This study investigated postoperative complications after surgery for medullary thyroid carcinoma (MTC) in Europe. Hypoparathyroidism, recurrent laryngeal nerve palsy and bleeding requiring reoperation occurred in 170 (26·2 per cent), 62 (13·7 per cent) and 17 (2·6 per cent) patients respectively. Complications after surgery for medullary thyroid carcinoma were procedure‐specific; the extent of surgery should therefore be a careful balance between unavoidable consequences of radical tumour resection against the risk of avoidable complications.
Hard to improve on excellence
Abstract
Background
Surgery is the curative therapy for patients with medullary thyroid carcinoma (MTC). In determining the extent of surgery, the risk of complications should be considered. The aim of this study was to assess procedure‐specific outcomes and risk factors for complications after surgery for MTC.
Methods
Patients who underwent thyroid surgery for MTC were identified in two European prospective quality databases. Hypoparathyroidism was defined by treatment with calcium/active vitamin D. Recurrent laryngeal nerve (RLN) palsy was diagnosed on laryngoscopy. Complications were considered at least transient if present at last follow‐up. Risk factors for at‐least transient hypoparathyroidism and RLN palsy were identified by logistic regression analysis.
Results
A total of 650 patients underwent surgery in 69 centres at a median age of 56 years. Hypoparathyroidism, RLN palsy and bleeding requiring reoperation occurred in 170 (26·2 per cent), 62 (13·7 per cent) and 17 (2·6 per cent) respectively. Factors associated with hypoparathyroidism were central lymph node dissection (CLND) (odds ratio (OR) 2·20, 95 per cent c.i. 1·04 to 4·67), CLND plus unilateral lateral lymph node dissection (LLND) (OR 2·78, 1·20 to 6·43), CLND plus bilateral LLND (OR 2·83, 1·13 to 7·05) and four or more parathyroid glands observed (OR 4·18, 1·46 to 12·00). RLN palsy was associated with CLND plus LLND (OR 4·04, 1·12 to 14·58) and T4 tumours (OR 12·16, 4·46 to 33·18). After compartment‐oriented lymph node dissection, N0 status was achieved in 248 of 537 patients (46·2 per cent).
Conclusion
Complications after surgery for MTC are procedure‐specific and may relate to the unavoidable consequences of radical dissection needed in some patients.
Resumen
Antecedentes
La cirugía es el tratamiento curativo para pacientes con carcinoma medular de tiroides (medullary thyroid carcinoma, MTC). A la hora de determinar le extensión de la cirugía, conviene considerar el riesgo de complicaciones. El objetivo de este estudio fue evaluar los resultados específicos del procedimiento quirúrgico y los factores de riesgo de las complicaciones tras la cirugía por un MTC.
Métodos
Los pacientes sometidos a cirugía tiroidea por MTC se identificaron a partir de dos bases de datos prospectivas de calidad europeas. El hipoparatiroidismo se definió como el tratamiento con calcio/vitamina D activa. La parálisis del nervio laríngeo recurrente (recurrent laryngeal nerve, RLN) se diagnosticó por laringoscopia. Las complicaciones se consideraron “como mínimo transitorias” si estaban presentes en el último seguimiento. Los factores de riesgo de hipoparatiroidismo como mínimo transitorio y parálisis del RLN se determinaron por análisis de regresión logística, con estimación de la razón de oportunidades (odds ratio, OR) y los i.c. del 95%.
Resultados
Se operaron un total de 650 pacientes en 69 centros, con una mediana de edad de 56 años. El hipoparatiroidismo, la parálisis del RLN y la hemorragia que precisó reoperación se presentaron en 170 (26,2%), 62 (62/451; 13,7%) y 17 (2,6%) pacientes, respectivamente. Los factores asociados a hipoparatiroidismo fueron la disección ganglionar cervical central (central lymph node dissection, CLND) (OR 2,20 (1,04‐4,67)), CLND más vaciaje ganglionar lateral de un lado (lateral lymph node dissection, LLND) (2,78 (1,20‐6,43)), CLND más LLND bilateral (2,83 (1,13‐7,05)) y la observación de cuatro o más glándulas paratiroideas (4,18 (1,46‐12,00)). La parálisis del RLN se asoció a CLND más LLND (4,04 (1,12‐14,58) y tumores T4 (12,16 (4,46‐33,18)). Tras un vaciaje ganglionar orientado a los compartimentos ganglionares, un 46,2% (248/537) de los pacientes tenían estadio N0.
Conclusión
Las complicaciones tras la cirugía por un MTC son específicas del procedimiento, algunas de las cuales son inevitables como consecuencia de la disección radical que precisan algunos pacientes.
Introduction
Medullary thyroid carcinoma (MTC) is a calcitonin‐producing neuroendocrine tumour originating from thyroid C‐cells. The estimated incidence is less than 0·5 per 100 000 people1–4. Patients with MTC often present with advanced lymphatic spread5–7. Ten‐year disease‐specific survival rates range from 75 to 82 per cent in population‐based cohorts1,8,9. Operative resection is the cornerstone of curative treatment10,11. The American Thyroid Association guidelines10 recommend total thyroidectomy (TT) plus central lymph node dissection (CLND) as the minimum strategy in patients with a preoperative diagnosis of MTC. Ipsilateral and/or contralateral lateral lymph node dissections (LLNDs) might be performed depending on clinical or ultrasonographic findings or calcitonin levels10.
When deciding on the optimal extent of surgery, the oncological benefit should be weighed against the risk of surgical complications. In a population‐based study12, hypoparathyroidism or recurrent laryngeal nerve (RLN) palsy occurred in 12·3 per cent after surgery for all types of thyroid cancer. Studies including patients with MTC reported any form of hypoparathyroidism and RLN palsy in 5–27·6 per cent and 2·5–9·5 per cent respectively6,13–17. These studies were hampered by a limited sample size (most studies comprising less than 150 patients) and retrospective design. Patients were generally included over a long time span and mostly from expert centres, calling into question the generalizability of the outcomes6,13–17. Risk factors for complications were not addressed, although analysis of these factors is pivotal for adequate preoperative patient counselling and decision‐making regarding the extent of surgery. The aim of the present study was to assess procedure‐specific risk factors for complications in patients with previously untreated MTC in a large European cohort registered in the European database EUROCRINE® and the Scandinavian Quality Register for Thyroid, Parathyroid and Adrenal Surgery (SQRTPA).
Methods
All surgical procedures for thyroid diseases were extracted from two multicentre quality registries for endocrine surgical procedures: SQRTPA and EUROCRINE®. Duplicate records were identified and excluded. Adults and children who underwent primary thyroid surgery for previously untreated (no thyroid surgery in medical history) and histopathologically proven MTC between January 2004 and September 2019 were included in the study. Patients without histopathologically proven MTC were excluded, as were those who had prophylactic surgery owing to hereditary disease without MTC in the resection specimen.
Scandinavian Quality Register for Thyroid, Parathyroid and Adrenal Surgery
The SQRTPA, a prospective online database, has recorded endocrine surgical procedures from all hospitals performing thyroid surgery in Sweden since its initiation in 2004. Data collection is supervised by the principal surgeon in each centre. The registry is supported by the Swedish Association of Endocrine Surgeons and the Swedish Association of Otorhinolaryngology and Head and Neck Surgery. Data quality control is undertaken at a national level by means of annual audits by an external auditor, which has proven good data quality with an error rate of less than 5 per cent. At present, 37 centres participate in SQRTPA.
EUROCRINE ® database
EUROCRINE® is an online prospective database that has registered endocrine surgical procedures from centres across Europe since 2015. EUROCRINE® was started as a project within the Health Programme of the European Union in 2013 to improve morbidity and mortality in patients undergoing surgical treatment of endocrine tumours. EUROCRINE® is managed by the EUROCRINE® Society, based in Vienna, and with a council with representation from 13 participating national endocrine surgical societies and the European Society of Endocrine Surgeons. In 2017, the SQRTPA was moved to the EUROCRINE® platform. A personal registration is needed to enter data in EUROCRINE®, which is supplied to the principal investigator at each participating centre. Data collection is supervised by the principal surgeon at each site. Currently the EUROCRINE® database is used by 94 departments and clinics (https://www.eurocrine.eu). The owner of the platform is Region Skåne in Sweden.
Participating centres obtain informed consent from individual patients and pseudonymized (anonymized) data are stored under General Data Protection Regulation. This study was approved by the Ethical Committee of Lund University (numbers 2018/723 and 2019‐00689), and the council and steering committee of EUROCRINE® and SQRTPA respectively.
Data collection and clinical definitions
Data regarding demography, preoperative evaluation, extent of surgery including lymph node dissection (LND), intraoperative findings, histopathology and complications (in hospital and during follow‐up) were extracted. Duration of operation was defined as the interval from skin incision to skin closure. Duration of hospital stay was defined as number of days from the date of surgery until date of discharge. Tumours were staged according to the seventh or eighth edition of the AJCC/UICC TNM classification, depending on the year of surgery. For analysis, the TNM classifications were combined18. The lymph node ratio was calculated by dividing the number of metastatic lymph nodes by the total number of resected lymph nodes.
Surgical groups
To assess procedure‐specific complications, patients were grouped according to the type of thyroid resection and extent of LND. Patients who did not undergo TT, including those who underwent bilateral (subtotal) thyroid resection, were considered to have had less than TT with or without concurrent LND. Patients for whom no formal LND was registered were regarded as having not been subjected to LND, regardless of the presence of any lymph nodes in the histological specimen. CLND included unilateral or bilateral central dissection, and patients undergoing LLND were all assumed to have undergone CLND.
Outcome definitions
The duration of follow‐up differed for patients across registries and follow‐up was also incomplete for some patients. Therefore, it was not possible to determine the true rate of permanent complications (hypoparathyroidism and RLN palsy); instead, rates of transient and at least transient complications were calculated. At least transient complications were considered the main outcome of the study. Complications could be reported during the hospital stay, at discharge, at first follow‐up at 1–6 weeks after discharge, and at a possible second follow‐up visit at around 6 months after surgery.
Hypoparathyroidism
Hypoparathyroidism was defined by a report in the registers of the prescription of calcium (oral or intravenous) or active vitamin D (1,25‐dihydroxy‐cholecalciferol) after surgery. Patients receiving treatment at discharge or at the first follow‐up visit were considered to have had transient hypoparathyroidism if no treatment was prescribed at a later follow‐up visit. Patients on medication at second follow‐up and those on medication at the first follow‐up without additional follow‐up data were considered to have at least transient hypoparathyroidism. Patients without medication at discharge and thereafter were considered not to have hypoparathyroidism. For patients without follow‐up data after discharge, it could not be determined whether hypoparathyroidism was present. Indications for parathyroidectomy –hereditary MTC plus simultaneous primary hyperparathyroidism or parathyroidectomy owing to tumour infiltration – were not registered.
Recurrent laryngeal nerve palsy
RLN palsy was diagnosed based on findings at laryngoscopy during the hospital stay, at first follow‐up and at a possible second follow‐up visit. Patients with an abnormal laryngoscopy followed by a normal laryngoscopy were considered to have had transient RLN palsy. Patients with an abnormal laryngoscopy that was not followed by a normal laryngoscopy were considered to have at least transient RLN palsy. The presence or absence of preoperative RLN paresis was recorded in patients who underwent preoperative laryngoscopy. Any resection of the RLN because of tumour infiltration was recorded.
Bleeding and other complications
Postoperative bleeding was defined as haemorrhage requiring reoperation. Other complications were also recorded, regardless of severity. Postoperative mortality was assessed in the first 30 days after surgery.
Statistical analysis
Continuous variables are reported as median (i.q.r.) and categorical variables as counts with percentages. Differences in complications between patients undergoing TT without compartment‐oriented LND versus TT plus compartment‐oriented LND were analysed using the Mann–Whitney U test for continuous data and χ2 or Fisher's exact test for categorical variables. Groups that underwent TT plus CLND versus TT plus CLND plus LLND were also compared. The rate of N0 to N1a and N1b, number of resected lymph nodes, number of metastatic lymph nodes and lymph node ratio, in relation to extent of LND (CLND and LLND), was also determined. The reasons for LND (prophylactic, diagnostic or therapeutic based on the surgeon's judgement) was compared between groups.
Univariable and multivariable logistic regression analyses were undertaken to identify factors associated with at least transient hypoparathyroidism and at least transient RLN palsy. Odds ratios (ORs) with 95 per cent confidence intervals were calculated. Potential confounders were selected based on clinical reasoning. For hypoparathyroidism, age, sex, type of thyroid surgery, type of LND, T, N, M category, parathyroid excision, parathyroid reimplantation and number of parathyroid glands observed during surgery were included in the model. For RLN palsy, type of LND, T category and the use of intraoperative nerve monitoring (IONM) were included. Patients who had TT, bilateral resection or lobectomy plus resection of contralateral lobe were regarded as having bilateral surgery. All other thyroid procedures were regarded as unilateral thyroid surgery. Patients with N0 and Nx tumours and those with M0 and Mx disease were grouped as N0/Nx and M0/Mx for logistic regression analyses. Patients without follow‐up data regarding treatment for hypoparathyroidism were excluded from analysis of hypoparathyroidism, as were those with a preoperative diagnosis of RLN palsy from the evaluation of RLN palsy.
Missing data were considered as missing at random, and therefore handled by multivariable imputation by chained equations using 20 iterations and creating 25 data sets19. Age, sex, type of thyroid surgery, type of LND, duration of operation, T, N and M categories, tumour size, number of resected and metastatic lymph nodes, and year of surgery were used for multiple imputation of missing data for variables used in logistic regression with the primary outcome hypoparathyroidism or RLN palsy20. Outcomes (hypoparathyroidism and RLN palsy) were not imputed. Outcome‐specific variables were added; for the analysis of hypoparathyroidism, parathyroid resection, parathyroid reimplantation and number of parathyroid glands observed during surgery were added. For the analysis of RLN palsy, IONM, number of nerves identified, intraoperative suspicion of RLN damage and its cause (intentional versus unintentional) were included. Pooled ORs with 95 per cent confidence intervals were calculated using Rubin's rules21. Complete‐case and multiple imputation analyses were performed. Two‐sided P < 0·050 was considered statistically significant. Statistical analyses were done using SPSS® version 25.0 (IBM, Armonk, New York, USA).
Results
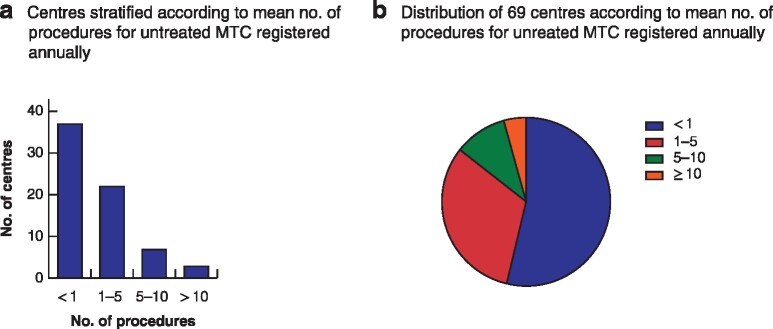
A total of 118 centres registered 59 356 unique procedures; 1119 procedures (1·9 per cent) were registered for MTC or C‐cell hyperplasia, of which 650 (1·1 per cent of total) were for previously untreated MTC (Fig. 1). These procedures were registered in 69 centres (58 per cent) with a median of 0·7 (i.q.r. 0·3–2·2) primary procedures for MTC annually; these centres reported 70 (40–213) procedures annually for thyroid diseases. More than half of the centres (37, 53 per cent) reported performing less than one primary procedure for previously untreated MTC annually (Fig. 2).
Fig. 1.
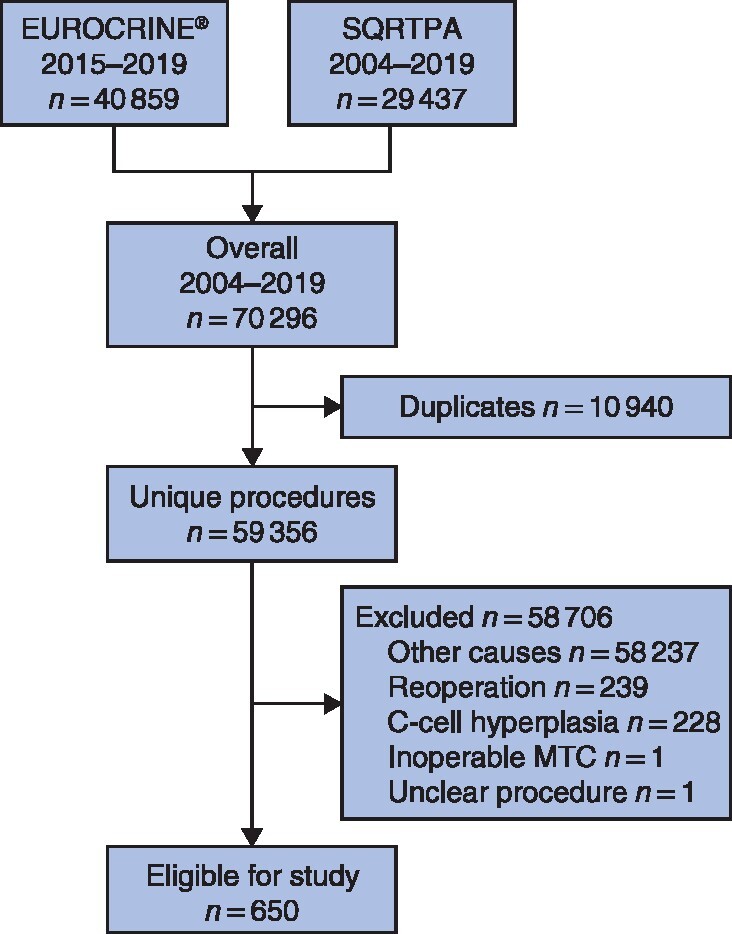
Study flow chart
SQRTPA, Scandinavian Quality Register for Thyroid, Parathyroid and Adrenal Surgery; MTC, medullary thyroid carcinoma.
Fig. 2.
Distribution of procedures for previously untreated medullary thyroid carcinoma between centres
a Number of centres according to annual number of procedures for previously untreated medullary thyroid carcinoma (MTC); b distribution of centres according to mean annual number of procedures for previously untreated MTC.
Baseline characteristics and investigations
Patients underwent surgery at a median age of 56 (i.q.r. 43–66, range 1–85) years and 23 patients (3·5 per cent) were younger than 18 years (Table 1). The majority of patients were female (62·3 per cent). Most patients (556, 85·5 per cent) underwent TT. A compartment‐oriented LND was performed in 537 patients (82·6 per cent).
Table 1.
Baseline and surgical characteristics
| No. of patients* (n = 650) | |
|---|---|
| Age (years) † | 56·0 (43·0–66·0) |
| Sex ratio (M : F) | 245 : 405 |
| Primary tumour diagnosis | |
| MTC | 627 (96·5) |
| Other with MTC as secondary diagnosis | 18 (2·8) |
| Mixed MTC/FTC | 5 (0·8) |
| Preoperative diagnosis (based on cytology and/or biopsy) | |
| Not performed | 214 (32·9) |
| Non‐diagnostic/inadequate | 11 (1·7) |
| Benign | 34 (5·2) |
| Atypia/follicular lesion of unknown significance | 13 (2·0) |
| Follicular neoplasm | 27 (4·2) |
| Suspicious for malignancy | 56 (8·6) |
| Malignant | 295 (45·4) |
| Indication for surgery | |
| Thyrotoxicosis | 9 (1·4) |
| Compression symptoms | 11 (1·7) |
| Excluding malignancy | 123 (18·9) |
| Malignancy | 494 (76·0) |
| Other indication | 13 (2·0) |
| Thyroid surgery | |
| Unilateral lobectomy of thyroid gland | 65 (10·0) |
| Unilateral resection of thyroid gland | 4 (0·6) |
| Bilateral resection of thyroid gland | 22 (3·4) |
| Total thyroidectomy | 556 (85·5) |
| Lobectomy and resection of contralateral lobe | 2 (0·3) |
| Other | 1 (0·2) |
| Lymph node dissection | |
| None | 90 (13·8) |
| Excision of lymph nodes | 23 (3·5) |
| CLND | 287 (44·2) |
| CLND plus unilateral LLND | 168 (25·8) |
| CLND plus bilateral LLND | 82 (12·6) |
| No. of parathyroid glands identified during surgery † | 3 (2–4) |
| 0 | 10 (1·5) |
| 1 | 51 (7·8) |
| 2 | 126 (19·4) |
| 3 | 146 (22·5) |
| 4 | 316 (48·6) |
| 5 | 1 (0·2) |
| Parathyroid reimplantation | 196 (30·2) |
| Parathyroid resection (n = 623) | 38 (6·1) |
| Intraoperative nerve monitoring (n = 578) | 498 (86·2) |
With percentages in parentheses unless indicated otherwise;
values are median (i.q.r.). MTC, medullary thyroid carcinoma; FTC, follicular thyroid carcinoma; CLND, central lymph node dissection; LLND, lateral lymph node dissection.
Hypoparathyroidism
In all, 170 patients (26·2 per cent) had at least transient and 91 (14·0 per cent) had transient hypoparathyroidism. Of the 170 patients with at least transient hypoparathyroidism, 47 (27·6 per cent) had 6‐month follow‐up data available.
A total of 223 patients had 6‐month follow‐up. Of these, 137 (61·4 per cent) had no hypoparathyroidism, 39 (17·5 per cent) had transient hypoparathyroidism and 47 (21·1 per cent) had at least transient hypoparathyroidism.
Recurrent laryngeal nerve palsy
Of 53 patients (53 of 521, 10·2 per cent) with RLN damage suspected during surgery, intentional damage was documented in 16 and unintentional damage in 24; the type of damage was not specified for the remaining 13 patients.
Some 199 patients (30·6 per cent) did not have laryngoscopy after surgery, so RLN function could be assessed in 451 (69·4 per cent). Of these, 62 (13·7 per cent) had at least transient and 15 (3·3 per cent) had transient RLN palsy. Four patients had bilateral palsy, at least transient in three and transient in one, of whom all had IONM. Intraoperative RLN damage was intentional in two of these patients. Of the 199 patients who did not undergo postoperative laryngoscopy, IONM was used in 113 of 162 patients (69·8 per cent) for whom nerve monitoring data were available.
Of 475 patients who underwent preoperative laryngoscopy, seven (1·5 per cent) had a documented RLN paresis, of whom three had a T4 tumour, three a T3 tumour and one a T1 tumour. Of these patients with preoperative vocal cord palsy, five had at least transient RLN palsy documented and one transient RLN palsy; the patient with a T1 tumour had normal findings at laryngoscopy during follow‐up.
Hospital stay, complications and mortality
Median duration of hospital stay was 2 (i.q.r. 1–4) days. One patient died within 30 days (0·2 per cent). Seventeen patients (2·6 per cent) had rebleeding that required reoperation. Any other complication was noted in 17 patients (2·6 per cent) and wound infections were reported in five patients (0·8 per cent), exclusively after compartment‐oriented LND.
Procedure‐specific outcomes and complications
Procedure‐specific outcomes are reported in Table 2. The occurrence of at least transient hypoparathyroidism and at least transient RLN palsy increased with the extent of thyroid surgery and LND. At least transient hypoparathyroidism and at least transient RLN palsy were observed most frequently after TT plus CLND plus bilateral LLND, in 27 (35 per cent) and 14 (26 per cent) patients respectively.
Table 2.
Procedure‐specific intraoperative and postoperative outcomes
| Less than TT (n = 35) | Less than TT + any LND (n = 59) | TT without LND (n = 55) | TT + LNE (n = 18) | TT + CLND (n = 251) | TT + CLND + unilateral LLND (n = 155) | TT + CLND + bilateral LLND (n = 77) | |
|---|---|---|---|---|---|---|---|
| Intraoperative outcomes | |||||||
| Duration of operation (min) (n = 603)* | 78 (55–105) | 105 (71–154) | 101 (80–126) | 150 (95–169) | 118 (85–145) | 220 (165–300) | 240 (184–370) |
| No. of parathyroid glands identified | |||||||
| 0 | 5 (14) | 2 (3) | 0 (0) | 0 (0) | 2 (0·8) | 1 (0·6) | 0 (0) |
| 1 | 18 (51) | 8 (14) | 4 (7) | 0 (0) | 5 (2·0) | 11 (7·1) | 5 (6) |
| 2 | 9 (26) | 24 (41) | 7 (13) | 4 (22) | 30 (12·0) | 37 (23·9) | 15 (19) |
| 3 | 1 (3) | 11 (19) | 17 (31) | 6 (33) | 50 (19·9) | 38 (24·5) | 23 (30) |
| 4 | 2 (6) | 14 (24) | 27 (49) | 8 (44) | 163 (64·9) | 68 (43·9) | 34 (44) |
| 5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0·4) | 0 (0) | 0 (0) |
| Parathyroid reimplantation | 5 (14) | 6 (10) | 3 (5) | 5 (28) | 68 (27·1) | 74 (47·7) | 35 (45) |
| IONM used (n = 578) | 18 (62) | 43 (78) | 39 (78) | 11 (85) | 212 (88·3) | 123 (93·9) | 52 (87) |
| No. of RLNs identified | |||||||
| 0 | 0 (0) | 2 (3) | 0 (0) | 0 (0) | 2 (0·8) | 3 (1·9) | 0 (0) |
| 1 | 32 (91) | 28 (47) | 1 (2) | 0 (0) | 1 (0·4) | 3 (1·9) | 1 (1) |
| 2 | 3 (9) | 29 (49) | 54 (98) | 18 (100) | 248 (98·8) | 149 (96·1) | 76 (99) |
| RLN damage (n = 521) | |||||||
| No | 18 (5) | 47 (85) | 40 (95) | 13 (100) | 208 (94·5) | 98 (83·8) | 44 (80) |
| Unilateral | 1 (5) | 8 (15) | 2 (5) | 0 (0) | 12 (5·5) | 19 (16·2) | 10 (18) |
| Bilateral | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Postoperative complications | |||||||
| Duration of hospital stay (days) (n = 502)* | 1·5 (1–2) | 2 (1–3) | 2 (1–2) | 2 (1–3) | 2 (1–3) | 3 (2–5) | 4 (2–6) |
| Calcium therapy (n = 627) | |||||||
| Oral only | 1 (3) | 8 (15) | 8 (15) | 7 (30) | 74 (29·6) | 56 (36·8) | 28 (41) |
| Intravenous only | 0 (0) | 1 (2) | 1 (2) | 0 (0) | 1 (0·4) | 1 (0·7) | 1 (1) |
| Oral and intravenous | 0 (0) | 1 (2) | 1 (2) | 1 (6) | 4 (1·6) | 7 (4·6) | 7 (10) |
| Treatment at discharge | |||||||
| Calcium only | 0 (0) | 1 (2) | 3 (5) | 3 (17) | 9 (3·6) | 21 (13·5) | 8 (10) |
| Active vitamin D only | 0 (0) | 2 (3) | 0 (0) | 1 (6) | 3 (1·2) | 4 (2·6) | 3 (4) |
| Calcium and active vitamin D | 1 (3) | 7 (12) | 9 (16) | 2 (11) | 102 (40·6) | 52 (33·5) | 27 (35) |
| Hypoparathyroidism | |||||||
| No (or during hospital stay) | 34 (97) | 48 (81) | 39 (71) | 11 (61) | 132 (52·6) | 78 (50·3) | 34 (44) |
| At discharge, no follow‐up | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (3·6) | 4 (2·6) | 0 (0) |
| Transient – recovered | 1 (3) | 5 (8) | 6 (11) | 3 (17) | 35 (13·9) | 25 (16·1) | 16 (21) |
| At least transient | 0 (0) | 6 (10) | 10 (18) | 4 (11) | 75 (29·9) | 48 (31·0) | 27 (35) |
| Laryngoscopy | 23 (66) | 36 (61) | 32 (58) | 9 (50) | 186 (74·1) | 112 (72·3) | 53 (69) |
| RLN palsy (n = 451) | |||||||
| No | 21 (91) | 27 (75) | 30 (94) | 9 (100) | 165 (88·7) | 84 (75·0) | 38 (72) |
| Transient – recovered | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 6 (3·2) | 7 (6·3) | 1 (2) |
| At least transient | 2 (9) | 9 (25) | 1 (3) | 0 (0) | 15 (8·1) | 21 (18·8) | 14 (26) |
| Bleeding requiring reoperation | 1 (3) | 0 (0) | 2 (4) | 0 (0) | 5 (2·0) | 7 (4·5) | 2 (3) |
| Wound infection (n = 642) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (0·8) | 1 (0·7) | 2 (3) |
| Other complication (any grade) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (1·2) | 11 (7·1) | 3 (4) |
Values in parentheses are percentages unless indicated otherwise; *values are median (i.q.r.). Bilateral subtotal/near‐total thyroidectomy was considered as less than total thyroidectomy (TT). LND, lymph node dissection; LNE, lymph node excision; CLND, central lymph node dissection; LLND, lateral lymph node dissection; IONM, intraoperative nerve monitoring; RLN, recurrent laryngeal nerve.
Patients who underwent TT plus compartment‐oriented LND significantly more often had at least transient hypoparathyroidism (31·1 versus 19 per cent) and RLN palsy (14·2 versus 2 per cent) than those who had TT without compartment‐oriented LND (Table S1, supporting information).
The rate of at least transient hypoparathyroidism was similar after TT plus CLND only and TT plus CLND plus LLND (Table S2, supporting information). Patients in the TT plus CLND plus LLND group more often developed at least transient RLN palsy than patients who had TT plus CLND only (21·2 versus 8·1 per cent).
Of the 242 patients who had TT plus CLND without LLND, at least transient hypoparathyroidism occurred in seven of 26 patients (27 per cent) who underwent unilateral CLND compared with 68 of 216 (31·5 per cent) who had bilateral CLND.
Lymph node dissections and histopathological outcomes
Histopathological data for the whole cohort are summarized in Table 3. Most patients underwent LND for prophylactic reasons in the CLND group, and for therapeutic reasons in the CLND plus LLND group (Table 4). The number of MTC‐positive lymph nodes increased with the extent of LND. Thus, N0 disease was observed in 176 patients (61·3 per cent) who had CLND and in 72 (28·8 per cent) who underwent CLND plus LLND. Overall, 248 patients (46·2 per cent) undergoing a compartment‐oriented LND had no lymph node metastases. N1b disease was observed in 124 patients (49·6 per cent) who had CLND plus LLND.
Table 3.
Histopathological data
| No. of patients* (n = 650) | |
|---|---|
| Tumour size (mm) (n = 580) † | 13 (7–25) |
| No. of lymph nodes resected (n = 490) † | 10 (4–25) |
| No. of tumour‐positive lymph nodes (n = 531) † | 0 (0–3) |
| Lymph node ratio (n = 455) † | 0 (0–0·23) |
| T category | |
| T1a | 213 (32·8) |
| T1b | 186 (28·6) |
| T2 | 111 (17·1) |
| T3 | 76 (11·7) |
| T4a | 29 (4·5) |
| T4b | 3 (0·5) |
| Tx | 3 (0·5) |
| Unknown | 29 (4·5) |
| N category | |
| N0 | 283 (43·5) |
| N1a | 106 (16·3) |
| N1b | 130 (20·0) |
| Nx | 88 (13·5) |
| Unknown | 43 (6·6) |
| M category | |
| M0 | 359 (55·2) |
| M1 | 37 (5·7) |
| Mx | 162 (24·9) |
| Unknown | 92 (14·2) |
| Tumour stage | |
| I | 289 (44·5) |
| II | 81 (12·5) |
| III | 97 (14·9) |
| IVA | 108 (16·6) |
| IVB | 1 (0·2) |
| IVC | 37 (5·7) |
| Unknown | 37 (5·7) |
With percentages in parentheses unless indicated otherwise;
values are median (i.q.r.).
Table 4.
Lymph node dissections and histopathological findings
| Lymph node excision (n = 23) | CLND (n = 287) | CLND + unilateral LLND (n = 168) | CLND + bilateral LLND (n = 82) | |
|---|---|---|---|---|
| Reason for lymph node dissection (n = 490) | ||||
| Prophylactic | 1 (7) | 191 (70·5) | 47 (33·6) | 22 (34) |
| Diagnostic | 10 (67) | 31 (11·4) | 11 (7·9) | 9 (14) |
| Therapeutic | 4 (27) | 49 (18·1) | 82 (58·6) | 33 (52) |
| N category | ||||
| N0 | 12 (52) | 176 (61·3) | 49 (29·2) | 23 (28) |
| N1a | 4 (17) | 65 (22·6) | 23 (13·7) | 11 (13) |
| N1b | 1 (4) | 5 (1·7) | 81 (48·2) | 43 (52) |
| Nx | 2 (9) | 22 (7·7) | 3 (1·8) | 1 (1) |
| Unknown | 4 (17) | 19 (6·6) | 12 (7·1) | 4 (5) |
| No. of lymph nodes resected (n = 424) * | 3 (1–7) | 8 (5–12) | 27·5 (16·5–45·8) | 35·5 (21·8–68·3) |
| No. of tumour‐positive lymph nodes (n = 466) * | 0 (0–3) | 0 (0–1) | 3 (0–14) | 3 (0–16) |
| Lymph node ratio (n = 455) * | 0 (0–0·75) | 0 (0–0) | 0·15 (0–0·45) | 0·14 (0–0·34) |
Values in parentheses are percentages unless indicated otherwise;
values are median (i.q.r.). CLND, central lymph node dissection; LLND, lateral lymph node dissection.
Factors associated with at least transient hypoparathyroidism
Risk factors for at least hypoparathyroidism are shown in Table 5. Thirteen patients (2·0 per cent) had no follow‐up data available and were excluded from the analysis. Overall, 125 patients (19·6 per cent), of whom 47 had at least transient hypoparathyroidism, had data missing for any variable: T category (4·7 per cent), N category (6·4 per cent), M category (14·1 per cent) and parathyroid resection (4·2 per cent); these data were imputed.
Table 5.
Risk factors for hypoparathyroidism identified by univariable and multivariable regression analyses
| Hypoparathyroidism‡ |
Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| Yes (n = 170) | No (n = 467) | Crude odds ratio* | P | Adjusted odds ratio* | P | |
| Age (years) † | 56 (43–67) | 56 (43–66) | 1·00 (0·99, 1·01) | 0·954 | 1·00 (0·99, 1·01) | 0·790 |
| Sex | ||||||
| M | 62 (36·5) | 181 (38·8) | 1·00 (reference) | 1·00 (reference) | ||
| F | 108 (63·5) | 286 (61·2) | 1·10 (0·77, 1·59) | 0·599 | 1·04 (0·69, 1·55) | 0·864 |
| Thyroid surgery | ||||||
| Unilateral | 5 (2·9) | 64 (13·7) | 1·00 (reference) | 1·00 (reference) | ||
| Bilateral | 165 (97·1) | 403 (86·3) | 5·24 (2·08, 13·26) | < 0·001 | 2·05 (0·72, 5·79) | 0·177 |
| Lymph node dissection | ||||||
| None | 10 (5·9) | 80 (17·1) | 1·00 (reference) | 1·00 (reference) | ||
| Excision | 5 (2·9) | 18 (3·9) | 2·22 (0·68, 7·30) | 0·188 | 1·53 (0·43, 5·46) | 0·511 |
| CLND | 78 (45·9) | 200 (42·8) | 3·12 (1·54, 6·33) | 0·002 | 2·20 (1·04, 4·67) | 0·040 |
| CLND + unilateral LLND | 50 (29·4) | 114 (24·4) | 3·51 (1·68, 7·33) | 0·001 | 2·78 (1·20, 6·43) | 0·017 |
| CLND + bilateral LLND | 27 (15·9) | 55 (11·8) | 3·93 (1·76, 8·76) | 0·001 | 2·83 (1·13, 7·05) | 0·026 |
| T category | ||||||
| T1 | 100 (58·8) | 306 (65·5) | 1·00 (reference) | 1·00 (reference) | ||
| T2 | 38 (22·4) | 79 (16·9) | 1·48 (0·94, 2·33) | 0·093 | 1·52 (0·92, 2·49) | 0·101 |
| T3 | 21 (12·4) | 59 (12·6) | 1·07 (0·61, 1·86) | 0·824 | 1·16 (0·61, 2·23) | 0·649 |
| T4 | 11 (6·5) | 23 (4·9) | 1·56 (0·72, 3·37) | 0·262 | 1·99 (0·75, 5·24) | 0·166 |
| N category | ||||||
| N0/Nx | 104 (61·2) | 280 (60·0) | 1·00 (reference) | 1·00 (reference) | ||
| N1a | 29 (17·1) | 85 (18·2) | 0·93 (0·57, 1·50) | 0·754 | 0·71 (0·42, 1·20) | 0·197 |
| N1b | 37 (21·8) | 102 (21·8) | 0·99 (0·64, 1·54) | 0·964 | 0·58 (0·31, 1·09) | 0·090 |
| M category | ||||||
| M0/Mx | 153 (90·0) | 437 (93·6) | 1·00 (reference) | 1·00 (reference) | ||
| M1 | 17 (10·0) | 30 (6·4) | 1·60 (0·81, 3·16) | 0·179 | 2·15 (0·90, 5·10) | 0·084 |
| Parathyroid resection | ||||||
| No | 159 (93·5) | 441 (94·4) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 11 (6·5) | 26 (5·6) | 1·20 (0·57, 2·49) | 0·632 | 0·87 (0·40, 1·89) | 0·716 |
| Parathyroid reimplantation | ||||||
| No | 102 (60·0) | 342 (73·2) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 68 (40·0) | 125 (26·8) | 1·82 (1·26, 2·64) | 0·001 | 1·35 (0·90, 2·01) | 0·146 |
| No. parathyroid glands | ||||||
| 0 or 1 | 5 (2·9) | 56 (12·0) | 1·00 (reference) | 1·00 (reference) | ||
| 2 | 22 (12·9) | 101 (21·6) | 2·44 (0·88, 6·80) | 0·088 | 1·73 (0·59, 5·09) | 0·319 |
| 3 | 37 (21·8) | 108 (23·1) | 3·84 (1·43, 10·31) | 0·008 | 2·50 (0·86, 7·32) | 0·094 |
| 4 or 5 | 106 (62·4) | 202 (43·3) | 5·88 (2·29, 15·11) | < 0·001 | 4·18 (1·46, 12·00) | 0·008 |
Values in parentheses are percentages, except
95 per cent confidence intervals and
values are median (i.q.r.).
Data after multiple imputation. There were missing data for T category (4·7 per cent), N category (6·4 per cent), M category (14·1 per cent) and parathyroid resection (4·2 per cent). Multivariable analysis included all variables listed in the table. CLND, central lymph node dissection; LLND, lateral lymph node dissection.
In univariable analysis, bilateral thyroid surgery, CLND, CLND plus unilateral LLND, CLND plus bilateral LLND, parathyroid reimplantation, and the identification of three, four or five parathyroid glands during surgery were associated with at least transient hypoparathyroidism. After adjusting for potential confounders, CLND (OR 2·20, 95 per cent c.i. 1·04 to 4·67), CLND plus unilateral LLND (OR 2·78, 1·20 to 6·43), CLND plus bilateral LLND (OR 2·83, 1·13 to 7·05), and four or five parathyroid glands identified during surgery (OR 4·18, 1·46 to 12·00) were independently associated with hypoparathyroidism. Complete‐case analysis yielded similar results, albeit with wider 95 per cent confidence intervals owing to reduced statistical power (Table S3, supporting information). Of the patients with complete data, 15 of 87 (17 per cent) classified as Nx developed at least transient hypoparathyroidism compared with 82 of 277 (29·6 per cent) with disease classified as N0.
Factors associated with at least transient laryngeal nerve palsy
No preoperative RLN palsy was diagnosed and at least one postoperative laryngoscopy was undertaken in 445 patients. Data on use of IONM and T category were missing for 35 (7·9 per cent) and 24 (5·4 per cent) patients respectively, and were therefore imputed.
Factors associated with at least transient RLN palsy in univariable analysis were CLND plus LLND, T3 tumours, T4 tumours, N1a disease, N1b disease, M1 disease and suspected intraoperative nerve damage (Table S4, supporting information). Considering the relatively small number of events (57), a limited number of variables could be included in the multivariable analysis. Patients who had CLND plus LLND (OR 4·04, 95 per cent c.i. 1·12 to 14·58) and those with T4 tumours (OR 12·16, 4·46 to 33·18) had an increased risk of least transient RLN palsy, after adjustment for IONM use (Table 6). Fifty‐nine patients (13·3 per cent) were excluded from the complete‐case analysis because of missing data, of whom eight were diagnosed with RLN palsy, leaving only 49 patients with RLN palsy. Results for T category were similar to those of the multiple imputation analysis, whereas the point estimate for CLND plus LLND decreased slightly (OR 2·93, 0·79 to 10·78), probably owing to the limited number of outcomes (Table S5, supporting information).
Table 6.
Risk factors for recurrent laryngeal nerve palsy identified by univariable and multivariable regression analyses
| RLN palsy† |
Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| Yes (n = 57) | No (n = 388) | Crude odds ratio* | P | Adjusted odds ratio* | P | |
| Lymph node dissection | ||||||
| None/excision | 3 (5) | 63 (16·2) | 1·00 (reference) | 1·00 (reference) | ||
| CLND | 20 (35) | 186 (47·9) | 2·26 (0·65, 7·86) | 0·200 | 2·82 (0·76, 10·39) | 0·120 |
| CLND + LLND | 34 (60) | 139 (35·8) | 5·14 (1·52, 17·36) | 0·008 | 4·04 (1·12, 14·58) | 0·033 |
| T category | ||||||
| p1 | 25 (44) | 277 (71·4) | 1·00 (reference) | 1·00 (reference) | ||
| p2 | 9 (16) | 59 (15·2) | 1·59 (0·68, 3·71) | 0·287 | 1·34 (0·55, 3·25) | 0·520 |
| p3 | 9 (16) | 41 (10·6) | 2·48 (1·08, 5·71) | 0·033 | 2·12 (0·88, 5·11) | 0·095 |
| p4 | 14 (26) | 11 (2·8) | 14·14 (5·49, 36·39) | < 0·001 | 12·16 (4·46, 33·18) | < 0·001 |
| Intraoperative nerve monitoring | ||||||
| No | 5 (9) | 31 (8·0) | 1·00 (reference) | 1·00 (reference) | ||
| Yes | 52 (91) | 357 (92·0) | 0·99 (0·32, 3·05) | 0·983 | 1·12 (0·32, 3·96) | 0·857 |
Values in parentheses are percentages, except
95 per cent confidence intervals.
Data after multiple imputation. There were missing data for T category (5·4 per cent) and intraoperative nerve monitoring (7·9 per cent). Seven patients with recurrent laryngeal nerve (RLN) palsy diagnosed on preoperative laryngoscopy were excluded. Multivariable analysis included all variables listed in the table. CLND, central lymph node dissection; LLND, lateral lymph node dissection.
Discussion
In this study, at least transient hypoparathyroidism was observed in 26·2 per cent and at least transient RLN palsy in 13·7 per cent of patients after primary surgery for MTC. After adjusting for patient, tumour and surgical characteristics, CLND, CLND plus unilateral LLND and CLND plus bilateral LLND, compared with no LND, were independently associated with at least transient hypoparathyroidism. In addition, four or five parathyroid glands observed, compared with none or one, were associated with at least transient hypoparathyroidism, after adjusting for multiple variables including more aggressive disease and more extensive operation. CLND plus LLND compared with no compartment‐oriented LND, and T4 compared with T1 tumours, were associated with at least transient RLN palsy after adjustment for IONM use. Surgery for MTC is associated with a substantial risk of complications, and the risk is related not only to tumour stage but also to the extent of surgery.
Hypoparathyroidism is the most common complication after thyroid surgery, and is particularly relevant because permanent hypoparathyroidism is associated with an increased risk of morbidity and mortality and decreased quality of life22–25. The rate of at least transient hypoparathyroidism was 26·2 per cent here; permanent rates could not be established owing to differences in follow‐up practices. In a cohort of patients undergoing TT (22 per cent for malignancy) who were followed prospectively after surgery, 17·8 per cent had hypoparathyroidism after 1 month, of whom approximately 75 per cent had recovered after 1 year26. If figures were similar in the present cohort, the rate of permanent hypoparathyroidism would be in the range of 7 per cent. This is markedly higher than the reported rate of 1 per cent in a systematic review27. Underlying disparities in case mix, definitions, surgeon volume and extent of surgery might account for the differences. It should be noted that results from (population‐based) registry data generally differ from those reported from expert centres.
Adequate management to preserve in situ functioning parathyroid glands is important to prevent hypoparathyroidism26,28–30. Despite anatomical and embryological knowledge, and use of magnifying glasses and extracapsular dissection techniques to prevent accidental resection and injury, preservation of intact vascularized parathyroid glands remains difficult owing to the complex anatomy31–33. Intraoperative visualization of more parathyroid glands increased the risk of at least transient hypoparathyroidism in the present study. This finding points to the risk of damage to the parathyroid vascularization during compartment‐oriented LND. To prevent inadvertent resection and accidental devascularization, intraoperative parathyroid near‐infrared fluorescence imaging is a promising technique34–37, but use of such adjuncts was not reported in the registries and could not therefore be investigated here.
As postoperative biochemical cure is associated with survival, extensive compartment‐oriented LND is advised5,6,10,15,17,33. The policy of some centres regarding inclusion of LLND is based solely on preoperative calcitonin levels without an anatomical substrate within the respective compartment. The present study showed that, after adjusting for patient, tumour and surgical characteristics, compartment‐oriented LND almost tripled the risk of hypoparathyroidism. This is in line with a previous report17 that patients with hypoparathyroidism had more resected lymph nodes.
Strikingly, no lymph node metastases were reported (N0) in 176 patients (61·3 per cent) who had CLND, 49 (29·2 per cent) who had unilateral LLND and 23 (28 per cent) who underwent bilateral LLND, suggesting that 46·2 per cent of patients in the present series underwent unnecessary LND. These numbers might be higher because data on the presence of lymph node metastases was unknown or missing for 62 patients (11·5 per cent). Similar observations have been documented by others. Of 101 patients undergoing TT plus CLND plus LLND, 45 (44·6 per cent) had N0 disease and 59 (58·4 per cent) had no metastases in the lateral compartments15. Two other series6,14 of patients who had TT plus at least CLND reported N0 disease in 27·6 and 56·3 per cent respectively. In the present study, the postoperative tumour burden was not evaluated biochemically, so the biochemical cure/complication rate could not be established.
The presence or absence of lymph node metastases and their subsequent compartment cannot always be predicted reliably by tumour size, calcitonin or carcinoembryonic antigen levels, desmoplastic stromal reaction, ultrasonography or number of observed lymph node metastases5–7,10,15,17,38–40. Preoperative and intraoperative factors guiding the surgical strategy, such as calcitonin levels, were unknown for the present cohort. Nevertheless, it can be assumed that surgeons chose the best strategy for each patient. There is a need for better diagnostic tools to select patients for extensive LND. Recent insights in colorectal carcinoma have shown that some tumours metastasize before becoming clinically detectable (‘born bad’) and that such tumours harbour early drivers that can be used as biomarkers41. Similar markers are needed for MTC to tailor the extent of surgery. Given the rarity and aggressiveness of MTC, determination of the correct surgical strategy – avoiding both overtreatment and undertreatment – as well as the procedure itself demands profound knowledge of the tumour, surgical experience and adequate hospital resources.
RLN palsy was considered at least transient in 62 patients (13·7 per cent) in this study and was associated with CLND plus LLND and T4 tumours. A population‐based study42 reported early vocal cord palsy (1–6 weeks after surgery) in 4·1 per cent and permanent palsy in 1·2 per cent, which is considerably lower than the rate in the present study, probably because of differences in extent of surgery and extent of tumour. No relationship was observed between the use of IONM and RLN palsy in the present analysis. Although IONM reduced the risk of permanent RLN palsy in a population‐based cohort42, a systematic review and meta‐analysis43 of RCTs did not report the superiority or inferiority of IONM over visual inspection.
The incidence of postoperative haemorrhage requiring reoperation was relatively low (2·6 per cent), but is higher than in studies reporting general thyroid surgery (1·7–2·1 per cent) and lower than in an MTC series of extensive surgery (3·3 per cent)17,42,44–46.
The major strength of this study is the large cohort of patients who had primary surgery for MTC covering hospitals in multiple European countries. Patients were included from 2004 up to and including 2019, so the study accurately reflects current practice. Data, including complications, were collected from routine care with the aim of improving patient outcomes, instead of from administrative databases designed for other purposes47,48. In addition, multivariable analysis was performed to investigate the effect of extent of surgery, with adjustment for patient and disease factors.
This study also has several limitations. Registry data are prone to missing data and typing and coding errors. As data on individual patients were deidentified upon registration, missing data could not be retrieved and were therefore handled using multiple imputation, which is currently considered the best method49,50. Differences in duration of follow‐up were observed in both registries and so at least transient complications were used as proxies for permanent complications. Laryngoscopy was not performed routinely in all patients in all centres. Therefore, some patients with RLN palsy might have been missed. Laryngoscopy was probably performed selectively (in patients with signs or symptoms of RLN palsy or in those with loss of IONM signal) and, in the absence of routine evaluation of RLN function by IONM and/or laryngoscopy, rates of RLN palsy might not be comparable across centres42,44. Postoperative hypoparathyroidism was determined based on oral medication with active vitamin D and/or calcium; information on levels of parathyroid hormone was not available in the quality registers used in the present study. In addition, in several centres the policy might have been to treat patients routinely with calcium or active vitamin D during the perioperative phase. This could have led to overestimation of the transient hypoparathyroidism rate. Neither database captured data on inadvertent resection of parathyroid glands and this could therefore not be analysed. Other complications after neck surgery, such as chyle leak, injury of spinal accessory, vagus or phrenic nerves, sympathetic chain, brachial or cutaneous cervical plexus, were not collected systematically in the registries51,52. No postoperative calcitonin levels were available, which makes true evaluation of long‐term oncological outcome impossible. Patients with N0 and Nx tumours and those with M0 and Mx disease were included in N0 and M0 categories for logistic regression analyses. Surgeon volume and experience, which are important predictors of complications after thyroid surgery53–55, were not registered. A minimum of 25 TTs should be performed annually to reduce complications, and this number is probably even higher for compartment‐oriented LNDs56.
Surgery for MTC is associated with a high risk of complications and warrants specialized care by experienced endocrine surgeons. The small number of operations for MTC in many institutions in the present study suggests that further centralization might be necessary to improve outcomes. More extensive surgery increases the risk of complications. The high rate of N0 disease after lymph node dissection underscores the need for adequate patient counselling, and the need for improved prognostic factors that can be used to select patients for more extensive surgery.
Collaborators
Members of the EUROCRINE® Council: M. Almquist (Skåne University Hospital and Lund University, Lund, Sweden); M. Barczynski (Jagiellonian University Medical College, Kraków, Poland); L. Brunaud (University of Lorraine, Centre Hospitalier Régional Universitaire Nancy, Brabois Hospital, Nancy, France); T. Clerici (Cantonal Hospital of St Gallen, St Gallen, Switzerland); M. H. Hansen (University Hospital of North Norway, Tromsø, Norway); M. Iacobone (University of Padua, Padua, Italy); Ö. Makay (Ege University Hospital, Izmir, Turkey); F. F. Palazzo (Hammersmith Hospital and Imperial College Healthcare NHS Trust, Hammersmith Hospital, London, UK); N. Muñoz‐Pérez (Virgen de las Nieves University Hospital, Granada, Spain); M. Raffaelli (Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico, Università Cattolica del Sacro Cuore, Rome, Italy); P. Riss (Medical University of Vienna, Vienna, Austria); S. van Slycke (Onze‐Lieve‐Vrouw Clinic Aalst, Aalst, Belgium); M. R. Vriens (University Medical Centre Utrecht, Utrecht, the Netherlands).
Supplementary Material
Acknowledgements
D.J.v.B. undertook this research while on the Sten Tibblin Fellowship, which was funded by Novartis Health Alliance. M.A. was funded by Skåne University Hospital Funds, and the Anna‐Lisa and Sven Lundgren Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No preregistration exists for the study reported in this article. Because of the sensitive nature of the data collected for this study, the authors do not wish to make the data publicly available.
Disclosure: The authors declare no conflict of interest.
Contributor Information
the EUROCRINE® Council:
M Almquist, M Barczynski, L Brunaud, T Clerici, M H Hansen, M Iacobone, Ö Makay, F F Palazzo, N Muñoz‐Pérez, M Raffaelli, P Riss, S van Slycke, and M R Vriens
Members of the EUROCRINE® Council are co‐authors of this article and can be found under the heading Collaborators.
Supporting information
Additional supporting information can be found online in the Supporting Information section at the end of the article.
References
- 1. Opsahl EM, Akslen LA, Schlichting E, Aas T, Brauckhoff K, Hagen AI. et al. Trends in diagnostics, surgical treatment, and prognostic factors for outcomes in medullary thyroid carcinoma in Norway: a nationwide population‐based study. Eur Thyroid J 2019;8:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathiesen JS, Kroustrup JP, Vestergaard P, Stochholm K, Poulsen PL, Rasmussen ÅK. et al. Incidence and prevalence of sporadic and hereditary MTC in Denmark 1960–2014: a nationwide study. Endocr Connect 2018;7:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Husson O, Haak HR, Van Steenbergen LN, Nieuwlaat WA, Van Dijk BAC, Nieuwenhuijzen GAP. et al. Rising incidence, no change in survival and decreasing mortality from thyroid cancer in The Netherlands since 1989. Endocr Relat Cancer 2013;20:263–271. [DOI] [PubMed] [Google Scholar]
- 4. Lise M, Franceschi S, Buzzoni C, Zambon P, Falcini F, Crocetti E. et al. Changes in the incidence of thyroid cancer between 1991 and 2005 in Italy: a geographical analysis. Thyroid 2012;22:27–34. [DOI] [PubMed] [Google Scholar]
- 5. Moley JF, DeBenedetti MK.. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg 1999;229:880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chandeze MM, Noullet S, Faron M, Trésallet C, Godiris‐Petit G, Tissier F. et al. Can we predict the lateral compartment lymph node involvement in RET‐negative patients with medullary thyroid carcinoma? Ann Surg Oncol 2016;23:3653–3659. [DOI] [PubMed] [Google Scholar]
- 7. Machens A, Hauptmann S, Dralle H.. Prediction of lateral lymph node metastases in medullary thyroid cancer. Br J Surg 2008;95:586–591. [DOI] [PubMed] [Google Scholar]
- 8. Mathiesen JS, Kroustrup JP, Vestergaard P, Stochholm K, Poulsen PL, Rasmussen ÅK. et al. Survival and long‐term biochemical cure in medullary thyroid carcinoma in Denmark 1997–2014: a nationwide study. Thyroid 2019;29:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuo EJ, Sho S, Li N, Zanocco KA, Yeh MW, Livhits MJ.. Risk factors associated with reoperation and disease‐specific mortality in patients with medullary thyroid carcinoma. JAMA Surg 2018;153:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF. et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fagin JA, Wells SA.. Biologic and clinical perspectives on thyroid cancer. N Engl J Med 2016;375:1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papaleontiou M, Hughes DT, Guo C, Banerjee M, Haymart MR.. Population‐based assessment of complications following surgery for thyroid cancer. J Clin Endocrinol Metab 2017;102:2543–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosato L, Avenia N, Bernante P, De Palma M, Gulino G, Nasi PG. et al. Complications of thyroid surgery: analysis of a multicentric study on 14 934 patients operated on in Italy over 5 years. World J Surg 2004;28:271–276. [DOI] [PubMed] [Google Scholar]
- 14. Polistena A, Sanguinetti A, Lucchini R, Galasse S, Monacelli M, Avenia S. et al. Timing and extension of lymphadenectomy in medullary thyroid carcinoma: a case series from a single institution. Int J Surg 2017;41:S70–S74. [DOI] [PubMed] [Google Scholar]
- 15. Scollo C, Baudin E, Travagli JP, Caillou B, Bellon N, Leboulleux S. et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab 2003;88:2070–2075. [DOI] [PubMed] [Google Scholar]
- 16. Toniato A, Boschin IM, Piotto A, Pelizzo MR, Guolo A, Foletto M. et al. Complications in thyroid surgery for carcinoma: one institution's surgical experience. World J Surg 2008;32:572–575. [DOI] [PubMed] [Google Scholar]
- 17. Machens A, Dralle H.. Biomarker‐based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab 2010;95:2655–2663. [DOI] [PubMed] [Google Scholar]
- 18. Young Park S, Young Cho Y, In Kim H, Choe JH, Kim JH, Kim JS. et al. Clinical validation of the prognostic stage groups of the eighth‐edition TNM staging for medullary thyroid carcinoma. J Clin Endocrinol Metab 2018;103:4609–4616. [DOI] [PubMed] [Google Scholar]
- 19. Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG. et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;339:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moons KGM, Donders RART, Stijnen T, Harrell FE.. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 2006;59:1092–1101. [DOI] [PubMed] [Google Scholar]
- 21. Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley and Sons: New York, 1987. [Google Scholar]
- 22. Mitchell DM, Regan S, Cooley MR, Lauter KB, Vrla MC, Becker CB. et al. Long‐term follow‐up of patients with hypoparathyroidism. J Clin Endocrinol Metab 2012;97:4507–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergenfelz A, Nordenström E, Almquist M.. Morbidity in patients with permanent hypoparathyroidism after total thyroidectomy. Surgery 2020;167:124–128. [DOI] [PubMed] [Google Scholar]
- 24. Almquist M, Ivarsson K, Nordenström E, Bergenfelz A.. Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. Br J Surg 2018;105:1313–1318. [DOI] [PubMed] [Google Scholar]
- 25. Cusano NE, Rubin MR, McMahon DJ, Irani D, Tulley A, Sliney J. et al. The effect of PTH(1–84) on quality of life in hypoparathyroidism. J Clin Endocrinol Metab 2013;98:2356–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villarroya‐Marquina I, Sancho J, Lorente‐Poch L, Gallego‐Otaegui L, Sitges‐Serra A.. Time to parathyroid function recovery in patients with protracted hypoparathyroidism after total thyroidectomy. Eur J Endocrinol 2018;178:103–111. [DOI] [PubMed] [Google Scholar]
- 27. Edafe O, Antakia R, Laskar N, Uttley L, Balasubramanian SP.. Systematic review and meta‐analysis of predictors of post‐thyroidectomy hypocalcaemia. Br J Surg 2014;101:307–320. [DOI] [PubMed] [Google Scholar]
- 28. Lorente‐Poch L, Sancho JJ, Ruiz S, Sitges‐Serra A.. Importance of in situ preservation of parathyroid glands during total thyroidectomy. Br J Surg 2015;102:359–367. [DOI] [PubMed] [Google Scholar]
- 29. Sitges‐Serra A, Ruiz S, Girvent M, Manjón H, Dueñas JP, Sancho JJ.. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg 2010;97:1687–1695. [DOI] [PubMed] [Google Scholar]
- 30. Orloff LA, Wiseman SM, Bernet VJ, Fahey TJ, Shaha AR, Shindo ML. et al. American Thyroid Association statement on postoperative hypoparathyroidism: diagnosis, prevention, and management in adults. Thyroid 2018;28:830–841. [DOI] [PubMed] [Google Scholar]
- 31. Wang C. The anatomic basis of parathyroid surgery. Ann Surg 1976;183:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flament JB, Delattre JF, Pluot M.. Arterial blood supply to the parathyroid glands: implications for thyroid surgery. Anat Clin 1982;3:279–287. [Google Scholar]
- 33. Sheahan P, Mehanna R, Basheeth N, Murphy MS.. Is systematic identification of all four parathyroid glands necessary during total thyroidectomy? A prospective study. Laryngoscope 2013;123:2324–2328. [DOI] [PubMed] [Google Scholar]
- 34. McWade MA, Paras C, White LM, Phay JE, Solórzano CC, Broome JT. et al. Label‐free intraoperative parathyroid localization with near‐infrared autofluorescence imaging. J Clin Endocrinol Metab 2014;99:4574–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benmiloud F, Godiris‐Petit G, Gras R, Gillot JC, Turrin N, Penaranda G. et al. Association of autofluorescence‐based detection of the parathyroid glands during total thyroidectomy with postoperative hypocalcemia risk: results of the PARAFLUO multicenter randomized clinical trial. JAMA Surg 2019:E1–E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F.. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 2016;103:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vidal Fortuny J, Sadowski SM, Belfontali V, Guigard S, Poncet A, Ris F. et al. Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br J Surg 2018;105:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Machens A, Dralle H.. Surgical cure rates of sporadic medullary thyroid cancer in the era of calcitonin screening. Eur J Endocrinol 2016;175:219–228. [DOI] [PubMed] [Google Scholar]
- 39. Scheuba C, Kaserer K, Kaczirek K, Asari R, Niederle B.. Desmoplastic stromal reaction in medullary thyroid cancer – an intraoperative ‘marker’ for lymph node metastases. World J Surg 2006;30:853–859. [DOI] [PubMed] [Google Scholar]
- 40. Koperek O, Scheuba C, Puri C, Birner P, Haslinger C, Rettig W. et al. Molecular characterization of the desmoplastic tumor stroma in medullary thyroid carcinoma. Int J Oncol 2007;31:59–67. [PubMed] [Google Scholar]
- 41. Hu Z, Ding J, Ma Z, Sun R, Seoane JA, Scott Shaffer J. et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat Genet 2019;51:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bergenfelz A, Salem AF, Jacobsson H, Nordenström E, Almquist M, Wallin GW. et al. Risk of recurrent laryngeal nerve palsy in patients undergoing thyroidectomy with and without intraoperative nerve monitoring. Br J Surg 2016;103:1828–1838. [DOI] [PubMed] [Google Scholar]
- 43. Cirocchi R, Arezzo A, D'Andrea V, Abraha I, Popivanov GI, Avenia N. et al. Intraoperative neuromonitoring versus visual nerve identification for prevention of recurrent laryngeal nerve injury in adults undergoing thyroid surgery. Cochrane Database Syst Rev 2019; (1)CD012483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bergenfelz A, Jansson S, Kristoffersson A, Mårtensson H, Reihnér E, Wallin G. et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3660 patients. Langenbecks Arch Surg 2008;393:667–673. [DOI] [PubMed] [Google Scholar]
- 45. Promberger R, Ott J, Kober F, Koppitsch C, Seemann R, Freissmuth M. et al. Risk factors for postoperative bleeding after thyroid surgery. Br J Surg 2012;99:373–379. [DOI] [PubMed] [Google Scholar]
- 46. Salem FA, Bergenfelz A, Nordenström E, Dahlberg J, Hessman O, Lundgren CI. et al. Evaluating risk factors for re‐exploration due to postoperative neck hematoma after thyroid surgery: a nested case–control study. Langenbecks Arch Surg 2019;404:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iezzoni LI. Assessing quality using administrative data content of administrative databases. Ann Intern Med 1997;127:666–674. [DOI] [PubMed] [Google Scholar]
- 48. Duclos A, Lifante JC.. Hospital administrative data should not be used to study thyroid surgery outcomes. Ann Surg 2018;267:78. [DOI] [PubMed] [Google Scholar]
- 49. Janssen KJM, Donders ART, Harrell FE, Vergouwe Y, Chen Q, Grobbee DE. et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol 2010;63:717–727. [DOI] [PubMed] [Google Scholar]
- 50. van der Heijden GJMG, Donders ART, Stijnen T, Moons KGM.. Imputation of missing values is superior to complete case analysis and the missing‐indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol 2006;59:1102–1109. [DOI] [PubMed] [Google Scholar]
- 51. Shaha AR. Editorial: complications of neck dissection for thyroid cancer. Ann Surg Oncol 2008;15:397–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Porterfield JR, Factor DA, Grant CS.. Operative technique for modified radical neck dissection in papillary thyroid carcinoma. Arch Surg 2009;144:567–574. [DOI] [PubMed] [Google Scholar]
- 53. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R.. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg 1998;228:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duclos A, Peix JL, Colin C, Kraimps JL, Menegaux F, Pattou F. et al. Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ 2012;344:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maruthappu M, Gilbert BJ, El‐Harasis MA, Nagendran M, McCulloch P, Duclos A. et al. The influence of volume and experience on individual surgical performance: a systematic review. Ann Surg 2015;261:642–647. [DOI] [PubMed] [Google Scholar]
- 56. Adam MA, Thomas S, Youngwirth L, Hyslop T, Reed SD, Scheri RP. et al. Is there a minimum number of thyroidectomies a surgeon should perform to optimize patient outcomes? Ann Surg 2017;265:402–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.