Abstract
This prospective nationwide cohort study examined the feasibility of a watchful-waiting protocol for non-functional pancreatic neuroendocrine tumours (NF-pNET) of 2 cm or smaller. In total, 8 of 76 patients (11 per cent) with a NF-pNET no larger than 2 cm showed significant tumour progression (more than 0.5 cm/year) during 17 months of follow-up, of whom two opted for resection. No patient developed metastases. Quality of life was poorer than in the reference population. Watchful waiting seems a safe alternative to upfront surgery in patients with a NF-pNET no larger than 2 cm, although longer follow-up is necessary.
Introduction
Non-functional pancreatic neuroendocrine tumours (NF-pNETs) are rare neoplasms, often detected incidentally. The prognosis varies and is largely dependent on the Ki-67 proliferation index, presence of a genetic syndrome, lymph node involvement, and tumour size1–4. Resection of pancreatic lesions is associated with significant morbidity, and may include long-term complications such as new-onset diabetes and exocrine pancreatic insufficiency5,6. Clearly, the potential survival benefit obtained with surgery needs to outweigh the morbidity associated with pancreatic surgery. This explains the current controversy regarding small (2 cm or less) asymptomatic NF-pNETs, for which some advocate surgery and others suggest a conservative approach7–11.
Based on retrospective data, guidelines advise watchful waiting for NF-pNETs of 2 cm or smaller, but provide no clear recommendation on the required follow-up4,12. Therefore, the objective of this study was to prospectively evaluate disease-related outcomes and quality of life (QoL) after implementation of a nationwide, watchful-waiting programme for NF-pNETs no larger than 2 cm. The study also study sought to evaluate the feasibility of the proposed follow-up protocol, as well as adherence to the protocol in participating centres.
Methods
This was an interim analysis of the multicentre prospective PANDORA study of the Dutch Pancreatic Cancer Group. Full details of the study design, methods employed, and statistical analysis can be found in Appendix S1. All patients with sporadic, asymptomatic NF-pNETs of 2 cm or smaller were included if they met the eligibility criteria, in particular absence of nodal and/or distant metastases. The trial was registered in the Netherlands Trial Register (NL6510).
Patients were enrolled into a watchful-waiting protocol to monitor tumour progression (Fig. 1). Surgical resection was recommended if patients developed symptoms, tumour growth exceeding 0.5 cm/year, total tumour size greater 2 cm, pathological lymph node enlargement, vascular involvement or infiltration into surrounding organs, or pancreatic duct dilatation, or if the patient expressed a strong preference for operation.
Fig. 1.
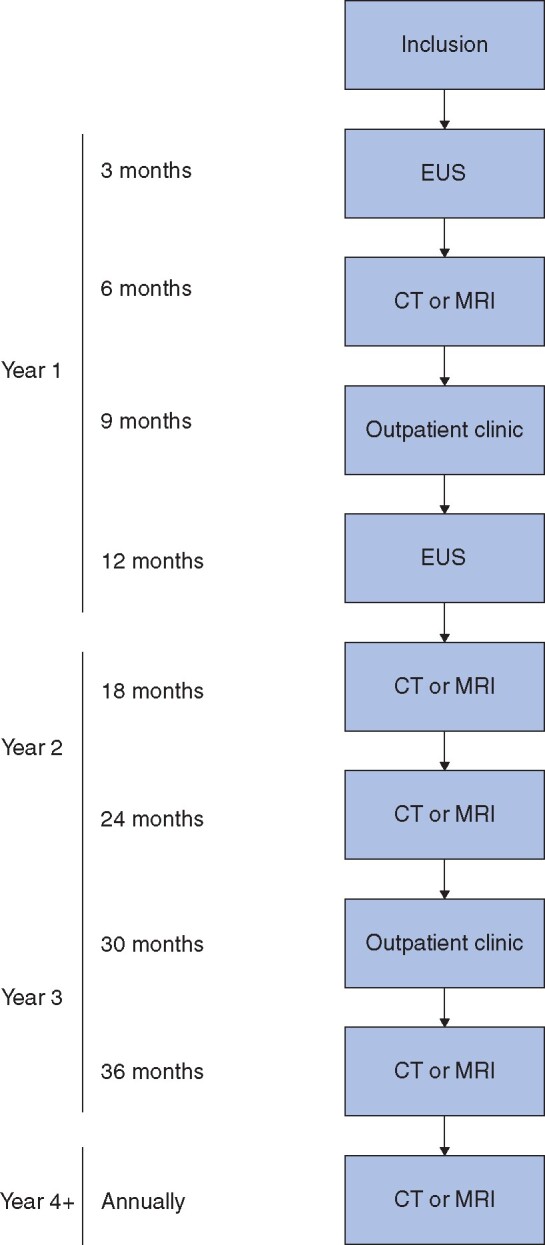
Follow-up protocol for watchful waiting of patients with a non-functional pancreatic neuroendocrine tumour of 2 cm or smaller included in the PANDORA study
EUS, endoscopic ultrasonography.
Results
Between 1 January 2017 and 29 February 2020, a total of 76 patients with a NF-pNET no larger than 2 cm were included. Baseline characteristics are summarized in Table S1. During a median follow-up of 17 (i.q.r. 8–35) months, 68 participants (89 per cent) had no signs of tumour progression. Eight patients (11 per cent) showed tumour progression exceeding 0.5 cm/year, and two also had a final tumour size of more than 2.0 cm. No other tumours larger than 2.0 cm were noted, and 21 patients (28 per cent) had tumours smaller than 1.0 cm. Characteristics of patients with progression are shown in Table 1 and Table S1.
Table 1.
Follow-up of patients with progressive non-functional pancreatic neuroendocrine tumours
|
No. of patients*
(n = 8) |
|
|---|---|
| Clinical characteristics | |
| Tumour size at last follow-up (cm)† | 1.2 (0.7) |
| Time to progression (months)‡ | 17 (13–30) |
| Duration of follow-up (months)‡ | 24 (9–61) |
| Developed symptoms | 0 |
| Developed metastases | |
| None | 7 |
| Lymph node + peritoneal | 1 |
| Surgical resection | |
| Type of surgery | |
| Spleen-preserving distal pancreatectomy | 1 |
| Enucleation | 1 |
| Surgical approach | |
| Open | 1 |
| Laparoscopic | 0 |
| Clavien–Dindo ≥ grade III complications | 1 |
| Postoperative histopathology | |
| Positive lymph nodes | 1 |
| Ki-67 index (%) | |
| < 3 | 1 |
| 3–20 | 1 |
| > 20 | 0 |
*Unless indicated otherwise; values are
†s.d. and
‡median (i.q.r.).
Overall, six patients (8 per cent) underwent surgery during follow-up (Table S2). Two patients had surgery because of significant tumour growth, detected after 3 and 10 months of follow-up. One patient had tumour progression of 0.8 cm in 1 year (from 1.8 to 2.6 cm). Gallium-68 DOTATATE PET–CT showed two enlarged lymph nodes (aortocaval and para-aortal). During surgery, one unexpected peritoneal deposit was identified and the patient underwent laparoscopic enucleation of the primary tumour with lymphadenectomy and removal of the peritoneal lesion. The final histopathological diagnosis was a pNET of 2.0 cm, with a Ki-67 index of 5–10 per cent, two positive lymph nodes and, indeed, peritoneal metastasis. Two new lymph nodes were detected 11 months after surgery, for which somatostatin analogue therapy was started.
The second patient showed tumour progression exceeding 0.5 cm (from 1.0 to 1.7 cm) within 3 months of follow-up and as a result underwent surgery. The final histopathological diagnosis showed a pNET of 1.7 cm, R0 resection, with a Ki-67 index of less than 3 per cent, and 0 of 17 positive lymph nodes. The patient is currently asymptomatic at 11 months’ follow-up without signs of disease progression.
Three patients had a pNET resected despite lacking an indication according to the study protocol. Of these, two underwent spleen-resecting distal pancreatectomy, citing fear of disease progression as the predominant reason for requesting surgery. One patient underwent laparoscopic spleen-resecting distal pancreatectomy because the surgeon did not support the decision for watchful waiting and advocated tumour resection. All three patients had a pNET on final histopathology.
A fourth patient had a pNET enucleated owing to uncertainty regarding the pNET diagnosis on delayed (contrast-enhanced) endoscopic ultrasonography (EUS) at 8 months’ follow-up. The final histopathological report showed an intravascular pyogenic granuloma, but no pNET.
In total, four patients died, all from non-pNET-related causes.
Although the study protocol recommended confirmation of the diagnosis to by at least 2 different imaging modalities, only one type of imaging was used at the time of diagnosis in 19 patients (25 per cent). Thirty-two patients (42 per cent) had two, and 25 (33 per cent) had three or more imaging modalities to confirm the diagnosis. Only 17 patients (22 per cent) underwent EUS at the suggested 3-month time point. Instead, patients opted for CT (31, 41 per cent), MRI (16, 21 per cent), or no imaging at all (12, 16 per cent). At 6 and 12 months, 21 (28 per cent) and 11 (15 per cent) patients did not undergo any imaging.
QoL scores on the European Organisation for Research and Treatment of Cancer QLQ-C30 questionnaire were statistically significantly worse at baseline for the study population compared with the mean of the reference population regarding emotional functioning (83.9 versus 89.0; P = 0.042), nausea and vomiting (6.9 versus 2.7; P = 0.037), dyspnoea (18.8 versus 7.1; P = 0.004), and insomnia (22.9 versus 14.0; P = 0.046) (Fig. S1).
Discussion
This multicentre prospective cohort study, which evaluated watchful waiting for NF-pNETs no larger than 2 cm, found that short-term follow-up is both safe and feasible. A small proportion of patients showed tumour progression. Application of a watchful-waiting protocol successfully prevented surgery in over 9 of 10 patients. Furthermore, heterogeneity in pNET management, despite use of a study protocol, was observed in this study, along with poor QoL at the time of diagnosis.
The present finding of slow tumour progression supports previous studies9,11,13–17 of NF-pNETs of 2 cm or smaller, which advised wait-and-see in certain patients. In contrast, other authors18 have recommended upfront surgery for all pNETs, as even small lesions may have malignant characteristics that could impair survival. Importantly, patients with malignant tumour features were excluded from the present study, and, even when significant tumour growth occurred, six of eight patients with tumour progression refused surgery and opted to continue watchful waiting. Collectively, these results indicate that, under strict criteria, patients with a NF-pNET no larger than 2 cm can safely be treated conservatively.
In turn, it is clear that implementation of this novel watchful-waiting approach to pNET is challenging19–21. As is common in investigator-driven multicentre studies, not all centres adhered strictly to the follow-up protocol. EUS was included at 3 and 12 months of follow-up to reduce the number of scans per patient, and so that multiple imaging modalities could confirm tumour size stability. It also provided an immediate opportunity to perform fine-needle aspiration (FNA) if there was doubt regarding tumour origin. However, EUS was considered a high burden for patients, and was frequently rejected by both patients and physicians. In addition, not all patients underwent the suggested CT or MRI at 6 and 12 months’ follow-up, as the interval after diagnosis was deemed too short by some physicians. A reduction in the follow-up protocol has been made by the study group, whereby the EUS examination at 3 months is suggested only for patients who have not undergone EUS previously. In future studies, EUS FNA could also be used to examine other tumour characteristics.
A potential pitfall of a wait-and-see approach is late detection of disease spread. This was the case in one patient in the present study who underwent surgery for rapid tumour progression, in whom peritoneal metastases were diagnosed during surgery. The sensitivity of CT, MRI, and DOTATATE PET–CT is known to be low for (small) peritoneal metastases22,23. However, the optimal timing of adjuvant treatment for metastases in pNET is unknown, and treatment in the absence of radiologically measurable disease is usually not recommended. To truly evaluate the oncological safety of watchful waiting of pNET, longer follow-up is necessary. Nevertheless, it is important to report these short-term findings, because they give insight into the obstacles of implementation of new guidelines, as well as the pitfalls regarding treatment indication and sensitivity of imaging techniques. QoL was poorer at baseline in the study population than that of the reference population, but the results are too premature for conclusions to be drawn on the exact reason for this difference.
The authors further recommend improved patient support during the first years of watchful waiting. The PANDORA study is continuing to evaluate long-term outcomes of a wait-and-see approach for NF-pNETs no larger than 2 cm.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Supplementary Material
References
- 1. Genç CG, Jilesen AP, Partelli S, Falconi M, Muffatti F, van Kemenade FJ. et al. A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann Surg 2018;267:1148–1154 [DOI] [PubMed] [Google Scholar]
- 2. Merath K, Bagante F, Beal EW, Lopez-Aguiar AG, Poultsides G, Makris E. et al. Nomogram predicting the risk of recurrence after curative-intent resection of primary non-metastatic gastrointestinal neuroendocrine tumors: an analysis of the U.S. Neuroendocrine Tumor Study Group. J Surg Oncol 2018;117:868–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY. et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg 2008;247:490–500 [DOI] [PubMed] [Google Scholar]
- 4. Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M. et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016;103:153–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Rooij T, Van Hilst J, Van Santvoort H, Boerma D, Van Den Boezem P, Daams F. et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg 2019;269:2–9 [DOI] [PubMed] [Google Scholar]
- 6. van Hilst J, De Rooij T, Bosscha K, Brinkman DJ, Van Dieren S, Dijkgraaf MG. et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 2019;4:199–207 [DOI] [PubMed] [Google Scholar]
- 7. Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS.. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J Gastrointest Surg 2015;19:117–123 [DOI] [PubMed] [Google Scholar]
- 8. Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA.. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol 2014;21:3515–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaujoux S, Partelli S, Maire F, D'Onofrio M, Larroque B, Tamburrino D. et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab 2013;98:4784–4789 [DOI] [PubMed] [Google Scholar]
- 10. Partelli S, Cirocchi R, Crippa S, Cardinali L, Fendrich V, Bartsch DK. et al. Systematic review of active surveillance versus surgical management of asymptomatic small non-functioning pancreatic neuroendocrine neoplasms. Br J Surg 2017;104:34–41 [DOI] [PubMed] [Google Scholar]
- 11. Sallinen V, Haglund C, Seppanen H.. Outcomes of resected nonfunctional pancreatic neuroendocrine tumors: do size and symptoms matter? Surgery 2015;158:1556–1563 [DOI] [PubMed] [Google Scholar]
- 12. Howe JR, Merchant NB, Conrad C, Keutgen XM, Hallet J, Drebin JA. et al. The North American Neuroendocrine Tumor Society consensus paper on the surgical management of pancreatic neuroendocrine tumors. Pancreas 2020;49:1–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL. et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery 2012;152:965–974 [DOI] [PubMed] [Google Scholar]
- 14. Sadot E, Reidy-Lagunes DL, Tang LH, Do RK, Gonen M, D'Angelica MI. et al. Observation versus resection for small asymptomatic pancreatic neuroendocrine tumors: a matched case–control study. Ann Surg Oncol 2016;23:1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Partelli S, Gaujoux S, Boninsegna L, Cherif R, Crippa S, Couvelard A. et al. Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). JAMA Surg 2013;148:932–939 [DOI] [PubMed] [Google Scholar]
- 16. Rosenberg AM, Friedmann P, Del Rivero J, Libutti SK, Laird AM.. Resection versus expectant management of small incidentally discovered nonfunctional pancreatic neuroendocrine tumors. Surgery 2016;159:302–309 [DOI] [PubMed] [Google Scholar]
- 17. Sallinen VJ, Le Large TYS, Tieftrunk E, Galeev S, Kovalenko Z, Haugvik SP. et al. Prognosis of sporadic resected small (≤ 2 cm) nonfunctional pancreatic neuroendocrine tumors—a multi-institutional study. HPB (Oxford) 2018;20:251–259 [DOI] [PubMed] [Google Scholar]
- 18. Ricci C, Casadei R, Taffurelli G, Pacilio CA, Campana D, Ambrosini V. et al. Sporadic small (</= 20 mm) nonfunctioning pancreatic neuroendocrine neoplasm: is the risk of malignancy negligible when adopting a more conservative strategy? A systematic review and meta-analysis. Ann Surg Oncol 2017;24:2603–2610 [DOI] [PubMed] [Google Scholar]
- 19. Mintziras I, Keck T, Werner J, Fichtner-Feigl S, Wittel U, Senninger N. et al. Implementation of current ENETS guidelines for surgery of small (</= 2 cm) pancreatic neuroendocrine neoplasms in the German surgical community: an analysis of the prospective DGAV StuDoQ|Pancreas Registry. World J Surg 2019;43:175–182 [DOI] [PubMed] [Google Scholar]
- 20. Partelli S, Mazza M, Andreasi V, Muffatti F, Crippa S, Tamburrino D. et al. Management of small asymptomatic nonfunctioning pancreatic neuroendocrine tumors: limitations to apply guidelines into real life . Surgery 2019;166:157–163 [DOI] [PubMed] [Google Scholar]
- 21. Mansour JC, Chavin K, Morris-Stiff G, Warner SG, Cardona K, Fong ZV. et al. Management of asymptomatic, well-differentiated PNETs: results of the Delphi consensus process of the Americas Hepato-Pancreato-Biliary Association. HPB (Oxford) 2019;21:515–523 [DOI] [PubMed] [Google Scholar]
- 22. Bastiaenen VP, Klaver CEL, Kok NFM, de Wilt JHW, de Hingh I, Aalbers AGJ. et al. Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases; the COLOPEC 2 randomized multicentre trial. BMC Cancer 2019;19:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komek H, Ansal Balci T, Can C.. Efficacy of galium-68 DOTATATE PET/CT in the detection of metastasis rate of well-differentiated gastroenteropancreatic neuroendocrine tumors. Asia Ocean J Nucl Med Biol 2019;7:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


