Abstract
Background
Despite the fact that primary percutaneous catheter drainage has become standard practice, some patients with pancreatic fistula after pancreatoduodenectomy ultimately undergo a relaparotomy. The aim of this study was to compare completion pancreatectomy with a pancreas-preserving procedure in patients undergoing relaparotomy for pancreatic fistula after pancreatoduodenectomy.
Methods
This retrospective cohort study of nine institutions included patients who underwent relaparotomy for pancreatic fistula after pancreatoduodenectomy from 2005–2018. Furthermore, a systematic review and meta-analysis were performed according to the PRISMA guidelines.
Results
From 4877 patients undergoing pancreatoduodenectomy, 786 (16 per cent) developed a pancreatic fistula grade B/C and 162 (3 per cent) underwent a relaparotomy for pancreatic fistula. Of these patients, 36 (22 per cent) underwent a completion pancreatectomy and 126 (78 per cent) a pancreas-preserving procedure. Mortality was higher after completion pancreatectomy (20 (56 per cent) versus 40 patients (32 per cent); P = 0.009), which remained after adjusting for sex, age, BMI, ASA score, previous reintervention, and organ failure in the 24 h before relaparotomy (adjusted odds ratio 2.55, 95 per cent c.i. 1.07 to 6.08). The proportion of additional reinterventions was not different between groups (23 (64 per cent) versus 84 patients (67 per cent); P = 0.756). The meta-analysis including 33 studies evaluating 745 patients, confirmed the association between completion pancreatectomy and mortality (Mantel–Haenszel random-effects model: odds ratio 1.99, 95 per cent c.i. 1.03 to 3.84).
Conclusion
Based on the current data, a pancreas-preserving procedure seems preferable to completion pancreatectomy in patients in whom a relaparotomy is deemed necessary for pancreatic fistula after pancreatoduodenectomy.
Based on the present cohort study and meta-analysis, a pancreas-preserving procedure seems to be the preferred surgical strategy whenever possible during relaparotomy for pancreatic fistula after pancreatoduodenectomy.
Introduction
Postoperative pancreatic fistula is among the most notorious complications after pancreatoduodenectomy as it is associated with a high morbidity and mortality rate1. Primary percutaneous catheter drainage has become standard practice in the management of a clinically relevant pancreatic fistula. However, percutaneous catheter drainage is not successful in all patients and a small subset ultimately undergo a relaparotomy2. An international survey showed good agreement between surgeons on the indication for relaparotomy when image-guided percutaneous catheter drainage of fluid collections is not technically feasible3.
During relaparotomy, different strategies are possible: surgical drainage (intra-abdominal lavage and placement of drains); repair or redo of the pancreatic anastomosis; salvage pancreatogastrostomy; and completion pancreatectomy4. Completion pancreatectomy is the most aggressive strategy which aims to remove completely the focus of intra-abdominal leakage and associated inflammation. Downsides of this procedure are the additional inflammatory stress from the extensive surgical procedure and subsequent possible deterioration of organ failure, technical difficulty resulting in blood loss, risk of damaging other structures and pancreatic exocrine and endocrine insufficiency. On the other hand, pancreas-preserving procedures might not be sufficient and thereby lead to further clinical deterioration including multiple organ failure, more reinterventions and prolonged hospital stay5,6. Few studies have been performed on the clinical outcomes of different surgical strategies in patients with pancreatic fistula after pancreatoduodenectomy4.
The aim of this study was to evaluate surgical strategies (completion pancreatectomy versus a pancreas-preserving procedure) in patients undergoing relaparotomy for pancreatic fistula after pancreatoduodenectomy. Additionally, a systematic review and meta-analysis were performed to summarize the available evidence on this topic.
Methods
Study design and patient selection
This was a retrospective multicentre cohort study of the Dutch Pancreatic Cancer Group7 in which nine institutions participated. The need for informed consent was waived by the Medical Ethics Committee of the Leiden University Medical Centre. This study was performed in accordance with the Declaration of Helsinki and reported according to the STROBE criteria8.
All patients undergoing relaparotomy for pancreatic fistula after pancreatoduodenectomy from 2005 to 2018 were included. The indication for relaparotomy was assessed by three independent authors (J.V.G., D.K., J.S.D.M.) and discrepancies were resolved by consensus. Patients were identified using the prospective Dutch Pancreatic Cancer Audit (2013–2018). Participation in the Dutch Pancreatic Cancer Audit is mandatory for all institutions performing pancreatic surgery in the Netherlands9. In addition, an existing database2 containing patients with severe pancreatic fistula after pancreatoduodenectomy (8 institutions, 2005–2013) was evaluated.
Data collection
Data were extracted from the Dutch Pancreatic Cancer Audit and through systematic evaluation of medical records using a predefined case record form. Variables of interest included: patient-related variables (gender, age, BMI, pathology, preoperative biliary drainage, ASA score); surgery-related variables (type and duration of surgery, pancreatic anastomosis, vascular resection, additional organ resection, blood loss); postoperative variables (postoperative complications, reinterventions, organ failure, Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, systemic inflammatory response syndrome (SIRS), duration of admission to the intensive care unit (ICU), Clavien–Dindo classification of surgical complications, removal of abdominal drain, duration of hospital stay, postoperative mortality); and follow-up variables (new-onset postoperative exocrine insufficiency and diabetes mellitus, and adjuvant therapy).
Definitions
Postoperative pancreatic fistula was defined and classified according to the International Study Group of Pancreatic Surgery criteria10. Death was defined as death during the index admission up to 3 months after discharge. Organ failure was defined as one or more of the following: respiratory organ failure (partial pressure of oxygen less than 60 mmHg despite a fraction of inspired oxygen of 0.3 or need for mechanical ventilation), circulatory organ failure (systolic blood pressure less than 90 mmHg despite adequate fluid resuscitation or need for inotropic support) or renal organ failure (creatinine level greater than 2.0 mg/dl after rehydration or need for haemofiltration or haemodialysis). APACHE II score and SIRS criteria were scored 24 h before and 24 h after initial relaparotomy11,12. SIRS was considered in cases of two or more positive criteria12. Other pancreatic-specific complications (postpancreatectomy haemorrhage, bile leakage, delayed gastric emptying) were defined and classified according to the International Study Group of Pancreatic Surgery or Liver Surgery definitions13–15. Only grade B and C were reported as these are generally considered as clinically relevant. Duration of pancreatic fistula was calculated as time from pancreatoduodenectomy to removal of last abdominal catheter in patients undergoing a pancreas-preserving procedure. New-onset postoperative exocrine pancreatic insufficiency and diabetes mellitus were defined as need for oral pancreatic enzyme supplementation or antidiabetics within 3 months after discharge, not present before pancreatoduodenectomy. All data were collected which were available from the medical charts (from index admission up to 3 months after discharge).
Outcomes and comparison
The primary outcome was death (defined as death during the index admission up to 3 months after discharge). Secondary outcomes included organ failure and APACHE II score in the 24 h after initial relaparotomy, the number and type of additional reinterventions after initial relaparotomy, duration of ICU stay, duration of hospital stay, new-onset postoperative exocrine pancreatic insufficiency and diabetes mellitus, duration of pancreatic fistula in patients undergoing a pancreas-preserving procedure and proportion of patients with pancreatic cancer receiving adjuvant therapy.
Patients were divided into two groups based on the surgical strategy during the initial relaparotomy for pancreatic fistula: completion pancreatectomy versus pancreas-preserving procedure. A sensitivity analysis over time was performed stratified by period (2005–2008, 2009–2012, 2013–2015 and 2016–2018).
Statistical analysis
Statistical analysis was performed with SPSS Statistics for Windows™, version 23.0 (IBM, Armonk, New York, USA). Continuous variables with a skewed distribution were presented as median (i.q.r.) and compared using the Mann–Whitney U test. Categorical variables were presented as numbers (percentages) and compared using χ2 or Fisher’s Exact tests, as appropriate. Multivariable logistic regression analysis for mortality was conducted to adjust for theoretical confounding factors with sufficient available data (sex, age, BMI, ASA score, reintervention before initial relaparotomy and organ failure in the 24 h before initial relaparotomy). Results are given as odds ratios with 95 per cent confidence intervals. All tests were two-sided and statistical significance was defined as P < 0.050.
Systematic review and meta-analysis
A systematic literature search (Supplementary material) was performed according to the PRISMA guidelines16. The databases of PubMed, MEDLINE, Embase, Web of Science and COCHRANE Library were searched for full-text, English-written studies. Titles, abstracts and full-text articles were screened by two independent authors (J.V.G., D.K.) for eligibility. Studies were included if patients were described who underwent relaparotomy for pancreatic fistula after pancreatoduodenectomy. Literature reviews and case reports were excluded. Data extraction was performed using a standardized form with study characteristics and postoperative outcomes (mortality, duration of hospital stay, ICU admission, organ failure and additional reinterventions). The risk of bias was determined using the ROBINS-I tool for cohort studies17. A meta-analysis was performed for death (completion pancreatectomy versus pancreas-preserving procedure) using Review Manager (RevMan version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The I2 statistic was used to assess heterogeneity between studies. An I2 value of greater than 50 per cent was considered as substantial heterogeneity. The Mantel–Haenszel random-effects model was used to calculate pooled effects. A fixed-effects model was used for sensitivity analysis.
Results
Baseline characteristics
Of the 4877 patients undergoing pancreatoduodenectomy, 786 (16 per cent) developed a pancreatic fistula grade B/C and 162 (3 per cent of all; 21 per cent of those with a pancreatic fistula) underwent a relaparotomy for pancreatic fistula (Fig. 1). During initial relaparotomy for pancreatic fistula, completion pancreatectomy was performed in 36 (22 per cent) patients and a pancreas-preserving procedure in 126 (78 per cent) patients (Table 1). Strategies during an initial pancreas-preserving procedure included 80 patients (63 per cent) who had surgical drainage, 20 patients (16 per cent) with attempt to repair the pancreatic anastomosis, 21 patients (17 per cent) disconnection of the pancreatic anastomosis with preservation of the remnant and five patients (4 per cent) redo of the pancreatic anastomosis. Patients undergoing completion pancreatectomy were older (median 70 (i.q.r. 66–73) versus 64 (i.q.r. 58–71) years; P = 0.025). In the completion pancreatectomy group, 13 patients (36 per cent) were ASA III–IV compared with 26 (21 per cent) patients in the pancreas-preserving group (P = 0.055).
Fig. 1.
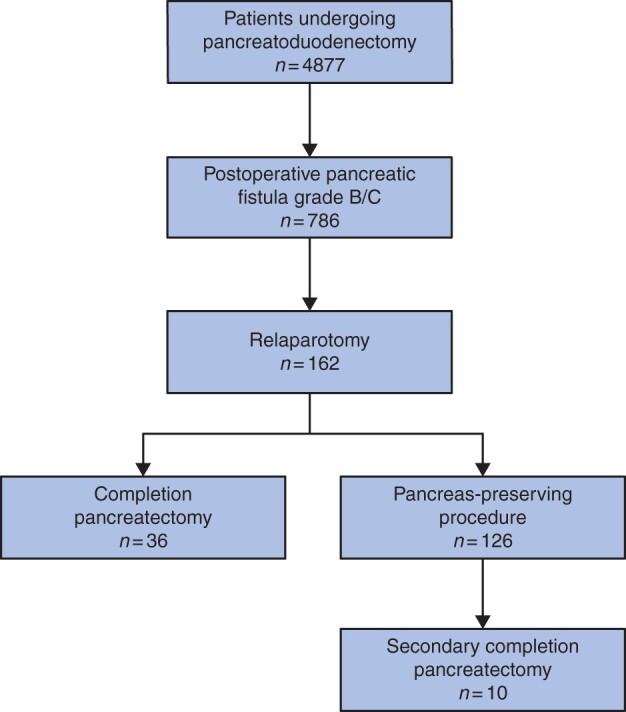
Flow chart of patient selection
Table 1.
Baseline characteristics by surgical strategy for pancreatic fistula
| Characteristics | Completion pancreatectomy (n = 36) | Pancreas-preserving procedure (n = 126) | P |
|---|---|---|---|
| Baseline at time of index surgery | |||
| Sex (female) | 8 (22) | 36 (28.6) | 0.450 |
| Age* | 70 (66, 73) | 64 (58, 71) | 0.025 |
| BMI*† | 26.8 (24.2, 28.9) | 26.1 (23.4, 28.7) | 0.447 |
| ASA (III–IV) | 13 (36) | 26 (20.6) | 0.055 |
| Type of resection | 0.302 | ||
| Whipple | 11 (31) | 28 (22.2) | |
| PPPD | 25 (66) | 96 (77.8) | |
| Vascular resection | 4 (11) | 7 (5.6) | 0.243 |
| Additional organ resection | 4 (11) | 16 (12.7) | 0.789 |
| Pancreatic anastomosis | 0.111 | ||
| Duct-to-mucosa PJ | 28 (78) | 113 (89.7) | |
| Duct-to-mucosa PG | 0 | 1 (0.8) | |
| Dunking PJ | 8 (22) | 12 (9.5) | |
| Pathology | 0.786 | ||
| Pancreatic cancer/pancreatitis | 12 (33) | 39 (31.0) | |
| Other | 24 (67) | 87 (69.0) | |
| Baseline at time of initial relaparotomy | |||
| Previous reintervention | 17 (47) | 57 (45.2) | 0.833 |
| Radiological intervention | 15 (42) | 52 (41.3) | 0.966 |
| Relaparotomy | 5 (14) | 7 (5.6) | 0.092 |
| Previous reintervention for PPH | 6 (17) | 12 (9.5) | 0.229 |
| Radiological intervention for PPH | 5 (14) | 10 (12.6) | 0.277 |
| Relaparotomy for PPH | 1 (3) | 2 (1.6) | 0.640 |
| Organ failure 24 h before† | |||
| No | 19 (53) | 68 (54.8) | 0.035 |
| Single | 6 (17) | 39 (31.5) | |
| Multiple | 11 (31) | 17 (13.7) | |
| Highest APACHE II score 24 h before*† | 14 (10, 18) | 12 (8, 15) | 0.055 |
| Postoperative day of initial relaparotomy for POPF* | 10 (4, 14) | 9 (6, 14) | 0.521 |
Continuous variables all compared using the Mann, Whitney U test. Categorical variables all compared using χ2. Except: vascular resection, additional organ resection, previous reintervention for postpancreatectomy haemorrhage (PPH), relaparotomy for PPH, which were compared with Fisher’s exact tests. Values in parentheses are percentages unless indicated otherwise
*values are median (i.q.r.).
†Missing data: BMI (n = 6), organ failure 24 h before (n = 2), highest Acute Physiology And Chronic Health Evaluation (APACHE) II score 24 h before (n = 14). PPPD, pylorus-preserving pancreatoduodenectomy; PJ, pancreatojejunostomy; PG, pancreatogastrostomy; POPF, postoperative pancreatic fistula.
Patients undergoing completion pancreatectomy more often had single or multiple organ failure 24 h before the initial relaparotomy (P = 0.035). The highest APACHE II score within the 24 h before the initial relaparotomy (median 14 (i.q.r. 10–18) versus 12 (i.q.r. 8–15); P = 0.055), the proportion of reinterventions before the initial relaparotomy (17 patients (47 per cent) versus 57 patients (45 per cent); P = 0.833) and the proportion of reinterventions for postpancreatectomy haemorrhage before the initial relaparotomy (6 patients (17 per cent) versus 12 patients (10 per cent); P = 0.229) did not differ significantly between groups. The timing of initial relaparotomy also did not differ (median on postoperative day 10 (i.q.r. 4–14) versus 9 (i.q.r. 6–14); P = 0.521). Other details regarding baseline characteristics, reinterventions and disease severity before initial relaparotomy are shown in Table S1.
Main outcomes
Main outcomes are summarized in Table 2. Patients undergoing completion pancreatectomy had a higher mortality rate, compared with patients undergoing a pancreas-preserving procedure (20 patients (56 per cent) versus 40 patients (32 per cent); P = 0.009). At multivariable analysis, adjusting for sex, age, BMI, ASA score, previous reintervention and organ failure in the 24 h before relaparotomy, completion pancreatectomy was associated with fatal outcome (adjusted odds ratio 2.55, 95 per cent c.i. 1.07 to 6.08; Table 3).
Table 2.
Main outcomes by surgical strategy for pancreatic fistula
| Outcomes | Completion pancreatectomy (n = 36) | Pancreas-preserving procedure (n = 126) | P |
|---|---|---|---|
| Death | 20 (56) | 40 (31.7) | 0.009 |
| Organ failure 24 h after initial relaparotomy † | 0.165 | ||
| No | 6 (17) | 34 (27.4) | |
| Single | 5 (14) | 26 (21.0) | |
| Multiple | 25 (69) | 64 (51.6) | |
| Highest APACHE II score 24 h after initial relaparotomy * † | 18 (15, 23) | 15 (11, 18) | <0.001 |
| ICU admission | 35 (97) | 107 (84.9) | 0.048 |
| Duration of ICU admission (days)* | 13 (3, 32) | 7 (2, 17) | 0.091 |
| Additional reintervention after initial relaparotomy | 23 (64) | 84 (66.7) | 0.756 |
| Radiological intervention | 16 (44) | 71 (56.3) | 0.206 |
| Relaparotomy | 14 (39) | 40 (31.7) | 0.423 |
| Secondary completion pancreatectomy | – | 10 (7.9) | |
| Additional reintervention for PPH after initial relaparotomy | 6 (17) | 21 (16.7) | >0.999 |
| Radiological intervention for PPH | 2 (6) | 12 (9.5) | 0.455 |
| Relaparotomy for PPH | 4 (11) | 10 (7.9) | 0.550 |
| Duration of hospital stay (days)* | 38 (24, 61) | 53 (31, 66) | 0.042 |
| Duration of hospital stay in survivors (days)* | 55 (31, 70) | 56 (40, 71) | 0.592 |
| New-onset postoperative pancreatic exocrine insufficiency in survivors † | – | 32 (43.2) | – |
| New-onset postoperative diabetes mellitus in survivors † | – | 19 (25.7) | – |
Continuous variables all compared using the Mann, Whitney U test. Categorical variables all compared using χ2. Except: additional reintervention for postpancreatectomy haemorrhage (PPH) after initial relaparotomy, radiological intervention for PPH, additional reintervention for PPH after initial relaparotomy, relaparotomy for PPH, which were compared with Fisher’s exact tests. Values in parentheses are percentages unless indicated otherwise
*values are median (i.q.r.).
†Missing data: organ failure 24 h after (n = 2), highest APACHE II score 24 h after (n = 28), new-onset postoperative pancreatic exocrine insufficiency (n = 14), new-onset postoperative diabetes mellitus (n = 14), APACHE, Acute Physiology And Chronic Health Evaluation; ICU, intensive care unit.
Table 3.
Multivariable analysis for fatal outcome
| Strategy during initial relaparotomy, pancreas-preserving | Odds ratio | P |
|---|---|---|
| Completion pancreatectomy | 2.55 (1.07, 6.08) | 0.035 |
| Sex | ||
| Male | Reference | |
| Female | 1.97 (0.87, 4.44) | 0.104 |
| Age | 1.08 (1.03, 1.13) | 0.002 |
| BMI* | 1.02 (0.93, 1.12) | 0.702 |
| ASA score | ||
| I–II | Reference | |
| III–IV | 0.89 (0.38, 2.07) | 0.785 |
| Previous reintervention | ||
| No | Reference | |
| Yes | 1.12 (0.56, 2.38) | 0.707 |
| Organ failure 24 h before* | ||
| No | Reference | |
| Single organ | 1.15 (0.49, 2.69) | 0.755 |
| Multiple organ | 2.47 (0.91, 6.68) | 0.075 |
Continuous variables all compared using the Mann, Whitney U test. Categorical variables all compared using χ2. Except: additional reintervention for postpancreatectomy haemorrhage (PPH) after initial relaparotomy, radiological intervention for PPH, additional reintervention for PPH after initial relaparotomy, relaparotomy for PPH, which were compared with Fisher’s exact tests. Values in parentheses are 95% confidence intervals.
*Missing data: BMI or organ failure 24 h before (n = 7).
There was no difference in the number of postoperative abdominal catheters after initial relaparotomy between groups (median 2 (i.q.r. 1–2) versus 2 (i.q.r. 2–3); P = 0.119; 10 per cent missing data). Patients undergoing completion pancreatectomy had higher APACHE II scores within the 24 h after initial relaparotomy (median 18 (i.q.r. 15–23) versus 15 (i.q.r. 11–18); P < 0.001), whereas single or multiple organ failure (P = 0.165) did not differ. The proportion of additional reintervention after initial relaparotomy was not different (23 patients (64 per cent) versus 84 patients (67 per cent); P = 0.756). Out of 126 initial pancreas-preserving procedures, 10 (8 per cent) patients ultimately underwent completion pancreatectomy. The proportion of additional reinterventions for postpancreatectomy haemorrhage after initial relaparotomy did not differ between groups (6 patients (17 per cent) versus 21 patients (17 per cent); P > 0.999). In surviving patients, duration of hospital stay did not differ (median 55 (i.q.r. 31–70) versus 56 (i.q.r. 40–71) days; P = 0.592). In surviving patients undergoing a pancreas-preserving procedure, 32 patients (43 per cent) developed new-onset postoperative pancreatic exocrine insufficiency and 19 patients (26 per cent) developed new-onset diabetes mellitus.
Other outcomes
Median time to resolution of postoperative pancreatic fistula was 47 (i.q.r. 25–69) days in patients undergoing a pancreas-preserving procedure (Table S2). One of five (20 per cent) surviving pancreatic cancer patients who underwent a completion pancreatectomy received adjuvant therapy, compared with one of 25 patients (4 per cent) in the pancreas-preserving group (P = 0.314). Other details regarding disease severity, reinterventions and other postoperative outcomes after initial relaparotomy are given in Table S2.
Sensitivity analysis by period
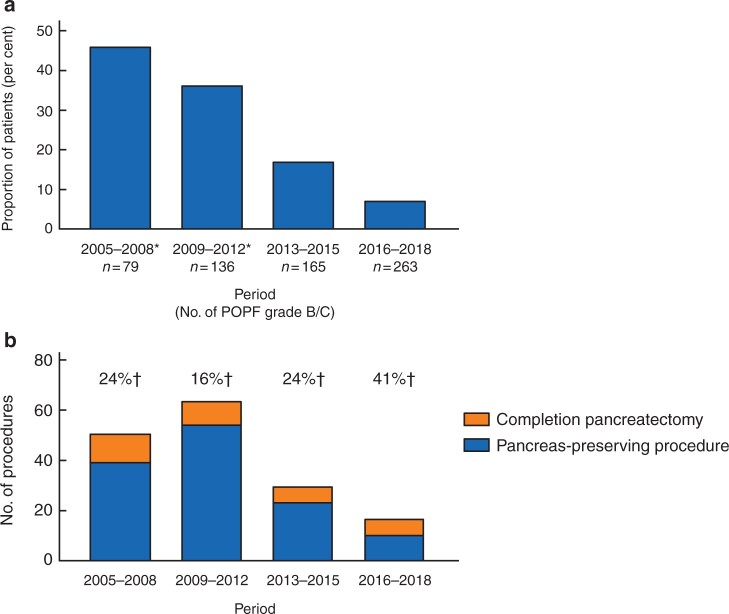
The sensitivity analysis stratified by period showed a linear decrease in proportion of patients undergoing relaparotomy for pancreatic fistula (P < 0.001) and no linear change in proportion of patients undergoing completion pancreatectomy or a pancreas-preserving procedure (P = 0.228) (Fig. 2). The sensitivity analysis stratified by period also showed a higher mortality rate after completion pancreatectomy compared with a pancreas-preserving procedure in all four periods (Table S3).
Fig. 2.
Sensitivity analysis
a Proportion of patients undergoing relaparotomy for postoperative pancreatic fistula (POPF). P < 0.001 for χ2 for linear trend. b Proportion of patients undergoing completion pancreatectomy or a pancreas-preserving procedure during relaparotomy for pancreatic fistula. P = 0.228 for χ2 for linear trend. *Data from six of nine institutions; †numbers indicate the percentage of patients undergoing completion pancreatectomy.
Systematic review and meta-analysis
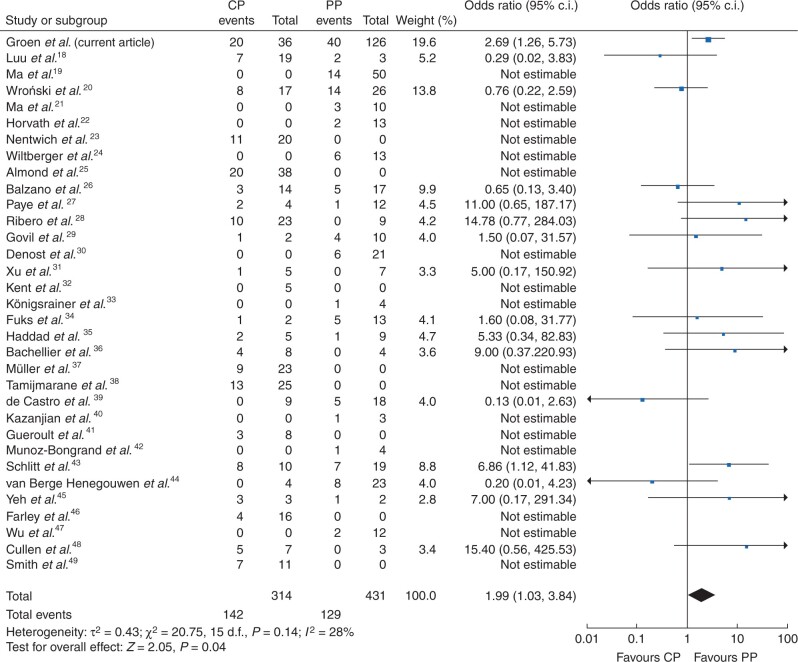
The literature search identified 763 unique studies. After screening titles, abstracts and full texts, 35 studies were included, which reported on patients undergoing relaparotomy for pancreatic fistula after pancreatoduodenectomy (Fig. S1 and Table S4). All included studies, except one, were retrospective in design and the number of included patients ranged from three to 57. Five out of 35 studies were graded as having moderate overall risk of bias, mainly due to confounding and lack of defining outcomes; the remaining studies did not provide sufficient information to determine the risk of bias in one or more domains of the ROBINS-I tool (Table S5). The meta-analysis consisted of 32 studies (583 patients) and the present study, with a total of 745 patients undergoing completion pancreatectomy or a pancreas-preserving procedure for pancreatic fistula. Mortality rate ranged from 0 to 100 per cent and completion pancreatectomy was associated with death (random-effects model, odds ratio 1.99, 95 per cent c.i. 1.03 to 3.84, P = 0.040; I2 = 28 per cent; Fig. 3). The funnel plot showed a symmetrical scatter around the mean (Fig. S2). Sensitivity analysis showed a similar association between completion pancreatectomy and death (fixed-effects model, odds ratio 1.94, 95 per cent c.i. 1.27 to 2.97; I2 = 28 per cent; Fig. S3).
Fig. 3.
Forest plot of death after initial relaparotomy by surgical strategy for pancreatic fistula: completion pancreatectomy (CP) versus pancreas-preserving (PP) procedure (random-effects model)18–49
Twenty-two surgical strategies during relaparotomy were described with varying definitions (Table S6). Overall mean/median duration of hospital stay ranged from 15–62 days (23 studies and the present study), ICU admission after relaparotomy ranged from 38–100 per cent (5 studies and the present study), organ failure after relaparotomy ranged from 25–83 per cent (7 studies and the present study) and relaparotomy after relaparotomy ranged from 0–100 per cent (15 studies and the present study).
Discussion
The present cohort study found that one in five patients with a postoperative pancreatic fistula grade B/C after pancreatoduodenectomy underwent a relaparotomy. Completion pancreatectomy was independently associated with a doubling of mortality rate, compared with a pancreas-preserving procedure. The meta-analysis of 33 cohort studies confirmed this finding. Patients undergoing completion pancreatectomy had a higher APACHE II score within the 24 h after relaparotomy, whereas there was no difference in the proportion of additional reinterventions or duration of hospital stay.
The rate of pancreatic fistula grade B/C in this study was fairly comparable to previous studies (16 versus 9–11 per cent)1,50, as was the rate of relaparotomy for pancreatic fistula (21 versus 17–37 per cent)1,50. A recent study showed large variation in overall reoperation rate (6–17 per cent) between several pancreatic surgery registries in the USA and Europe51. The paradigm shift to percutaneous catheter drainage as primary management of pancreatic fistula and advances in interventional radiology probably explain the linear decrease in proportion of patients undergoing relaparotomy over the study period. The systematic review of studies from 1992–2020 shows that a variety of 22 surgical strategies are used or have been used in clinical practice during relaparotomy for pancreatic fistula. It remains unknown what the exact considerations are and it is likely that personal experience and preference influence the surgeon’s choice. Completion pancreatectomy has been associated with a longer duration of surgery and more blood loss5,52, and a higher APACHE II score after relaparotomy in this study, which suggest that a completion pancreatectomy has a significant impact on the clinical condition of the patient. These factors should be considered when deciding to proceed with a completion pancreatectomy or a pancreas-preserving procedure53.
The high mortality rate after completion pancreatectomy may be explained by more severe tissue injury and inflammatory response in already critically ill patients. This effect was seen in a randomized trial in patients with necrotizing pancreatitis and secondary infection in which primary open necrosectomy was compared with a minimally invasive step-up approach54 and in a matched cohort study in patients with pancreatic fistula in which relaparotomy was compared with catheter drainage as primary treatment2. Randomized trials on surgical strategies during relaparotomy for pancreatic fistula after pancreatoduodenectomy are not currently available. Such a trial would be difficult to perform as this critically ill population is increasingly rare, and it seems unlikely that surgeons will accept that the surgical strategy in this population is randomized55. Although the systematic review summarized the evidence on this topic, it should be noted that the included studies were all small, observational and heterogeneous. Despite the fact that the indications for relaparotomy may have varied and changed over time, mortality rates were higher after completion pancreatectomy in all four periods in the sensitivity analysis.
A theoretical advantage of completion pancreatectomy is that it removes the source of inflammation, thereby possibly decreasing the risk of additional reinterventions5,52. The present and previous studies2,54 did not show fewer reinterventions after completion pancreatectomy. Furthermore, the risk of postpancreatectomy haemorrhage after the relaparotomy and required reinterventions was not different between the groups (17 versus 17 per cent). Possibly, the actions applied by the surgeons were usually sufficient to prevent erosion of the peripancreatic vascular structures by leaking pancreatic enzymes56. A recent study showed that pancreatic fistula and postpancreatectomy haemorrhage can develop independently and have a major impact on organ failure and mortality57. The Dutch Pancreatic Cancer Group is currently analysing the data of the nationwide PORSCH trial to investigate whether early recognition and a minimally invasive step-up approach for pancreatic fistula after pancreatic resection decreases the risk of postpancreatectomy haemorrhage, organ failure and mortality58. Of note, the present study was not designed to promote relaparotomy over percutaneous catheter drainage as primary management of pancreatic fistula and the authors emphasize that a minimally invasive step-up approach should be the preferred strategy.
Little is known about new-onset pancreatic exocrine insufficiency. One study reported a rate of 67 per cent (43 per cent in the present study)59. More studies reported on new-onset diabetes mellitus, ranging 26–50 per cent (26 per cent in the present study)52,59–62. A recent meta-analysis showed an acceptable rate of diabetes-related morbidity and levels of HbA1c 1 year after elective or emergency total pancreatectomy63. Unfortunately, these data were not available for the present study. In the previously mentioned meta-analysis, diarrhoea was the most frequent symptom (24 per cent), which may be caused by pancreatic exocrine insufficiency or autonomic denervation of the bowel due to the extent of the resection63. In the Netherlands, initiatives like the PACAP-1 trial are aimed at improving pancreatic enzyme replacement therapy in patients with pancreatic cancer64.
The results of the present study should be interpreted in light of some limitations. First, some data were collected retrospectively and this holds the risk of information and classification bias. The data extracted from the prospective Dutch Pancreatic Cancer Audit have been validated previously for data accuracy9. Second, due to the observational design of this study, confounding by indication is an important potential bias as the surgeon’s decision to perform a completion pancreatectomy or pancreas-preserving procedure is based on the experience and personal preferences of the surgeon and the clinical and surgical context of the patient. For example, patients with completion pancreatectomy were older and more often had multiple organ failure. Inherent differences between patients undergoing completion pancreatectomy compared with a pancreas-preserving procedure may partly explain the observed results. The multivariable analysis was limited by the sample size and could only adjust for a few possible confounders. Also, data of some other possible confounders, for example blood loss and the use of antibiotics1, were not sufficiently available. Due to these limitations, residual confounding cannot be ruled out and results should be interpreted with caution. Strengths of this study include the detailed data of disease severity and reinterventions before and after the initial relaparotomy and the systematic review of available evidence.
Funding
This work was supported by the Bas Mulder Award from the Alpe d'HuZes foundation/Dutch Cancer Society (UL2015-7665). The funding source had no role in the study design, collection, analyses or interpretation of the data, drafting of the manuscript or the decision to publish.
Supplementary Material
Acknowledgements
The authors thank J.W.S. (Walaeus Library, Leiden University Medical Centre, Leiden, the Netherlands) for composing the literature search. J.V.G. and F.J.S. contributed equally and share first authorship. H.C.v.S. and J.S.D.M. equally and share senior authorship. Study conception and design; data analysis and interpretation; manuscript drafting and revising; final approval of manuscript: all authors. The data sets used during this study are available on reasonable request from the corresponding author.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online
References
- 1. McMillan MT, Vollmer CM Jr, Asbun HJ, Ball CG, Bassi C, Beane JD. et al. The characterization and prediction of ISGPF grade C fistulas following pancreatoduodenectomy. J Gastrointest Surg 2016;20:262–276. [DOI] [PubMed] [Google Scholar]
- 2. Smits FJ, van Santvoort HC, Besselink MG, Batenburg MCT, Slooff RAE, Boerma D. et al. ; Dutch Pancreatic Cancer Group. Management of severe pancreatic fistula after pancreatoduodenectomy. JAMA Surg 2017;152:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melloul E, Raptis DA, Clavien PA, Lesurtel M; European–African Hepato-Pancreato-Biliary Association. Poor level of agreement on the management of postoperative pancreatic fistula: results of an international survey. HPB (Oxford )2013;15:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou YM, Zhou X, Wan T, Xu D, Si XY.. An evidence-based approach to the surgical interventions for severe pancreatic fistula after pancreatoduodenectomy. Surgeon 2018;16:119–124. [DOI] [PubMed] [Google Scholar]
- 5. Balzano G, Pecorelli N, Piemonti L, Ariotti R, Carvello M, Nano R. et al. Relaparotomy for a pancreatic fistula after a pancreaticoduodenectomy: a comparison of different surgical strategies. HPB (Oxford )2014;16:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ.. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg 2005;92:1117–1123. [DOI] [PubMed] [Google Scholar]
- 7. Strijker M, Mackay TM, Bonsing BA, Bruno MJ, van Eijck CHJ, de Hingh I. et al. ; Dutch Pancreatic Cancer Group. Establishing and coordinating a nationwide multidisciplinary study group: lessons learned by the Dutch Pancreatic Cancer Group. Ann Surg 2020;271:e102–e104. [DOI] [PubMed] [Google Scholar]
- 8. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499. [DOI] [PubMed] [Google Scholar]
- 9. van Rijssen LB, Koerkamp BG, Zwart MJ, Bonsing BA, Bosscha K, van Dam RM. et al. ; Dutch Pancreatic Cancer Group. Nationwide prospective audit of pancreatic surgery: design, accuracy, and outcomes of the Dutch Pancreatic Cancer Audit. HPB (Oxford) 2017;19:919–926. [DOI] [PubMed] [Google Scholar]
- 10. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M. et al. ; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584–591. [DOI] [PubMed] [Google Scholar]
- 11. Knaus WA, Draper EA, Wagner DP, Zimmerman JE.. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–829. [PubMed] [Google Scholar]
- 12. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 13. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L. et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–688. [DOI] [PubMed] [Google Scholar]
- 14. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR. et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–768. [DOI] [PubMed] [Google Scholar]
- 15. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ. et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007;142:20–25. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 17. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG. et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luu AM, Krasemann L, Fahlbusch T, Belyaev O, Janot-Matuschek M, Uhl W, Braumann C.. Facing the surgeon's nightmare: Incidence and management of postoperative pancreatic fistulas grade C after pancreaticoduodenectomy based on the updated definition of the International Study Group of Pancreatic Surgery (ISGPS). J Hepatobiliary Pancreat Sci 2020;27:171–181. [DOI] [PubMed] [Google Scholar]
- 19. Ma T, Bai X, Chen W, Lao M, Jin G, Zheng K, Fu D, Yang F, Qin R, Li X, Lou W, Zhang L, Jiang K, Wu P, Shao C, Liu A, Yang Y, Ma Y, Wu H, Liang T.. Surgical management and outcome of grade-C pancreatic fistulas after pancreaticoduodenectomy: A retrospective multicenter cohort study. Int J Surg 2019;68:27–34. [DOI] [PubMed] [Google Scholar]
- 20. Wroński M, Cebulski W, Witkowski B, Guzel T, Karkocha D, Lech G, Słodkowski M.. Surgical management of the grade C pancreatic fistula after pancreatoduodenectomy. HPB (Oxford). 2019;21:1166–1174. [DOI] [PubMed] [Google Scholar]
- 21. Ma T, Bai X, Chen W, Li G, Lao M, Liang T.. Pancreas-preserving management of grade-C pancreatic fistula and a novel bridging technique for repeat pancreaticojejunostomy: An observational study. Int J Surg 2018;52:243–247. [DOI] [PubMed] [Google Scholar]
- 22. Horvath P, Beckert S, Nadalin S, Königsrainer A, Königsrainer I.. Pancreas-preserving surgical management of grade-C pancreatic fistulas after pancreatic head resection by external wirsungostomy. Langenbecks Arch Surg 2016;401:457–462. [DOI] [PubMed] [Google Scholar]
- 23. Nentwich MF, El Gammal AT, Lemcke T, Ghadban T, Bellon E, Melling N, Bachmann K, Reeh M, Uzunoglu FG, Izbicki JR, Bockhorn M.. Salvage Completion Pancreatectomies as Damage Control for Post-pancreatic Surgery Complications: A Single-Center Retrospective Analysis. World J Surg. 2015;39:1550–1556. [DOI] [PubMed] [Google Scholar]
- 24. Wiltberger G, Schmelzle M, Tautenhahn HM, Krenzien F, Atanasov G, Hau HM, Moche M, Jonas S.. Alternative treatment of symptomatic pancreatic fistula. J Surg Res 2015;196:82–89. [DOI] [PubMed] [Google Scholar]
- 25. Almond M, Roberts KJ, Hodson J, Sutcliffe R, Marudanayagam R, Isaac J, Muiesan P, Mirza D.. Changing indications for a total pancreatectomy: perspectives over a quarter of a century. HPB (Oxford). 2015;17:416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balzano G, Pecorelli N, Piemonti L, Ariotti R, Carvello M, Nano R, Braga M, Staudacher C.. Relaparotomy for a pancreatic fistula after a pancreaticoduodenectomy: a comparison of different surgical strategies. HPB (Oxford). 2014;16:40–45. doi: 10.1111/hpb.12062. Epub 2013 Feb 20. PMID: 23458209; PMCID: PMC3892313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paye F, Lupinacci RM, Kraemer A, Lescot T, Chafaï N, Tiret E, Balladur P.. Surgical treatment of severe pancreatic fistula after pancreaticoduodenectomy by wirsungostomy and repeat pancreatico-jejunal anastomosis. Am J Surg 2013;206:194–201. [DOI] [PubMed] [Google Scholar]
- 28. Ribero D, Amisano M, Zimmitti G, Giraldi F, Ferrero A, Capussotti L.. External tube pancreatostomy reduces the risk of mortality associated with completion pancreatectomy for symptomatic fistulas complicating pancreaticoduodenectomy. J Gastrointest Surg 2013;17:332–338. [DOI] [PubMed] [Google Scholar]
- 29. Govil S. Salvage pancreaticogastrostomy for pancreatic fistulae after pancreaticoduodenectomy. Indian J Gastroenterol. 2012;31:263–266. [DOI] [PubMed] [Google Scholar]
- 30. Denost Q, Pontallier A, Rault A, Ewald JA, Collet D, Masson B, Sa-Cunha A.. Wirsungostomy as a salvage procedure after pancreaticoduodenectomy. HPB (Oxford). 2012;14:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu J, Dai X, Bu X, Gao F, Zhang X.. Pancreaticojejunal bridge-anastomosis: a novel option for surgeon to preserve pancreatic body and tail in urgent reoperation for intra-abdominal massive hemorrhage after pancreaticoduodenectomy. World J Surg 2010;34:2457–2462. [DOI] [PubMed] [Google Scholar]
- 32. Kent TS, Callery MP, Vollmer CM Jr.. The bridge stent technique for salvage of pancreaticojejunal anastomotic dehiscence. HPB (Oxford). 2010;12:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Königsrainer I, Zieker D, Beckert S, Glatzle J, Schroeder TH, Heininger A, Nadalin S, Königsrainer A.. A pancreas-preserving technique for the management of symptomatic pancreatic anastomotic insufficiency refractory to conservative treatment after pancreas head resection. Langenbecks Arch Surg 2010;395:693–696. [DOI] [PubMed] [Google Scholar]
- 34. Fuks D, Piessen G, Huet E, Tavernier M, Zerbib P, Michot F, Scotte M, Triboulet JP, Mariette C, Chiche L, Salame E, Segol P, Pruvot FR, Mauvais F, Roman H, Verhaeghe P, Regimbeau JM.. Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg 2009;197:702–709. [DOI] [PubMed] [Google Scholar]
- 35. Haddad LB, Scatton O, Randone B, Andraus W, Massault PP, Dousset B, Soubrane O.. Pancreatic fistula after pancreaticoduodenectomy: the conservative treatment of choice. HPB (Oxford). 2009;11:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bachellier P, Oussoultzoglou E, Rosso E, Scurtu R, Lucescu I, Oshita A, Jaeck D.. Pancreatogastrostomy as a salvage procedure to treat severe postoperative pancreatic fistula after pancreatoduodenectomy. Arch Surg 2008;143:966–970. [DOI] [PubMed] [Google Scholar]
- 37. Müller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, Breisch-Girbig D, Ceyhan GO, Büchler MW.. Is there still a role for total pancreatectomy? Ann Surg 2007;246:966-974. [DOI] [PubMed] [Google Scholar]
- 38. Tamijmarane A, Ahmed I, Bhati CS, Mirza DF, Mayer AD, Buckels JA, Bramhall SR.. Role of completion pancreatectomy as a damage control option for post-pancreatic surgical complications. Dig Surg 2006;23:229–34. [DOI] [PubMed] [Google Scholar]
- 39. de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ.. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg 2005;92:1117-23. [DOI] [PubMed] [Google Scholar]
- 40. Kazanjian KK, Hines OJ, Eibl G, Reber HA.. Management of pancreatic fistulas after pancreaticoduodenectomy: results in 437 consecutive patients. Arch Surg 2005;140:849–854. [DOI] [PubMed] [Google Scholar]
- 41. Gueroult S, Parc Y, Duron F, Paye F, Parc R.. Completion pancreatectomy for postoperative peritonitis after pancreaticoduodenectomy: early and late outcome. Arch Surg 2004;139:16–9. [DOI] [PubMed] [Google Scholar]
- 42. Munoz-Bongrand N, Sauvanet A, Denys A, Sibert A, Vilgrain V, Belghiti J.. Conservative management of pancreatic fistula after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg 2004;199:198–203. [DOI] [PubMed] [Google Scholar]
- 43. Schlitt HJ, Schmidt U, Simunec D, Jäger M, Aselmann H, Neipp M, Piso P.. Morbidity and mortality associated with pancreatogastrostomy and pancreatojejunostomy following partial pancreatoduodenectomy. Br J Surg 2002;89:1245–1251. [DOI] [PubMed] [Google Scholar]
- 44. van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ.. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg 1997;185:18–24. [DOI] [PubMed] [Google Scholar]
- 45. Yeh TS, Jan YY, Jeng LB, Hwang TL, Wang CS, Chen SC, Chao TC, Chen MF. Pancreaticojejunal anastomotic leak after pancreaticoduodenectomy–multivariate analysis of perioperative risk factors. J Surg Res 1997;67:119–125. [DOI] [PubMed] [Google Scholar]
- 46. Farley DR, Schwall G, Trede M.. Completion pancreatectomy for surgical complications after pancreaticoduodenectomy. Br J Surg 1996;83:176–179. [PubMed] [Google Scholar]
- 47. Wu CC, Hwang CR, Yeh DC, Hwang YC, Liu TJ, P'eng FK.. Treatment for dehiscence of pancreaticojejunostomy after pancreaticoduodenectomy: is resection of the residual pancreas necessary? Hepatogastroenterology 1996;43:271–274. [PubMed] [Google Scholar]
- 48. Cullen JJ, Sarr MG, Ilstrup DM.. Pancreatic anastomotic leak after pancreaticoduodenectomy: incidence, significance, and management. Am J Surg 1994;168:295–298. [DOI] [PubMed] [Google Scholar]
- 49. Smith CD, Sarr MG, vanHeerden JA.. Completion pancreatectomy following pancreaticoduodenectomy: clinical experience. World J Surg 1992;16:521–524. [DOI] [PubMed] [Google Scholar]
- 50. Hackert T, Hinz U, Pausch T, Fesenbeck I, Strobel O, Schneider L. et al. Postoperative pancreatic fistula: we need to redefine grades B and C. Surgery 2016;159:872–877. [DOI] [PubMed] [Google Scholar]
- 51. Mackay TM, Gleeson EM, Wellner UF, Williamsson C, Busch OR, Groot Koerkamp B. et al. Transatlantic registries of pancreatic surgery in the United States of America, Germany, the Netherlands, and Sweden: comparing design, variables, patients, treatment strategies, and outcomes . Surgery 2021;169:396–402. [DOI] [PubMed] [Google Scholar]
- 52. Wroński M, Cebulski W, Witkowski B, Guzel T, Karkocha D, Lech G. et al. Surgical management of the grade C pancreatic fistula after pancreatoduodenectomy. HPB (Oxford) 2019;21:1166–1174. [DOI] [PubMed] [Google Scholar]
- 53. Bressan AK, Wahba M, Dixon E, Ball CG.. Completion pancreatectomy in the acute management of pancreatic fistula after pancreaticoduodenectomy: a systematic review and qualitative synthesis of the literature. HPB (Oxford) 2018;20:20–27. [DOI] [PubMed] [Google Scholar]
- 54. van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH. et al. ; Dutch Pancreatitis Study Group. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491–1502. [DOI] [PubMed] [Google Scholar]
- 55. Oberkofler CE, Hamming JF, Staiger RD, Brosi P, Biondo S, Farges O. et al. Procedural surgical RCTs in daily practice: do surgeons adopt or is it just a waste of time? Ann Surg 2019;270:727–734. [DOI] [PubMed] [Google Scholar]
- 56. Hanna-Sawires RG, Groen JV, Klok FA, Tollenaar R, Mesker WE, Swijnenburg RJ. et al. Outcomes following pancreatic surgery using three different thromboprophylaxis regimens. Br J Surg 2019;106:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smits FJ, Verweij ME, Daamen LA, van Werkhoven CH, Goense L, Besselink MG. et al. Impact of complications after pancreatoduodenectomy on mortality, organ failure, hospital stay, and readmission: analysis of a nationwide audit. Ann Surg 2020; DOI: 10.1097/SLA.0000000000003835. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 58. Smits FJ, Henry AC, van Eijck CH, Besselink MG, Busch OR, Arntz M. et al. ; Dutch Pancreatic Cancer Group. Care after pancreatic resection according to an algorithm for early detection and minimally invasive management of pancreatic fistula versus current practice (PORSCH-trial): design and rationale of a nationwide stepped-wedge cluster-randomized trial. Trials 2020;21:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma T, Bai X, Chen W, Lao M, Jin G, Zheng K. et al. Surgical management and outcome of grade-C pancreatic fistulas after pancreaticoduodenectomy: a retrospective multicenter cohort study. Int J Surg 2019;68:27–34. [DOI] [PubMed] [Google Scholar]
- 60. Bachellier P, Oussoultzoglou E, Rosso E, Scurtu R, Lucescu I, Oshita A. et al. Pancreatogastrostomy as a salvage procedure to treat severe postoperative pancreatic fistula after pancreatoduodenectomy. Arch Surg 2008;143:966–970. [DOI] [PubMed] [Google Scholar]
- 61. Govil S. Salvage pancreaticogastrostomy for pancreatic fistulae after pancreaticoduodenectomy. Indian J Gastroenterol 2012;31:263–266. [DOI] [PubMed] [Google Scholar]
- 62. Paye F, Lupinacci RM, Kraemer A, Lescot T, Chafaï N, Tiret E. et al. Surgical treatment of severe pancreatic fistula after pancreaticoduodenectomy by wirsungostomy and repeat pancreatico-jejunal anastomosis. Am J Surg 2013;206:194–201. [DOI] [PubMed] [Google Scholar]
- 63. Scholten L, Stoop TF, Del Chiaro M, Busch OR, van Eijck C, Molenaar IQ. et al. ; Dutch Pancreatic Cancer Group. Systematic review of functional outcome and quality of life after total pancreatectomy. Br J Surg 2019;106:1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mackay TM, Smits FJ, Latenstein AEJ, Bogte A, Bonsing BA, Bos H. et al. ; Dutch Pancreatic Cancer Group. Impact of nationwide enhanced implementation of best practices in pancreatic cancer care (PACAP-1): a multicenter stepped-wedge cluster randomized controlled trial. Trials 2020;21:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.