DCD transplantation20.
Supplementary Material
Acknowledgements
J.A.R. and J.L.R. are joint first authors. C.W. and G.P. are joint senior authors. J.A.R., J.L.R., C.W. and G.P. made a substantial contribution to the conception and writing of this manuscript. J.A.R., J.L.R., A.F., S.P., S.C. and G.D. made substantial contribution to data collection. J.A.R. analysed the data. All authors reviewed, contributed to and commented on the final draft of the manuscript.
Funding
The authors would like to acknowledge support from the National Institute for Health Research (NIHR) Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The University of Cambridge has received salary support in respect of C.J.E.W. from the NHS in the East of England through the Clinical Academic Reserve. J.R. was supported by a National Institute of Health Research (NIHR) Academic Clinical Lectureship.
Disclosure. The authors of this manuscript have no conflicts of interest to disclose.
Supplementary material
Supplementary material is available at BJS online
References
- 1. Esmeijer K, Hoogeveen EK, van den Boog PJM, Konijn C, Mallat MJK, Baranski AG. et al. Superior long-term survival for simultaneous pancreas–kidney transplantation as renal replacement therapy: 30-year follow-up of a nationwide cohort. Diabetes Care 2020;43:321–328 [DOI] [PubMed] [Google Scholar]
- 2. Barlow AD, Saeb-Parsy K, Watson CJE.. An analysis of the survival outcomes of simultaneous pancreas and kidney transplantation compared to live donor kidney transplantation in patients with type 1 diabetes: a UK Transplant Registry study. Transpl Int 2017;30:884–892 [DOI] [PubMed] [Google Scholar]
- 3. NHSBT. Annual Report on Pancreas and Islet Transplantation. 2019. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/16776/nhsbt-pancreas-and-islet-transplantation-annual-report-2018-2019.pdf (accessed 7 June 2020)
- 4. Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Urban R, Fox A. et al. OPTN/SRTR 2017 annual data report: pancreas. Am J Transplant 2019;19:124–183 [DOI] [PubMed] [Google Scholar]
- 5. Hodgson R, Young AL, Attia MA, Lodge JPA.. Impact of a national controlled donation after circulatory death (DCD) program on organ donation in the United Kingdom: a 10-year study. Am J Transplant 2017;17:3172–3182 [DOI] [PubMed] [Google Scholar]
- 6. Bellingham JM, Santhanakrishnan C, Neidlinger N, Wai P, Kim J, Niederhaus S. et al. Donation after cardiac death: a 29-year experience. Surgery 2011;150:692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qureshi MS, Callaghan CJ, Bradley JA, Watson CJ, Pettigrew GJ.. Outcomes of simultaneous pancreas–kidney transplantation from brain-dead and controlled circulatory death donors. Br J Surg 2012;99:831–838 [DOI] [PubMed] [Google Scholar]
- 8. Watson CJE, Hunt F, Messer S, Currie I, Large S, Sutherland A. et al. In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transplant 2019;19:1745–1758 [DOI] [PubMed] [Google Scholar]
- 9. Miñambres E, Suberviola B, Dominguez-Gil B, Rodrigo E, Ruiz-San Millan JC, Rodríguez-San Juan JC. et al. Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant 2017;17:2165–2172 [DOI] [PubMed] [Google Scholar]
- 10. Hessheimer AJ, Coll E, Torres F, Ruíz P, Gastaca M, Rivas JI. et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol 2019;70:658–665 [DOI] [PubMed] [Google Scholar]
- 11. Summers DM, Watson CJ, Pettigrew GJ, Johnson RJ, Collett D, Neuberger JM. et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int 2015;88:241–249 [DOI] [PubMed] [Google Scholar]
- 12. Kopp WH, Verhagen MJJ, Blok JJ, Huurman VAL, de Fijter JW, de Koning EJ. et al. Thirty years of pancreas transplantation at Leiden University Medical Center. Transplantation 2015;99:e145–e151 [DOI] [PubMed] [Google Scholar]
- 13. Muthusamy ASR, Mumford L, Hudson A, Fuggle SV, Friend PJ.. Pancreas transplantation from donors after circulatory death from the United Kingdom. Am J Transplant 2012;12:2150–2156 [DOI] [PubMed] [Google Scholar]
- 14. Kopp WH, de Vries E, de Boer J, Putter H, Schareck W, Samuel U. et al. Donor risk indices in pancreas allocation in the Eurotransplant region. Transpl Int 2016;29:921–929 [DOI] [PubMed] [Google Scholar]
- 15. Reid AWN, Harper S, Jackson CH, Wells AC, Summers DM, Gjorgjimajkoska O. et al. Expansion of the kidney donor pool by using cardiac death donors with prolonged time to cardiorespiratory arrest. Am J Transplant 2011;11:995–1005 [DOI] [PubMed] [Google Scholar]
- 16. Mittal S, Gilbert J, Friend PJ.. Donors after circulatory death pancreas transplantation. Curr Opin Organ Transplant 2017;22:372–376 [DOI] [PubMed] [Google Scholar]
- 17. Hakeem A, Chen J, Iype S, Clatworthy MR, Watson CJE, Godfrey EM. et al. Pancreatic allograft thrombosis: suggestion for a CT grading system and management algorithm. Am J Transplant 2018;18:163–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Demiselle J, Augusto JF, Videcoq M, Legeard E, Dubé L, Templier F. et al. Transplantation of kidneys from uncontrolled donation after circulatory determination of death: comparison with brain death donors with or without extended criteria and impact of normothermic regional perfusion. Transpl Int 2016;29:432–442 [DOI] [PubMed] [Google Scholar]
- 19. Nadalin S, Girotti P, Königsrainer A.. Risk factors for and management of graft pancreatitis. Curr Opin Organ Transplant 2013;18:89–96 [DOI] [PubMed] [Google Scholar]
- 20. Taylor R, Allen E, Richards JA, Goh MA, Neuberger J, Collett D. et al. Survival advantage for patients accepting the offer of a circulatory death liver transplant. J Hepatol 2019;70:855–865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.