Abstract
Background
Uncertainty exists regarding the clinical relevance of programmed cell death ligand 1 (PD-L1) expression in breast cancer.
Methods
A systematic review was performed in accordance with PRISMA guidelines. Observational studies that compared high versus low expression of PD-L1 on breast cancer cells were identified. Log hazard ratios (HRs) for disease-free and overall survival and their standard errors were calculated from Kaplan–Meier curves or Cox regression analyses, and pooled using the inverse-variance method. Dichotomous variables were pooled as odds ratios (ORs) using the Mantel–Haenszel method.
Results
Sixty-five studies with 19 870 patients were included; 14 404 patients were classified as having low and 4975 high PD-L1 expression. High PD-L1 was associated with achieving a pathological complete response following neoadjuvant chemotherapy (OR 3.30, 95 per cent confidence interval 1.19 to 9.16; P < 0.01; I2 = 85 per cent). Low PD-L1 expression was associated with human epidermal growth factor receptor 2 (OR 3.98, 1.81 to 8.75; P < 0.001; I2 = 96 per cent) and luminal (OR 14.93, 6.46 to 34.51; P < 0.001; I2 = 99 per cent) breast cancer subtypes. Those with low PD-L1 had favourable overall survival rates (HR 1.30, 1.05 to 1.61; P = 0.02; I2 = 85 per cent).
Conclusion
Breast cancers with high PD-L1 expression are associated with aggressive clinicopathological and immunohistochemical characteristics and are more likely to achieve a pathological complete response following neoadjuvant chemotherapy. These breast cancers are, however, associated with worse overall survival outcomes.
Uncertainty currently exists regarding the relevance of programmed cell death ligand 1 (PD-L1) in breast cancer. This updated systematic review and meta-analysis of 65 studies illustrates the predictive role of PD-L1 in determining pathological response rates to neoadjuvant chemotherapy, as well as its inherent value in predicting survival outcomes in breast oncology.
Lay summary
This is a pooled analysis assessing the relevance of a biological marker known as programmed cell death ligand 1 (PD-L1) from almost 20 000 patients diagnosed with breast cancer. The main results from this analysis suggest that high levels of PD-L1 are associated with worse survival but better response to chemotherapy for patients diagnosed with breast cancer. This may be used prospectively to enhance outcomes for patients with breast cancer.
Introduction
Breast cancer is the most commonly diagnosed cancer and leading cause of cancer death in women worldwide. There has been an increasing incidence of breast cancer in recent decades, but breast cancer-associated mortality is decreasing1. This emerging trend is likely due to an increased understanding of breast cancer biology, as well as excellent progress in the development of breast cancer diagnostics and treatments2,3. Breast cancer treatment has evolved in the molecular area such that each subtype is managed in a manner complementary to its underlying biological drivers. Breast cancer management currently involves various combinations of surgical, chemical, radiation, hormonal and targeted therapies, although novel therapeutic avenues including immune oncology are currently being explored4.
Programmed cell death 1 (PD-1) plays a critical role in cancer immunotherapy; PD-1 is a receptor expressed on the surfaces of various immune cells such as T, B, and natural killer cells, which are responsible for regulation of cell death and apoptosis. Programmed cell death ligand 1 (PD-L1) (also referred to as B7-H1 or CD274) is a complementary ligand of PD-1 which is expressed on the exterior surface of cancer cells and recruited immune cells, such as macrophages and dendritic cells. PD-L1 suppresses the immunological cascade attacking the cancer cells by inducing apoptosis of local T cells, and propagating tumour proliferation as a consequence5,6. Manipulation of the PD-1/PD-L1 pathway by monoclonal antibodies in cancers has provided promising therapeutic approaches. Various reports7–10 have implied a strong correlation between PD-L1 expression and poor prognosis, particularly in malignant melanoma, and non-small cell lung, colorectal, and bladder carcinomas.
The advent of immune oncology, including immunomodulatory therapies such as immune checkpoint inhibitors (ICI), appears to hold particular promise for future clinical oncological practice, although the current role of PD-L1 assessment in breast cancer remains unclear. Traditionally, breast cancer was not considered an especially immunogenic tumour. However, recent developments have shown that some aggressive triple negative breast cancers (TNBC) are immunogenic, exhibit chemo-resistance and have a poor prognosis11,12. These cancers have been shown to express molecules identified as targets for immunotherapy13. Previous meta-analyses14,15 provided conflicting evidence concerning the prognostic value of PD-L1 expression for those diagnosed with invasive breast cancer, and its role as a predictive marker in neoadjuvant chemotherapy (NACT) and immunotherapy has yet to be fully elucidated. Consequently, the relevance and overall role of PD-L1 assessment in breast cancer management remains unclear.
The main aim of the present study was to perform an updated systematic review and meta-analysis evaluating the relationship between PD-L1 expression and routine clinicopathological and immunohistochemical characteristics in patients diagnosed with breast cancer, irrespective of molecular subtype. Other aims were to determine the prognostic value of PD-L1 status regarding oncological and survival outcomes, and its value as a predictor of response to current multimodal management of breast cancer.
Methods
Search strategy
A systematic review was undertaken in accordance with the PRISMA checklist16. Local institutional ethical approval was not required. An electronic search was performed of the PubMed MEDLINE, Embase, and Scopus databases on 13 June 2020 for relevant studies that would be suitable for inclusion. The search was performed for the following headings: (PD-L1 OR B7-H1 OR CD274 OR ‘programmed cell death 1 ligand 1’ OR ‘PD-L1 costimulatory protein’ OR ‘B7 homolog 1’ OR ‘B7-H1 antigen’ OR ‘CD274 antigen’) AND (“breast cancer” OR “breast neoplasms” OR “breast tumour” OR “breast carcinoma” OR “cancer of breast” OR “human mammary neoplasm” OR “human mammary carcinoma”). Only manuscripts published in the English language were included. Inclusion was not restricted based on year of publication. All titles were initially screened, and studies deemed appropriate had abstracts and full texts reviewed.
Inclusion and exclusion criteria
Studies meeting the following criteria were included: studies on patients with histologically confirmed primary breast cancer; studies that evaluated tumour expression of PD-L1 in breast cancer tissue; and studies investigating the correlation between PD-L1 and clinicopathological parameters, treatment characteristics, pathological complete response (pCR) rates in breast tissue following NACT, disease-free survival (DFS) or overall survival (OS). Exclusion criteria were: studies only describing PD-L1 levels on tumour-infiltrating lymphocytes (TILs); studies in which patients were treated with PD-1/PD-L1-targeting therapies; review articles; studies including fewer than five patients or case reports; editorial articles; and conference abstracts.
Data extraction and quality assessment
The literature search was undertaken by two independent reviewers using the predesigned search strategy. Duplicate studies were removed manually. Each reviewer read the retrieved manuscripts to ensure that all inclusion criteria were met, before extracting the following data: first author name, year of publication, study design, country, level of evidence, study title, number of patients, patient PD-L1 status, clinicopathological characteristics, treatment characteristics, and survival data. Data specific to patient outcomes and survival (expressed as hazard ratios (HRs), with 95 per cent c.i. and P values) were extracted directly from tables and study text. Risk-of-bias and methodological quality assessment was performed in accordance with the Newcastle–Ottawa scale17. In the event of discrepancies in opinion between the reviewers, a third reviewer was asked to arbitrate.
Statistical analysis
PD-L1 expression and clinicopathological and treatment characteristics were presented as proportions using descriptive statistics. DFS and OS were expressed as HRs, and were considered the primary analytical endpoints. HRs and corresponding confidence intervals were retrieved directly for use in this meta-analysis. Either fixed- or random-effects models were applied on the basis of whether significant heterogeneity (I2 over 50 per cent) existed between studies included in each analysis. Symmetry of funnel plots was used to assess publication bias. Statistical heterogeneity was determined using I2 statistics. P < 0.050 was considered statistically significant. Statistical analysis was done using Review Manager (RevMan) version 5.4 (Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Literature search
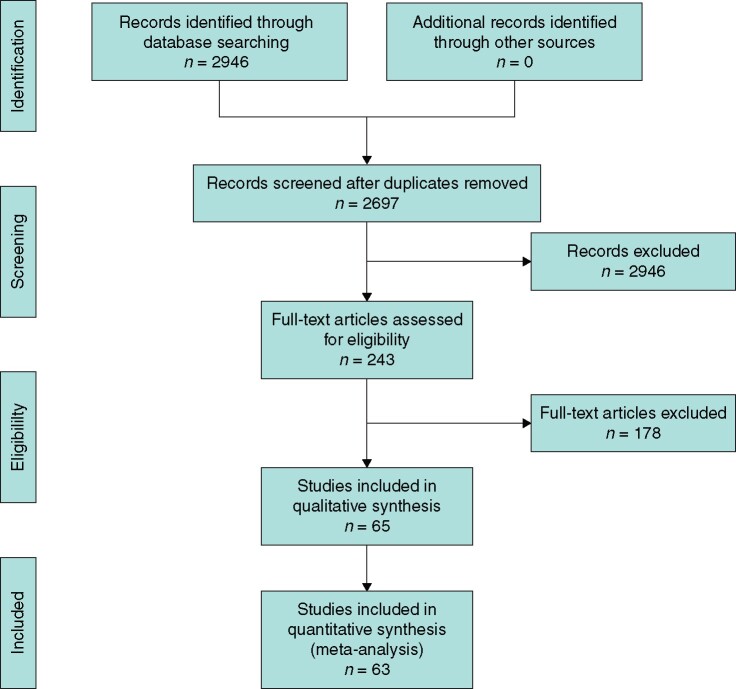
The initial electronic search resulted in a total of 2946 studies. After removal of 249 duplicates, the remaining 2697 titles were screened for relevance, and the abstracts and full text of 243 of these were assessed for eligibility. Overall, 65 clinical studies were included in the systematic review18–82, and 63 in the meta-analyses (Fig. 1). Individual studies included in the analysis are outlined in Table S1.
Fig. 1.
PRISMA diagram showing selection of studies for review
Study characteristics
There were 19 870 patients included; of these, 14 404 patients had low PD-L1 expression on breast cancer cells, and 4975 had high PD-L1 expression. The median age at diagnosis was 52 years and studies had a median follow-up of 75.0 months. Molecular subtype was reported for 17 684 patients (89.0 per cent); 8532 had oestrogen receptor (ER)-positive disease (luminal type) (48.3 per cent), 6548 had TNBC (37.0 per cent), and 2604 human epidermal growth factor receptor 2 (HER2)-positive (HER2+) breast cancer (14.7 per cent).
Clinicopathological and immunohistochemical data
Increased tumour grade (P < 0.001), increased tumour stage (P < 0.001), ER negativity (P < 0.001), progesterone receptor (PgR) negativity (P < 0.001), HER2 positivity (P = 0.001), and Ki-67 proliferation index exceeding 14 per cent (P < 0.001) were all independently associated with PD-L1 status (all Fisher’s exact test) (Tables S2–S4).
High PD-L1 expression on breast tumour cells was associated with grade 3 tumours (odds ratio (OR) 2.16, 95 per cent c.i. 1.64 to 2.83; P < 0.01; I2 = 82 per cent), ER negativity (OR 2.29, 1.54 to 3.41; P < 0.001; I2 = 89 per cent), PgR negativity (OR 2.44, 1.69 to 3.51; P < 0.001; I2 = 83 per cent), and Ki-67 index greater than 14 per cent (OR 2.12, 1.23 to 3.65; P = 0.007; I2 = 89 per cent) ( Fig. S1). Fig. S2 provides details for all clinicopathological and immunohistochemical variables that failed to reach statistical significance in meta-analyses.
Breast cancer molecular subtypes
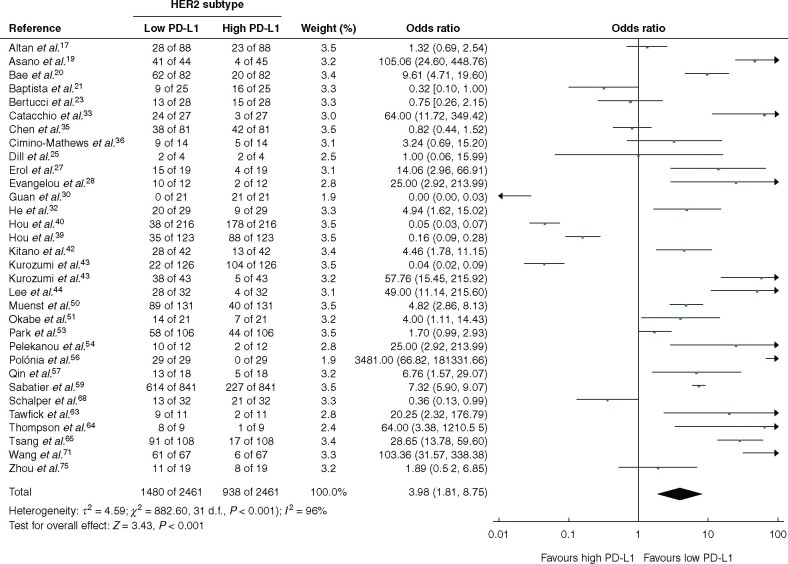
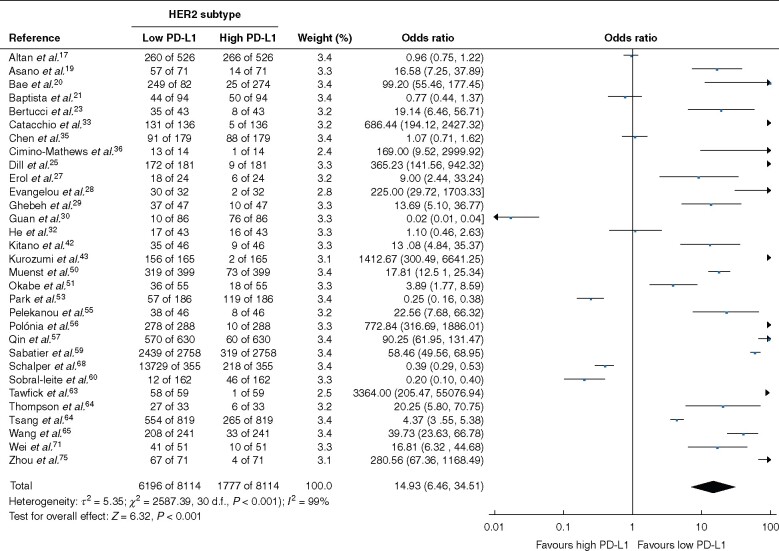
Low PD-L1 expression was associated with the HER2 (OR 3.98, 95 per cent c.i. 1.81 to 8.75; P < 0.001; I2 = 96 per cent) and luminal (OR 14.93, 6.46 to 34.51; P < 0.001; I2 = 99 per cent) molecular subtypes (Figs 2 and 3). PD-L1 expression was not associated with TNBC (OR 0.98, 0.74 to 1.30; P = 0.90; I2 = 97 per cent), and failed to inform patient prognosis in terms of DFS or OS for patients with TNBC (Fig. S2d,g,h). Performing subgroup survival analyses for the luminal and HER2 molecular subgroups was not feasible owing to lack of available data.
Fig. 2.
Association between programmed cell death ligand 1 expression and human epidermal growth factor receptor 2-amplified molecular subtype of breast cancer
A Mantel–Haenszel random-effects model was used for meta-analysis. Odds ratios are shown with 95 per cent confidence intervals. PD-L1, programmed cell death ligand 1. Kurozumi et al. evaluated two independent patient cohorts hence being twice in this analysis.
Fig. 3.
Association between programmed cell death ligand 1 expression and luminal molecular subtype type of breast cancer
A Mantel–Haenszel random-effects model was used for meta-analysis. Odds ratios are shown with 95 per cent confidence intervals. PD-L1, programmed cell death ligand 1.
Treatment characteristics
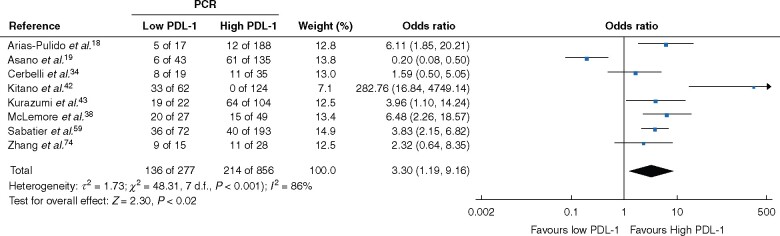
Thirteen studies reported on breast cancer management using treatment with NACT and PD-L1 expression. Eleven studies reported patients achieving a pCR in the breast following NACT, eight of which were suitable for meta-analysis. Patients with high PD-L1 expression were more likely to achieve a pCR after NACT (OR 3.30, 1.19 to 9.16; P < 0.01; I2 = 85 per cent) (Fig. 4).
Fig. 4.
Association between programmed death ligand 1 expression and likelihood of achieving a pathological complete response after neoadjuvant chemotherapy
A Mantel–Haenzel random-effects model was used for meta-analysis. Odds ratios are shown with 95 per cent confidence intervals. pCR, pathological complete response; PD-L1, programmed cell death ligand 1.
Three studies reported adjuvant chemotherapy prescription with respect to PD-L1. Adjuvant chemotherapy was prescribed for 759 of 952 patients (79.7%) with low PD-L1 expression and 333 of 543 (61.3%) with high PD-L1 expression (P < 0.001). Details regarding adjuvant radiotherapy and PD-L1 expression was recorded in three studies; 90 of 952 patients with low PD-L1 expression and 88 of 543 with high PD-L1 expression received radiotherapy (P < 0.001). Only AiErken and colleagues19 reported results regarding PD-L1 status and surgical management; all 70 patients with PD-L1-positive disease underwent mastectomy, whereas 136 of 145 (93.8 per cent) with PD-L1-negative disease underwent mastectomy (P = 0.027). Treatment characteristics and associations between PD-L1 and responses to NACT are outlined in Tables S5 and S6.
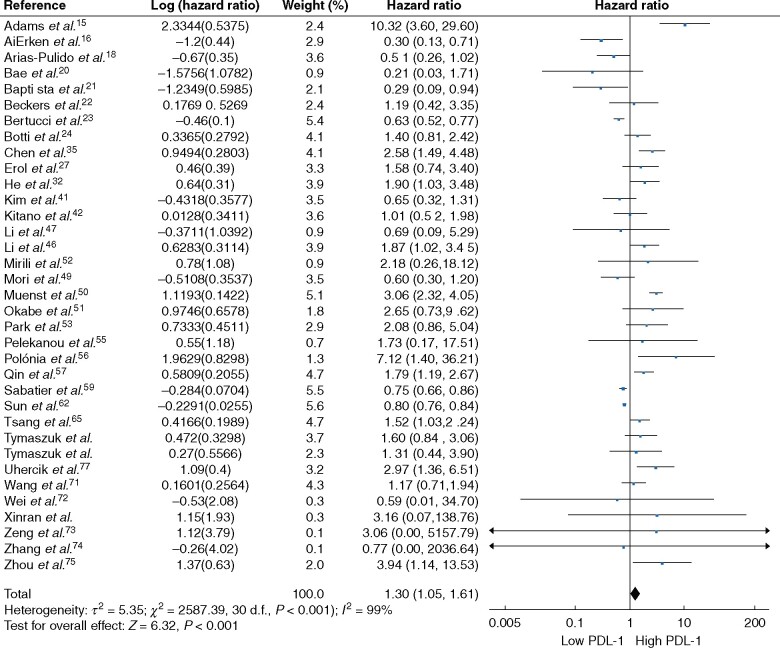
Survival outcomes
Patients with cancers expressing high levels of PD-L1 on breast tumour cells had worse OS rates than those with low PD-L1 expression (HR 1.30, 95 per cent c.i. 1.05 to 1.61; P = 0.02). There was significant heterogeneity between the 35 included independent patient cohorts (I2 = 85 per cent; P < 0.001) (Fig. 5).
Fig. 5.
Association between programmed death ligand 1 expression and overall survival in breast cancer
An inverse-variance random-effects model was used for meta-analysis. Hazard ratios are shown with 95 per cent confidence intervals.
There was no increased risk of breast cancer recurrence for patients with high PD-L1 expression versus those with low PD-L1 expression on breast cancer cells (HR 1.11, 0.86 to 1.44; P = 0.41). Significant heterogeneity was present between the 26 patient cohorts analysed for DFS (I2 = 88 per cent; P < 0.001) (Fig. S2e). Furthermore, there was no increased risk of disease recurrence after 5 years for those with high PD-L1 expression (HR 0.86, 0.64 to 1.17; P = 0.34; I2 = 73 per cent, P < 0.001) (Fig. S2f).
Discussion
The present analysis of 19 870 patients with breast cancer investigated the prognostic value of PD-L1 expression on breast cancer cells. The most important clinical findings are the increased likelihood of achieving a pCR in tumours expressing high levels of PD-L1 on cancer cells, as well as the favourable OS outcomes for patients with breast cancers expressing low PD-L1 levels. Breast oncology has evolved in concordance with the molecular era, such that biomarkers, genetic testing, and genomic assays are used to personalize treatment regimens and provide valuable prognostic information; immunomodulatory strategies are a promising approach to enhance outcomes even further83,84. This study provides clarity pertaining to the clinical role of PD-L1 immunohistochemical testing; incorporation of this biomarker into routine histopathological evaluation of diagnostic tissue biopsies may aid clinical and therapeutic decision-making in the neoadjuvant setting, and further reduce prognostic uncertainty of a breast cancer diagnosis.
Evidence from this meta-analysis suggests that increased PD-L1 expression by cancer cells indicates that patients are likely to achieve a pCR on NACT. A pCR has been recognized as an excellent indicator of prognosis and survival in breast cancer in a number of prospective studies85, with patients attaining a pCR typically outperforming their counterparts in terms of survival. These results support the clinical importance of clarifying PD-L1 status on core tissue biopsy in the neoadjuvant setting in those being considered for preoperative systemic chemotherapy. Furthermore, recent prospective studies such as the IMPASSION 130 and Keynote 522 trials86,87 have outlined the role of confirming PD-L1 status on tissue biopsy in both early-stage and metastatic settings, when gauging whether immunotherapeutic agents, such as anti-PD-L1 drugs, will be indicated. These novel immunotherapies currently rely on clarification of PD-L1 status88, and their potential for enhancing oncological and survival outcomes for even the most aggressive of TNBCs has been described87. This data, reported in tandem with these large prospective studies, support routine PD-L1 detection in breast cancer immunohistochemical tissue analysis, given the robust prognostic value PD-L1 offers within the breast oncological paradigm, as well as its potential role as a prospective therapeutic target.
In contrast, the results shown here from a pooled analysis of 33 independent patient cohorts highlight a significant survival benefit associated with breast cancer diagnoses harbouring low PD-L1 expression on cancer cells. These observations are consistent with two previous meta-analyses14,89, both of which suggested that high PD-L1 expression is correlated with poor prognosis. These results imply that PD-L1 could perhaps be considered as a reliable predictor of OS in breast cancer, even though this is incongruent with observations associating PD-L1 expression to pCR, a recognised surrogate for survival85. While this inconsistency creates ambiguity over the interpretation of PD-L1 expression and its supplementary value in breast oncology, perhaps increased PD-L1 expression provides a novel avenue of investigation to establish the rationale surrounding the cohort of patients who paradoxically successfully achieve a pCR, yet fail to derive meaningful long-term survival benefit from therapy, as alluded to in several prospective analyses85.
Although PD-L1 provides excellent prognostic information regarding pCR and patient mortality, its relevance as an indicator of disease recurrence is less conclusive. Both previous meta-analyses reported PD-L1 as a predictive biomarker of DFS, a finding that was not replicated here, and uncertainty is further potentiated as PD-L1 failed to predict DFS within TNBC. Huang and colleagues described PD-L1 positivity correlating with earlier time to breast cancer recurrence in patients managed with breast-conserving surgery followed by radiotherapy90, further complicating the application of PD-L1 in breast oncology. There is a temptation to implicate the aggressive clinicopathological characteristics associated with increased PD-L1 expression on cancer cells as providing the rationale for worse OS. In the absence of disease recurrence, however, the present data fail to support this. Data surrounding a systemic role of PD-L1 suggest that host immunological factors and other complex factors may contribute, as upregulation of PD-L1 suppresses T lymphocyte function overall, increasing patient susceptibility to infection and inflammation91. Moreover, simple measures of PD-L1 expression does not capture differential enrichments across patients, tumour and immune cell subtypes, as well as the spatial proximity of these cell types in tissues. These relational features may be critical to further evaluating the complex stimulatory and inhibitory processes that depends on the interplay between individual cells in the tumour microenvironment, and the immune system.92 In the interim, prognostic information provided by PD-L1 assessment concerning pCR and mortality is explicit, and supports its potential value as a breast cancer biomarker.
In this meta-analysis, increased PD-L1 expression correlated with aggressive clinicopathological and immunohistochemical tumour features, such as grade 3 tumours, ER and PgR negativity, as well as Ki-67 proliferation greater than 14 per cent. Uncontrolled proliferation is a hallmark of oncogenesis93, and consequently, routine Ki-67 proliferation staining is performed to appraise the proportion of tumour cells actively proliferating at a molecular level94. Ki-67 indices, as well as the mitotic component of Nottingham histopathological grading systems, are of crucial clinical relevance in modern histopathological reporting95. Moreover, ER and PgR negativity both significantly correlated with PD-L1 expression in this analysis, indicating that PD-L1 status provides data somewhat compatible with routine prognostic molecular variables. This complements the findings concerning OS, particularly when historical data connect ER and PgR negativity, increased histological grade, and Ki-67 proliferation to worse clinical outcomes in breast cancer96–98. Linking increased PD-L1 expression with aggressive microscopic tumour characteristics and immunohistochemical features encourages hypotheses that assessment of PD-L1 would be best reserved for those with triple-negative and ER-positive/PgR-negative disease, whose responses to systemic treatments fall short of their ER-positive/PgR-positive and HER2-positive counterparts. Perhaps ICIs have the potential to bridge the current gap in clinical outcomes between these molecular subtypes, and the authors await the results of the IMPASSION 130 and Keynote 522 trials with great anticipation86,87.
The results of the present study lead to a congruent message: evaluating PD-L1 status appears advantageous in reducing prognostic uncertainty for patients diagnosed with breast cancer. Reduced PD-L1 in the luminal and HER2+ molecular subtypes corresponds with their more favourable prognoses64; however, the challenge remains in establishing the scientific reasoning for such results. Within the tumour microenvironment, interplay between PD-1 expressed on TILs and its complementary ligand (expressed on both cancer and immune cells) appears to suppress and dampen the cytotoxic anticancer effect of local inflammatory cells99. Consequently, cancer development may be unopposed by the host immune system, even in more immunogenic breast cancer subtypes such as TNBC, leading to development of more aggressive tumour biology100. This provides a rationale for the strong associations between high PD-L1 expression and aggressive clinicopathological characteristics, and moreover, the worse survival outcomes in the long term. On the contrary, the paradigm is evolving such that positive PD-L1 expression may provide the potential for novel therapeutic avenues for patients in both early and metastatic settings86,87, which perhaps suggests PD-L1 positivity may develop into a favourable parameter in certain circumstances. In the interim,the results of this meta-analysis demonstrate high PD-L1 expression expression is an adverse prognostic marker in its current unmanipulated state, as well as having indispensable value in predicting those likely to achieve a pCR during NACT.
Despite efforts to ascertain accurate and comprehensive information with clinical relevance, several limitations should be considered when interpreting the results of this analysis. The study was based solely on studies published in the English language, and the vast majority of included studies were conducted retrospectively, providing low-to-moderate levels of evidence. The included studies provided inconsistent definitions and cut-offs defining PD-L1 status, and there was considerable variation in determining PD-L1 expression, such as in detection antibodies used and preparation of tissues, and human error in reporting. Perhaps expert consensus, such as those proposed annually by the St Gallen panel or the recent standardised methodology to assess TILs in breast cancer101, may shed light on the most appropriate means of evaluating PD-L1 expression. Survival analysis was not available for 58 per cent of included studies (38 of 65), limiting the conclusions that can be drawn from these analyses. The paucity of studies reporting on HER2+ or luminal molecular subtypes in isolation limited independent molecular subtype analyses of PD-L1 status and survival outcomes. This review focused solely on PD-L1 expression by cancer cells, and provides no prognostic information regarding the role of PD-L1 status on tumour microenvironmental immune cells; debate about which is more critical in predicting clinical outcomes and response to ICI is ongoing. Moreover, as previously mentioned simple measures of PD-L1 expression does not capture the complexity of the relational features within the tumour microenvironment that may be critical to further evaluating the complex and dynamic dual tumour stimulatory and inhibitory processes of the immune system on breast cancer tumourogenesis and establishing the basis for novel individualised cancer immunotherapies, including ICIs.
Disclosure. The authors have no conflicts of interest to declare.
Supplementary material
Supplementary material is available at BJS online.
Supplementary Material
References
- 1. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34 [DOI] [PubMed] [Google Scholar]
- 2. Meisel JL, Venur VA, Gnant M, Carey L.. Evolution of targeted therapy in breast cancer: where precision medicine began. Am Soc Clin Oncol Educ Book 2018;38:78–86 [DOI] [PubMed] [Google Scholar]
- 3. Baldassarre G, Belletti B.. Molecular biology of breast tumors and prognosis. F1000Res 2016;5:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Waks AG, Winer EP.. Breast cancer treatment: a review. JAMA 2019;321:288–300 [DOI] [PubMed] [Google Scholar]
- 5. Zou W, Wolchok JD, Chen L.. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schütz F, Stefanovic S, Mayer L, von Au A, Domschke C, Sohn C. et al. PD-1/PD-L1 pathway in breast cancer. Oncol Res Treat 2017;40:294–297 [DOI] [PubMed] [Google Scholar]
- 7. Ma J, Chi D, Wang Y, Yan Y, Zhao S, Liu H. et al. Prognostic value of PD-L1 expression in resected lung adenocarcinoma and potential molecular mechanisms. J Cancer 2018;9:3489–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, He M, Zhou Y, Yang C, Wei S, Bian X. et al. The prognostic and clinicopathological roles of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. Front Pharmacol 2019;10:139–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu L, Sun J, Wang L, Li Z, Wang L, Li Z. et al. Prognostic and clinicopathological significance of PD-L1 in patients with bladder cancer: a meta-analysis. Front Pharmacol 2019;10:962–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yun S, Park Y, Moon S, Ahn S, Lee K, Park HJ. et al. Clinicopathological and prognostic significance of programmed death ligand 1 expression in Korean melanoma patients. J Cancer 2019;10:3070–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams S, Gatti-Mays ME, Kalinsky K, Korde LA, Sharon E, Amiri-Kordestani Let al. . Current Landscape of Immunotherapy in Breast Cancer. JAMA Oncol 2019;5:1205–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teng MWL, , Ngiow SF, , Ribas A, , Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res 2015;75:2139–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mittendorf EA, , Philips AV, , Meric-Bernstam F, , Qiao N, , Wu Y, , Harrington S et al. PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol Res 2014;2:361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang W, Ran R, Shao B, Li H.. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res Treat 2019;178:17–33 [DOI] [PubMed] [Google Scholar]
- 15. Matikas A, Zerdes I, Lövrot J, Richard F, Sotiriou C, Bergh J. et al. Prognostic implications of PD-L1 expression in breast cancer: systematic review and meta-analysis of immunohistochemistry and pooled analysis of transcriptomic data. Clin Cancer Res 2019;25:5717–5726 [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ga Wells BS, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P.. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 1 July 2020)
- 18. Adams TA, Vail PJ, Ruiz A, Mollaee M, McCue PA, Knudsen ES. et al. Composite analysis of immunological and metabolic markers defines novel subtypes of triple negative breast cancer. Mod Pathol 2018;31:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. AiErken N, Shi HJ, Zhou Y, Shao N, Zhang J, Shi Y. et al. High PD-L1 expression is closely associated with tumor-infiltrating lymphocytes and leads to good clinical outcomes in Chinese triple negative breast cancer patients. Int J Biol Sci 2017;13:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Altan M, Kidwell KM, Pelekanou V, Carvajal-Hausdorf DE, Schalper KA, Toki MI. et al. Association of B7-H4, PD-L1, and tumor infiltrating lymphocytes with outcomes in breast cancer. NPJ Breast Cancer 2018;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arias-Pulido H, Cimino-Mathews A, Chaher N, Qualls C, Joste N, Colpaert C. et al. The combined presence of CD20 + B cells and PD-L1 + tumor-infiltrating lymphocytes in inflammatory breast cancer is prognostic of improved patient outcome. Breast Cancer Res Treat 2018;171:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Morisaki T. et al. Prediction of treatment responses to neoadjuvant chemotherapy in triple-negative breast cancer by analysis of immune checkpoint protein expression. J Transl Med 2018;16:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bae SB, Cho HD, Oh MH, Lee JH, Jang SH, Hong SA. et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer 2016;19:242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baptista MZ, Sarian LO, Derchain SFM, Pinto GA, Vassallo J.. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol 2016;47:78–84 [DOI] [PubMed] [Google Scholar]
- 25. Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K. et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016;69:25–34 [DOI] [PubMed] [Google Scholar]
- 26. Bertucci F, Finetti P, Colpaert C, Mamessier E, Parizel M, Dirix L. et al. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget 2015;6:13 506–13 519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Botti G, Collina F, Scognamiglio G, Rao F, Peluso V, De Cecio R. et al. Programmed death ligand 1 (PD-L1) tumor expression is associated with a better prognosis and diabetic disease in triple negative breast cancer patients. Int J Mol Sci 2017;18:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dill EA, Dillon PM, Bullock TN, Mills AM.. IDO expression in breast cancer: an assessment of 281 primary and metastatic cases with comparison to PD-L1. Mod Pathol 2018;31:1513–1522 [DOI] [PubMed] [Google Scholar]
- 29. Doğukan R, Uçak R, Doğukan FM, Tanık C, Çitgez B, Kabukcuoğlu F. et al. Correlation between the expression of PD-L1 and clinicopathological parameters in triple negative breast cancer patients. Eur J Breast Health 2019;15:235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erol T, İmamoğlu NE, Aydin B, Taşkiran ZE, Esendağli G, Kösemehmetoğlu K. et al. Primary tumor resection for initially staged IV breast cancer: an emphasis on programmed death-ligand 1 expression, promoter methylation status, and survival. Medicine (Baltimore) 2019;98:e16773-e16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evangelou Z, Papoudou-Bai A, Karpathiou G, Kourea H, Kamina S, Goussia A. et al. PD-L1 expression and tumor-infiltrating lymphocytes in breast cancer: clinicopathological analysis in women younger than 40 years old. In Vivo 2020;34:639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghebeh H, Tulbah A, Mohammed S, ElKum N, Amer SMB, Al-Tweigeri T. et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer 2007;121:751–758 [DOI] [PubMed] [Google Scholar]
- 33. Guan H, Lan Y, Wan Y, Wang Q, Wang C, Xu L. et al. PD-L1 mediated the differentiation of tumor-infiltrating CD19(+) B lymphocytes and T cells in invasive breast cancer. Oncoimmunology 2016;5:e1075112–e1075112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo L, Li W, Zhu X, Ling Y, Qiu T, Dong L. et al. PD-L1 expression and CD274 gene alteration in triple-negative breast cancer: implication for prognostic biomarker. SpringerPlus 2016;5:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He J, Huo L, Ma J, Zhao J, Bassett RL, Sun X. et al. Expression of programmed death ligand 1 (PD-l1) in posttreatment primary inflammatory breast cancers and clinical implications. Am J Clin Pathol 2018;149:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Catacchio I, Silvestris N, Scarpi E, Schirosi L, Scattone A, Mangia A. et al. Intratumoral, rather than stromal, CD8+ T cells could be a potential negative prognostic marker in invasive breast cancer patients. Trans Oncol 2019;12:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cerbelli B, Pernazza A, Botticelli A, Fortunato L, Monti M, Sciattella P. et al. PD-L1 expression in TNBC: a predictive biomarker of response to neoadjuvant chemotherapy? BioMed Res Int 2017;2017:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen S, Wang RX, Liu Y, Yang WT, Shao ZM.. PD-L1 expression of the residual tumor serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Int J Cancer 2017;140:1384–1395 [DOI] [PubMed] [Google Scholar]
- 39. Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A. et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 2016;47:52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hirakata T, Fujii T, Kurozumi S, Katayama A, Honda C, Yanai K. et al. FDG uptake reflects breast cancer immunological features: the PD-L1 expression and degree of TILs in primary breast cancer. Breast Cancer Res Treat 2020;181:331–338 [DOI] [PubMed] [Google Scholar]
- 41. Hou Y, Nitta H, Parwani AV, Li Z.. PD-L1 and CD8 are associated with deficient mismatch repair status in triple-negative and HER2-positive breast cancers. Hum Pathol 2019;86:108–114 [DOI] [PubMed] [Google Scholar]
- 42. Hou Y, Nitta H, Wei L, Banks PM, Lustberg M, Wesolowski R. et al. PD-L1 expression and CD8-positive T cells are associated with favorable survival in HER2-positive invasive breast cancer. Breast J 2018;24:911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hou Y, Nitta H, Wei L, Banks PM, Parwani AV, Li Z. et al. Evaluation of immune reaction and PD-L1 expression using multiplex immunohistochemistry in HER2-positive breast cancer: the association with response to anti-HER2 neoadjuvant therapy. Clin Breast Cancer 2018;18:e237–e244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim HS, Do SI, Kim DH, Apple S.. Clinicopathological and prognostic significance of programmed death ligand 1 expression in Korean patients with triple-negative breast carcinoma. Anticancer Res 2020;40:1487–1494 [DOI] [PubMed] [Google Scholar]
- 45. Kitano A, Ono M, Yoshida M, Noguchi E, Shimomura A, Shimoi T. et al. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open 2017;2:e000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kurozumi S, Inoue K, Matsumoto H, Fujii T, Horiguchi J, Oyama T. et al. Clinicopathological values of PD-L1 expression in HER2-positive breast cancer. Sci Rep 2019;9:16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee DW, Ryu HS, Jin MS, Lee KH, Suh KJ, Youk J. et al. Immune recurrence score using 7 immunoregulatory protein expressions can predict recurrence in stage I–III breast cancer patients. Br J Cancer 2019;121:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee J, Kim DM, Lee A.. Prognostic role and clinical association of tumor-infiltrating lymphocyte, programmed death ligand-1 expression with neutrophil–lymphocyte ratio in locally advanced triple-negative breast cancer. Cancer Res Treat 2019;51:649–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li M, Li A, Zhou S, Xu Y, Xiao Y, Bi R. et al. Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer 2018;18:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li X, Wetherilt CS, Krishnamurti U, Yang J, Ma Y, Styblo TM. et al. Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am J Clin Pathol 2016;146:496–502 [DOI] [PubMed] [Google Scholar]
- 51. Lou J, Zhou Y, Huang J, Qian X.. Relationship between PD-L1 expression and clinical characteristics in patients with breast invasive ductal carcinoma. Open Medicine (Wars) 2017;12:288–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N. et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017;8:15 584–15 592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA. et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014;146:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Okabe M, Toh U, Iwakuma N, Saku S, Akashi M, Kimitsuki Y. et al. Predictive factors of the tumor immunological microenvironment for long-term follow-up in early stage breast cancer. Cancer Sci 2017;108:81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mirili C, Paydas S, Kilic EB, Seydaoglu G, Ogul A, Gokcay S. et al. Prognostic significance of EGFR, MUC1 and PD-L1 expressions in cases with triple negative breast cancer. J BUON 2020;25:159–167 [PubMed] [Google Scholar]
- 56. Park IH, Kong SY, Ro JY, Kwon Y, Kang JH, Mo HJ. et al. Prognostic implications of tumor-infiltrating lymphocytes in association with programmed death ligand 1 expression in early-stage breast cancer. Clin Breast Cancer 2016;16:51–58 [DOI] [PubMed] [Google Scholar]
- 57. Pelekanou V, Barlow WE, Nahleh ZA, Wasserman B, Lo YC, von Wahlde MK. et al. Tumor-infiltrating lymphocytes and PD-L1 expression in pre- and posttreatment breast cancers in the SWOG S0800 phase II neoadjuvant chemotherapy trial. Mol Cancer Ther 2018;17:1324–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pelekanou V, Carvajal-Hausdorf DE, Altan M, Wasserman B, Carvajal-Hausdorf C, Wimberly H. et al. Effect of neoadjuvant chemotherapy on tumor-infiltrating lymphocytes and PD-L1 expression in breast cancer and its clinical significance. Breast Cancer Res 2017;19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Polónia A, Pinto R, Cameselle-Teijeiro JF, Schmitt FC, Paredes J.. Prognostic value of stromal tumour infiltrating lymphocytes and programmed cell death-ligand 1 expression in breast cancer. J Clin Pathol 2017;70:860–867 [DOI] [PubMed] [Google Scholar]
- 60. Qin T, Zeng YD, Qin G, Xu F, Lu JB, Fang WF. et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 2015;6:33 972–33 981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ren X, Wu H, Lu J, Zhang Y, Luo Y, Xu Q. et al. PD1 protein expression in tumor infiltrated lymphocytes rather than PDL1 in tumor cells predicts survival in triple-negative breast cancer. Cancer Biol Ther 2018;19:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR. et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015;6:5449–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sobral-Leite M, Van de Vijver K, Michaut M, van der Linden R, Hooijer GKJ, Horlings HM. et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology 2018;7:e1509820–e1509820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A.. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol 2009;20:628–635 [DOI] [PubMed] [Google Scholar]
- 65. Sun WY, Lee YK, Koo JS.. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J Transl Med 2016;14:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tawfik O, Kimler BF, Karnik T, Shehata P.. Clinicopathological correlation of PD-L1 expression in primary and metastatic breast cancer and infiltrating immune cells. Hum Pathol 2018;80:170–178 [DOI] [PubMed] [Google Scholar]
- 67. Thompson ED, Taube JM, Asch-Kendrick RJ, Ogurtsova A, Xu H, Sharma R. et al. PD-L1 expression and the immune microenvironment in primary invasive lobular carcinomas of the breast. Mod Pathol 2017;30:1551–1560 [DOI] [PubMed] [Google Scholar]
- 68. Tsang JYS, Au WL, Lo KY, Ni YB, Hlaing T, Hu J. et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat 2017;162:19–30 [DOI] [PubMed] [Google Scholar]
- 69. Tung N, Garber JE, Hacker MR, Torous V, Freeman GJ, Poles E. et al. Prevalence and predictors of androgen receptor and programmed death-ligand 1 in BRCA1-associated and sporadic triple-negative breast cancer. NPJ Breast Cancer 2016;2:16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tymoszuk P, Charoentong P, Hackl H, Spilka R, Müller-Holzner E, Trajanoski Z. et al. High STAT1 mRNA levels but not its tyrosine phosphorylation are associated with macrophage infiltration and bad prognosis in breast cancer. BMC Cancer 2014;14:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L. et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014;20:2773–2782 [DOI] [PubMed] [Google Scholar]
- 72. Van Berckelaer C, Rypens C, van Dam P, Pouillon L, Parizel M, Schats KA. et al. Infiltrating stromal immune cells in inflammatory breast cancer are associated with an improved outcome and increased PD-L1 expression. Breast Cancer Res 2019;21:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang X, Liu Y.. PD-L1 expression in tumor infiltrated lymphocytes predicts survival in triple-negative breast cancer. Pathol Res Pract 2020;216:152802. [DOI] [PubMed] [Google Scholar]
- 74. Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. et al. PD-L1 and intratumoral immune response in breast cancer. Oncotarget 2017;8:51 641–51 651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wei L, Wu N, Wei F, Li F, Zhang Y, Liu J. et al. Prognosis significance of indoleamine 2,3-dioxygenase, programmed death ligand-1 and tumor-infiltrating immune cells in microenvironment of breast cancer. Int Immunopharmacol 2020;84:106506. [DOI] [PubMed] [Google Scholar]
- 76. Zeng Y, Wang CL, Xian J, Ye Q, Qin X, Tan YW. et al. Positive correlation between programmed death ligand-1 and p53 in triple-negative breast cancer. Onco Targets Ther 2019;12:7193–7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang L, Wang XI, Ding J, Sun Q, Zhang S.. The predictive and prognostic value of Foxp3+/CD25+ regulatory T cells and PD-L1 expression in triple negative breast cancer. Ann Diagn Pathol 2019;40:143–151 [DOI] [PubMed] [Google Scholar]
- 78. Zhou T, Xu D, Tang B, Ren Y, Han Y, Liang G. et al. Expression of programmed death ligand-1 and programmed death-1 in samples of invasive ductal carcinoma of the breast and its correlation with prognosis. Anticancer Drugs 2018;29:904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhu X, Zhang Q, Wang D, Liu C, Han B, Yang JM. et al. Expression of PD-L1 attenuates the positive impacts of high-level tumor-infiltrating lymphocytes on prognosis of triple-negative breast cancer. Cancer Biol Ther 2019;20:1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Uhercik M, Sanders AJ, Owen S, Davies EL, Sharma AK, Jiang WG. et al. Clinical significance of PD1 and PDL1 in human breast cancer. Anticancer Res 2017;37:4249–4254 [DOI] [PubMed] [Google Scholar]
- 81. McLemore LE, Janakiram M, Albanese J, Shapiro N, Lo Y, Zang X. et al. An Immunoscore using PD-L1, CD68, and tumor-infiltrating lymphocytes (TILs) to predict response to neoadjuvant chemotherapy in invasive breast cancer. Appl Immunohistochem Mol Morphol 2018;26:611–619 [DOI] [PubMed] [Google Scholar]
- 82. Li F, Ren Y, Wang Z.. Programmed death 1 ligand 1 expression in breast cancer and its association with patients' clinical parameters. J Cancer Res Ther 2018;14:150–154 [DOI] [PubMed] [Google Scholar]
- 83. Hamdan D, Nguyen TT, Leboeuf C, Meles S, Janin A, Bousquet G. et al. Genomics applied to the treatment of breast cancer. Oncotarget 2019;10:4786–4801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McVeigh TP, Kerin MJ.. Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer (Dove Med Press) 2017;9:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–172 [DOI] [PubMed] [Google Scholar]
- 86. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H. et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–2121 [DOI] [PubMed] [Google Scholar]
- 87. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J. et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020;382:810–821 [DOI] [PubMed] [Google Scholar]
- 88. Cyprian FS, Akhtar S, Gatalica Z, Vranic S.. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: a new clinical paradigm in the treatment of triple-negative breast cancer. Bosn J Basic Med Sci 2019;19:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li S, Chen L, Jiang J.. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Huang R, Cui Y, Guo Y.. Programmed cell death protein-1 predicts the recurrence of breast cancer in patients subjected to radiotherapy after breast-preserving surgery. Technol Cancer Res Treat 2018;17:1533033818793425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Keren L, , Bosse M, , Marquez D, , Angoshtari R, , Jain S, , Varma S et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell 2018;174:1373–1387.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gutschner T, Diederichs S.. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol 2012;9:703–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA.. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010;11:174–183 [DOI] [PubMed] [Google Scholar]
- 95. van Diest PJ, van der Wall E, Baak JPA.. Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol 2004;57:675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M. et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 2013;139:539–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schwartz AM, Henson DE, Chen D, Rajamarthandan S.. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: a study of 161 708 cases of breast cancer from the SEER Program. Arch Pathol Lab Med 2014;138:1048–1052 [DOI] [PubMed] [Google Scholar]
- 98. Boland MR, Ryan ÉJ, Dunne E, Aherne TM, Bhatt NR, Lowery AJ. et al. Meta-analysis of the impact of progesterone receptor status on oncological outcomes in oestrogen receptor-positive breast cancer. Br J Surg 2019;107:33–43 [DOI] [PubMed] [Google Scholar]
- 99. Kurozumi S, Fujii T, Matsumoto H, Inoue K, Kurosumi M, Horiguchi J. et al. Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer. Med Mol Morphol 2017;50:185–194 [DOI] [PubMed] [Google Scholar]
- 100. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800 [DOI] [PubMed] [Google Scholar]
- 101. Salgado R, , Denkert C, , Demaria S, , Sirtaine N, , Klauschen F, , Pruneri G et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.