Abstract
Conventional cancer therapies such as chemotherapy are non-selective and induce immune system anergy, which lead to serious side effects and tumor relapse. It is a challenge to prime the body’s immune system in the cancer-bearing subject to produce cancer antigen-targeting antibodies, as most tumor-associated antigens are expressed abundantly in cancer cells and some of normal cells. This study illustrates how hapten-based pre-immunization (for anti-hapten antibodies production) combined with cancer receptor labeling with hapten antigen constructs can elicit antibody-dependent cellular phagocytosis (ADCP). Thus, the hapten antigen 2,4-dinitrophenol (DNP) was covalently combined with a cancer receptor-binding dipeptide (IYIY) to form a dipeptide-hapten construct (IYIY-DNP, MW = 1322.33) that targets the tropomyosin receptor kinase C (TrkC)-expressed on the surface of metastatic cancer cells. IYIY-DNP facilitated selective association of RAW264.7 macrophages to the TrkC expressing 4T1 cancer cells in vitro, forming cell aggregates in the presence of anti-DNP antibodies, suggesting initiation of anti-DNP antibody-dependent cancer cell recognition of macrophages by the IYIY-DNP. In in vivo, IYIY-DNP at 10 mg/kg suppressed growth of 4T1 tumors in DNP-immunized BALB/c mice by 45% (p < 0.05), when comparing the area under the tumor growth curve to that of the saline-treated DNP-immunized mice. Meanwhile, IYIY-DNP at 10 mg/kg had no effect on TrkC-negative 67NR tumor-bearing mice immunized with DNP. Tumor growth suppression activity of IYIY-DNP in DNP-immunized mice was associated with an increase in the anti-DNP IgG (7.3 × 106 ± 1.6 U/mL) and IgM (0.9 × 106 ± 0.07 U/mL) antibodies after five cycles of DNP treatment, demonstrated potential for hapten-based pre-immunization then treatment with IYIY-DNP to elicit ADCP for improved immunotherapy of TrkC expressing cancers.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-022-03147-y.
Keywords: Dinitrophenol, Immunotherapy, Antibody-dependent cellular phagocytosis, TrkC, Ligand-hapten conjugate, Active targeting
Introduction
Chemotherapy has been associated with immune cell anergy and immune editing of the tumor microenvironment [1]. Suppression of the body’s immune system against residual tumor tissue post-chemotherapy impedes the therapeutic outcomes and increases relapse risk. Elicitation of antibody-dependent cellular phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC) against tumor tissue may complement chemotherapy to give better treatment outcomes. ADCP is a phenomenon whereby the body’s humoral immune system is induced to produce antibodies that target selective tumor-associated antigens on the cancer cell surface; binding of these antibodies to the cell surface antigens via their Fab subsequently attracts the association of macrophages to the cancer cells (via the interaction of the Fc gamma receptor), followed by the internalization and degradation of the cancer cells through phagosome acidification [2–5]. ADCC is a mechanism whereby effector immune cells kill the antibody-bounded target cells expressing tumor antigen.
Use of ADCP and ADCC has given positive outcomes in cancer models [6], but priming the body’s humoral immune system to produce substantial antibodies titers that selectively target the tumor tissue remains challenging. This may be because many of the targetable receptors and tumor-associated antigens are ubiquitously expressed on cancer- and normal-cell surfaces. We postulated that ADCP and ADCC may be efficiently induced in tumor-bearing subjects by immunizing them against specific hapten antigen, thereafter exposing the immunized subjects to hapten antigens conjugated with a cancer cell receptor-targeting small molecule ligand. Such cancer cell-targeting hapten antigen is anticipated to selectively bind the cancer cells and occupy its surface with hapten antigen. Hapten is a conventional agent in cancer immunotherapy by triggering the production of anti-hapten antibodies from B cells against hapten protein. In clinical setting, the hapten-based vaccination has been studied in melanoma patients and revealed the development of an inflammatory response in metastatic sites post-hapten-vaccination [7], with high infiltration of T lymphocytes into tumor microenvironment [8]. Recently, few studies have used the hapten-based tumor targeted conjugates to increase the tumor clearance, as studied in uPAR receptor [9] and VEGF receptor [10] expressing cancer, through ADCP mechanism. This approach is different from the monoclonal antibodies based immunotherapy that act as checkpoint. Monoclonal antibodies bind to the checkpoint protein to prevent anergy of activated T lymphocytes in tumor microenvironment, without stimulating antibody production from B cells.
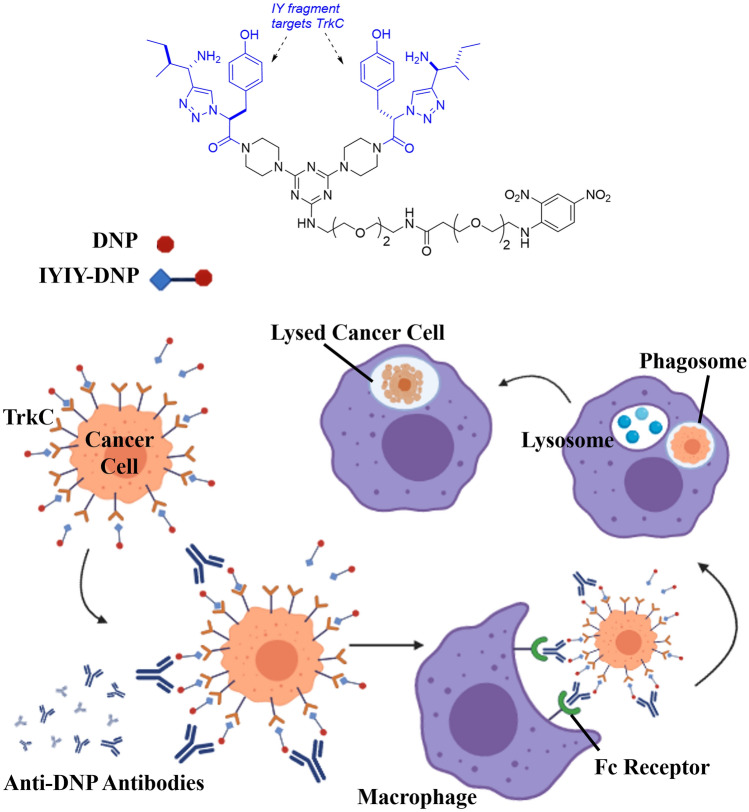
Hence in the present work, we conjugate a tropomyosin receptor kinase C (TrkC) receptor-binding dipeptide (IYIY) to the hapten antigen 2, 4-dinitrophenol (DNP) to explore such a possibility. The receptor featured in this work is one of the tropomyosin receptor kinase (Trk) neurotrophin receptors, TrkC. TrkC is highly expressed in breast cancer [11], neuroblastoma [12], medulloblastoma [13] and colorectal cancers [14] and is associated with cancer cell proliferation and metastasis. Previously, a TrkC receptor-binding dipeptide (IYIY) synthesized by our group [15] was conjugated with photosensitizers for targeted-anticancer photodynamic therapy [16–18]. The role of antibodies to trigger ADCP, ADCC and other complement-dependent cytotoxicity is notable. In the study described here, the 2,4-dinitrophenol (DNP) hapten molecule was conjugated to the IYIY to form a conjugate (IYIY-DNP) intended to target TrkC receptors to study one of the antibodies-mediated function, macrophage-based anticancer ADCP (Fig. 1).
Fig. 1.
Schematic showing the IYIY-DNP structure, and the way it is intended to initiate antibody-dependent cellular phagocytosis (ADCP) mechanism against TrkC expressing cancer cells
Experimental materials and methods
Synthesis of conjugates IYIY-DNP and YIYI-DNP
All reactions were carried out under an argon atmosphere. Reagents were purchased at a high commercial quality (typically 97% or higher) and used without further purification, unless otherwise stated. DNP-PEG2-acid (Catalog# BP-20563) was purchased from BroadPharm (San Diego, CA, USA). High-field NMR spectra were recorded with Bruker Avance at 500 MHz for 1H and 125 MHz for 13C, and were calibrated using residual non-deuterated solvent as an internal reference (CDCl3: 1H NMR = 7.24, 13C NMR = 77.0, MeOD: 1H NMR = 3.30, 13C NMR = 49.0, DMSO-d6: 1H NMR = 2.50, 13C NMR = 39.5). The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, dd = double doublet, dt = double triplet, dq = double quartet, m = multiplet, br = broad. Electrospray ionization mass spectrometry (ESI–MS) data were collected on triple-stage quadrupole instrument in a positive mode. Flash chromatography was performed using silica gel (230–400 mesh). LC–MS analyses were collected from Agilent 1260 Infinity Quaternary LC and Agilent 6120 Quadrupole LC/MS modules using Poroshell 120 EC-C18 2.7 µM (4.6 × 50 mm) column in 5–95% CH3CN/water gradient with 0.1% formic acid over 10 min. Prep HPLC was performed on Agilent 1260 Infinity in 50–90 CH3CN/water gradient with 0.1% TFA over 20 min.
Conjugates and cell lines
Conjugates IYIY-DNP and YIYI-DNP were synthesized as described [19]. Murine breast cancer 4T1 cell line and macrophage RAW264.7 cell line were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA), and breast carcinoma cell line 67NR was obtained from Barbara Ann Karmanos Cancer Institute (Detroit, MI, USA). Both 4T1 and 67NR cell lines were cultured in RPMI medium, whereas RAW246.7 macrophage cell line was cultured in DMEM, supplemented with 10% of fetal bovine serum (FBS) and 1% penicillin- streptomycin solution. All cell lines were maintained at 37 °C in a 5% CO2 incubator.
In vitro cytotoxicity assay
4T1, 67NR and RAW246.7 cell lines were, respectively, cultured in 96 wells plate at a density of 5000 cells per well for 24 h. Conjugates IYIY-DNP and YIYI-DNP were dissolved in DMSO. Each cell line was incubated with either conjugate at concentrations ranging from 0.3 to 30 µM, for 2 and 24 h. The media was removed from the wells; then, 10 µl of 5 mg/ml MTT solution was added into each well and incubated for 4 h in 37 °C. The medium was then removed from the wells, and 100 µl of DMSO was added to solubilize the formazan crystals. The viability of cells was determined by using SpectraMax® M4 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) at 570 nm absorbance [16]. The percentage of viability of each treatment group in different cell lines was calculated as (OD treated/OD control) × 100%.
In vitro antibody-dependent cellular phagocytosis (ADCP) assay
RAW 246.7 macrophage cell line has been employed for ADCP assay [20, 21]. Suspended RAW246.7 cells treated with 200 U/ml IFN-γ (Cell Signaling Technology, Danvers, MA, USA) [22] were stained with 10 µl of DiO dye (Abs: 484 nm; Em: 501 nm) for 45 min at 37 °C. 4T1 and 67NR cancer cells were stained with 10 µl of DiD dye (Abs: 644 nm; Em: 665 nm) for 45 min at 37 °C. The stained cells were then centrifuged at 1500 rpm for 5 min. ADCP assay was performed by suspending cancer cells (4T1, 67NR) at a density of 2.5 × 104 in phenol-red free RPMI media containing cross species antibodies [9, 23, 24] of 100 nM anti-DNP antibodies (rabbit polyclonal KLH IgG, Invitrogen #A6430) and 10 µM of IYIY-DNP or YIYI-DNP conjugates, followed by 1 × 105 of RAW 246.7 macrophage cells. The mixture then incubated at 37 °C for 2 h. The cells interaction and phagocytosis were observed using a confocal microscope (Leica Tcs Sp5 Ii, Wetzlar, Germany).
Animal model
Female 6–8 weeks old wild-type Balb/c mice were purchased from Animal Experimental Unit (AEU), University of Malaya, Malaysia, for in vivo studies. These mice were kept at the animal facility of the Management and Science University, Shah Alam, Malaysia. All animal experiments were performed according to protocols approved by The University of Malaya Faculty of Medicine Institutional Animal Care and Use Committee (Approval ID: AUP2019/335) and Management & Science University Ethics Committee (Approval ID: MSU-RMC-02/FR01/08/L3/019).
In vivo acute toxicity
Mice (n = 2) were injected intravenously with IYIY-DNP and YIYI-DNP at doses up to 30 mg/kg. Toxicity was observed based on the Berlin test of typical symptoms such as apathy, horrent fur, behavior changes, and weight loss for two weeks [16].
In vivo 2,4-dinitrophenol-keyhole limpet hemocyanin (DNP-KLH) immunization and IgG and IgM quantification
Healthy Balb/c mice were immunized with 1 mg/ml DNP-Keyhole Limpet Hemocyanin (DNP-KLH) (Sigma-Aldrich, St. Louis, MO, USA) in PBS with complete Freund’s adjuvant at the ratio of 1:1. After a week, the immunized mice were then boosted with the same dose of DNP-KLH suspended in PBS-incomplete Freund’s adjuvant at the same ratio. A week after administering the booster dose, the mice were bled retro-orbitally for blood collection and the anti-DNP IgG and anti-DNP IgM antibody level were quantified using ELISA (Mouse Anti-DNP IgG ELISA, Mouse Anti-DNP IgM ELISA, Life Diagnostics, West Goshen, PA, USA) following the manufacturer guidelines.
Tumor cell inoculation and treatment in mice
DNP-KLH immunized mice were randomly divided into four groups (IYIY-DNP, YIYI-DNP, DNP and control saline), and orthotopically injected with either 4T1 or 67NR breast carcinoma cell lines into the mammary fat pad at a density of 5 × 105 tumor cells in 0.1 ml of media. Conjugates IYIY-DNP, YIYI-DNP and DNP were dissolved in a cocktail of 2.5% ethanol and 2.5% Cremophore EL, then resuspended using saline to a volume of 0.2 ml. The immunized mice bearing 4T1 or 67NR tumor with the volume of 60–80 mm3 were intravenously injected with either 10 mg/kg IYIY-DNP, 10 mg/kg YIYI-DNP, or 1.4 mg/kg DNP (equivalent to 10 mg/kg IYIY-DNP) (n = 6 for each group) every alternate day for 5 cycles. For control groups, DNP-KLH immunized mice and non-immunized mice were treated with saline. The tumor dimension was measured every two days using a caliper (TESA Technology, Renens, Switzerland). The tumor volume, mm3, was calculated using the formula [(L × W2)/2], where L is the longest dimension and W is the shortest dimension. The blood was drawn retro-orbitally from the mice at the next day of five cycles of treatment (day 10) to quantify the level of anti-DNP IgG and IgM antibody using ELISA.
Statistical analysis
All data were analyzed statistically using SPSS, nonparametric Mann–Whitney U-Test to compare between groups in the case of violation of normality assumption. One-way ANOVA with Tukey’s Multiple Comparisons were used to compare means among the two groups of samples. Student’s t test was used to analyze between two groups. Mean differences were considered statistically significant when the p value was less than 0.05.
Results
Synthesis of conjugates IYIY-DNP and YIYI-DNP
A modified route was used to synthesize IY-IY targeting group S1 (IY-IY-NH2) and YI-YI non-targeting control S4 (YI-YI-NH2) on a large scale as previously reported [25]. The reactive groups on S1 and S2 (DNP-PEG2-acid) were protected with Boc and t-butyl groups to inhibit cross-reactivity during the synthesis. Compound S1 and S2 contained a reactive amine handle that allowed easy conjugation with the activated form of PEG2-DNP-carboxylic acid (S3). The protected groups were cleaved at the last step in acidic conditions to give the desired compound, IYIY-DNP and YIYI-DNP that were purified by Prep HPLC. The synthesis scheme and characterization are described in Fig S1.
In vitro cytotoxicity assay
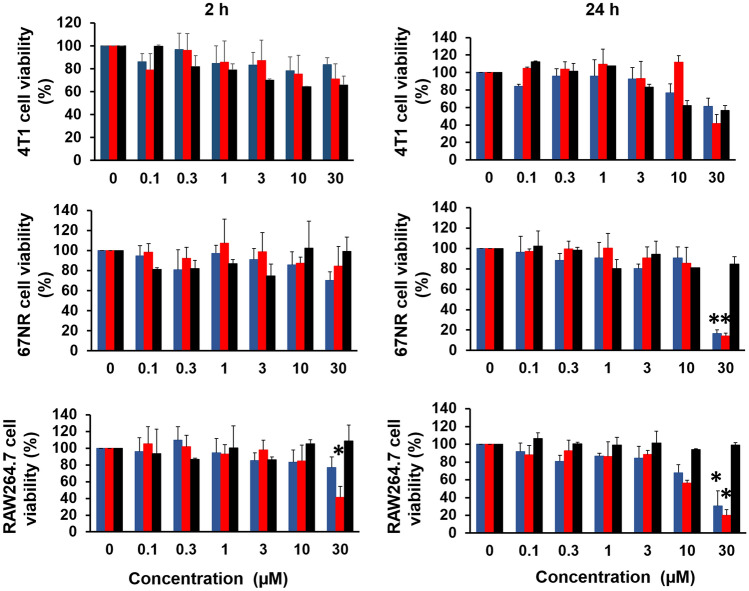
First, the toxicity of the conjugates and the parent DNP was tested in short (2 h) and long (24 h) incubation times against TrkC expressing 4T1 breast carcinoma cells, non-TrkC expressing 67NR cells and RAW264.7 macrophage cells. Based on the result obtained, 2 h incubation with conjugates or DNP did not induce toxicity against the cell lines up to 30 µM. However, the conjugates induced a dose-dependent reduction in cell viability when incubated for 24 h, and yielded significantly reduced cell viability at the concentration of 30 µM for 67NR and RAW264.7 cells (Fig. 2). Hence, 10 µM was chosen as the concentration for subsequent ADCP assay with 2 h of incubation.
Fig. 2.
Percentage cell viabilities for a TrkC expressing 4T1 cells, b non-TrkC expressing 67NR cells and c RAW264.7 macrophage cells after 2 h and 24 h incubation with different concentrations of IYIY-DNP, YIYI-DNP and DNP. Data represent mean ± SEM from three independent experiments (n = 3). *p < 0.05, IYIY-DNP and YIYI-DNP vs DNP using one-way ANOVA (Tukey’s test)
In Vitro antibody-dependent cellular phagocytosis (ADCP)
IYIY-DNP was designed to trigger phagocytes to phagocytose TrkC + tumor cells bound with IYIY-DNP and anti-DNP antibody. This assay was performed by co-culturing TrkC + 4T1 or TrkC- 67NR cells with RAW264.7 macrophage cells, together with IYIY-DNP or YIYI-DNP in the presence of anti-DNP antibodies. As shown in Fig. 3a, TrkC + 4T1 and RAW264.7 cells interacted to form clusters in the presence of IYIY-DNP and anti-DNP antibody, but not for the scrambled control YIYI-DNP (Fig. 3b). TrkC- 67NR cell was used to confirm the ability of IYIY-DNP in inducing interaction between macrophages and tumor cells. As shown in Fig. 3c, d, neither IYIY-DNP nor YIYI-DNP promoted cells interaction. This indicated that IYIY-DNP bound to TrkC + 4T1 cells, and the anti-DNP antibody recruited the RAW264.7 cells to target the former. In the absence of anti-DNP antibodies, IYIY-DNP, YIYI-DNP-treated 4T1 and 67NR existed as individual cells with no cell–cell interaction or cluster formation (Fig S2, S3).
Fig. 3.
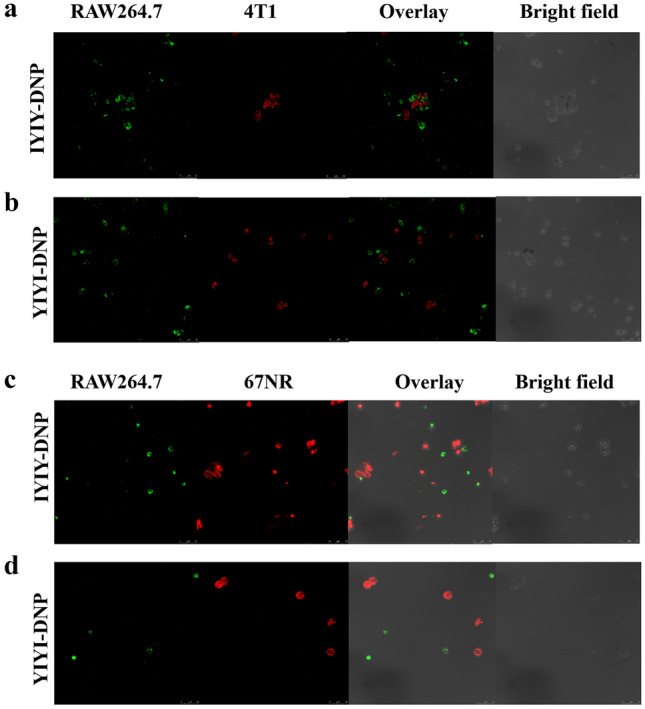
In vitro antibody-dependent cellular phagocytosis in the presence of 0.1% of anti-DNP antibodies. RAW264.7 macrophages were labelled with green dye whereas cancer cells (4T1, 67NR) were labelled with red dye. a 4T1 with 10 µM IYIY-DNP, b 4T1 with 10 µM YIYI-DNP, c 67NR with 10 µM IYIY-DNP, d 67NR with 10 µM YIYI-DNP. Data shown are representative of at least five fields, three independent experiments (n = 3) with similar results
In Vivo acute toxicity test
IYIY-DNP, YIYI-DNP and parent DNP at doses of 10, 20 and 30 mg/kg were administered intravenously for acute toxicity studies. Mice treated with IYIY-DNP at 30 mg/kg died after 1 min of administration, whereas mice treated with YIYI-DNP at 30 mg/kg were inactive and showed movement difficulties with horrent fur for about 40 min before becoming normal again. The behavior of mice administered with 20 mg/kg IYIY-DNP was the same as YIYI-DNP at 30 mg/kg and became normal after 20 min. However, mice administered with 10 mg/kg IYIY-DNP tolerated the conjugate well, and DNP at 4.2 mg/kg (equivalent to 30 mg/kg of conjugate) did not cause movement difficulties, or weight loss in the mice up to 14 days observation (Fig. 4).
Fig. 4.
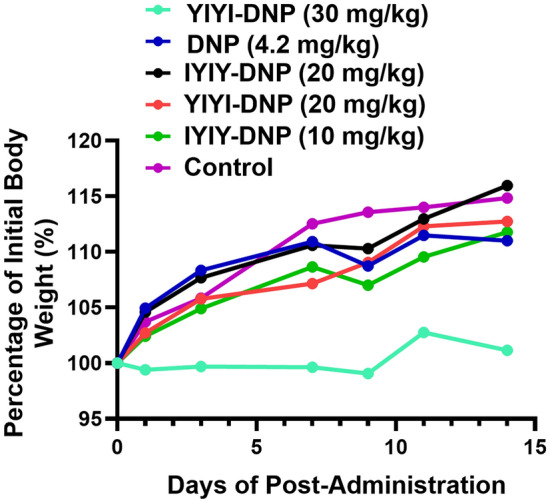
Bodyweight of the healthy Balb/c female mice administered intravenously with IYIY-DNP and YIYI-DNP and DNP at selected doses. Data represent the average body weight (g) of two mice per treatment group
In Vivo antitumor efficacy
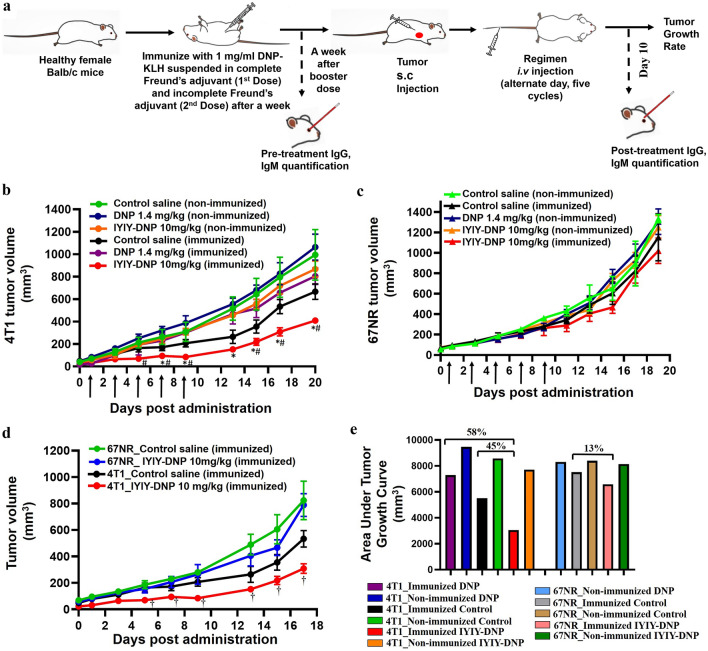
For the antitumor efficacy study as planned in Fig. 5a, mice were first immunized with two doses (1 week apart) of 2,4-dinitrophenol-Keyhole Limpet Hemocyanin (DNP-KLH), followed by tumor cell inoculation. Prior to evaluating the anti-tumor efficacy of the conjugates, the quantities of anti-DNP antibodies produced in the mice post-DNP-KLH immunization were quantified. After immunization with DNP-KLH in complete and incomplete Freund’s adjuvants, blood was drawn retro-orbitally from mice and both the anti-DNP IgG and anti-DNP IgM antibodies levels were quantified. Immunized mice had about 1.5–8.4 × 106 U/mL of anti-DNP IgG antibody whereas no anti-DNP IgG antibody was found in non-immunized mice. Anti-DNP IgM antibody was positive for immunized mice, at 0.7 to 6 × 106 U/mL, and a lower amount of 0.2 to 0.4 × 106 U/mL in non-immunized mice (Fig S4a).
Fig. 5.
In vivo antitumor efficacy study with a schematic diagram on the workflow. Antitumor efficacy of IYIY-DNP conjugates and DNP in b TrkC + 4T1 and c TrkC- 67NR tumor-bearing mice. d Antitumor efficacy of IYIY-DNP in 4T1 and 67NR tumor-bearing mice. e Percentage of tumor reduction based on area under the tumor growth curve of IYIY-DNP and DNP in 4T1 and 67NR tumor-bearing mice, compared to respective immunized control mice. Arrow indicated the treatment given. The graph showed the mean ± SEM of tumor volume, n = 6. *p < 0.05 for IYIY-DNP- vs DNP-treated immunized mice, # p < 0.05 for IYIY-DNP vs immunized control saline, using nonparametric Mann–Whitney U-test. †p < 0.05 for 4T1 IYIY-DNP vs 67NR IYIY-DNP, using nonparametric Mann–Whitney U-test
The DNP-KLH immunized mice bearing TrkC + 4T1 tumor ranging from 60 to 80 mm3 volume were randomly divided into groups for treatment with the conjugate 10 mg/kg of IYIY-DNP, 1.4 mg/kg of DNP (equivalent to 10 mg/kg IYIY-DNP) and control saline. The mice were treated with IYIY-DNP, DNP or saline on alternate days for five cycles. The experiment was repeated in non-immunized 4T1 tumor-bearing mice. As shown in Fig. 5b, IYIY-DNP-treated mice significantly (p < 0.05) reduced tumor growth compared to immunized control mice at day 5 (69.6 ± 12.8 mm3 vs 164.1 ± 34.6 mm3, p = 0.018), day 7 (94.3 ± 16.6 mm3 vs 172.3 ± 30.8 mm3, p = 0.045) and day 9 (84.9 ± 18.6 mm3 vs 207.3 ± 32.6 mm3, p = 0.018) post-initial treatment. The tumor volume in the IYIY-DNP-treated mice was the lowest among all the groups up to day-20 of observation. Conversely, tumor volume of DNP-treated immunized and non-immunized mice had no significant differences compared to control saline groups.
As IYIY-DNP showed the best antitumor activity in 4T1 mice; hence, it was used to confirm the selective antitumor efficacy in TrkC- 67NR model. As shown in Fig. 5c, IYIY-DNP-treated 67NR tumor-bearing mice had comparable tumor volume with non-immunized mice. For ease of comparison, antitumor efficacies of IYIY-DNP in both TrkC + and TrkC- tumors are plotted in Fig. 5d. IYIY-DNP showed significant tumor regression in the TrkC + 4T1 tumor, compared to IYIY-DNP 67NR-treated mice from day-5 onwards. Area under the curve (AUC) was calculated to compare the overall tumor volume among groups in 4T1 and 67NR. As shown in Fig. 5e, IYIY-DNP-treated group has 45% lower tumor volume compared to immunized control mice. This confirms the selectivity of IYIY-DNP in targeting TrkC, in agreement with the enhanced efficacy observed in TrkC expressing cancer.
Blood was drawn retro-orbitally from the mice at day-10 (the day after completion of five treatment cycles) to quantify levels of anti-DNP IgG and IgM after five cycles of treatment. The results revealed that anti-DNP IgG had increased to levels higher than pre-treatment and the level of IgM (Fig S4). A graph analysis was plotted to compare the anti-DNP IgG and IgM antibodies in pre- and post-treatment, as shown in Fig. 6. IgG and IgM antibodies in pre-treatment samples averaged 3.0 × 106 ± 0.4 U/mL and 2.7 × 106 ± 0.2 U/mL, respectively. On average, there were 58% (p = 0.002) increments in the IgG antibody in the mice that received IYIY-DNP compared to pre-treatment. For controls, DNP-treated mouse showed 74% increase in the IgG, while the IgG in the saline-treated mice was 78% (p = 0.003), both compared to pre-treatment. On the other hand, anti-DNP IgM antibodies were reduced in post-treatment among compared to pre-treatment; levels of IgM in IYIY-DNP, DNP and the saline-treated group were 67% (p < 0.0001), 81% (p < 0.0001) and 79% (p < 0.0001), respectively.
Fig. 6.
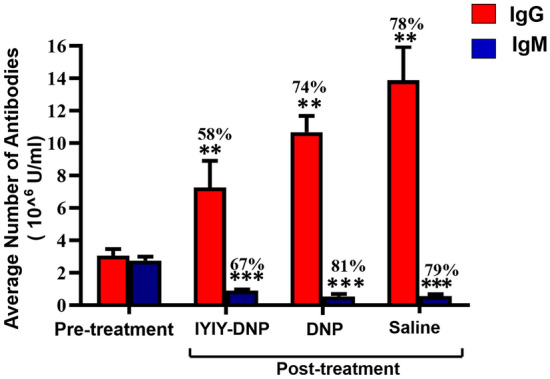
Average number of anti-DNP IgG and IgM antibodies calculated based on individual mouse in pre-treatment and post-treatment. The graph represents mean ± SEM of (n = 18) in pre-treatment, IYIY-DNP, DNP, and saline-treated mice. Values represent percentages of increased (IgG) and decreased (IgM) compared to pre-treatment. **p < 0.005 for post-treatment IYIY-DNP, DNP and control saline vs pre-treatment, ***p < 0.0001 for post-treatment IYIY-DNP, DNP and control saline vs pre-treatment, using independent T test
Discussion
We report the first combined use of hapten-based pre-immunization and targeted-labelling of cancer cells with TrkC-binding-dipeptide-hapten antigen constructs (IYIY-DNP) for eliciting ADCP in a metastatic mammary cancer model. IYIY-DNP (MW = 1322.33), a small construct consisting of 2, 4-dinitrophenol (DNP) chemically combined to a TrkC-binding dipeptide of facilitated selective association of RAW264.7 macrophages to the TrkC expressing 4T1 cancer cells in vitro, and suppressed growth of 4T1 tumor in IYIY-DNP-immunized BALB/c mice by 36.1% at 10 mg/kg dose (p < 0.05, compared to that of the DNP-immunized saline control). The tumor growth suppression activity by the IYIY-DNP in DNP-immunized mice was associated with an increase in the anti-DNP IgG (7.3 × 106 ± 1.6 U/mL) and IgM (0.9 × 106 ± 0.07 U/mL) antibodies after five cycles of DNP treatment.
DNP hapten has been reported to be toxic; it apparently acts by decreasing the electrochemical gradients that are essential for oxidative phosphorylation across the mitochondrial membrane [26]. Hence, an in vitro toxicity tests were carried out to determine the safety threshold of DNP and its conjugate: up to 10 µM of the conjugates or DNP were non-toxic to cell lines treated for 2 h and 24 h. However, toxicity occurred at 30 µM when incubated for 24 h for both conjugates, but not for DNP. Cytotoxicities of the conjugates at 30 µM at 24 h were not dependent on the TrkC expression of the cells, as the RAW 264.7 macrophages and TrkC negative 67NR cells had lower viability compared to TrkC expressing 4T1, perhaps due to prolonged incubation time, triggering non-selective binding to cells. This observation is similar to our previous studies using a TrkC targeted photosensitizer conjugate, whereby increased toxicity with increasing incubation time was observed regardless of the TrkC status [16]. Shorter incubation times can probe for selectivity in active targeting; hence, 2 h was used as the incubation time for the ADCP assay. For in vivo toxicity test, IYIY-DNP was neurotoxic to the mouse, indicated by the contraction of the limb muscle, jerking and seizure. The lethal dose (30 mg/kg) and maximum tolerated dose (20 mg/kg) of the IYIY-DNP in vivo were observed to be the same as our previous study using IYIY-I2-BODIPY [16]. This indicated that the observed toxicity is due to the IYIY-ligand, which is postulated to bind the conjugate to TrkC of the neuronal cells and induces neuronal excitatory and necrosis [27].
Antibody levels are crucial for ADCP and directly determine the antitumor efficacy of the conjugate. There was an increase in anti-DNP IgG antibodies in IYIY-DNP, YIYI-DNP and DNP-treated mice compared to the pre-treatment. This phenomenon has been observed before in conjugates containing antigenic DNP conjugates; these prime more memory B cells to produce anti-DNP IgG for defense [28]. Levels of IgM antibodies appeared higher in pre-treatment and decreased after five cycles of conjugates or DNP administration, accompanied by increased IgG. IgM is the first line defense antibody in acute exposure, and IgM becomes less prevalent than IgG following antigen exposure [29, 30]. High IgG in the saline-treated group is a common phenomenon of IgG production post-immunization, where the antibodies levels may increase [31]. Among the three groups, IYIY-DNP-treated mice have on average lower anti-DNP IgG antibodies in the blood and we postulated that this might be due to their infiltration to the tumor microenvironment post-IYIY-DNP treatment. Conversely, there was no selectivity against TrkC + tumor cells in non-targeted YIYI-DNP, DNP and saline-treated groups; hence, the antibodies in these groups remained high.
Tumor growth in the IYIY-DNP-treated group was delayed throughout the first five cycles, but the tumors grew rapidly once the treatment was stopped. The reasons for this contrast might be depletion of antigenic DNP in the body, which reduced the priming of B cells to produce anti-DNP IgG, as well as the possibility of IgG depletion from binding to IYIY-DNP-cancer cell complex. Thus, continuous administration of the conjugates might stabilize the disease from progressing. When conducted in non-immunized mice, both IYIY-DNP and DNP did not induce tumor ablation in both 4T1 and 67NR bearing mice, suggesting that IYIY-DNP is selective against TrkC + cells in hapten DNP-immunized mice. In comparison, non-immunized monoclonal antibody-based targeted therapy such as trastuzumab [32], alemtuzumab [33], rituximab [34] directly target surface molecules HER2, CD52 and CD20, respectively, on cancer cells. These mAb not only induce targeted cell death by blocking ligand-receptor interaction that are essential for survival, but are also reported to mediate ADCP, ADCC and complement-dependent cytotoxicity (CDC) [35]. We postulate that ADCC and CDC might also be triggered in our therapeutic approach due to the abundance of the targeted receptors, which could produce antibodies against the targeted antigen. Further in-depth studies on the ADCC and CDC of IYIY-DNP are warranted.
In conclusion, this study demonstrates macrophage-dependent ADCP may be induced in tumor-bearing subjects through the combined use of hapten-based pre-immunization and targeted-labelling of cancer cells by tumor cell-binding-dipeptide-hapten antigen constructs. IYIY-DNP targets TrkC + metastatic mammary tumors, probably through the induction of ADCP. Looking ahead, immune profiling of T cells and B cells post-treatment with the IYIY-DNP may be warranted to understand mechanisms of ADCP facilitation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was funded by the Ministry of Higher Education Malaysia Fundamental Research Grant Scheme (FRGS/1/2020/SKK0/MSU/02/1), NIH R01EY029645, the Texas A&M University T3-Grants Program (246292-00000), and the National Cancer Council Malaysia (MAKNA) Cancer Research Award (CRA) 2017
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kevin Burgess, Email: burgess@chem.tamu.edu.
Chin Siang Kue, Email: cskue@msu.edu.my.
References
- 1.Bernal-Estévez D, Sánchez R, Tejada RE, Parra-López C (2016) Chemotherapy and radiation therapy elicits tumor specific T cell responses in a breast cancer patient. BMC cancer. 16:591. Doi: 10.1186/s12885-016-2625-2 [DOI] [PMC free article] [PubMed]
- 2.Li H, Somiya M, Kuroda Si (2021) Enhancing antibody-dependent cellular phagocytosis by Re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials. 268:120601. Doi: 10.1016/j.biomaterials.2020.120601 [DOI] [PubMed]
- 3.Graziano RF, Engelhardt JJ (2019) Role of FcγRs in antibody-based cancer therapy. Current Top Microbiol Immunol 423:13–34. Doi: 10.1007/82_2019_150 [DOI] [PubMed]
- 4.Kurdi AT, Glavey SV, Bezman NA et al. (2018) Antibody-dependent cellular phagocytosis by macrophages is a novel mechanism of action of Elotuzumab. Mol Cancer Ther. 17:1454–1463. Doi: 10.1158/1535-7163.MCT-17-0998 [DOI] [PMC free article] [PubMed]
- 5.Kamen L, Myneni S, Langsdorf C, Kho E, Ordonia B, Thakurta T, Zheng K, Song A, Chung S (2019) A novel method for determining antibody-dependent cellular phagocytosis. J Immunol methods. 468:55–60. Doi: 10.1016/j.jim.2019.03.001 [DOI] [PubMed]
- 6.Tsao L-C, Crosby EJ, Trotter TN et al. (2019) CD47 blockade augmentation of trastuzumab antitumor efficacy dependent on antibody-dependent cellular phagocytosis. JCI Insight. 4:e131882. Doi: 10.1172/jci.insight.131882 [DOI] [PMC free article] [PubMed]
- 7.Berd D, Sato T, Maguire HC, Kairys J, Mastrangelo MJ (2004) Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J Clin Oncol 22:403–415. Doi: 10.1200/JCO.2004.06.043 [DOI] [PubMed]
- 8.Berd D, Maguire HC, Jr., Mastrangelo MJ, Murphy G (1994) Activation markers on T cells infiltrating melanoma metastases after therapy with dinitrophenyl-conjugated vaccine. Cancer Immunol Immunother 39:141–147. Doi: 10.1007/bf01533378 [DOI] [PMC free article] [PubMed]
- 9.Rullo AF, Fitzgerald KJ, Muthusamy V, Liu M, Yuan C, Huang M, Kim M, Cho AE, Spiegel DA (2016) Re-engineering the immune response to metastatic cancer: antibody-recruiting small molecules targeting the urokinase receptor. Angew Chem Int Ed Engl 55:3642–3646. Doi: 10.1002/anie.201510866 [DOI] [PMC free article] [PubMed]
- 10.Schrand B, Clark E, Levay A, Capote AR, Martinez O, Brenneman R, Castro I, Gilboa E (2018) Hapten-mediated recruitment of polyclonal antibodies to tumors engenders antitumor immunity. Nature Commun 9:3348. Doi: 10.1038/s41467-018-05566-x [DOI] [PMC free article] [PubMed]
- 11.Jin W (2020) Roles of TrkC Signaling in the regulation of tumorigenicity and metastasis of cancer. Cancers. 12:147. Doi: 10.3390/cancers12010147 [DOI] [PMC free article] [PubMed]
- 12.Brodeur GM, Minturn JE, Ho R, Simpson AM, Iyer R, Varela CR, Light JE, Kolla V, Evans AE (2009) Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 15:3244–3250. Doi: 10.1158/1078-0432.CCR-08-1815 [DOI] [PMC free article] [PubMed]
- 13.Friedrich C, Shalaby T, Oehler C et al. (2017) Tropomyosin receptor kinase C (TrkC) expression in medulloblastoma: relation to the molecular subgroups and impact on treatment response. Child's Nervous System ChNS Official J Int Soc Pediatric Neurosurg 33:1463–1471. Doi: 10.1007/s00381-017-3506-y [DOI] [PubMed]
- 14.Kim MS, Suh KW, Hong S, Jin W (2017) TrkC promotes colorectal cancer growth and metastasis. Oncotarget 8:41319–41333. Doi: 10.18632/oncotarget.17289 [DOI] [PMC free article] [PubMed]
- 15.Chen D, Brahimi F, Angell Y, Li YC, Moscowicz J, Saragovi HU, Burgess K (2009) Bivalent peptidomimetic ligands of TrkC are biased agonists and selectively induce neuritogenesis or potentiate neurotrophin-3 trophic signals. ACS Chem Biology 4:769–781. Doi: 10.1021/cb9001415 [DOI] [PMC free article] [PubMed]
- 16.Kue CS, Kamkaew A, Lee HB, Chung LY, Kiew LV, Burgess K (2015) Targeted PDT agent eradicates TrkC expressing tumors via photodynamic therapy (PDT). Molecular Pharm 12:212–222. Doi: 10.1021/mp5005564 [DOI] [PMC free article] [PubMed]
- 17.Kue CS, Kamkaew A, Voon SH, Kiew LV, Chung LY, Burgess K, Lee HB (2016) Tropomyosin receptor kinase C Targeted delivery of a peptidomimetic ligand-photosensitizer conjugate induces antitumor immune responses following photodynamic therapy. Sci Rep 6:37209. Doi: 10.1038/srep37209 [DOI] [PMC free article] [PubMed]
- 18.Kue CS, Kamkaew A, Burgess K, Kiew LV, Chung LY, Lee HB (2016) Small molecules for active targeting in cancer. Med Res Rev 36:494–575. Doi: 10.1002/med.21387 [DOI] [PubMed]
- 19.Chen D, Brahimi F, Angell Y, Li Y-C, Moscowicz J, Saragovi HU, Burgess K (2009) Bivalent peptidomimetic ligands of TrkC are biased agonists and selectively induce neuritogenesis or potentiate neurotrophin-3 trophic signals. ACS Chem Biol 4:769–781. Doi: 10.1021/cb9001415 [DOI] [PMC free article] [PubMed]
- 20.Benonisson H, Sow HS, Breukel C et al. (2018) FcγRI expression on macrophages is required for antibody-mediated tumor protection by cytomegalovirus-based vaccines. Oncotarget 9:29392–29402. Doi: 10.18632/oncotarget.25630 [DOI] [PMC free article] [PubMed]
- 21.Bakalar MH, Joffe AM, Schmid EM, Son S, Podolski M, Fletcher DA (2018) Size-dependent segregation controls macrophage phagocytosis of antibody-opsonized targets. Cell 174:131–142.e13. Doi: 10.1016/j.cell.2018.05.059 [DOI] [PMC free article] [PubMed]
- 22.He H, Li W, Chen S-Y, Zhang S, Chen Y-T, Hayashida Y, Zhu Y-T, Tseng SCG (2008) Suppression of activation and induction of apoptosis in RAW264.7 cells by amniotic membrane extract. Invest Ophthalmol Vis Sci 49:4468–4475. Doi: 10.1167/iovs.08-1781 [DOI] [PMC free article] [PubMed]
- 23.Rane D, Carlson EJ, Yin Y, Peterson BR (2020) Fluorescent detection of peroxynitrite during antibody-dependent cellular phagocytosis. Methods Enzymol 640:1–35. Doi: 10.1016/bs.mie.2020.04.001 [DOI] [PMC free article] [PubMed]
- 24.Unkeless JC, Kaplan G, Plutner H, Cohn ZA (1979) Fc-receptor variants of a mouse macrophage cell line. In: Proceedings of the National Academy of Sciences of the United States of America 76:1400–1404. Doi: 10.1073/pnas.76.3.1400 [DOI] [PMC free article] [PubMed]
- 25.Yang Z, Usama SM, Li F, Burgess K, Li Z (2018) A zwitterionic near-infrared dye linked TrkC targeting agent for imaging metastatic breast cancer. Medchemcomm 9:1754–1760. Doi: 10.1039/c8md00190a [DOI] [PMC free article] [PubMed]
- 26.Janz DM. Dinitrophenols. In: Wexler P, editor. Encyclopedia of Toxicology. 3. Oxford: Academic Press; 2014. pp. 177–178. [Google Scholar]
- 27.Koh JY, Gwag BJ, Lobner D, Choi DW (1995) Potentiated necrosis of cultured cortical neurons by neurotrophins. Science (New York, NY) 268:573–575. Doi: 10.1126/science.7725105 [DOI] [PubMed]
- 28.Manz RA, Hauser AE, Hiepe F, Radbruch A (2005) Maintenance of serum antibody levels. Annu Rev Immunol 23:367–386. Doi: 10.1146/annurev.immunol.23.021704.115723 [DOI] [PubMed]
- 29.Díaz-Zaragoza M, Hernández-Ávila R, Viedma-Rodríguez R, Arenas-Aranda D, Ostoa-Saloma P (2015) Natural and adaptive IgM antibodies in the recognition of tumor-associated antigens of breast cancer (Review). Oncol Rep 34:1106–1114. Doi: 10.3892/or.2015.4095 [DOI] [PMC free article] [PubMed]
- 30.Keyt BA, Baliga R, Sinclair AM, Carroll SF, Peterson MS (2020) Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies 9. Doi: 10.3390/antib9040053 [DOI] [PMC free article] [PubMed]
- 31.Ayres JA, Barraviera B, Calvi SA, Carvalho NR, Peraçoli MTS. Antibody and cytokine serum levels in patients subjected to anti-rabies prophylaxis with serum-vaccination. J Venom Animals Toxins Including Trop Dis. 2006;12:435–455. [Google Scholar]
- 32.Shi Y, Fan X, Deng H et al. (2015) Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages. J Immunol 1402891. Doi: 10.4049/jimmunol.1402891 [DOI] [PubMed]
- 33.Golay J, Manganini M, Rambaldi A, Introna M. Effect of alemtuzumab on neoplastic B cells. Haematologica. 2004;89:1476–1483. [PubMed] [Google Scholar]
- 34.VanDerMeid KR, Elliott MR, Baran AM, Barr PM (2018) cellular cytotoxicity of next-generation CD20 monoclonal antibodies. 6:1150–1160. Doi: 10.1158/2326-6066.cir-18-0319 [DOI] [PubMed]
- 35.Golay J, Taylor RP (2020) The role of complement in the mechanism of action of therapeutic anti-cancer mAbs. Antibodies 9 Doi: 10.3390/antib9040058 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.