Abstract
Background
Autoimmune mechanisms represent a novel category for causes of seizures and epilepsies in humans, and LGI1‐antibody associated limbic encephalitis occurs in cats.
Hypothesis/Objectives
To investigate the presence of neural antibodies in dogs with epilepsy or dyskinesia of unknown cause using human and murine assays modified for use in dogs.
Animals
Fifty‐eight dogs with epilepsy of unknown cause or suspected dyskinesia and 57 control dogs.
Methods
Serum and CSF samples were collected prospectively as part of the diagnostic work‐up. Clinical data including onset and seizure/episode type were retrieved from the medical records. Screening for neural antibodies was done with cell‐based assays transfected with human genes for typical autoimmune encephalitis antigens and tissue‐based immunofluorescence assays on mouse hippocampus slices in serum and CSF samples from affected dogs and controls. The commercial human und murine assays were modified with canine‐specific secondary antibody. Positive controls were from human samples.
Results
The commercial assays used in this study did not provide unequivocal evidence for presence of neural antibodies in dogs including one dog with histopathologically proven limbic encephalitis. Low titer IgLON5 antibodies were present in serum from one dog from the epilepsy/dyskinesia group and in one dog from the control group.
Conclusion and Clinical Importance
Specific neural antibodies were not detected using mouse and human target antigens in dogs with epilepsy and dyskinesia of unknown origin. These findings emphasize the need for canine‐specific assays and the importance of control groups.
Keywords: animal model, autoimmune epilepsy, dyskinesia, epileptic seizure, idiopathic epilepsy, movement disorder, neural antibodies, neuroinflammation, sleep disorder
Abbreviations
- AB
antibody
- ADHD
attention‐deficit/hyperactivity disorder
- AMPAR
α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor
- APE
antibody prevalence in epilepsy
- AQP4
aquaporin‐4
- BBB
blood‐brain barrier
- CASPR2
contactin‐associated protein‐like 2
- CBA
cell‐based assay
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DCC
deleted in colorectal carcinoma
- DPPX
dipeptidyl‐peptidase‐like protein 6
- EEG
electroencephalography
- FEPSO
feline complex partial seizures with orofacial involvement
- FLAIR
fluid attenuation inversion recovery
- GABAAR
γ‐aminobutyric acid receptor A
- GABABR
γ‐aminobutyric acid receptor B
- GAD65
anti‐glutamic acid decarboxylase 65‐kDa isoform
- GlyR
glycine receptor
- IF
immunofluorescence
- Ig
immunoglobulin
- IgG
immunoglobulin‐G
- IgLON5
immunoglobulin LON5
- IL‐17
interleukin 17
- IVETF
International Veterinary Epilepsy Task Force
- LE
limbic encephalitis
- LGI1
leucine‐rich glioma‐inactivated protein 1
- mGlu5
metabotropic glutamate‐5 receptor
- MRI
magnetic resonance imaging
- MUE
meningoencephalomyelitis of unknown etiology
- NMDAR
N‐methyl‐d‐aspartate receptor
- TBA
tissue‐based assay
- VGKC
voltage‐gated potassium channel
1. INTRODUCTION
Epilepsy is the most common chronic central nervous system (CNS) disease in dogs affecting 0.43% to 0.82% of dogs admitted to veterinary practices. 1 , 2 , 3 , 4 A genetic cause is frequently suspected in dogs with idiopathic epilepsy, but causal epilepsy genes are only identified for rare genetic idiopathic epilepsies with an onset in young dogs corresponding to human childhood and juvenile epilepsies. 5 The etiology of idiopathic epilepsy with onset in adult dogs remains largely unknown. There might be a complex and multifactorial etiology with an interaction of multiple genetic risk variants and environmental factors. 6
Autoimmune mechanisms have increasing relevance in humans as a distinct etiologic category offering new therapeutic approaches. 7 , 8 , 9 , 10 Characteristic clinical features are sudden onset of psychiatric symptoms with rapid progression to epileptic seizures or movement disorders which could be associated with signal changes on magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) pleocytosis, and the presence of neural antibodies in serum or CSF. 11 Further proof for the involvement of autoimmune mechanisms comes from positive outcomes of therapeutic trials with immunosuppressives. 9 , 12 Therefore, the ILAE epilepsy classification includes immune epilepsy as a distinct entity, 13 and the ILAE autoimmunity and inflammation task force recommend distinguishing between autoimmune‐associated epilepsy and acute symptomatic seizures secondary to autoimmune encephalitis. 9
In animals, there is unequivocal evidence for autoimmune mechanisms in cats with limbic encephalitis (LE), orofacial seizures, and suggestive MRI and pathology findings associated with antibodies directed against leucine‐rich glioma inactivated protein 1 (LGI1). The disorder in cats thus parallels anti‐LGI1 LE in humans. 14 , 15 , 16 , 17 A further report refers to a cat with LE in association with deleted colorectal carcinoma (DCC, also known as netrin‐1 receptor) autoantibodies. 18 Reports on dogs are scarce. 19 , 20 , 21 , 22 N‐methyl‐d‐aspartate receptor (NMDAR) antibodies are suggested in the CSF of two dogs with meningoencephalitis of unknown etiology (MUE) and one dog with multifocal signs of neurologic disease and an abscessed maxillary tooth, and in the CSF of a polar bear (Ursus maritimus) that drowned because of epileptic seizures and NMDAR‐antibody (AB) mediated encephalitis. 20 , 23 Furthermore, positive effects of prostaglandin G/H synthase 2 (PTGS2; also known as cyclooxygenase‐2, COX‐2) inhibitors in single dogs with IE and increased T helper 17 cells (Th17) support neuroinflammatory mechanisms in idiopathic epilepsy in dogs. 24 , 25
This study aims to screen for neural antibodies in dogs with epilepsy or suspected dyskinesia of unknown origin with commercial diagnostic murine and human assays, modified for use in dogs. We hypothesized that we would detect cross‐reacting antibodies.
2. MATERIALS AND METHODS
This study was performed with institutional ethical approval (#125‐16‐05‐2018). The study was designed as a prospective case‐control study. Serum and CSF samples from 120 dogs, 63 dogs with epilepsy or suspected dyskinesia and 57 controls, were collected prospectively and screened for neural antibodies with commercially available murine and human assays.
Inclusion criteria were epilepsy or dyskinesia of unknown cause required a tier II diagnostic work‐up in line with International Veterinary Epilepsy Task Force (IVETF) tier II recommendations. 26 Dogs were included in the epilepsy group if ≥2 generalized or focal epileptic seizures occurred ≥24 hours apart. 26 Paroxysmal dyskinesia was considered based on characteristic clinical features of episodes and review of video footage. 27 Episodes which were difficult to classify were also considered in this group (Table 1). We encouraged recruitment of dogs with unusual features of epilepsy/suspected dyskinesia, for example, subtle MRI signal intensity changes of undefined significance, mild unexplained CSF pleocytosis, other non‐neurologic comorbidities suggestive of inflammation/autoimmunity or behavioral changes (not postictal or adverse effects of medications). 28 Also, few drug‐resistant dogs from breeds predisposed to epilepsy and seven dogs with idiopathic epilepsy and increased interleukin 17 (IL‐17) levels (published previously 24 ) were included. Exclusion criteria were a diagnosis or suspicion of genetic epilepsy/dyskinesia because of affected family members or obvious structural brain disease, for example, neoplasia and meningoencephalitis. Information on clinical data (age, breed, sex, onset, phenotype of seizures or episodes, comorbidities) and diagnostic findings (MRI, CSF) were extracted from the medical records. Additional telephone interviews were conducted if deemed necessary to validate information. Histopathological examination was carried out on one dog.
TABLE 1.
Clinical characteristics, diagnostic work‐up, and samples for neural antibodies in pet dogs with epilepsy or dyskinesia and controls.
| No. dogs | Additional features | Diagnostic tests | Samples | Neural antibody testing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute onset a | Behavior changes | Immune‐mediated comorbidity b | MRI | CT | CSF | EEG | Serum | CSF | Serum only | CSF only | CSF/serum pairs | ||
| Epilepsy/dyskinesia group (n = 58) | 30 | 17 | 18 | 52 | 1 | 48 | 12 | 57 | 38 | 20 | 1 | 37 | |
| Epileptic seizures | 38 | 18 | 14 | 11 | 38 | 1 | 35 | 4 | 37 | 27 | 11 | 1 | 26 |
| Tonic‐clonic epileptic seizures | 32 | 15 | 11 | 10 | 32 | — | 29 | 2 | 31 | 22 | 10 | 1 | 21 |
| Generalized | 14 | 7 | 3 | 4 | 14 | — | 12 | — | 13 | 10 | 4 | 1 | 9 |
| Focal to bilateral tonic‐clonic | 9 | 3 | 3 | 4 | 9 | — | 8 | 2 | 9 | 5 | 4 | — | 5 |
| Additional focal seizures | 9 | 5 | 5 | 2 | 9 | — | 9 | — | 9 | 7 | 2 | — | 7 |
| Focal epileptic seizures | 6 | 3 | 3 | 1 | 6 | 1 | 6 | 2 | 6 | 5 | 1 | — | 5 |
| Suspected dyskinesia/unclassified | 20 | 12 | 3 | 7 | 14 | — | 13 | 8 | 20 | 11 | 9 | — | 11 |
| Dystonia | 2 | 1 | — | — | 2 | — | 2 | 1 | 2 | 2 | — | — | 2 |
| Unclassified tremor/myoclonus/episodes | 6 | 2 | 1 | 2 | 6 | — | 5 | 1 | 6 | 3 | 3 | — | 3 |
| Idiopathic head tremor | 12 | 9 | 2 | 5 | 6 | — | 6 | 6 | 12 | 6 | 6 | — | 6 |
| Control group (n = 57) | — | — | — | 17 | 5 | 17 | — | 46 | 17 | 40 | 11 | 6 | |
| Neurologic disease | 22 | — | — | — | 17 | 5 | 17 | — | 11 | 17 | 5 | 11 | 6 |
| Compressive myelopathy c | 14 | — | — | — | 10 | 5 | 11 | — | 6 | 11 | 3 | 8 | 3 |
| Lumbosacral disease | 3 | — | — | — | 3 | — | 2 | — | 1 | 2 | 1 | 2 | — |
| Fibrocartilagenous embolism | 1 | — | — | — | 1 | — | — | — | 1 | — | 1 | — | — |
| Meningitis/meningoencephalitis | 2 | — | — | — | 1 | — | 2 | — | 2 | 2 | — | — | 2 |
| Idiopathic vestibular disease | 1 | — | — | — | 1 | — | 1 | — | — | 1 | — | 1 | — |
| Unexplained lameness | 1 | — | — | — | 1 | — | 1 | — | 1 | 1 | — | — | 1 |
| Non‐neurologic disease | 25 | — | — | — | — | — | — | — | 25 | — | 25 | — | — |
| Cardiologic | 7 | — | — | — | — | — | — | — | 7 | — | 7 | — | — |
| Metabolic/endocrine/gastroenterologic | 6 | — | — | — | — | — | — | — | 6 | — | 6 | — | — |
| Trauma | 4 | — | — | — | — | — | — | — | 4 | — | 4 | — | — |
| Dental | 4 | — | — | — | — | — | — | — | 4 | — | 4 | — | — |
| Orthopedic | 3 | — | — | — | — | — | — | — | 3 | — | 3 | — | — |
| Dermatologic | 1 | — | — | — | — | — | — | — | 1 | — | 1 | — | — |
| Healthy | 10 | — | — | — | — | — | — | — | 10 | — | 10 | — | — |
| Preventative health care | 8 | — | — | — | — | — | — | — | 8 | — | 8 | — | — |
| Blood donor | 2 | — | — | — | — | — | — | — | 2 | — | 2 | — | — |
| All dogs (n = 115) | 30 | 17 | 18 | 69 | 6 | 65 | 12 | 103 | 55 | 60 | 12 | 43 | |
Onset was described in detail in 54 dogs. An acute onset was defined by ≥2 days with epileptic seizures or other episodes in the first 4 weeks, or cluster seizures or episodes or status epilepticus at onset.
One dog had a history of surgical removal of an abdominal teratoma and encephalomyelitis 2 years before the onset of epilepsy (not counted here). An immune‐mediated comorbidity was an exclusion criteria for control dogs.
Intervertebral disc herniation (n = 9), subarachnoid diverticula (n = 3), disc‐associated cervical spondylomyelopathy (n = 2).
Control dogs had other neurological diseases but no seizures or dyskinesia (22 dogs; Table 1), non‐neurological disease (25 dogs) or were healthy dogs presenting for preventative health care or as blood donors (10 dogs). Care was taken to exclude dogs with a history of epileptic seizures, neoplasia, or recent treatment with glucocorticoids as controls.
Serum and CSF were collected as part of the routine diagnostic work‐up. MRI was performed in all dogs with epilepsy or dyskinesia except six dogs with clinical signs and videos suggestive of idiopathic head tremor syndrome described previously (Table 1). 29 Cerebrospinal fluid was collected under general anesthesia by atlanto‐occipital puncture. Routine CSF analysis (total nucleated cell counts, cytospin differential cell count, protein content, infectious disease tests as indicated) was performed. In most cases, CSF and serum (100 μL) was shipped to the immunodiagnostic laboratory, specialized for neural AB testing in human medicine, for immediate AB testing after arrival. Additional archived samples, which had been stored at −20°C or −80°C until testing, were transported frozen on dry ice.
Screening for neural antibodies was done on a tissue‐based assay (TBA) on unfixed sagittal mouse brain slices (hippocampus, brain stem, and cerebellum; Euroimmun, Lübeck, Germany), and, secondly, by a commercial cell‐based assay (CBA) biochip containing human embryonic kidney (HEK) cells transfected with human genes for different antigens of interest (Euroimmun, Lübeck, Germany): glutamic acid decarboxylase (GAD65; intraneural) and the neural surface antigens N‐methyl‐d‐aspartate receptor (NMDAR), γ‐aminobutyric acid receptor B (GABABR), α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor‐2 (AMPAR1/2), dipeptidyl‐peptidase‐like protein 6 (DPPX), leucine‐rich glioma inactivated protein 1 (LGI1), contactin‐associated protein‐like 2 (CASPR2), glycine receptor (GlyR: now known as GLR), metabotropic glutamate‐5 receptor (mGlu5; now known as GRM5) in all dogs and IgLON5 (42 dogs with epilepsy or dyskinesia, 55 controls). GABAAR‐transfected HEK cells were not available at the time of the study. Secondary AB was a polyclonal rabbit anti‐dog immunoglobulin‐G (IgG) AB directed against canine IgG heavy and light chains (catalog no. 304‐065‐003; Jackson ImmunoResearch/Dianova) conjugated with red Alexa Fluor 594 used at a dilution of 1:100 and incubated for 30 minutes at room temperature; nuclei were counterstained with Hoechst 33342, 1:10 000. Slides were embedded with 1,4‐Diazabicyclo[2.2.2]octan. IgG positivity was confirmed with a specific anti‐canine IgG directed against the Fc fragment (Jackson ImmunoResearch, 304‐545‐008, 1:100), conjugated with the green Alexa 488 dye. Human samples with different AB reactivities served as positive control. 8
2.1. Validation
For validation of negative TBA test results, four samples with immunofluorescence (IF) staining results suspicious of neuropil binding on the initial testing by TBA and 1 sample with a positive CBA but negative TBA were re‐tested together with 16 chosen negative samples from the epilepsy/dyskinesia group. TBA was performed by two examiners (masked for review) which were blinded to the initial test results and applied stringent criteria. Samples were rated in three categories: (1) negative; (2) questionable neuropil/diffuse staining; and (3) neuropil. A positive test result required a staining, specific for neuropil and comparable with positive samples from the literature 30 and own human results with confirmed CBA positivity. Observer agreement was evaluated by Cohen kappa. For further validation of negative test results for NMDAR ABs on CBA, western blots of canine and murine brain lysates were incubated with a commercial anti‐NMDAR‐antibody (rabbit polyclonal anti‐NMDAR1; 1:1000; #AB‐0889; ABnostics, Dossenheim, Germany) with proposed specificity against canine NMDAR to identify canine NMDAR protein. For methodological details see File S1.
3. RESULTS
One hundred twenty dogs were recruited for the study. Five dogs, all with negative neural AB test results, were subsequently excluded because of genetic dyskinesia (1 dog, phosphoenolpyruvate carboxykinase deficiency 31 ), suspicion on genetic epilepsy (2 dogs), progressive neurologic disease (1 dog), and incomplete MRI study (1 dog with epilepsy). Thus, samples from 115 dogs (58 epilepsy and dyskinesia cases, 57 controls; 43 serum‐CSF pairs, 60 sera, 12 CSF) were included in the analysis (Table 1).
3.1. Clinical characteristics of the epilepsy/dyskinesia group
The epilepsy/dyskinesia group consisted of 58 dogs (33 males; 25 females; median age at study inclusion 3.75 years). Median age of onset was 2.5 years for epilepsy (range 0.4‐11.7 years) and 2.0 years for suspected dyskinesia (range 0.3‐10.5 years). Onset was described in detail in 54 dogs and was fairly acute in 55.6% (30/54) with ≥2 days with epileptic seizures or other episodes, cluster seizures or episodes or status epilepticus in the first 4 weeks (Table 1); status epilepticus (2 dogs), cluster seizures (9 dogs) or cluster episodes (5 dogs) occurred already on the first day in 29.6% (16/54). Behavioral changes, which occurred independently from the seizures or episodes and unrelated to medication, were reported in 29.3% (17/58). One dog had a history of surgical removal of an abdominal teratoma and encephalomyelitis 2 years before the onset of epilepsy with tonic‐clonic seizures but the original report or specimens were unavailable for review. Other comorbidities with a presumed immune‐mediated etiology were reported in 18 dogs (31.0%; 18/58). Details on the clinical phenotype are presented in File S2.
3.2. MRI and CSF findings
Subtle MRI changes were evident in 53.8% of the dogs (28/52). Brain MRI revealed regional T2‐ or T2‐FLAIR hyperintensity in 13 dogs (13/52; 25%). Hyperintense signal changes appeared in the hippocampus, temporal or piriform lobes of one or both hemispheres (11 dogs), cerebellum and brainstem (1 dog), and as leukoaraiosis in one 9‐year‐old dog. Contrast enhancement was noted in five of these dogs on corresponding regions. Other findings were variable degrees of ventriculomegaly or asymmetry (14 dogs) and soft tissue attenuating/fluid material in both tympanic bullae in one dog. Analysis of CSF (48 dogs) revealed mild CSF pleocytosis in four dogs with total nucleated cell counts from 7 to 37 cells/μL (reference range 0‐5 cells/μL). There was marked CSF pleocytosis (389/μL) and increased protein content (0.82 g/L; reference range <0.3 g/L) in a fifth dog. 32 Histopathologic examination indicated limbic encephalitis in this dog. CSF analysis was unremarkable in the others.
3.3. Screening for neural antibodies with TBA
Tissue‐based assays utilizing unfixed sagittal mouse brain slices which were intended as a screening test for antibodies to new neuronal antigens showed negative results for all samples from affected dogs and controls.
Validation of test results: We were unable to confirm initial suspicion on positive IF labeling in five dogs from the epilepsy/dyskinesia group (3 CSF, 2 serum samples, including the dog with a history of teratoma) when the same samples were re‐examined by blinded investigators applying stringent evaluation criteria. Thereby, re‐evaluation of 21 samples from the epilepsy/dyskinesia group (11 CSF, 10 serum) including the five dogs with questionable positive results on initial examination and 16 dogs with initially negative test results by two experienced examiners (CIB, CGB) which had been blinded to the initial test results, showed substantial agreement (Cohen kappa = 0.67) between examiners (CGB, CIB) on interpretation of IF and classification of samples (Table 2). Finally, none of the investigated materials produced a staining that could be rated as “neuropil AB.” 33
TABLE 2.
Interobserver agreement on evaluation of TBA: Validation showed substantial agreement (Cohen kappa = 0.67).
| CGB | |||||
|---|---|---|---|---|---|
| Negative | Questionable | Neuropil | Total | ||
| CIB | Negative | 11 | 1 | 0 | 12 |
| Questionable | 2 | 7 | 0 | 9 | |
| Neuropil | 0 | 0 | 0 | 0 | |
| Total | 13 | 8 | 0 | 21 | |
| Agreement | 13 | 8 | 0 | 18 | |
| By chance | 7.43 857 143 | 4.57 142 857 | 0 | 12 | |
3.4. Screening for specific neural antibodies with CBA
Cell‐based assays which express human antigens provided negative results for each tested neural AB in all dogs except low serum AB titers of IgLON5 antibodies (1:40) in one dog with unclassified episodes from the epilepsy/dyskinesia group (Figure 1), but also in one dog from the control group. The case dog was a 3‐year‐old female Golden Retriever with unremarkable MRI and CSF analysis which presented for further investigation of episodes of abnormal spontaneous arousal from sleep with howling, urination, defecation followed by signs of hind limb paresis. The control dog was a 9‐year‐old German Wirehaired Pointer presenting for further evaluation of severe osteoarthritis of the elbow joint. The owner denied any signs of seizures, movement disorders, sleep‐related movements, or disturbed sleep on follow‐up phone calls. Follow‐up time was 2 years in this dog.
FIGURE 1.
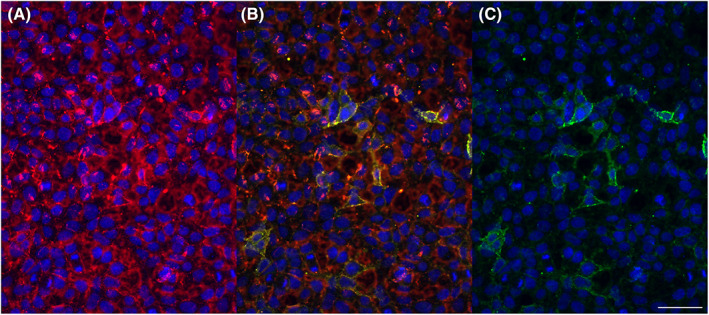
Canine anti‐IgLON5 antibodies. Immunofluorescence studies: Diluted serum (1:20) from a dog from the epilepsy/dyskinesia group (Golden Retriever, female, 3 years) is incubated with IgLON5‐transfected human embryonic kidney (HEK) cells. Binding of antibodies to cell surfaces is visualized by two different secondary anti‐canine antibodies coupled with immunofluorescence dyes and subsequently overlaid. The results demonstrate that there are canine anti‐IgLON5 IgG antibodies. Endpoint titration gave a titer of 1:40 (not shown). (A) anti‐canine IgG heavy and light chain (a sensitive secondary antibody), coupled with the red Alexa 594 dye. (B) overlay; yellow: double‐stainings. (C) anti‐canine IgG Fc fragment (the more specific secondary antibody), coupled with the green Alexa 488 dye. Bar: 25 μm.
Both, CSF and serum were tested in the dog with a histopathological diagnosis of limbic encephalitis and the dog with a history of teratoma, and CBA provided negative results. Our attempts to further validate negative CBA test results for NMDAR antibodies on canine brain failed because the commercial NMDAR antibody did not show any specific binding in dogs while it did so in mice (File S3).
4. DISCUSSION
Autoimmune epilepsy is a new etiologic category of epilepsy in humans, and epilepsy of unknown cause is a frequent diagnosis in dogs. 13 , 34 Further investigation in dogs is warranted considering the potential implications for treatment and the high proportion of dogs with epilepsy of unknown etiology and a sudden onset in adult dogs. 4 Therefore, in this study, we investigated neural AB profiles in serum and CSF of dogs with epilepsy and dyskinesia of unknown etiology with available commercial murine and human assays, which were adapted for the use in dogs. We aimed to include dogs with clinical features frequently associated with neural antibodies and response to immunotherapy in humans and in line with suggested diagnostic criteria in humans. 11 , 35
The study did not detect neural antibodies with commercial murine and human diagnostic assays in all but two dogs which showed low titers of IgLON5 ABs in serum in CBA expressing human antigen. This antibody appeared of uncertain relevance, because cell‐based assays showed IgLON5 antibodies in one dog from the epilepsy/dyskinesia group and one dog from the control group. Signs of episodic pelvic limb paresis in the IgLON5 AB‐positive dog occurred only in temporal context with sleep, that is, on awakening or associated with paradoxical arousal which is to some degree reminiscent of the frequent reports of sleep disorders in humans with IgLON5 antibodies. 36 , 37 We were unable to provide unequivocal classification of the episodes as dyskinesia or epileptic seizures by EEG or to perform polysomnography because of their infrequent occurrence but considering the association with involuntary urination and defecation an epileptic seizure could also have occurred. IgLON5 disease in humans is a tauopathy frequently characterized by sleep problems and signs of bulbar dysfunction as dysphagia, progressive supranuclear palsy, dysautonomia, cognitive decline, with additional signs of a movement disorder during daytime, such as distal myoclonus, chorea, limb or oromandibular dystonia, hand tremor, and facial spasm reported in up to 64%. 36 , 37 , 38 We also found IgLON5 antibodies in one control dog without any signs of epileptic seizures, movement, or sleep disorder on questioning of the dog owner. This demonstrates the importance of case‐control studies to be able to judge the specificity and the pathogenic potential of neural antibodies.
The study was designed as a screening study using available human and murine assays. The study did not provide evidence for neural antibodies including NMDAR antibodies in dogs with epilepsy of unknown cause or dyskinesia with consideration of autoimmune epilepsy with human and murine assays. Thus, with the present diagnostic methods the question remains yet unresolved whether autoimmune epilepsy is a rare condition in dogs or whether the applied test methods developed for use in humans are not reliable for investigation of dogs. Considering the selection of the dogs, 1 concern is that the results could have been negative because of methodological issues, that is, that dog antibodies did not bind to human and mouse neural antigens. This hypothesis is in line with other authors' negative findings when investigating glial fibrillary acidic protein (GFAP) autoantibodies in dogs with similar methods. 21 Furthermore, use of human immune assays for detection of antibodies to new antigenic targets in dogs is significantly limited by the lack of canine positive controls, and, therefore, we were required to use positive human samples with different AB reactivities as controls to ensure reliable staining. Another explanation might be that more recently discovered antigens like GABAAR were not available at the time of testing. Recently, we described a 1‐year‐old male intact Cavalier King Charles Spaniel with GABAAR AB, serum titer 1:320, CSF titer 1:2 and a corresponding staining pattern on mouse brain. This dog recovered upon steroid treatment from its acutely emerging and progressive signs like tonic‐clonic epileptic seizures, episodic hyperexcitability alternating with episode of stupor, and intermittent circling behavior. 22
Even though the respective genes have amino acid sequences that are to ≥94% identical between humans and dogs, 20 our results highlight the need for cross‐species validation of antibodies and target antigen epitopes, considering that the specificity of the AB is a critical part of IF‐based methods, and the requirement to confirm positive results from IF and diagnostic assays with other or multiple detection methods. We attempted this with western blots but surprisingly the commercial NMDAR antibody failed to recognize canine NMDAR antigen despite being marketed for dogs (Figure S3). It is a drawback that further confirmation, for example, with TBA failed in our IgLON5 AB positive dogs, and other detection methods, for example, gel electrophoresis was not pursued. 39 In the present study, all samples were tested in a two‐step procedure, first by TBA, a screening test for unknown autoantibodies on unfixed sagittal mouse brain slices, and second by CBA, assuming that the TBA could serve as a screening test for yet unknown autoantibodies. 8 However, the TBA has been recently questioned in humans, based on demonstration of a weak correlation between CBA and TBA with no neuropil staining in the TBA despite a clear positive CBA, which also reflects our results in the IgLON5‐positive dogs. A similar discrepancy between weakly positive CBA and negative TBA in dogs with MUE was recently described by others evaluating GFAP antibodies with TBA and CBA in dogs. 21 Furthermore, we were unable to provide evidence for reliable neuropil staining on blinded re‐evaluation of samples with TBA. Likewise, in humans, diagnosis of autoimmune CNS disease has only been achieved in a few cases with neuropil staining in the TBA. 8 As a consequence, the presence of yet unknown autoantibodies in dogs with epilepsy or dyskinesia remains to be elucidated with different methodology, as the TBA on its own is not an appropriate test for finding new antibodies and confirming results of CBA.
Previously, others had explored a subset of neural antibodies (NMDAR1, AMPAR1, AMPAR2, GABABR1, GABABR2, CASPR2, LGI1, and DPPX) in 32 dogs with CNS disease, including four dogs with epilepsy, with a commercially available CBA expressing human antigen and indirect immunofluorescence assay, with postulated AB specificity because of high homology of the amino acid sequences between humans and dogs. 20 The authors reported on NMDAR1 antibodies in CSF of three dogs (9.4%). They used a less stringent approach classifying samples from two dogs with MUE with diffuse staining in CBA as positive while staining was convincing in one dog with tooth root abscesses and multifocal signs of neurologic disease which were responsive to immunotherapy but in the absence of MRI or CSF changes indicative of meningoencephalitis. 20 Pan et al reported on high prevalences of NMDAR AB in different mammalian species, including dogs and cats, ranging from 4% in dogs <4 years of age to 30% in dogs >11 years. 40 These authors did not consequently differentiate between Ig classes; thus comparison to our data is difficult. Differences are in part because of the fact that Pan et al used a protein‐A‐antibody detection method that did not differentiate between IgA and IgG antibodies. 40 In contrast we used anti‐canine IgG antibody as a secondary antibody, because only IgG NMDAR ABs are shown to be of clinical relevance in humans. 41 Interestingly, Pan et al emphasized that the immunologically relevant portions of the NMDAR were identical across species. 40 Furthermore results might also depend on the interpretation by the authors. Misinterpretation of non‐specific cell staining as indicating antibody‐positivity has been criticized previously. 42 We rated immunofluorescence in a stringent way. Figure 1C of the present article demonstrates the lack of background staining with a more specific anti‐IgG AB in the modified cell‐based assay.
Our affected group included dogs with epilepsy of unknown etiology or suspected dyskinesia, and we included dogs with unusual features, for example, new onset and rapid progression in adult dogs, behavioral and autoimmune comorbidities, reports on MRI signal changes in some of the dogs and inflammatory CSF changes in few dogs. Furthermore, nearly one‐third of the dog owners reported notable behavioral changes. In three dogs the onset of behavioral changes which were described by the owners as restlessness, hyperactivity, lack of concentration, and inattention was concurrent with the onset or progression of the epilepsy suggesting a shared pathophysiology between seizures and behavioral changes. 43 In humans, sudden onset of alteration of behavior (delusions, psychosis, catatonia) and cognition combined with abnormal movements (eg, orofacial dyskinesia) are frequently seen in patients with AB against NMDAR1. 7 , 44 , 45 The AB prevalence in epilepsy score (APE score) includes aside from new‐onset epileptic seizures, neuropsychiatric changes, for example, agitation, aggressiveness, emotional lability, as common symptoms of patients with positive AB‐status (up to 78.3%), and additionally rapidly progressive mental status changes within 1 to 6 weeks. 46
At this stage, it is premature to exclude autoimmune mechanisms in dogs with epilepsy and dyskinesia of unknown origin based on negative test results for neural antibodies with assays expressing murine and human neural antigens, considering that we included one dog with epilepsy and a history of a teratoma, which is frequently associated with NMDAR1 antibodies in humans. 47 We also included one dog with a histopathologic diagnosis of limbic encephalitis, which is frequently associated with LGI1 or CASPR2 antibodies in humans. 13 , 30 We were unable to identify neural AB, including LGI1 AB, in the 1 histopathologically confirmed case with LE. Low titer IgLON5 antibodies were present in 1 case and 1 control dog. Although we appreciated some parallels in clinical manifestations to IgLON5 disease in humans the finding of a low positive titer in a control dog argues against a significant association with neurologic disease in dogs. Lastly, AB status is not necessarily needed to assume immune‐mediated LE in humans and 7% of patients with clinical and MRI features of LE, CSF, and serum remain negative for neural antibodies. 48 Furthermore, autoimmune encephalitis with epileptic seizures can also present with normal brain MRI or CSF results in humans and cats. 7 , 16 , 49 , 50
5. CONCLUSION
Our results highlight the need for the development of canine species‐specific assays for neural antibodies and also the need for inclusion of control groups to assess whether there is a significant association with disease.
CONFLICT OF INTEREST DECLARATION
Andrea Fischer receives sponsoring support from Purina Deutschland GmBH (Euskirchen, Germany) for the Ludwig‐Maximilians‐Universitat epilepsy consulting unit and from Vetoquinol GmbH (Ismaning, Germany) for the Ludwig‐Maximilians‐Universitat veterinary mobility center. No other authors declare a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the ethical commission of the veterinary faculty of Ludwig‐Maximilians‐Universitat Munich (No. 125‐16‐05‐2018).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
File S1. Methods western blot.
File S2. Details on the clinical phenotype.
Figure S3. Western blot of canine and murine brain lysate. Western blots demonstrating failure of the antibody to bind to NMDAR1 protein in canine brain samples while there is expression of NMDAR1 protein (100 kDa) in murine hippocampus (lane 5‐6), and murine amygdala (lane 7‐8) samples. ACTB (45 kDa) was used as reference protein.
ACKNOWLEDGMENT
Funding provided by GkF Gesellschaft zur Förderung Kynologischer Forschung e.V, 2018.
Hemmeter L, Bien CG, Bien CI, et al. Investigation of the presence of specific neural antibodies in dogs with epilepsy or dyskinesia using murine and human assays. J Vet Intern Med. 2023;37(4):1409‐1417. doi: 10.1111/jvim.16744
REFERENCES
- 1. Tauber C, Fischer A. Epidemiologische Untersuchungen zum Vorkommen von Epilepsie bei Hund und Katze in der Kleintierpraxis (Dissertation). LMU München: Tierärztliche Fakultät 2017;130.
- 2. Erlen A, Potschka H, Volk HA, Sauter‐Louis C, O'Neill DG. Seizure occurrence in dogs under primary veterinary care in the UK: prevalence and risk factors. J Vet Intern Med. 2018;32(5):1665‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heske L, Nødtvedt A, Jäderlund KH, Berendt M, Egenvall A. A cohort study of epilepsy among 665,000 insured dogs: incidence, mortality and survival after diagnosis. Vet J. 2014;202(3):471‐476. [DOI] [PubMed] [Google Scholar]
- 4. Kearsley‐Fleet L, O'Neill DG, Volk HA, Church DB, Brodbelt DC. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet Rec. 2013;172(13):338. [DOI] [PubMed] [Google Scholar]
- 5. Wielaender F, Sarviaho R, James F, et al. Generalized myoclonic epilepsy with photosensitivity in juvenile dogs caused by a defective DIRAS family GTPase 1. Proc Natl Acad Sci U S A. 2017;114(10):2669‐2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belanger JM, Heinonen T, Famula TR, et al. Validation of a chromosome 14 risk haplotype for idiopathic epilepsy in the belgian shepherd dog found to be associated with an insertion in the RAPGEF5 gene. Genes (Basel). 2022;13(7):1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long‐term outcome in patients with anti‐NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bien CG, Bien CI, Dogan Onugoren M, et al. Routine diagnostics for neural antibodies, clinical correlates, treatment and functional outcome. J Neurol. 2020;267(7):2101‐2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steriade C, Britton J, Dale RC, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune‐associated epilepsy: conceptual definitions. Epilepsia. 2020;61(7):1341‐1351. [DOI] [PubMed] [Google Scholar]
- 10. Quek AML, Britton JW, McKeon A, et al. Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol. 2012;69(5):582‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jang Y, Kim DW, Yang KI, et al. Clinical approach to autoimmune epilepsy. J Clin Neurol. 2020;16(4):519‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klang A, Schmidt P, Kneissl S, et al. IgG and complement deposition and neuronal loss in cats and humans with epilepsy and voltage‐gated potassium channel complex antibodies. J Neuropathol Exp Neurol. 2014;73(5):403‐413. [DOI] [PubMed] [Google Scholar]
- 15. Pakozdy A, Gruber A, Kneissl S, Leschnik M, Halasz P, Thalhammer JG. Complex partial cluster seizures in cats with orofacial involvement. J Feline Med Surg. 2011;13(10):687‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pakozdy A, Glantschnigg U, Leschnik M, et al. EEG‐confirmed epileptic activity in a cat with VGKC‐complex/LGI1 antibody‐associated limbic encephalitis. Epileptic Disord. 2014;16(1):116‐120. [DOI] [PubMed] [Google Scholar]
- 17. Pakozdy A, Halasz P, Klang A, et al. Suspected limbic encephalitis and seizure in cats associated with voltage‐gated potassium channel (VGKC) complex antibody. J Vet Intern Med. 2013;27(1):212‐214. [DOI] [PubMed] [Google Scholar]
- 18. Hasegawa D, Ohnishi Y, Koyama E, et al. Deleted in colorectal cancer (netrin‐1 receptor) antibodies and limbic encephalitis in a cat with hippocampal necrosis. J Vet Intern Med. 2019;33(3):1440‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pancotto TE, Rossmeisl JHJ. A case of stiff dog syndrome associated with anti‐glutamic acid decarboxylase antibodies. J Clin Mov Disord. 2017;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stafford EG, Kortum A, Castel A, et al. Presence of cerebrospinal fluid antibodies associated with autoimmune encephalitis of humans in dogs with neurologic disease. J Vet Intern Med. 2019;33(5):2175‐2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rozental AJ, McGrath S, Mooney AP, et al. Evaluation of cell‐based and tissue‐based immunofluorescent assays for detection of glial fibrillary acidic protein autoantibodies in the cerebrospinal fluid of dogs with meningoencephalitis of unknown origin and other central nervous system disorders. Am J Vet Res. 2021;82(2):132‐137. [DOI] [PubMed] [Google Scholar]
- 22. Huenerfauth EI, Bien CG, Bien C, Volk HA, Meyerhoff N. Case report: anti‐GABA (A) receptor encephalitis in a dog. Front Vet Sci. 2022;9:886711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pruss H, Leubner J, Wenke NK, Czirjak GA, Szentiks CA, Greenwood AD. Anti‐NMDA receptor encephalitis in the polar bear (Ursus maritimus) Knut. Sci Rep. 2015;5:12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freundt‐Revilla J, Maiolini A, Carlson R, et al. Th17‐skewed immune response and cluster of differentiation 40 ligand expression in canine steroid‐responsive meningitis‐arteritis, a large animal model for neutrophilic meningitis. J Neuroinflammation. 2017;14(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fischer A, Hülsmeyer VI, Munoz Schmieder VP, et al. Cyclooxygenase‐2 inhibition as an add‐on strategy in drug resistant epilepsy‐a canine translational study. Front Vet Sci. 2022;9:864293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Risio L, Bhatti SFM, Munana K, et al. International veterinary epilepsy task force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cerda‐Gonzalez S, Packer RA, Garosi L, et al. International veterinary canine dyskinesia task force ECVN consensus statement: terminology and classification. J Vet Intern Med. 2021;35(3):1218‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagendran A, McConnell JF, De Risio L, et al. Peri‐ictal magnetic resonance imaging characteristics in dogs with suspected idiopathic epilepsy. J Vet Intern Med. 2021;35(2):1008‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shell LG, Berezowski J, Rishniw M, Nibblett BM, Kelly P. Clinical and breed characteristics of idiopathic head tremor syndrome in 291 dogs: a retrospective study. Vet Med Int. 2015;2015:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97(2):839‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nessler J, Hug P, Mandigers PJJ, et al. Mitochondrial PCK2 missense variant in Shetland sheepdogs with paroxysmal exercise‐induced dyskinesia (PED). Genes (Basel). 2020;11(7):774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Terlizzi R, Platt SR. The function, composition and analysis of cerebrospinal fluid in companion animals: part II – analysis. Vet J. 2009;180(1):15‐32. [DOI] [PubMed] [Google Scholar]
- 33. Ances BM, Vitaliani R, Taylor RA, et al. Treatment‐responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128(8):1764‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berendt M, Farquhar RG, Mandigers PJJ, et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dubey D, Singh J, Britton JW, et al. Predictive models in the diagnosis and treatment of autoimmune epilepsy. Epilepsia. 2017;58(7):1181‐1189. [DOI] [PubMed] [Google Scholar]
- 36. Gaig C, Graus F, Compta Y, et al. Clinical manifestations of the anti‐IgLON5 disease. Neurology. 2017;88(18):1736‐1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaig C, Iranzo A, Cajochen C, et al. Characterization of the sleep disorder of anti‐IgLON5 disease. Sleep. 2019;42(9):133. [DOI] [PubMed] [Google Scholar]
- 38. Honorat JA, Komorowski L, Josephs KA, et al. IgLON5 antibody: neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa‐Molinar E. Controls for immunohistochemistry: the histochemical society's standards of practice for validation of immunohistochemical assays. J Histochem Cytochem. 2014;62(10):693‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pan H, Oliveira B, Saher G, et al. Uncoupling the widespread occurrence of anti‐NMDAR1 autoantibodies from neuropsychiatric disease in a novel autoimmune model. Mol Psychiatry. 2019;24(10):1489‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hara M, Martinez‐Hernandez E, Ariño H, et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology. 2018;90(16):1386‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Komorowski L, Stoecker W, Fraune J, Probst C. Letter to the editor re: “Pitfalls in the detection of N‐methyl‐d‐aspartate‐receptor (NMDA‐R) antibodies” accurate evaluation of anti‐NMDA‐R cell‐based assay avoids reporting of false positive results. Clin Biochem. 2017;50(7‐8):458‐459. [DOI] [PubMed] [Google Scholar]
- 43. Packer RMA, McGreevy PD, Salvin HE, Valenzuela MJ, Chaplin CM, Volk HA. Cognitive dysfunction in naturally occurring canine idiopathic epilepsy. PLoS One. 2018;13(2):e0192182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kayser MS, Titulaer MJ, Gresa‐Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti‐N‐methyl‐d‐aspartate receptor encephalitis. JAMA Neurol. 2013;70(9):1133‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dubey D, Kothapalli N, McKeon A, et al. Predictors of neural‐specific autoantibodies and immunotherapy response in patients with cognitive dysfunction. J Neuroimmunol. 2018;323:62‐72. [DOI] [PubMed] [Google Scholar]
- 46. Dubey D, Alqallaf A, Hays R, et al. Neurological autoantibody prevalence in epilepsy of unknown etiology. JAMA Neurol. 2017;74(4):397‐402. [DOI] [PubMed] [Google Scholar]
- 47. Chefdeville A, Treilleux I, Mayeur ME, et al. Immunopathological characterization of ovarian teratomas associated with anti‐N‐methyl‐D‐aspartate receptor encephalitis. Acta Neuropathol Commun. 2019;7(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Graus F, Escudero D, Oleaga L, et al. Syndrome and outcome of antibody‐negative limbic encephalitis. Eur J Neurol. 2018;25(8):1011‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baumgartner A, Rauer S, Mader I, Meyer PT. Cerebral FDG‐PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260(11):2744‐2753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1. Methods western blot.
File S2. Details on the clinical phenotype.
Figure S3. Western blot of canine and murine brain lysate. Western blots demonstrating failure of the antibody to bind to NMDAR1 protein in canine brain samples while there is expression of NMDAR1 protein (100 kDa) in murine hippocampus (lane 5‐6), and murine amygdala (lane 7‐8) samples. ACTB (45 kDa) was used as reference protein.


