Abstract
Background
Previous studies have suggested improved efficiency and patient outcomes with 125I seed compared with hookwire localization (HWL) in breast-conserving surgery, but high-level evidence of superior surgical outcomes is lacking. The aim of this multicentre pragmatic RCT was to compare re-excision and positive margin rates after localization using 125I seed or hookwire in women with non-palpable breast cancer.
Methods
Between September 2013 and March 2018, women with non-palpable breast cancer eligible for breast-conserving surgery were assigned randomly to preoperative localization using 125I seeds or hookwires. Randomization was stratified by lesion type (pure ductal carcinoma in situ (DCIS) or other) and study site. Primary endpoints were rates of re-excision and margin positivity. Secondary endpoints were resection volumes and weights.
Results
A total of 690 women were randomized at eight sites; 659 women remained after withdrawal (125I seed, 327; HWL, 332). Mean age was 60.3 years in the 125I seed group and 60.7 years in the HWL group, with no difference between the groups in preoperative lesion size (mean 13.2 mm). Lesions were pure DCIS in 25.9 per cent. The most common radiological lesion types were masses (46.9 per cent) and calcifications (28.2 per cent). The localization modality was ultrasonography in 65.5 per cent and mammography in 33.7 per cent. The re-excision rate after 125I seed localization was significantly lower than for HWL (13.9 versus 18.9 per cent respectively; P = 0.019). There were no significant differences in positive margin rates, or in specimen weights and volumes.
Conclusion
Re-excision rates after breast-conserving surgery were significantly lower after 125I seed localization compared with HWL. Registration number: ACTRN12613000655741 (http://www.ANZCTR.org.au/).
This is the first study to confirm a significant improvement in re-excision rate for breast conserving surgery (BCS) using radioactive seed rather than hookwire guidance. 659 patients were randomised to undergo BCS with iodine125 seed or hookwire localisation. Re-excision rates were significantly lower in the seed group. Involved margin rates were also lower, although this did not reach statistical significance. BCS with radioactive seed rather than wire guidance does give superior patient outcomes.
Introduction
The number of non-palpable breast cancers requiring preoperative image-guided localization continues to increase as a result of more screen-detected cancers and neoadjuvant chemotherapy1,2.
Hookwire localization (HWL) has been used widely since the 1970s, but is associated with high positive margin (20–40 per cent) and re-excision (30–50 per cent) rates3–5. Other disadvantages of HWL include technical difficulties (such as wire transection and migration), inefficient use of radiology bookings, and impact on theatre time6–8.
Radioguided occult lesion localization using 125I seeds is the most widely used non-wire localization technique, involving a radiologist placing a 4.5×0.8-mm titanium seed containing 125I into the lesion under image guidance. The 27-keV photons emitted are detected at surgery using the γ probe already used widely for sentinel node biopsy. Use of radioactive 125I seeds decouples the localization procedure from surgery, thereby improving scheduling and efficiency8. The seed provides precise real-time three-dimensional intraoperative surgical guidance, potentially resulting in improved surgical outcomes. Retrospective cohort studies and individual RCTs have been discordant, and a recent meta-analysis9 of five previous RCTs failed to show a significant reduction in positive margins (involved or very close) or re-excision rates.
The primary objectives of this study were to compare positive radial margin and re-excision rates following initial breast-conserving surgery (BCS) in patients with biopsy-proven non-palpable invasive or in situ breast cancer randomized to either 125I seed or HWL localization.
Methods
Women who were candidates for BCS were recruited into this prospective multicentre RCT (Australian New Zealand Clinical Trials Registry number 12613000655741) between September 2013 and March 2018. Ethical approval was obtained from all hospital sites, and the study was undertaken according to the National Statement on Ethical Conduct in Human Research 200710. The study was performed at eight tertiary care institutions: Royal Perth (RPH), Sir Charles Gairdner (SCGH), Fiona Stanley, and St John of God Subiaco Hospitals in Perth; Monash Hospital, Melbourne; Robina, Gold Coast University Hospitals, Gold Coast; Westmead Hospital, Sydney; and Waikato Hospital, New Zealand. Fifty breast surgeons and 64 radiologists were involved. Before study commencement, the lead sites (RPH and SCGH) conducted two pilot studies to test seed-handling protocols and train multidisciplinary team members11,12. Thereafter at least one surgeon and one pathologist from each external site participated in a training course for 125I seed handling. Radiological insertion of iodine seeds is similar to placing a breast marker and does not require special training. Each site handled radioactive seeds according to state safety regulations, and individualized institutional seed-handling protocols were developed.
Inclusion and exclusion criteria
Study inclusion criteria included: age 18 years and over, histologically confirmed non-palpable in situ cancer including pleomorphic lobular carcinoma in situ or invasive non-palpable breast cancer requiring localization, including neoadjuvant chemotherapy responders and women undergoing planned BCS. Multifocal and bilateral disease were allowed. Exclusion criteria were male sex, pregnancy or lactation, multicentric disease, contraindications to BCS, and classical lobular carcinoma in situ only.
Randomization
After obtaining written consent, each participant was randomized to lesion localization using either 125I seed or HWL. Centrally concealed computer-generated block randomization was performed via a secure online database. Randomization was stratified according to study site and core biopsy histopathology (ductal carcinoma in situ (DCIS) only and other malignant pathology) of the index lesion. Participants with multifocal or bilateral lesions received the same localization method for all lesions. To satisfy local radiation protection guidelines, the number of seeds per participant was restricted to two, with combined activity of both seeds below 4 MBq. Typical radiation doses have been published elsewhere13.
Lesion localization
Localization was performed with either mammographic (grid, stereo, or tomosynthesis) or ultrasound guidance. Seed placement was performed using 18-G needles containing an 125I seed, up to 8 days before surgery. HWL was done on the morning of surgery, by insertion of a 9-cm modified Kopans hookwire.
Two wires or seeds were used to bracket larger (above 20 mm) lesions at the discretion of the radiologist. The position of seeds and wires was assessed on two-view mammograms after insertion, and, if considered unsatisfactory following discussion with the surgeon, another hookwire was inserted; a further seed was not inserted to avoid surgical confusion. Participants scheduled to have sentinel node biopsy underwent sentinel node mapping (predominantly guided by 99mTc radiotracer) according to site-specific protocols.
Surgical procedure and retrieval of seeds
A standard dual-energy γ probe was used to detect and remove the breast lesion and seed(s), and, where appropriate, the sentinel node. Blue dye alone was used for sentinel node localization at one trial site. The surgeon aimed to resect a cylindrical volume of tissue including the marker clip and seed with a radiological resection margin of 10 mm beyond the identified tumour border, or, in patients with complete clinical and radiological response after neoadjuvant chemotherapy, a 10-mm radius surrounding the marker clip. Routinely, resections were performed from skin to pectoralis fascia. When multiple seeds were used for bracketing14, a block of tissue was resected to encompass seeds and an adequate margin.
Retrieval of seeds was confirmed by presence of an125I signal in the specimen and absence of this signal in the surgical bed, together with visualization of the seed on intraoperative specimen radiography (IOSR). Strict seed-tracking protocols were maintained. Specimens were oriented using sutures and markers such as large titanium LIGA® clips (Ethicon, Somerville, NJ, USA), to aid radiographic margin identification (large clips were used to avoid confusion with seeds on the specimen radiograph).
Intraoperative re-excision was performed when the margin was deemed close, based on the IOSR report or surgical suspicion. Frozen-section/immediate pathological margin assessment was not done. Seeds were retrieved by the pathologist from the fresh or fixed specimen15, and specimens were inked, sectioned, and processed with standard histopathological protocols. Specimens were weighed and measured before fixation. The volume of the main resection specimen was estimated using the formula for volume of a cube.
Self-reported ease of use
Radiologists and surgeons were asked to rate their ease of use of 125I seed localization and HWL using a 7-point Likert scale.
Adjuvant treatment
Postoperative management of the participants was decided by the local multidisciplinary tumour board, using individual site definitions for involved and close tumour margins, tumour biology, patient factors such as age, expected adjuvant treatment, and whether or not further margin excision was possible.
Data and statistical analysis
Before the RCT, an audit at two tertiary referral centres in Western Australia found a re-excision rate of 30 per cent, comparable to that in the worldwide literature5. Based on this, a power calculation with the aim of detecting a reduction in re-excision rates from 30 to 20 per cent with a significance level of 5 per cent (two-tailed) and 90 per cent power was done. A sample size of 293 participants per arm was required and, to allow for an attrition rate of 10 per cent in each study arm, a total sample size of 650 women was specified a priori.
A breast level analysis was performed for positive margin and re-excision rates. Tumour size was measured as the maximum dimension of the largest invasive focus. The weight and dimensions of the initial breast specimen were used to calculate specimen volume. Weights and dimensions of additional margins excised during surgery were not added to the initial specimen. The closest radial margin including additional cavity shaves taken at the first operation was used for analysis.
Demographic and clinical characteristics were summarized using mean(s.d.) values for continuous variables, and frequencies and percentages for categorical variables. Logistic regression using generalized estimating equations (PROC GENMOD in SAS® version 9.4; SAS Institute, Cary, NC, USA) was used to test for differences between arms in primary (re-excision rates and positive margins) and secondary (specimen weight and volume) outcomes, allowing for correlations within stratified blocks. Statistical comparisons we made using the Z test. Additional adjustment for known confounders (number of cavity shaves taken; lesion size on imaging; participant age; in situ only versus invasive with/without in situ disease at core biopsy) was undertaken and effect estimates were compared in the main analysis, with assessment of linearity for continuous variables based on restricted cubic splines. The primary analysis was by intention-to-treat and a supplementary per-protocol analysis was also undertaken.
Distributions of difficulty scores for localization and excision were summarized within study arms as median (i.q.r.) values, and compared with the Wilcoxon rank sum test (PROC NPAR1WAY in SAS®). Comparisons of difficulty scores were stratified by modality of localization (mammography versus ultrasonography) as a post hoc subgroup analysis. All tests of statistical significance were two-sided. The level chosen for statistical significance was P < 0.050.
Results
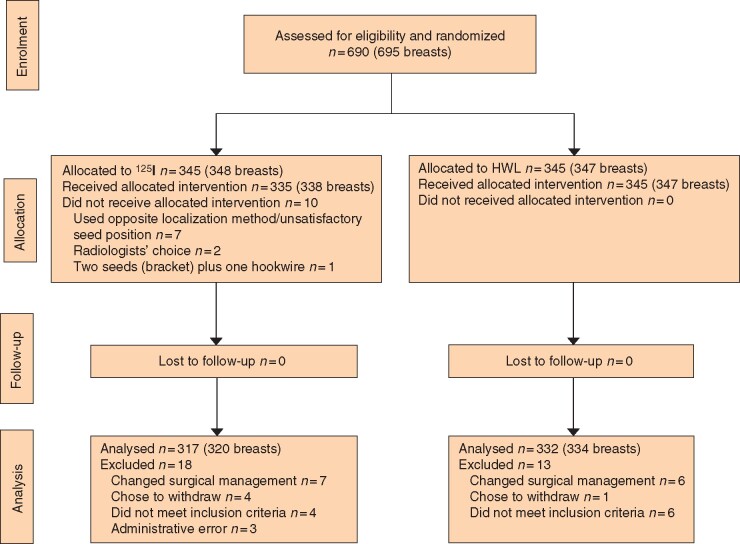
A total of 690 participants gave written informed consent and 31 were withdrawn after randomization (18 from the 125I seed arm and 13 from the HWL arm), for reasons shown in the CONSORT diagram (Fig. 1). After withdrawals, 327 participants (330 breasts) remained in the 125I seed arm and 332 participants (334 breasts) in the HWL arm. In the 125I seed group, seven participants underwent HWL due to suboptimal seed position, two had HWL at radiologist discretion, and one woman with multifocal disease on imaging underwent bracketing with two seeds and a HWL. All participants in the HWL arm received their allocated localization device.
Fig. 1.
CONSORT diagram for the trial
HWL, hookwire localization.
Participant demographics and imaging features are shown in Table 1. Mean patient age was 60.7 years in the HWL group and 60.3 years in the 125I seed group. The mean size of the lesions in both groups was identical (13.2 mm). Some 25.9 per cent of the index lesions were pure DCIS on core biopsy, with the remainder being invasive disease with or without an in situ component. Insertion of seed or wire was performed using ultrasound guidance in most cases (65.5 per cent), with mammographic guidance techniques (stereotactic, grid, tomosynthesis) used in 33.7 per cent. Most participants had only one wire or seed inserted for lesion localization, but 40 had more than one seed (18 patients) or wire (22) inserted to bracket either a single large lesion (greater than 20 mm) or an apparent multifocal lesion on preoperative imaging.
Table 1.
Patient and imaging characteristics
| Wire (n = 332; 334 breasts, 340 lesions) | Seed (n = 327; 330 breasts, 333 lesions) | Total (n = 659; 664 breasts, 673 lesions) | |
|---|---|---|---|
| Age (years)* | 60.7(10.4) | 60.3(10.2) | 60.5(10.3) |
| Radiological lesion size (mm)*† | 13.2(8.9) | 13.2(8.9) | 13.2(8.9) |
| Sentinel node biopsy performed | |||
| Yes | 245 (73.4) | 244 (73.9) | 489 (73.6) |
| No | 89 (26.6) | 86 (26.1) | 175 (26.4) |
| Neoadjuvant therapy | |||
| Yes | 8 (2.4) | 11 (3.4) | 19 (2.9) |
| No | 324 (97.6) | 316 (96.6) | 640 (97.1) |
| Mammogram findings | |||
| Clip only | 4 (1.2) | 5 (1.5) | 9 (1.3) |
| Calcification | 90 (26.5) | 100 (30.0) | 190 (28.2) |
| Distortion | 18 (5.3) | 18 (5.4) | 36 (5.3) |
| Localized increased density | 31 (9.1) | 38 (11.4) | 69 (10.3) |
| Mass | 151 (44.4) | 138 (41.4) | 289 (42.9) |
| Mass and calcification | 14 (4.1) | 13 (3.9) | 27 (4.0) |
| Visible, not specified | 5 (1.5) | 7 (2.1) | 12 (1.8) |
| Not visible | 27 (7.9) | 14 (4.2) | 41 (6.1) |
| Ultrasound findings | |||
| Visible | 248 (72.9) | 245 (73.6) | 493 (73.3) |
| Not visible | 52 (15.3) | 46 (13.8) | 98 (14.6) |
| Not done | 40 (11.8) | 42 (12.6) | 82 (12.2) |
| Preoperative MRI | |||
| Yes | 25 (7.4) | 33 (9.9) | 58 (8.6) |
| No | 315 (92.6) | 300 (90.1) | 615 (91.4) |
| Guidance method for localization | |||
| Mammography | 113 (33.2) | 114 (34.2) | 227 (33.7) |
| Ultrasonography | 226 (66.5) | 215 (64.6) | 441 (65.5) |
| Not specified | 1 (0.3) | 4 (1.2) | 5 (0.7) |
| Lesions where bracketing used | |||
| Yes | 26 (7.6) | 22 (6.6) | 48 (7.1) |
| No | 314 (92.4) | 311 (93.4) | 625 (92.9) |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.). †Radiological size not recorded for eight breasts (hookwire, 2; seed, 6); size derived from largest measurement on mammography or ultrasonography, and tumour size summed for eight breasts with multifocal tumours.
Final pathology
The final pathology characteristics of the localized lesions are reported in Table 2 with 24.7 per cent being DCIS only and the remainder having invasive disease, with or without DCIS, with no difference between the 125I seed and HWL groups. The most common mammographic lesion types were a mass (with or without microcalcification) (46.9 per cent) or microcalcifications (28.2 per cent) (Table 1). Of the 591 lesions assessed with ultrasonography, 493 (83.4 per cent) were visible. In nine cases a biopsy marker clip was the only visible target for preoperative localization; four had had a complete response to neoadjuvant treatment, with no residual malignancy on final histopathology, and in one the lesion had been removed completely by vacuum-assisted diagnostic core biopsy. Preoperative MRI was performed infrequently (8.6 per cent of cases), with no difference in incidence between the two study arms.
Table 2.
Tumour characteristics
| Wire (n = 334 breasts) | Seed (n = 330 breasts) | Total (n = 664 breasts) | |
|---|---|---|---|
| Tumour category | |||
| pTis | 80 (24.0) | 83 (25.2) | 163 (24.5) |
| pT1a/b | 98 (29.3) | 86 (26.1) | 184 (27.7) |
| pT1c | 124 (37.1) | 111 (33.6) | 235 (35.4) |
| pT2 | 31 (9.3) | 45 (13.6) | 76 (11.4) |
| pT3 | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| No residual tumour | 0 (0) | 4 (1.2) | 4 (0.6)† |
| Nodal status | |||
| NX | 81 (24.3) | 77 (23.3) | 158 (23.8) |
| N0 | 217 (65.0) | 221 (67.0) | 438 (66.0) |
| N1 | 31 (9.3) | 30 (9.1) | 61 (9.2) |
| N2 | 4 (1.2) | 2 (0.6) | 6 (0.9) |
| N3 | 1 (0.3) | 0 (0) | 1 (0.2) |
| Pathological classification* | |||
| DCIS only | 80 (24.0) | 84 (25.5) | 164 (24.7) |
| Invasive only | 96 (28.7) | 105 (31.8) | 201 (30.3) |
| Invasive + DCIS | 158 (47.3) | 141 (42.7) | 299 (45.0) |
Values in parentheses are percentages. *Pathological classification from core biopsy for four breasts with no residual tumour (ductal carcinoma in situ (DCIS) only, 1; invasive only, 1; invasive + DCIS, 2). †Pathological complete response to neoadjuvant therapy (3 breasts); tumour completely removed at core biopsy (1 breast).
Surgical outcomes
The rates of involved margins and reoperation, and specimen weights and volumes are summarized in Table 3. On intention-to-treat and per-protocol analyses, no statistically significant differences were observed in rates of positive margins in 125I seed (5.5 per cent) and HWL (7.8 per cent) groups (P = 0.183). A significant reduction in the re-excision rate was observed for 125I seed localization compared with HWL (13. 9 versus 18.9 per cent respectively; P = 0.019). The weights and volumes of the main tissue specimens (where recorded) were similar for the two groups.
Table 3.
Surgical and specimen outcomes by localizing modality
| Seed (n=330) | Wire (n=334) | Seed versus wire | P | |
|---|---|---|---|---|
| Surgical outcomes | Odds ratio | |||
| Reoperation (%) | 13.9 (10.7, 18.0) | 18.9 (14.8, 23.8) | 0.70 (0.52, 0.94) | 0.019‡ |
| Positive margins (%) | 5.5 (3.5, 8.4) | 7.8 (5.4, 11.0) | 0.68 (0.39, 1.20) | 0.183‡ |
| Specimen outcomes | Difference | |||
| Estimated weight (g)* | 44.7 (36.3, 53.1) | 40.4 (34.1, 46.6) | 4.3 (−1.5, 10.2) | 0.146‡ |
| Estimated volume (ml)† | 86.4 (74.0, 98.8) | 3.9 (73.1, 94.6) | 2.6 (−4.5, 9.6) | 0.476‡ |
Values in parentheses are 95 per cent confidence intervals. *Weight missing for 63 breasts (seed, 28; wire, 35). †Estimated volume missing for 11 breasts (seed, 7; wire, 4). ‡ Z test.
The magnitude of estimates for surgical outcomes, and statistical significance of the differences between groups, were not changed substantially by adjusting for co-variables, or when per-protocol analysis was undertaken (data not shown). The incidence of additional intraoperative cavity margin excision was similar for the two groups. Over half of participants in 125I seed and HWL groups had one or more additional intraoperative cavity margins taken (57.3 and 57.8 per cent respectively), predominantly due to apparent close margins on IOSR (more than 98 per cent). A similar number of participants in each group underwent sentinel node biopsy (73.4 per cent for 125I seed versus 73.9 per cent for HWL). Failure to identify a sentinel node using 99mTc radiotracer was reported in two cases (1 in each study arm).
Adverse events
Suboptimal positioning of seed or wire requiring corrective wire insertion occurred in 9 and 13 cases respectively. In one malpositioned hookwire, the wire tip was embedded in the pectoral muscle and transected during surgery. The wire tip could not be removed and subsequently migrated into the pleural cavity. No further surgery was performed. There were no reported cases of seed migration after insertion. All lesions and seeds were removed successfully. There were several cases in which the seed was observed to separate from the tissue specimen during surgery, particularly when it had been placed at the superficial aspect of the lesion. One case of seed loss between excision and obtaining a specimen radiograph occurred early in the trial; the surgeon had experienced difficulty in using the γ probe and the radiologist mistook a LIGA® clip attached to an orientation suture for a seed on IOSR. By the time the pathologist reported that the seed was missing, theatres had been cleared and the seed could not be found. One episode of seed transection by the pathologist occurred during removal from the specimen. No other adverse events were reported.
Results of user self-reporting
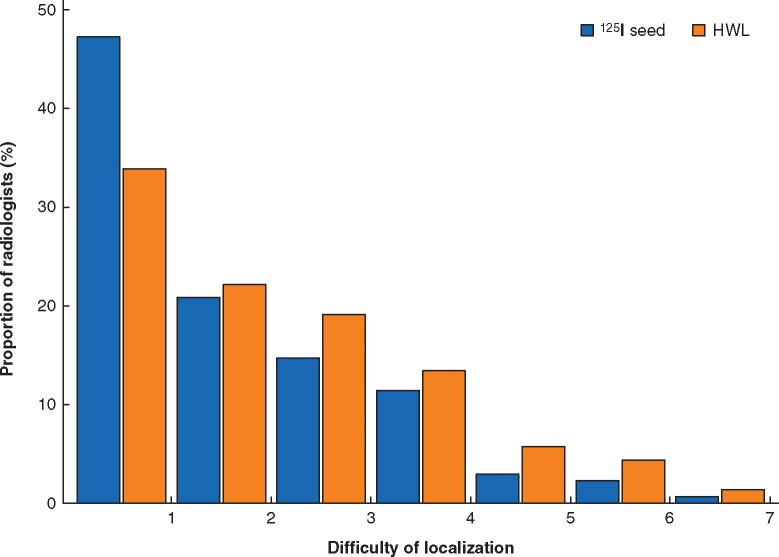
The degrees of difficulty for 125I seed and HWL were self-reported by a subset of radiologists and surgeons on a scale of 1 (not at all difficult) to 7 (very difficult) (Fig. 2). Radiologist-reported difficulty of localization was the same for 125I seed localization and HWL (median 2 (i.q.r. 1–3)), indicating that the majority of radiologists reported difficulty below the scale mid-point, regardless of whether seed or wire was used. There was, however, a statistically significant difference in the distributions of ratings by localizing device (P < 0.001). A rating of 1 was reported more frequently by radiologists using125I seed, and ratings greater than 1 were reported more frequently by radiologists using HWL (Fig. 2).
Fig. 2.
Radiologists’ degree of difficulty of localization using seed versus hookwire
125I seed localization (n=307) versus hookwire localization (HWL) (n=298). Difficulty of localization: 1, not at all difficult; 7, very difficult.
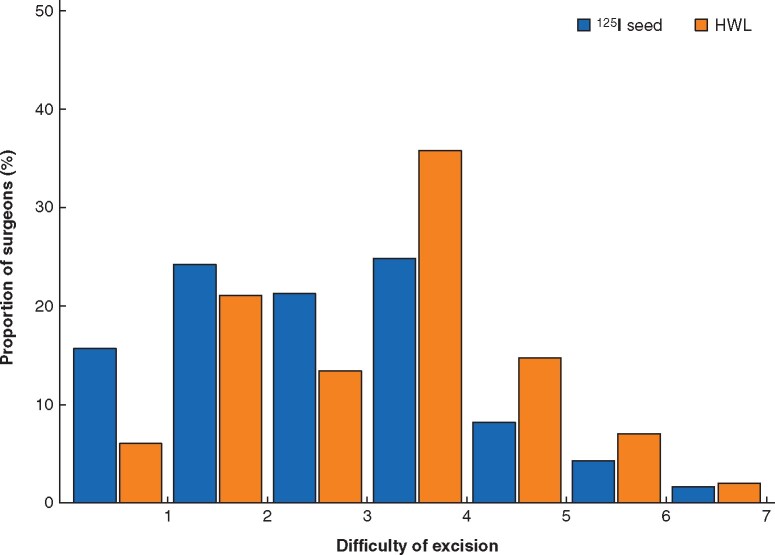
Self-reported difficulty of excision by surgeons was lower for 125I seed (median 3 (i.q.r. 2–4)) than for HWL (4 (2–4)) (P < 0.001). Surgeons using 125I seed were more likely to report difficulty below the scale mid-point; whereas surgeons using HWL were more likely to report difficulty at or above the scale mid-point (Fig. 3).
Fig. 3.
Surgeons’ degree of difficulty of excision using seed versus hookwire
125I seed localization (n=307) versus hookwire localization (HWL) (n=298). Difficulty of excision: 1, not at all difficult; 7, very difficult.
Discussion
Limitations in HWL of breast lesions have led to the development of several non-wire localization methods. Radioactive seed localization using 125I seeds was first described in 2001, with subsequent cohort studies8,11,16,17 showing that both radiologists and surgeons found seeds easier to use, with improved efficiency, convenience, and patient preference. Retrospective studies indicated equivalent or lower re-excision rates, but improved surgical outcomes have not been shown by subsequent RCTs18–20.
The present RCT is the first to show a significant reduction in re-excision rates with 125I seed localization compared with HWL (13.9 versus 18.9 per cent; P = 0.019), a finding that persisted after adjustment for co-variables (number of cavity shaves taken, lesion size on imaging, and presence of in situ only versus invasive disease with or without an in situ component). Although most trial sites used a radial margin of less than 2 mm rather than ‘no tumour on ink’ as the main indicator for re-excision, decisions for reoperation were dependent on locally agreed institutional protocols plus individual decisions discussed at tumour boards.
Although a statistically significant reduction in the proportion of positive margins (tumour on ink) was not demonstrated in this study, it should be noted that the observed difference was in the expected direction (5.5 per cent for 125I seed versus 7.8 per cent for HWL), and that the study was powered primarily to detect a reduction in the reoperation rate rather than positive margins. The positive margin rates for 125I seed versus HWL in this study were similar to those of other reports indicating a 1–3 per cent difference18–20. Langhans and colleagues19. considered a radial margin width of less than 2 mm as positive, which could possibly account for their higher margin positivity rate. On the other hand, Lovrics et al.18. and Bloomquist and co-workers20. both defined a positive margin as ‘tumour on ink’.
The finding in the present study that there was no significant differences in mean weights and volumes of the main excision specimens in 125I seed and HWL groups is consistent with results of previous RCTs. Lesion size on preoperative imaging is likely to influence the weight and volume of tissue surgically excised. The mean radiological lesion size in the study by Langhans et al.19 was 9 mm for both 125I seed and HWL, smaller than the mean lesion size of 13 mm in the present study. All lesion types were included in the present study, whereas Langhans and colleagues19 excluded lesions not visible on ultrasound imaging and those removed with an oncoplastic procedure.
Both radiologists and surgeons found 125I seed easier to use than HWL, echoing findings from an initial pilot study11. Improved ease of use for 125I seed localization was noted particularly by surgeons, as shown in other studies18,21. For radiologists, insertion of a seed is similar to placing a marker clip after a breast biopsy, and thus no additional skills are required. Instances of suboptimal positioning of either seeds or wires requiring corrective action during the study were infrequent; however, it is important to note that patients with a malpositioned seed inserted days before surgery require a corrective HWL on the day of surgery. An important disadvantage of HWL is the potential for wire migration and transection. By contrast, as in other studies22, there were no cases of seed migration in the present study. Although procedure times were not measured, other investigators18,21 have noted that surgeons found no difference or that 125I seed was faster. Other advantages of 125I seed localization include significantly lower levels of patient anxiety and pain16, and improved efficiency related to decoupling of radiological and surgical lists with elimination of avoidable delays8,20,23,24.
The results of this study add high-level evidence to other published literature showing that the use of 125I seed localization rather than HWL in BCS of non-palpable breast cancer gives superior surgical outcomes9.
The present study has limitations. Data regarding tumour grade, hormone receptor, extensive intraductal component and lymphovascular invasion were not collected. The randomization makes it unlikely, however, that the groups were unbalanced for these factors. Moreover, a universally applied definition for clear margins was not applied during the study. Before publication of the Society of Surgical Oncology–American Society for Radiation Oncology (SSO–ASTRO) margin guidelines (for invasive disease in March 2014 and for DCIS in August 2016)25,26, no consensus existed. From late 2016 onwards, when the majority of patient recruitment took place, the SSO–ASTRO margin definitions were in use at all sites bar one. The reduced re-excision rate observed in the 125I seed arm is therefore applicable to contemporary surgical practice. Furthermore, as with most surgical intervention trials, it was not possible to blind the treating team to the intervention received by the patient, and this may have biased decisions regarding re-excision at tumour boards. Given the size of the trial and involvement of multiple centres, systematic use of different treatment protocols for 125I seed localization versus HWL was unlikely.
Some practical issues need to be overcome to enable widespread implementation. There is considerable variability in the regulations regarding handling of low-activity 125I seeds between countries and even between states, with some insisting that a medical physicist accompany the seed throughout its journey. In Australia, the Australasian College of Physical Scientists and Engineers in Medicine is currently lobbying radiation councils in each state for the development of uniform guidelines that reflect the low-risk nature of 125I seeds. Moreover, access to a radiation safety officer to assist with seed dispensing and disposal processes is needed. Finally, reduced re-excision rates and improvements in utilization of radiology and theatre lists are likely to lead to improved cost-effectiveness.
Recent publications have highlighted the potential advantages of using other non-wire preoperative lesion localization techniques that avoid ionizing radiation, such as magnetic seeds and radiofrequency devices27. Although the lack of exposure to ionizing radiation and the need for radioactive substance tracking make these options attractive, issues that warrant consideration include the lack of mature, large-scale efficacy data, the large size of the implantable device (for example, 12 mm for the Savi Scout®, Merit Medical, South Jordan, UT, USA) and associated metallic artefacts (such as the Magseed®, Endomagnetics Ltd, St John’s Innovation Park, Cambridge, UK), which may preclude the use of MRI to monitor neoadjuvant treatment27. It is important to note that 125I seed localization utilizes existing technology, as most γ probes already used for sentinel node detection can also detect 125I, whereas alternative non-wire techniques mandate the purchase of proprietary-owned detector technology and consumables.
This RCT found a significant difference in re-excision rates with the use of 125I seeds versus HWL. Importantly, this was demonstrated in a multicentre real-world setting involving a large number of radiologists and surgeons, and a broad group of patients without the exclusions imposed in other studies19,28. The pragmatic nature of these data ensures that 125I seed localization is ready to implement in many breast practices.
Collaborators
Trial coordination and data management: S. Aggarwal, C. Lizama, L. Debry, J. Newton, E. Boland, R. Singer, N. Perera, N. Foster, S. Rule (RPH, SCGH, FSH), N. Johansen, C. May (St John of God Hospital (SJOG)); A. Singh (Monash); S. Black (Robina); M. Kabir (Westmead); J. Scarlet, H. Flay (Waikato).
Radiologists: S. Madhala (SJOG); L. Du, R. Alzuhairy (Robina); M. Nasreddine, S. Grayson (Westmead); M. Robert, D. Dissanayake (Fiona Stanley Hospital (FSH)); D. Balog, J. Dumble (Waikato); S. Bose, M. Bennett, R. Dhillon, G. Lo, G. Porter (SCGH); M. Pahuja (Monash).
Medical imaging technologists: C. Madeley, M. Kessell (RPH); N. Webb (FSH); V. Mallett-Smith (Waikato); L. Rattigan (SCGH).
Surgeons: C. Saunders (RPH, SJOG, FSH), J. French, F. Meybodi, J. Hsu (Westmead); W.-C. Yeow, L. Jackson (SJOG); J. Cid-Fernandez, V. Singh, M. Yew (RPH); I. Campbell, L. Hayes, J. Creighton, A. Stewart (Waikato); R. Kamyab, F. Abdul Aziz, K. Ponniah, A. Yeo, P. Thirunavukkarasu (SCGH); J. Fox, C. Ooi, C. Tsan, F. Loh, J. Morgan, M. Walker, J. Senior (Monash); R. Liang (Robina).
Pathologists/pathology assistants: M.-A. Koh (Robina); H. Mahajan, S. Chou (Westmead); B. Cooke, M. Gera (SJOG); G. Lanham, D. Bromwich (Waikato); G. Sterrett, F. Frost (SCGH); B. Kumar (Monash).
Nurses: T. Liu (Westmead); B. Pisano, G. McCallum (SJOG); L. Banez, K.Libre (Waikato); F. Morcombe, C. Fletcher (SCGH); T. Pitts (Monash); preadmissions: H. Taylforth (SJOG).
Practice manager: S. Del Dosso; Co-ordinator: G. Meloncelli (Sprague, Kam, Glancy and Partners, SJOG).
Medical physicists and nuclear medicine technologists: J. Burrage, D. Hudson, A. Reed (RPH, FSH); K. Mugabe (Waikato), B. Allen (Waikato); J. Bradley (Monash); D. Carrick (Robina); D. Skerrett (Westmead); N. Reynders (SJOG); M. McGibbons, T. Rourke, O. Luddington (SCGH).
In-kind support: Department of Radiology, Royal Perth Hospital; Medical Technology and Physics, Sir Charles Gairdner Hospital; Westmead Breast Cancer Institute; Department of Nuclear Medicine, Westmead Hospital.
Acknowledgements
This study was funded by grants from the following: State Health Research Advisory Council, Royal Perth Hospital Medical Research Foundation, Cancer Council of Western Australia, St John of God Foundation, Ladybird Foundation (Western Australia); Southern Trust, Grassroots Trust, Lion Foundation, Waikato Breast Cancer Research Trust, Jumble Around (New Zealand); Clinical Excellence Unit, Queensland Health (Robina and Gold Coast Hospital and Health Service).
125I seed kits were supplied at cost price by Isoaid LLC and AlphaXRT Pty Ltd.
M.L.M. was supported by a Western Australian Health Translation Network Early Career Fellowship and the Australian Government’s Medical Research Future Fund as part of the Rapid Applied Research Translation programme, and a National Breast Cancer Foundation Investigator Initiated Research Scheme grant (IIRS-20-011).
Disclosure. Iodine 125 seed kits used in this trial were manufactured by Isoaid LLC, 7824 Clark Moody Blvd, Port Richey, FL 34668 USA. D.B.T. provided advice to Isoaid in their application to the Australian Therapeutic Goods Administration, for the use of iodine seeds for breast lesion localisation. D.B.T. does not have any financial relationships with either Isoaid or AlphaXRT Pty Ltd, the company that imports iodine seed kits for sale in Australia. The authors declare no conflict of interest.
References
- 1. Cady B, Stone MD, Schuler JG, Thakur R, Wanner MA, Lavin PT. The new era in breast cancer: invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg 1996;131:301–308 [DOI] [PubMed] [Google Scholar]
- 2. Koo JH, Kim E-K, Moon HJ, Yoon JH, Park VY, Kim MJ. Comparison of breast tissue markers for tumor localization in breast cancer patients undergoing neoadjuvant chemotherapy. Ultrasonography 2019;38:336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pleijhuis RG, Graafland M, de Vries J, Bart J, de Jong JS, van Dam GM. Obtaining adequate surgical margins in breast-conserving therapy for patients with early-stage breast cancer: current modalities and future directions. Ann Surg Oncol 2009;16:2717–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chadwick D, Shorthouse A. Wire-directed localization biopsy of the breast: an audit of results and analysis of factors influencing therapeutic value in the treatment of breast cancer. Eur J Surg Oncol 1997;23:128–133 [DOI] [PubMed] [Google Scholar]
- 5. Ballal H, Taylor DB, Bourke AG, Latham B, Saunders CM. Predictors of re-excision in wire-guided wide local excision for early breast cancer: a Western Australian multi-centre experience. ANZ J Surg 2015;85:540–545 [DOI] [PubMed] [Google Scholar]
- 6. Christie D, Bonar F, Hammond B, Boyages J. Thoracotomy as a complication of hookwire localization of a breast lump. Breast 1996;5:331 [Google Scholar]
- 7. Davis PS, Wechsler RJ, Feig SA, March DE. Migration of breast biopsy localization wire. AJR Am J Roentgenol 1988;150:787–788 [DOI] [PubMed] [Google Scholar]
- 8. Sharek D, Zuley ML, Zhang JY, Soran A, Ahrendt GM, Ganott MA. Radioactive seed localization versus wire localization for lumpectomies: a comparison of outcomes. AJR Am J Roentgenol 2015;204:872–877 [DOI] [PubMed] [Google Scholar]
- 9. Wang GL, Tsikouras P, Zuo HQ, Huang MQ, Peng L, Bothou A et al. Radioactive seed localization and wire guided localization in breast cancer: a systematic review and meta-analysis. J BUON 2019;24:48–60 [PubMed] [Google Scholar]
- 10.Commonwealth of Australia. National Statement on Ethical Conduct in Human Research 2007. Canberra: The National Health and Medical Research Council, the Australian Research Council and Universities Australia, 2018
- 11. Taylor D, Bourke A, Westcott E, Burrage J, Latham B, Riley P et al. Radioguided occult lesion localisation using iodine-125 seeds (‘ROLLIS’) for removal of impalpable breast lesions: first Australian experience. J Med Imaging Radiat Oncol 2015;59:411–420 [DOI] [PubMed] [Google Scholar]
- 12. Bourke AG, Taylor DB, Westcott E, Hobbs M, Saunders C. Iodine-125 seeds to guide removal of impalpable breast lesions: radio-guided occult lesion localization—a pilot study. ANZ J Surg. 2017;87:E178–E182 [DOI] [PubMed] [Google Scholar]
- 13. Reed AJ, Kim JH, Burrage JW. Development and application of a simple method for calculating breast dose from radio-guided occult lesion localisation using iodine-125 seeds (ROLLIS). Phys Med Biol 2019;64:075020. [DOI] [PubMed] [Google Scholar]
- 14. Al-Hilli Z, Glazebrook KN, McLaughlin SA, Chan DM, Robinson KT, Giesbrandt JG et al. Utilization of multiple I-125 radioactive seeds in the same breast is safe and feasible: a multi-institutional experience. Ann Surg Oncol 2015;22:3350–3355 [DOI] [PubMed] [Google Scholar]
- 15. Dessauvagie B, Frost F, Sterrett G, Hardie M, Parry J, Latham B et al. Handling of radioactive seed localisation breast specimens in the histopathology laboratory: the Western Australian experience. Pathology 2015;47:21–26 [DOI] [PubMed] [Google Scholar]
- 16. Ong JS, Teh J,, Saunders C, Bourke AG, Lizama C, Newton J et al. Patient satisfaction with Radioguided Occult Lesion Localisation using Iodine-125 Seeds (‘ROLLIS’) versus conventional hookwire localisation. Eur J Surg Oncol 2017;43:2261–2269 [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y, Seely J, Cordeiro E, Hefler J, Thavorn K, Mahajan M et al. Radioactive seed localization versus wire-guided localization for nonpalpable breast cancer: a cost and operating room efficiency analysis. Ann Surg Oncol 2017;24:3567–3573 [DOI] [PubMed] [Google Scholar]
- 18. Lovrics PJ, Goldsmith CH, Hodgson N, McCready D, Gohla G, Boylan C et al. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol 2011;18:3407–3414 [DOI] [PubMed] [Google Scholar]
- 19. Langhans L, Tvedskov TF, Klausen TL, Jensen MB, Talman ML, Vejborg I et al. Radioactive seed localization or wire-guided localization of nonpalpable invasive and in situ breast cancer: a randomized, multicenter, open-label trial. Ann Surg 2017;266:29–35 [DOI] [PubMed] [Google Scholar]
- 20. Bloomquist EV, Ajkay N, Patil S, Collett AE, Frazier TG, Barrio AV. A randomized prospective comparison of patient‐assessed satisfaction and clinical outcomes with radioactive seed localization versus wire localization. Breast J 2016;22:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gray RJ, Salud C, Nguyen K, Dauway E, Friedland J, Berman C et al. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Ann Surg Oncol 2001;8:711–715 [DOI] [PubMed] [Google Scholar]
- 22. Alderliesten T, Loo CE, Pengel KE, Rutgers EJ, Gilhuijs KG, Vrancken Peeters MJ. Radioactive seed localization of breast lesions: an adequate localization method without seed migration. Breast J 2011;17:594–601 [DOI] [PubMed] [Google Scholar]
- 23. R Liang. New Technology Funding and Evaluation Program Evaluation Report. Queensland: The State of Queensland, 2017 [Google Scholar]
- 24. Pearson R, Milligan R, Cain H. Radioactive iodine-125 seed localisation of breast carcinoma in advance of the day of surgery reduces pre-operative anxiety levels. Eur J Surg Oncol 2017;43:S7 [Google Scholar]
- 25. Morrow M, Van Zee KJ, Solin LJ, Houssami N, Chavez-MacGregor M, Harris JR et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J Clin Oncol 2016;34:4040–4046 Central PMCID: 5477830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J et al. Society of Surgical Oncology–American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014;32:1507–1515 [DOI] [PubMed] [Google Scholar]
- 27. Hayes MK. Update on preoperative breast localization. Radiol Clin North Am 2017;55:591–603 [DOI] [PubMed] [Google Scholar]
- 28. van Riet YE, Jansen FH, van Beek M, van de Velde CJ, Rutten HJ, Nieuwenhuijzen GA. Localization of non-palpable breast cancer using a radiolabelled titanium seed. Br J Surg 2010;97:1240–1245 [DOI] [PubMed] [Google Scholar]