Background.
Kidney transplants from small pediatric donors are considered marginal and often transplanted as dual grafts. This study aimed to compare long-term outcomes between recipients of single kidney transplants (SKTs) and dual en bloc kidney transplants (EBKTs) from small pediatric donors.
Methods.
Data were obtained from the Australia and New Zealand Dialysis and Transplant Registry. All adult recipients of kidney transplants from donors aged ≤5 y were identified. The primary outcome of interest was death-censored graft survival by donor type. The secondary outcomes were early graft loss, delayed graft function, serum creatinine posttransplantation, acute rejection, and patient survival.
Results.
There were 183 adult recipients of kidney transplants from donors aged ≤5 y old. Of these, 60 patients had EBKT grafts, 79 patients had SKT grafts, and 44 patients had grafts of unknown type. Compared with SKT donors, EBKT donors had lower mean age (P < 0.001) and body weight (P < 0.001). There was no significant difference in death-censored graft survival between the groups, with median survival of 23.8 y (interquartile range 21.2–25) in the EBKT cohort and 21.8 y (11.6–26.8) in the SKT cohort (hazard ratio 1.3; 95% confidence interval, 0.59-2.64; P = 0.56). EBKT grafts had lower acute rejection rates than SKT grafts (P = 0.014). There was no significant difference observed between groups with respect to early graft loss, delayed graft function, posttransplantation serum creatinine posttransplantation, or patient survival.
Conclusions.
EBKT and SKTs from small pediatric donors are associated with excellent long-term graft survival rates.
Because of long waiting times on transplantation lists, there has been increased utilization of marginal deceased donor kidney transplants in Australia and New Zealand.1 The use of transplants from small pediatric donors was historically controversial because these grafts were associated with poor outcomes compared with adult donor organs.2,3 These grafts were seen as technically difficult with anastomoses between small blood vessels and larger recipient vessels.3 Early literature demonstrated that kidneys from small pediatric donors were associated with a significant risk of graft thrombosis when transplanted into paediatric4 and adult recipients.2
An additional concern is that reduced kidney mass from small donor kidneys may result in hyperfiltration injury because of size mismatch and raised intraglomerular pressures.5 One way to mitigate this risk is through the use of en bloc kidney transplant (EBKT)—a technique that involves keeping both kidneys attached to the aorta and inferior vena cava and using these as conduits in the recipient.6 The predominant method of en bloc implantation in Australia and New Zealand involves the vascular anastomosis of these larger donor patches to the recipient iliac vessels. This can be performed via the classical extra-peritoneal approach or even through a laparotomy approach‚ which has been shown to yield excellent long-term outcomes.7 Studies have demonstrated that EBKTs from pediatric donors have excellent short-term and long-term outcomes.8-14 The influence of this literature on surgical practice has seen the introduction of protocol-based decision-making for EBKT when donors are very small, often using age or weight criteria.14,15 Although the use of EBKT from pediatric donors may be safe, this method carries a considerable opportunity cost in that 2 potentially viable kidneys are transplanted into a single recipient.
There are minimal data available to clinicians to guide decision-making as to when to use EBKT or single kidney transplants (SKTs) from pediatric donors. Transplant centers have developed various age- and weight-based protocols;15,16 however, the ensuing long-term outcomes have not been comprehensively examined. The aims of this study, therefore, were to examine the outcomes of kidney transplants from young (≤5 y old) pediatric donors and compare these outcomes between EBKT and SKT.
MATERIAL AND METHODS
Study Design
This retrospective, multicenter, observational cohort study used de-identified data obtained from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry. It was approved by the local Human Research Ethics Committee (2018/QMS/44000). All adult recipients of pediatric kidney transplants from donors aged 5 y or younger between 1963 (Registry inception) and 2016 were included. They were then categorized according to whether they received EBKT or SKT. Recipients were excluded if they met any of the following criteria: unknown graft type with respect to EBKT or SKT, multiple organ transplants, or prior kidney transplantation.
Data Collection
Recipient and donor characteristics were recorded at the time of transplantation. Recipient characteristics included age, gender, weight, height, primary kidney disease, comorbid diseases (pulmonary disease, coronary artery disease, peripheral vascular disease, cerebrovascular disease, hypertension, and diabetes mellitus), dialysis duration, and time to transplant from dialysis commencement. Donor characteristics included age, sex, weight, height, cause of death, terminal creatinine, and blood group. Transplantation details recorded were the date of transplantation, ischemic time, and HLA mismatch. Transplant era was defined according to year of transplant (1989–1999 or 2000–2016) and only years with known graft types were included. The dividing mark corresponded to the time point when mycophenolate was used in place of azathioprine as maintenance immunosuppression.
Clinical Outcomes
The primary outcome was death-censored graft survival. Secondary outcomes were early graft loss (defined as graft failure within 28 d of kidney transplantation), delayed graft function (DGF; defined by ANZDATA as requiring dialysis within first 72 h of transplantation), posttransplantation serum creatinine over time (at 1-, 5-, and 10-y posttransplantation), acute rejection, and patient survival.
Statistical Analysis
Results were expressed as proportion (%), mean (SD), or median (interquartile range [IQR]), as appropriate. The demographics of EBKT and SKT groups were compared using Fisher exact, Chi-square, unpaired t tests, or 2-sample Wilcoxon rank-sum tests depending on data type and distribution. Death-censored graft and patient survival times were assessed by Kaplan-Meier analysis and multivariable Cox proportional hazards regression (with the EBKT group as the reference group). A secondary Kaplan-Meier analysis of graft survival times was performed after separating donors by weight at transplant (<20 kg or ≥ 20 kg). After separating donors by weight, graft survival between EBKT and SKT was compared using log-rank tests. Donor-related covariates included in the multivariate Cox regression models were age, weight, height, donor-to-recipient weight ratio, and graft type (EBKT/SKT).
A secondary competing risks analysis with death as a competing event was also performed to analyze the relationship between graft survival and graft type. Chi-square tests were used to determine if the type of graft received was significantly associated with the risk of DGF. Multivariable logistic regression analyses that included the type of graft and either donor age or donor weight were also completed to determine a relationship with DGF risk. A similar multivariable logistic regression was undertaken that included type of graft and either recipient weight or age. The relationship between graft survival and transplant center volume, with hospitals classified as having performed <15 small donor transplants or >15 small donor transplants, was assessed using Cox regression analysis. Similarly, an interaction between early graft loss and center experience was assessed using Chi-square tests.
Chi-square and Fisher exact tests were utilized to assess the relationship between graft type and acute rejection at any time posttransplantation. Chi-square and Fisher exact tests were also used to assess for an association between graft type and transplant era (1989–1999 or 2000–2016). The effect of transplant era and graft type on graft survival was also assessed using a Cox proportional hazards regression. A mixed-effects linear regression model with a random intercept for each patient was used to compare the long-term serum creatinine values between EBKT and SKT recipients. The relationship between early graft loss and graft type was assessed using Chi-square and Fisher exact tests.
Statistical analysis was conducted with the Stata software program, version number 15.1 (College Station, TX). P values of less than 0.05 were considered statistically significant.
RESULTS
Study Population
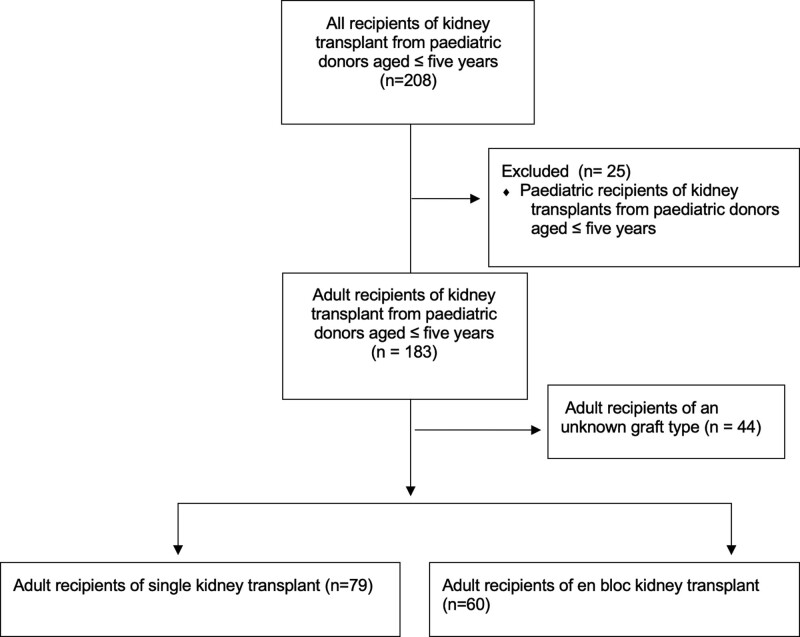
During the study period (1963–2016), a total of 208 patients received a kidney transplant from a deceased pediatric donor aged 5 y old or younger across 20 transplant centers in Australia and New Zealand (Figure 1). Once pediatric recipients were excluded (n = 25), there were 183 adult recipients remaining. There were 7 donors aged younger than 12 mo. The graft type (EBKT or SKT) was recorded in 139 recipients (SKT n = 79, EBKT n = 60). The transplant dates for patients with a known graft type ranged from 1989 to 2016. There were 129 DBD donors and 10 donation after circulatory death donors recorded during the study period (Table 1). Compared with SKT donors, the EBKT donors were significantly younger and had lower body weights and heights (Table 1). All donors aged younger than 12 mo (n = 7) had a recorded graft type of EBKT. The donor weight to recipient weight ratio was lower for the EBKT donor/recipient group. Otherwise, recipient and transplant characteristics were similar between the 2 groups (Table 2). The median follow-up time for the total cohort (n = 183) was 7.14 y (IQR 2.14–16.50). The median follow-up times for the EBKT and SKT groups were 6.73 y (IQR 2.96–12.21) and 9.47 y (IQR 3.11–17.52), respectively. Of the participating transplant hospitals, only 4 had performed more than 15 small pediatric donor transplants.
FIGURE 1.
Flow diagram demonstrating study population.
TABLE 1.
Characteristics for Australian and New Zealand donors aged ≤5 y old (1989–2016).
| EBKT (n = 60) | SKT (n = 79) | P | |
|---|---|---|---|
| Donor age, y | 2.0 (1.3) | 4.4 (0.9) | <0.001 |
| Female donors | 25 (42%) | 34 (43%) | 1.00 |
| Donor weight, kg | 13.6 (3.7) | 20.1 (4.0) | <0.001 |
| Donor height, cm | 88.6 (17.3) | 108.6 (16.1) | 0.016 |
| Donor weight: recipient weight ratio | 0.2 (0.1) | 0.3 (0.1) | <0.001 |
| Donor: creatinine—terminal, µmol/L | 46.5 (32.5) | 46.5 (20.2) | 1.00 |
| DCD donors | 4 | 6 | 0.83 |
Data are presented as mean (SD) for continuous measures and n (%) for categorical measures.
DCD, donation after circulatory death; EBKT, en bloc kidney transplant; SKT, single kidney transplant.
TABLE 2.
Characteristics of Australian and New Zealand recipients who received kidney transplants from donors aged ≤5 y old.
| EBKTs (n = 60) | SKTs (n = 79) | P | |
|---|---|---|---|
| Age at transplant, y | 44.7 (13.4) | 45.9 (12.9) | 0.67 |
| Female recipients | 27 (45%) | 37 (47%) | 0.86 |
| Recipient weight at transplant, kg | 71.6 (16.2) | 70.5 (14.4) | 0.18 |
| Recipient height, cm | 170.2 (9.6) | 166.7 (10.0) | 0.061 |
| Recipient peripheral vascular disease at transplant | 3 (5%) | 3 (4%) | 0.81 |
| Recipient diabetes at transplant | 7 (12%) | 11 (14%) | 0.77 |
| Recipient coronary artery disease at transplant | 7 (12%) | 5 (6%) | 0.75 |
| Time to transplant from commencement of renal replacement therapy, y | 3.7 (2.8) | 3.4 (3.5) | 0.62 |
| HLA mismatch | 3.4 (1.7) | 3.1 (1.8) | 0.33 |
| Total ischemia to nearest hour | 13.9 (6.1) | 15.4 (5.8) | 0.16 |
Data are presented as mean (SD) for continuous measures and n (%) for categorical measures.
EBKT, en bloc kidney transplant; SKT, single kidney transplant.
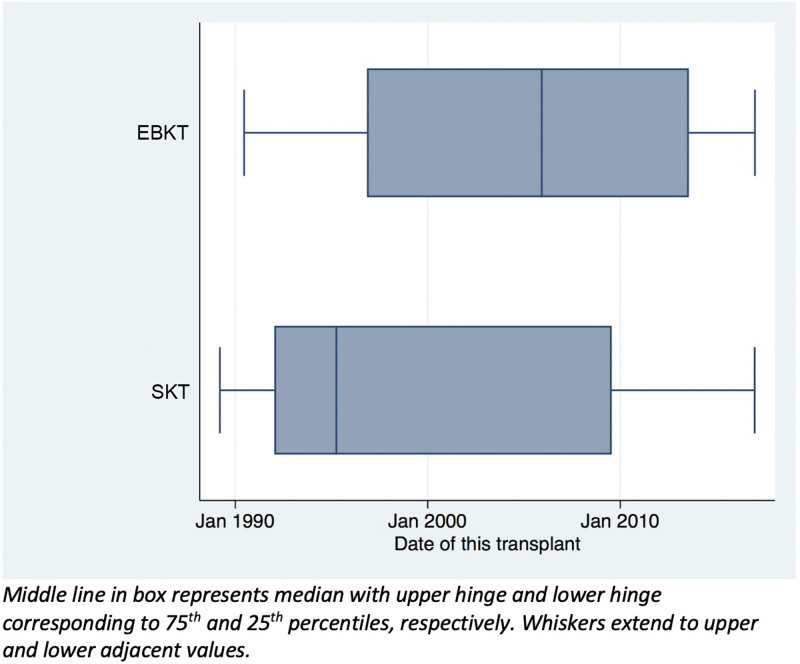
The distribution of types of graft varied over the observed period (1989–2016). There were significantly more EBKT grafts from the year 2000 onward compared with SKTs (P < 0.01) (Figure 2). Mean donor weights before 2000 and from 2000 onwards were 17.8 kg (±4.51 kg) and 16.8 kg (±5.45 kg), respectively (P = 0.23). When comparing grafts in the 2000–2016 era, donors of EBKT grafts were still significantly smaller on average than SKT donors with average weights of 13.2 kg (±3.5) and 21.1 kg (±4.1), respectively (P < 0.001).
FIGURE 2.
Box plot of distribution of each type of kidney transplant over time from pediatric donors aged ≤5 y. Middle line in box represents median with upper hinge and lower hinge corresponding to 75th and 25th percentiles, respectively. Whiskers extend to upper and lower adjacent values. EBKTS, en bloc kidney transplant; SKT, singe kidney transplant.
Primary Outcomes
Death-censored Graft Survival
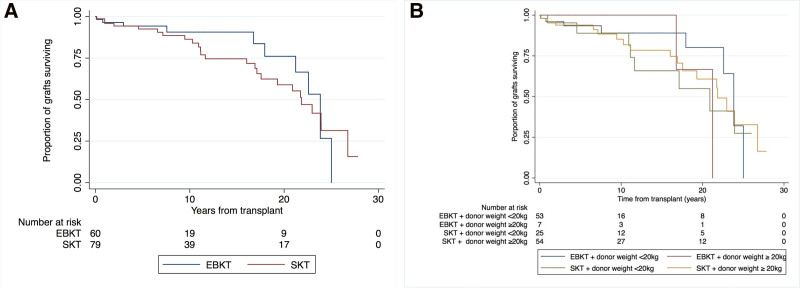
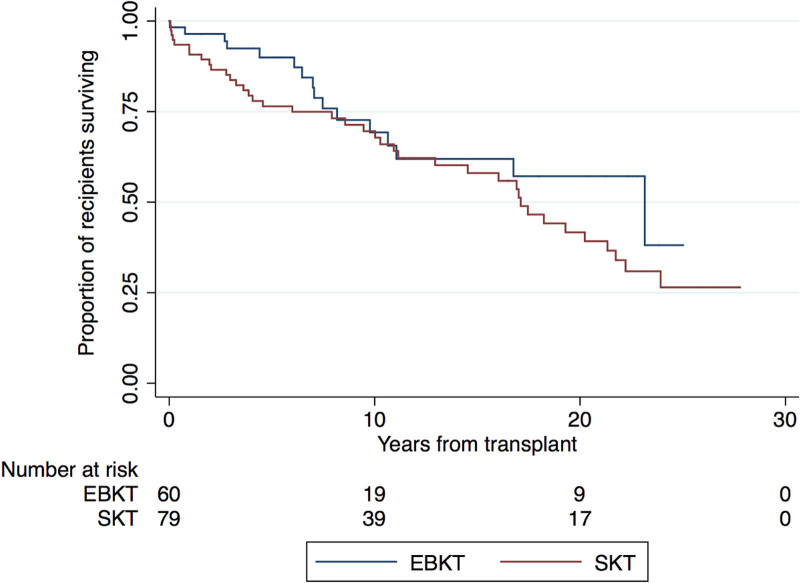
Overall, there were 35 cases of graft failure. Median death-censored graft survival rates in the EBKT and SKT cohorts were 23.8 y (IQR 21.2–25) and 21.8 y (IQR 11.6–26.8), respectively (Figure 3A). There was no significant difference in graft survival between the EBKT and SKT groups (unadjusted hazard ratio [HR] 1.30; 95% confidence interval [CI], 0.59-2.64; P = 0.56). Respective death-censored graft survival for EBKT and SKT were 96% versus 96% at 1 y, 94% versus 93% at 5 y, and 91% versus 87% at 10 y.
FIGURE 3.
Death-censoed graft survival outcomes for recipients of small paediatric donor kidneys. A) EBKT versus SKT B) EBKT versus SKT, stratified by weight and donor group. EBKT, en bloc kidney transplant; SKT, single kidney transplant.
Using multivariable Cox proportional hazards model analysis, again there was no significant difference in graft survival between EBKT and SKT recipients (HR 1.3; 95% CI, 0.4-4.09; P = 0.680). Furthermore, death-censored graft survival was not associated with donor age, height, weight, or donor-to-recipient weight ratio. A subgroup analysis revealed that there was no significant difference in graft loss between EBKT and SKT for smaller-weight (≤20 kg) donors (P = 0.51). Similarly, there was no difference in risk of graft loss between groups for donors weighing >20 kg (P = 0.69). Figure 3B shows Kaplan-Meier survival times for EBKT and SKT grafts, stratified by weight.
Using a univariate competing risks regression analysis, graft type was not significantly associated with graft failure (HR 1.46; 95% CI, 0.73-2.93; P = 0.29). Graft failure events overall were more common in the 1989–1999 era compared with the 2000–2016 era (HR 0.405; 95% CI, 0.17-0.95; P = 0.039). However, no significant interaction was observed between the type of graft and transplant era with respect to graft failure (P = 0.97). There was no significant difference in graft survival between the <15 small donor transplant hospitals or ≥15 small donor transplant hospitals (HR 1.57; 95% CI, 0.95-2.61; P = 0.080).
Secondary Outcomes
Early Graft Loss
Fifteen (10.8%) patients experienced early graft failure. Seven of these cases were EBKT grafts (11.7%, n = 60) and 8 were in the SKT cohort (10.1%, n = 79). There was no significant difference in early graft loss rate between these 2 cohorts (P = 0.77). There were 7 (5%) cases where the cause of graft failure was not documented in the ANZDATA Registry, all except one of these cases occurred in the 2000–2016 era. Eight (5.8%) patients experienced early graft failure with an identified cause of graft loss. There were 5 cases of graft loss because to either renal artery thrombosis (n = 2) or renal vein thrombosis (n = 3). There was 1 case of primary hemorrhage causing graft loss at day 0. The other causes of graft loss were a complication of drug therapy (SKT graft) and hemolytic uremic syndrome (EBKT graft). The median time to a graft-ending thrombotic event was 3 d (IQR: 1–7 d). These vascular events, stratified by weight, are noted in Table 3. All graft-ending cases of thrombosis occurred before 2000 except 1 EBKT from a 7 kg donor. Donor weight was significantly associated with early graft loss where an increase in donor weight decreased the odds ratio of early graft loss (odds ratio 0.89; 95% CI, 0.53-1.01; P = 0.049). The rate of early graft loss observed in lower-volume centers was not significantly different from that observed in higher-volume centers (P = 0.238).
TABLE 3.
Vascular events causing graft loss in recipients of known organ type.
| Donor weight class (kg) | Transplant type | Total vascular complications | Renal artery thrombosis | Renal vein thrombosis | Primary hemorrhage |
|---|---|---|---|---|---|
| <10 | EBKT = 5 | 1 | 1 (EBKT, 2014) | ||
| SKT = 0 | |||||
| 10.0–14.9 | EBKT = 28 | 1 | 1 (EBKT, 1999) | ||
| SKT = 5 | |||||
| 15.0–19.9 | EBKT = 20 | 3 | 1 (SKT, 1996) | 1 (SKT, 1990) | 1 (EBKT, 1992) |
| SKT = 20 | |||||
| ≥20 | EBKT = 7 | 1 (SKT, 1992) | |||
| SKT = 54 |
EBKT, en bloc kidney transplant; SKT, single kidney transplant.
DGF
There were 24 (17%) cases that required dialysis within 72 h of transplantation. The type of graft received was not significantly associated with DGF (χ2 [1, n = 110] = 0.001; P = 0.966). Multivariable logistic regression analysis did not identify a significant relationship between DGF and either graft type or donor characteristics; however, regarding recipient characteristics, higher weight at time of transplantation had a significant association with DGF (odds ratio 1.03; 95% CI, 1.00-1.06; P = 0.034).
Posttransplantation Serum Creatinine Concentration
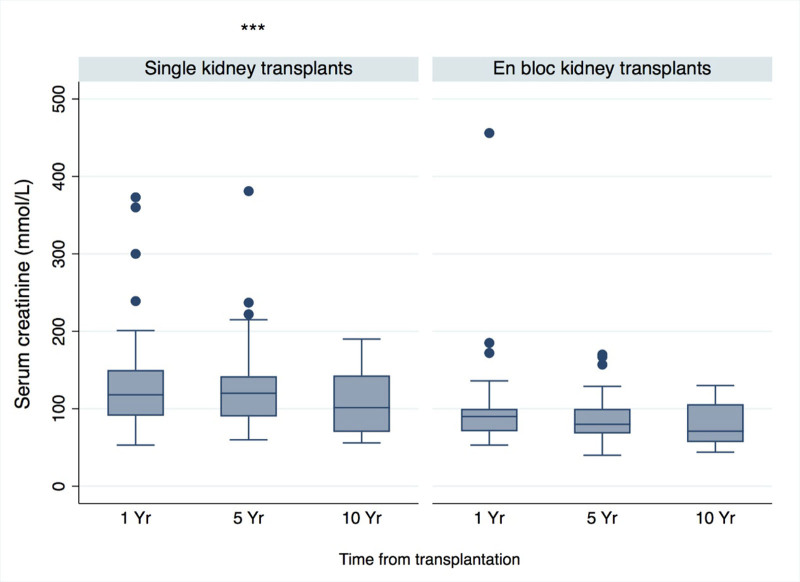
The mean serum creatinine concentrations across all recipients of small pediatric donor transplants were 116 µmol/L at 1 y (n = 109), 110 µmol/L at 5 y (n = 72), and 97 µmol/L at 10 y posttransplant (n = 39). The overall mean serum creatinine observed for SKT grafts was 126 µmol/L (SD 58) compared with 91 µmol/L (SD 47) for EBKT grafts. Creatinine levels by graft type are shown in Figure 4. EBKT recipients had significantly lower long-term creatinine levels (coefficient −34.7; 95% CI, −48.68 to −20.65; P ≤ 0.01). The proportions of recipients with serum creatinine values ≤150 µmol/L were 82% (n, SKT = 43/60, EBKT = 46/49), 89% (n, SKT = 31/39, EBKT = 29/32), and 84% (n, SKT = 18/24, EBKT = 14/14) at 1-, 5-, and 10-y posttransplantation, respectively.
FIGURE 4.
Serum creatinine values for recipients of small pediatric donor kidney transplants over time. Boxplot for serum creatinine over time, by type of donor organ received. Middle line in box represents median with upper hinge and lower hinge corresponding to 75th and 25th percentiles, respectively. Whiskers extend to upper and lower adjacent values with outside values represented by dots. *** indicates significantly higher overall serum creatinine values across the SKT group. EBKT, en bloc kidney transplant; SKT, single kidney transplant.
Acute Rejection
Sixteen (11.5%) patients experienced episodes of biopsy-proven acute rejection occurring at any time point posttransplantation. The majority of these occurred in the year 2000 or later. EBKT grafts had lower acute rejection rates than SKT grafts (2 [3%] versus 14 [18%], P = 0.014).
Patient Survival
A total of 55 (40%) patients died during the study (EBKT n = 16, SKT n = 39). The most common causes of death were malignant disease (n = 16, 29%) and infectious diseases (n = 9, 16%). Median patient survival posttransplant was 18.2 y (IQR 7.92–inestimable). Patient survival rates at 1, 5, and 10 y for EBKT versus SKT were 96% versus 83%, 90% versus 66%, and 59% versus 54%, respectively. No significant differences in survival were observed between the EBKT and SKT (HR 1.48; 95% CI, 0.83-2.66; P = 0.185; Figure 5).
FIGURE 5.
Kaplan-Meier patient survival estimates (all-cause mortality) for recipients of dual en bloc and single kidney grafts from small pediatric donors. EBKT, en bloc kidney transplant; SKT, single kidney transplant.
For the SKT group, 35 (90%) deaths occurred pre-2000. For the EBKT group, 11 (69%) deaths occurred in the earlier time period. When analysis was limited to the post-2000 era, the patient survival rates at 1, 5, and 10 y for EBKT versus SKT were 97% versus 96%, 92 versus 88%, and 77% versus 88%, and no significant differences in survival were observed between groups (HR 0.78; 95% CI, 0.21-2.93; P = 0.71).
DISCUSSION
This registry analysis shows that overall graft survival for recipients of small pediatric kidney transplants is excellent with death-censored median graft survival exceeding 20 y and not significantly different between EBKT and SKT groups. Despite this, the use of pediatric donors aged 5 y or younger for kidney transplantation remains uncommon in Australia and New Zealand. Implantation by way of EBKT has increased in the more recent era. EBKT donors were generally smaller with significantly lower ages, heights, and weights. The risk of early graft loss was 10.3%. The specific risk of a technical vascular complication was at least 4.3% with the majority of these graft-ending complications occurring within the first week posttransplantation and before the year 2000. DGF rates were low and not different between groups. Long-term graft function, as measured by serum creatinine concentration, was excellent in both groups although significantly lower in the EBKT recipients. Size-related donor factors were not found to be associated with long-term graft survival but were associated with a significantly higher risk of early graft loss. Recipient weight at time of transplant did correlate with graft survival.
To our knowledge, the current study is among the overall longest-reported follow-up for small pediatric donors and confirms an emerging consensus that the use of kidney grafts from small pediatric donors is safe and effective. The 2020 ANZDATA Registry publication noted an overall graft survival in Australia of 94% at 1 y and 83% at 5 y.17 For extended criteria donors, the overall graft survival in Australia was 95% at 1 y and 81% at 5 y. Comparing these data to the current study’s results, the grafts from small pediatric donors have superior survival outcomes. This is consistent with other studies that have demonstrated that EBKT from small pediatric donors have excellent graft survival rates that are often comparable to or better than standard-criteria adult donors.8,14,16,18,19 Although not a statistically significant difference, it is of note that the 5 and 10 y patient survival for the overall SKT recipient group was considerably lower than both EBKT recipients and overall Australian recipients. This is likely explained by an era effect because, when pre-2000 transplants were excluded from analysis, the patient survival results for SKT recipients were comparable to the EBKT recipients.
There is limited consensus on when to surgically split kidneys and perform SKT, but studies have overall shown SKT from small pediatric donors yields better long-term outcomes than extended criteria and even standard-criteria donors in some studies.15,18,20,21 Direct comparisons have reinforced that EBKT has better long-term survival than single kidney grafts.3,8,18 In contrast, our study included the largest cohort of small pediatric donors and showed similar outcomes between EBKT and SKT recipients, although the findings were limited by differences in donor ages and weights.
The numbers of early graft loss events in the current study were similar between both EBKT and SKT groups and predominantly occurred in smaller-weight donors. Other registry-based studies have shown similar rates of early technical graft loss.18 It has been previously demonstrated that SKT grafts <10 kg have a higher risk of graft failure compared with weight-matched EBKT grafts and the majority of this risk was attributed to early technical failure.21 Larger studies with more low-weight donors have found that donor weight, EBKT, and center experience were associated with better 1 y graft survival, which was predominantly driven by differences in early graft loss.18 It should be noted that these studies mostly utilized datasets that predated 2010 and so may not be generalizable to contemporary practice. Fewer graft failure events were observed in the 2000–2016 period‚ and this may be a function of improved center experience with small pediatric donors.
There is no globally accepted size criterion that mandates the splitting of a pair of small pediatric donor kidneys. The 2021 Transplantation Society of Australia and New Zealand Clinical Guidelines for Organ Transplantation from Deceased Donors suggest a weight cutoff of 20 kg for guiding decisions regarding use of en bloc kidneys versus split kidneys.22 It is for this reason that the current study analyzed outcomes based above or below this weight cutoff. It is likely that experience with small donors varies between institutions and somewhat contributes to heterogenous approaches to splitting kidneys. Furthermore, a donor weight of <20 kg has been used as the definition of small pediatric donors by other authors.23 Our study, while limited by small numbers, demonstrates that if smaller-weight donor grafts do not thrombose in the early postoperative period, they are likely to exhibit reasonable long-term survival. However, there is a need to balance risk with benefit. This concept was further expanded by Maluf et al18 who demonstrated that, particularly in experienced centers, a net gain in overall transplant years was achieved by splitting kidneys from donors >10–12 kg. This was associated with only a small trade-off in risk for increased early graft loss.
The contemporary shift toward EBKT in Australia and New Zealand mirrors similar trends seen in the United States.8 There was no difference in donor weights in the pre- and post-2000 cohorts so this may represent an increasing caution among transplant clinicians when utilizing small pediatric kidneys. Consideration needs to be given to the loss of opportunity by using more EBKT, but decisions need to be made within individual clinical units regarding the accepted threshold for splitting. Generally, most centers in the study had limited experience with small pediatric donors; the majority of hospitals performed <15 transplantations since ANZDATA records began. Based on the current study, it remains uncertain as to whether or not there is a relationship between number of small pediatric transplants performed and graft outcome.
Over an analysis period between 1997 and 2014, approximately 1 in 5 deceased donor kidney transplant recipients in Australia or New Zealand required dialysis within 72 h of transplantation.24 Grafts from small pediatric donors in our study showed a slightly lower rate of such DGF (17%), with no difference between types of graft. Smaller, contemporary studies have similarly found no difference between DGF rates for EBKT and SKT15,25 although other studies have shown DGF rates to be significantly higher in SKT cohorts.8,26 Recipient weight at transplant as a risk factor for DGF in pediatric donor grafts has been well characterized previously.27,28
Although overall rates of acute rejection in small pediatric donor kidneys were low, there was a significantly higher risk in SKT. This is unlikely explained by an era effect as most acute rejection episodes were recorded in the more contemporary period (after 2000). HLA mismatch was not different between groups. These data may be biased in that clinicians are often reluctant to biopsy EBKT as this procedure is thought to be associated with a higher risk of complications. Another possible explanation is that there may be variable institutional practices regarding immunosuppression treatments for single kidneys—for example, reduced calcineurin inhibitor dosing to avoid toxicity may predispose to higher risk of rejection. Further research is required to better delineate how clinicians modify medical management for small grafts.
The concept of hyperfiltration injury in small donor kidneys stems from early investigations.29 It has subsequently been demonstrated that single kidneys from pediatric donors undergo a rapid, compensatory hypertrophy following transplantation and thus the caution regarding small nephron mass may be misplaced.30 Although EBKT grafts show higher rates of postoperative proteinuria initially (compared with deceased adult donor grafts), this difference resolves over long-term follow-up.7 On a cellular level, murine models have shown younger donor kidneys to have superior capacity to repair peritransplant injuries and maintain organ mass.31 Although SKT did have an overall higher average serum creatinine, the magnitude of difference from EBKT grafts was of questionable clinical significance. Unfortunately, proteinuria data were not available for correlation. Few large studies have systematically evaluated proteinuria as an outcome, but a direct comparison of single kidneys from donors aged <5 y and 5–10 y showed no significant difference in posttransplant proteinuria.32 EBKT versus SKT comparisons have also shown no difference in measured proteinuria levels posttransplant.25 In EBKT recipient cohorts, proteinuria found in patients receiving organs from donors <5 kg subsided within the first year posttransplant.33 Taking all this together with the demonstrated excellent long-term outcomes, residual concerns regarding small nephron masses in small pediatric donor kidneys should be allayed.
A strength of this study was the inclusion of all adult recipients of small pediatric donor kidney transplants from 20 centers in Australia and New Zealand over a period of 53 y. Compared with similar studies, the sample size was similar or larger and the follow-up time was often longer.23 However, this retrospective registry study was limited by a restricted range of variables collected and a lack of granular data in relation to surgical expertise, local center protocols (eg, peritransplant anticoagulation), selection criteria used to determine whether or not to split kidneys or use en bloc kidneys, preservation method, urologic complications, warm ischemic times, primary method of vascular anastomosis for the EBKT group, and recipient blood pressure. The cause of early graft loss was not documented in all cases. ANZDATA is a voluntary registry and there was no external auditing process, such that the possibility of coding bias could not be excluded. The observed event rates were low, which limited estimate precision and the extent to which multivariable adjustment could be performed. The possibility of indication bias with residual confounding could not be excluded. A further prospective trial that addresses these missing data is required to inform clinical decision-making regarding small pediatric donors.
CONCLUSIONS
Although there is an increased risk of graft loss from pediatric donors ≤5 y during the early postoperative period, this study has demonstrated excellent long-term graft survival rates. Clinicians should thus be encouraged to utilize the pediatric donor pool where possible and not regard such grafts as marginal. Further research is required to better inform clinical decision-making regarding when to split pediatric donor kidneys, particularly in the 15–20 kg body weight range.
ACKNOWLEDGMENTS
We acknowledge the substantial contributions of the entire Australian and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients) in providing information for and maintaining the ANZDATA and Australian Peritonitis registries. The ANZDATA Registry is funded by the Australian Organ and Tissue Donation and Transplantation Authority, the New Zealand Ministry of Health, and Kidney Health Australia. The authors acknowledge the statistical support received through the Metro South Health Biostatistics Service.
Footnotes
D.W.J. has received consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care, consultancy fees from Astra Zeneca, Bayer, BI and Lilly, Vifor, and AWAK, speaker’s honoraria and travel sponsorships from ONO Pharmaceutical Co. Ltd., and travel sponsorships from Amgen. He is a current recipient of an Australian National Health and Medical Research Council (NHMRC) Leadership Investigator Grant. A.K.V. is a current recipient of an NHMRC Emerging Leadership Investigator Grant and a Queensland Advancing Clinical Research Fellowship. The other authors declare no conflicts of interest.
J.G.E., E.G.R., J.P., D.W.J., and N.I. were involved in the research design. J.G.E., J.P., and N.I. were involved in the writing of the article. J.G.E. was involved in the performance of the research. J.G.E., E.G.R., A.K.V., D.J., J.P., D.W.J., and N.I. were involved in data analysis, including interpretation. J.G.E., E.G.R., S.C., M.R., A.K.V., D.J., V.K., A.G., J.P., D.W.J., and N.I. were involved in the revision of the article and critical analysis of outcomes and conclusions.
REFERENCES
- 1.ANZDATA Registry. 41st Report, Chapter 6: Australian Transplant Waiting List. Australia and New Zealand Dialysis and Transplant Registry; 2019. [Google Scholar]
- 2.Gourlay W, Stothers L, McLoughlin MG, et al. Transplantation of pediatric cadaver kidneys into adult recipients. J Urol. 1995;153:322–5 6. [DOI] [PubMed] [Google Scholar]
- 3.Bresnahan BA, McBride MA, Cherikh WS, et al. Risk factors for renal allograft survival from pediatric cadaver donors: an analysis of United Network for Organ Sharing data. Transplantation. 2001;72:256–261. [DOI] [PubMed] [Google Scholar]
- 4.Churchill BM, Sheldon CA, McLorie GA, et al. Factors influencing patient and graft survival in 300 cadaveric pediatric renal transplants. J Urol. 1988;140(5, Part 2):1129–1133. [DOI] [PubMed] [Google Scholar]
- 5.Balamuthusamy S, Paramesh A, Zhang R, et al. The effects of body mass index on graft survival in adult recipients transplanted with single pediatric kidneys. Am J Nephrol. 2009;29:94–101. [DOI] [PubMed] [Google Scholar]
- 6.Meakins JL, Smith EJ, Alexander JW. En bloc transplantation of both kidneys from pediatric donors into adult patients. Surgery. 1972;71:72–75. [PubMed] [Google Scholar]
- 7.Thomusch O, Tittelbach-Helmrich D, Meyer S, et al. Twenty-year graft survival and graft function analysis by a matched pair study between pediatric en bloc kidney and deceased adult donors grafts. Transplantation. 2009;88:920–925. [DOI] [PubMed] [Google Scholar]
- 8.Dharnidharka VR, Stevens G, Howard RJ. En-bloc kidney transplantation in the United States: an analysis of United Network of Organ Sharing (UNOS) data from 1987 to 2003. Am J Transplant. 2005;5:1513–1517. [DOI] [PubMed] [Google Scholar]
- 9.Troppmann C, Santhanakrishnan C, Fananapazir G, et al. Pediatric en bloc kidney transplantation from very small (≤10 kg) donation after circulatory death (versus brain death) donors: single-center matched-pair analysis of 130 transplants. Am J Transplant. 2018;18:2811–2817. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Cotterell A, Behnke M, et al. Pediatric en bloc kidney transplantation: comparable outcomes to standard criteria deceased and living donor kidney transplantation in adult recipients. Transplantation. 2014;98:505. [DOI] [PubMed] [Google Scholar]
- 11.Hiramoto JS, Freise CE, Randall HR, et al. Successful long-term outcomes using pediatric en bloc kidneys for transplantation. Am J Transplant. 2002;2:337–342. [DOI] [PubMed] [Google Scholar]
- 12.Hafner-Giessauf H, Mauric A, Müller H, et al. Long-term outcome of en bloc pediatric kidney transplantation in adult recipients—up to 22 years of center experience. Ann Transplant. 2013;18:101–107. [DOI] [PubMed] [Google Scholar]
- 13.Considine SW, Davis NF, McLoughlin LC, et al. Long-term outcomes of en-bloc renal transplantation from paediatric donors into adult recipients. Surgeon. 2019;17:1–5. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Fisher RA, Cotterell AH, et al. En bloc kidney transplantation from pediatric donors: comparable outcomes with living donor kidney transplantation. Transplantation. 2011;92:564–569. [DOI] [PubMed] [Google Scholar]
- 15.Al-Shraideh Y, Farooq U, El-Hennawy H, et al. Single vs dual (en bloc) kidney transplants from donors ≤ 5 years of age: a single center experience. World J Transplant. 2016;6:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhayana S, Kuo YF, Madan P, et al. Pediatric en bloc kidney transplantation to adult recipients: more than suboptimal? Transplantation. 2010;90:248–254. [DOI] [PubMed] [Google Scholar]
- 17.ANZDATA Registry. 43rd Report, Chapter 7: Kidney Transplantation. Australia and New Zealand Dialysis and Transplant Registry; 2020. [Google Scholar]
- 18.Maluf DG, Carrico RJ, Rosendale JD, et al. Optimizing recovery, utilization and transplantation outcomes for kidneys from small, ≤20 kg, pediatric donors. Am J Transplant. 2013;13:2703–2712. [DOI] [PubMed] [Google Scholar]
- 19.Sureshkumar KK, Habbach A, Tang A, et al. Long-term outcomes of pediatric en bloc compared to living donor kidney transplantation: a single-center experience with 25 years follow-up. Transplantation. 2018;102:e245–e248. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Ramanathan R, Behnke M, et al. Single pediatric kidney transplantation in adult recipients: comparable outcomes with standard-criteria deceased-donor kidney transplantation. Transplantation. 2013;95:1354–1359. [DOI] [PubMed] [Google Scholar]
- 21.Kayler LK, Magliocca J, Kim RD, et al. Single kidney transplantation from young pediatric donors in the United States. Am J Transplant. 2009;9:2745–2751. [DOI] [PubMed] [Google Scholar]
- 22.The Transplantation Society of Australia and New Zealand. Clinical Guidelines for Organ Transplantation from Deceased Donors. Version 1.8. TSANZ; 2021. [Google Scholar]
- 23.Damji S, Callaghan CJ, Loukopoulos I, et al. Utilisation of small paediatric donor kidneys for transplantation. Pediatr Nephrol. 2019;34:1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim WH, Johnson DW, Teixeira-Pinto A, et al. Association between duration of delayed graft function, acute rejection, and allograft outcome after deceased donor kidney transplantation. Transplantation. 2019;103:412–419. [DOI] [PubMed] [Google Scholar]
- 25.Fayek SA, Ali MS, Hasham L, et al. Expanding the envelope: favorable outcomes utilizing kidneys from small pediatric donors (≤ 15 kg). Transplant Proc. 2018;50:3204–3210. [DOI] [PubMed] [Google Scholar]
- 26.Jin X, Hu JM, Liu YG, et al. A multicenter clinical study of single-kidney transplantation vs en bloc transplantation with kidneys from deceased pediatric donors. Transplant Proc. 2019;51:3252–3258. [DOI] [PubMed] [Google Scholar]
- 27.Ojo AO, Wolfe RA, Held PJ, et al. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968–974. [DOI] [PubMed] [Google Scholar]
- 28.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes JM, Steinmuller DR, Streem SB, et al. The development of proteinuria and focal-segmental glomerulosclerosis in recipients of pediatric donor kidneys. Transplantation. 1991;52:813–817. [DOI] [PubMed] [Google Scholar]
- 30.Uemura T, Liang J, Khan A, et al. Outcomes of transplantation of single pediatric renal allografts equal to or more than 6 cm in length. Transplantation. 2010;89:710–713. [DOI] [PubMed] [Google Scholar]
- 31.Melk A, Schmidt BMW, Braun H, et al. Effects of donor age and cell senescence on kidney allograft survival. Am J Transplant. 2009;9:114–123. [DOI] [PubMed] [Google Scholar]
- 32.Zhang R, Paramesh A, Florman S, et al. Long-term outcome of adults who undergo transplantation with single pediatric kidneys: how young is too young? Clin J Am Soc Nephrol. 2009;4:1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wijetunga I, Ecuyer C, Martinez-Lopez S, et al. Renal transplant from infant and neonatal donors is a feasible option for the treatment of end-stage renal disease but is associated with increased early graft loss. Am J Transplant. 2018;18:2679–2688. [DOI] [PubMed] [Google Scholar]