Abstract
Background
Limited recent data exists regarding discospondylitis in dogs.
Hypothesis/Objectives
(i) Describe the signalment, clinical and imaging findings, etiologic agents, treatment, and outcome of dogs with discospondylitis, (ii) determine diagnostic agreement between radiographs, CT, and MRI with regard to the presence of discospondylitis and its location, and (iii) determine risk factors for relapse and progressive neurological deterioration.
Animals
Three hundred eighty‐six dogs.
Methods
Multi‐institutional retrospective study. Data extracted from medical records were: signalment, clinical and examination findings, diagnostic results, treatments, complications, and outcome. Potential risk factors were recorded. Breed distribution was compared to a control group. Agreement between imaging modalities was assessed via Cohen's kappa statistic. Other analyses were performed on categorical data, using cross tabulations with chi‐squared and Fisher's exact tests.
Results
Male dogs were overrepresented (236/386 dogs). L7‐S1 (97/386 dogs) was the most common site. Staphylococcus species (23/38 positive blood cultures) were prevalent. There was a fair agreement (κ = 0.22) between radiographs and CT, but a poor agreement (κ = 0.05) between radiographs and MRI with regard to evidence of discospondylitis. There was good agreement between imaging modalities regarding location of disease. Trauma was associated with an increased risk of relapse (P = .01, OR: 9.0, 95% CI: 2.2‐37.0). Prior steroid therapy was associated with an increased risk of progressive neurological dysfunction (P = .04, OR: 4.7, 95% CI: 1.2‐18.6).
Conclusions and Clinical Importance
Radiograph and MRI results could be discrepant in dogs with discospondylitis. Prior trauma and corticosteroids could be associated with relapse and progressive neurological dysfunction, respectively.
Keywords: computed tomography, disc, infection, radiology, vertebral
Abbreviations
- AKC
American Kennel Club
- CSF
cerebrospinal fluid
- CT
computed tomography
- MRI
magnetic resonance imaging
1. INTRODUCTION
Discospondylitis is an infection of the intervertebral disc and adjacent vertebral body endplates. 1 The most common route of infection is hematogenous spread from the site of primary infection via lymphatic drainage and subsequent arterial spread. 2 Other proposed routes of infection include trauma, migrating foreign bodies, and iatrogenic inoculation. Management of discospondylitis is guided by a limited number of research studies that typically involve either a small sample size or a single geographic region. 3 , 4 , 5 , 6 Many of these studies are over a decade old and predate the routine addition of MRI and CT to radiographs in the diagnostic approach to suspected discospondylitis. The prognosis for discospondylitis is guarded to good, and therapeutic recommendations are largely anecdotal. 3
The objectives of our study were to document the clinical features of discospondylitis in a group of dogs across a wide geographic region, with additional focuses on (i) the level of agreement between various imaging modalities in identifying lesions consistent with discospondylitis, and (ii) risk factors for disease relapse or progressive neurological dysfunction.
2. MATERIALS AND METHODS
2.1. Case identification
Cases were identified by searching the medical record systems at 8 institutions for the following terms: “discospondylitis,” “diskospondylitis,” and “vertebral end plate infection” from 01‐Jan‐2010 to 31‐Dec‐2022. Dogs were included if they had a diagnosis of discospondylitis and there was radiographic, computed tomography (CT), or magnetic resonance imaging (MRI) evidence of discospondylitis. Characteristic radiographic features of discospondylitis included irregular endplate lysis with extension into the vertebral body, collapse of the intervertebral disc space, and sclerosis peripheral to the endplate lysis. 7 Characteristic CT features of discospondylitis included endplate erosion, osteolysis, and periosteal proliferation adjacent to the intervertebral disc spaces. 8 Characteristic MRI features of discospondylitis were STIR hyperintensity of the intervertebral discs and endplates, or endplates, contrast enhancement of the intervertebral discs, and increased T2 and decreased T1 signal intensity of soft tissues ventral to vertebral bodies. 4 , 9 , 10 All cases were diagnosed and managed by a board‐certified veterinary neurologist or an intern/resident under the guidance of a veterinary neurologist.
2.2. Data collection
Medical records were analyzed, data extracted for each dog were: signalment, breed, clinical signs, neurological examination findings, clinicopathologic test results, diagnostic imaging results, treatments, complications, and outcome. Medical records were also searched for the potential risk factors (based on a small group consensus) of: trauma (eg, vehicular trauma and bite wounds), neoplasia, dental disease, preexisting steroid therapy, and before spinal surgical procedures. Other risk factors were also recorded when specifically stated in each medical record. The affected intervertebral disc site(s) were recorded based on each imaging modality.
2.3. Diagnostic imaging
Data on radiographic, CT, and MRI features of discospondylitis were recorded. Where multiple imaging modalities were used concurrently, the radiology reports of each of the imaging modality were recorded separately and subsequently compared.
2.4. Treatment and outcome
Treatment protocols were recorded, including antibiotic dosage and duration of treatment. If surgery was performed, the procedure was also recorded. Assessment of dog outcome was obtained by review of clinical history, neurological examination, and imaging findings. Clinical and imaging findings were evaluated separately. Euthanasia and death were recorded. A relapse was defined as recurrence of clinical signs after a normal clinical and neurological examination, without an other identified cause of clinical signs.
2.5. Statistical analysis
Signalment, clinical signs, neuroanatomical localization, diagnostic imaging, treatment, and outcome were summarized in a descriptive manner. All statistical analyses were conducted using commercial software (IBM SPSS Statistics for Windows, Version 28.0.1.1, Armonk, New York). To determine either over‐ or underrepresentation of a particular breed, the study group was compared to AKC registration data from 2009, which is the most recent publicly available data. 11 Standardized residuals from a crosstabulation of breed and data source (AKC vs study group) were performed. Where a breed was not represented in both the AKC and study groups, comparative analyses were not reported. Mixed breed dogs were excluded from this specific analysis. Agreement between imaging modalities was assessed using Cohen's kappa statistic. For Cohen's kappa statistic, a κ of 0.01 to 0.20 was considered none to slight agreement, a κ of 0.21 to 0.40 was considered fair agreement, a κ of 0.41 to 0.60 was considered moderate agreement, a κ of 0.61 to 0.80 was considered substantial agreement, and a κ > 0.80 was considered almost perfect agreement. 12 All other analyses were performed on categorical data, using crosstabulations with chi‐squared and Fisher's exact tests. Missing data was excluded from analysis. For all tests P ≤ .05 was considered significant.
3. RESULTS
3.1. Animals
A total of 386 animals were included in analysis. The median age of enrolled dogs was 7 years (range, 2 months to 15 years). Thirty‐nine dogs were aged <1 year of age, 30 dogs were ≥1 year of age but <2 years of age, 33 dogs were ≥2 years but <4 years of age, 76 dogs were ≥4 years but <7 years of age, 94 dogs were ≥7 years but <10 years of age, and 114 dogs were ≥10 years of age. One hundred fifty were female (120 spayed and 30 intact females) and 236 were male (135 castrated and 101 intact males). The most common dog breed was mixed breed (70), followed by Labrador Retriever (50), German Shepherd Dog (34), Boxer (21), French Bulldog (14), Doberman Pinscher (13), English Bulldog (9), Great Dane (9), Golden Retriever (8), Rottweiler (7), Weimaraner (7), English Springer Spaniel (6), Staffordshire Bull Terrier (6), Welsh Corgi (5), Maltese (5), Mastiff (5), Miniature Pinscher (5), Dachshund (4), Miniature Schnauzer (4), Pit Bull (4), Shih Tzu (4), Toy Poodle (4), Vizsla (4), Yorkshire Terrier (4), Border Collie (3), Bulldog (3), Cocker Spaniel (3), English Setter (3), Greyhound (3), Jack Rusell Terrier (3), Siberian Husky (3), Newfoundland (3), Airedale Terrier (2), American Bulldog (2), Beagle (2), Boston Terrier (2), Bullmastiff (2), Cane Corso (2), Dalmatian (2), Irish Wolfhound (2), Pomeranian (2), Pug (2), Shetland Sheepdog (2), and 1 each of: Akita, Anatolian Shepherd, Australian Cattle Dog, Australian Shepherd, Bassett Hound, Belgian Malinois, Belgian Tervuren, Bernese Mountain Dog, Briard, Brussels Griffon, Bull Terrier, Catahoula, Chihuahua, Coonhound, Dandie Dinmont Terrier, Dogue De Bordeaux, English Mastiff, German Shorthair Pointer, Giant Schnauzer, Great Pyrenees, Hovawart, Italian Bracco, Lurcher, Miniature Schnauzer, Norwich Terrier, Old English Sheepdog, Patterdale Terrier, Pekingese, Pointer, Polish Lowland Sheepdog, Poodle, Presa Canario, Rhodesian Ridgeback, Standard Poodle, Standard Schnauzer, Toy Fox Terrier, Treeing Walker Coonhound, Wheaten Terrier. Weight was not recorded.
Breeds significantly overrepresented in our study group, with ratios of breed as a proportion of the study group compared to the proportion of the AKC group occupied by the same breed, indicated by the term “ratio” were: Greyhound (ratio: 42.4, P < .001), Polish Lowland Sheepdog (ratio: 20.5, P < .001), Dandie Dinmont Terrier (ratio: 19.6, P < .001), Staffordshire Bull Terrier (ratio: 10.7, P < .001), English Setter (ratio: 42.4, P < .001), Briard (ratio: 7.5, P = .02), Irish Wolfhound (ratio: 4.2, P = .03), French Bulldog (ratio: 3.2, P < .001), Weimaraner (ratio: 2.5, P = .01), Doberman Pinscher (ratio: 2.1, P = .005), German Shepherd Dog (ratio: 1.4, P = .05), and Australian Cattle Dog (ratio: 1.3, P < .001). Breeds significantly underrepresented in our study group were Golden Retriever (ratio: 0.4, P = .02), Dachshund (ratio: 0.3, P = .02), Yorkshire Terrier (ratio: 0.18, P = <.001), German Shorthair Pointer (ratio: 0.2, P = .04), American Bulldog (ratio: 0.1, P = .001), Chihuahua (ratio: 0.1, P = .01), Beagle (ratio: 0.1, P < .001), and Standard Poodle (ratio: 0.1, P = .003).
3.2. Clinical signs and neuroanatomical localization
The duration of clinical signs before presentation could not be definitively determined in all records. Of the available data, 20 of 134 dogs (15%) had clinical signs reported for <24 hours duration, 23 of 134 dogs (17%) had clinical signs reported of 24 to 72 hours duration, and 91 of 134 dogs (68%) had a >72‐hour duration of clinical signs before presentation.
At the time of evaluation, 245 of 386 dogs (63.5%) had overt signs of pain reported in their history or physical exam, 55 of 386 (14.2%) had a decreased appetite, and 55 of 386 dogs (14.2%) were lethargic. One hundred eighteen of the 386 dogs (30.6%) were reported to have paresis. Sixty of 118 dogs (50.8%) were ambulatory paraparetic, while 30 of 118 dogs (25.4%) were nonambulatory paraparetic. Six of 118 dogs (5.1%) were ambulatory tetraparetic, and 8 of 118 dogs (6.8%) were nonambulatory tetraparetic. The remaining paretic dogs (14/118) could not be further characterized based on the available medical records. Thirty‐one of 386 dogs (8.0%) were reported to have a temperature above 103.5 °F (39.7°C). Thirteen of 233 dogs (5.6%) had a C1‐C5 neuroanatomiclocalization, 6 of 233 dogs (2.6%) had a C6‐T2 neuroanatomiclocalization, 93 of 233 dogs (39.9%) had a T3‐L3 neuroanatomiclocalization, 68 of 233 dogs (29.2%) had a L4‐S3 neuroanatomiclocalization, and 51 of 233 dogs (21.9%) were determined to be multifocal. Neuroanatomiclocalization was not specifically recorded in the remaining cases.
3.3. Diagnostic imaging
Information on affected sites were available for 362 of 386 dogs (93.8%). The most commonly affected intervertebral disc site was L7‐S1 (97/362 dogs, 26.8%). Other commonly affected sites included L1‐L2 (17/362 dogs, 4.7%), L2‐L3 (11/362 dogs, 3.0%), T13‐L1 (10/362 dogs, 2.8%), C6‐C7 (9/362 dogs, 2.5%), T9‐T10 (8/362 dogs, 2.2%), T12‐T13 (7/362 dogs, 1.9%), T4‐T5 (6/362 dogs, 1.7%), T10‐T11 (6/362 dogs, 1.7%), L3‐L4 (6/362 dogs, 1.7%), T3‐T4 (5/362 dogs, 1.4%), T5‐T6 (5/362 dogs, 1.4%), L6‐L7 (5/362 dogs, 1.4%). Less than 5 dogs were affected at each of the following sites: C2‐C3, C3‐C4, C4‐C5, C5‐C6, C7‐T1, T1‐T2, T2‐T3, T6‐T7, T7‐T8, T8‐T9, T11‐T12, L4‐L5, and L5‐L6. Multifocal lesions were prevalent and reported in 134 of 362 dogs (37.0%).
One hundred sixty‐five dogs had ≥1 imaging modality performed at the time of initial diagnosis. Diagnostic imaging findings agreed regarding affected site(s) in 99 of 165 cases (60.0%). Two dogs with equivocal radiographic findings had definitive evidence of disease on MRI. One dog had an L7‐S1 lesion suspected on radiographs that was not reported on MRI, and 1 dog had multifocal lesions detected on CT but not on MRI. Seven of 165 dogs (4.2%) had additional lesions detected on advanced imaging (CT and MR, or MRI) when compared to radiographs alone, and 55 of 165 dogs (33.3%) had no evidence of discospondylitis on radiographs but had evidence of disease on CT (18/55 dogs, 33%), MRI (35/55 dogs, 63%), or both (2/55 dogs, 4%). Four of these 165 dogs (2.4%) had no radiographic or CT abnormalities but had lesions on MRI alone. Cases representing discrepancies in imaging findings between radiographs and MRI are shown in Figures 1, 2, 3.
FIGURE 1.
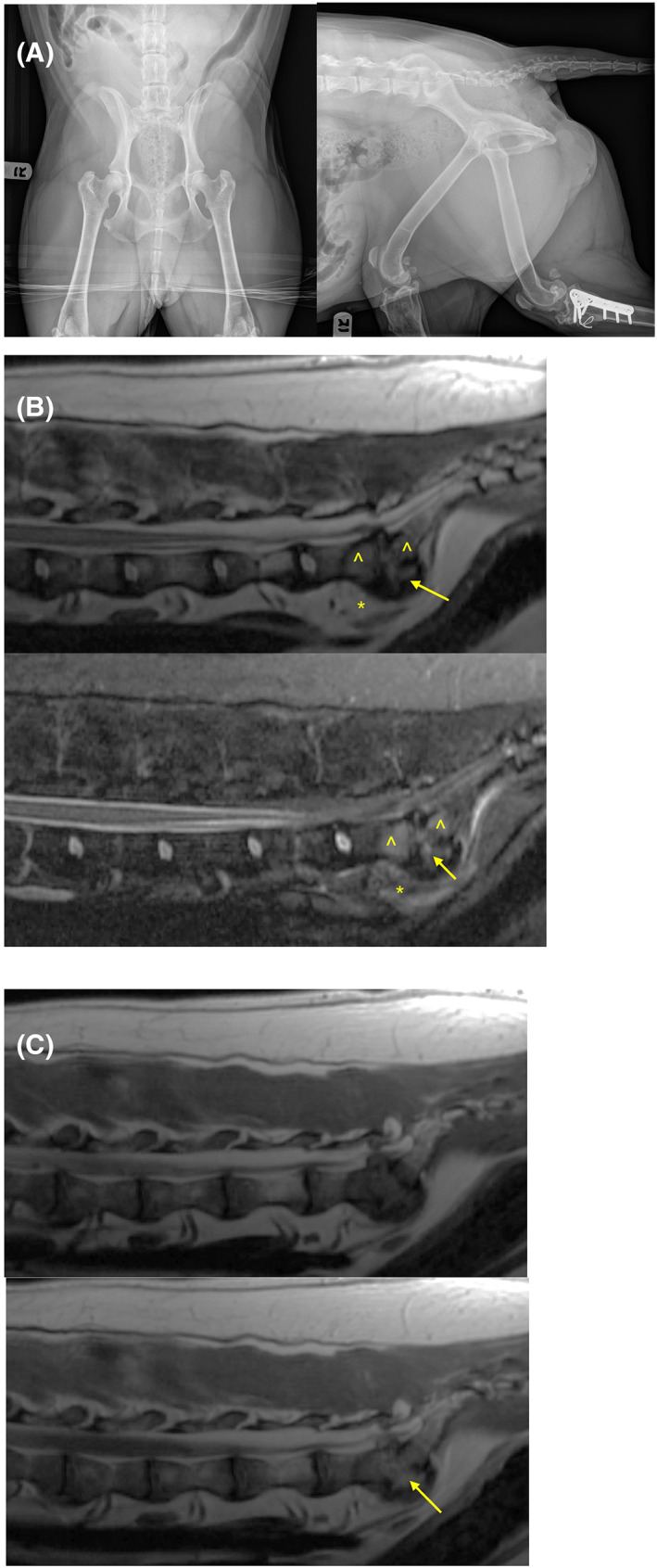
(A‐C) Representative images of a 7‐year‐old female spayed Staffordshire bull terrier with discospondylitis. Advanced imaging such as MRI might detect lesions that are not evident on standard radiographs. (A) Ventrodorsal and right lateral radiographs revealed no evidence of lytic lesions of the lumbosacral disc space. (B) Midline sagittal T2‐weighted (top) and short tau inversion recovery (STIR) (bottom) MRI images of the lumbosacral spine revealed hyperintensity within the L7‐S1 intervertebral disc (arrow), adjacent vertebral endplates (^), and adjacent soft tissues (*). (C) Midline sagittal T1‐weighted (top) and T1‐weight postcontrast (bottom) MRI images of the lumbosacral spine revealed moderate contrast enhancement (arrow) of the L7‐S1 intervertebral disc, adjacent vertebral endplates which were irregular, and adjacent soft tissues.
FIGURE 2.
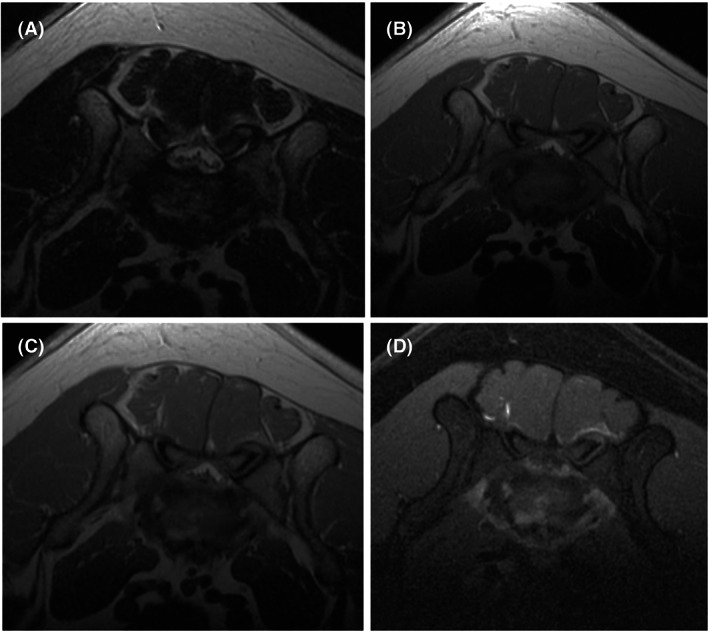
(A‐D) Representative axial MRI images of the 7‐year‐old female spayed Staffordshire bull terrier with discospondylitis from Figure 1. T2‐weighted (A), T1‐weighted (B), T1‐weighted postcontrast (C), and T1‐weight postcontrast fat saturated (D) images are at the level of the L7‐S1 intervertebral disc revealed moderate T2 hyperintensity and contrast enhancement of the irregular vertebral endplates adjacent to the L7‐S1 intervertebral disc. Fat saturated postcontrast images (D) help to accentuate contrast enhancement.
FIGURE 3.
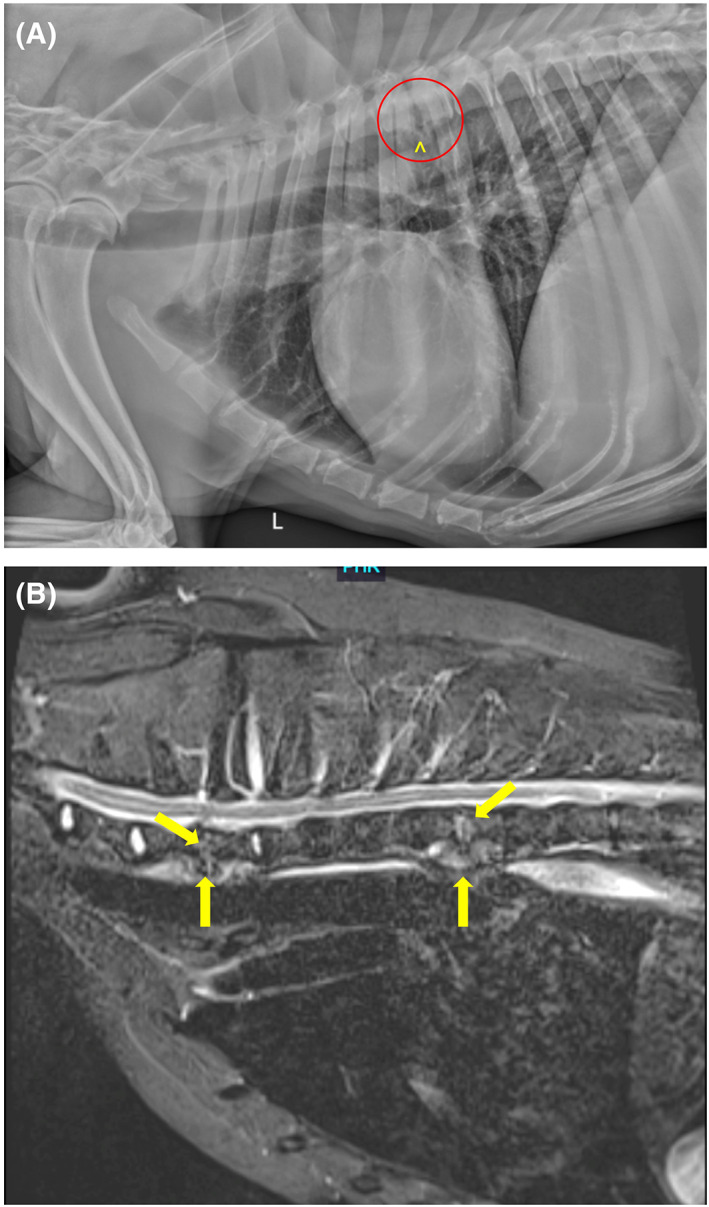
(A and B) Representative images of a 9‐year‐old male neutered Labrador Retriever with discospondylitis. Advanced imaging such as MRI may detect more lesions than are evident on routine radiographs. (A) A left lateral radiographic projection reveals lysis of the caudal endplate of T5 and the cranial endplate of T6 (within red circle), along with spondylosis deformans at T5‐T6 (^). (B) A midline STIR image of the cranial thoracic spine reveals irregularity and hyperintensity of the C7‐T1 and T5‐T6 intervertebral discs and adjacent cranial and caudal vertebral endplates (arrows).
Cohen's weighted kappa statistic revealed fair agreement (κ = 0.22) between the presence or absence of lesions on radiographs and CT, but only none to slight agreement between radiographs and MRI (κ = 0.05), as well as between CT and MRI (κ = 0.06). Agreement on anatomic location of disease was much greater than agreement on presence or absence of disease. For location of disease, a moderate agreement was noted between radiographs and CT (κ = 0.51), and between radiographs and MRI (κ = 0.53). Substantial agreement on location of disease was also noted between CT and MRI (κ = 0.72).
Of the 55 dogs without radiographic lesions but with lesions identified on CT or MRI, repeat imaging during dog follow‐up was available for 20 dogs. Despite lack of lesions of the first examination, 11 of 20 dogs (55%) had follow‐up radiographs performed as the primary therapeutic monitoring tool. Of these dogs, 5/11 (46%) had radiographic lesions which subsequently became inactive after a median of 172 days (range, 115‐300 days). Seven of 20 (35%) dogs had CT utilized for disease monitoring, and where data was available, lesions became inactive after a median of 120 days (range, 90‐150 days). Two of 20 dogs (10%) had MRI utilized for disease monitoring, and lesions became inactive after 75 and 168 days, respectively.
3.4. Cerebrospinal fluid analysis and culture
Thirty‐four dogs had a cerebrospinal fluid (CSF) sample collected. Intracellular bacteria were detected in 2 of 34 samples (6%). A mononuclear pleocytosis was seen in 7 of 34 dogs (21%), a neutrophilic pleocytosis was seen in 3 of 34 dogs (9%), 2 of 34 dogs (6%) had a mixed inflammatory pleocytosis, and a lymphocytic pleocytosis was seen in 2 of 34 dogs (6%). No abnormalities were seen in 12 of 34 samples (35%), and findings attributed to blood contamination were seen in an additional 6 of 34 (18%) CSF samples. Cytology of 1 of the 34 CSF samples (3%) was reportedly consistent with osteoblastic proliferation and osteolysis. Twenty dogs had CSF cultures performed, of which 4 (20%) were positive for bacterial growth. Staphylococcus spp. were cultured in 2 dogs (2/4 positive cultures, 50%), while Enterobacter cloacae and Pasturella spp. were cultured in 1 CSF sample each (1/4 positive cultures each, 25%). There was no significant difference in CSF culture positivity between institutions (P = .11).
3.5. Additional infectious disease screening
One hundred thirty‐six dogs (136/386 dogs) were screened for brucellosis via a rapid slide agglutination (RSAT) or modified RSAT with 2‐mercaptoethanol (2ME‐RSAT), of which 9/136 dogs (6.6%) had positive test results. This included intact male dogs (4/9 positive results, 44%), neutered male dogs (4/9 positive results, 44%), and a spayed female dog (1/9 positive results, 11%). There was no significant difference in brucella positivity results between institutions (P = .62). Blood cultures were performed in 143 dogs, of which 38 (27%) were positive. The cultured organism was not recorded for 1 dog. Staphylococcus spp. (23/38, 61%) were most commonly identified, while Streptococcus spp. (4/38, 11%), and Pasteurella species (3/38, 8%) were identified less frequently. One each (3%) of the following organisms were also cultured: Brucella canis, Eggerthella lentum, Enterococcus spp., Erysipelothrix rhusiopathiae, Escherichia coli, Pseudomonas spp., and Psychrobacter phenylpyruvicus. Urine cultures were performed in 277 dogs, of which 77 (27.8%) were positive. Eleven of 77 (14%) urine cultures grew multiple infectious agents. Staphylococcus spp. (30/77, 39%) were most commonly identified, while Escherichia coli (22/77, 29%), Streptococcus spp. (10/77, 13%), Enterococcus spp. (8/77, 10%), and Pseudomonas aeruginosa (3/77, 4%) were identified less frequently. One each (1%) of: Aerococcus viridans, Aspergillus spp., Brucella canis, Pasturella spp., and an unknown mold were also cultured. There was no significant difference in urine culture positivity between institutions (P = .2). Fungal cultures were performed in 38 of 386 dogs (9.8%), of which 8 (21.0%) were positive. Aspergillus spp. were cultured in 6 dogs (75% of positive cultures). One of the 8 positive cases (13%) grew Candida parapsillosis. One case (13%) had a positive fungal culture, but the causative agent was not reported. Aspergillus urine antigen tests were positive in 1 further dog. Five of 7 dogs with Aspergillosis were German Shepherd Dogs. One dog's culture yielded Candida parapsilosis while another dog had an unknown fungus cultured. There was no significant difference in fungal culture positivity between institutions (P = .6). Intervertebral disc cultures were performed in 63 of 386 dogs (16.3%), of which 26 (41%) were positive. Eighteen of 26 (69%) were positive for Staphylococcus spp. Two of 26 (8%) grew Pseudomonas aeruginosa, and 2/26 (8%) grew Escherichia coli. One each (4%) of the following were also cultured from intervertebral disc cultures: Brucella canis, Cutibacterium acnes, Erysipelothrix rhusiopathiae, Streptococcus canis. There was no significant difference in disc culture positivity between institutions (P = .08).
3.6. Identified risk factors
Trauma was recorded in the history of 15/386 dogs (3.9%), while neoplasia and dental disease were recorded as potential risk factors in 11 of 386 dogs (2.9%), and 19 (4.9%) dogs, respectively. Twenty‐five of 386 (10.4%) dogs had a history of spinal surgery before diagnosis of discospondylitis, and 25 of 386 (6.5%) dogs had a history of steroid therapy.
3.7. Treatment
Two hundred eighty‐four dogs (73.6%) were treated with medical management, while 40 of 386 dogs (10.4%) also underwent surgical procedures. Eighteen of 386 dogs (4.7%) were euthanized at the time of diagnosis. The remaining dogs had no data on treatment in the available medical records. Four of 40 dogs (10%) undergoing surgery had a sublumbar abscess drained, 2 of 40 dogs (5%) underwent stabilization of a vertebral subluxation, and the remainder of the dogs underwent a variety of laminectomy and discectomy procedures, or discectomy procedures. Two of 40 dogs (5%) that underwent surgery were subsequently euthanized. Dogs that had preexisting dental disease were more likely to have a surgical intervention than those without preexisting dental disease (P = .05). No other risk factors were associated with surgical intervention including steroid therapy (P = .75), before spinal surgeries (P = .18), trauma (P = .23), and cancer (P = .11). Antibiotics administered were: cephalexin (157/285 dogs, 55.1%), amoxicillin clavulanic acid or ampicillin sulbactam (103/285 dogs, 36.1%), enrofloxacin (56/285 dogs, 18.6%), clindamycin (22/285 dogs, 7.7%), cefpodoxime (19/285 dogs, 6.7%), sulfadimethoxine/trimethoprim or sulfadimethoxine/ormetoprim (11/285 dogs, 3.9%), doxycycline (10/285 dogs, 3.5%), and metronidazole (7/285 dogs, 2.5%). Less than 5 of 285 (<1.8%) dogs each received: amoxicillin, cefazolin, chloramphenicol, ciprofloxacin, levofloxacin, marbofloxacin, meropenem, minocycline, nitrofurantoin, and rifampin. Antifungal agents administered were: itraconazole (5/285 dogs, 1.8%), terbinafine (3/285 dogs, 1.1%), ketoconazole (2/285 dogs, 0.7%), and fluconazole (1/285 dogs, 0.35%). Antimicrobial treatment duration ranged from 1 to 84 weeks. The median duration of therapy was 16 weeks.
3.8. Complications and outcome
Eighteen dogs were euthanized at time of diagnosis. Fifteen of the 386 (3.9%) dogs had concurrent empyema. Follow‐up status was reported in 101/386 cases (26.2%). During known follow‐up, 12 of 101 dogs (11.9%) had a clinical relapse while an additional 12 dogs (11.9%) developed progressive neurological deterioration. Seven of these 12 dogs (58%) were treated with empiric antibiotics, while the rest were treated based upon results of blood (1 dog), urine (2 dogs), disc (1 dog) or fungal (1 dog) culture. Three of 12 dogs that relapsed (25%) were German Shepherds. One of these German Shepherds (1/3, 33%) had fungal disease. Nine of the 12 relapsed dogs (75%) had multifocal lesions, while 2/12 dogs (17%) had disease at L7‐S1, and 1/12 dogs (8%) had disease at T13‐L1. Four of the 12 relapsed dogs (33%) were treated with amoxicillin clavulanic acid, 3/12 dogs (25%) were treated with cephalexin, and the remaining 5 dogs each received different antimicrobial agent(s). A clear timeline to relapse was reported in 10 of 12 dogs (83%) and ranged from 1 week to 60 months. The median time to relapse was 12 months. Potential reasons for disease relapse were reported in 6 of 12 dogs. In 4/6 cases (67%) relapse occurred following antimicrobial discontinuation, in 1/6 dogs (17%) relapse occurred after the dog went into estrus, and 1/6 dogs (17%) relapsed after inconsistent medication administration. Dogs with a history of trauma were more likely to relapse than those without prior trauma (P = .01, OR: 9.0, 95% CI: 2.2‐37.0). Dogs that had preexisting steroid therapy were more likely to develop progressive neurologic dysfunction than those dogs that did not have preexisting steroid therapy (P = .04, OR: 4.7, 95% CI: 1.2‐18.6). No other risk factors were associated with development of a complication. Insufficient data was available to compare duration of antibiotic treatment to risk of relapse.
4. DISCUSSION
In this study, we report the clinical features, comparative imaging findings, treatment, and outcome of a large cohort of dogs diagnosed with discospondylitis across a large geographic region. Similar to previous studies, male dogs were overrepresented (ratio: 2 : 1), with a 1.6 : 1 ratio of males to females in our study. 3 , 13 The median age of affected dogs in our study was 7 years of age. Dogs >10 years of age were the most commonly affected age group, as seen in an earlier study. 3 , 14 This could be due to the increased frequency of bacteruria and other abnormalities in older dogs, which could predispose to discospondylitis. 15 This contrasts with the results of 2 studies with smaller sample sizes. 6 , 13 German Shepherd dogs and Doberman Pinschers were overreported in our study, as with a previous large‐scale study. 3 German Shepherd dogs were common in those with fungal etiologies. Other predisposed breeds in our study included Greyhound, Polish Lowland Sheepdog, Dinmont Terrier, Staffordshire Bull Terrier, English Setter, Briard, Irish Wolfhound, French Bulldog, Weimaraner, and Australian Cattle Dog. To our knowledge these are newly reported breed predispositions. Most underrepresented breeds were small breeds, with the exception of Golden Retrievers, German Shorthair Pointers, and Standard Poodles.
Most dogs in the study presented with nonspecific clinical signs such as pain, decreased appetite, and lethargy, as seen in earlier studies. 3 Approximately 8% of dogs in our study were febrile, thus a lack of a fever should not rule out an infectious etiology. It is possible that this low frequency was due to prereferral treatments such as antibiotics and nonsteroidal anti‐inflammatory drugs. Most dogs had a T3‐L3 neurolocalization, fewer had an L4‐S3 neurolocalization, and even fewer had a multifocal neurolocalization. The most common neurolocalization site differed from the most common imaging confirmed sites of lesions. This could be due to differences in the onset of clinical signs vs development of imaging findings, or alternatively the possibility that not all lesions were associated with clinical signs.
Staphylococcus species were the most commonly isolated bacterial species on blood culture as with earlier studies (46%‐59%). 3 , 13 Blood culture positivity was slightly lower than previously reported. 3 Other common bacteria included Streptococcus and Pasturella species. Escherichia coli was found on 1 blood culture, though it was frequently identified on urine culture. Urine culture positivity was 28%. Disc culture positivity was greater than that of blood and urine cultures at 43%. Fourteen of 386 (4%) dogs required disc culture to identify the offending organism.
Several dogs were screened for brucellosis. It is often recommended to use signalment, geographic location, and history to guide the decision to screen for brucellosis. Indeed sexually intact male dogs and dogs in the southeast or southwest United States are predisposed to Brucella canis‐associated discospondylitis. 16 , 17 In contrast to these earlier reports, our study noted several sexually altered dogs with positive screening tests for discospondylitis with no apparent geographical predisposition. This highlights the need to consider brucellosis in any dog diagnosed with discospondylitis, regardless of reproductive status. Fungal cultures were uncommonly performed in our study (<2% of dogs) and they were frequently negative (84%). However, performing additional fungal screening (eg, aspergillosis testing/routine fungal culture) is important because identification of a fungal organism would alter treatment. This might be of particular importance in dogs with evidence of concurrent systemic disease, as aspergillosis commonly affects multiple body systems. 18
Intervertebral disc cultures were performed in 63 of 386 (16.3%) dogs. Samples for intervertebral disc culture can either be collected via open surgical approaches or percutaneously guided by ultrasound, computed tomography, or fluoroscopy. The most common indications for intervertebral disc sampling include dogs with negative urine or blood cultures, or lack of clinical response to broad spectrum antimicrobial therapy. Although an open surgical approach provides a superior visual field of the affected disc and facilitates collection of a sizeable sample for culture and biopsy, associated risks include those associated with prolonged general anesthesia, hemorrhage, vertebral destabilization, and postoperative neurological worsening. Potential complications of percutaneous sampling of the intervertebral disc include inadvertent damage to the spinal cord, spinal nerves, or large vessels, worsening pain, progression of neurological deficits, and seeding of bacteria from the disc into the epidural space, subarachnoid space or both, however, these complications are reported to be rare in the currently available literature. 19 , 20 One aim of our study was to determine whether geographical differences existed with regard to infectious disease positivity. Our study found no significant differences in culture results between institutions, although this could have been affected by sample sizes that could be too low to allow for some comparisons.
While many studies have reported radiographic, CT, and MRI features of discospondylitis in dogs, data on comparative imaging findings is more limited. 6 , 8 , 9 , 13 , 21 In our study, a radiographic diagnosis of discospondylitis had a fair agreement with a CT diagnosis, but a reduced level of agreement with MRI. This suggests the importance of pursuing advanced imaging when radiographs alone do not show evidence of lesions. This is likely because there is a delay between the onset of clinical signs and development of radiographic lesions. 5 , 22 Indeed 55 of 165 dogs (33.3%) in our study had no radiographic evidence of discospondylitis but did have evidence of disease on CT (18/165 dogs, 10.1%), MRI (35/165 dogs, 21.0%), or both (2/165 dogs, 1.2%). Four dogs had no radiographic or CT abnormalities but had lesions detected on MRI. When evaluating agreement between imaging modalities with regard to lesion site, there was significant agreement between imaging modalities, particularly between CT and MRI. This could be of particular relevance to cases with financial limitations. It is however important to note that 2 dogs with equivocal radiographic findings had definitive evidence of disease on MRI, and 7 dogs had additional lesions detected on advanced imaging (CT and MRI) when compared to radiographs alone. Thus, advanced imaging might still provide benefit in some cases.
Radiographs are frequently recommended to monitor recovery from discospondylitis and could be used to guide duration of antibiotic treatment. 23 Radiographic changes could however lag behind clinical improvement. 23 One group of particular interest to clinicians are those dogs with no initial lesions on radiographs but with lesions on advanced imaging. In these cases, clinicians could recommend using the same imaging modality used in initial lesion identification for continued monitoring of disease. While this approach is both logical and ideal, repeat advanced imaging can be cost prohibitive for many clients. Findings from advanced imaging modalities such as MRI might also have no meaningful association with clinical status. 24 In this study, we noted 5 dogs that initially had normal radiographs, who later developed lesions consistent with discospondylitis. As such, radiographs could be a suitable monitoring tool in dogs whose owners are financially restricted, though, it should be noted that any lesion improvement on follow‐up imaging could be indicative of either successful treatment or variability in diagnostic sensitivity between imaging modalities.
Treatment was largely consistent with earlier recommendations (eg, empiric cephalexin), with cephalexin being the most commonly prescribed antibiotic. Another common empiric choice was amoxicillin/clavulanic acid. If an infectious organism is not identified and empiric treatment is started, response to therapy could guide changes in antibiotic selection for example, failure to improve might lead to escalation of antibiotic spectrum. When available, culture results should be used to guide antibiotic selection. Unfortunately, follow‐up data was insufficient to accurately compare duration of antimicrobial treatment with the risk for clinical relapse.
Surgical intervention was utilized in a minority of dogs, with laminectomy and discectomy, or disectomy, procedures being most common, though 4 dogs had a sublumbar abscess drained and 2 dogs underwent surgical stabilization of a vertebral subluxation. Surgical decompression is indicated in cases with evidence of marked spinal cord compression or vertebral column instability on advanced imaging. 25 , 26 Some conditions that have traditionally been considered surgical emergencies (eg, empyema) could also be managed medically. 27 Dogs that had preexisting dental disease were statistically more likely to have surgery than those without preexisting dental disease. While the exact reason for this is unknown, it could be due to the presence of a more significant bacteremia in dogs with dental disease and more severe resultant discospondylitis compared to those without dental disease.
Dogs that had relevant trauma were more likely to experience clinical relapse than those without a history of trauma. Potential explanations for this could include destabilization of infected sites, direct inoculation of pathogens leading to a higher bacterial/fungal load, reduced penetration of antibiotics into the site of infection (scar tissue), chronic inflammation by traumatic injury propagating pain or neurological deficits, or a type 1 error due to multiple comparisons. One dog relapsed during estrus, which could be due to increased progesterone levels, as this hormone diminishes maturation of dendritic cells in dogs, leading to a decreased immune response to pathogens. 28 It is unknown whether a multifocal distribution of lesions is associated with a higher risk of relapse in dogs, due to the small number of relapsing dogs in the study and the commonality of multifocal lesions at diagnosis in our study group. Additionally, dogs that had preexisting steroid therapy were more likely to develop progressive neurologic dysfunction compared to those without previous steroid therapy. Potential explanations for this include clinician bias in using steroid therapy in more severe cases, or higher organism load due to immunosuppression, although these are speculations. Preexisting dental disease was associated with an increased need for surgical intervention, which could be due to potential bacteremia as previously discussed.
This study was limited by its retrospective nature, nonstandardized diagnostic and therapeutic approaches, nonstandardized case follow‐up, and loss of many cases to follow up which could influence the study's results. To evaluate whether breeds were overrepresented publicly available data from the American Kennel Club (breed registrations) was used as a control group. While most institutions were located in the United States, a small number of cases were also included from 2 collaborating institutions in Europe, thus AKC data might not accurately represent a reference standard for all dogs in this study. This could have implications on our data due to potential regional differences in breed distribution. Additionally comparison between imaging techniques was based on diagnostic imaging reports and imaging studies were not reread for the purpose of the study. Statistically significant results in this study could also be as a result of type 1 error associated with multiple comparisons. A Bonferroni or similar correction was not applied to mitigate the risk of introducing type 2 error given the group size for some comparisons.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Several dogs received off‐label antibiotics as part of their treatment protocols. These are retrospectively reported in this study.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Cassie Van Hoof and Nicole Davis were funded as part of an NIH summer research grant program: NIH‐5T35OD016477‐20. The authors thank Kathryn Winger DVM, DACVIM (Neurology) for consultation on initial study design and internal review of the manuscript before external peer‐review.
Van Hoof C, Davis NA, Carrera‐Justiz S, et al. Clinical features, comparative imaging findings, treatment, and outcome in dogs with discospondylitis: A multi‐institutional retrospective study. J Vet Intern Med. 2023;37(4):1438‐1446. doi: 10.1111/jvim.16785
Cassie Van Hoof and Nicole A. Davis contributed equally as co‐first authors.
REFERENCES
- 1. Thomas WB. Diskospondylitis and other vertebral infections. Vet Clin North Am Small Anim Pract. 2000;30:169‐182. vii. [DOI] [PubMed] [Google Scholar]
- 2. Wiley AM, Trueta J. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg Br. 1959;41‐B:796‐809. [DOI] [PubMed] [Google Scholar]
- 3. Burkert BA, Kerwin SC, Hosgood GL, Pechman RD, Fontenelle JP. Signalment and clinical features of diskospondylitis in dogs: 513 cases (1980‐2001). J Am Vet Med Assoc. 2005;227:268‐275. [DOI] [PubMed] [Google Scholar]
- 4. Harris JM, Chen AV, Tucker RL, Mattoon JS. Clinical features and magnetic resonance imaging characteristics of diskospondylitis in dogs: 23 cases (1997‐2010). J Am Vet Med Assoc. 2013;242:359‐365. [DOI] [PubMed] [Google Scholar]
- 5. Kornegay JN, Barber DL. Diskospondylitis in dogs. J Am Vet Med Assoc. 1980;177:337‐341. [PubMed] [Google Scholar]
- 6. Hurov L, Troy G, Turnwald G. Diskospondylitis in the dog: 27 cases. J Am Vet Med Assoc. 1978;173:275‐281. [PubMed] [Google Scholar]
- 7. Widmer W, Thrall D. Canine and feline vertebrae. In: Thrall D, ed. Textbook of Veterinary Diagnostic Radiology. 7th ed. St. Louis, MO: Elsevier; 2018:249‐270. [Google Scholar]
- 8. Gomes SA, Targett M, Lowrie M. Computed tomography features of discospondylitis in dogs. J Vet Intern Med. 2022;36:2123‐2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalo‐Orden JM, Altonaga JR, Orden MA, et al. Magnetic resonance, computed tomographic and radiologic findings in a dog with discospondylitis. Vet Radiol Ultrasound. 2000;41:142‐144. [DOI] [PubMed] [Google Scholar]
- 10. Gendron K, Doherr M, Gavin P, et al. Magnetic resonance imaging characterization of vertebral entplate changes in the dog. Vet Radiol Ultrasound. 2012;53:50‐56. [DOI] [PubMed] [Google Scholar]
- 11. American Kennel Club . Dog Registration Statistics (1991‐2008) . Accessed June 06, 2022. Available online: http://images.akc.org/pdf/archives/AKCregstats_1991-2008.pdf
- 12. McHugh M. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276‐282. [PMC free article] [PubMed] [Google Scholar]
- 13. Davis M, Dewey C, Walker M, et al. Contrast radiographic features in canine bacterial discospondylitis: a multicenter, retrospective study of 27 cases. J Am Anim Hosp Assoc. 2000;36:81‐85. [DOI] [PubMed] [Google Scholar]
- 14. Coelho C, Adeodato A, Brock G, et al. Canine breeds predisposed to develop diskospondylitis: a retrospective study of 181 cases (2009‐2018). Ars Veterinaria. 2020;36:321‐327. [Google Scholar]
- 15. Lulich JP, Osborne CA, Bartges JW, et al. Canine lower urinary tract disorders. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. Philadelphia: WB Saunders; 2000:1748. [Google Scholar]
- 16. Kerwin S, Lewis D, Hribernik T, Partington B, Hosgood G, Eilts BE. Diskospondylitis associated with Brucella canis infection in dogs: 14 cases (1980‐1991). J Am Vet Med Assoc. 1992;201:1253‐1257. [PubMed] [Google Scholar]
- 17. Long C, Burgers E, Copple C, et al. Brucella canis discospondylitis in 33 dogs. Front Vet Sci. 2022;9:1043610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schultz RM, Johnson EG, Wisner ER, Brown NA, Byrne BA, Sykes JE. Clinicopathologic and diagnostic imaging characteristics of systemic aspergillosis in 30 dogs. J Vet Intern Med. 2008;22:851‐859. [DOI] [PubMed] [Google Scholar]
- 19. Laborda‐Vidal P, Martin M, Orts‐Porcar M, et al. Computed tomography‐guided fine needle biopsies of vertebral and paravertebral lesions in small animals. Animals (Basel). 2022;12:1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fischer A, Mahaffey M, Oliver J. Fluoroscopically guided percutaneous disk aspiration in 10 dogs with diskospondylitis. J Vet Intern Med. 1997;11:284‐287. [DOI] [PubMed] [Google Scholar]
- 21. Carrera I, Sullivan M, McConnell F, et al. Magnetic resonance imaging features of discospondylitis in dogs. Vet Radiol Ultrasound. 2011;52:125‐131. [DOI] [PubMed] [Google Scholar]
- 22. Ruoff C, Kerwin S, Taylor A. Diagnostic imaging of discospondylitis. Vet Clin Small Anim. 2018;48:85‐94. [DOI] [PubMed] [Google Scholar]
- 23. Shamir M, Tavor N, Aizenberg T. Radiographic findings during recovery from discospondylitis. Vet Radiol Ultrasound. 2001;42:496‐503. [DOI] [PubMed] [Google Scholar]
- 24. de Freitas M, Vettorato E, Scarpante E, et al. Retrospective preliminary assessment of routine follow‐up low‐field magnetic resonance imaging in dogs presumptively diagnosed with discospondylitis. Front Vet Sci. 2022;9:880038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lavely J, Vernau K, Vernau W, et al. Spinal epidural empyema in seven dogs. Vet Surg. 2006;35:176‐185. [DOI] [PubMed] [Google Scholar]
- 26. Cabassu J, Moissonnier P. Surgical treatment of a vertebral fracture associated with a hematogenous osteomyelitis in a dog. Vet Comp Orthop Traumatol. 2007;20:227‐230. [DOI] [PubMed] [Google Scholar]
- 27. Monforte‐Monteiro S, Gallucci A, Rousset N, et al. Medical management of spinal epidural empyema in five dogs. J Am Vet Med Assoc. 2016;249:1180‐1186. [DOI] [PubMed] [Google Scholar]
- 28. Wijewardana V, Sugiura K, Wijesekera DP, et al. Effect of ovarian hormones on maturation of dendritic cells from peripheral blood monocytes in dogs. J Vet Med Sci. 2015;77:771‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]


