Abstract
Background
The effect of immediate total-body CT (iTBCT) on health economic aspects in patients with severe trauma is an underreported issue. This study determined the cost-effectiveness of iTBCT compared with conventional radiological imaging with selective CT (standard work-up (STWU)) during the initial trauma evaluation.
Methods
In this multicentre RCT, adult patients with a high suspicion of severe injury were randomized in-hospital to iTBCT or STWU. Hospital healthcare costs were determined for the first 6 months after the injury. The probability of iTBCT being cost-effective was calculated for various levels of willingness-to-pay per extra patient alive.
Results
A total of 928 Dutch patients with complete clinical follow-up were included. Mean costs of hospital care were €25 809 (95 per cent bias-corrected and accelerated (bca) c.i. €22 617 to €29 137) for the iTBCT group and €26 155 (€23 050 to €29 344) for the STWU group, a difference per patient in favour of iTBCT of €346 (€4987 to €4328) (P = 0.876). Proportions of patients alive at 6 months were not different. The proportion of patients alive without serious morbidity was 61.6 per cent in the iTBCT group versus 66.7 per cent in the STWU group (difference −5.1 per cent; P = 0.104). The probability of iTBCT being cost-effective in keeping patients alive remained below 0.56 for the whole group, but was higher in patients with multiple trauma (0.8–0.9) and in those with traumatic brain injury (more than 0.9).
Conclusion
Economically, from a hospital healthcare provider perspective, iTBCT should be the diagnostic strategy of first choice in patients with multiple trauma or traumatic brain injury.
The effect of immediate total-body CT (iTBCT) on health economic aspects in patients with severe trauma is an underreported issue. This study (REACT-2) determined the cost-effectiveness of iTBCT compared with conventional radiological imaging with selective CT during the initial trauma evaluation. Economically, from a hospital healthcare provider perspective, iTBCT should be the diagnostic strategy of first choice in patients with multiple trauma or traumatic brain injury.
Total body CT cost effective
Resumen
Antecedentes
El efecto de la tomografía computarizada inmediata de todo el cuerpo (immediate total-body CT, iTBCT) sobre los aspectos económicos de la salud en pacientes con traumatismos graves es un tema con información limitada. Este estudio determinó el coste-efectividad de la exploración iTBCT en comparación con las imágenes radiológicas convencionales y la CT selectiva (evaluación estándar, standard work-up, STWU) durante la evaluación inicial del trauma.
Métodos
En este ensayo clínico aleatorizado y multicéntrico, los pacientes adultos con una alta sospecha de lesiones graves, una vez ingresados en el hospital, se asignaron al azar a una exploración iTBCT o STWU. Los costes de atención médica hospitalaria se determinaron durante los primeros seis meses posteriores al trauma. La probabilidad de que iTBCT fuera coste-efectiva se calculó para varios niveles de disposición a pagar por cada paciente adicional vivo.
Resultados
Se incluyeron un total de 928 pacientes holandeses con seguimiento clínico completo. Los costes medios de la atención hospitalaria fueron 25.809€ (95% bcaCI: 22.617€ a 29.137€) para el grupo iTBCT y 26.155€ (95% bcaCI: 23.050€ a 29.344€) para el grupo STWU, una diferencia de 346€ por paciente en favor de iTBCT (95% bcaCI: 4.987€ a 4.328€; P = 0,876). El porcentaje de pacientes vivos a los seis meses no fue diferente. La diferencia en el porcentaje de pacientes vivos sin morbilidad grave fue del 61,6% en el grupo iTBCT versus 66,7% en el grupo STWU (-5,1%, P = 0,104). La probabilidad de que iTBCT fuese costo-eficiente para mantener a los pacientes en vida se mantuvo por debajo de 0,56 en todo el grupo, sin embargo, fue mayor en pacientes politraumatizados (0,8-0,9) y en pacientes con lesión cerebral traumática (más de 0,9).
Conclusión
Desde la perspectiva económica del proveedor de atención médica hospitalaria, la tomografía computarizada inmediata de todo el cuerpo debería ser la estrategia diagnóstica de primera elección en pacientes con traumatismos múltiples o traumatismos craneoencefálicos.
Introduction
Immediate total-body CT (iTBCT) during initial trauma assessment was recently evaluated clinically against conventional imaging supplemented with selective CT (standard work-up), as its best alternative1. Outcome measures included (in-hospital) mortality, times to end of imaging and diagnosis, radiation exposure, safety, and hospital costs. Although the REACT-2 multicentre RCT showed reduced times to diagnosis and end of imaging in the trauma room, no gain in reducing mortality was observed1. iTBCT increased the observed minimum level of radiation exposure, but, simultaneously, excessive exposure of 25 mSv or more became unlikely, whereas such high levels were still frequently observed for standard work-up. More readmissions during the first 6 months after trauma were observed for the iTBCT group. This evidence is neither very supportive, nor very discouraging to hospital managers and medical professionals in taking investment decisions in favour of iTBCT in the trauma room.
A further relevant, and yet underexposed, issue of iTBCT for injured patients involves the health economic aspects. Alongside the REACT-2 trial, a health economic evaluation was conducted to inform hospital healthcare managers and professionals in the Netherlands about the cost-effectiveness of iTBCT of patients suspected of being severely injured, with the standard work-up as its comparator.
Methods
The design of the REACT-2 multicentre RCT of iTBCT versus standard work-up for patients with potential major trauma (ClinicalTrials.gov: registration number NCT01523626) has been reported previously1,2. The study was approved by the institutional review boards at all participating centres, of which four resided in the Netherlands and one in Switzerland. Injured adult patients with compromised vital parameters and clinically suspected of life-threatening injury or severe injury mechanisms were enrolled. See Appendix S1 for inclusion and exclusion criteria for the study.
Eligible patients were assigned randomly to either iTBCT without previous conventional imaging or to standard work-up in a 1 : 1 ratio, with stratification for centre. With permission of the institutional review board, the injured patient or their legal representative was informed about the REACT-2 trial at the first convenient moment after trauma work-up. Following written informed consent, medical data and patient-reported outcomes were gathered. In the absence of written informed consent despite all efforts, medical data were still gathered (again, with permission) and reported, but these living patients were excluded from the intention-to-treat analyses of patient-reported outcomes.
Imaging strategies
The CT scanner was located in the trauma room or an adjacent room. The protocol for the iTBCT group consisted of a two-step acquisition (from vertex to pubic symphysis) without gantry angulations, starting with head and neck non-enhanced CT (NECT) with arms alongside the body. The second scan covered chest, abdomen and pelvis. The preferred technique for the second scan was with a split-bolus intravenous contrast of the body directly after raising the arms alongside the head, if not precluded by injury. The radiologist decided on the use of contrast and, if so, in which phase it was applied.
In the standard work-up group, chest and pelvic X-rays and focused assessment with sonography in trauma (FAST) ultrasound imaging were performed during the Advanced Trauma Life Support® (ATLS®; American College of Surgeons, Chicago, IL, USA) primary survey. After further assessment and resuscitation during the secondary survey, selective CT could be done of individual body regions with (segmented) acquisition of the respective body segments (possibly turning cumulatively into a whole body scan as well). Worldwide, the standard radiological trauma work-up is performed according to ATLS® guidelines3.
Type of health economic evaluation, outcomes, perspectives and time horizon
The economic evaluation of iTBCT of potentially severely injured patients was performed as a cost-effectiveness analysis, with the costs per patient alive (with or without serious morbidity) and the costs per patient alive without serious morbidity at the end of a 6-month follow-up as distinct outcome measures. All Dutch patients with a known health status at the end of follow-up were included. Patients were classified into one of six stages, ordered by increasing severity: ‘recovered’, ‘still recovering without remaining handicap’, ‘still recovering with remaining handicap’, ‘handicapped, stable’, ‘handicapped, progressive’, and ‘deceased’. Serious morbidity (or worse) was defined as ‘still recovering with remaining handicap’, or any stage that was more severe. The cost-effectiveness analysis was performed from a hospital healthcare perspective to assist hospital managers in deciding how to provide in-hospital trauma care efficiently.
Conform study protocol2, the time horizon for all analyses was restricted to 6 months after trauma. With a time horizon of 6 months, no discounting of costs and effects was done to account for time preferences.
Cost components, resources and unit costing
Hospital costs included the costs of initial trauma care, ICU stay and general ward stay during the index admission, including all diagnostic (such as imaging, function tests, laboratory tests) and therapeutic (such as intubation, surgery, radiographic intervention, rehabilitation) procedures. This health economic evaluation also covered inpatient and outpatient hospital consultations, repeat hospital admissions, and diagnostic and therapeutic procedures during 6 months of follow-up. Costs of stay in a nursing home or rehabilitation centre (other than rehabilitation in the index hospital) were not included.
Data on healthcare volume in the Dutch index hospitals (during both initial and repeat hospital stays) were gathered uniformly from the hospital information systems with the help of local back-office managers. If no information could be obtained from this database, the patient and/or their general practitioner were contacted by telephone by one of the authors or a research nurse. If a patient was transferred to another hospital after initial admission, data from this hospital admission (duration of inpatient stay, therapeutic interventions, imaging procedures) and on subsequent outpatient visits were also included in the analysis.
Unit costs of different costs components were taken from the Dutch costing guideline for healthcare research4. However, as trauma care is centralized regionally in highly specialized centres and the Dutch hospitals participating in this trial were all affiliated academically, unit costing levels for care in university hospitals were selected where appropriate. The unit costs for major healthcare components were: €627 for a hospital inpatient day on the general ward; €2380 for a day in the ICU; and €141 for an inpatient or outpatient hospital consultation. For a day in the medium-care facility, a unit cost of €1254 was used (doubling the unit costs of the general ward and about half the costs of a day in the ICU). All unit costs for diagnostic and therapeutic procedures were determined in one of the participating academic centres, and ranged from less than €1 for a single blood test to several tens of thousands of euros for complex surgery; the average costs per procedure, including back-office costs, were slightly higher than €25 in this group of patients with multiple injury.
Unit costs were expressed in euros for the base year 2013 during the study period; unit costs from other calendar years were price-indexed using national general consumer price indices as published by Statistics Netherlands5.
Analysis sets, demographics and economic analysis
Originally, the trial was intended to run as a full international trial including trauma centres from the Netherlands, Switzerland and the USA. Unfortunately, although trauma surgeons in a large US trauma centre were able and willing to participate, the associated radiologists decided not to contribute for financial reasons. Late replacement by a centre in the UK became unworkable, because of the lengthy institutional review board procedure for this particular patient group. In addition, as costing data were available only partially for the Swiss institution, the economic analysis was restricted to the patient data set (89.3 per cent of all patients) relevant for decision-making in the Netherlands.
Normally and non-normally distributed continuous data are reported with mean(s.d.) and median (i.q.r.) values respectively. Differences in case mix between study arms after exclusion of patients from the Swiss institution and Dutch patients with unknown health status at the end of follow-up were assessed with the independent-samples t test or Mann–Whitney U test for continuous data, and with χ2 and Fisher’s exact tests for categorical variables as appropriate, to detect possible attrition bias.
Differences in costs and health outcomes between iTBCT and standard work-up of injured patients were assessed by calculating the 95 per cent c.i. of the mean differences after correction for bias, and using accelerated non-parametric bootstrapping, drawing 5000 samples of the same size as the original sample separately for each subgroup (see below) and with replacement6. Incremental cost-effectiveness ratios (ICERs) were calculated, expressing the extra costs per extra patient alive and per extra patient alive and without serious morbidity. Cost-effectiveness planes of differences in costs by differences in health outcomes were drawn, again after non-parametric bootstrapping. The corresponding cost-effectiveness acceptability curves were derived to show the probability of iTBCT being cost-effective for a range of values of the societal willingness to pay for health improvement.
A point-estimated scenario analysis was performed with a more stringent definition of ‘being alive at 6 months without serious morbidity’ by including only patients who had recovered fully. Another point-estimated scenario analysis was performed to account for potentially missing data in 7.1 and 8 per cent of patients for whom non-observed volumes and costs of diagnostic and therapeutic procedures, respectively, in outpatient hospital consultations were set to zero in the main analysis. In the alternative scenario, non-observed volumes and costs for patients were set to the means per treatment group, based on available data.
Preplanned subgroup analyses were performed for patients with multiple injury, defined as having an Injury Severity Score (ISS) of at least 16, and for those with severe traumatic brain injury (TBI), defined as having a Glasgow Coma Scale (GCS) score no greater than 8 on admission and an Abbreviated Injury Scale head score of 3 or above. The above-mentioned bootstrapping procedures were stratified for multiple injury and severe TBI status to maintain consistency between the main analyses and preplanned subgroup analyses.
All analyses were performed on an intention-to-treat basis. Microsoft® Access® 2010 (Microsoft, Redmond, WA, USA) and SPSS® version 24 (IBM, Armonk, NY, USA) were the software platforms used. P < 0.050 was considered statistically significant.
Results
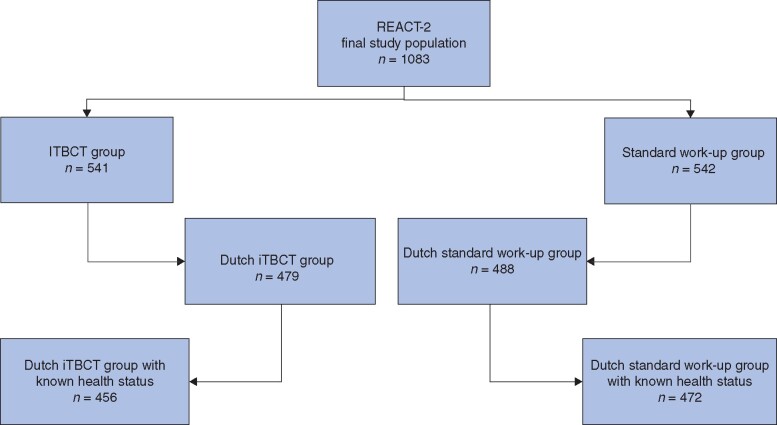
Patient enrolment began on 22 April 2011, and ended on 1 January 2014. A total of 1083 injured patients were included in the clinical analysis set1, and 928 patients were included in the cost-effectiveness analyses (Fig. 1).
Fig. 1.
Selected patients from the REACT-2 multicentre RCT
Of the 541 patients in the immediate total-body CT (iTBCT) group, 62 Swiss patients were excluded and a further 23 Dutch patients had no known health status at 6 months, leaving 456 patients available for cost-effectiveness analysis. Of the 542 patients in the standard work-up group, 54 Swiss patients were excluded and the health status of 16 Dutch patients was unknown, leaving 472 patients available for cost-effectiveness analysis.
Table 1 shows baseline demographics and clinical characteristics of the 928 patients in the cost-effectiveness analysis set. Median age was 43 (i.q.r. 26–59) years, 76.4 per cent of the patients were men, 98.0 per cent presented with blunt trauma, and 66.3 per cent had multiple injury. The median ISS was 21 (i.q.r. 10–30). Randomization groups were comparable for all characteristics.
Table 1.
Baseline characteristics of patients with known health status at end of follow-up
| iTBCT (n = 456) | Standard work-up (n = 472) | P ¶ | |
|---|---|---|---|
| Age (years)* | 42 (27–59) | 44 (25–59) | 0.936# |
| Male sex | 348 (76.3) | 361 (76.5) | 0.952 |
| Blunt trauma | 445 (97.6) | 464 (98.3) | 0.656** |
| Mechanism of blunt trauma | n=445 | n=464 | 0.453 |
| Fall from height | 134 (30.1) | 149 (32.1) | |
| MVC, patient as occupant | 187 (42.0) | 176 (37.9) | |
| MVC, patient as cyclist | 46 (10.3) | 52 (11.2) | |
| MVC, patient as pedestrian | 23 (5.2) | 35 (7.5) | |
| Other | 55 (12.4) | 52 (11.2) | |
| AIS score ≥3 | |||
| Head | 224 (49.1) | 203 (43.0) | 0.062 |
| Chest | 198 (43.4) | 182 (38.6) | 0.132 |
| Abdomen | 44 (9.6) | 63 (13.3) | 0.078 |
| Extremities | 125 (27.4) | 139 (29.4) | 0.492 |
| ISS* | 22 (10–33) | 21 (9–30) | 0.276# |
| Multiple trauma† | 315 (69.1) | 300 (63.6) | 0.075# |
| TBI‡ | 165 (36.2) | 143 (30.3) | 0.057# |
| TRISS (survival probability)*§ | 0.92 (0.61–0.98) | 0.93 (0.68–0.98) | 0.403# |
Values in parentheses are percentages unless indicated otherwise; *values are median (i.q.r.). †Defined as an Injury Severity Score (ISS) of 16 or above. ‡Defined as a Glasgow Coma Scale score below 9 at presentation and an Abbreviated Injury Scale (AIS) score for the head of 3 or more. §There were 279 patients in the immediate total-body CT (iTBCT) group and 273 in the standard work-up group. MVC, motor vehicle collision; TBI, traumatic brain injury; TRISS, Trauma and Injury Severity Score. ¶χ2 test, except #Mann–Whitney U test and **Fisher’s exact test.
Differences in volumes and costs
Table 2 shows differences in costs of the cost-effectiveness analysis set of 928 patients. The 456 patients in the iTBCT group spent 11.4 (95 per cent bias-corrected and accelerated (bca) c.i. 9.9 to 13.1) days on the general ward, 3.6 (3.0 to 4.3) days in the ICU, and 0.8 (0.5 to 1.0) days in the medium care unit (MCU), costing €7171 (95 per cent bca c.i. €6216 to €8241), €8560 (€7088 to €10 155) and €941 (€652 to €1273) respectively. On average, a patient spent 15.8 (13.9 to 17.8) days in hospital, at a cost of €16 671 (€14 553 to €18 929).
Table 2.
Costs
| Mean costs (€) |
Differences in costs (€) | P § | ||
|---|---|---|---|---|
| iTBCT (n = 456) | Standard work-up (n = 472) | |||
| Hospital admission days (all patients) | 16 671 (14 553, 18 929) | 16 860 (14 559, 19 228) | −189 (−3519, 3124) | 0.914 |
| Diagnostic and therapeutic procedures (all patients) | 8790 (7333, 10 406) | 8909 (7686, 10 260) | −119 (−2103, 1861) | 0.907 |
| Specialist consultation (all patients) | 1168 (1073, 1269) | 1144 (1059, 1233) | 25 (−109, 160) | 0.717 |
| Total hospital costs | ||||
| All patients | 25 809 (22 617, 29 137) | 26 155 (23 050, 29 344) | −346 (−4987, 4328) | 0.876 |
| Patients with multiple injury† | 32 093 (27 881, 36 919) | 35 063 (30 547, 39 999) | −2970 (−9839, 3756) | 0.391 |
| Patients with TBI‡ | 33 393 (28 370, 38 766) | 36 352 (30 344, 42 719) | −2959 (−11 201, 4990) | 0.468 |
Values in parentheses are 95 per cent bias-corrected and accelerated confidence intervals. †Defined as an Injury Severity Score of 16 or above. ‡Defined as a Glasgow Coma Scale score below 9 at presentation and an Abbreviated Severity Scale score for the head of 3 or more. iTBCT, immediate total-body CT; TBI, traumatic brain injury. §P values calculated with 95 per cent bias-corrected and accelerated confidence intervals.
In contrast, the 472 patients in the standard work-up group spent 9.7 (95 per cent bca c.i. 8.5 to 10.9) days on the general ward, 4.2 (3.5 to 5.1) days in the ICU, and 0.6 (0.4 to 0.8) days in the MCU, costing €6081 (95 per cent bca c.i. €5348 to €6812), €10 029 (€8221 to €12 061), and €749 (€499 to €1057) respectively. On average, a patient spent 14.5 (12.8 to 16.2) days in the hospital at a cost of €16 860 (€14 559 to €19 228) (Table 2).
iTBCT was associated with about half a day less in the ICU than standard work-up (difference −0.6, 95 per cent bca c.i. −1.8 to 0.5) days, and nearly 2 days more on the general ward (difference 1.7, −0.2 to 3.8) days. The resulting savings, −€189 (95 per cent bca c.i. −€3519 to €3124), were not significant (P = 0.914) (Table 2).
Mean numbers of diagnostic and therapeutic procedures performed were 349.5 (95 per cent bca c.i. 292.4 to 420.2) for 418 patients in the iTBCT group, and 329.5 (282 to 382) for 436 patients in the standard work-up group. Corresponding costs were €8790 (95 per cent bca c.i. €7333 to €10 406) versus €8909 (€7686 to €10 260) respectively. The difference of −€119 (−€2103 to €1861) was not significant (P = 0.907) (Table 2).
On average, 422 patients who had iTBCT received 8.3 (95 per cent bca c.i. 7.6 to 9.0) specialist consultations at a mean cost of €1168 (95 per cent bca c.i. €1073 to €1269). Some 440 patients in the standard work-up group received 8.1 (7.5 to 8.8) specialist consultations at a mean cost of €1144 (€1059 to €1233). The difference between the groups, €25 (−€109 to €160), was not significant (P = 0.717) (Table 2).
On average, total hospital costs during the 6 months after injury were €25 809 (95 per cent bca c.i. €22 617 to €29 137) for the 456 patients in the iTBCT group and €26 155 (€23 050 to €29 344) for the 472 patients having standard work-up. The difference in favour of iTBCT, a saving of €346 (−€4987 to €4328), was not significant (P = 0.876) (Table 2).
Differences in health
Table 3 shows differences in health for the 928 patients in the cost-effectiveness analysis set. At 6 months of follow-up, 764 patients (82.3 per cent) had survived, 82.0 per cent (374 of 456) in the iTBCT group and 82.6 per cent (390 of 472) in the standard work-up group. The difference of 0.6 per cent surviving patients in favour of standard work-up was not significant (χ2 = 0.06, P = 0.808).
Table 3.
Six-month survival and morbidity
| iTBCT | Standard work-up | Difference (%) | P ‡ | |
|---|---|---|---|---|
| 6-month survival | ||||
| All patients | 374 of 456 (82.0) | 390 of 472 (82.6) | −0.6 | 0.808 |
| Patients with multiple trauma | 238 of 315 (75.6) | 221 of 300 (73.7) | 1.9 | 0.590 |
| Patients with TBI | 101 of 165 (61.2) | 78 of 143 (54.5) | 6.7 | 0.237 |
| Alive without serious morbidity | ||||
| All patients | 281 of 456 (61.6) | 315 of 472 (66.7) | −5.1 | 0.104 |
| Patients with multiple trauma* | 156 of 315 (49.5) | 158 of 300 (52.7) | −3.1 | 0.436 |
| Patients with TBI† | 58 of 165 (35.2) | 58 of 143 (40.6) | −5.4 | 0.329 |
Values in parentheses are percentages. *Defined as an Injury Severity Score of 16 or above. †Defined as a Glasgow Coma Scale score below 9 at presentation and an Abbreviated Severity Scale score for the head of 3 or more. iTBCT, immediate total-body CT; TBI, traumatic brain injury. ‡χ2 test.
The proportion of patients alive and without serious morbidity was 61.6 per cent (281 of 456) in the iTBCT group and 66.7 per cent (315 of 472) in the standard work-up group. The difference of 5.1 per cent in favour of the standard work-up group was not significant (χ2 = 2.64, P = 0.104). If the more stringent definition was used, and ‘still recovering without remaining handicap’ at the end of the 6 months was also considered as serious morbidity, the proportions dropped considerably to 36.6 per cent (167 of 456) for iTBCT and 39.2 per cent (185 of 472) for standard work-up; the difference of 2.6 per cent was not significant (χ2 = 0.65, P = 0.419).
Incremental cost-effectiveness
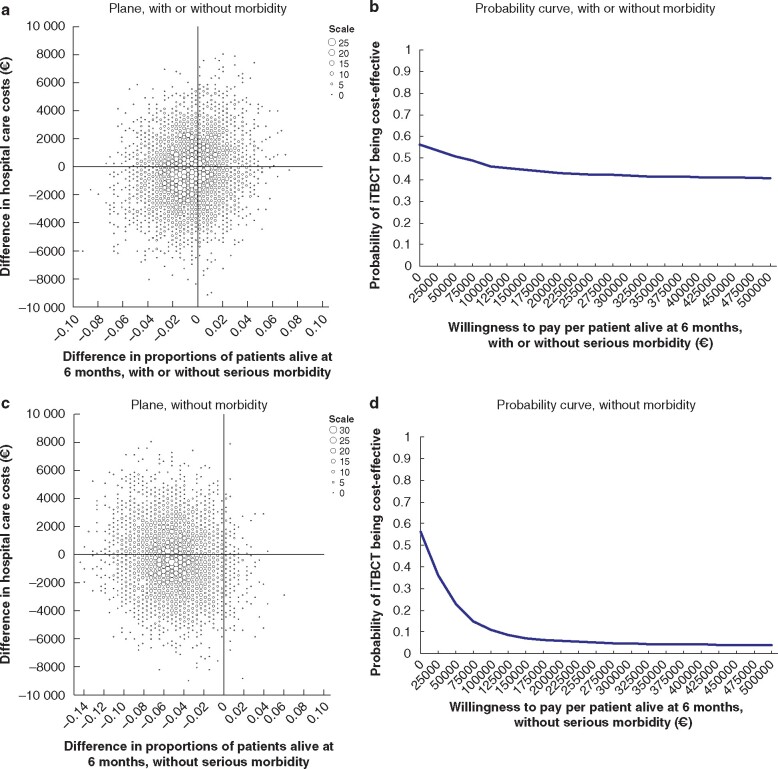
Based on the point estimates and considered from a hospital healthcare perspective, iTBCT saved €56 761 per life lost and €6765 per lost patient alive without serious morbidity. The cost-effectiveness planes and corresponding cost-effectiveness acceptability curves are shown in Fig. 2a–d.
Fig. 2.
Cost-effectiveness of immediate total-body CT versus standard work-up for all patients
a,c Cost-effectiveness plane based on 5000 bootstrap resamples showing differences in hospital healthcare costs and proportions of patients alive at 6 months with or without serious morbidity (a) and without serious morbidity (c) between immediate total-body CT (iTBCT) and standard workup. Larger dots represent higher bootstrap counts (scale legend). iTBCT may be more costly and more effective (upper right quadrant), more costly and less effective (upper left), cheaper and less effective (lower left), or cheaper and more effective (lower right). b,d Cost-effectiveness acceptability curve showing the probability of iTBCT being cost-effective for different values of willingness to pay up to €500 000 per patient alive at 6 months with or without serious morbidity (b) and without serious morbidity (d).
iTBCT was cost saving in 56.2 per cent of cases and kept patients alive more effectively for at least 6 months in 39.9 per cent (irrespective of serious morbidity) or 3.5 per cent (without serious morbidity) of all bootstraps. The probability of iTBCT being cost-effective ranged from 56.2 to 40.9 per cent, depending on the societal willingness to pay up to €500 000 per patient alive for at least 6 months after injury. The probability of iTBCT being cost-effective ranged from 56.2 to 3.9 per cent, depending on the societal willingness to pay up to €500 000 per patient alive at 6 months after injury without serious morbidity.
Scenario analyses
Under the more stringent definition, iTBCT saved €13 452 per lost patient who had fully recovered at 6 months after injury. Assuming non-zero, mean values per treatment group for non-observed volumes and costs of diagnostic and therapeutic procedures as well as outpatient hospital consultations, the base case results decreased by 17.9 per cent to €46 590 per life lost, €5553 per lost patient alive without serious morbidity, and €11 042 per lost patient who was fully recovered at 6 months after injury.
Subgroup of patients multiple injury
On average, total hospital costs in the first half-year after trauma were €32 093 (95 per cent bca c.i. €27 881 to €36 919) for 315 patients with multiple injury who had iTBCT and €35 063 (€30 547 to €39 999) for 300 patients with multiple injury who underwent standard work-up. The difference in favour of iTBCT, a saving of €2970 (−€9839 to €3756), was not significant (P = 0.391) (Table 2).
At 6 months of follow-up, 459 (74.6 per cent) of the 615 patients with multiple injury had survived, 238 (75.6 per cent) in the iTBCT group and 221 (73.7 per cent) in the standard work-up group. The difference of 1.9 per cent in favour of iTBCT was not significant (χ2 = 0.29, P = 0.590).
The proportion of patients with multiple injury alive at 6 months without serious morbidity was 49.5 per cent (156 of 315) in the iTBCT group and 52.7 per cent (158 of 300) in the standard work-up group. The difference of 3.1 per cent in favour of the standard work-up group was not significant (χ2 = 0.61; P = 0.436).
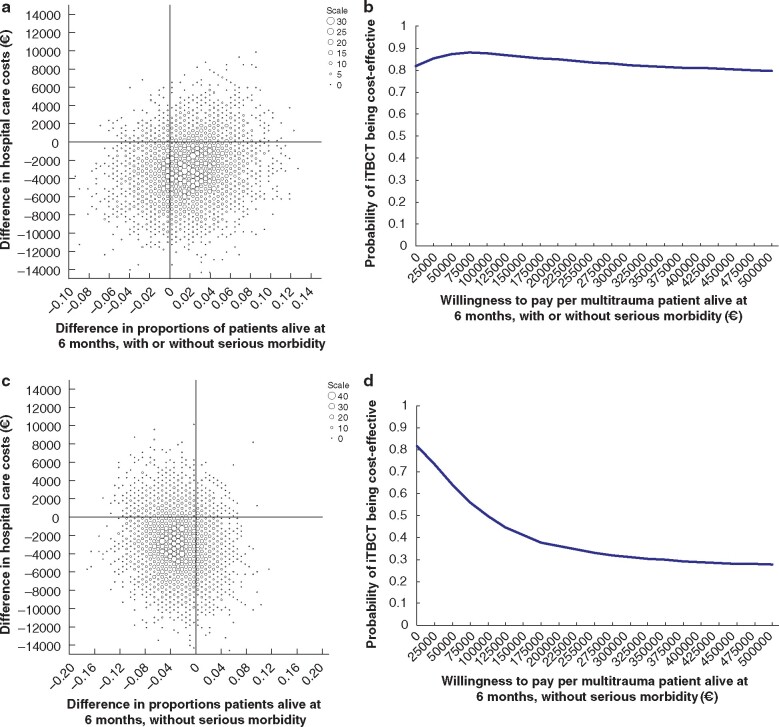
Based on the point estimates and considered from a hospital healthcare perspective, iTBCT saved €157 235 per multiple trauma life gained and €94 500 per lost patient with multiple injury alive without serious morbidity. The cost-effectiveness planes and corresponding cost-effectiveness acceptability curves are shown in Fig. 3a–d.
Fig. 3.
Cost-effectiveness of immediate total-body CT versus standard work-up in patients with multiple injury
a,c Cost-effectiveness plane based on 5000 bootstrap resamples showing differences in hospital healthcare costs and proportions of patients alive at 6 months with or without serious morbidity (a) and without serious morbidity (c) between immediate total-body CT (iTBCT) and standard workup. Larger dots represent higher bootstrap counts (scale legend). iTBCT may be more costly and more effective (upper right quadrant), more costly and less effective (upper left), cheaper and less effective (lower left), or cheaper and more effective (lower right). b,d Cost-effectiveness acceptability curve showing the probability of iTBCT being cost-effective for different values of willingness to pay up to €500 000 per patient alive at 6 months with or without serious morbidity (b) and without serious morbidity (d).
Among patients with multiple injury, iTBCT was cost-saving in 81.7 per cent and kept patients alive more effectively for at least 6 months in 72.7 per cent (irrespective of serious morbidity) or 22.0 per cent (without serious morbidity) of all bootstraps. The probability of iTBCT being cost-effective ranged from 88.0 to 79.6 per cent, depending on the societal willingness to pay up to €500 000 per patient with multiple trauma alive for at least 6 months after injury. The probability of iTBCT being cost-effective ranged from 81.7 to 27.7 per cent, depending on the societal willingness to pay up to €500 000 per patient with multiple trauma alive at 6 months after injury without serious morbidity.
In contrast, for 313 patients with a single injury, and based on point estimates, iTBCT (141 patients) was dominated by the standard work-up (172 patients), with non-significantly increased hospital care costs of €1153 (95 per cent bca c.i. −€3813 to €5588; P = 0.637), and non-significantly decreased numbers of patients alive by −1.8 per cent (χ2 = 1.01, P = 0.315) or numbers of patients alive without serious morbidity by −2.6 per cent (χ2 = 0.60, P = 0.439).
Subgroup of patients with traumatic brain injury
On average, total hospital costs in the first half-year after injury were €33 393 (95 per cent bca c.i. €28 370 to €38 766) for 165 patients with TBI who had iTBCT and €36 352 (€30 344 to €42 719) for 143 patients with TBI who underwent standard work-up. The difference in favour of iTBCT, a saving of €2959 (−€11 201 to €4990), was not significant (P = 0.468) (Table 2).
At 6 months of follow-up, 179 (58.1 per cent) of the 308 patients with TBI had survived, 101 (61.2 per cent) in the iTBCT group and 78 (54.5 per cent) in the standard work-up group. The difference of 6.7 per cent in favour of iTBCT was not significant (χ2 = 1.40, P = 0.237).
The proportion of patients with TBI alive at 6 months without serious morbidity was 35.2 per cent (58 of 165) in the iTBCT group and 40.6 per cent (58 of 143) in the standard work-up group. The difference of 5.4 per cent in favour of the standard work-up group was not significant (χ2 = 0.95, P = 0.329).
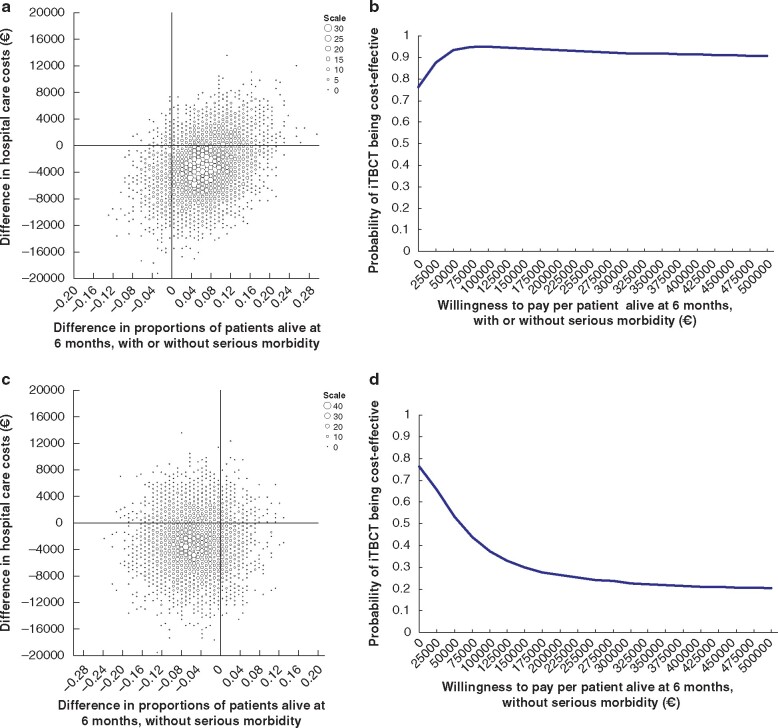
Based on the point estimates and considered from a hospital healthcare perspective, iTBCT saved €44 385 per gained patient with TBI alive and €54 716 per lost patient with TBI alive without serious morbidity. The cost-effectiveness planes and corresponding cost-effectiveness acceptability curves are shown in Fig. 4a–d.
Fig. 4.
Cost-effectiveness of immediate total-body CT versus standard work-up in patients with traumatic brain injury
a,c Cost-effectiveness plane based on 5000 bootstrap resamples showing differences in hospital healthcare costs and proportions of patients alive at 6 months with or without serious morbidity (a) and without serious morbidity (c) between immediate total-body CT (iTBCT) and standard workup. Larger dots represent higher bootstrap counts (scale legend). iTBCT may be more costly and more effective (upper right quadrant), more costly and less effective (upper left), cheaper and less effective (lower left), or cheaper and more effective (lower right). b,d Cost-effectiveness acceptability curve showing the probability of iTBCT being cost-effective for different values of willingness to pay up to €500 000 per patient alive at 6 months with or without serious morbidity (b) and without serious morbidity (d).
The effect of immediate total-body CT (iTBCT) on health economic aspects in patients with severe trauma is an underreported issue. This study (REACT-2) determined the cost-effectiveness of iTBCT compared with conventional radiological imaging with selective CT during the initial trauma evaluation. Economically, from a hospital healthcare provider perspective, iTBCT should be the diagnostic strategy of first choice in patients with multiple trauma or traumatic brain injury.
Among the 308 patients with TBI, iTBCT was cost-saving in 76.3 per cent and kept patients alive more effectively for at least 6 months in 88.0 per cent (irrespective of serious morbidity) or 16.9 per cent (without serious morbidity) of all bootstraps. The probability of iTBCT being cost-effective ranged from 94.9 to 90.8 per cent, depending on societal willingness to pay up to €500 000 per patient with TBI alive for at least 6 months after injury. The probability of iTBCT being cost-effective ranged from 76.3 to 20.4 per cent, depending on societal willingness to pay up to €500 000 per patient with TBI alive at 6 months after injury without serious morbidity.
In contrast, for 620 patients without TBI and based on point estimates, iTBCT (291 patients) in comparison with standard work-up (329 patients) non-significantly decreased hospital care costs by −€241 (95 per cent bca c.i. −€5632 to €5461; P = 0.941), numbers of patients alive by −1.0 per cent (χ2 = 0.301, P = 0.583) or numbers of patients alive without serious morbidity by −1.5 per cent (χ2 = 0.194, P = 0.659).
Discussion
The REACT-2 trial generally demonstrated that iTBCT and standard radiological imaging in injured patients after major trauma have comparable outcomes at 6 months in terms of hospital care costs and proportions of patient alive and patients alive without serious morbidity. However, the cost-effectiveness analysis from the hospital care provider perspective suggested that iTBCT was more efficient than the standard work-up in keeping patients with multiple injury and those with TBI alive for at least 6 months, given the per patient cost savings of almost €3000 and survival rates that were slightly, although not significantly, higher by 1.9 and 6.7 per cent respectively. Hence, from a health economic viewpoint, iTBCT was the strategy of first choice in at least three of every four injured patients.
The role of iTBCT is more debatable when the cost savings are offset against the non-significantly lower rates of patients alive at 6 months without serious morbidity (−3.1 per cent for multiple trauma and 5.4 per cent for TBI subgroups compared with standard work-up). The diagnostic strategy of first choice then becomes dependent on the societal willingness to pay to prevent serious morbidity. Results have been reported for willingness-to-pay levels up to half a million euros; above the €500 000 plateau, the probability of iTBCT being cost-effective tends to freeze. The higher the willingness to pay, the lower the probability of iTBCT being cost-effective.
These results of iTBCT being more efficient in keeping both patients with multiple injury and those with TBI alive, while coming under debate as the preferred strategy to prevent serious morbidity, are paradoxical. Further data analysis revealed that more than 80 per cent of patients with serious morbidity at 6 months will remain handicapped, but are actually still recovering. At 6 months after injury, the worst that could have happened (death) had already happened; progressive handicap was observed infrequently (1 per cent in the TBI subgroup). Therefore, iTBCT could well have its place in the diagnostic work-up of patients with multiple injury and those with TBI, thereby placing most emphasis on the survival rates in combination with the cost savings in these target subpopulations. As these results involve for subgroups of injured patients, this also stresses the need to apply the most adequate set of indication criteria available to preselect patients with multiple trauma and/or TBI. Taking an investment decision on iTBCT near or at the trauma room should be discussed within major level-1 trauma centres in the Netherlands.
The absence of statistically significant differences in health outcomes between iTBCT and standard work-up may have originated from the high proportion of patients (40–50 per cent) in the standard work-up group who received sequential segmental CT scans of all body regions, comprising a TBCT scan in the end. The standard work-up does not lag behind in effectiveness, and continuing the standard work-up cannot be considered unethical based on the present results.
A cost-utility analysis, with the costs per quality-adjusted life-year (QALY) as outcome, was also planned alongside the REACT-2 trial, but analyses could be performed only in a convenience subsample of 615 patients, including all deceased patients and only living patients who reported their quality-of-life status during follow-up. In this convenience sample with low external validity, only marginal, near zero, differences in QALYs (less than 0.007 across all subgroups; data available on request) in favour of iTBCT were observed. The cost-utility analysis was considered uninformative, in addition to the cost-effectiveness analysis reported in this paper.
Van Vugt and colleagues7 reported a reduction in direct medical costs with iTBCT, probably owing to faster work-up times that reduced personnel costs during the trauma room assessment. This analysis7, however, did not relate the costs to effectiveness in terms of survival or morbidity. The cost-utility analysis by Lee et al.8 focused on a simulation for less injured patients (median ISS 5, GCS score 14 or 15) and concluded TBCT to be cost-effective as it reduced the need for clinical observation of patients who had selective CT. The present study focused on cost-effectiveness in terms of mortality and morbidity reduction in more severely injured patients, and cannot therefore be compared with the results reported by Lee and co-workers8.
The time horizon of this cost-effectiveness analysis was 6 months after injury. Most health economic costing guidelines suggest a lifetime horizon as the base-case scenario. However, trauma care for severely injured patients is often the beginning of a time-consuming trajectory towards optimal recovery, with very heterogeneous patterns of follow-up care, especially in elderly patients who often have co-existing morbidity. Moreover, diagnostic strategies preceding trauma care are applied at the very beginning of these trajectories, and the extent to which longer-term healthcare consumption and health outcomes are attributable to the initially chosen diagnostic approach remains to be determined. In addition, in absence of a clear absolute difference in health outcomes, a time horizon of 6 months seems defensible in practice.
Care should be taken when extrapolating these study results to other countries, because of differences in demography, geographical accessibility to trauma centers, and financing of health care9. Hopefully though, the randomized design, stratified by treatment centre, and with highly comparable iTBCT and standard work-up groups in terms of patient characteristics and survival probability based on trauma severity scores, may inspire hospital managers to redesign their local in-hospital diagnostic trauma work-up logistics, if they have not already done so.
From a hospital healthcare provider perspective, economically iTBCT should be the diagnostic strategy of first choice for patients with multiple injury or TBI in trauma centres.
Collaborators
J. S. Luitse and T. Schepers (Trauma Unit, Department of Surgery, Amsterdam UMC, Amsterdam, the Netherlands); L. F. M. Beenen (Department of Radiology, Amsterdam UMC, Amsterdam, the Netherlands), T. N. Tromp and M. Brink (Trauma Unit, Department of Surgery, Radboud UMC, Nijmegen, the Netherlands); M. El Moumni and J. S. Harbers (Trauma Unit, Department of Surgery, University Medical Centre Groningen, Groningen, the Netherlands); P. Patka, D. den Hartog and T. Hagenaars (Trauma Unit, Department of Surgery, Erasmus MC, University Medical Centre Rotterdam, Rotterdam, the Netherlands).
Supplementary material
Supplementary material is available at BJS online.
Supplementary Material
Acknowledgements
The authors thank the following institutions and persons: ZonMw, the Netherlands Organization for Health Research and Development, for providing an unrestricted grant for the REACT-2 trial (ZonMw grant number 171102023); G. P. Clerx, T. N. Tromp, B. Bos, E. Baard, B. Visser, C. Bathelt and S. Purschke, research nurses at the participating sites, for their efforts in including patients and data completion; J. C. J. Noordegraaf, R. Siemons, C. van Ooijen, H. C. R. Nanninga, H. Hollander, B. J. Ponit, P. van Moorsel and L. van Moorsel, for gathering data on healthcare volume; M. J. A. M. Russchen and M. R. Wirtz, research students, for their assistance in data completion.
Disclosure. The authors declare no conflict of interest.
Contributor Information
REACT-2 study group:
J S Luitse, T Schepers, L F M Beenen, T N Tromp, M Brink, M El Moumni, J S Harbers, P Patka, D den Hartog, and T Hagenaars
References
- 1. Sierink JC, Treskes K, Edwards MJ, Beuker BJ, den Hartog D, Hohmann J et al. Immediate total-body CT scanning versus conventional imaging and selective CT scanning in patients with severe trauma (REACT-2): a randomised controlled trial. Lancet 2016;388:673–683 [DOI] [PubMed] [Google Scholar]
- 2. Sierink JC, Saltzherr TP, Beenen LF, Luitse JS, Hollmann MW, Reitsma JB et al. A multicenter, randomized controlled trial of immediate total-body CT scanning in trauma patients (REACT-2). BMC Emerg Med 2012;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Surgeons Committee on Trauma. ATLS, Advanced Trauma Life Support Program for Doctors. Chicago: ACS, 2018.
- 4. Hakkaart-Van Roijen L, Tan SS, Bouwmans CAM (eds). [Guideline for Costing Research. Methods and Standard Unit Costs for Economic Evaluation in Health Care (actualized version edition).] Rotterdam: Erasmus University, 2010. [Google Scholar]
- 5.CBS StatLine. [Consumer Prices; September 2014.] http://Statline.cbs.nl/StatWeb/publications/?PA=71311ned (accessed 11 February 2016).
- 6. Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219–3236 [DOI] [PubMed] [Google Scholar]
- 7. van Vugt R, Kool DR, Brink M, Dekker HM, Deunk J, Edwards MJ. Thoracoabdominal computed tomography in trauma patients: a cost-consequences analysis. Trauma Mon 2014;19:e19219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee WS, Parks NA, Garcia A, Palmer BJ, Liu TH, Victorino GP. Pan computed tomography versus selective computed tomography in stable, young adults after blunt trauma with moderate mechanism: a cost-utility analysis. J Trauma Acute Care Surg 2014;77:527–533 [DOI] [PubMed] [Google Scholar]
- 9. Haslam NR, Bouamra O, Lawrence T, Moran CG, Lockey DJ. Time to definitive care within major trauma networks in England. BJS Open 2020;4:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.