Abstract
PURPOSE:
The traditional standard of care for Graves’ ophthalmopathy (GO) is glucocorticoid therapy, which is associated with many long-term side effects. The aim of this systematic review and meta-analysis was to compare the traditional therapy to novel monoclonal antibodies (e.g. rituximab [RTX], teprotumumab, and tocilizumab [TCZ]).
METHODS:
We searched the Medline, Embase, and Cochrane Central Register of Controlled Trials databases. We included randomized controlled trials (RCTs) that compared different monoclonal antibodies (e.g. RTX, teprotumumab, and TCZ) with glucocorticoids or placebo in patients with GO. We evaluated the clinical activity score (CAS), proptosis, subjective diplopia using the Gorman score, quality of life (QoT), adverse events, change in lid fissure, NOSPECS score, and TSH receptor antibody (TRAb) levels. The odds ratio (OR) was used to represent dichotomous outcomes. The continuous outcomes were represented as standardized mean difference (SMD). Data were pooled using the inverse variance weighting method. Risk of bias was assessed using the revised Cochrane risk-of-bias tool for randomized trials.
RESULTS:
Six (n = 571) RCTs were deemed eligible. The different monoclonal antibodies were significantly more efficacious than glucocorticoid/placebo in terms of reduction in CAS (SMD = −1.44, 95% confidence interval (CI): −1.91–−0.97, P < 0.00001, I2 = 74%), change in proptosis (SMD = −4.96, 95% CI: −8.02–−1.89, P = 0.002, I2 = 99%), QoL (SMD = 2.64, 95% CI: 0.50–4.79, P = 0.02, I2 = 97%), and Gorman score for diplopia (OR = 3.42, 95% CI: 1.62–7.22, P = 0.001, I2 = 8%). However, monoclonal antibodies have shown higher rates of adverse events (OR = 2.91, 95% CI: 1.12–7.56, P = 0.03, I2 = 62%). No significant difference was found with respect to lid fissure, NOSPECS, and TRAb levels.
CONCLUSION:
This meta-analysis demonstrated that monoclonal antibodies were associated with more favorable clinical outcomes than standard steroid therapy or placebo, especially with regard to CAS, change in proptosis, diplopia, and QoL, with teprotumumab being superior. In addition, only minor safety concerns were identified with monoclonal antibodies though less worrisome than using traditional steroids.
Keywords: Graves’, ophthalmopathy, Graves’, orbitopathy, rituximab, teprotumumab, thyroid eye disease, tocilizumab
INTRODUCTION
Graves’ ophthalmopathy (GO), also known as thyroid eye disease (TED), is an autoimmune thyroid-related ocular manifestation characterized by pain, double or decreased vision, and exophthalmos.[1,2] It is also the most prevalent Graves’ disease (GD) extrathyroidal manifestation. The incidence of GO in the general population is around 0.1%–0.3%; however, this proportion rises to 20%–40% in patients with GD.[2,3] The pathogenesis of GO is not fully understood, but cross-reactivity between thyroid and orbital tissue might be the mechanism behind the disease due to the presence of autoantibodies in the orbital tissues.[4,5] In patients with GD, these antibodies are not only present in the hyperthyroid state but also can persist in euthyroid or hypothyroid states when patients are under antithyroid treatments.[6] Changes in ocular performance and significant disfigurement such as lid retraction, periorbital tissue swelling, and fixed gaze are common adverse effects of GO that may disturb daily activities, thus negatively impacting the patients’ quality of life (QoL).[3]
In clinical practice, the traditional standard of care for GO is glucocorticoid therapy. The benefits of systemic glucocorticoid treatment include the relief of acute GO symptoms, improved optical nerve function, and improved patient QoL.[3] Long-term use of corticosteroids, on the other hand, can cause steroid-related side effects that include weight gain, hyperglycemia, hypertension, osteoporosis, and liver damage.[7] In addition to these systemic adverse effects, steroids have many ocular side effects, including glaucoma, cataract formation, delayed wound healing, and an increased susceptibility to infections.[8] Furthermore, symptoms of GO may worsen when the amount of glucocorticoids is gradually reduced. Hence, additional studies into the pathophysiology of GO are required to develop a more commonly utilized therapy with fewer adverse effects.[3]
Recently, novel pharmaceutical agents have been widely evaluated for the management of GO. Monoclonal antibodies like tocilizumab (TCZ), rituximab(RTX), and teprotumumab are among the most important developments (TCZ). A recent meta-analysis, exploring the efficacy and safety of RTX, concluded that for individuals with moderate-to-severe GO, RTX is a generally safe and effective therapy that outperforms glucocorticoids and saline.[9] Two other randomized controlled trials (RCTs) showed that teprotumumab had superior results than placebo in terms of proptosis, clinical activity score (CAS), double vision, and QoL.[1,10] However, none of the published studies collectively determined the role of different monoclonal antibody drugs in the management of GO compared to steroids.
The aim of this systematic review and meta-analysis was to provide an exhaustive evaluation of the efficacy and safety of monoclonal antibodies (e.g. RTX, teprotumumab, and TCZ) compared to steroid therapy for the management of GO with respect to CAS, proptosis, and subjective diplopia using the Gorman score, QoL, lid fissure, NOSPECS score, TSH receptor antibody (TRAb) levels, and adverse events.
METHODS
This systematic review was conducted in accordance with a prespecified protocol registered in PROSPERO (CRD42021266175). The report of this review was in the light of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis checklist.[11]
Eligibility criteria
Population: Adult individuals with moderate-to-severe and active GO with CAS ≥3 out of 7. Intervention: Monoclonal antibody administered intravenously (IV) (i.e. RTX or teprotumumab or TCZ). Comparison: IV administered glucocorticoids or placebo. Placebo-controlled trials were included in order to reach a larger sample size as well as to include the most RCTs for the different monoclonal antibodies, as up to our knowledge, no studies compared teprotumumab or TCZ to glucocorticoids. Outcomes: CAS, proptosis, subjective diplopia score using the Gorman scale, QoT, adverse events, change in lid fissure, NOSPECS score, and TRAb levels. Study design: RCTs. We excluded trials that enrolled participants who received glucocorticoid therapy within the past month. We also excluded trials that included those who received radiotherapy.
Search strategy
The systematic search was performed on Medline, Embase, and the Cochrane Central Register of Controlled Trials from database inception to July 3, 2021, without any restriction on date or language. The complete search strategy is provided in the supplementary material. We manually searched the references of the included studies for potentially relevant RCTs missed during the systematic search.
Study selection and data extraction
Two reviewers performed title and abstract screening against the eligibility criteria, full-text assessment, and data extraction from eligible trials independently and in duplicate. Discrepancies were resolved through consensus or discussion with a third reviewer before the analyses were performed.
Meta-analysis
Data analysis was performed using RevMan (Review Manager) version 5.3 (Cochrane Collaboration). All statistical analyses were performed using the random-effects model. We adopted 95% as a confidence level and P < 0.05 as a threshold. The statistical heterogeneity was assessed using I2 and the P value of the Chi-square test. The continuous outcomes (CAS, change in proptosis, change in lid fissure, NOSPECS score, and TRAbs) were represented as standardized mean difference (SMD) and pooled using the inverse variance weighting method. The dichotomous outcomes (adverse events and Gorman diplopia score) were expressed as odds ratio (OR) and pooled using the inverse variance weighting method. We performed subgroup analysis based on the type of monoclonal antibody used: RTX, teprotumumab, and TCZ.
Risk-of-bias assessment
Two reviewers independently and in duplicate performed the risk-of-bias assessment for the included RCTs using the revised Cochrane risk-of-bias assessment tool. The potential of publication bias for each outcome was assessed by visual inspection of the funnel plot and assessment of symmetry. Evidence of publication bias is considered possible when it is asymmetrical.
RESULTS
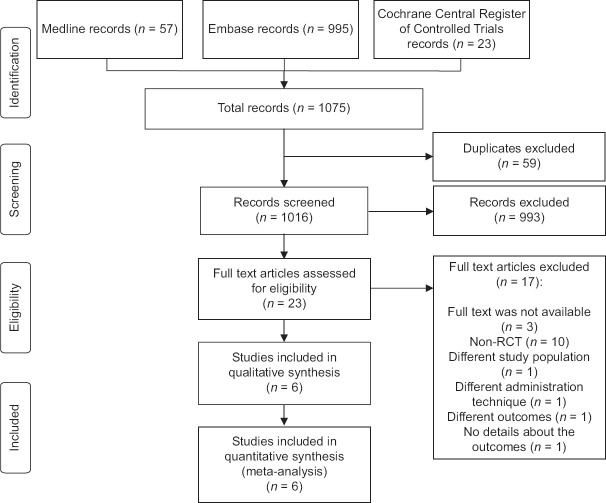
The flowchart of this systematic review, including the justification for exclusion of studies, is shown in Figure 1. In the literature search, we identified 1075 records, of which 59 duplicates were excluded. After screening the titles and abstracts, there were 23 potentially eligible studies assessed for inclusion. Only six RCTs, eventually, were eligible, and all of them were included in the meta-analysis. Three articles assessed RTX monoclonal antibody. Two studies evaluated the effect of teprotumumab monoclonal antibody. Only one study assessed TCZ monoclonal antibody.
Figure 1.
Study flow diagram. RCT: Randomized controlled trial
Trial characteristics
A total of 571 individuals were included in this systematic review. Of whom, 101 (17.6%) were randomized to RTX, 84 (14.7%) were randomly assigned to teprotumumab, and 15 (2.6%) were randomized to the TCZ group. The mean age ranged from 45 to 61 years for the control group and 47 to 57 years for the monoclonal antibody group. Table 1 shows the detailed characteristics of the included studies.
Table 1.
Characteristics of the included studies
| Study, Year | Intervention | Number of participants | Age (in years) | Measured outcomes | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control | Monoclonal antibody | Control | Monoclonal antibody | |||
| Li 2017 | Rituximab Vs (A) Iodine-131, (B) Iodine-131 and Methylprednisolone | A=72, B=70 | 75 | (A) 49.1±9.3, (B) 50.3±10.0 | 48.1±10.0 | Hyperthyroidism treatment outcomes, orbital volumetric analysis through CT imaging, proptosis, eyelid width, soft tissue swelling, conjunctival hyperemia, intraocular pressure, visual acuity, CAS, serum Th1 and Th2 cytokine levels, and adverse events. |
| Salvi 2015 | Rituximab Vs Methylprednisolone | 16 | 15 | 50.4±11.4 | 51.9±13.1 | CAS, proptosis, lid fissure, diplopia and eye muscle motility, and quality of life score, number of therapeutic responses, disease reactivation, and surgical procedures required during follow-up. |
| Stan 2015 | Rituximab Vs Placebo | 10 | 11 | 61.8±11.0 | 57.6±12.7 | CAS, success and failure rates, proportions showing clinically significant improvement in proptosis, lid fissure width, diplopia score, lagophthalmos and disease severity, orbital fat/muscle volume and quality-of-life. |
| Douglas 2020 | Teprotumumab Vs Placebo | 42 | 41 | 48.9±13.0 | 51.6±12.6 | Proptosis response, CAS, diplopia response, and Graves’ophthalmopathy-specific quality-of-life (GO-QOL). |
| Smith 2017 | Teprotumumab Vs Placebo | 44 | 43 | 54.2±13.0 | 51.6±10.6 | CAS, proptosis, GO-QOL questionnaire, and adverse events. |
| Perez- Moreiras 2018 | Tocilizumab Vs Placebo | 17 | 15 | 45.07 (IQR=38.9-50.5) | 47.5 (IQR=41.1-57.4) | CAS, patient global assessment (PtGA) of pain, quality of life evaluated by the generic SF-36 and GO-QoL, adverse events, death, and clinically significant changes in vital signs and laboratory tests. |
QOL: Quality of life, PtGA: Patient global assessment, CAS: Clinical activity score, GO-QoL: Graves’ ophthalmopathy-specific QOL, IQR: Interquartile range, CT: Computed tomography
Risk-of-bias assessment
Three of the six RCTs had an overall low risk of bias. Two RCTs had some concerns. One RCT had an overall high risk of bias due to deviation from the intended intervention. Figures 2 and 3 demonstrate the risk-of-bias assessment of the included RCTs.
Figure 2.
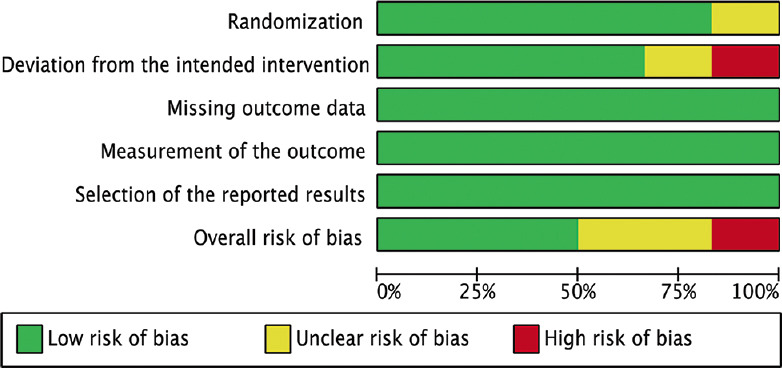
Risk-of-bias graph
Figure 3.
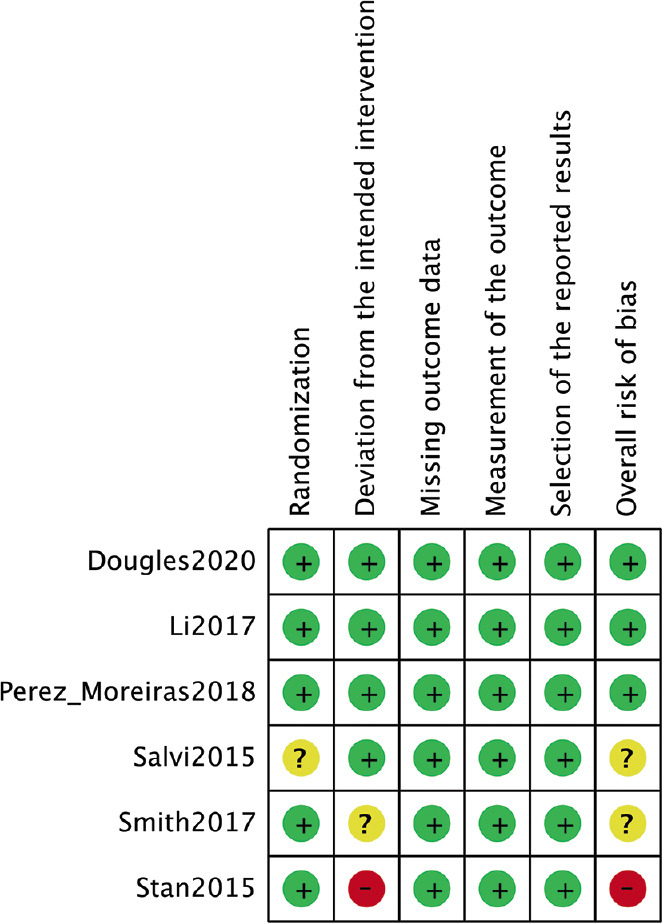
Risk-of-bias summary
Clinical activity score
All included RCTs reported on CAS (n = 571).[1,3,4,10,12,13] The monoclonal antibody groups showed a significant difference in CAS compared to glucocorticoid/placebo (SMD = −1.44, 95% confidence interval (CI): −1.91–−0.97, P < 0.00001, I2 = 74%). Three RCTs reported significantly better CAS in the RTX group in comparison to the glucocorticoid/placebo group (SMD = −1.51 points, 95% CI: −2.19–−0.82, P < 0.0001, I2 = 67%). Similarly, Douglas, 2020, and Smith, 2017, also found a significant difference in CAS between the teprotumumab and placebo groups (SMD = −1.46, 95% CI: −2.67–−0.25, P = 0.02, I2 = 92%). Moreover, Perez-Moreiras, 2018, also showed a significant difference in CAS between TCZ and placebo (SMD = −1.18, 95% CI: −1.94–−0.42, P = 0.002, I2 = not applicable) [Figure 4a]. The funnel plot was symmetrical on visual inspection, and no evidence of publication bias was noted [Figure 4b].
Figure 4.
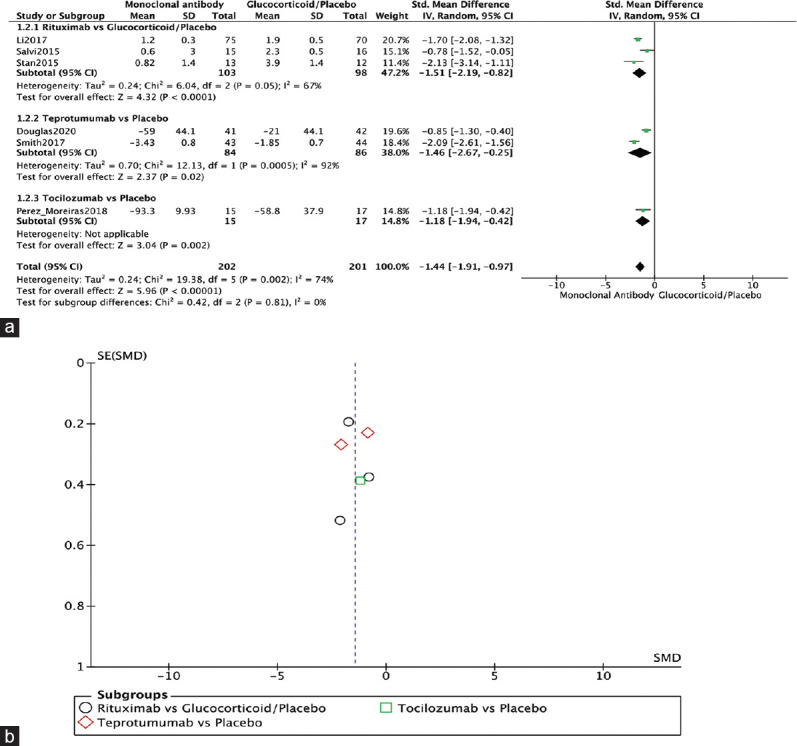
Clinical activity score. (a) Forest plot. (b) Funnel plot. CI: Confidence interval, IV: Inverse variance, RTX: Rituximab
Change in proptosis
Five RCTs reported on change in proptosis.[1,3,4,10,12] The monoclonal antibody group showed a significantly better outcome in terms of proptosis compared to glucocorticoid/placebo (SMD = −4.96, 95% CI: −8.02–−1.89, P = 0.002, I2 = 99%). Three RCTs showed no significant difference in change in proptosis between RTX and glucocorticoid/placebo (SMD = −0.29, 95% CI: −0.97–0.39, P = 0.40, I2 = 73%). On the contrary, Douglas, 2020, and Smith, 2017, reported a significantly better change in proptosis when administrating teprotumumab over placebo (SMD = −12.52, 95% CI: −13.92–−11.12, P < 0.00001, I2 = 0%) [Figure 5a]. The funnel plot was symmetrical on visual inspection, and no evidence of publication bias was noted [Figure 5b].
Figure 5.
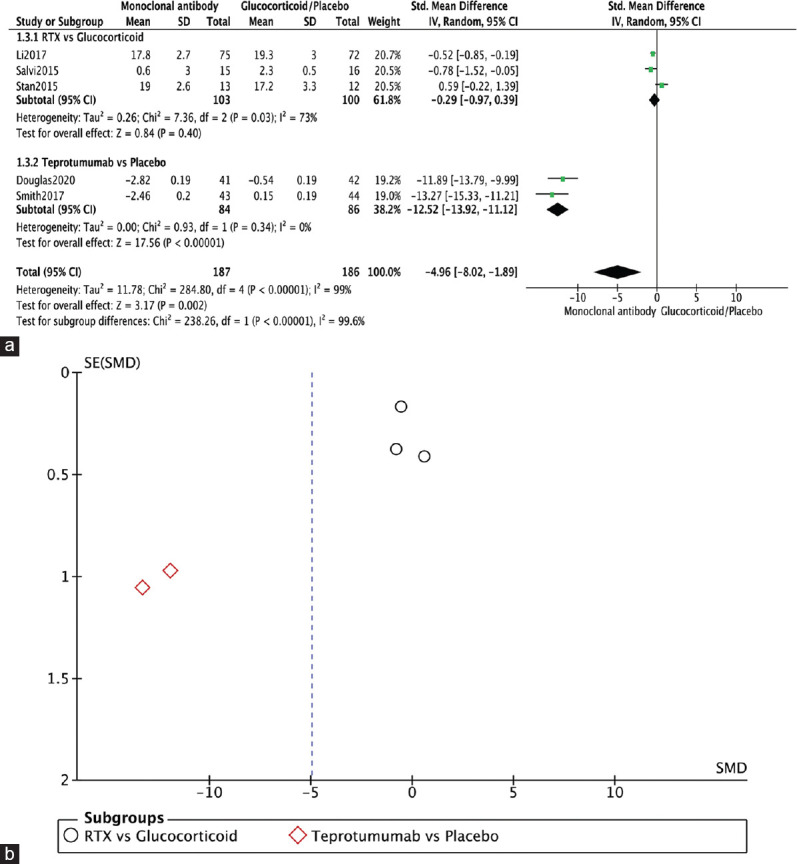
Change in proptosis. (a) Forest plot. (b) Funnel plot.
Gorman diplopia score
Three RCTs reported on Gorman diplopia score.[1,4,10] Monoclonal antibodies showed significantly better scores in the Gorman diplopia score in comparison to the glucocorticoid/placebo group (OR = 3.42, 95% CI: 1.62–7.22, P = 0.001, I2 = 8%). Two RCTs showed a significant improvement in Gorman diplopia score in teprotumumab over placebo therapy (OR = 4.31, 95% CI: 1.98–9.39, P = 0.0002, I2 = 0%). Salvi, 2015, compared RTX to glucocorticoids in terms of Gorman diplopia score and showed no significant difference between the two interventions (OR = 1.08, 95% CI: 0.18–6.44, P = 0.93, I 2 = not applicable) [Figure 6a]. The funnel plot was symmetrical on visual inspection, and no evidence of publication bias was noted [Figure 6b].
Figure 6.
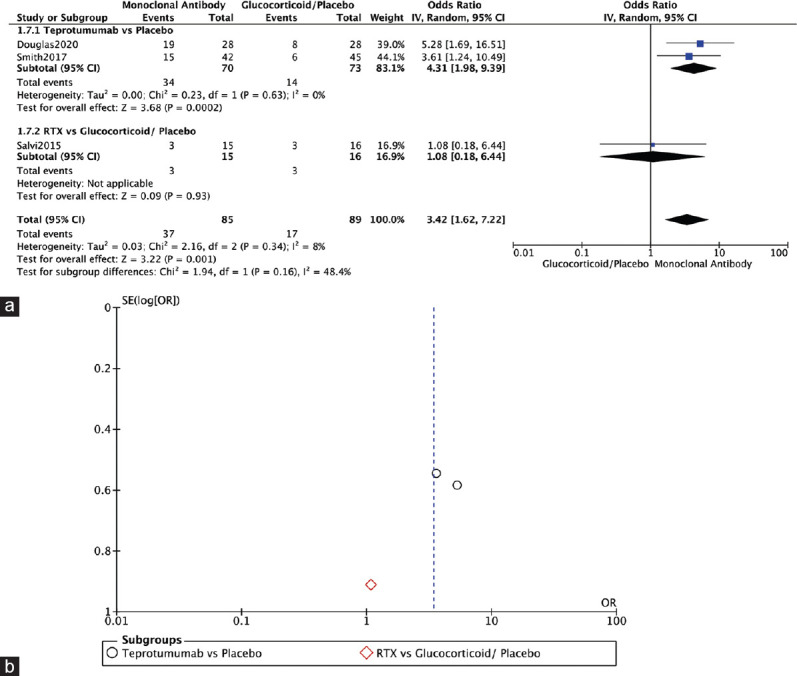
Gorman diplopia score. (a) Forest plot. (b) Funnel plot
Change in lid fissure
Two RCTs reported on change in lid fissure.[4,12] Both compared RTX to glucocorticoids (Li 2017) or placebo (Stan 2015) and showed no significant difference in change in lid fissure (SMD = −0.24, 95% CI: −0.54–0.06, P = 0.12, I2 = 0%) [Figure 7a]. The funnel plot was symmetrical on visual inspection, and no evidence of publication bias was noted [Figure 7b].
Figure 7.
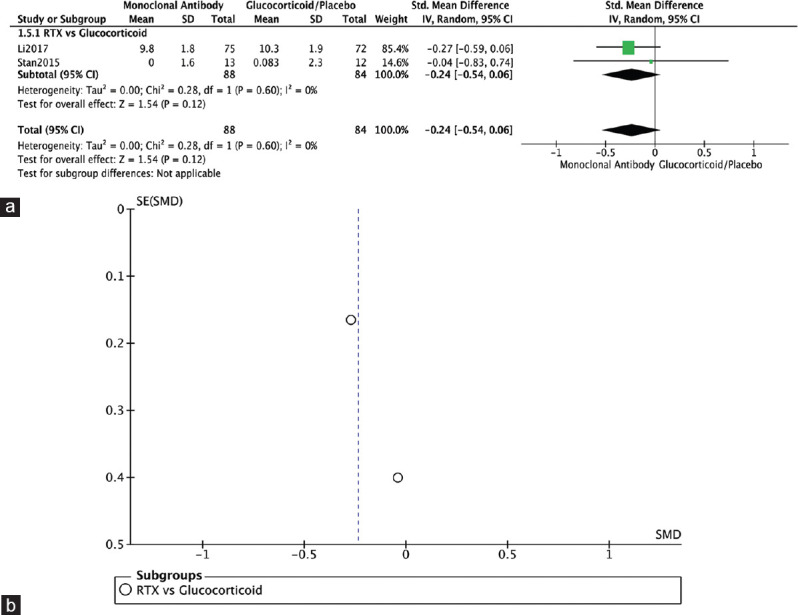
Change in lid fissure. (a) Forest plot. (b) Funnel plot
Adverse events
All RCTs reported on adverse events.[1,3,4,10,12,13] The monoclonal antibody group showed significantly higher rates of adverse events compared to the glucocorticoid or placebo group (OR = 2.91, 95% CI: 1.12–7.56, P = 0.03, I2 = 62%). For RTX, only Li 2017 reported less adverse events with RTX compared to steroids. In Salvi, 2015, and Stan, 2015, RTX was associated with more adverse events compared to the control groups (OR = 2.54, 95% CI: 0.40–16.15, P = 0.32, I2 = 78%). Teprotumumab was associated with significantly higher rates of adverse events compared to placebo (OR = 3.06, 95% CI: 1.16–8.09, P = 0.02, I2 = 0%). Similarly, in Perez-Moreiras, 2018, TCZ was associated with significantly high rates of adverse events (OR = 4.88, 95% CI: 1.06–22.38, P = 0.04, I2 = not applicable) [Figure 8a]. The funnel plot was symmetrical on visual inspection, and no evidence of publication bias was noted [Figure 8b].
Figure 8.
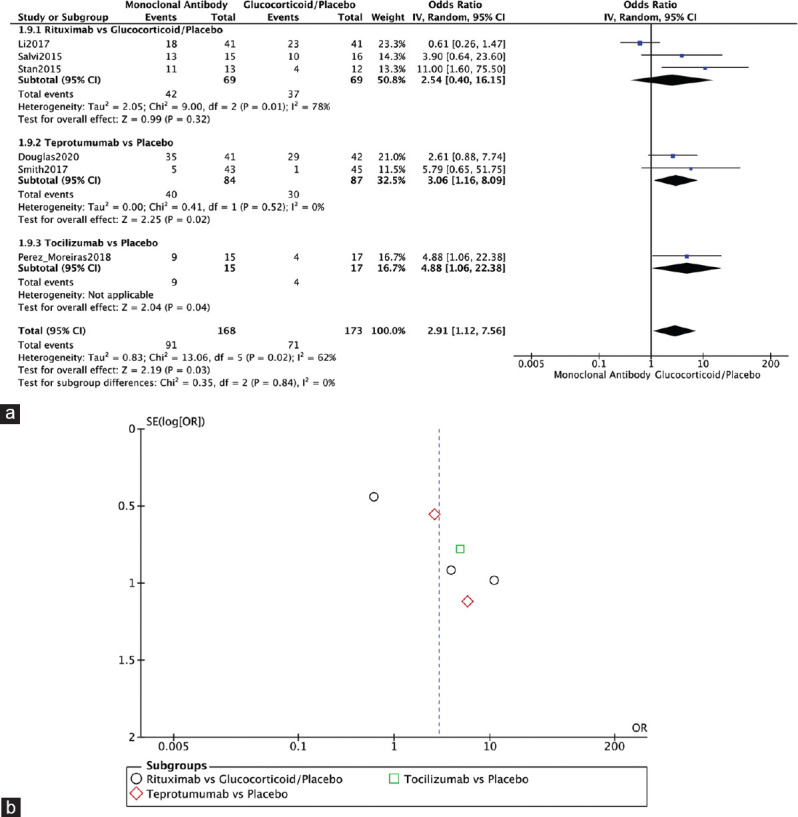
Adverse events. (a) Forest plot. (b) Funnel plot
NOSPECS score
Two RCTs reported on NOSPECS score.[4,12] Both compared RTX to glucocorticoids (Salvi, 2015) or placebo (Stan, 2015) and showed no significant difference in NOSPECS score (OR = 0.65, 95% CI: 0.18–2.42, P = 0.53, I2 = 0%) [Figure 9a]. The funnel plot was symmetrical on visual inspection, and no evidence of publication bias was noted [Figure 9b].
Figure 9.
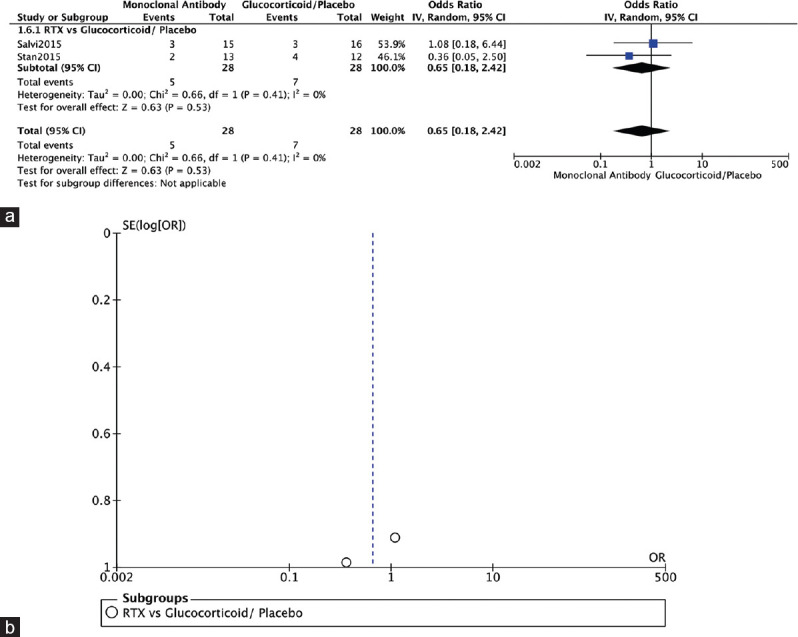
NOSPECS score. (a) Forest plot. (b) Funnel plot
Quality of life
Four RCTs reported on QoT.[1,10,12,13] The monoclonal antibody group showed significantly improved QoT (SMD = 2.64, 95% CI: 0.50–4.79, P = 0.02, I2 = 97%). Two RCTs showed a significant difference in QoL between teprotumumab and placebo (SMD = 4.52, 95% CI: 3.95–5.10, P < 0.00001, I2 = 0%). Perez-Moreiras, 2018, also showed a significant difference in QoL between TCZ and placebo (SMD = 1.19, 95% CI: 0.43–1.96, P = 0.002, I2 = not applicable). Stan, 2015, showed no significant difference between RTX and placebo (SMD = 0.33, 95% CI: −0.46–1.13, P = 0.41, I2 = not applicable) [Figure 10a]. The funnel plot was symmetrical on visual inspection, and no evidence of publication bias was noted [Figure 10b].
Figure 10.
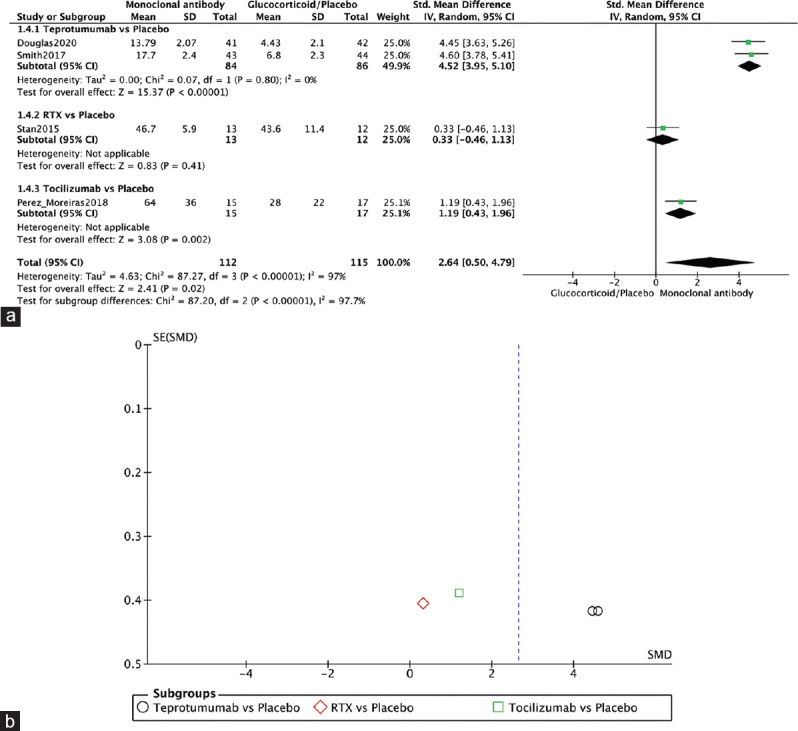
Quality of life. (a) Forest plot. (b) Funnel plot
TRAb levels
Two RCTs reported on TSH receptor antibodies (TRAbs).[4,12] In Salvi, 2015, RTX was found to be superior to IV methylprednisolone (IVMP) in reducing serum TRAb levels. In Stan 2015, however, no significant difference was found between RTX and placebo in this outcome. Overall, no significant difference was found in terms of reducing TRAb (SMD= −0.30, 95% CI: −1.19–0.59, P = 0.51, I2 = 64%) [Figure 11a]. The funnel plot was symmetrical on visual inspection, and no evidence of publication bias was noted [Figure 11b].
Figure 11.
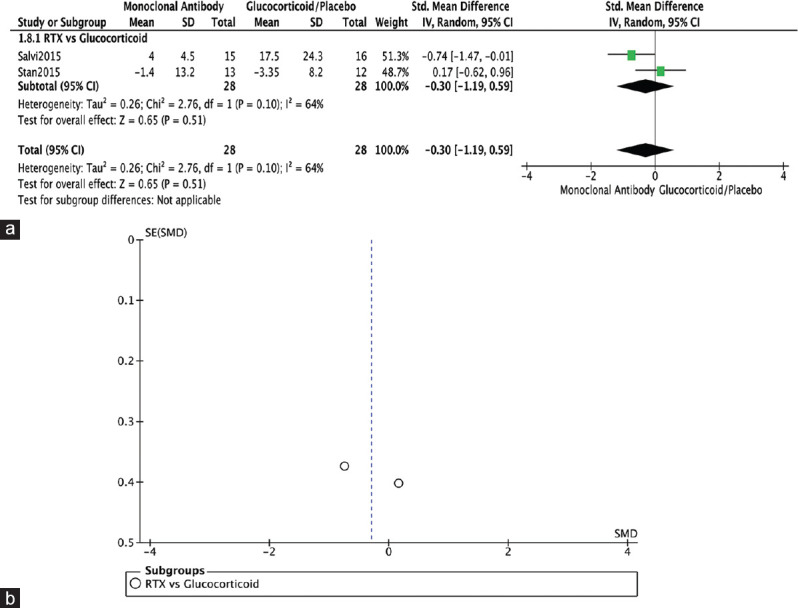
TRAb. (a) Forest plot. (b) Funnel plot. TRAb: TSH receptor antibody
DISCUSSION
Glucocorticoids have been the mainstay therapy of GO for decades. However, due to their potential hepatic and cardiovascular risks, safer and more effective treatments were sought. Among these, novel treatment options are monoclonal antibodies such as RTX, teprotumumab, and TCZ. RTX, which was first approved in 1997 for oncology patients, is an anti-CD20 monoclonal antibody. Many symptoms of GO are caused by B-cell-induced production of TSH receptor-directed immunoglobulins. RTX works by blocking the surface protein CD20 on B-lymphocytes, causing depletion of B-cells.[14,15] Teprotumumab has a different mechanism of action than RTX; it is a monoclonal antibody that blocks the insulin-like growth factor I receptor, a major contributor in GO.[1] TCZ is a monoclonal antibody targeted against IL-6 receptors which are important for the activation of B-cells and the development of antibody-producing plasma cells.[16]
This systematic review and meta-analysis of 6 RCTs representing 571 individuals with GO compared the safety and efficacy of the aforementioned monoclonal antibodies to the standard steroid therapy or placebo in treating GO. The pooled effect estimate showed a statistically significant difference between monoclonal antibodies and glucocorticoids/placebo in terms of CAS, adverse events, change in proptosis, QoL, and Gorman score for diplopia. However, no significant difference was found with respect to lid fissure, NOSPECS, and TRAb levels.
The CAS can be used to estimate disease activity based on the presence of pain, redness, and swelling. A reduction in CAS signifies higher therapeutic effects.[17] Our study demonstrated that RTX significantly improved the CAS. Similar findings were reported in a 2018 systematic review where there was a reduction from a mean baseline CAS of 5.5 to approximately 1.0.[18] Similarly, a reduction from 4.7 to 1.8 was reported in another study by Salvi et al. despite being a pilot study comparing only 20 patients.[19] Teprotumumab also showed a significant difference in reducing CAS compared to placebo. This was in accordance with a cohort study published in 2021, where a statistically significant reduction by 2.2 ± 1.4 in CAS was found.[20] For TCZ, Perez-Moreiras et al. showed similar results as the two other monoclonal antibodies.[13] Unfortunately, no other RCTs were done to compare the effect of TCZ on CAS in patients with GO. However, in an observational study published by the same author, an absolute response of CAS (meaning CAS = 0 or 1) was seen in 55% of patients, and a relative response (reduction ≥2 points) was seen in 90.9% of patients.[21] In a case report published in 2017, two patients with moderate-to-severe GO who received a maximal dose of IV glucocorticoids and underwent orbital decompression but had persistent ocular symptoms showed improved CAS after receiving TCZ therapy.[22] These findings most likely explain why these treatments were approved for GO; in other words, they reduce the disease activity.
Regarding the change in proptosis, our findings showed an overall better RTX effect on proptosis though with no significant difference to the standard of care or placebo. However, the results of the three individual RCTs were conflicting. Unlike the other two, Stan et al. showed better results when placebo was used.[12] This study, however, has a high risk of bias with regard to the deviation from the intended intervention, so results should be interpreted carefully. A 2018 systematic review of 293 patients supports our previously mentioned findings regarding the effect of RTX on proptosis.[9] Another systematic review of 167 showed a significant difference in terms of change in proptosis in favor of RTX. This difference could be explained by the nature of the studies included, as the latter systematic review included mostly observational studies.[18] Teprotumumab shows promising results, especially with regard to the change in proptosis, filling the gap left by RTX. A recently published meta-analysis of 16 studies with a total of 663 patients assessed changes in proptosis and diplopia in 3 different arm groups: teprotumumab, IVMP, and placebo. It showed a significantly greater improvement from baseline proptosis compared to IVMP.[23] Moreover, a retrospective series of nine patients with chronic TED, a mean proptosis reduction of 4.2 ± 2.8 mm in the more severely affected eyes and 2.6 ± 2.3 mm in the less affected eye, were reported. The results of these individual cases were slightly higher though still consistent with ours, which could be due to relatively small sample size.[24] To our knowledge, no RCTs evaluated proptosis using TCZ. NOSPECS classes assess the severity of the disease, including the presence of proptosis (no signs or symptoms; only signs, no symptoms; signs only; proptosis; eye muscle involvement; corneal involvement; and sight visual acuity reduction). A reduction was reported in two of the included studies. Both RCTs, Salvi et al. and Stan et al., evaluated RTX and showed similar yet nonsignificant results compared to IVMP or placebo, respectively.[4,12]
The Gorman scale is a subjective scoring system used to assess diplopia or double vision; a maximum score of 3 indicates constant diplopia, a score of 2 indicates inconstant diplopia, and a score of 1 or 0 indicates intermittent or no double vision. Improvement in diplopia using RTX was only assessed in one of the included RCTs, however, with no significant difference to glucocorticoids.[4] Very few studies evaluated the Gorman diplopia scale, perhaps due to its subjective nature. However, in a retrospective case series of 14 patients, only one of them showed improvement.[25] As for teprotumumab, both included RCTs have shown superior results in comparison to placebo. This was consistent with a 2022 meta-analysis that showed that teprotumumab was associated with more clinically relevant changes in diplopia and was more likely to have 1 grade or more reduction in diplopia compared to IVMP.[23]
Adverse events, including infusion reactions and hearing impairment, were overall lower in the groups receiving placebo than groups treated with different monoclonal antibodies. However, when IVMP was compared to RTX, conflicting results were reported depending on the adverse events measured. Li et al. found that symptoms, such as flushing and dyspepsia, were relatively low using RTX.[3] Salvi et al. found that minor adverse events (e.g. infusion reactions) occurred more with RTX, but major adverse events (e.g. increase in liver enzymes and HbA1c) occurred more with IVMP, with the exception of cytokine release syndrome (causing decreased vision).[4] The disparity in these results was also found in other systematic reviews and requires further investigation as risk/benefit ratio is yet to be established for RTX as a treatment of GO.[9] As for teprotumumab, the two included RCTs reported more adverse events in the treatment group. However, the majority of these events were considered minor (e.g. nausea, headache, dry skin, and hearing impairment); only one deemed major event (infusion reaction) occurred that was considered related to teprotumumab.[1,10] The first patient with chronic GO treated with teprotumumab reported no side effects apart from fatigue postinfusion that spontaneously resolved.[26] Otologic symptoms, such as tinnitus and ear plugging, were strongly associated with teprotumumab with most of them resolving after cessation of teprotumumab. However, for persistent or worsening sensorineural hearing loss, prior history of hearing loss may be a risk factor.[27] Perez-Moreiras et al. reported no significant adverse event in most patients treated with TCZ. However, one patient experienced a moderate increase in transaminases, and another had an acute pyelonephritis.[13] A 2021 case series reported hypercholesterolemia, neutropenia, elevated liver enzymes, and infusion reactions as possible side effects of TCZ. This study also highlights the potential benefits of administrating TCZ subcutaneously, as it may be less time and resource-consuming with comparable clinical efficacy to intravenous administration. However, further high-quality trials are needed.[28] In our study, glucocorticoids and placebo were joined in the same arm against the monoclonal antibodies for the sake of including a large sample size. However, this resulted in showing a safer margin with glucocorticoids compared to monoclonal antibodies, as it was combined with placebo, which is known to be safe, making steroids appear as safe as placebo which is inaccurate.
As for the change in lid fissure, it was only measured in two of the included studies, Li et al. and Stan et al., both comparing RTX to IVMP or placebo, respectively.[3,12] Other meta-analyses have not reported on this outcome; however. Regarding the QoL,it was evaluated in four out of the six RCTs included, using questionnaires that assess the effects of GO as patients perceived them in two aspects: visual functioning and the change in their appearance. Relatively considerable discrepancies were found for the three monoclonal antibodies, which may be due to the different QoL measurement tools used in each one. For teprotumumab, the 16-item GO-QOL questionnaire was used; for TCZ, the GO-QOL and SF-36 questionnaires were used; and for RTX, the SF-12 questionnaire, a shorter version of SF-36, was used. Only teprotumumab was found to significantly improve the QoL, as well as TCZ, though to a lesser extent. Better esthetic and functional outcomes (e.g. reduced proptosis and diplopia) and less side effects from therapy may be the main contributors to an improved QoL.
To this day, teprotumumab is the latest Food and Drug Administration (FDA)-approved drug for the treatment of GO. Douglas, 2020, which was named the OPTIC study, is one of two RCTs that played a central role in obtaining the FDA approval.[29] Recently, the OPTIC-X study, an extension of the OPTIC study, was published. It aimed to answer further questions about the safety and efficacy of additional treatment with teprotumumab. Patients from the OPTIC study who had a longer disease duration, who were initial nonresponders, and those with disease exacerbations received additional infusions of teprotumumab. Improvement in proptosis, CAS, diplopia, and QoL was almost mirroring the results of the OPTIC study, and overall results were very similar. Furthermore, no additional safety concerns were raised. Hence, the study demonstrated the effectiveness and safety of teprotumumab with extended treatment.[30]
As for the strengths of this systematic review and meta-analysis, this study is the first to compare three available monoclonal antibodies with standard glucocorticoid treatment or placebo. Also, systematic search according to prespecified strategies was performed and only RCTs were included, which in turn contributes to a higher level of evidence. Moreover, our study included a relatively large sample size when compared to other previous meta-analyses on this topic. Finally, this meta-analysis was done with respect to several primary and secondary outcomes in order to have a larger clinical picture.
We also acknowledge that this systematic review has limitations. First, only a small number of RCTs met our inclusion criteria. Second, not all of them reported on the secondary outcomes we aimed to measure, which might affect our results. Third, the risk of bias was not equal in all the included studies, especially with regard to deviation from the intended intervention, with one study having a high risk of bias. Finally, both studies evaluating teprotumumab were conducted by the same research team, thus additional studies are warranted to further validate the results.
CONCLUSION
Overall, this meta-analysis demonstrated that monoclonal antibodies were associated with more favorable clinical outcomes than standard steroid therapy or placebo, especially with regard to CAS, change in proptosis, diplopia, and QoL, with teprotumumab being superior. No significant difference was found with respect to lid fissure, NOSPECS, and TRAb levels. Moreover, only minor safety concerns were identified though fewer than traditional steroids. However, more high-quality RCTs comparing the different monoclonal antibodies (teprotumumab, RTX, and TCZ) with glucocorticoids are still warranted in order to have a better understanding of their efficacy and safety as well as to confirm the superiority of teprotumumab and, possibly, set it as the new standard of care for GO.
However, we recommend further studies to compare the different monoclonal antibodies to glucocorticoids in order to have a better understanding of their efficacy and safety.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EH, Perdok R, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382:341–52. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 2.Ugradar S, Kang J, Kossler AL, Zimmerman E, Braun J, Harrison AR, et al. Teprotumumab for the treatment of chronic thyroid eye disease. Eye (Lond) 2022;36:1553–9. doi: 10.1038/s41433-021-01593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Xiao Z, Hu X, Li Y, Zhang X, Zhang S, et al. The efficacy of rituximab combined with 131i for ophthalmic outcomes of graves'ophthalmopathy patients. Pharmacology. 2017;99:144–52. doi: 10.1159/000453618. [DOI] [PubMed] [Google Scholar]
- 4.Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves'orbitopathy: A randomized controlled study. J Clin Endocrinol Metab. 2015;100:422–31. doi: 10.1210/jc.2014-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shan SJ, Douglas RS. The pathophysiology of thyroid eye disease. J Neuroophthalmol. 2014;34:177–85. doi: 10.1097/WNO.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 6.Carballo MC, de Sa BP, Rocha DR, Arbex AK. Pathophysiology of graves'ophthalmopathy: A literature review. Open J Endocr Metab Dis. 2017;7:77–87. [Google Scholar]
- 7.Yasir M, Goyal A, Sonthalia S. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. [[Last accessed on 2022 Apr 28]]. Corticosteroid adverse effects. Available from:http://www.ncbi.nlm.nih.gov/books/NBK531462/ [PubMed] [Google Scholar]
- 8.Gaballa SA, Kompella UB, Elgarhy O, Alqahtani AM, Pierscionek B, Alany RG, et al. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv Transl Res. 2021;11:866–93. doi: 10.1007/s13346-020-00843-z. [DOI] [PubMed] [Google Scholar]
- 9.Shen WC, Lee CH, Loh EW, Hsieh AT, Chen L, Tam KW. Efficacy and safety of rituximab for the treatment of graves'orbitopathy: A meta-analysis of randomized controlled trials. Pharmacotherapy. 2018;38:503–10. doi: 10.1002/phar.2111. [DOI] [PubMed] [Google Scholar]
- 10.Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–61. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions:explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with graves'orbitopathy. J Clin Endocrinol Metab. 2015;100:432–41. doi: 10.1210/jc.2014-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, Perez-Pampin E, Romo Lopez A, Rodríguez Alvarez FM, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves orbitopathy: A randomized clinical trial. Am J Ophthalmol. 2018;195:181–90. doi: 10.1016/j.ajo.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 14.Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F, et al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol. 2006;154:511–7. doi: 10.1530/eje.1.02119. [DOI] [PubMed] [Google Scholar]
- 15.Pierpont TM, Limper CB, Richards KL. Past, present, and future of rituximab-the world's first oncology monoclonal antibody Therapy. Front Oncol. 2018;8:163. doi: 10.3389/fonc.2018.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strianese D, Rossi F. Interruption of autoimmunity for thyroid eye disease: B-cell and T-cell strategy. Eye (Lond) 2019;33:191–9. doi: 10.1038/s41433-018-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrio-Barrio J, Sabater AL, Bonet-Farriol E, Velázquez-Villoria Á, Galofré JC. Graves'ophthalmopathy: VISA versus EUGOGO classification, assessment, and management. J Ophthalmol. 2015;2015:249125. doi: 10.1155/2015/249125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Ning Q, Jin K, Xie J, Ye J. Does rituximab improve clinical outcomes of patients with thyroid-associated ophthalmopathy?A systematic review and meta-analysis. BMC Ophthalmol. 2018;18:46. doi: 10.1186/s12886-018-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvi M, Vannucchi G, Campi I, Currò N, Dazzi D, Simonetta S, et al. Treatment of graves'disease and associated ophthalmopathy with the anti-CD20 monoclonal antibody rituximab: An open study. Eur J Endocrinol. 2007;156:33–40. doi: 10.1530/eje.1.02325. [DOI] [PubMed] [Google Scholar]
- 20.Diniz SB, Cohen LM, Roelofs KA, Rootman DB. Early experience with the clinical use of teprotumumab in a heterogenous thyroid eye disease population. Ophthalmic Plast Reconstr Surg. 2021;37:583–91. doi: 10.1097/IOP.0000000000001959. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Moreiras JV, Varela-Agra M, Prada-Sánchez MC, Prada-Ramallal G. Steroid-resistant graves'orbitopathy treated with tocilizumab in real-world clinical practice: A 9-year single-center experience. J Clin Med. 2021;10:706. doi: 10.3390/jcm10040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sy A, Eliasieh K, Silkiss RZ. Clinical response to tocilizumab in severe thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2017;33:e55–7. doi: 10.1097/IOP.0000000000000730. [DOI] [PubMed] [Google Scholar]
- 23.Douglas RS, Dailey R, Subramanian PS, Barbesino G, Ugradar S, Batten R, et al. Proptosis and diplopia response with teprotumumab and placebo versus the recommended treatment regimen with intravenous methylprednisolone in moderate to severe thyroid eye disease: A meta-analysis and matching-adjusted indirect comparison. JAMA Ophthalmol. 2022;140:328–35. doi: 10.1001/jamaophthalmol.2021.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozzello DJ, Dallalzadeh LO, Liu CY. Teprotumumab for chronic thyroid eye disease. Orbit. 2022;41:539–46. doi: 10.1080/01676830.2021.1933081. [DOI] [PubMed] [Google Scholar]
- 25.Eid L, Coste-Verdier V, Longueville E, Ribeiro E, Nicolescu-Catargi B, Korobelnik JF. The effects of rituximab on graves'orbitopathy: A retrospective study of 14 patients. Eur J Ophthalmol. 2020;30:1008–13. doi: 10.1177/1120672119845224. [DOI] [PubMed] [Google Scholar]
- 26.Ozzello DJ, Kikkawa DO, Korn BS. Early experience with teprotumumab for chronic thyroid eye disease. Am J Ophthalmol Case Rep. 2020;19:100744. doi: 10.1016/j.ajoc.2020.100744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sears CM, Azad AD, Amarikwa L, Pham BH, Men CJ, Kaplan DN, et al. Hearing dysfunction after treatment with teprotumumab for thyroid eye disease. Am J Ophthalmol. 2022;240:1–13. doi: 10.1016/j.ajo.2022.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silkiss RZ, Paap MK, Roelofs KA, Agi J, Weis E. Treatment of corticosteroid-resistant thyroid eye disease with subcutaneous tocilizumab. Can J Ophthalmol. 2021;56:66–70. doi: 10.1016/j.jcjo.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Ting M, Ezra DG. Teprotumumab: A disease modifying treatment for graves'orbitopathy. Thyroid Res. 2020;13:12. doi: 10.1186/s13044-020-00086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douglas RS, Kahaly GJ, Ugradar S, Elflein H, Ponto KA, Fowler BT, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X study. Ophthalmology. 2022;129:438–49. doi: 10.1016/j.ophtha.2021.10.017. [DOI] [PubMed] [Google Scholar]