An intervention of non-supervised physical activity did not significantly improve recovery after breast cancer surgery.
Abstract
Background
The effect of preoperative physical activity on recovery and complications after primary breast cancer surgery is unknown. The objective of this trial was to evaluate whether a recommendation of non-supervised physical activity improved recovery after breast cancer surgery.
Methods
This parallel, unblinded, multicentre interventional trial randomized women in whom breast cancer surgery was planned. The intervention consisted of an individual recommendation of added aerobic physical activity (30 min/day), before and 4 weeks after surgery. The control group did not receive any advice regarding physical activity. The primary outcome was patient-reported physical recovery at 4 weeks after surgery. Secondary outcomes included mental recovery, complications, reoperations, and readmissions.
Results
Between November 2016 and December 2018, 400 patients were randomized, 200 to each group. Some 370 participants (180 intervention, 190 control) remained at 4 weeks, and 368 at 90 days. There was no significant difference in favour of the intervention for the primary outcome physical recovery (risk ratio (RR) 1.03, 95 per cent c.i. 0.95 to 1.13). There was also no difference for mental recovery (RR 1.05, 0.93 to 1.17) nor in mean Comprehensive Complication Index score (4.2 (range 0–57.5) versus 4.7 (0–58.3)) between the intervention and control groups.
Conclusion
An intervention with recommended non-supervised physical activity before and after breast cancer surgery did not improve recovery at 4 weeks after surgery. Registration number: NCT02560662 (http://www.clinicaltrials.gov).
Introduction
Breast cancer effects more than 2.1 million women worldwide every year1. The standard treatment is surgical excision followed by adjuvant treatment, including radiation therapy, chemotherapy and endocrine therapy2. Most breast cancers are removed using breast-conserving surgery, and axillary staging is performed using sentinel lymph node biopsy. For some patients the preferred surgical option is still mastectomy with or without reconstruction and axillary lymph node dissection (ALND)2.
Any surgical procedure is followed by a recovery phase, and adjuvant therapy for patients with breast cancer is initiated at the end of the recovery period. Complications after surgery or a prolonged recovery period can result in delaying the start of adjuvant treatment3, and this may affect the patient’s resilience to any adverse effects of such treatment4. Preoperative interventions to enhance recovery after surgery have gained attention; smoking and alcohol intake cessation have reported benefits5,6, and these strategies have been implemented into clinical routine.
Results from observational studies7,8 indicate that physical activity after a breast cancer diagnosis is associated with decreased breast cancer-specific and overall mortality. In patients undergoing adjuvant treatment for breast cancer, interventions with physical exercise have been reported to reduce fatigue9,10 and increase quality of life11–13.
Little is known about the use of physical activity as prehabilitation for improved recovery after breast cancer surgery. In an observational cohort study of patients who underwent breast cancer surgery, it was shown that those who undertook regular physical activity for at least 2–3 h per week had a higher probability of feeling physically recovered 3 weeks after surgery than physically inactive patients14. There are no reports of interventional studies examining the effects of prehabilitation on postoperative outcomes after breast cancer surgery15. An intervention enhancing recovery after breast cancer surgery would not only be of importance for the individual, but might also have an effect on resource consumption in society, taken the large number of patients with breast cancer.
The primary objective of this trial was to evaluate whether an intervention consisting of recommended physical activity before and after surgery improved physical recovery at 4 weeks after breast cancer surgery.
Methods
PhysSURG-B was a randomized, controlled, multicentre open-label trial. Patients were recruited at one university hospital and one county hospital in western Sweden. The trial was registered at ClinicalTrials.gov (NCT02560662) on 25 September 2015. The Regional Ethics Committee in Gothenburg, Sweden, approved the trial (522-15) on 14 September 2015 (trial protocol version 1.1, dated 12 June 2015; updated trial protocol v.1.3 in Appendix S1). Patient recruitment started on 2 November 2016 and ended on 14 December 2018. PhysSURG-B was designed within the Scandinavian Surgical Outcomes Research Group (http://www.ssorg.net).
Participants
Women aged 18 years or older and scheduled for surgery for confirmed or suspected breast cancer (index surgery) were recruited. The exclusion criteria were: inability to understand information provided, inability to perform the intervention, stage IV breast cancer at diagnosis, and receipt of neoadjuvant treatment. Patients were recruited at outpatient clinics at the time of diagnosis and when scheduled for surgery. Written informed consent was mandatory and patients could withdraw their consent at any time.
Randomization and masking
After receiving general information about the trial and providing written consent, participants were randomized through an online system with a 1 : 1 allocation in permutated blocks, to the intervention or control group. Patients were enrolled by their treating surgeon, and randomization was performed by a research nurse. The research nurse collected information in an electronic case report form regarding the surgery and secondary outcomes (duration of hospital stay, postoperative complications, reoperations, and readmissions). Neither research nurses nor participants were blinded because of the nature of the intervention. Patient allocation was, however, not actively communicated to healthcare personnel involved in routine care.
Procedures
The intervention took place before and after surgery. Participants in the intervention group were individually instructed by a physiotherapist to add 30 min of aerobic physical activity daily, before surgery (usually 2 weeks, ±1 week) and for 4 weeks after discharge from hospital. The physical activity was to be of medium intensity, resulting in shortness of breath, but retaining the ability to talk. The patients chose the type of activity, which was performed without supervision. To increase adherence, patients in the intervention group received a diary in which they were instructed to mark each day that they performed the recommended activity. They also received two follow-up telephone calls from the physiotherapist, one during the preoperative and one during the postoperative intervention period. The control group followed routine care and were merely informed about their group allocation after randomization; they did not receive any advice regarding physical activity. All participants, however, received standardized information from a physiotherapist regarding early mobilization and shoulder movement before discharge from hospital after surgery.
Outcomes
The primary outcome was physical recovery at 4 weeks after surgery, measured using self-administered questionnaires. The question, used in several previous studies14,16,17, was: ‘To what extent do you feel physically recovered after surgery?’. Response categories were: not applicable, ‘I don’t feel recovered at all’, 25 per cent recovered, 50 per cent recovered, 75 per cent recovered, and 100 per cent recovered. The primary outcome was dichotomized between 0–50 and 75–100 per cent according to the statistical analysis plan (Appendix S2). The question was expert-validated and face-validated in patients with breast cancer before use.
Secondary outcomes were self-reported mental recovery at 4 weeks after surgery (answering alternatives as described above for the primary outcome; these were validated in a similar manner), duration of hospital stay for index surgery, unplanned reoperations requiring general anaesthesia, unplanned readmissions, and complications (highest grade according to the Clavien–Dindo classification18,19, and Comprehensive Complication Index (CCI®registered and owned by the University of Zurich20), within 90 days after the index surgery. Planned reoperations and readmissions were those owing to a positive resection margin or completion ALND.
Data collection
Patient questionnaires were collected at three time points: before operation, after 4 weeks and at 12 months after surgery. The baseline questionnaire included general background questions on height, weight, lifestyle factors, co-morbidity, and socioeconomic factors used in previous studies of patients with cancer21–23. Physical and mental recovery was assessed using specific questions used in previous studies, including a cohort of patients with breast cancer14,16,17. The Saltin–Grimby Physical Activity Level Scale (SGPALS)24 was used to assess physical activity; this is a highly reliable and validated four-level single question form, associated with cardiovascular risk factors, morbidity, and mortality. The Alcohol Use Disorders Identification Test (AUDIT-C)25 was used to evaluate alcohol risk consumption (AUDIT-C score ≥ 4). The questionnaires can be accessed in Swedish on request.
Electronic case report forms for data such as ASA physical status grade, type of surgery, use of drainage, antibiotic and thromboembolic prophylaxis, duration of hospital stay, complications, reoperations, and readmissions were retrieved from medical records. Tumour characteristics (size, grade, oestrogen receptor status, progesterone receptor status, HER2 gene amplification, and nodal status) were retrieved from the Swedish national breast cancer registry. Baseline questionnaires were administered at inclusion and returned by mail. Before the postoperative questionnaires and return envelopes were sent by mail, all included patients received a letter, followed by a telephone call from a research nurse for renewed consent. Patients in the intervention group received a diary at their visit to the physiotherapist, and were instructed to mark each day of physical activity according to the recommendation with an X, to improve and allow evaluation of adherence to the intervention. Adverse events could be reported in the diary. The diary was sent to the secretariat together with the 4-week questionnaire. Time points for data collection are shown in a SPIRIT diagram (Table S1).
Statistical analysis
A sample size estimation was performed using the first 100 evaluable patients. This preplanned interim analysis was conducted by an independent committee that was unaware of the group allocation, and showed a 9 per cent difference in the primary endpoint between the two groups (88 versus 97 per cent). This was considered to be a true difference that was important to detect (if present), and for a two-sided test with 5 per cent significance, a total of 314 patients (157 evaluable patients per group) would yield a power of 80 per cent. Considering a detected drop-out frequency of 8 per cent, the study aimed to include 400 patients.
A statistical analysis plan was prespecified before accessing the data set (Appendix S2). The participants were analysed quantitatively according to randomization (intention-to-treat analysis). To account for low adherence to the recommended intervention, a post hoc per-protocol analysis was undertaken with inclusion of those who fulfilled the intervention, defined as recording at least five days/week of added physical activity during the intervention period. The two subgroups were identified by whether the patient had marked X for added physical activity in the diary ≥ 10 days before surgery and less than 20 days after surgery (subgroup 1), or ≥ 10 days before surgery and ≥ 20 days after surgery subgroup 2). No correction for multiplicity was performed. A statistical significance level of 5 per cent and 95 per cent confidence intervals were used.
For recovery, the risk ratio (RR) was calculated using Poisson regression with a robust error variance; a RR point estimate in favour of the intervention exceeded 1.00, whereas a value of less than 1.00 was in favour of the control26. All other secondary endpoints were tabulated. No imputation of missing values was done for the outcome variables. When there was at least one missing value for co-variables or factors in a multiple regression model, the default approach was listwise deletion, whereby the entire record was excluded from analysis.
The primary analysis was adjusted for the following variables: type of surgery (breast-conserving surgery versus mastectomy and ALND versus non-ALND), age as a continuous variable, and baseline physical activity level (grouped as inactive, low physical activity versus merged moderate–vigorous physical activity from the SGPALS). Statistical analysis was carried out using SAS® version 9.4 (SAS Institute, Cary, North Carolina, USA) and SPSS® version 24 (IBM, Armonk, New York, USA).
Results
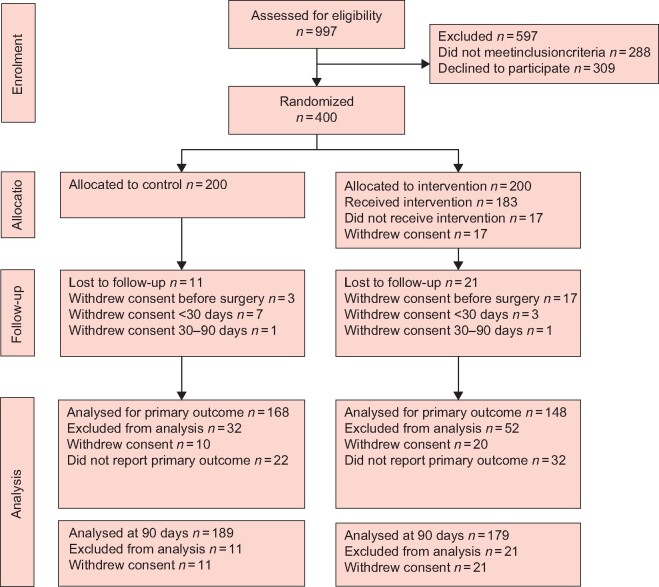
Patients were recruited between 2 November 2016 and 14 December 2018. Of 1254 screened patients, 997 were assessed for eligibility. Of these, 288 met the exclusion criteria and 309 declined to participate. The remaining 400 patients were randomized to intervention (200) and control (200) groups. After randomization, 20 participants (17 intervention, 3 control) withdrew consent during the preoperative intervention period and 10 others (3 intervention, 7 control) withdrew consent during the postoperative phase, and provided no information regarding the patient-reported outcomes. A total of 370 participants (180 intervention, 190 control) remained in the trial at the time of analysis of the primary endpoint; these patients constitute the cohort for the intention-to-treat analysis.
Of these, 318 of 370 participants (85.9 per cent) returned both the baseline and 4-week questionnaires; 149 of 180 (82.8 per cent) in the intervention group and 169 of 190 (88.9 per cent) in the control group (Fig. 1). The response rate was between 83 and 90 per cent for the individual questionnaires. Two patients, one in each group, withdrew consent between 30 and 90 days after surgery, leaving 368 participants (179 intervention, 189 control) for analysis.
Fig. 1.
CONSORT diagram for PhysSURG-B trial
Baseline characteristics were distributed evenly between the groups (Table 1). The median age was 62 (range 30–89) years and 218 participants (64.1 per cent) had one or more co-morbidities. The highest educational level (university or equivalent) was reported by 55.8 per cent. Assessment of preoperative physical activity level using the SGPALS showed that 71 patients (21.0 per cent) reported a moderate to vigorous level of activity. Risk consumption of alcohol was seen in 32.7 per cent of patients in the intervention group and 30.2 per cent in the control group (Table 1).
Table 1.
Baseline characteristics of study participants
| Control group (n = 200) | Intervention group (n = 200) | |
|---|---|---|
| Age (years) * | 63 (54–71; 38–89) | 61 (52–68; 30–84) |
| BMI (kg/m2) * | 25 (23–28; 19–39) | 25 (22–29; 18–48) |
| ASA physical status grade | ||
| I | 75 of 194 (38.7) | 92 of 179 (51.4) |
| II | 108 of 194 (55.7) | 83 of 179 (46.4) |
| III | 10 of 194 (5.2) | 4 of 179 (2.2) |
| IV | 1 of 194 (0.5) | 0 of 179 (0) |
| Missing | 6 | 21 |
| Co-morbidities | ||
| None | 63 of 175 (36.0) | 59 of 165 (35.8) |
| Any | 112 of 175 (64.0) | 106 of 165 (64.2) |
| Diabetes mellitus | 13 of 175 (7.4) | 10 of 165 (6.1) |
| Cardiovascular disease | 37 of 175 (21.1) | 28 of 165 (17.0) |
| Pulmonary disease | 5 of 175 (2.9) | 8 of 165 (4.8) |
| Psychiatric illness | 15 of 175 (8.6) | 15 of 165 (9.1) |
| Chronic pain | 12 of 175 (6.9) | 14 of 165 (8.5) |
| Missing | 25 | 35 |
| Active smoker | ||
| Yes | 12 of 173 (6.9) | 4 of 164 (2.4) |
| No | 161 of 173 (93.1) | 160 of 164 (97.6) |
| Missing | 27 | 36 |
| Alcohol consumption | ||
| AUDIT-C score < 4 | 115 of 171 (67.3) | 113 of 162 (69.8) |
| AUDIT-C score ⩾ 4† | 56 of 171 (32.7) | 49 of 162 (30.2) |
| Missing | 29 | 38 |
| Physical activity at baseline ‡ | ||
| 1 (inactive) | 28 of 175 (16.0) | 22 of 163 (13.5) |
| 2 (low) | 115 of 175 (65.7) | 102 of 163 (62.6) |
| 3–4 (moderate–vigorous) | 32 of 175 (18.3) | 39 of 163 (23.9) |
| Missing | 25 | 37 |
| Residence | ||
| Rural | 16 of 175 (9.1) | 16 of 164 (9.8) |
| Urban | 159 of 175 (90.9) | 148 of 164 (90.2) |
| Missing | 25 | 36 |
| Educational level | ||
| University or higher | 85 of 175 (48.6) | 104 of 164 (63.4) |
| ≤ 12 years | 90 of 175 (51.4) | 60 of 164 (36.6) |
| Missing | 25 | 37 |
| Relationship status | ||
| Married or in relationship | 118 of 175 (67.4) | 123 of 163 (75.5) |
| No relationship | 57 of 175 (32.6) | 40 of 163 (24.5) |
| Missing | 25 | 37 |
| Occupational status | ||
| Working full or part time | 101 of 176 (57.4) | 114 of 165 (69.1) |
| Not working | 75 of 176 (42.6) | 51 of 165 (30.9) |
| Missing | 24 | 35 |
Values in parentheses are percentages unless indicated otherwise.
Values are median (i.q.r.; range).
Risk consumption defined as Alcohol Use Disorders Identification Test (AUDIT-C) score ⩾4.
Measured using Saltin–Grimby Physical Activity Level Scale.
The majority of patients had breast-conserving surgery (79.2 per cent) and sentinel lymph node biopsy (88.7 per cent). Analysis of the invasive tumour characteristics showed that 87.4 per cent of patients had hormone receptor-positive disease and 24.0 per cent had positive lymph node status (Table 2).
Table 2.
Tumour and surgical characteristics
| Control group (n = 200) | Intervention group (n = 200) | |
|---|---|---|
| Type of breast surgery | ||
| Breast-conserving surgery | 154 of 197 (78.2) | 147 of 183 (80.3) |
| Bilateral surgery | 7 of 154 (4.5) | 3 of 147 (2.0) |
| Mastectomy | 43 of 197 (21.8) | 36 of 183 (19.7) |
| Bilateral surgery | 4 of 43 (9.3) | 6 of 36 (16.7) |
| Direct reconstruction | 4 of 43 (9.3) | 2 of 36 (5.6) |
| Missing | 3 | 17 |
| Type of axillary surgery | ||
| None | 12 of 197 (6.1) | 11 of 183 (6.0) |
| SLNB | 176 of 197 (89.3) | 161 of 183 (88.0) |
| ALND | 9 of 197 (4.6) | 11 of 183 (6.0) |
| Missing | 3 | 17 |
| Postoperative drains used | 37 (18.5) | 32 (17.4) |
| Tumour type | ||
| Invasive cancer | 180 of 197 (91.4) | 170 of 183 (92.9) |
| Cancer in situ | 17 of 197 (8.6) | 12 of 183 (6.6) |
| Other | 0 (0) | 1 of 183 (0.5) |
| Missing | 3 | 17 |
| Tumour size (mm) † | 16 (1–137) | 17 (0–108) |
| Oestrogen receptor-positive | 154 of 180 (85.6) | 152 of 170 (89.4) |
| Progesterone receptor-positive | 134 of 180 (74.4) | 130 of 170 (76.5) |
| HER2-positive | 19 of 180 (10.6) | 14 of 170(8.2) |
| Nodal metastasis (N+) | 37 of 180 (20.6) | 47 of 170 (27.6) |
| Change in SGPALS score (before versus after surgery) | ||
| Increase | 18 of 162 (11.1) | 25 of 142 (17.6) |
| Decrease | 37 of 162 (22.8) | 26 of 142 (18.3) |
| No change | 107 of 162 (66.0) | 91 of 142 (64.1) |
| Missing | 38 | 58 |
Values in parentheses are percentages unless indicated otherwise.
Values are median (range). ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; HER2, human epidermal growth factor receptor 2; SGPALS, Saltin–Grimby Physical Activity Level Scale.
Some 95 of 180 patients (52.8 per cent) in the intervention group and 90 of 190 (47.4 per cent) in the control group underwent day surgery. The mean duration of hospital stay was 1.2 days in control group compared with 1.1 day in the intervention group, including the day of surgery.
The physical activity diary was returned by 151 of 180 women (83.9 per cent) in the intervention group. The mean registered additional physical activity was 14 (median 10, range 0–46) days before surgery and 20 (21, 0–34) days after operation. Eighty-one participants (53.6 per cent) reported more than 10 days of activity before surgery, and 86 (56.9 per cent) reported more than 20 days of added activity after operation. To further describe the difference in level of physical activity, the change in SGPALS score was analysed for each individual, both at inclusion and 4 weeks after surgery (Table 2). In the intervention group, 64.1 per cent did not report any change in physical activity level, comparable to 66.0 per cent in the control group.
The primary outcome, physical recovery at 4 weeks after surgery, was reported by 316 participants (148 intervention, 168 control). Of these, 302 patients had all the data necessary for an adjusted analysis. In the intervention group, 130 patients (87.8 per cent) reached 75–100 per cent physical recovery compared with 143 (85.1 per cent) in the control group (RR 1.03, 95 per cent c.i. 0.95 to 1.13). Similar results were found in the adjusted analysis (RR 1.02, 0.93 to 1.11) (Table 3).
Table 3.
Recovery at 4 weeks after surgery
| Recovery (%) |
Risk ratio
|
|||
|---|---|---|---|---|
| Physical recovery | Mental recovery | |||
| 75–100 | Intention to treat | Crude | 1.03 (0.95, 1.13) | 1.05 (0.93, 1.17) |
| Adjusted* | 1.02 (0.93, 1.11) | 1.05 (0.94, 1.18) | ||
| 100 | Intention to treat | Crude | 1.14 (0.92, 1.40) | 1.03 (0.82, 1.31) |
| Adjusted* | 1.18 (0.97, 1.43) | 1.12 (0.88, 1.42) | ||
| 75–100 | Per protocol, subgroup 1 | Adjusted* | 1.04 (0.94, 1.15) | 1.08 (0.95, 1.23) |
| 75–100 | Per protocol, subgroup 2 | Adjusted* | 1.05 (0.93, 1.18) | 1.14 (0.99, 1.30) |
Values in parentheses are 95 per cent confidence intervals. Subgroup 1: ⩾ 10 days of physical activity before surgery and less than 20 days of physical activity after operation added according to diary; subgroup 2: ⩾ 10 days of physical activity before surgery and ⩾20 days of physical activity after surgery added according to diary. Risk ratios for intervention versus control are shown with 95% confidence intervals.
Adjusted for age, physical activity (Saltin–Grimby Physical Activity Level Scale) at baseline, and type of surgery (breast-conserving surgery or mastectomy and sentinel lymph node biopsy or axillary lymph node dissection).
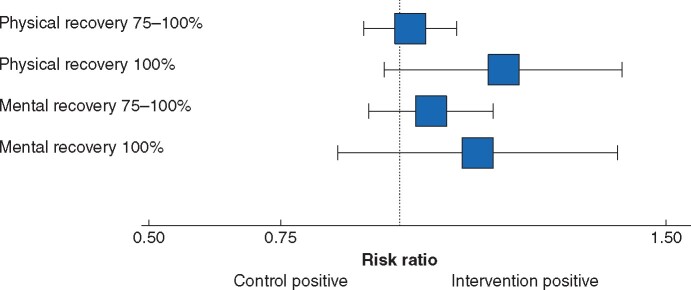
A supporting analysis for complete physical recovery (100 per cent) showed that 71 of 148 patients (48.0 per cent) in the intervention group and 76 of 168 (45.2 per cent) in the control group had a full physical recovery (RR 1.14, 0.92 to 1.40) (Fig. 2). The adjusted analysis yielded similar results (RR 1.18, 0.97 to 1.43). In the post hoc analyses of physical recovery (75–100 per cent recovery), the RR was 1.04 to 1.06 (not significant), with the largest effect size in subgroup 2 (Table 3 and Fig. S1).
Fig. 2.
Recovery at 4 weeks after surgery (intention-to-treat analysis)
Risk ratios for intervention versus control are shown with 95 per cent confidence intervals.
The crude RR between the groups for self-reported mental recovery (75–100 per cent) at 4 weeks was 1.05 (0.93–1.17), and the adjusted RR was 1.05 (0.94 to 1.18). Evaluation of complete mental recovery (100 per cent) showed similar results for the unadjusted (RR 1.03, 82 to 1.31) and adjusted (RR 1.12, 0.88 to 1.42) analyses (Table 3 and Fig. 2). In per-protocol analyses, the RR was 1.08–1.14 (not significant) for feeling 75–100 per cent mentally recovered, with the greatest effect size in subgroup 2.
Complications developed within 30 days of surgery in 28 of 180 patients (15.6 per cent) in the intervention group compared with 35 of 190 (18.4 per cent) in the control group. None of the participants had a grade IV complication, and grade III complications were documented in five patients in the control and one in the intervention group (Table S2). The mean CCI® score within 30 days after surgery was 2.3 (range 0–40.6) in the intervention group compared with 3.1 (0–42.7) in the control group (Table S3).
At 90 days after primary surgery, 368 patients remained for evaluation (179 intervention, 189 control). Complications were seen within 90 days of surgery in 42 of 179 patients (23.5 per cent) in the intervention group compared with 47 of 189 (24.9 per cent) in the control group. One patient in the control group had one grade IV complication; grade III complications were recorded for five patients in the control group and two in the intervention group (Table 4). The mean CCI® score at 90 days after operation was 4.2 (range 0–57.5) in the intervention group compared with 4.7 (range 0–58.3) in the control group (Table 4).
Table 4.
Secondary outcomes at 90 days after surgery
| Control (n = 189) | Intervention (n = 179) | |
|---|---|---|
| Complications (Clavien–Dindo grade) | ||
| I | 23 (12.1) | 23 (12.8) |
| II | 18 (9.5) | 17 (9.5) |
| IIIa | 1 (0.5) | 0 (0) |
| IIIb | 4 (2.1) | 2 (1.1) |
| IV | 1 (0.5) | 0 (0) |
| CCI® score* | 4.7 (0–58.3) | 4.2 (0–57.5) |
| Reoperations † | 8 (4.2) | 8 (4.5) |
| Readmissions † | 10 (5.3) | 15 (8.4) |
Values in parentheses are percentages unless indicated otherwise.
Values are mean (range). CCI®, Comprehensive Complication Index (range 0–100).
Unplanned.
In total, 68 reoperations were performed within 90 days of surgery. These included 52 planned procedures (owing to inadequate tumour margin or completing ALND) and 16 unplanned operations. Of the 16 unplanned procedures (8 in each group), nine were for complications such as infection or bleeding, one patient underwent a second-stage reconstruction and the remaining six procedures were for unrelated reasons (Table S4). The rate of unplanned readmissions within 90 days was 8.4 per cent (15 of 179) in the intervention group compared with 5.3 per cent (10 of 189) in the control group.
Discussion
PhysSURG-B is an RCT examining the effect of prehabilitation with physical activity before and after breast cancer surgery15. The hypothesis was that added physical activity would improve recovery and reduce complications after breast cancer surgery. No significant effect on the primary endpoint, self-reported physical recovery (75 per cent recovery and above) at 4 weeks after surgery, was found in the intervention group compared with the control group.
This could be because the hypothesis was incorrect, but other explanations include an insufficient intervention, poor adherence and/or that the primary endpoint lacked sensitivity for this patient group. It is reasonable to assume that a higher level of adherence could have been accomplished with supervised exercise, but with greater inclusion bias, increased cost and reduced feasibility. The intervention was chosen with this in mind, to be implementable in routine healthcare, if proven efficient. The diary showed lower adherence to the intervention than anticipated for this patient group, even though the type of activity was individualized by the physiotherapist, agreed with the patient, and expected to represent a window of opportunity for lifestyle changes.
There was also only a limited increase in the level of physical activity as measured using SGPALS, between baseline and 4 weeks after surgery in the intervention compared with the control group (Table 2). It is possible that patients diagnosed with, and operated for, breast cancer require supervised exercise or repeated instructions to undertake additional physical exercise, which could explain why the method had limited results. This highlights the drawback of recommendations regarding lifestyle changes, commonly used at a population level, and presented here for a group of patients with breast cancer. Tailored exercise intervention strategies based on fitness level, including supervised exercise and non-supervised exercise in the adjuvant setting, are expected to be further clarified through the ongoing EBBA-II (Energy Balance and Breast Cancer Aspects-II) trial (NCT02240836).
Limitations regarding objective measures concerning the type, amount and intensity of exercise, fulfilment of the recommendation, and difference in physical activity between the study groups are evident in the present study, aside from using the SGPALS instrument. Using accelerometers could have been useful for this purpose, but could also have introduced bias in the control group, blurring evaluation of the simple recommendation. The physical activity diary was not used to collect information about the physical activity performed, but acted primarily as a tool to enhance and evaluate adherence to the recommendation. The aim of this trial was not to assess the quantifiable dimensions of physical activity, and the authors acknowledge the loss of information about the actual physical activity performed. The objective was to evaluate whether an individual recommendation and follow-up was enough to influence recovery. The method was chosen in order to put minimal strain on the healthcare system and offer enough flexibility to be accepted by the majority of patients with breast cancer. The findings may provide useful information when planning new studies aiming to improve recovery of patients with breast cancer.
A low level of surgical morbidity after breast cancer surgery is typically coupled with a high level of recovery, resulting in a small-to-moderate improvement possible with any intervention. Self-reported recovery was chosen with the aim of evaluating a patient-reported outcome with relevance to clinical breast cancer management. The results showed that almost 90 per cent of all patients reached this level of recovery (75 per cent or more), and a ceiling effect can be expected to partially mitigate the effect of physical activity. A previous observational study18 revealed an association between physical activity level before surgery and physical recovery at 3 weeks after operation using the same question and same dichotomization, but at 6 weeks there was no such association. Unfortunately, this may indicate that the dichotomization for recovery (75–100 per cent) was appropriate at the 3-week follow-up in the observational study, but not at the 4-week follow-up in this RCT. The majority of patients undergo one or more modalities of adjuvant treatment, the start of which often coincides with the evaluation period, and this may have affected the feeling of recovery.
The strengths of this trial include the design, being an RCT with a large sample size. The ability to implement the intervention within the standard care of patients with breast cancer is another strength. The key assets of the trial also create the main limitations, including the outcome measure used and the intentionally non-supervised, non-measured physical activity, which precluded detailed analyses of adherence to, or specification of, added physical activity in relation to the recovery experienced.
The authors’ hypothesis was that a recommendation to undertake physical activity would improve recovery, but a significant effect was not shown. There may be several reasons for this finding, including a weak intervention or a ceiling effect. Another explanation might be that for breast cancer surgery, with rapid recovery and few surgical complications, the beneficial effect of physical activity may be related to improved resilience to the side-effects of the subsequent adjuvant therapy. These long-term effects as well as late effects after breast cancer treatment will be further analysed using 12-month data from the PhysSURG-B trial.
Supplementary Material
Acknowledgements
The authors are grateful to the participating hospitals, Skaraborg Hospital, Skövde, Skaraborg Hospital, Lidköping and Sahlgrenska University Hospital, Gothenburg. They thank T. Bengtsson, M. Magnusson, E. Larsson, M. Modin, E. Blomsterwall, and M. Fagevik Olsén for their contribution. The study was supported by Sahlgrenska University Hospital (ALFGBG-4307771, ALFGBG-718221), AFA Insurance (150072), the Swedish Cancer Society (CAN 2016/362), the Gothenburg Medical Society (GLS 779001, GLS 879101), Lions Cancer Research Fund of Western Sweden (LCV2018:28), Anna-Lisa and Bror Björnssons Foundation, and Knut and Alice Wallenberg Foundation.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
References
- 1.WHO. Breast Cancer. http://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (accessed 4 June 2018)
- 2. Moo TA, Sanford R, Dang C, Morrow M. Overview of breast cancer therapy. PET Clin 2018;13:339–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tevis SE, Steiman JG, Neuman HB, Greenberg CC, Wilke LG. Postoperative complications in combined gynecologic, plastic, and breast surgery: an analysis from National Surgical Quality Improvement Program. Breast J 2019;25:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinto A, Faiz O, Davis R, Almoudaris A, Vincent C. Surgical complications and their impact on patients’ psychosocial well-being: a systematic review and meta-analysis. BMJ Open 2016;6:e007224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomsen T, Villebro N, Moller AM. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev 2010; (7)CD002294. [DOI] [PubMed] [Google Scholar]
- 6. Eliasen M, Gronkjaer M, Skov-Ettrup LS, Mikkelsen SS, Becker U, Tolstrup JS et al. Preoperative alcohol consumption and postoperative complications: a systematic review and meta-analysis. Ann Surg 2013;258:930–942 [DOI] [PubMed] [Google Scholar]
- 7. Ogunleye AA, Holmes MD. Physical activity and breast cancer survival. Breast Cancer Res 2009;11:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA 2005;293:2479–2486 [DOI] [PubMed] [Google Scholar]
- 9. Mijwel S, Backman M, Bolam KA, Jervaeus A, Sundberg CJ, Margolin S et al. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat 2018;168:79–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juvet LK, Thune I, Elvsaas IKO, Fors EA, Lundgren S, Bertheussen G et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast 2017;33:166–177 [DOI] [PubMed] [Google Scholar]
- 11. Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev 2012;2012:CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baglia ML, Lin IH, Cartmel B, Sanft T, Ligibel J, Hershman DL et al. Endocrine-related quality of life in a randomized trial of exercise on aromatase inhibitor-induced arthralgias in breast cancer survivors. Cancer 2019;125:2262–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K,, Sweeney FC et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res 2018;20:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Onerup A, Angeras U, Bock D, Borjesson M, Fagevik Olsen M et al. The preoperative level of physical activity is associated to the postoperative recovery after elective cholecystectomy—a cohort study. Int J Surg 2015;19:35–41 [DOI] [PubMed] [Google Scholar]
- 15. Loughney LA, West MA, Kemp GJ, Grocott MP, Jack S. Exercise interventions for people undergoing multimodal cancer treatment that includes surgery. Cochrane Database Syst Rev 2018;2018:CD012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Onerup A, Bock D, Borjesson M, Fagevik Olsen M, Gellerstedt M et al. Is preoperative physical activity related to post-surgery recovery?—a cohort study of colorectal cancer patients. Int J Colorectal Dis 2016;31:1131–1140 [DOI] [PubMed] [Google Scholar]
- 17. Nilsson H, Angeras U, Bock D, Borjesson M, Onerup A, Fagevik Olsen M et al. Is preoperative physical activity related to post-surgery recovery? A cohort study of patients with breast cancer. BMJ Open 2016;6:e007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 20. Clavien PA, Vetter D, Staiger RD, Slankamenac K, Mehra T, Graf R et al. The comprehensive complication index (CCI®): added value and clinical perspectives 3 years ‘down the line’. Ann Surg 2017;265:1045–1050 [DOI] [PubMed] [Google Scholar]
- 21. Bock D, Angenete E, Bjartell A, Carlsson S, Steineck G, Stranne J et al. Habits and self-assessed quality of life, negative intrusive thoughts and depressed mood in patients with prostate cancer: a longitudinal study. Scand J Urol 2017;51:353–359 [DOI] [PubMed] [Google Scholar]
- 22. Steineck G, Bergmark K, Henningsohn L, al-Abany M, Dickman PW, Helgason A. Symptom documentation in cancer survivors as a basis for therapy modifications. Acta Oncol 2002;41:244–252 [DOI] [PubMed] [Google Scholar]
- 23. Johansson E, Steineck G, Holmberg L, Johansson JE, Nyberg T, Ruutu M et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol 2011;12:891–899 [DOI] [PubMed] [Google Scholar]
- 24. Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation 1968;38:1104–1115 [DOI] [PubMed] [Google Scholar]
- 25. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789–1795 [DOI] [PubMed] [Google Scholar]
- 26. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.