Abstract
The sequence and analysis of the capsular polysaccharide biosynthesis locus, PS B2, of Bacteroides fragilis 638R are described, and the sequence is compared with that of the PS B1 biosynthesis locus of B. fragilis NCTC 9343. Two genes of the region, wcgD and wcgC, are shown by complementation to encode a UDP-N-acetylglucosamine 2-epimerase and a UDP-N-acetylmannosamine dehydrogenase, respectively.
Bacteroides fragilis is the anaerobe most frequently isolated from intra-abdominal abscesses. The capsular polysaccharide complex (CPC) of the prototype strain, NCTC 9343, is the major virulence factor for abscess formation, and purified CPC induces the formation of abscesses in animal models (8). The CPC of strain NCTC 9343 is composed of two distinct polysaccharides, polysaccharide A (PS A) and polysaccharide B (PS B) (10). The repeating units of PS A and PS B each contain both positively and negatively charged groups (19). The presence of both types of charged groups is required for the induction of abscesses, and other bacterial polysaccharides that have been chemically modified to contain these charged groups also induce abscess formation (17, 18).
Studies with monoclonal antibodies (MAb) have demonstrated that the capsular polysaccharides of B. fragilis are heterogeneous and that the majority of strains examined produce a CPC composed of two distinct polysaccharides (9, 11). It is not known whether other strains of B. fragilis synthesize capsular polysaccharides containing both positively and negatively charged groups.
The sequence of the PS B biosynthesis locus of strain NCTC 9343 has been reported (2). Here, we report the sequence of the PS B biosynthesis region of the most-studied B. fragilis strain, 638R, which produces a CPC that is immunologically distinct from the CPC of strain NCTC 9343. In this study, we determined the genetic relatedness of the PS B biosynthesis regions of two immunologically diverse strains of B. fragilis and revealed that the 638R PS B locus contains genes whose products are involved in conferring charged groups to the polysaccharide.
The PS B biosynthesis locus of strain 638R was located by transposon mutagenesis as described previously (2). Bacterial strains and plasmids used in this study are listed in Table 1. Mutant 2-42 did not react with a MAb specific to the CPC of strain 638R, and the B. fragilis DNA at the junction of mutant 2-42 was cloned, creating plasmid pLEC6.1 (2). This plasmid was used as a probe to select pMJC10 from a 638R cosmid gene bank constructed by using a previously described protocol (2). Cosmid clones pLEC17, pLEC18, and pLEC19 were selected by using a probe consisting of an internal portion of orf7 of strain NCTC 9343, an open reading frame (ORF) found by PCR to be common to both strains (Fig. 1).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference and/or source |
|---|---|---|
| E. coli strains | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17 (rK− mK+) deoR thi-1 supE44 gyrA96 relA1 λ− | 1 |
| AB1133 | K-12 thr-1 leuB6 Δ(gpt-proA)66 hisG4 argE3 thi-1 rfbD1 lacY1 ara-14 galK2 xyl-5 mtl-1 mgl-51 rpsL31 kdgK51 supE44 | 7 |
| 21546 | AB1133 with rff::Tn10-46 | 7 |
| 21566 | AB1133 with rff::Tn10-66 | 7 |
| B. fragilis strains | ||
| NCTC 9343 | Type strain, appendix abscess | 5, ATCCa |
| 638R (TM4000) | Clinical | 12 |
| 2-42 | 638R PS B mutant with transposon insertion in wcg locus | 2 |
| Plasmids | ||
| pHC79 | Cosmid vector; Apr | 4 |
| pUC19 | Cloning vector; Apr | New England Biolabs |
| pLEC6.1 | 900-bp EcoRI-HindIII fragment (B. fragilis DNA) cloned in pBluescript; Apr | 2 |
| pMJC10 | 638R gene bank clone including orf3-wcgE cloned in pHC79; Apr | This study |
| pLEC17 | 638R gene bank clone including wcgM-orf7 cloned in pHC79; Apr | This study |
| pLEC18 | 638R gene bank clone including wcgJ-orf7 cloned in pHC79; Apr | This study |
| pLEC19 | 638R gene bank clone including wcgF-orf7 cloned in pHC79; Apr | This study |
| pWCGC | 1,424-bp PCR amplicon containing wcgC cloned in pUC19; Apr | This study |
| pWCGD | 1,518-bp PCR amplicon containing wcgD cloned in pUC19; Apr | This study |
| pWCGCD | 2,711-bp PCR amplicon containing wcgC-wcgD cloned in pUC19; Apr | This study |
ATCC, American Type Culture Collection.
FIG. 1.
Comparison of the PS B1 and PS B2 biosynthesis regions of B. fragilis NCTC 9343 and 638R. The direction of transcription of each ORF (boxes) is designated with an arrow. The ORFs that do not demonstrate homology with genes involved in polysaccharide biosynthesis are shaded. The area between the diagonal lines indicates where the two chromosomes diverge. Two stem-loop regions that may serve to terminate transcription in the 638R region are indicated, as are the locations of primers used to amplify wcgC and wcgD for complementation studies. The average G+C content of each ORF of the 638R region is indicated by a black bar. Cosmid clones and a PCR product used as sequencing templates are shown.
A total of 26,443 bp were sequenced, revealing 19 ORFs similar to products encoded by genes of other polysaccharide biosynthesis loci (Table 2). Comparison of the sequence of this region of the chromosome with that reported previously for strain NCTC 9343 showed that the genes involved in polysaccharide biosynthesis are distinct (Fig. 1). A region including 2,048 bp of DNA upstream of rmlA was sequenced from strain 638R and was found to be 99.1% identical to the region upstream of the PS B biosynthesis locus of strain NCTC 9343 (Fig. 1). The identity continues into rmlA and is 100% for 338 bp of rmlA before the two chromosomes begin to diverge. This complete divergence continues throughout the biosynthesis regions until just downstream of orf5, where the two chromosomes are 99.6% identical for the remainder of the DNA sequenced from both chromosomes (2,081 bp). To standardize the nomenclature, the polysaccharide that is synthesized from any B. fragilis strain by genes in this area of the chromosome is designated PS B. As these polysaccharides are genetically and immunologically distinct, an arabic numeral is placed after this designation; therefore, the polysaccharide synthesized by the wcf locus of NCTC 9343 is designated PS B1 and that synthesized by the 638R wcg locus is designated PS B2.
TABLE 2.
Identity of the products encoded by the wcg region with sequences in the GenBank database
| ORF | G+C content (%) | Size (amino acids) | Organism and gene | Gene product | % Identity/similaritya | Accession no. |
|---|---|---|---|---|---|---|
| rmlA | 38.1 | 294 | B. fragilis NCTC 9343, rmlA | Putative glucose 1-phosphate thymidyltransferase | 92/96 (1–294) | AF048749 |
| Shigella flexneri, rmlA | Glucose 1-phosphate thymidyltransferase | 67/80 (2–289) | D55213 | |||
| wzx | 28.8 | 509 | E. coli 0111, wzx | Putative flippase | 18/37 (21–351) | U13629 |
| Yersinia enterocolitica O:3, trsA | Putative flippase | 21/38 (13–359) | S51260 | |||
| wcgA | 30.3 | 369 | Vibrio cholerae O139, wbfL | Unknown | 31/45 (262–348) | Y07786 |
| wcgB | 31.3 | 315 | E. coli, kfiA | Unknown | 24/47 (65–174) | X77617 |
| Rhizobium tropici, nodC | N-acetylglucosaminyltransferase | 20/38 (67–167) | X98514 | |||
| wzy | 28.3 | 478 | Streptococcus pneumoniae, cap1H | Unknown | 24/52 (392–468) | Z83335 |
| wcgC | 34.6 | 408 | E. coli, wecC (formerly rffD) | UDP-N-acetylmannosamine dehydrogenase | 49/66 (6–400) | P27829 |
| S. aureus, cap5/8 O | UDP-N-acetylmannosamine dehydrogenase | 41/60 (1–400) | U73374 | |||
| U81973 | ||||||
| wcgD | 35.7 | 384 | E. coli, wecB (formerly rffE) | UDP-N-acetylglucosamine-2-epimerase | 55/71 (3–382) | P27828 |
| S. aureus, cap5/8 P | UDP-N-acetylglucosamine-2-epimerase | 47/64 (1–378) | U73374 | |||
| U81973 | ||||||
| wcgE | 30.4 | 363 | Synechocystis sp., icsA | Putative glycosyltransferase | 27/45 (178–334) | BA17441 |
| S. aureus, capJ | Unknown | 25/50 (213–338) | P39859 | |||
| wcgF | 32.9 | 143 | B. fragilis, wcgG | Unknown | 49/66 (9–136) | AF125164 |
| Vibrio anguillarum, orf15x4 | Unknown | 40/58 (6–131) | AF025396 | |||
| wcgG | 32.2 | 141 | B. fragilis, wcgF | Unknown | 49/66 (12–139) | AF125164 |
| V. anguillarum, orf15x4 | Unknown | 43/55 (23–133) | AF025396 | |||
| S. pneumoniae, cp13-1 | Unknown | 33/61 (55–96) | S21550 | |||
| wcgH | 32.9 | 366 | V. anguillarum, orf41x4 | Putative aminotransferase | 55/77 (1–365) | AF025396 |
| Streptomyces antibioticus, oleN2 | Putative transaminase | 41/61 (2–362) | AF055579 | |||
| wcgI | 29.7 | 331 | Leptospira borgpetersenii, orfH18 | Unknown | 35/54 (1–300) | AF078135 |
| Bacillus subtilis, ORF | Unknown | 33/52 (4–239) | D86418 | |||
| wcgJ | 41.4 | 341 | L. borgpetersenii, orfH1 | Unknown | 66/84 (3–339) | AF078135 |
| S. aureus, cap8E | Unknown | 63/80 (3–337) | U81973 | |||
| wcgK | 40.5 | 383 | Acholeplasma laidlawii, ORF | Putative nucleotide-binding protein | 66/84 (1–383) | U81973 |
| S. aureus, cap8F | Unknown | 55/68 (1–383) | U73374 | |||
| wcgL | 43.7 | 394 | S. aureus, cap8G | Unknown | 57/71 (17–393) | U73374 |
| L. borgpetersenii, orfH11 | Unknown | 46/64 (19–394) | AF078135 | |||
| Methanobacterium thermoautotrophicum, ORF | Putative UDP-N-acetylglucosamine 2-epimerase | 34/54 (18–305) | AAB85335 | |||
| wcgM | 37.6 | 402 | L. borgpetersenii, orfH12 | Unknown | 26/47 (22–402) | AF078135 |
| S. aureus, cap8L | Unknown | 24/45 (55–391) | U73374 | |||
| E. coli, wcaI | Putative glycosyltransferase | 20/41 (1–343) | P32057 | |||
| wcgN | 39.2 | 202 | Campylobacter jejuni, orfC | Putative UDP-galactose phosphate transferase | 61/68 (41–202) | AF001498 |
| Streptococcus agalactiae, cpsD | Putative UDP-galactose phosphate transferase | 42/55 (39–186) | Q04664 | |||
| wcgO | 44.8 | 194 | Caulobacter crescentus, lpsB | Putative UDP-N-acetylglucosamine acetyltransferase | 43/59 (52–190) | AF062345 |
| C. jejuni, orfD | Putative acetyltransferase | 27/53 (1–185) | AF001498 | |||
| wcgP | 49.2 | C. jejuni, orfF | Putative aminotransferase | 45/60 (24–395) | AF001497 | |
| M. thermoautotrophicum, ORF | Putative perosamine synthetase | 37/53 (32–395) | AE000818 | |||
| orfA6 | 38.3 | 167 | None | |||
| orfB6 | 35.2 | 519 | A. chroococcum, ORF | Unknown | 35/54 (7–519) | P24423 |
The identity and similarity percentages are based on the stated amino acid region (in parentheses) of the wcg product.
Like the NCTC 9343 wcf region, the ORFs of wcg are all transcribed from the same DNA strand, and most are tightly clustered; the exceptions are rmlA and wzx, which have a 321-bp gap between them. If transcription of the wcf and wcg regions were initiated upstream of rmlA (the first gene of each region), transcription would be driven by a promoter common to both loci.
Unlike the wcf region of NCTC 9343, which contains only genes whose products are similar to proteins involved in polysaccharide biosynthesis, the unique region of the 638R chromosome contains two additional ORFs flanking the 3′ end of the wcg region, which may not be involved in polysaccharide biosynthesis. OrfA6 is not similar to any sequences deposited in public databases. In addition, a region of 43 bp between wcgP and orfA6 contains a 20-bp inverted repeat that may terminate transcription downstream of wcgP. OrfB6 is similar to an ORF of Azotobacter chroococcum that is not contained in a polysaccharide biosynthesis locus and whose function is unknown (3).
Structural analysis of the two capsular polysaccharides of 638R (PS A2 and PS B2) is currently under way in our laboratory. A brief discussion of the putative gene products synthesized by the wcg locus and a prediction of some of the monosaccharides likely to be present in the repeating unit of PS B2 follows.
The four genes downstream of rmlA may have been acquired from a common ancestor; the G+C content of all these ORFs is low (averaging 29.3%) and is different from those of the ORFs immediately upstream and downstream (38.1 and 34.6%, respectively). The first and last ORFs of the four-gene block likely encode a flippase and a polymerase, respectively. These products are not similar to the flippase and polymerase of the NCTC 9343 wcf locus. Wzx contains 13 putative transmembrane regions and is similar to various other products believed to function as flippases (Table 2). Wzy, the putative polymerase, has 11 potential transmembrane regions. Of the two ORFs between wzx and wzy, a putative function for only one of these products (WcgB) can be proposed by homology analysis. Because of its limited similarity to NodC and the presence of the conserved amino acids by hydrophobic cluster analysis (16), this gene is designated a putative glycosyltransferase.
Three clustered genes, wcgJ to wcgL, encode products that, along their entire lengths, are highly similar to products encoded by a cluster of genes from the Staphylococcus aureus cap5 and cap8 regions (capE to capG). These Cap proteins have been predicted to be involved in the formation of deoxynucleoside triphosphate-N-acetylfucosamine (6, 15). A fourth gene adjacent to this cluster, wcgM, encodes a product that is similar to another product encoded by the S. aureus cap region, CapL. The similarity of WcgM to putative glycosyltransferases and the detection of the mandatory conserved aspartic acid residues by hydrophobic cluster analysis suggest that this product functions as a glycosyltransferase.
WcgC and WcgD are similar over their entire length to two products of the Escherichia coli wec region (formerly rff) and the S. aureus cap region. In E. coli, these products are necessary for the synthesis of enterobacterial common antigen (ECA) and have been shown to convert UDP-N-acetylglucosamine (UDP-GlcNAc) to UDP-N-acetylmannosaminuronic acid (UDP-ManNAcA) in a two-step process (7). WcgD is a homolog of WecB (RffE) and CapP. WecB is a UDP-GlcNAc 2-epimerase that converts UDP-GlcNAc to UDP-N-acetylmannosamine (UDP-ManNAc) (7, 14). WcgC is a homolog of CapO and WecC (RffD). WecC has been demonstrated to have dehydrogenase activity and catalyzes the conversion of UDP-ManNAc to UDP-ManNAcA (7), one of the biosynthesis precursors of ECA. The synthesis of ECA is restored to an E. coli wecB wecC mutant by cap5O and cap5P in trans (6).
Due to the requirement of a negatively charged group for abscess induction, the involvement of WcgC and WcgD in the production of UDP-ManNAcA (a negatively charged monosaccharide) from UDP-GlcNAc was investigated. wcgC was amplified as a 1,424-bp product with primers wcgC-F (5′ CATAGAATTCGGCTCACCCTTTATATCCTATAC) and wcgC-R (5′ CAGGGGATCCTTTTAATGGCTTCGGGAC). wcgD was amplified as a 1,518-bp product with primers wcgD-F (5′-CGAGGAATTCCTGACATTTTGGTTGTAGAGC) and wcgD-R (5′ CCAGGGATCCGGAACAAAAACAAAAGGACC). In addition, wcgC and wcgD were amplified as a single 2,711-bp product with primers wcgC-F and wcgD-R (Fig. 1). The PCRs were performed by using HiFi PCR Supermix (Life Technologies, Gaithersburg, Md.) with 30 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 3 min. The reaction products were run over a nucleotide removal column, digested with EcoRI and BamHI, gel purified, and cloned into EcoRI-BamHI-digested pUC19. This orientation allows for utilization of the vector-based promoter, while translation is initiated from within the cloned B. fragilis DNA. The ligations were first transformed into DH5α, and the correct clone from each ligation was selected (pWCGC [wcgC only], pWCGD [wcgD only], or pWCGCD [wcgC and wcgD]). The E. coli ECA mutants 21546 (wecC) and 21566 (wecB wecC) (7) were transformed with pUC19, pWCGC, pWCGD, or pWCGCD, and the synthesis of ECA was monitored by using the ECA-specific MAb 898 (13).
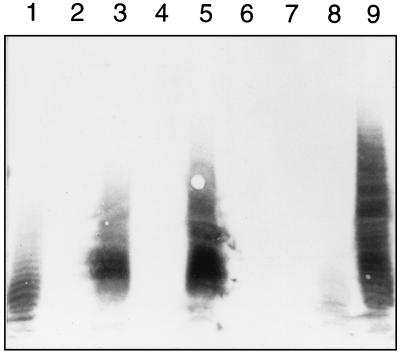
Figure 2 is a Western blot of the complementation results. Both pWCGC and pWCGCD were able to complement 21546 (wecC); however, pWCGD was not. These results demonstrate that wcgC encodes a dehydrogenase involved in the formation of UDP-ManNAcA.
FIG. 2.
Western immunoblot analysis of E. coli AB1133 and ECA mutants 21546 and 21566 containing B. fragilis wcg DNA in trans. Bacterial cultures were grown to an optical density at 600 nm of 0.8, the bacteria were pelleted by centrifugation and lysed with 1× loading buffer, and volumes of bacteria equivalent to 40 μl of culture were added to each well of the sodium dodecyl sulfate–12% polyacrylamide gel, transferred to a nylon membrane, and probed with MAb 898 (ECA specific). Lanes: 1, AB1133; 2, 21546 (pUC19); 3, 21546(pWCGC); 4, 21546(pWCGD); 5, 21546(pWCGCD); 6, 21566(pUC19); 7, 21566(pWCGC); 8, 21566(pWCGD); 9, 21566(pWCGCD).
wcgC alone was not able to complement 21566 (wecB wecC). The presence of wcgD in 21566 allowed a very slight amount of ECA to be produced (Fig. 2, lane 8). This result is consistent with that obtained by Kiser and Lee, using capP (wecB homolog) for complementation of this mutant (6). These authors attribute this ECA expression to low levels of dehydrogenase activity in 21566 that was not detected until high levels of a substrate, UDP-ManNAc, were introduced. When both wcgC and wcgD (pWCGCD) were supplied to mutant 21566, ECA synthesis was restored (Fig. 2, lane 9). As with complementation of mutant 21566 by cap5O and cap5P, ECA was overexpressed compared to the level of expression found in the wild type and likely reflects the presence of these genes in multiple copies. As WcgC is a functional homolog of WecC, this finding shows that WcgD is a UDP-GlcNAc 2-epimerase and can replace WecB in the synthesis of ECA in E. coli. These observations strongly suggest that the repeating unit of PS B2 contains ManNAcA (a negatively charged monosaccharide).
The wcg locus contains two genes, wcgH and wcgP, whose products display significant similarity to a variety of aminotransferases. WcgH and WcgP may each transfer a free amino group (positive charge) to the repeating unit of PS B2. Since both positively and negatively charged groups are integral to the biological activity of the polysaccharides, the enzymatic activity of these putative aminotransferases will be investigated in future studies.
In addition to wcgB and wcgM, two other genes, wcgE and wcgI, may also encode glycosyltransferases. WcgE is similar to putative glycosyltransferases, and both WcgE and WcgI contain the conserved aspartate residues detected by hydrophobic cluster analysis. WcgN is similar to many products that transfer galactose to an undecaprenol phosphate carrier as the first step in subunit assembly.
The data gathered by homology and complementation analyses suggest that the subunit of PS B2 contains five sugars, including one that is likely to be ManNAcA and one that may be similar to N-acetylfucosamine. The demonstration of dehydrogenase activity and of two genes whose products are similar to various aminotransferases further suggests that the repeating unit of PS B2 may contain both positively and negatively charged groups. Elucidation of the structure of PS B2 will provide a foundation from which to continue enzymatic analysis of various gene products of the wcg locus.
Nucleotide sequence accession number.
The sequence of the PS BZ biosynthesis locus of strain 638R, described herein, has been deposited in GenBank under accession no. AF125164.
Acknowledgments
We are grateful to P. D. Rick for bacterial strains and to D. Bitter-Suermann for MAb 898. We thank J. C. Lee and K. Kiser for helpful discussions, and J. McCoy for editorial services.
This work was supported by the Kass Fellowship and Public Health Service grants AI44193 and AI39576 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Bethesda Research Laboratories. BRL pUC host: E. coli DH5 alpha competent cells. Focus. 1986;8:9. [Google Scholar]
- 2.Comstock L E, Coyne M J, Tzianabos A O, Pantosti A, Onderdonk A B, Kasper D L. Analysis of a capsular polysaccharide biosynthesis locus of Bacteroides fragilis. Infect Immun. 1999;67:3525–3532. doi: 10.1128/iai.67.7.3525-3532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fallik E, Robson R L. Completed sequence of the region encoding the structural genes for the vanadium nitrogenase of Azotobacter chroococcum. Nucleic Acids Res. 1990;18:4616. doi: 10.1093/nar/18.15.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohn B, Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980;11:291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 5.Johnson J L. Taxonomy of the Bacteroides. I. Deoxyribonucleic acid homologies among Bacteroides fragilis and other saccharolytic Bacteroides species. Int J Syst Bacteriol. 1978;28:245–268. [Google Scholar]
- 6.Kiser K B, Lee J C. Staphylococcus aureus cap5O and cap5P genes functionally complement mutations affecting enterobacterial common-antigen biosynthesis in Escherichia coli. J Bacteriol. 1998;180:403–406. doi: 10.1128/jb.180.2.403-406.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meier-Dieter U, Starman R, Barr K, Mayer H, Rick P D. Biosynthesis of enterobacterial common antigen in Escherichia coli. J Biol Chem. 1990;265:13490–13497. [PubMed] [Google Scholar]
- 8.Onderdonk A B, Kasper D L, Cisneros R L, Bartlett J G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977;136:82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- 9.Pantosti A, Colangeli R, Tzianabos A O, Kasper D L. Monoclonal antibodies to detect capsular diversity among Bacteroides fragilis isolates. J Clin Microbiol. 1995;33:2647–2652. doi: 10.1128/jcm.33.10.2647-2652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantosti A, Tzianabos A O, Onderdonk A B, Kasper D L. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect Immun. 1991;59:2075–2082. doi: 10.1128/iai.59.6.2075-2082.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantosti A, Tzianabos A O, Reinap B G, Onderdonk A B, Kasper D L. Bacteroides fragilis strains express multiple capsular polysaccharides. J Clin Microbiol. 1993;31:1850–1855. doi: 10.1128/jcm.31.7.1850-1855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Privitera G, Dublanchet A, Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979;139:97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- 13.Rick P D, Mayer H, Neumayer B A, Wolski S, Bitter-Suermann D. Biosynthesis of enterobacterial common antigen. J Bacteriol. 1985;162:494–503. doi: 10.1128/jb.162.2.494-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sala R F, Morgan P M, Tanner M E. Enzymatic formation and release of a stable glycal intermediate: the mechanism of the reaction catalyzed by UDP-N-acetylglucosamine 2-epimerase. J Am Chem Soc. 1996;118:3033–3034. [Google Scholar]
- 15.Sau S, Bhasin N, Wann E R, Lee J C, Foster T J, Lee C Y. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143:2395–2405. doi: 10.1099/00221287-143-7-2395. [DOI] [PubMed] [Google Scholar]
- 16.Saxena I M, Brown R M, Jr, Fevre M, Geremia R A, Henrissat B. Multidomain architecture of β-glycosyl transferases: implications for mechanism of action. J Bacteriol. 1995;177:1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 18.Tzianabos A O, Onderdonk A B, Smith R S, Kasper D L. Structure-function relationships for polysaccharide-induced intra-abdominal abscesses. Infect Immun. 1994;62:3590–3593. doi: 10.1128/iai.62.8.3590-3593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzianabos A O, Pantosti A, Baumann H, Brisson J R, Jennings H J, Kasper D L. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 1992;267:18230–18235. [PubMed] [Google Scholar]