Abstract
Background
Rates of surgery and adjuvant therapy for breast cancer vary widely between breast units. This may contribute to differences in survival. This cluster RCT evaluated the impact of decision support interventions (DESIs) for older women with breast cancer, to ascertain whether DESIs influenced quality of life, survival, decision quality, and treatment choice.
Methods
A multicentre cluster RCT compared the use of two DESIs against usual care in treatment decision-making in older women (aged at least ≥70 years) with breast cancer. Each DESI comprised an online algorithm, booklet, and brief decision aid to inform choices between surgery plus adjuvant endocrine therapy versus primary endocrine therapy, and adjuvant chemotherapy versus no chemotherapy. The primary outcome was quality of life. Secondary outcomes included decision quality measures, survival, and treatment choice.
Results
A total of 46 breast units were randomized (21 intervention, 25 usual care), recruiting 1339 women (670 intervention, 669 usual care). There was no significant difference in global quality of life at 6 months after the baseline assessment on intention-to-treat analysis (difference –0.20, 95 per cent confidence interval (C.I.) –2.69 to 2.29; P = 0.900). In women offered a choice of primary endocrine therapy versus surgery plus endocrine therapy, knowledge about treatments was greater in the intervention arm (94 versus 74 per cent; P = 0.003). Treatment choice was altered, with a primary endocrine therapy rate among women with oestrogen receptor-positive disease of 21.0 per cent in the intervention versus 15.4 per cent in usual-care sites (difference 5.5 (95 per cent C.I. 1.1 to 10.0) per cent; P = 0.029). The chemotherapy rate was 10.3 per cent at intervention versus 14.8 per cent at usual-care sites (difference –4.5 (C.I. –8.0 to 0) per cent; P = 0.013). Survival was similar in both arms.
Conclusion
The use of DESIs in older women increases knowledge of breast cancer treatment options, facilitates shared decision-making, and alters treatment selection.
Trial registration numbers: EudraCT 2015-004220-61 (https://eudract.ema.europa.eu/), ISRCTN46099296 (http://www.controlled-trials.com).
This cluster randomized trial compared the use of a decision support tool with usual care in supporting clinical decision-making in older women with early breast cancer. Use of the tool enhanced levels of patient knowledge and altered decision preferences for both the choice between surgery versus primary endocrine therapy, and whether or not to have adjuvant chemotherapy.
Introduction
Breast cancer outcomes for older women are inferior to those of younger women because of the later stage at diagnosis and high rates of non-guideline-concordant care1–3. The UK lags behind other high-income European countries in survival outcomes for older women4. Age-related variation in practice between UK units is widespread owing to a lack of guidelines stratified by health and fitness and variation in clinician opinion5–7. Omission of surgery in favour of primary endocrine therapy occurs in up to 40 per cent of women over 70 years of age in the UK8, despite evidence of substantially improved rates of local control9 and a small long-term survival benefit for women who have surgery8,10. Surgery is, however, not always appropriate and may cause harm if used unnecessarily in very frail patients, as shown in a US study11 in which nursing home residents with breast cancer had high rates of morbidity, mortality, and functional decline following surgery.
Rates of adjuvant chemotherapy are also significantly lower in women aged over 70 years. Sixty-one per cent of patients aged 50–69 years with oestrogen receptor (ER)-negative disease receive chemotherapy versus 23 per cent of those aged over 70 years. Similar differences in chemotherapy rates are seen in women with ER-positive disease3.
The potential risks of adverse events from surgery12 or chemotherapy13,14 are increased in older patients, but breast cancer-specific and overall survival benefits exist for appropriately selected women. There is a lack of guidance on which thresholds should be applied in making these decisions. The International Society of Geriatric Oncology/European Society of Breast Cancer Specialists15 guidelines recommend surgery if the woman is fit, but patient automomy and right to choose must be respected if older women elect to have non-guideline-concordant care.
Decision-making in cancer care is now supported by a number of online algorithms, which permit stratification of treatment according to parameters such as stage and tumour biology, but no such tool exists for the choice of breast surgery plus adjuvant therapies versus primary endocrine therapy alone in older women. In addition, the tools available to support chemotherapy decision-making are based on trial data from younger women16–18 and typically do not take into account co-morbidities, so may be less applicable to older women19,20.
This study builds on previous work to develop decision support interventions (DESIs) to aid in shared decision-making by older women with breast cancer. These DESIs include two booklets21, brief decision aids, and a validated online decision algorithm (Age Gap Decision Tool©; https://agegap.shef.ac.uk/), based on UK cancer registry survival data, adjusted for co-morbidities and frailty8,22. The online tool produces personalized survival outcomes for women aged 70 years or above with operable breast cancer according to fitness, frailty, stage, treatment choice, and disease biology. The DESIs developed for this study used the preferred informational content, format, terminology, and media for women over 70 years of age, and were piloted extensively in this age group21,23,24.
The present study evaluated the impact of these DESIs on quality of life (QoL), survival, breast cancer treatment choice, and decision quality measures, in a multicentre cluster RCT (cRCT) across England and Wales. Trial centres were already recruiting to a multicentre, prospective cohort study of treatment and outcomes in women aged 70 years or above with early breast cancer (Age Gap study), which collected detailed data on baseline characteristics (a comprehensive geriatric assessment), treatments, and outcomes. This cRCT was nested within the Age Gap study. The trial was cluster randomized because the materials could not feasibly be given to, or withheld from, individually randomized clinicians or patients. Recruiting sites were randomized to either continue with usual care or were given training in the use of, and access to, the DESIs. Cluster randomization was stratified by primary endocrine therapy and chemotherapy rates to avoid bias.
The aims of the study were to evaluate the effects of the DESIs on patients’ QoL, survival (at a median follow-up of 36 months), decision quality, and on the two key treatment choices: surgery plus adjuvant endocrine therapy versus primary endocrine therapy, and adjuvant chemotherapy versus no chemotherapy. Secondary aims included assessment of the effects on patients’ coping and decision regret.
Methods
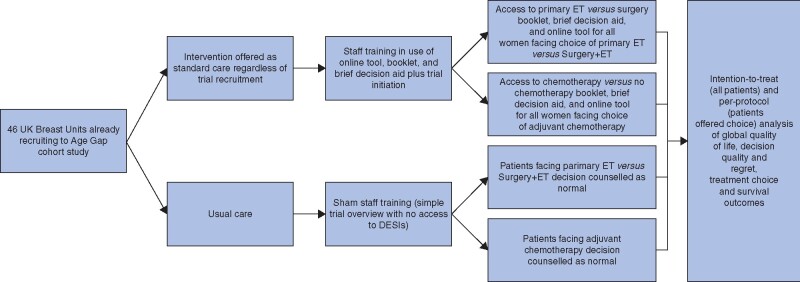
This was a multicentre, parallel-group, pragmatic, cRCT nested within a larger cohort study of older women (aged over 70 years) with early breast cancer (Age Gap study). Clusters (individual breast units) were randomized to either normal decision-making practices (relating to choice of surgery plus adjuvant endocrine therapy versus primary endocrine therapy, or whether to have adjuvant chemotherapy after surgery) or to training and use of the DESIs (Fig. 1). Trial reporting followed the cluster trial extension of the CONSORT guidelines25. The protocol for the trial has been published26. Ethics (IRAS:12LO1808) and research governance approvals were obtained. The trial was registered with EudraCT (2015-004220-61) and the ISRCTN registry (ISRCTN46099296).
Fig. 1.
Design of cluster RCT
DESI, decision support intervention; ET, endocrine therapy.
Recruitment sites
The study recruited from 46 breast units (Table S1) in England and Wales between December 2015 and June 2018.
Eligibility and exclusion criteria
Inclusion criteria were women aged 70 years or above at diagnosis with primary operable invasive breast cancer (TNM categories T1–3 N0–1 M0), who were able to read English. Exclusion criteria were: inoperable, locally recurrent or metastatic breast cancer; and a history of previous invasive breast cancer within 5 years.
Randomization
Centres (breast units) were subjected to 1 : 1 block randomization, stratified by high or low current primary endocrine therapy and chemotherapy rates. Centres were randomized either to have access to the DESIs and training in their use, or to continue with usual care.
Trial interventions
The DESIs included an online tool, booklet, and brief decision aid to support the decision regarding surgery plus adjuvant endocrine therapy versus primary endocrine therapy, or adjuvant chemotherapy versus no chemotherapy. The development of these tools has been reported previously21–24,27 and is summarized in Fig. S1.
Training package
Staff from usual-care and intervention sites attended a site initiation visit to explain the trial. Intervention sites were also given specific face-to-face or online training in the use of the two DESIs to support shared decision-making.
Trial outcomes
Data were collected at baseline, 6 weeks, and 6 months (Table S2).
Primary endpoint
QoL was assessed at 6 months after diagnosis using the generic cancer QoL measure, the European Organisation for the Research and Treatment of Cancer (EORTC) QoL questionnaire (QLQ) C3028. The primary outcome was the global health status score (questions 29 and 30) of the C30 at 6 months. Patients had the option to decline completion of QoL forms and the various decision quality forms if felt to be burdensome, and patients with significant cognitive impairment (recruited by proxy) were not expected to complete them.
Secondary outcome measures
Data from EORTC QLQ-C30, QLQ-BR23 (breast cancer-specific QoL)29, and QLQ-ELD14 (older person-specific QoL30) questionnaires were collected at 6 weeks and 6 months, and compared across all domains between usual-care and intervention centres.
Treatment choices were evaluated in certain subgroups of women (rates of surgery plus adjuvant endocrine therapy versus primary endocrine therapy in women with ER-positive cancers, chemotherapy rates in all patients and those whose cancer had a high recurrence risk). The appropriateness of treatment choice was assessed by comparison of the characteristics of allocated women (age, fitness, frailty, tumour characteristics) and survival metrics. To facilitate this assessment, all patients in the study, not just those who were actually offered the relevant choice, were included in the analysis.
Patient knowledge, if offered a choice of relevant treatments, was assessed using a bespoke eight-item questionnaire. Correct responses were assigned a score of 1, whereas incorrect and ‘unsure’ responses scored 0. Scores were measured before and 6 weeks after decision-making (Table S2).
CollaboRATE, a validated three-item shared decision-making measure31, was scored by patients on a 10-point scale ranging from 0 (no effort at all) to 9 (every effort made) to rate efforts to facilitate shared decision-making. Scores were summed and multiplied by 3.704 to transform into a scale of 0–100. This was applied to all women offered a choice and assessed at baseline after decision-making but before treatment commenced (Table S2).
Five items on a decision regret scale32 were rated from 1 (strongly agree) to 5 (strongly disagree); examples are ‘It was the right decision’ and ‘The choice did me a lot of harm’. This was given to all women offered a choice, and assessed at 6 weeks and 6 months after decision-making (Table S2).
The six-item Spielberger Short State–Trait Anxiety Inventory (STAI)33 was administered before treatment, and at 6 weeks and 6 months after decision-making (Table S2).
Women were asked to complete the Brief Illness Perceptions Questionnaire (BIPQ)34, a nine-item questionnaire measuring cognitive and emotional representations of cancer, at 6 weeks and 6 months after decision-making (Table S2).
Coping strategies in response to facing cancer treatment decisions were assessed using Brief COPE35 6 weeks and 6 months after decision-making (Table S2).
Overall and breast-cancer specific survival were derived from UK cancer registry returns (downloaded February 2020, median survival 36 months).
The study also collected data on the following baseline and treatment characteristics: age, Charlson Co-morbidity Index score36, Activities of Daily Living (ADL)37 and Instrumental ADL38, the Mini Mental State Examination score39, the abridged Patient Generated Subjective Global Assessment40,41, tumour stage42, tumour grade and biotype, and treatment.
Use of the online tool by clinicians was evaluated using an integrated registration system for number and duration of log-ins at each site. Information on use of the DESIs was collected directly from the case report form about the consultation.
Process evaluation
A formal process evaluation of the dose, reach, fidelity, adaptations, and participants’ responses to the DESIs was undertaken alongside the trial, and is in submission for publication separately.
Data monitoring and ethics
Source data verification was performed for 10 per cent of patients. An independent data monitoring and ethics committee reviewed the trial every 3–6 months.
Statistical analysis
The sample size calculation was based on the primary endpoint: global health status QoL score (questions 29 and 30 of EORTC QLQ-C30) at 6 months. It was assumed that 50 breast units would be randomized and would recruit a set number of women per cluster43, based on a standard deviation of 21 for women aged 70 years or more with breast cancer, with a clinically significant difference set at 7 points (medium standardized effect size of 0.33)44. An intracluster correlation estimate of 0.03 with 90 per cent power, 5 per cent significance, and 20 per cent loss to follow-up yielded a sample size requirement of 50 clusters and 650 patients (13 per cluster; 325 per group).
QoL outcomes were analysed using a general linear model, with coefficients estimated by generalized estimating equations with robust standard errors, and an exchangeable correlation matrix to allow for the clustered nature of the data. Survival was analysed using Cox proportional hazards regression with site clustering incorporated via a shared frailty model. Survival time was censored on 30 February 2020. Sites with fewer than 10 participants were combined into one cluster for these analyses. Treatment decision was tabulated by arm and visualized using funnel plots45. QoL, survival, and treatment choice were analysed on an intention-to-treat basis. QoL and survival analyses were repeated on a planned per-protocol basis among patients who were offered a treatment choice and excluding intervention sites that did not adopt the Age Gap tools. Measures of decision quality and knowledge were confined to women who were offered a relevant treatment choice. Other outcomes are presented descriptively. Time trends for the percentage of patients opting for primary endocrine therapy and chemotherapy compared with historical practice in the same unit (recorded from February 2013 for the cohort study in which the cRCT was nested) were presented visually using a Lowess smoother46.
Results
Training in and use of DESIs in intervention sites
Training in DESI use was received by 229 healthcare professionals at intervention sites, with 44 attending a 2-h workshop and their colleagues completing on-site training (lasting 15–120 (median 60) min). An educational video was also available online, which was viewed 227 times.
Site randomization and patient recruitment
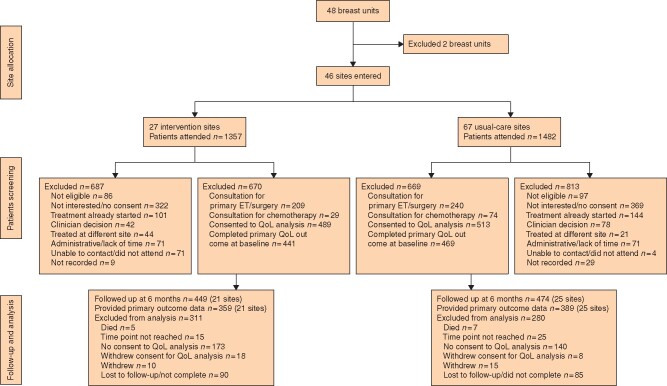
The trial randomized 48 centres (23 to DESI, 25 to usual care) (Table S1). Two clusters dropped out before recruiting any women (both in intervention arm), leaving 46 clusters (21 intervention, 25 usual care) in the intention-to-treat analysis. The 46 clusters recruited 1339 patients (670 intervention, 669 usual care) between 1 December 2015 and 6 June 2018 (Fig. 2).
Fig. 2.
CONSORT diagram for the trial. (ET, endocrine therapy; QoL, quality of life).
The median cluster size was 26 (range 1–96); three clusters recruited fewer than five patients and seven centres fewer than 10. QoL form completion was optional and this resulted in a total of 441 patients (in the 21 clusters) in the intervention arm completing baseline QoL forms and 469 (in the 25 clusters) in the usual-care arm. Not all of these completed the 6-month follow-up forms; 359 QoL forms were available at 6 months in the intervention arm and 389 in the usual-care arm (Fig. 2).
The median age of participants was 77 (i.q.r. 70–96) years. Patients in the intervention and usual-care arms were well matched for baseline health and fitness, tumour stage, and biology (Table 1).
Table 1.
Baseline characteristics of patients by randomized group (46 clusters or breast units)
| Intervention (21 clusters) | Usual care (25 clusters) | Overall (46 clusters) | |
|---|---|---|---|
| No. of patients | 670 | 669 | 1339 |
| Age (years) | |||
| Mean(s.d.) | 78 (6) | 77 (6) | 78 (6) |
| Median (i.q.r.; range) | 77 (73–82; 70–98) | 76 (72–81; 69–102) | 77 (70–96; 69–102) |
| 70–74 | 239 (35.7) | 260 (38.9) | 499 (37.3) |
| 75–79 | 196 (29.3) | 197 (29.4) | 393 (29.4) |
| 80–84 | 131 (19.6) | 133 (19.9) | 264 (19.7) |
| 85–89 | 70 (10.4) | 57 (8.5) | 127 (9.5) |
| ≥ 90 | 34 (5.1) | 22 (3.3) | 56 (4.2) |
| Participation level (QoL form completion) | |||
| Full (QoL forms) | 489 (73.0) | 513 (76.7) | 1002 (74.8) |
| Partial (elected no QoL forms) | 156 (23.3) | 138 (20.6) | 294 (22.0) |
| Consultee participation (cognitively unable to complete QoL forms) | 25 (3.7) | 18 (2.7) | 43 (3.2) |
| aPG-SGA score (nutrition) | n = 519 (77.5) | n = 565 (84.5) | n = 1084 (81.0) |
| Mean(s.d.) | 1.6 (2.6) | 1.1 (2.1) | 1.4 (2.4) |
| Median (i.q.r.; range) | 0 (0–2; 0–16) | 0 (0–1; 0–14) | 0 (0–2; 0–16) |
| Barthel ADL index score (frailty) | n = 564 (84.2) | n = 607 (90.7) | n = 1171 (87.5) |
| Mean(s.d.) | 96.0 (10.5) | 97.1 (7.7) | 96.6 (9.2) |
| Median (i.q.r.; range) | 100 (95–100; 5–100) | 100 (100–100; 20–100) | 100 (95–100; 5–100) |
| Barthel ADL index risk category (ADL score)* | |||
| No dependency (100) | 408 (60.9) | 479 (71.6) | 887 (66.2) |
| Mild dependency (95) | 86 (12.8) | 56 (8.4) | 142 (10.6) |
| Moderate/severe dependency (≤ 90) | 84 (12.5) | 84 (12.6) | 168 (12.5) |
| Missing | 92 (13.7) | 50 (7.5) | 142 (10.6) |
| IADL score | n = 538 (80.3) | n = 607 (90.7) | n = 1145 (85.5) |
| Mean(s.d.) | 7.4(1.3) | 7.5(1.2) | 7.5(1.2) |
| Median (i.q.r.; range) | 8 (7–8; 0–8) | 8 (8–8; 0–8) | 8 (8–8; 0–8) |
| IADL risk category (IADL score)* | |||
| No dependency (8) | 411 (61.3) | 484 (72.3) | 895 (66.8) |
| Mild dependency (7) | 69 (10.3) | 61 (9.1) | 130 (9.7) |
| Moderate/severe dependency (≤ 6) | 83 (12.4) | 76 (11.4) | 159 (11.9) |
| Missing | 107 (16.0) | 48 (7.2) | 155 (11.6) |
| CCI score | n = 618 (92.2) | n = 646 (96.6) | n = 1264 (94.4) |
| Mean(s.d.) | 4.7 (1.6) | 4.5 (1.5) | 4.6 (1.6) |
| Median (i.q.r.; range) | 4 (3–6; 3–11) | 4 (3–5; 3–15) | 4 (3–5; 3–15) |
| CCI risk category | |||
| 0 or 1 co-morbidity | 504 (75.2) | 543 (81.2) | 1047 (78.2) |
| ≥ 2 or more co-morbidities | 114 (17.0) | 105 (15.7) | 219 (16.4) |
| Missing | 52 (7.8) | 21 (3.1) | 73 (5.5) |
| MMSE score | n = 385 (57.5) | n = 423 (63.2) | n = 808 (60.3) |
| Mean(s.d.) | 28.4(2.3) | 28.0(3.0) | 28.2(2.7) |
| Median (i.q.r.; range) | 29 (28–30; 11–30) | 29 (27–30; 11–30) | 29 (28–30; 11–30) |
| Cognitive status (MMSE score) | |||
| Normal function (25–30) | 593 (88.5) | 578 (86.4) | 1171 (87.5) |
| Mild impairment (20–24) | 56 (8.4) | 68 (10.2) | 124 (9.3) |
| Moderate impairment (15–19) | 3 (0.4) | 12 (1.8) | 15 (1.1) |
| Severe (11–14) | 18 (2.7) | 11 (1.6) | 29 (2.2) |
| ECOG performance status score | |||
| 0: fully active | 405 (60.4) | 454 (67.9) | 859 (64.2) |
| 1: restricted in physically strenuous activity | 149 (22.2) | 140 (20.9) | 289 (21.6) |
| 2: ambulatory and capable of all self-care | 40 (6.0) | 26 (3.9) | 66 (4.9) |
| 3: capable of limited self-care | 25 (3.7) | 19 (2.8) | 44 (3.3) |
| 4: Completely disabled | 1 (0.1) | 1 (0.1) | 2 (0.1) |
| Missing | 50 (7.5) | 29 (4.3) | 79 (5.9) |
| No. of medications | |||
| ≤3 | 276 (41.2) | 269 (40.2) | 545 (40.7) |
| ≥4 | 318 (47.5) | 290 (43.3) | 608 (45.4) |
| Missing | 76 (11.3) | 110 (16.4) | 186 (13.9) |
Values in parentheses are percentages unless indicated otherwise.
Dependency categories incorporate partially complete questionnaires where category is defined unambiguously from completed questions. Missing data regarding cognition status were augmented with a diagnosis of dementia on the Charlson Co-morbidity Index (CCI) score or partial/consultee participation (verified at site as indicative of dementia). QoL, quality of life; aPG-SGA, abridged Patient Generated Subjective Global Assessment; ADL, Activities of Daily Living; IADL, Instrumental ADL; MMSE, Mini-Mental State Examination; ECOG, Eastern Cooperative Oncology Group.
Patients offered treatment choice and use of DESIs
A total of 319 women were offered a choice of surgery plus endocrine therapy versus primary endocrine therapy alone.
Online algorithm usage data showed that 221 healthcare professionals were given log-in codes and 494 individual log-in events were recorded to the DESI tool. Approximately half of intervention sites used the DESI tool regularly and the other half rarely. Use of the DESI tool for surgery plus endocrine therapy versus primary endocrine therapy alone was much greater than that for the chemotherapy DESI, reflecting low rates of discussion about chemotherapy among older women. A total of 209 patients had a discussion about surgery plus endocrine therapy versus primary endocrine therapy alone, and 27 a discussion about having chemotherapy or not. The online tool printout (Fig. S1c,d) was given to 81 patients, booklets (Fig. S1e,f) to 95, and the brief decision aid to 86 (Fig. S1g). Use of the various components of the DESIs is summarized in Table S3.
Primary outcome
The primary outcome, global QoL score, was similar between usual-care and intervention sites at 6 months (mean difference –0.20, 95 per cent c.i. –2.69 to 2.29; P = 0.900) in intention-to-treat analysis (Table 2). QoL, was, however, better among participants who had relevant treatment choice consultations (planned per-protocol analysis) in the intervention arm compared with the usual-care arm (mean difference 3.96, 0.10 to 7.82; P = 0.044). No differences in QoL were evident at 6 weeks in either intention-to-treat or per-protocol analysis.
Table 2.
Quality-of-life (global health status) at baseline, and 6 weeks and 6 months after treatment decision-making
| Intervention (n = 670) |
Usual care (n = 669) |
Difference † | ICC ‡ | P § | ||||
|---|---|---|---|---|---|---|---|---|
| n | QoL score* | n | QoL score* | |||||
| Intention-to-treat analysis of all patients regardless of whether offered treatment choice | ||||||||
| Baseline | 441 (65.8) | 74.7 (18.8) | 469 (70.1) | 76.0 (19.3) | ||||
| 6 weeks | 385 (57.5) | 69.2 (18.0) | 425 (63.5) | 69.5 (19.9) | –0.23 (–2.96, 2.50) | 0.00 | 0.868 | |
| 6 months | 359 (53.6) | 69.0 (19.1) | 389 (58.1) | 68.9 (19.9) | –0.20 (–2.69, 2.29) | –0.01 | 0.900 | |
| Per-protocol analysis (only patients offered treatment choice) | ||||||||
| Baseline | 149 (22.2) | 74.2 (19.8) | 211 (31.5) | 76.4 (18.6) | ||||
| 6 weeks | 132 (19.7) | 68.7 (16.9) | 187 (28.0) | 68.5 (19.6) | 0.25 (–3.83, 4.33) | 0.00 | 0.906 | |
| 6 months | 123 (18.4) | 70.7 (17.4) | 172 (25.7) | 66.8 (20.1) | 3.96 (0.10, 7.82) | –0.01 | 0.044 | |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.) and
values in parentheses are 95 per cent confidence intervals.
Intraclass correlation coefficient (ICC): within-hospital cluster term estimated from marginal generalized estimating equations with exchangeable correlation matrix.
Intraclass correlation coefficient test.
Secondary outcomes
Treatment choice
Table 3 summarizes treatment choices by study arm. Overall, 124 of 591 patients (21.0 per cent) with an ER-positive tumour underwent primary endocrine therapy at intervention sites, compared with 88 of 570 (15.4 per cent) at usual-care centres (difference 5.5 (95 per cent c.i. 1.1 to 10.0) per cent; P = 0.029). Similarly, the rate of uptake of adjuvant chemotherapy was lower among intervention sites than usual-care sites: 69 of 670 (10.3 per cent) versus 99 of 669 14.8 per cent) (difference –4.5 (–8.0 to 0) per cent; P = 0.013). A similar, albeit not statistically significant, difference was evident in chemotherapy uptake among those who had cancers with a high recurrence risk (node-positive, high grade, ER-negative, human epidermal growth factor receptor 2-amplified, high Oncotype DX recurrence score): 66 of 267 (24.7 per cent) versus 92 of 310 (29.7 per cent) (difference –5.0 (–12.2 to 2.3) per cent; P = 0.183). Funnel plots for treatment choice by site are shown in Fig. S2a (primary endocrine therapy) and 2b (chemotherapy). These show more variation in primary endocrine therapy rates regardless of site compared with variation in chemotherapy, but no particular pattern between the intervention and usual-care sites.
Table 3.
Treatment choice by site
| Intervention (21 clusters) | Usual care (25 clusters) | Overall (46 clusters) | P * | |
|---|---|---|---|---|
| Treatment decision-making: surgery versus primary endocrine thrapy | ||||
| All patients | 670 | 669 | 1339 | |
| Underwent consultation | 209 (31.2) | 240 (35.9) | 449 (33.5) | |
| Offered treatment choice | 168 (25.1) | 151 (22.6) | 319 (23.8) | |
| Treatment received | 0.009 | |||
| Surgery | 526 (78.5) | 547 (81.8) | 1073 (80.1) | |
| Primary endocrine therapy | 124 (18.5) | 89 (13.3) | 213 (15.9) | |
| Other/not treated | 20 (3.0) | 33 (4.9) | 53 (4.0) | |
| Patients with ER-positive disease | n = 591 | n = 570 | n = 1161 | |
| Underwent consultation | 197 (33.3) | 215 (37.7) | 412 (35.5) | |
| Offered treatment choice | 157 (26.6) | 140 (24.6) | 297 (25.6) | |
| Treatment received | 0.029 | |||
| Surgery | 451 (76.3) | 459 (80.5) | 910 (78.4) | |
| Primary endocrine therapy | 124 (21.0) | 88 (15.4) | 212 (18.3) | |
| Other/not treated | 16 (2.7) | 23 (4.0) | 39 (3.4) | |
| Treatment decision-making: chemotherapy after surgery | ||||
| All patients after surgery | n = 526 | n = 547 | n = 1073 | |
| Underwent consultation | 27 (5.1) | 67 (12.2) | 94 (8.8) | |
| Offered treatment choice | 21 (4.0) | 56 (10.2) | 77 97.2) | |
| Received chemotherapy | 69 (10.3) | 99 (14.8) | 168 (12.5) | 0.013 |
| High-risk patients | n = 267 | n = 310 | n = 577 | |
| Underwent consultation | 18 (6.7) | 61 (19.7) | 79 (13.7) | |
| Offered treatment choice | 17 (6.4) | 53 (17.1) | 70 (12.1) | |
| Received chemotherapy | 66 (24.7) | 92 (29.7) | 158 (27.4) | 0.183 |
Values in parentheses are percentages. ER, oestrogen receptor.
*Intraclass correlation coefficient: test.
Comparison of treatment choice change compared with historical practice
The main Age Gap cohort study within which the cRCT was nested started recruiting in February 2013, and 19 of the 21 cRCT intervention sites were included. The rate of primary endocrine therapy for women with ER-positive cancers after the cRCT intervention was compared with the historical primary endocrine therapy rate in the Age Gap cohort study to validate the cRCT findings. Similar analysis of chemotherapy rates was performed.
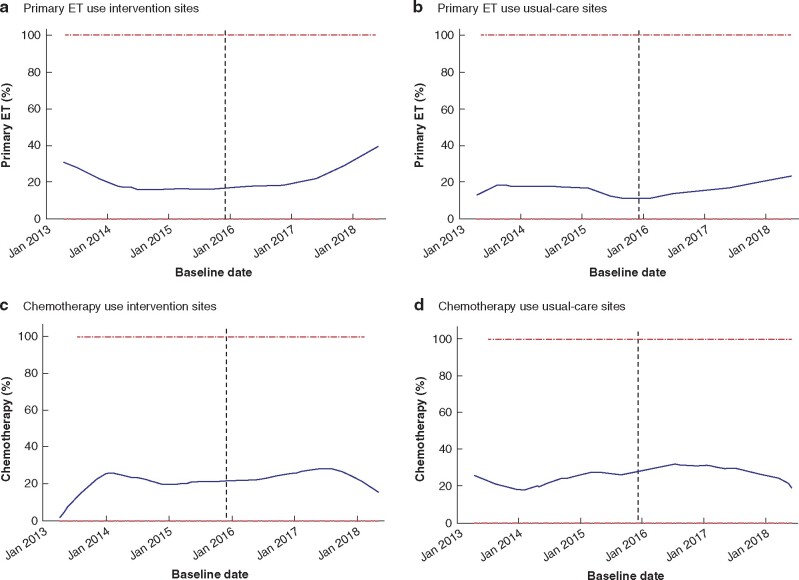
As a post hoc analysis, the proportions of participants who received primary endocrine therapy and chemotherapy were compared graphically before and after starting the cRCT. At RCT intervention sites, the proportion of patients treated with primary endocrine therapy reduced slightly in the years before the start of the RCT, but rose thereafter. By contrast, usual-care sites showed modest increases (Fig. 3a,b). Little change was apparent in the uptake of chemotherapy (Fig. 3c,d). This is in keeping with the findings of the randomized intervention versus usual-care analysis.
Fig. 3.
Lowess smoother plots 46
a,b Proportion of patients with oestrogen receptor-positive disease who had primary endocrine therapy (ET) and c,d proportion of high-risk patients after surgery who underwent adjuvant chemotherapy, at RCT a,c intervention and b,d usual-care sites. Dashed line denotes time at which first patient recruited during RCT phase. Blue line is the percentage of patients having the intervemtion of interest. Bandwidth 0.4 for all parts.
CollaboRATE results
Of the 319 patients undergoing a consultation for choice of primary endocrine therapy versus surgery or chemotherapy versus no chemotherapy, 148 (71 intervention, 77 usual care) completed the CollaboRATE tool. Patients reported a high quality of shared decision-making, with a median score of 100 in both arms (P = 0.729, Mann–Whitney U test) (Table S4).
Decision regret, anxiety, and perception of cancer
Of the 396 patients offered a choice, only 85 (18.9 per cent) completed the decision regret questionnaire. Decision regret scores did not differ significantly between usual-care and intervention sites. The STAI was also completed by a minority of patients. Among those who did, the scores were similar at 6 weeks and at 6 months. Similarly, the BIPQ was completed in only a small number of consultations and scores did not differ significantly (Table S4).
Knowledge and preferences
A total of 449 patients (209 intervention, 240 usual care) consulted a clinician regarding surgery plus endocrine therapy versus primary endocrine therapy alone, and 103 (27 intervention, 67 usual care) attended a consultion regarding chemotherapy. Table S5 summarizes participant knowledge, attitudes, and influences for 125 patients (67 intervention, 58 usual care) who completed the knowledge and preferences questionnaires for surgery plus endocrine therapy versus primary endocrine therapy. The questions were answered more knowledgeably by patients in the intervention arm (median scores 5 versus 3 of a possible 8; P < 0.001, Mann–Whitney test U test) and a greater percentage of participants in the intervention arm stated that they knew the available options (94 versus 74 per cent; P = 0.003) and associated advantages (91 versus 76 per cent; P = 0.054). There were no significant differences in the proportion of participants stating that they knew their preferred option (96 per cent intervention, 91 per cent usual care) or felt ready to make an informed decision (99 versus 90 per cent) (Table S5). For questions on knowledge of chemotherapy, the number of respondents was too small for meaningful analysis.
Survival
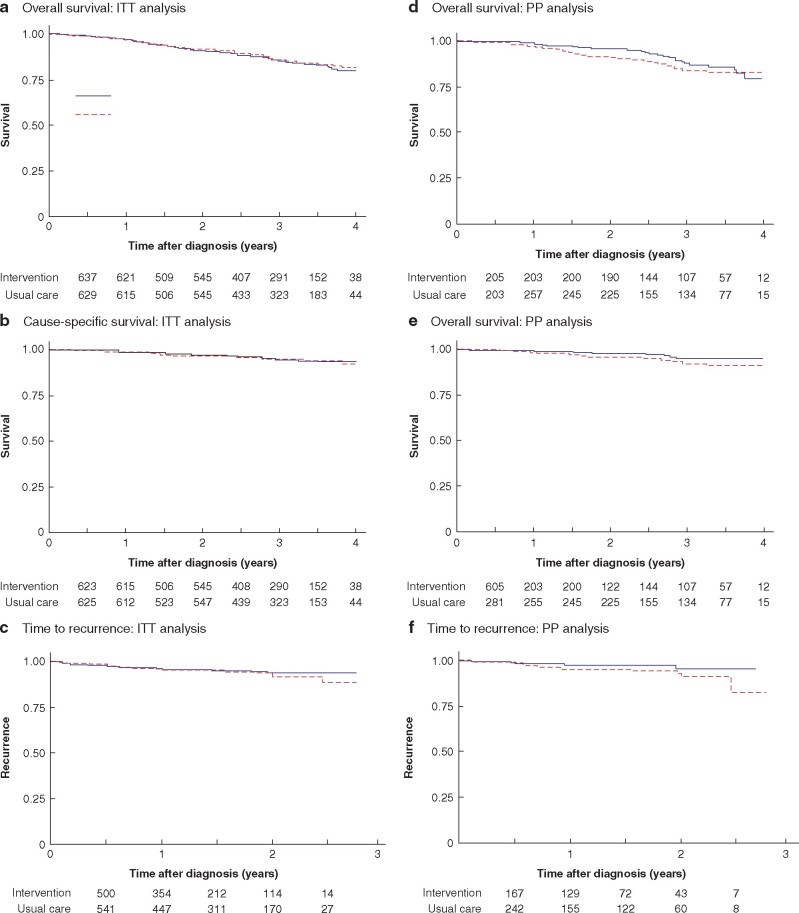
Median follow-up was 36 months among participants alive at the end of study. Survival was similar in the two arms, with an estimated 2-year survival rate of just over 90 per cent in both arms and a hazard ratio of 1.07 (95 per cent c.i. 0.80 to 1.43; P = 0.633) in favour of the usual-care arm (Fig. 4a). Of the 184 deaths (94 in intervention, 90 usual care), two-thirds were unrelated to breast cancer, giving a hazard ratio for breast cancer-specific survival of 0.88 (0.54 to 1.44; P = 0.609) in favour of the intervention arm (Fig. 4b and Table 4). Sixty patients (25 intervention, 35 usual care) experienced recurrence (hazard ratio 0.86, 0.51 to 1.43; P = 0.558) (Fig. 4c). Results of survival analyses on a per-protocol basis are shown in Fig. 4d–f.
Fig. 4.
Overall survival, cause-specific survival and time to recurrence
Intention-to-treat (ITT) analysis of a overall survival (hazard ratio (HR) 1.07, 95 per cent c.i. 0.80 to 1.43; P = 0.633), b cause-specific survival (HR 0.86, 0.54 to 1.44; P = 0.609), and c time to recurrence (HR 0.86, 0.51 to 1.43; P = 0.558); per-protocol (PP) analysis of d overall survival (HR 0.81, 0.49 to 1.33; P = 0.404), e cause-specific survival (HR 0.56, 0.24 to 1.29; P = 0.171), and f time to recurrence (HR 0.48, 0.17 to 1.31; P = 0.150).
Table 4.
Survival outcomes according to usual-care and intervention site allocation
| Intervention (21 clusters) | Usual care (25 clusters) | Overall (46 clusters) | |
|---|---|---|---|
| Survival data available | 644 | 641 | 1285 |
| Alive at 2 years | |||
| Yes | 584 (90.7) | 588 (91.7) | 1172 (91.2) |
| No | 60 (9.3) | 53 (8.3) | 113 (8.8) |
| Alive at end of follow-up | |||
| Yes | 550 (85.4) | 551 (86.0) | 1101 (85.7) |
| No | 94 (14.6) | 90 (14.0) | 184 (14.3) |
| Reason for death | |||
| Breast cancer | 29 (31) | 34 (38) | 63 (34.2) |
| Other reason | 57 (61) | 51 (57) | 108 (58.7) |
| Not known | 8 (9) | 5 (6) | 13 (7.1) |
Values in parentheses are percentages.
Two women died (1 in each arm) within 30 days of surgery (1 under local and 1 under general anaesthetic); both women were in their 90s and death certificates cited metastatic breast cancer as the primary cause, although a surgical contribution to the deaths cannot be excluded.
Quality of treatment allocation
Characteristics of patients who chose primary endocrine therapy or surgery plus endocrine therapy, and those who had chemotherapy or not, between between intervention and usual-care sites did not differ significantly (Table S6).
Discussion
This large multicentre cRCT evaluated the effects of two DESIs on clinical decision-making in older women with operable breast cancer. In intention-to-treat analysis, use of the DESI tools did not affect global QoL at 6 months, but the tools were intended only for women for whom either of the relevant choices was actually offered. Preplanned per-protocol analysis of women offered either choice showed a small improvement in QoL. Treatment choices were altered, with lower rates of surgery and chemotherapy at intervention sites. Patient knowledge was enhanced by use of the DESIs, suggesting that they improve informed decision-making.
The trial intended to recruit 50 sites, and a fixed and equal number of participants at each site. Although it managed to randomize only 46 sites, it did achieve its sample size target by allowing differential recruitment at the randomized sites. In addition, the observed variation in the primary outcome and the intraclass correlation coefficients were lower than anticipated in the sample size calculation, thus preserving the power of the trial.
The trial design included all women, regardless of whether the tool was relevant, because treatment choice was also under study. Consequently, clinicians were invited to use the tool to inform their thresholds for treatment selection, which could occur only if the study was not limited to those ultimately offered a choice.
Analysis of survival outcomes at a median of 36 months’ follow-up showed minimal survival difference between study arms in intention-to-treat analysis, although the per-protocol analysis did show some separation of the survival curves, indicating a small potential survival benefit among participants who underwent treatment consultations as intended by the intervention.
Use of the DESIs was embedded into normal practice for the whole breast unit (cluster) and was available for use by all women, whether or not in the trial, to ensure clinician familiarity and enhance enrolment. In addition, the protocol was designed to allow women to opt out of QoL and decision quality measurements, in order to reduce participant burden in the older and often frail group of women who are considered candidates for primary endocrine therapy. However, this strategy reduced the power of the study for certain outcomes.
A number of decision tools have been developed to enhance the quality of decision-making for patients with breast cancer (reviewed by Nicholas and colleagues47). These have explored various domains of care, such as type of primary surgery to have48, whether to accept adjuvant chemotherapy49, or whether to undergo reconstructive surgery50. None has explored the key decision between surgery or primary endocrine therapy in older women. Studies have shown that decision support tools can improve the quality of decision-making in breast cancer. The iCanDecide tool, for example, enhanced decision quality in women with early-stage breast cancer relating to which type of locoregional therapy to undergo51. Improved levels of knowledge to support decision-making have been shown in trials52. Although there has been a general trend by medical professionals to reduce rates of primary endocrine therapy as new evidence is emerging to show that this may result in inferior survival outcomes53, the aim of a DESI is to support autonomy and informed decision-making by the patient based on their own priorities and wishes54. It is known that older women value QoL and maintenance of independence more highly than younger women, who value length of life most highly55.
Patients vary in their preferred level of involvement in decision-making. Some desire passive involvement, others active engagement, and some collaborative discussions56. Older women tend to be slightly more passive in their decision-making than younger patients24, but most still prefer some level of shared decision-making56.
One of the limitations of the online component of the DESIs is that it only provides data on survival metrics. Previous work by the authors’ group has shown that older women highly value the preservation of QoL55 and independence57, both of which are negatively influenced to varying degrees by surgery58,59 and chemotherapy60–62. The main Age Gap cohort study63 has data on over 3400 older women in terms of the impact of these therapies on QoL and functional capacity. It also has detailed data on adverse events from therapy. Risk-adjusted analysis of these more nuanced outcomes will be undertaken and added to the online tool in future to give older women a better idea of the holistic impact of therapy and how it meets their priorities.
A detailed process evaluation was run alongside this study (reported separately), which found favourable feedback from clinicians and patients about the DESIs, but limited use by site, and evidence about adaptations in their use that can inform further implementation and evaluations (manuscript submitted). Use of the DESIs in women who were offered a choice was lower than anticipated, which in itself is an outcome from the study and demonstrates the challenges of introducing complex interventions into clinical practice. Despite face-to-face training and a training video on the study website, and regular contact with centres throughout the study period, staff changes, the sheer numbers of staff in National Health Service clinics, staff preferences, lack of IT in consulting rooms, and security firewalls in some Trusts’ IT systems were all implicated in this low usage. The low response rate to some of the questionnaires relating to decision quality metrics may also be a source of bias, with women potentially selectively agreeing to complete these if they had either a particularly positive or negative experience.
The online tools, booklets, and brief decision aids have been made available openly (https://agegap.shef.ac.uk/) following granting of UK Medicines and Healthcare products Regulatory Agency approval. It is hoped that the DESIs will be used by clinicians to support shared decision-making with older women facing these two choices, that the tool will be further developed in the future with 10-year survival and QoL outcomes, and that it will be updated periodically to remain valid for the changing population demography of the UK.
The Age Gap DESIs influenced QoL and levels of knowledge about the risks and benefits of breast cancer treatment in older women with breast cancer. They also affected treatment selection, especially among those offered choices, suggesting that informed decision-making was supported.
Funding
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (grant reference number RP-PG-1209-10071). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Supplementary Material
Acknowledgements
The authors acknowledge the contributions of all staff at recruiting sites (Table S1) and thank all patients who consented to take part in the study. The trial sponsor was Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust (Doncaster Royal Infirmary, Doncaster, UK). T.R. and S.J.W. are NIHR Senior Investigators, and J.M. is an NIHR Clinical Lecturer.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Presented in part to the British Association of Surgical Oncology Annual Scientific Meeting, November 2019, London, UK
References
- 1. Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C.. Older women with operable breast cancer are less likely to have surgery. Br J Surg 2007;94:1209–1215 [DOI] [PubMed] [Google Scholar]
- 2. Wyld L, Garg DK, Kumar ID, Brown H, Reed MW.. Stage and treatment variation with age in postmenopausal women with breast cancer: compliance with guidelines. Br J Cancer 2004;90:1486–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jauhari Y, Gannon M, Medina J, Cromwell D, Horgan K, Dodwell D.. National Audit of Breast Cancer in Older Patients Annual Report. Healthcare Quality Improvement Partnership, London, UK: Royal College of Surgeons of England. 2018.
- 4. Derks MGM, Bastiaannet E, Kiderlen M, Hilling DE, Boelens PG, Walsh PM. et al. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: a population-based cohort study from the EURECCA Breast Cancer Group. Br J Cancer 2018;119:121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan J, Richards P, Ward S, Francis M, Lawrence G, Collins K. et al. Case-mix analysis and variation in rates of non-surgical treatment of older women with operable breast cancer. Br J Surg 2015;102:1056–1063 [DOI] [PubMed] [Google Scholar]
- 6. Morgan JL, Collins K, Robinson TG, Cheung KL, Audisio R, Reed MW. et al. Healthcare professionals' preferences for surgery or primary endocrine therapy to treat older women with operable breast cancer. Eur J Surg Oncol 2015;41:1234–1242 [DOI] [PubMed] [Google Scholar]
- 7. Morgan JL, Walters SJ, Collins K, Robinson TG, Cheung KL, Audisio R. et al. What influences healthcare professionals' treatment preferences for older women with operable breast cancer? An application of the discrete choice experiment. Eur J Surg Oncol 2017;43:1282–1287 [DOI] [PubMed] [Google Scholar]
- 8. Ward SE, Richards P, Morgan J, Holmes GR, Broggio J, Collins K. et al. Omission of surgery in older women with early breast cancer has an adverse impact on breast cancer specific survival. Br J Surg 2018;105:1454–1463 [DOI] [PubMed] [Google Scholar]
- 9. Morgan J WL, Collins KA, Reed MW.. Surgery versus primary endocrine therapy for operable primary breast cancer in elderly women (70 years plus). Cochrane Database Syst Rev 2014;(1)CD004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan JL, Reed MW, Wyld L.. Primary endocrine therapy as a treatment for older women with operable breast cancer—a comparison of randomised controlled trial and cohort study findings. Eur J Surg Oncol 2014;40:676–684 [DOI] [PubMed] [Google Scholar]
- 11. Tang V, Zhao S, Boscardin J, Sudore R, Covinsky K, Walter LC. et al. Functional status and survival after breast cancer surgery in nursing home residents. JAMA Surg 2018;153:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rocco N, Rispoli C, Pagano G, Rengo G, Compagna R, Danzi M. et al. Breast cancer surgery in elderly patients: postoperative complications and survival. BMC Surg 2013;13:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Colleoni M, Price KN, Castiglione-Gertsch M, Gelber RD, Coates AS, Goldhirsch A.. Mortality during adjuvant treatment of early breast cancer with cyclophosphamide, methotrexate, and fluorouracil. International Breast Cancer Study Group. Lancet 1999;354:130–131 [DOI] [PubMed] [Google Scholar]
- 14. Muss HB, Berry DA, Cirrincione C, Budman DR, Henderson IC, Citron ML. et al. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B experience. J Clin Oncol 2007;25:3699–3704 [DOI] [PubMed] [Google Scholar]
- 15. Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I. et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol 2012;13:e148–e60. [DOI] [PubMed] [Google Scholar]
- 16. Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence G. et al. PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 2010;12:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olivotto IA, Bajdik CD, Ravdin PM, Speers CH, Coldman AJ, Norris BD. et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 2005;23:2716–2725 [DOI] [PubMed] [Google Scholar]
- 18. Al-Refaie WB, Vickers SM, Zhong W, Parsons H, Rothenberger D, Habermann EB.. Cancer trials versus the real world in the United States. Ann Surg 2011;254:438–442 [DOI] [PubMed] [Google Scholar]
- 19. de Glas NA, Bastiaannet E, Engels CC, de Craen AJ, Putter H, van de Velde CJ. et al. Validity of the online PREDICT tool in older patients with breast cancer: a population-based study. Br J Cancer 2016;114:395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Glas NA, van de Water W, Engelhardt EG, Bastiaannet E, de Craen AJ, Kroep JR. et al. Validity of Adjuvant! Online program in older patients with breast cancer: a population-based study. Lancet Oncol 2014;15:722–729 [DOI] [PubMed] [Google Scholar]
- 21. Lifford KJ, Edwards A, Burton M, Harder H, Armitage F, Morgan JL. et al. Efficient development and usability testing of decision support interventions for older women with breast cancer. Patient Prefer Adherence 2019;13:131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ward SE, Holmes GR, Ring A, Richards PD, Morgan JL, Broggio JW. et al. Adjuvant chemotherapy for breast cancer in older women: an analysis of retrospective English cancer registration data. Clin Oncol 2019;31:444–452 [DOI] [PubMed] [Google Scholar]
- 23. Burton M, Collins KA, Lifford KJ, Brain K, Wyld L, Caldon L. et al. The information and decision support needs of older women (>75 yrs) facing treatment choices for breast cancer: a qualitative study. Psychooncology 2015;24:878–884 [DOI] [PubMed] [Google Scholar]
- 24. Burton M, Kilner K, Wyld L, Lifford KJ, Gordon F, Allison A. et al. Information needs and decision-making preferences of older women offered a choice between surgery and primary endocrine therapy for early breast cancer. Psychooncology 2017;26:2094–2100 [DOI] [PubMed] [Google Scholar]
- 25. Campbell MK, Piaggio G, Elbourne DR, Altman DG; CONSORT Group. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. [DOI] [PubMed] [Google Scholar]
- 26. Collins K, Reed M, Lifford K, Burton M, Edwards A, Ring A. et al. Bridging the age gap in breast cancer: evaluation of decision support interventions for older women with operable breast cancer: protocol for a cluster randomised controlled trial. BMJ Open 2017;7:e015133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lifford KJ, Witt J, Burton M, Collins K, Caldon L, Edwards A. et al. Understanding older women's decision making and coping in the context of breast cancer treatment. BMC Med Inform Decis Mak 2015;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ. et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376 [DOI] [PubMed] [Google Scholar]
- 29. Sprangers MA, Groenvold M,, Arraras JI, Franklin J, te Velde A, Muller M. et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol 1996;14:2756–2768 [DOI] [PubMed] [Google Scholar]
- 30. Johnson C, Fitzsimmons D, Gilbert J, Arrarras JI, Hammerlid E, Bredart A. et al. Development of the European Organisation for Research and Treatment of Cancer quality of life questionnaire module for older people with cancer: the EORTC QLQ-ELD15. Eur J Cancer 2010;46:2242–2252 [DOI] [PubMed] [Google Scholar]
- 31. Barr PJ, Thompson R, Walsh T, Grande SW, Ozanne EM, Elwyn G.. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res 2014;16:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E. et al. Validation of a decision regret scale. Med Decis Making 2003;23:281–292 [DOI] [PubMed] [Google Scholar]
- 33. Marteau TM, Bekker H.. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31:301–306 [DOI] [PubMed] [Google Scholar]
- 34. Broadbent E, Petrie KJ, Main J, Weinman J.. The brief illness perception questionnaire. J Psychosom Res 2006;60:631–637 [DOI] [PubMed] [Google Scholar]
- 35. Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med 1997;4:92–100 [DOI] [PubMed] [Google Scholar]
- 36. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 37. Mahoney FI, Barthel DW.. Functional evaluation: the Barthel index. Md State Med J 1965;14:61–65 [PubMed] [Google Scholar]
- 38. Lawton MP, Brody EM.. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186 [PubMed] [Google Scholar]
- 39. Folstein MF, Folstein SE, McHugh PR.. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 40. Read JA, Crockett N, Volker DH, MacLennan P, Choy ST, Beale P. et al. Nutritional assessment in cancer: comparing the Mini-Nutritional Assessment (MNA) with the scored Patient-Generated Subjective Global Assessment (PGSGA). Nutr Cancer 2005;53:51–56 [DOI] [PubMed] [Google Scholar]
- 41. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996;12:S15–S19 [DOI] [PubMed] [Google Scholar]
- 42. Hortobagyi GNC, D'Orsy J, Edge C, Mittendorf S, Rugo E, Solin H. et al. A breast cancer staging. American joint committee on cancer. Cancer Staging Manual 2018:589–636 [Google Scholar]
- 43. Campbell MJ, Walters SJ.. How to Design, Analyse and Report Cluster Randomised Trials in Medicine and Health Related Research. Chichester: Wiley, 2014. [Google Scholar]
- 44. Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM.. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. J Clin Oncol 2011;29:89–96 [DOI] [PubMed] [Google Scholar]
- 45. Spiegelhalter DJ. Funnel plots for comparing institutional performance. Stat Med 2005;24:1185–1202 [DOI] [PubMed] [Google Scholar]
- 46.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829–836 [Google Scholar]
- 47. Nicholas Z, Butow P, Tesson S, Boyle F.. A systematic review of decision aids for patients making a decision about treatment for early breast cancer. Breast 2016;26:31–45 [DOI] [PubMed] [Google Scholar]
- 48. Goel V, Sawka CA, Thiel EC, Gort EH, O'Connor AM.. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Med Decis Making 2001;21:1–6 [DOI] [PubMed] [Google Scholar]
- 49. Whelan T, Sawka C, Levine M, Gafni A, Reyno L, Willan A. et al. Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node-negative breast cancer. J Natl Cancer Inst 2003;95:581–587 [DOI] [PubMed] [Google Scholar]
- 50. Heller L, Parker PA, Youssef A, Miller MJ.. Interactive digital education aid in breast reconstruction. Plast Reconstr Surg 2008;122:717–724 [DOI] [PubMed] [Google Scholar]
- 51. Hawley ST, Li Y, An LC, Resnicow K, Janz NK, Sabel MS. et al. Improving breast cancer surgical treatment decision making: the I can decide randomized clinical trial. J Clin Oncol 2018;36:659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Whelan T, Levine M, Willan A, Gafni A, Sanders K, Mirsky D. et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA 2004;292:435–441 [DOI] [PubMed] [Google Scholar]
- 53. de Boer AZ, de Glas NA, de Mheen PJM, Dekkers OM, Siesling S, de Munck L. et al. Effect of omission of surgery on survival in patients aged 80 years and older with early-stage hormone receptor-positive breast cancer. Br J Surg 2020;107:1145–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hall DE, Prochazka AV, Fink AS.. Informed consent for clinical treatment. CMAJ 2012;184:533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L.. Quality of life versus length of life considerations in cancer patients: a systematic literature review. Psychooncology 2019;28:1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morgan JL, Burton M, Collins K,, Lifford KJ, Robinson TG, Cheung KL. et al. The balance of clinician and patient input into treatment decision-making in older women with operable breast cancer. Psychooncology 2015;24:1761–1766 [DOI] [PubMed] [Google Scholar]
- 57. Husain LS, Collins K, Reed M, Wyld L.. Choices in cancer treatment: a qualitative study of the older women's (>70 years) perspective. Psychooncology 2008;17:410–416 [DOI] [PubMed] [Google Scholar]
- 58. Euhus DM, Addae JK, Snyder CF, Canner JK.. Change in health-related quality of life in older women after diagnosis of a small breast cancer. Cancer 2019;125:1807–1814 [DOI] [PubMed] [Google Scholar]
- 59. Swanick CW, Lei X, Xu Y, Shen Y, Goodwin NA, Smith GL. et al. Long-term patient-reported outcomes in older breast cancer survivors: a population-based survey study. Int J Radiat Oncol Biol Phys 2018;100:882–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Quinten C, Kenis C, Hamaker M, Coolbrandt A, Brouwers B, Dal Lago L. et al. The effect of adjuvant chemotherapy on symptom burden and quality of life over time; a preliminary prospective observational study using individual data of patients aged >/=70 with early stage invasive breast cancer. J Geriatr Oncol 2018;9:152–162 [DOI] [PubMed] [Google Scholar]
- 61. Chandler Y, Jayasekera J, Schechter C, Isaacs C, Cadham C, Mandelblatt J.. Simulation of chemotherapy effects in older breast cancer patients with high recurrence scores. J Natl Cancer Inst 2019;112:574–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hurria A, Soto-Perez-de-Celis E, Allred JB, Cohen HJ, Arsenyan A, Ballman K. et al. Functional decline and resilience in older women receiving adjuvant chemotherapy for breast cancer. J Am Geriatr Soc 2019;67:920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Morgan J WL, Collins KA, Reed MW. et al. Breast cancer surgery in older women: outcomes of the Bridging Age Gap in Breast Cancer study. Br J Surg 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.