Abstract
Staphylococcus aureus synthesizes C30 carotenoids. Their formation involves the introduction of three double bonds, which is catalyzed by a single enzyme. This enzyme, 4,4′-diapophytoene desaturase from S. aureus, was overexpressed in Escherichia coli and purified in one step by affinity chromatography, and then the protein was characterized with respect to substrate specificity, cofactor requirement, and oligomerization.
Carotenoids originate in the terpenoid biosynthetic pathway. Their most typical feature is their orange to red color. Most carotenoids contain 40 carbon atoms, but in some species, carotenoids with only 30 carbon atoms (diapocarotenoids) are detected. Carotenoids are present in the nonphotosynthetic bacteria Streptococcus faecium (12), Staphylococcus aureus (2), and Methylobacterium rhodinum (11) and in the genera of the photosynthetic heliobacteria (10). The carotenoid biosynthesis pathway in S. aureus is reported to be similar to the typical C40 pathway, starting with the condensation of two molecules of the prenyl precursor farnesyl diphosphate and yielding diapophytoene (3). A subsequent desaturation involves a 4,4′-diapophytoene desaturase, with diaponeurosporene as the product. Two different genes, crtM and crtN from S. aureus, have been cloned and identified (13). In this paper, we report the heterologous expression of crtN from S. aureus in Escherichia coli, the purification of the resulting diapophytoene desaturase, and a determination of the enzyme’s biochemical properties.
E. coli JM101 cells grown in Luria-Bertani medium (7) were used as hosts for protein expression and for the synthesis of C30 carotenoid substrates. Plasmid pACCRT-M was constructed by digesting plasmid pUG10 (13) and ligating the resulting crtM gene fragment into the vector pACYC184 (6); it was then used to produce diapophytoene. Carotenoids were extracted and purified as described in reference 5. Plasmid pQECRT-N was constructed by PCR amplification of the crtN gene from the plasmid pUG10 (13) and ligation of the resulting fragment into the expression vector pQE30. With this plasmid, it was possible to express a functional diapophytoene desaturase with an N-terminal extension of six histidine molecules. The resulting protein was purified by immobilized metal affinity chromatography with Talon resin (Clontech), following the manufacturer’s manual. Size exclusion chromatography was performed by using a Biologic fast protein liquid chromatograph (Bio-Rad) with a Biosil SEC 400 column (Bio-Rad) and 200 mM phosphate buffer (pH 6.6) at a flow rate of 0.7 ml min−1. The elution profile was monitored with a UV detector at 280 nm. In vitro assays were performed as described earlier (5) with diapophytoene or other C30 carotenes.
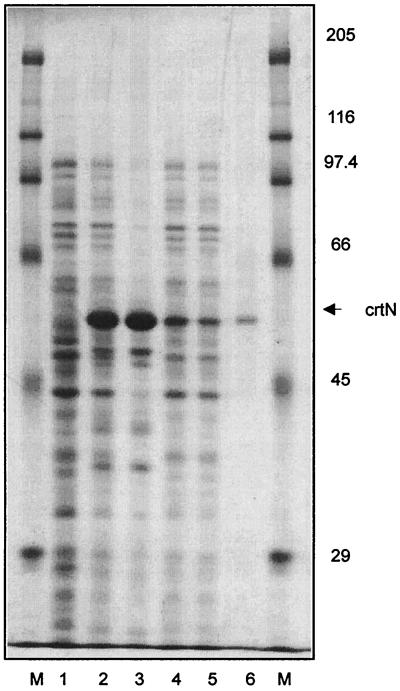
The purification of carotenogenic enzymes is facilitated when substantial protein amounts are generated by overexpression in a heterologous system (9). In addition, the construction of the expression plasmid offers the possibility of extending the reading frame by a short sequence suitable for affinity purification. For the diapophytoene desaturase from S. aureus, this cloning strategy resulted in a polypeptide composed of 525 amino acids. Including the six-histidine motif, an N-terminal extension of the original protein with 13 additional amino acids was obtained. Upon the transformation of E. coli with pQECRT-N, overexpression was observed as a protein band which was absent in a control (Fig. 1, lane 1). The calculated apparent molecular mass was 52 kDa. The solubilization of membrane-bound carotenogenic enzymes with detergents very often results in the loss of enzyme activity. Therefore, we broke the cells under high-pressure conditions. As described in previous publications (5, 9), a soluble fraction which contains a substantial portion of the expressed enzyme is obtained after centrifugation (Fig. 1, lane 1). The soluble supernatant obtained after French press treatment was used directly for immobilized metal affinity chromatography. After washing steps, a homogenous protein was eluted from the column with an imidazole concentration of 100 mM (Fig. 1, lane 6). Table 1 quantitates the purification of diapophytoene desaturase. The expression of pQECRT-N yielded 160 mg of diapophytoene desaturase per liter of cell suspension, which was 28.8% of the total protein content. More than half of the enzyme was found in a soluble form in the supernatant.
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel that shows the expression and purification of the recombinant diapophytoene desaturase from S. aureus. Lanes: M, molecular marker; 1, whole cells of transformants with pQE30; 2, whole cells of transformants with pQECRT-N; 3, membranes; 4, supernatant; 5, flowthrough Talon affinity resin; 6, fraction eluted with 100 mM imidazole.
TABLE 1.
Purification of the diapophytoene desaturase from S. aureus
| Step | Vol (ml) | Amt (mg) of:
|
Relative CrtN content (%) | Sp act (ng/mg · h) | Recovery (%) | |
|---|---|---|---|---|---|---|
| Protein | CrtN protein | |||||
| Cells | 5 | 28 | 8 | 28.8 | 100 | |
| Membranes | 5 | 11 | 5.6 | 51 | 70 | |
| Supernatant | 5 | 16 | 2.56 | 18.8 | 33.2 | 32 |
| Flowthrough | 5 | 14 | 1.86 | 13.3 | 23.3 | |
| Purified CrtN | 3.5 | 0.056 | 0.054 | 97.2 | 300 | 0.7 |
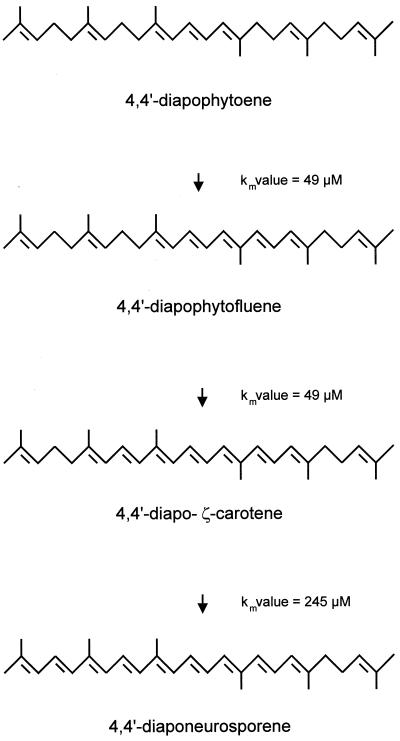
Figure 2 shows the reaction sequence which is catalyzed by the crtN gene product. Three double bonds are introduced into diapophytoene, with diapophytofluene and diapo-ζ-carotene as intermediates and diaponeurosporene as the end product. All these carotenoids were converted by diapophytoene desaturase, and their Km values were determined. Km values were 49 μM for diapophytoene, 10 μM for diapophytofluene, and 245 μM for diapo-ζ-carotene. The entire desaturation sequence is dependent on flavine adenine dinucleotide as a cofactor and is inhibited by diphenylamine (data not shown).
FIG. 2.
Desaturation pathway and substrates for the in vitro formation of diaponeurosporene by diapophytoene desaturase.
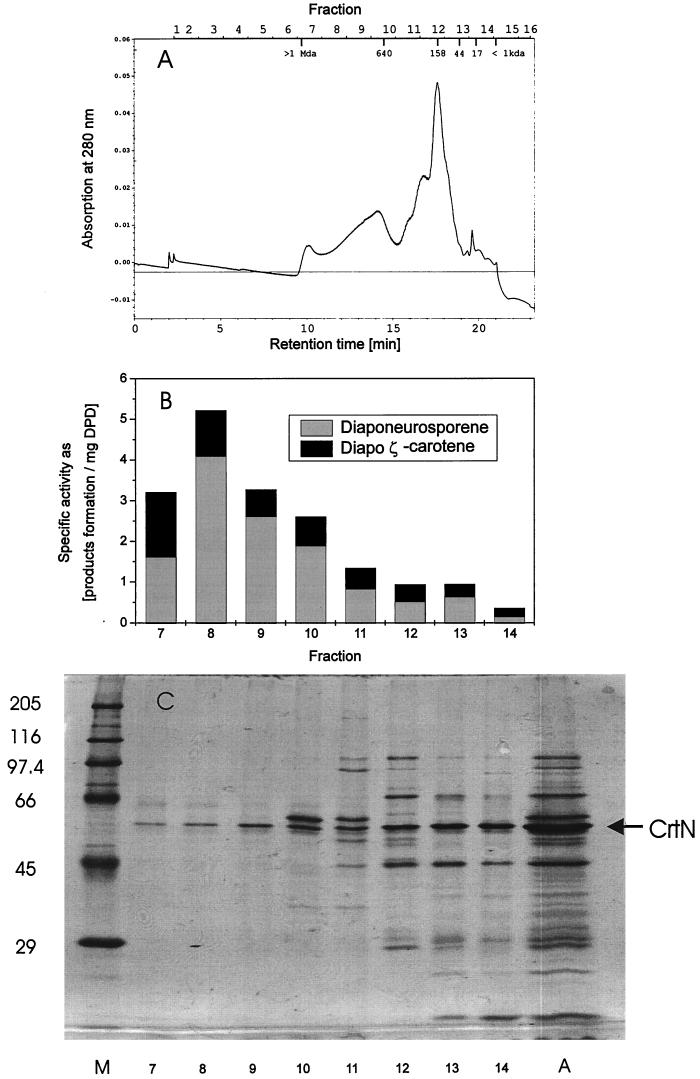
A possible dimerization of bacterial (1) and plant-type (4) carotenoid desaturases is under discussion. Therefore, a partially purified diapophytoene desaturase was fractionated by size exclusion chromatography. Figure 3A shows the corresponding elution profile of the proteins from a silica-based size exclusion column. In each fraction, the specific diapophytoene desaturase activity was determined, and the amounts of synthesized diapo-ζ-carotene and end-product diapophytoene are indicated (Fig. 3B). Substantial activity was found in fractions 7 to 13, which ranged in size from 40 to 1,000 kDa, but was highest in fraction 8, which comprised proteins and protein aggregates of 800 kDa. Regardless of the aggregation size of diapophytoene desaturase, the ratio of diaponeurosporene to diapo-ζ-carotene is more or less the same. A sodium dodecyl sulfate-polyacrylamide gel which separated the proteins under denaturing conditions shows that fractions 7 to 9 had the highest specific activity and that the diapophytoene desaturase monomeric band, at 52 kDa, is the dominating protein (Fig. 3C).
FIG. 3.
Size exclusion chromatography of an enriched diapophytoene desaturase fraction. (A) Elution profile at 280 nm. (B) Corresponding specific activities (milligrams of product formation/milligrams of diapophytoene desaturase) of the fractions. (C) Protein separation on a denaturating polyacrylamide gel of 500 μl of each fraction after concentration. Lane M, marker proteins; lane A, aliquot of 5 μl of initial partially purified protein preparation prior to size exclusion chromatography.
Purified diapophytoene desaturase carries out a three-step desaturation, with diapophytofluene and diapo-ζ-carotene as the intermediates. The reaction sequence is shown in Fig. 2. When the number of conjugated carbon double bonds, which are thought to be part of the substrate recognition structures for carotene desaturases (8), increases from three in diapophytoene to five in diapophytofluene, the Km value is not affected. Therefore, an efficient conversion to diapo-ζ-carotene is ensured. However, the Km value for the final step is much higher than those for the previous ones. Nevertheless, the carotenoid pathway in S. aureus (2) and the in vitro reaction are not substantially restricted at the level of diapo-ζ-carotene conversion. This may be explained by the oligomerization of diapophytoene desaturase, which is important for the optimum activity of this enzyme. It can be assumed that diapophytoene desaturase forms a complex in the membrane that keeps the substrate carotenoids bound until all three double bonds are introduced in the desaturation process. In this case, only small amounts of intermediates accumulate in vitro. Under these conditions, diapo-ζ-carotene, with seven conjugated double bonds that lower the affinity to the enzyme, is efficiently converted to the end product, diaponeurosporene. As hardly any other proteins were present in the fractions with the highest specific activity (Fig. 3C), the aggregation of diapophytoene desaturase with itself must be assumed. However, the possibility that lipids are part of this complex cannot be excluded.
Acknowledgments
We thank F. Götz, Universität Tübingen, for providing us with the crtN gene.
REFERENCES
- 1.Bartley G E, Schmidhauser T J, Yanofsky C, Scolnik P A. Carotenoid desaturases from Rhodobacter capsulatus and Neurospora crassa are structurally and functionally conserved and contain domains homologous to flavoprotein disulfide oxidoreductases. J Biol Chem. 1990;265:16020–16024. [PubMed] [Google Scholar]
- 2.Marshall J H, Wilmoth G J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol. 1981;147:900–913. doi: 10.1128/jb.147.3.900-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall J H, Wilmoth G J. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: evidence from a study of mutants. J Bacteriol. 1981;147:914–919. doi: 10.1128/jb.147.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pecker I, Chamovitz D, Linden H, Sandmann G, Hirschberg J. A single polypeptide catalysing the conversion of phytoene to ζ-carotene is transcriptionally regulated during tomato fruit ripening. Proc Natl Acad Sci USA. 1992;89:4962–4966. doi: 10.1073/pnas.89.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raisig A, Bartley G, Scolnik P, Sandmann G. Purification in an active state and properties of the 3-step phytoene desaturase from Rhodobacter capsulatus overexpressed in Escherichia coli. J Biochem. 1996;119:559–564. doi: 10.1093/oxfordjournals.jbchem.a021278. [DOI] [PubMed] [Google Scholar]
- 6.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 8.Sandmann G. Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem. 1994;223:7–24. doi: 10.1111/j.1432-1033.1994.tb18961.x. [DOI] [PubMed] [Google Scholar]
- 9.Sandmann G. High level expression of carotenogenic genes for enzyme purification and biochemical characterization. Pure Appl Chem. 1997;69:2163–2168. [Google Scholar]
- 10.Takaichi S, Inoue K, Akaike M, Kobayashi M, Oh-oka H, Madigan M T. The major carotenoid in all known species of heliobacteria is the C30 carotenoid 4,4′-diaponeurosporene, not neurosporene. Arch Microbiol. 1997;168:277–281. doi: 10.1007/s002030050499. [DOI] [PubMed] [Google Scholar]
- 11.Taylor R F. Bacterial triterpenoids. Microbiol Rev. 1984;48:181–198. doi: 10.1128/mr.48.3.181-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor R F, Davies B H. Triterpenoid carotenoids and related lipids. The triterpenoid carotenes of Streptococcus faecium UNH 564P. Biochem J. 1974;139:751–760. doi: 10.1042/bj1390751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieland B, Feil C, Gloria-Maercker E, Thumm G, Lechner M, Bravo J-M, Poralla K, Götz F. Genetic and biochemical analyses of the biosynthesis of the yellow carotenoid 4,4′-diaponeurosporene of Staphylococcus aureus. J Bacteriol. 1994;176:7719–7726. doi: 10.1128/jb.176.24.7719-7726.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]