Abstract
Home noninvasive ventilation (HNIV) improves outcomes in different disease categories. In this article, we discuss indications for when and how to initiate HNIV in COPD, obesity hypoventilation syndrome (OHS) and neuromuscular disorders (NMD). While in COPD, significant diurnal hypercapnia and high-intensity HNIV are essential ingredients for success, in NMD and OHS, early respiratory changes are best detected during sleep through oxy-capnography associated (or not) with respiratory polygraphy. In COPD and OHS, it is crucial to consider the coexistence of obstructive sleep apnoea because treatment with continuous positive airway pressure may be the simplest and most effective treatment that should be proposed even in hypercapnic patients as first-line therapy. In NMD, the need for continuous HNIV and eventual switching to tracheostomy ventilation makes this group's management more challenging. Achieving successful HNIV by improving quality of sleep, quality of life and keeping a good adherence to the therapy is a challenge, above all in COPD patients. In OHS patients, on top of HNIV, initiation of other interventions such as weight loss management is crucial. More resources should be invested in improving all these aspects. Telemonitoring represents a promising method to improve titration and follow-up of HNIV.
Tweetable abstract
Home NIV improves outcomes in different disease categories. However, there are certain specificities essential for success. CPAP may be sufficient in OHS, whereas in COPD and NMD, NIV is needed to normalise arterial carbon dioxide tension. https://bit.ly/3pZYQKx
Introduction
It is clear that the patient population eligible for home noninvasive ventilation (HNIV) is increasing worldwide [1, 2]. The identification of potential candidates for HNIV may be a challenging task. First, before starting HNIV, it is essential to ask three main questions: 1) Does the patient have a disease known to cause ventilatory failure? 2) Does the patient have symptoms suggesting hypoventilation? 3) Does the patient have physiological abnormalities confirming hypoventilation?
Sometimes, due to disease progression, ventilatory dependency may increase and ventilatory support must be adapted accordingly. Monitoring the quality of ventilation is essential to assess the quality and effectiveness of HNIV [3].
In this article, we review the three most important disease groups that are candidates for HNIV: COPD, obesity hypoventilation syndrome (OHS) and neuromuscular disorders (NMD).
Criteria for considering studies for this review
A literature search of Medline/PubMed of HNIV articles published between 2000 and 2020 was performed. The search items included “home non-invasive ventilation”, “chronic respiratory failure”, “chronic obstructive pulmonary disease”, “obesity-hypoventilation syndrome” and “amyotrophic lateral sclerosis”.
Chronic ventilatory support in COPD
When to start
Indication for HNIV in COPD patients with chronic hypercapnic respiratory failure is supported by a few studies with contrasting results. A recent Task Force supported by the European Respiratory Society considered two possible indications for HNIV treatment in COPD: 1) in stable chronic hypercapnic patients, and 2) for persistent hypercapnia after 2–4 weeks following an exacerbation of COPD needing mechanical ventilation [4]. Both indications were defined as “conditional recommendation with low certainty evidence” due to the few, not homogeneous studies.
In the stable chronic hypercapnic patient
Data pooled from randomised studies were not able to show a significant reduction of mortality and hospitalisation rate in this population. Probably the main reason was that not all the studies reached the aim of significantly reducing the arterial carbon dioxide tension (PaCO2) level. However, they showed that HNIV could significantly improve dyspnoea score, 6-min walking distance and quality of life. Because of the important social burden of these outcomes, the panel of experts decided upon a conditional recommendation. On the other hand, it is already known that dyspnoea and exercise tolerance are important predictors of mortality in COPD patients [5], which supports the indication to make a trial of HNIV, possibly targeting the setting to obtain a significant reduction of diurnal PaCO2. However, because of the possible high cost-effectiveness of the treatment, a follow-up at 6–12 months should be mandatory to verify the achievement of the purpose.
After an episode of acute hypercapnic respiratory failure
In this subgroup of patients, two recent studies with similar design showed different results in terms of effect of HNIV on mortality and hospitalisation rate [6, 7]. The most important explanation for the apparently contrasting results was the different timing in enrolment of patients. In the study by Murphy et al. [6], HNIV was shown to reduce the rate of deaths or hospitalisation in patients with persistent hypercapnia 2–4 weeks after the acute phase. On the other hand, by enrolling the patients at the discharge from hospitalisation, as in the study by Struik et al. [7], there is a risk of over-treatment due to possible spontaneous recovery from hypercapnia, as already described by Costello et al. [8].
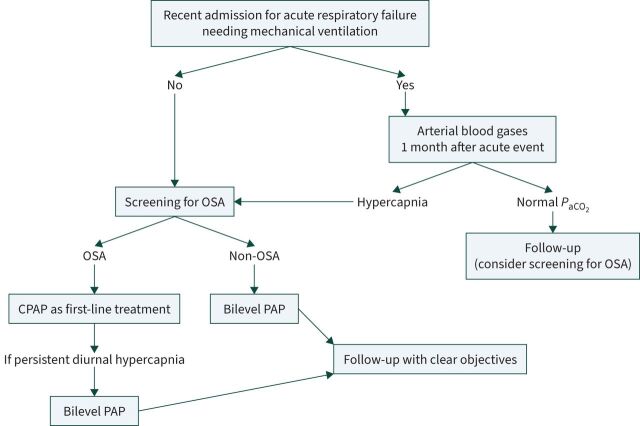
Similar indications came from the American Thoracic Society guidelines that followed, on HNIV in chronic hypercapnic COPD patients [9], which interestingly highlighted the importance of screening the patient for obstructive sleep apnoea (OSA) before starting HNIV. In fact, the prevalence of overlap with OSA was found to reach 50% in patients coming from an acute exacerbation of COPD [10]. This seems to be of great importance, because patients with overlap syndrome may benefit from continuous positive airway pressure (CPAP) to reverse hypercapnia. Figure 1 summarises practical suggestions in the approach to chronic hypercapnic COPD.
FIGURE 1.
Chronic hypercapnic respiratory failure and indications for home noninvasive ventilation. Hypercapnia was defined as arterial carbon dioxide tension (PaCO2) >50 mmHg. OSA: obstructive sleep apnoea; CPAP: continuous positive airway pressure; PAP: positive airway pressure.
How to ventilate the hypercapnic COPD patient: ventilation mode and setting
As raised by the Task Force panel, inhomogeneity of results from randomised controlled trials might be in part due to the different ventilation settings used, which were not always targeted to normalise PaCO2. This may explain the absence of improvement or only slight improvement of outcomes in some studies. For this reason, some years ago, high-intensity (HI) ventilation was proposed as a mode able to induce a significantly higher reduction of PaCO2 when compared with usual settings, defined as low-intensity (LI) [11]. HI ventilation was a pressure-targeted ventilation that aimed to reach the highest tolerated inspiratory pressure and a high back-up rate (very close to the patient's spontaneous respiratory rate) and was able to significantly decrease PaCO2 or reach normocapnia. In a randomised crossover trial, Dreher et al. [11] showed that HI ventilation was able to increase forced expiratory volume in 1 s and was associated with a better adherence than LI ventilation. However, no studies compared the long-term outcomes of these two ventilatory modes. A physiological study, albeit limited by the small number of patients enrolled, raised the issue that the main determinant of PaCO2 reduction is the high inspiratory pressure, irrespective of respiratory rate [12]. The physiological background may be the very high inspiratory drive that COPD patients show [13], which may be overcome by a high rise-time and high inspiratory pressure. For the same reason, the type of ventilator used can also make a difference because of significant differences in pressurisation behaviour, as recently shown by a bench and clinical study [14]. Flow waveform recorded by the built-in software of ventilators may help to better titrate these parameters. However, HI ventilation may be responsible for hyperinflation and induce ineffective efforts and “de-ventilation dyspnoea”. Ensuring a short inspiratory time with a high sensitivity of cycling criteria and/or setting a low maximal inspiratory time may be useful to avoid this problem [15]. Again, detailed data from ventilators are useful to detect this problem.
It should be considered that the definition of hypercapnia in COPD patients is very different among the different studies, as is the definition of the aim of noninvasive ventilation (NIV). Independently from the baseline degree of hypercapnia, a normalisation of elevated PaCO2 levels is unlikely to be achieved in all COPD patients even under high inspiratory positive airway pressure levels.
Managing overlapped OSA
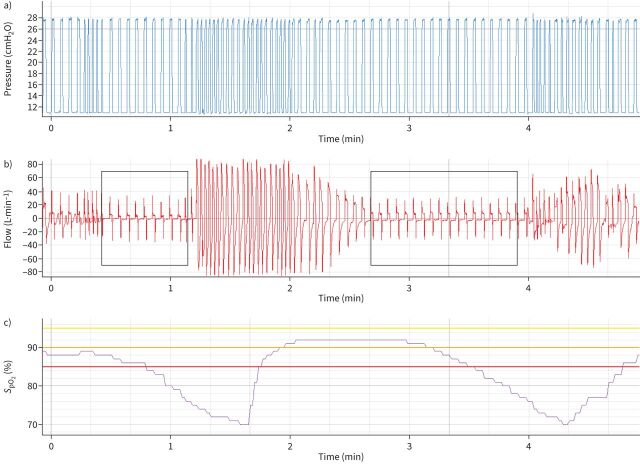
As mentioned, screening for OSA should be performed in COPD patients before starting HNIV (figure 1). This screening should use polygraphy. Some years ago, Marin et al. [15] showed that patients with overlapped COPD and OSA had a significantly higher risk of exacerbations than COPD patients with the same level of obstruction and using the same therapy. In these overlap patients, the use of CPAP significantly improved mortality and exacerbation rate. Moreover, CPAP is also able to significantly reduce PaCO2 to normocapnia in overlap patients with chronic hypercapnic respiratory failure [16]. In these patients, the main mechanism determinant of hypercapnia is probably the sleep disturbances, which, added to the increased respiratory load of the obstructive disease, lead to nocturnal and, consequently, diurnal hypoventilation. For all these reasons, a trial of CPAP treatment in overlap patients, even with hypercapnic respiratory failure, should be suggested because, in some cases, it may allow a reduction of costs (in countries where there is a reimbursement for both, CPAP is cheaper) and it may be easier to set. In fact, NIV needs a deeper monitoring of detailed data (pressure and flow curves) or polygraphy to correctly titrate expiratory positive airway pressure (EPAP) on the upper airway obstructive events and avoid ineffective ventilation (figure 2). Moreover, in COPD patients, above all with a prevalent emphysema, due to the significant reduction in the elastic recoil, hyperinflation induced by NIV may worsen the patient's symptoms, resulting in so-called “de-ventilation dyspnoea” [15]. When CPAP is not able to reduce PaCO2 level or is not well tolerated, a bilevel positive airway pressure is necessary. No study has compared the effect of CPAP and NIV in overlap COPD and OSA patients with hypercapnic respiratory failure.
FIGURE 2.
a) Pressure, b) flow and c) oxygen saturation measured by pulse oximetry (SpO2) data from a patient with chronic hypercapnic COPD ventilated with bilevel positive airway pressure. In the areas delimited by black boxes on the flow signal, there is an abrupt and intermittent flow reduction, as a consequence of upper airway obstruction. Despite the ventilator delivering the pre-set pressure support, probably in back-up rate, almost no volume is received by the patient. As a consequence, a significant oxygen desaturation is evident after 15 s.
Hybrid modes, such as volume-targeted pressure-controlled modes, have not shown improved clinical outcomes when compared to bilevel mode [17]. Moreover, the high respiratory drive of COPD patients may contribute to generating high tidal volumes and, consequently, may induce a lower assistance and ineffective matching with the patient's inspiratory flow.
Where to initiate HNIV in chronic COPD patients is still a debated topic. Setting of ventilators is not simple, high pressure is not always well tolerated and effective reduction of PaCO2 is not simple to reach and to monitor at home. There are few data comparing titration performed in the hospital with home titration. In a multicentre study enrolling 67 patients, home titration was showed to be non-inferior in terms of ability to significantly reduce the diurnal PaCO2 and improve quality of life, allowing a significant cost saving [18]. However, the follow-up of this study was only at 6 months. More data are needed to understand if home titration may ensure a good adherence to the therapy and may be effective in terms of long-term outcomes.
Follow-up of the hypercapnic COPD patient on HNIV
Achieving the target outcomes of HNIV represents a challenge in COPD patients with chronic hypercapnic respiratory failure. A recent real-life study showed that, among different causes of chronic respiratory failure with indication for HNIV, COPD patients represent a subgroup in which outcomes such as improvements in quality of life, sleep quality, adherence and reduction of PaCO2 are reached on average in 30–60% of patients [19]. Nevertheless, adherence to the therapy is very important because it significantly affects the efficacy of therapy. Many studies assumed a cut-off of 4 h per day, from data coming from other pathologies underlying chronic respiratory failure. However, a recent study showed that in hypercapnic COPD patients, longer daily usage of HNIV was associated with a longer time to death, with the most significant improvement with a usage between 12 and 16 h per day [20]. For these reasons and because of the low certainty of recommendation to use HNIV in chronic hypercapnic COPD, strict monitoring should be mandatory to avoid useless costs for the health system. Therefore, it is very important not only to treat the hypercapnia, but also to have a clear aim for the individual patient (i.e. reduction of exacerbations, improvement of symptoms, exercise tolerance, or quality of life), which should be clarified with a standardised timing in the follow-up. Reaching a previously defined outcome may be a reason to continue to use HNIV despite persistent hypercapnia.
Built-in software for downloading of recorded data is available in almost all ventilators in the market and represents the best and cheapest way to monitor HNIV. This provides long-term data about adherence to the therapy (up to some years) with details about interruptions of therapy and leaks for each day. Moreover, pressure and flow waveforms are available for the last 3–7 days, according to the ventilators used.
Telemonitoring is a promising tool to improve HNIV outcomes in COPD patients and reduce patient admission [21], although more prospective large multicentre studies are required for standardisation and validation.
Chronic ventilatory support in OHS
OHS is defined by daytime hypercapnia (i.e. PaCO2 >45 mmHg) associated with a body mass index (BMI) >30 kg·m−2, without any other disorder that may explain hypercapnia [22]. Given the obesity pandemic [23], OHS has become the most common cause of HNIV support initiation [24].
When to start chronic ventilatory support in patients with OHS
Initiation of HNIV after an acute respiratory failure is associated with a worse outcome than when initiated electively [25]. Therefore, screening for OHS in obese patients is needed. Such screening is challenging, as arterial blood gas measurements are not commonly performed in general practices. Therefore, the measurement of serum bicarbonate level has been suggested, using a cut-off of 27 mmol·L−1 [22, 26]. However, such a screening strategy led to a high false-positive rate [27], given numerous treatments with an impact on the level of bicarbonates [28]. Other studies have reported an increased likelihood of OHS with increased BMI, neck circumference and haemoglobin A1c [29, 30]. In the largest cohort, the only factor associated with OHS in multivariate analysis was the apnoea–hypopnoea index (AHI) [30].
OSA is a key factor for the onset and development of OHS. Severe OSA (AHI ≥30 events·h−1) is frequently seen in the OHS population [31]. Hence, performing an overnight sleep study is crucial for adequate management of patients at risk of OHS. As obese patients are more likely to report excessive daytime sleepiness [32], an overnight sleep study should be performed in patients with obesity, especially prior to undergoing surgery. Undiagnosed OHS is associated with an increased risk of post-operative complications [33] and with an increased mortality [34, 35].
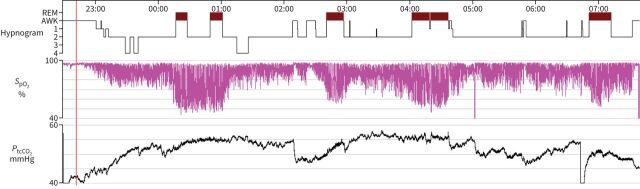
In patients with OHS who are electively initiating HNIV, the decision to initiate will depend on the presence or absence of severe OSA. In patients with an AHI <30 events·h−1, HNIV initiation is superior to lifestyle intervention for PaCO2 reduction [36]. Initiation of HNIV also reduces hospitalisation in patients adherent to ventilatory support use [37]. In patients with an AHI ≥30 events·h−1 (figure 3), CPAP should now be considered as the first-line treatment as its short-term and long-term efficacy is similar to HNIV [31, 38]. To date, there is no predictive factor of CPAP failure or a consensual definition of CPAP. Switching to HNIV should be considered in patients with persistent hypercapnia despite sufficient adherence to CPAP, and may be considered in patients not tolerating CPAP [39].
FIGURE 3.
Sleep study showing severe obstructive sleep apnoea and nocturnal hypoventilation. SpO2: oxygen saturation measured by pulse oximetry; PtcCO2: transcutaneous carbon dioxide tension.
Unfortunately, most patients with OHS have an episode of acute respiratory failure before being established on HNIV [25, 40]. These patients differ from those managed electively as they have a higher mortality [25], especially if they are not established on HNIV following their acute respiratory failure [41]. Henceforth, guidelines now advocate for every patient to be discharged with HNIV [26]. As none of the randomised controlled trials assessing the benefit of HNIV were in patients following an acute respiratory failure, the best management of patients established on HNIV after an acute event remains to be determined. As patients with OHS and acute respiratory failure often have acute heart failure [42], an accurate sleep study cannot be performed during the initial inpatient stay to assess whether the patient has severe OSA (AHI ≥30 events·h−1) or non-severe OSA (AHI <30 events·h−1). To determine whether the patient should continue HNIV or whether switching to CPAP would be preferable, patients should be re-evaluated within 3 months following discharge [26].
How to start chronic ventilatory support in patients with OHS
Unlike COPD or NMD, patients with OHS have a good prognosis [25, 37] and benefit from chronic ventilatory support regarding their quality of life and their quality of sleep [43, 44]. Therefore, outpatient HNIV setup is a safe option in this population. Several studies have assessed the efficacy of outpatient chronic ventilatory support in patients with restrictive disease including patients with OHS [45, 46]. HNIV approaches may have a lower cost than in-hospital approaches [46] but findings vary greatly depending on national healthcare organisation [47].
In patients with OHS, a pressure support ventilation is generally used, with a respiratory rate set at 2 breaths·min−1 less than the daytime respiratory rate, to avoid emergence of central events [48]. As patients with OHS have a decreased lung compliance with restrictive ventilatory defect, the level of pressure support is usually higher than that used in other diseases [25]. Given the high prevalence of OSA in patients with OHS [31], a high level of EPAP is required to overcome upper airway obstruction. For patients previously on positive airway pressure, an EPAP set 2 cmH2O below the pressure used previously seems to achieve similar efficacy in ventilatory support [39]. As for patients with OSA [49], a nasal interface should be used preferentially. However, in daily practice, most centres are using oronasal interfaces [25, 50].
In patients with OHS, automated modes of ventilation have been more largely evaluated than in other disease groups. Although some concerns were raised with those modes only including a targeted volume [51], those that also include an auto-EPAP titration for upper airway obstruction have satisfactory results. Compared with manually titrated modes, auto-EPAP with targeted volume modes have shown similar efficacy on the control of sleep disordered breathing, on sleep quality, on PaCO2 reduction and on improvement in health-related quality of life [47, 52, 53]. The main benefit of these automated modes of ventilation is the reduction in the time required for HNIV initiation [47, 53].
Follow-up of patients with OHS established on chronic ventilatory support
Objectives of chronic ventilatory support are to normalise daytime PaCO2, to control upper airway obstruction and to reduce acute admission for respiratory failure. These goals are usually achievable in patients with OHS. Normalisation of daytime PaCO2 should be achieved in most patients 3–6 months following initiation of chronic ventilatory support [36–38, 43, 44, 54, 55]. Control of upper airway obstruction may be monitored using data from ventilator built-in software [3]. However, the accuracy of these data has not been validated for all ventilator manufacturers [56] and such data should not be relied upon in the presence of leaks [57]. Therefore, overnight monitoring using oximetry and/or capnography should be performed [58].
Once these objectives are achieved, physicians should consider whether the patients can be stepped down from ventilatory support to positive airway pressure. Positive airway pressure is cheaper and more comfortable than ventilatory support. Few studies have assessed such a strategy, but it appears that around 20% of patients will fail such step-down for various reasons: recurrence of hypoventilation, less comfort using positive airway pressure, or acute respiratory failure [59, 60]. However, as 80% of patients succeed stepping down from ventilatory support to positive airway pressure, such trials should be performed in patients with OHS established on chronic ventilatory support. It may be performed a year after initiation of chronic ventilatory support.
Chronic ventilatory support in NMD
Many NMD can result in chronic ventilatory failure and benefit from home ventilation (table 1) [61]. It is well known that patients with NMD can have both inspiratory and expiratory muscle weakness and so apart from NIV they also need cough assistance (which is beyond the scope of this review) [62].
TABLE 1.
List of the most frequent neuromuscular disorders
| Duchenne and Becker muscular dystrophy |
| Steinert myotonic dystrophy |
| Amyotrophic lateral sclerosis |
| Spinal muscular atrophies |
| Limb-girdle muscular dystrophy |
| Facioscapulohumeral muscular dystrophy |
| Post-polio syndrome |
| Myasthenia gravis |
The specificities of the different NMD will not be covered in this review. Amyotrophic lateral sclerosis (ALS), an example of a rapidly evolving disease, requires a specific approach particularly for the bulbar involvement, which may render noninvasive respiratory aids less effective [63]. Discussing respiratory support management in ALS may make it easier to manage all other NMD.
When to start chronic ventilatory support in patients with ALS
According to the American Academy of Neurology guidelines [64], the presence of hypoventilation symptoms (e.g. orthopnoea), a maximal inspiratory pressure (PImax) <60 cmH2O/sniff nasal inspiratory pressure (SNIP) <40 cmH2O, or a forced vital capacity (FVC) <50% constitute an indication to start ventilatory support. In the absence of PImax and SNIP, a positional change in FVC of >20% (from sitting to supine) is a specific marker of diaphragm weakness, and supports the initiation of HNIV [65]. Specific hypoventilation scores [66], sleep disordered breathing questionnaires [67] and dyspnoea scales [68] may also be used in clinical practice to indicate initiation of HNIV.
Daytime hypercapnia is a late sign in NMD; nocturnal hypercapnia is preferred as an earlier marker of hypoventilation and should be sought even in patients with normal sleep oximetry levels [69]. Transcutaneous carbon dioxide tension (PtcCO2) devices to measure nocturnal PaCO2 still have some technical challenges and measurement failure rates, requiring more supervision when measured at home. However, they are preferred by patients and families [70].
Although there are no consensual definitions of nocturnal hypoventilation, a 10-mmHg increase in PtcCO2 above baseline, a PtcCO2 >49 mmHg for >10% of the total recording time, or a PtcCO2 peak >55 mmHg are criteria proposed by experienced centres in NMD [71].
Particularly in ALS, Boentert et al. [72] have described four different nocturnal oxy-capnography patterns: 1) patients with intermittent oxygen saturation without nocturnal hypercapnia (suggestive of pure OSA); 2) rapid eye movement (REM) sleep desaturation and hypercapnia; 3) desaturation and hypercapnia present during both non-REM and REM sleep; and 4) nocturnal hypercapnia without any significant changes of nocturnal oxygen saturation.
Sleep polygraphy or polysomnography may be also useful to detect underlying OSA [72]. In the study by Boentert et al. [72], AHI was ≥5 events·h−1 in 114 (45.6%), ≥15 events·h−1 in 41 (16.4%) and ≥30 events·h−1 in 17 (6.8%) patients with ALS. Median AHI and prevalence of sleep apnoea were significantly higher in male patients and in individuals with preserved bulbar function [72]. Moreover, the presence of underlying OSA has been associated with a worse prognosis in ALS [73].
Induced upper airway obstruction in ALS
In NMD, as in other disorders, ventilator settings can be adjusted to ensure sufficient inspiratory assistance to obtain normal daytime and nocturnal PaCO2. In the majority of cases, oronasal masks are used for HNIV initiation, but they may induce upper airway obstruction [74, 75]. Induced upper airway obstruction occurs more commonly in ALS than in other NMD and may have an impact on long-term outcomes [76]. A step-by-step approach has been proposed to solve this challenging event [77], starting with an interface change (from oronasal to nasal mask), an adjustment in bed position (with the use of a pillow for example, to avoid the position in which events occur), a change of the settings (increase in the EPAP levels up to 14 cmH2O if tolerated) or eventually a titration assisted with video-laryngoscopy [78]. In fact, the development of a standardised protocol of HNIV titration assisted with video-laryngoscopy [79] will allow a more complete understanding of the pathophysiology of upper airway obstruction and a better resolution of these challenging cases.
How to succeed with continuous HNIV in NMD: the case for mouthpiece ventilation
When a NMD patient starts to need more than nocturnal HNIV, an interface rotation strategy must be implemented [80]. Full face masks can be used as an alternative in case of pressure ulcers, at least as a temporary solution [81].
When ventilator dependency is >10 h·day−1, a second life support ventilator should be offered [61]. When diurnal ventilatory support is needed and the patient retains sufficient bulbar function (with sufficient orofacial strength), mouthpiece ventilation (MPV) should be proposed [82, 83]. ALS is the NMD where MPV may become ineffective earlier [83]. According to Bédard and McKim [84], the ability to generate a maximum insufflation capacity–FVC difference, reach a peak cough flow with lung volume recruitment of 180 L·min−1 and a revised ALS Functional Rating Scale bulbar subscale score of ≥6 points suggest the patient with ALS may maintain MPV.
Predictors of need for tracheostomy ventilation in ALS
According to Bach et al. [85], tracheostomy ventilation (TV) should be offered to fully dependent patients that cannot maintain oxygen saturation >95% in spite of well-controlled continuous HNIV and well-tailored mechanical in-exsufflation. According to this study [85], if not tracheotomised, this patient subgroup, which is characterised by poorer glottic function, will eventually die within 2 months.
Long-term tracheostomy ventilation: outcomes
In the 2001 Eurovent survey, 24% of NMD patients ventilated at home in Europe were under TV [86], especially in longer established centres (like Denmark and the Netherlands). Survival after TV in ALS is typically >30 months [87–89], so switching from NIV to TV extends survival in ALS [90], and this option should be kept in mind after formal elective patient and caregiver education [91]. For patients under TV that have impaired communication, complementary use of eye-tracking assistive devices improves quality of life for patients and reduces caregivers’ burden [92]. More sophisticated technologies like fully implanted brain–computer interfaces may allow for communication even in the locked-in ALS TV patients [93].
Conclusions
In conclusion, HNIV has been shown to improve outcomes in chronic hypercapnic respiratory failure, whatever the underlying pathophysiology. However, indications to start are quite different, nocturnal hypoventilation detection being the best time to recommend HNIV for NMD and OHS patients. The settings may also be different, with the suggestion of using high inspiratory pressure in COPD patients. In all cases, waveform detail monitoring allows the improvement of titration of HNIV, above all when upper airway obstruction is present. In overlap patients with OSA (COPD and OHS) a trial with CPAP as first-line therapy may be cost saving and better tolerated, with a similar outcome to bilevel modes. Outcomes of HNIV are not only merely the reduction of PaCO2, but also improvement of quality of life, quality of sleep, access to healthcare resources and mortality. The efficacy of HNIV in improving these outcomes has been shown to be different according to the underlying pathologies. Follow-up of these chronic care patients is a challenge and further studies should address telemonitoring to improve adherence and outcome of HNIV in all chronic hypercapnic respiratory diseases.
Key points
Capnography is essential to detect nocturnal hypoventilation.
COPD patients with persistent hypercapnia may be candidates for HNIV.
Patients with acute exacerbations of COPD should be recommended for HNIV if hypercapnia persists after 2–4 weeks.
Screening for OSA in COPD and OHS patients may allow treatment with CPAP as first-line therapy.
Built-in software is crucial to monitor adherence to the therapy and presence of leaks and upper airway obstruction as main causes of asynchronies and ineffective therapy.
Patient–ventilator matching is essential in COPD and residual obstructive events are a challenge in NMD (especially in ALS).
Maintaining an effective method of communication is a primary requirement for achieving a reasonable health-related quality of life in ALS patients using tracheostomy ventilation.
Telemonitoring should be developed to improve follow-up of home ventilated patients.
Footnotes
Conflict of interest: A. Carlucci has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Philips Respironics, Breas, Fisher & Paykel and Medicair; support for attending meetings and/or travel from Fisher & Paykel; and reports participation on a Data Safety Monitoring Board or Advisory Board for Breas. M. Patout reports grants or contracts from Fisher & Paykel, Resmed and Asten Santé; consulting fees from Philips Respironics, Resmed, Asten Santé and GSK; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Philips Respironics, Asten Santé, Resmed, Air Liquide Medical, SOS Oxygène, Antadir, Chiesi and Jazz Pharmaceutical; support for attending meetings and/or travel from Asten Santé; participation on a Data Safety Monitoring Board or Advisory Board for Resmed, Philips Respironics and Asten Santé; stock or stock options in Kernel Biomedical; and receipt of equipment, materials, drugs, medical writing, gifts or other services from Philips Respironics, Resmed and Fisher & Paykel. J.C. Winck reports payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Philips Respironics, Sentec, Breas, Vitalaire, Fisher & Paykel and NIPPON Gases; and support for attending meetings and/or travel from Vitalaire.
References
- 1.Ribeiro Baptista B, Baptiste A, Granger B, et al. Growth of home respiratory equipment from 2006 to 2019 and cost control by health policies. Respir Med Res 2022; 82: 100930. doi: 10.1016/j.resmer.2022.100930 [DOI] [PubMed] [Google Scholar]
- 2.Janssens JP, Derivaz S, Breitenstein E, et al. Changing patterns in long-term noninvasive ventilation: a 7-year prospective study in the Geneva Lake area. Chest 2003; 123: 67–79. doi: 10.1378/chest.123.1.67 [DOI] [PubMed] [Google Scholar]
- 3.Borel JC, Palot A, Patout M. Technological advances in home non-invasive ventilation monitoring: reliability of data and effect on patient outcomes. Respirology 2019; 24: 1143–1151. doi: 10.1111/resp.13497 [DOI] [PubMed] [Google Scholar]
- 4.Ergan B, Oczkowski S, Rochwerg B, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J 2019; 54: 1901003. doi: 10.1183/13993003.01003-2019 [DOI] [PubMed] [Google Scholar]
- 5.Cote CG, Pinto-Plata VM, Marin JM, et al. The modified BODE index: validation with mortality in COPD. Eur Respir J 2008; 32: 1269–1274. doi: 10.1183/09031936.00138507 [DOI] [PubMed] [Google Scholar]
- 6.Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA 2017; 317: 2177–2186. doi: 10.1001/jama.2017.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Struik FM, Sprooten RTM, Kerstjens HAM, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax 2014; 69: 826–834. doi: 10.1136/thoraxjnl-2014-205126 [DOI] [PubMed] [Google Scholar]
- 8.Costello R, Deegan P, Fitzpatrick M, et al. Reversible hypercapnia in chronic obstructive pulmonary disease: a distinct pattern of respiratory failure with a favorable prognosis. Am J Med 1997; 102: 239–244. doi: 10.1016/S0002-9343(97)00017-X [DOI] [PubMed] [Google Scholar]
- 9.Macrea M, Oczkowski S, Rochwerg B, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e74–e87. doi: 10.1164/rccm.202006-2382ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schreiber A, Cemmi F, Ambrosino N, et al. Prevalence and predictors of obstructive sleep apnea in patients with chronic obstructive pulmonary disease undergoing inpatient pulmonary rehabilitation. COPD 2018; 15: 265–270. doi: 10.1080/15412555.2018.1500533 [DOI] [PubMed] [Google Scholar]
- 11.Dreher M, Storre JH, Schmoor C, et al. High-intensity versus low-intensity non-invasive ventilation in patients with stable hypercapnic COPD: a randomised crossover trial. Thorax 2010; 65: 303–308. doi: 10.1136/thx.2009.124263 [DOI] [PubMed] [Google Scholar]
- 12.Murphy PB, Brignall K, Moxham J, et al. High pressure versus high intensity noninvasive ventilation in stable hypercapnic chronic obstructive pulmonary disease: a randomized crossover trial. Int J Chron Obstruct Pulmon Dis 2012; 7: 811–818. doi: 10.2147/COPD.S36151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He BT, Lu G, Xiao SC, et al. Coexistence of OSA may compensate for sleep related reduction in neural respiratory drive in patients with COPD. Thorax 2017; 72: 256–262. doi: 10.1136/thoraxjnl-2016-208467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalmolda C, Florez P, Corral M, et al. Does the efficacy of high intensity ventilation in stable COPD depend on the ventilator model? A bench-to-bedside study. Int J Chron Obstruct Pulmon Dis 2022; 17: 155–164. doi: 10.2147/COPD.S327994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 2010; 182: 325–331. doi: 10.1164/rccm.200912-1869OC [DOI] [PubMed] [Google Scholar]
- 16.Toraldo DM, De Nuccio F, Nicolardi G. Fixed-pressure nCPAP in patients with obstructive sleep apnea (OSA) syndrome and chronic obstructive pulmonary disease (COPD): a 24-month follow-up study. Sleep Breath 2010; 14: 115–123. doi: 10.1007/s11325-009-0291-1 [DOI] [PubMed] [Google Scholar]
- 17.Arellano-Maric MP, Gregoretti C, Duiverman M, et al. Long-term volume-targeted pressure-controlled ventilation: sense or nonsense? Eur Respir J 2017; 49: 1602193. doi: 10.1183/13993003.02193-2016 [DOI] [PubMed] [Google Scholar]
- 18.Duiverman ML, Vonk JM, Bladder G, et al. Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: a randomised controlled trial. Thorax 2020; 75: 244–252. doi: 10.1136/thoraxjnl-2019-213303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolly G, Razakamanantsoa L, Fresnel E, et al. Defining successful non-invasive ventilation initiation: data from a real-life cohort. Respirology 2021; 26: 1067–1075. doi: 10.1111/resp.14118 [DOI] [PubMed] [Google Scholar]
- 20.Schwarz EI, Mackie M, Weston N, et al. Time-to-death in chronic respiratory failure on home mechanical ventilation: a cohort study. Respir Med 2020; 162: 105877. doi: 10.1016/j.rmed.2020.105877 [DOI] [PubMed] [Google Scholar]
- 21.Jiang W, Wang L, Song Y. Titration and follow-up for home noninvasive positive pressure ventilation in chronic obstructive pulmonary disease: the potential role of tele-monitoring and the Internet of things. Clin Respir J 2021; 15: 705–715. doi: 10.1111/crj.13352 [DOI] [PubMed] [Google Scholar]
- 22.Randerath W, Verbraecken J, Andreas S, et al. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J 2017; 49: 1600959. doi: 10.1183/13993003.00959-2016 [DOI] [PubMed] [Google Scholar]
- 23.GBD 2015 Obesity Collaborators , Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377: 13–27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantero C, Adler D, Pasquina P, et al. Long-term noninvasive ventilation in the Geneva Lake area: indications, prevalence, and modalities. Chest 2020; 158: 279–291. doi: 10.1016/j.chest.2020.02.064 [DOI] [PubMed] [Google Scholar]
- 25.Patout M, Lhuillier E, Kaltsakas G, et al. Long-term survival following initiation of home non-invasive ventilation: a European study. Thorax 2020; 75: 965–973. doi: 10.1136/thoraxjnl-2019-214204 [DOI] [PubMed] [Google Scholar]
- 26.Mokhlesi B, Masa JF, Brozek JL, et al. Evaluation and management of obesity hypoventilation syndrome. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2019; 200: e6–e24. doi: 10.1164/rccm.201905-1071ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borel JC, Guerber F, Jullian-Desayes I, et al. Prevalence of obesity hypoventilation syndrome in ambulatory obese patients attending pathology laboratories. Respirology 2017; 22: 1190–1198. doi: 10.1111/resp.13051 [DOI] [PubMed] [Google Scholar]
- 28.Jullian-Desayes I, Borel JC, Guerber F, et al. Drugs influencing acid base balance and bicarbonate concentration readings. Expert Rev Endocrinol Metab 2016; 11: 209–216. doi: 10.1586/17446651.2016.1147951 [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Guo J, Liang Y, et al. Obesity hypoventilation syndrome in bariatric surgery patients: an underestimated disease. Surg Obes Relat Dis 2022; 18: 894–901. doi: 10.1016/j.soard.2022.02.017 [DOI] [PubMed] [Google Scholar]
- 30.Tran K, Wang L, Gharaibeh S, et al. Elucidating predictors of obesity hypoventilation syndrome in a large bariatric surgery cohort. Ann Am Thorac Soc 2020; 17: 1279–1288. doi: 10.1513/AnnalsATS.202002-135OC [DOI] [PubMed] [Google Scholar]
- 31.Masa JF, Corral J, Alonso ML, et al. Efficacy of different treatment alternatives for obesity hypoventilation syndrome. Pickwick study. Am J Respir Crit Care Med 2015; 192: 86–95. doi: 10.1164/rccm.201410-1900OC [DOI] [PubMed] [Google Scholar]
- 32.Slater G, Pengo MF, Kosky C, et al. Obesity as an independent predictor of subjective excessive daytime sleepiness. Respir Med 2013; 107: 305–309. doi: 10.1016/j.rmed.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 33.Chindamporn P, Wang L, Bena J, et al. Obesity-associated sleep hypoventilation and increased adverse post-operative bariatric surgery outcomes in a large clinical retrospective cohort. J Clin Sleep Med 2022; 18: 2793–2801. doi: 10.5664/jcsm.10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaw R, Bhateja P, Paz Y Mar H, et al. Postoperative complications in patients with unrecognized obesity hypoventilation syndrome undergoing elective noncardiac surgery. Chest 2016; 149: 84–91. doi: 10.1378/chest.14-3216 [DOI] [PubMed] [Google Scholar]
- 35.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116: 1–7. doi: 10.1016/j.amjmed.2003.08.022 [DOI] [PubMed] [Google Scholar]
- 36.Masa JF, Corral J, Caballero C, et al. Non-invasive ventilation in obesity hypoventilation syndrome without severe obstructive sleep apnoea. Thorax 2016; 71: 899–906. doi: 10.1136/thoraxjnl-2016-208501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masa JF, Benitez I, Sanchez-Quiroga MA, et al. Long-term noninvasive ventilation in obesity hypoventilation syndrome without severe OSA: the Pickwick randomized controlled trial. Chest 2020; 158: 1176–1186. doi: 10.1016/j.chest.2020.03.068 [DOI] [PubMed] [Google Scholar]
- 38.Masa JF, Mokhlesi B, Benítez I, et al. Long-term clinical effectiveness of continuous positive airway pressure therapy versus non-invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open-label, randomised controlled trial. Lancet 2019; 393: 1721–1732. doi: 10.1016/S0140-6736(18)32978-7 [DOI] [PubMed] [Google Scholar]
- 39.Ishak A, Ramsay M, Hart N, et al. BPAP is an effective second-line therapy for obese patients with OSA failing regular CPAP: a prospective observational cohort study. Respirology 2020; 25: 443–448. doi: 10.1111/resp.13674 [DOI] [PubMed] [Google Scholar]
- 40.Lee WY, Mokhlesi B. Diagnosis and management of obesity hypoventilation syndrome in the ICU. Crit Care Clin 2008; 24: 533–549. doi: 10.1016/j.ccc.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 41.Mokhlesi B, Masa JF, Afshar M, et al. The effect of hospital discharge with empiric noninvasive ventilation on mortality in hospitalized patients with obesity hypoventilation syndrome. An individual patient data meta-analysis. Ann Am Thorac Soc 2020; 17: 627–637. doi: 10.1513/AnnalsATS.201912-887OC [DOI] [PubMed] [Google Scholar]
- 42.Chebib N, Nesme P, Freymond N, et al. Acute respiratory failure in obesity-hypoventilation syndrome managed in the ICU. Respir Care 2019; 64: 1545–1554. doi: 10.4187/respcare.06901 [DOI] [PubMed] [Google Scholar]
- 43.Piper AJ, Wang D, Yee BJ, et al. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax 2008; 63: 395–401. doi: 10.1136/thx.2007.081315 [DOI] [PubMed] [Google Scholar]
- 44.Borel JC, Tamisier R, Gonzalez-Bermejo J, et al. Noninvasive ventilation in mild obesity hypoventilation syndrome: a randomized controlled trial. Chest 2012; 141: 692–702. doi: 10.1378/chest.10-2531 [DOI] [PubMed] [Google Scholar]
- 45.Hazenberg A, Kerstjens HAM, Prins SCL, et al. Initiation of home mechanical ventilation at home: a randomised controlled trial of efficacy, feasibility and costs. Respir Med 2014; 108: 1387–1395. doi: 10.1016/j.rmed.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 46.van den Biggelaar RJM, Hazenberg A, Cobben NAM, et al. A randomized trial of initiation of chronic noninvasive mechanical ventilation at home vs in-hospital in patients with neuromuscular disease and thoracic cage disorder: the Dutch Homerun trial. Chest 2020; 158: 2493–2501. doi: 10.1016/j.chest.2020.07.007 [DOI] [PubMed] [Google Scholar]
- 47.Murphy PB, Patout M, Arbane G, et al. Cost-effectiveness of outpatient versus inpatient non-invasive ventilation setup in obesity hypoventilation syndrome: the OPIP trial. Thorax 2023; 78: 24–31. doi: 10.1136/thorax-2021-218497 [DOI] [PubMed] [Google Scholar]
- 48.Contal O, Adler D, Borel JC, et al. Impact of different backup respiratory rates on the efficacy of noninvasive positive pressure ventilation in obesity hypoventilation syndrome: a randomized trial. Chest 2013; 143: 37–46. doi: 10.1378/chest.11-2848 [DOI] [PubMed] [Google Scholar]
- 49.Andrade RGS, Viana FM, Nascimento JA, et al. Nasal vs oronasal CPAP for OSA treatment: a meta-analysis. Chest 2018; 153: 665–674. doi: 10.1016/j.chest.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 50.Lebret M, Leotard A, Pepin JL, et al. Nasal versus oronasal masks for home non-invasive ventilation in patients with chronic hypercapnia: a systematic review and individual participant data meta-analysis. Thorax 2021; 76: 1108–1116. doi: 10.1136/thoraxjnl-2020-215613 [DOI] [PubMed] [Google Scholar]
- 51.Janssens JP, Metzger M, Sforza E. Impact of volume targeting on efficacy of bi-level non-invasive ventilation and sleep in obesity-hypoventilation. Respir Med 2009; 103: 165–172. doi: 10.1016/j.rmed.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 52.Orr JE, Coleman J, Criner GJ, et al. Automatic EPAP intelligent volume-assured pressure support is effective in patients with chronic respiratory failure: a randomized trial. Respirology 2019; 24: 1204–1211. doi: 10.1111/resp.13546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patout M, Gagnadoux F, Rabec C, et al. AVAPS-AE versus ST mode: a randomized controlled trial in patients with obesity hypoventilation syndrome. Respirology 2020; 25: 1073–1081. doi: 10.1111/resp.13784 [DOI] [PubMed] [Google Scholar]
- 54.Howard ME, Piper AJ, Stevens B, et al. A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome. Thorax 2017; 72: 437–444. doi: 10.1136/thoraxjnl-2016-208559 [DOI] [PubMed] [Google Scholar]
- 55.Murphy PB, Davidson C, Hind MD, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax 2012; 67: 727–734. doi: 10.1136/thoraxjnl-2011-201081 [DOI] [PubMed] [Google Scholar]
- 56.Georges M, Adler D, Contal O, et al. Reliability of apnea-hypopnea index measured by a home bi-level pressure support ventilator versus a polysomnographic assessment. Respir Care 2015; 60: 1051–1056. doi: 10.4187/respcare.03633 [DOI] [PubMed] [Google Scholar]
- 57.Contal O, Vignaux L, Combescure C, et al. Monitoring of noninvasive ventilation by built-in software of home bilevel ventilators: a bench study. Chest 2012; 141: 469–476. doi: 10.1378/chest.11-0485 [DOI] [PubMed] [Google Scholar]
- 58.Janssens JP, Borel JC, Pepin JL, et al. Nocturnal monitoring of home non-invasive ventilation: the contribution of simple tools such as pulse oximetry, capnography, built-in ventilator software and autonomic markers of sleep fragmentation. Thorax 2011; 66: 438–445. doi: 10.1136/thx.2010.139782 [DOI] [PubMed] [Google Scholar]
- 59.Arellano-Maric MP, Hamm C, Duiverman ML, et al. Obesity hypoventilation syndrome treated with non-invasive ventilation: is a switch to CPAP therapy feasible? Respirology 2020; 25: 435–442. doi: 10.1111/resp.13704 [DOI] [PubMed] [Google Scholar]
- 60.Patout M, Dantoing E, De Marchi M, et al. Step-down from non-invasive ventilation to continuous positive airway pressure: a better phenotyping is required. Respirology 2020; 25: 456. doi: 10.1111/resp.13746 [DOI] [PubMed] [Google Scholar]
- 61.Wolfe LF, Benditt JO, Aboussouan L, et al. Optimal NIV Medicare access promotion: patients with thoracic restrictive disorders: a technical expert panel report from the American College of Chest Physicians, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society. Chest 2021; 160: e399–e408. doi: 10.1016/j.chest.2021.05.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winck JC, Goncalves MR, Lourenco C, et al. Effects of mechanical insufflation-exsufflation on respiratory parameters for patients with chronic airway secretion encumbrance. Chest 2004; 126: 774–780. doi: 10.1378/chest.126.3.774 [DOI] [PubMed] [Google Scholar]
- 63.Sancho J, Martínez D, Bures E, et al. Bulbar impairment score and survival of stable amyotrophic lateral sclerosis patients after noninvasive ventilation initiation. ERJ Open Res 2018; 4: 00159-2017. doi: 10.1183/23120541.00159-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009; 73: 1218–1226. doi: 10.1212/WNL.0b013e3181bc0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lechtzin N, Wiener CM, Shade DM, et al. Spirometry in the supine position improves the detection of diaphragmatic weakness in patients with amyotrophic lateral sclerosis. Chest 2002; 121: 436–442. doi: 10.1378/chest.121.2.436 [DOI] [PubMed] [Google Scholar]
- 66.Dorst J, Behrendt G, Ludolph AC. Non-invasive ventilation and hypercapnia-associated symptoms in amyotrophic lateral sclerosis. Acta Neurol Scand 2019; 139: 128–134. doi: 10.1111/ane.13043 [DOI] [PubMed] [Google Scholar]
- 67.Steier J, Jolley CJ, Seymour J, et al. Screening for sleep-disordered breathing in neuromuscular disease using a questionnaire for symptoms associated with diaphragm paralysis. Eur Respir J 2011; 37: 400–405. doi: 10.1183/09031936.00036210 [DOI] [PubMed] [Google Scholar]
- 68.Vogt S, Schreiber S, Kollewe K, et al. Dyspnea in amyotrophic lateral sclerosis: the Dyspnea-ALS-Scale (DALS-15) essentially contributes to the diagnosis of respiratory impairment. Respir Med 2019; 154: 116–121. doi: 10.1016/j.rmed.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 69.Georges M, Nguyen-Baranoff D, Griffon L, et al. Usefulness of transcutaneous PCO 2 to assess nocturnal hypoventilation in restrictive lung disorders. Respirology 2016; 21: 1300–1306. doi: 10.1111/resp.12812 [DOI] [PubMed] [Google Scholar]
- 70.Shi J, Chiang J, Ambreen M, et al. Ambulatory transcutaneous carbon dioxide monitoring for children with neuromuscular disease. Sleep Med 2023; 101: 221–227. doi: 10.1016/j.sleep.2022.10.028 [DOI] [PubMed] [Google Scholar]
- 71.Orlikowski D, Prigent H, Quera Salva MA, et al. Prognostic value of nocturnal hypoventilation in neuromuscular patients. Neuromuscul Disord 2017; 27: 326–330. doi: 10.1016/j.nmd.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 72.Boentert M, Glatz C, Helmle C, et al. Prevalence of sleep apnoea and capnographic detection of nocturnal hypoventilation in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2018; 89: 418–424. doi: 10.1136/jnnp-2017-316515 [DOI] [PubMed] [Google Scholar]
- 73.Quaranta VN, Carratu P, Damiani MF, et al. The prognostic role of obstructive sleep apnea at the onset of amyotrophic lateral sclerosis. Neurodegener Dis 2017; 17: 14–21. doi: 10.1159/000447560 [DOI] [PubMed] [Google Scholar]
- 74.Schellhas V, Glatz C, Beecken I, et al. Upper airway obstruction induced by non-invasive ventilation using an oronasal interface. Sleep Breath 2018; 22: 781–788. doi: 10.1007/s11325-018-1640-8 [DOI] [PubMed] [Google Scholar]
- 75.Vrijsen B, Buyse B, Belge C, et al. Upper airway obstruction during noninvasive ventilation induced by the use of an oronasal mask. J Clin Sleep Med 2014; 10: 1033–1035. doi: 10.5664/jcsm.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Georges M, Attali V, Golmard JL, et al. Reduced survival in patients with ALS with upper airway obstructive events on non-invasive ventilation. J Neurol Neurosurg Psychiatry 2016; 87: 1045–1050. doi: 10.1136/jnnp-2015-312606 [DOI] [PubMed] [Google Scholar]
- 77.Georges M, Perez T, Rabec C, et al. Proposals from a French expert panel for respiratory care in ALS patients. Respir Med Res 2022; 81: 100901. doi: 10.1016/j.resmer.2022.100901 [DOI] [PubMed] [Google Scholar]
- 78.Sayas Catalán J, Jiménez Huerta I, Benavides Mañas P, et al. Videolaryngoscopy with noninvasive ventilation in subjects with upper-airway obstruction. Respir Care 2017; 62: 222–230. doi: 10.4187/respcare.04784 [DOI] [PubMed] [Google Scholar]
- 79.Conde B, Martins N, Brandao M, et al. Upper airway video endoscopy: assessment of the response to positive pressure ventilation and mechanical in-exsufflation. Pulmonology 2019; 25: 299–304. doi: 10.1016/j.pulmoe.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 80.Scala R, Accurso G, Ippolito M, et al. Material and technology: back to the future for the choice of interface for non-invasive ventilation – a concise review. Respiration 2020; 99: 800–817. doi: 10.1159/000509762 [DOI] [PubMed] [Google Scholar]
- 81.Belchior I, Goncalves MR, Winck JC. Continuous noninvasive ventilation delivered by a novel total face mask: a case series report. Respir Care 2012; 57: 449–453. doi: 10.4187/respcare.01275 [DOI] [PubMed] [Google Scholar]
- 82.Garuti G, Nicolini A, Grecchi B, et al. Open circuit mouthpiece ventilation: concise clinical review. Rev Port Pneumol 2014; 20: 211–218. doi: 10.1016/j.rppneu.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 83.Gonçalves MR, Bach JR, Ishikawa Y, et al. Continuous noninvasive ventilatory support outcomes for patients with neuromuscular disease: a multicenter data collaboration. Pulmonology 2021; 27: 509–517. doi: 10.1016/j.pulmoe.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 84.Bédard ME, McKim DA. Daytime mouthpiece for continuous noninvasive ventilation in individuals with amyotrophic lateral sclerosis. Respir Care 2016; 61: 1341–1348. 10.4187/respcare.04309. [DOI] [PubMed] [Google Scholar]
- 85.Bach JR, Bianchi C, Aufiero E. Oximetry and indications for tracheotomy for amyotrophic lateral sclerosis. Chest 2004; 126: 1502–1507. doi: 10.1378/chest.126.5.1502 [DOI] [PubMed] [Google Scholar]
- 86.Lloyd-Owen SJ, Donaldson GC, Ambrosino N, et al. Patterns of home mechanical ventilation use in Europe: results from the Eurovent survey. Eur Respir J 2005; 25: 1025–1031. 10.1183/09031936.05.00066704. [DOI] [PubMed] [Google Scholar]
- 87.Kaub-Wittemer D, von Steinbüchel N, Wasner M, et al. Quality of life and psychosocial issues in ventilated patients with amyotrophic lateral sclerosis and their caregivers. J Pain Symptom Manage 2003; 26: 890–896. doi: 10.1016/S0885-3924(03)00323-3 [DOI] [PubMed] [Google Scholar]
- 88.Vianello A, Arcaro G, Palmieri A, et al. Survival and quality of life after tracheostomy for acute respiratory failure in patients with amyotrophic lateral sclerosis. J Crit Care 2011; 26: 329.e7–329.e14. doi: 10.1016/j.jcrc.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 89.Lo Coco D, Marchese S, La Bella V, et al. The amyotrophic lateral sclerosis functional rating scale predicts survival time in amyotrophic lateral sclerosis patients on invasive mechanical ventilation. Chest 2007; 132: 64–69. doi: 10.1378/chest.06-2712 [DOI] [PubMed] [Google Scholar]
- 90.Dreyer P, Lorenzen CK, Schou L, et al. Survival in ALS with home mechanical ventilation non-invasively and invasively: a 15-year cohort study in west Denmark. Amyotroph Lateral Scler Frontotemporal Degener 2014; 15: 62–67. doi: 10.3109/21678421.2013.837929 [DOI] [PubMed] [Google Scholar]
- 91.McKim DA, King J, Walker K, et al. Formal ventilation patient education for ALS predicts real-life choices. Amyotroph Lateral Scler 2012; 13: 59–65. doi: 10.3109/17482968.2011.626053 [DOI] [PubMed] [Google Scholar]
- 92.Hwang CS, Weng HH, Wang LF, et al. An eye-tracking assistive device improves the quality of life for ALS patients and reduces the caregivers’ burden. J Mot Behav 2014; 46: 233–238. doi: 10.1080/00222895.2014.891970 [DOI] [PubMed] [Google Scholar]
- 93.Vansteensel MJ, Pels EGM, Bleichner MG, et al. Fully implanted brain–computer interface in a locked-in patient with ALS. N Engl J Med 2016; 375: 2060–2066. doi: 10.1056/NEJMoa1608085 [DOI] [PMC free article] [PubMed] [Google Scholar]