Abstract
High-resolution respirometry methods allow for the assessment of oxygen consumption by the electron transfer systems within cells, tissue samples, and isolated mitochondrial preparations. As mitochondrial integrity is compromised by the process of cryopreservation, these methods have been limited to fresh samples. Here we present a simple method to assess the activity of mitochondria respiratory complexes I and II in previously cryopreserved murine skeletal muscle tissue homogenates, as well as previously frozen D. melanogaster, as a function of oxygen consumption.
Introduction
As the field of biomedical research continues to expand throughout the 21st century, the use of frozen tissue in research laboratories will continue to expand. This increases the accessibility of clinically relevant samples to researchers and institutions where fresh samples might not be available. Sample freezing has consistently proven to be effective when making assessments of genomes, transcriptomes and proteomes of a given sample [1].
However, sample freezing is not always compatible with certain research interests, including mitochondrial respiration. Mitochondria are a fundamental organelle in eukaryotic organisms, where they are responsible for a wide range of biosynthetic and metabolic processes. This includes haem biosynthesis [2], calcium signalling [3], and apoptosis regulation [4]. However, they are most widely studied for the capacity to produce the biological energy currency of ATP, through the coupling of oxidative phosphorylation with the electron transfer system.
The electron transfer system is composed of a series of enzyme complexes in the inner mitochondrial membrane that oxidise a range of metabolites, including NADH derived from the TCA cycle [5], fatty acids via beta-oxidation [6], succinate [7], proline [8], and glycerol-3-phosphate in mammals [9]. Electrons derived from these species are funnelled into the Q-junction (co-enzyme Q), before the sequential movement through complex III, cytochrome c and finally respiratory complex IV, where they reduce molecular oxygen in a system that has been well-reviewed [10]. As a part of this process, complexes I, III, and IV pump protons across the inner mitochondrial membrane to generate the proton motive force that drives ATP synthesis via ATP synthase [11].
Classical studies by Chance and Williams in the 1950s used platinum microelectrode chemistry to assess oxygen consumption, with simultaneous spectrophotometric assays to assays ATP production via DNPH oxidation [12]. The current leading instruments for assessing mitochondrial oxygen consumption are based on either fluorometric measurement of oxygen (SeaHorse) [13, 14], or Clark electrodes within polarographic oxygen sensors (Oroboros Oxygraph-O2k) [15]. In particular the Oroboros Oxygraph-O2k allows for dynamic high-resolution respirometry assessments across tissues, cells, and mitochondrial isolates in response to titrations of substrates, uncouplers, and inhibitors of mitochondrial respiratory function (SUIT protocols) [16].
One limiting factor of existing SUIT protocols for the Oroboros Oxygraph-O2k, and respirometry assessments made with other instruments, is the inability to assess the respiratory profile or frozen tissue samples. This is due to the inactivation of the TCA cycle and the rupturing of the mitochondrial outer membrane during the process of sample freezing [17–19]. However, there are established enzyme assays that can measure the activity of mitochondrial enzyme complexes [20].
Recent studies have described methods to assess mitochondrial oxygen consumption in cryopreserved samples [21], alongside new respirometry protocols in different instruments [22]. Therefore, we sought to establish a method to measure the activity of mitochondrial respiratory complexes I and II as a function of oxygen consumption, in the Oroboros Oxygraph-O2k. NADH is directly oxidised by complex I, and succinate is commonly used in respirometry to assess complex II activity, so we tested these substrates in homogenates of previously frozen mouse tissue and Drosophila.
Methods
Mouse husbandry
Skeletal muscle sourced from 71-week-old female C57BL/6J mice was sourced from Charles River. Samples were snap frozen and stored at -80°C.
Animals were bred and housed in accordance with strict Home Office stipulated conditions. The overall programme of work (in respect to the original UK Home Office Project Licence application) is reviewed by the Animal Welfare and Ethical Review Body at the University of Nottingham and then scrutinised by the UK Home Office Inspectorate before approval by the Secretary of State. Individual study protocols link to the overarching Home Office Project Licence and are made available to the Named Animal Care and Welfare Officer, the Named Veterinary Surgeon (both are members of the AWERB), the animal care staff and the research group. The Project Licence Number for the breeding and maintenance of this genetically altered line of mice is PPL 40/3576. The mice are typically group housed and maintained within solid floor cages containing bedding and nesting material with additional environmental enrichment including chew blocks and hiding tubes. Cages are Individually Ventilated Cage Units within a barrier SPF unit to maintain biosecurity. Animals are checked daily by a competent and trained animal technician. Any animal giving cause for concern such as subdued behaviour, staring coat, loss of weight or loss of condition will be humanely killed using a Home Office approved Schedule 1 method of killing.
Drosophila husbandry
Drosophila melanogaster strain w1118 (males) were used in this study, housed in glass vials Fly food (Quick Mix Medium, Blades Biological) was added to the vial, to a depth of 1 cm, and 3 mL of distilled water was added; it was left for one minute, and then a small sprinkle of yeast was added. The Drosophila were maintained at 25°C, and the food was rehydrated with 150 uL of H2O every 24 hours. Flies were either frozen at -20 or -80. The study was reviewed and approved by the University of Nottingham SVMS local area ethics committee (#3091 200203 10 February 2020).
Skeletal muscle sample preparation
1, 5, 10, and 25 mg of skeletal muscle tissue from 71-week-old female mice was mechanically homogenised in 300 μL of MiRO5 buffer (Oroboros Instruments; 0.5 mM EGTA, 3 mM MgCl2, 60 mM lactobionic acid, 20 mM taurine, 10 mMKH2PO4, 20 mM HEPES, 110 mM D-sucrose, 1 g/L BSA, pH 7.1). The homogenate was spun down at 850 g (10 mins, 4°C) to remove the insoluble fraction. The subsequent lysate was added to 2ml volume chambers in the Oroboros O2k-FluoRespirometer. Technical replicates were used for this study (N = 5).
Drosophila sample preparation
Five flies were mechanically homogenised in 500 μL of MiR05 buffer. The homogenate was spun at 850 g (10 mins, 4°C) to clear insoluble material. The supernatant was added to the O2k-FluoRespirometer. Biological replicates were used for this study, with samples frozen at -20 (n = 3) or -80 (n = 6) used in this study.
High-resolution respirometry
A substrate, uncoupler, inhibitor, titration (SUIT) protocol was used to assess the uncoupled mitochondrial oxygen consumption capacity16. Briefly, 5 μL of cytochrome c was titrated into each chamber and a baseline value was reached, this was followed by a titration of 10 μL of NADH (10 mM) and the peak specific flux value was marked, then 20 μL of succinate (1 M), 1 μL of rotenone (1 mM), and 1 μL of antimycin A (5 mM) which is used for background correction.
Data analyses were performed in GraphPad Prism version 9.3.1.
Results
The mitochondrial respiratory capacity of frozen mouse skeletal muscle homogenate
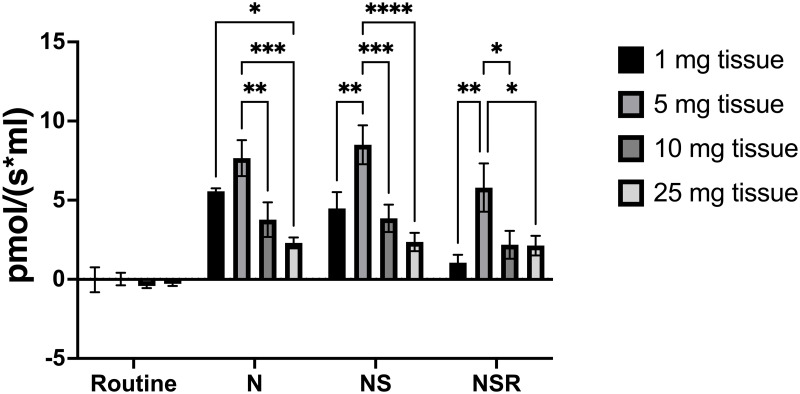
Different masses of skeletal muscle tissue were homogenised before being assessed for their mitochondrial respiratory capacity in response to NADH and succinate titrations (Figs 1 and 2). In line with particular SUIT protocols for the Oxygraph-O2k we followed a procedure in which N-linked respiration was assessed (NADH), followed by NS-linked respiration (NADH and succinate), and then S-linked respiration (NADH, succinate, rotenone). All tissues masses that we assessed (1, 5, 10, and 25 mg) showed oxygen consumption in response to substrate titration, and the 5 mg tissue sample lysate produced the strongest oxygen consumption signal (Table 1).
Fig 1. Complex I and complex II-linked mitochondrial oxygen consumption of frozen mouse skeletal muscle.
Mitochondrial respiration was stimulated in either 1, 5, 10, or 25 mg mouse skeletal muscle tissue homogenates with titrations of NADH (N) and succinate (S), before being inhibited by rotenone (R), and antimycin A for background correction. Technical replicates, N = 5, 2-way ANOVA, Turkey’s multiple comparison test, * P = 0.033, ** P = 0.002, *** P < 0.001.
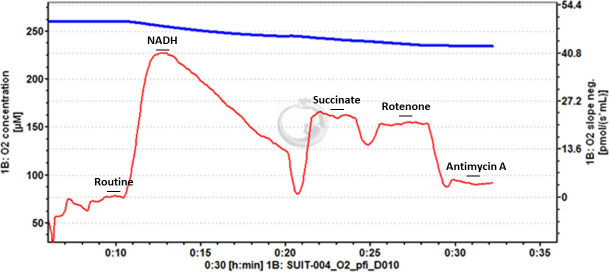
Fig 2. Example trace of frozen mouse skeletal muscle.
Stepwise titrations of NADH and succinate stimulate oxygen flux, which is inhibited by rotenone and antimycin A titrations. Titration of NADH leads to a peak of oxygen flux, before progressive decrease.
Table 1. Specific flux values of mouse skeletal muscle oxygen consumption.
Mean specific flux values (pmol/(s*mL)) in 1, 5, 10, and 25 mg lysates of skeletal mouse muscle tissue in response to sequential titrations of substrates (NADH, succinate) and inhibitors (rotenone). N = 5, (SEM).
| Specific flux of substrate and inhibitor titrations (pmol/(s*mL)) | Mouse skeletal muscle | |||
|---|---|---|---|---|
| 1 mg | 5 mg | 10 mg | 25 mg | |
| Routine | -0.029 (0.784) | 0.016 (0.396) | -0.415 (0.136) | -0.281 (0.135) |
| NADH | 5.565 (0.181) | 7.659 (1.139) | 3.769 (1.097) | 2.304 (0.345) |
| NADH, succinate | 4.475 (1.032) | 8.501 (1.228) | 3.852 (0.863) | 2.358 (0.579) |
| NADH, succinate, rotenone | 1.046 (0.508) | 5.795 (1.527) | 2.181 (0.876) | 2.13 (0.618) |
The mitochondrial respiratory capacity of frozen Drosophila (flies) after homogenisation
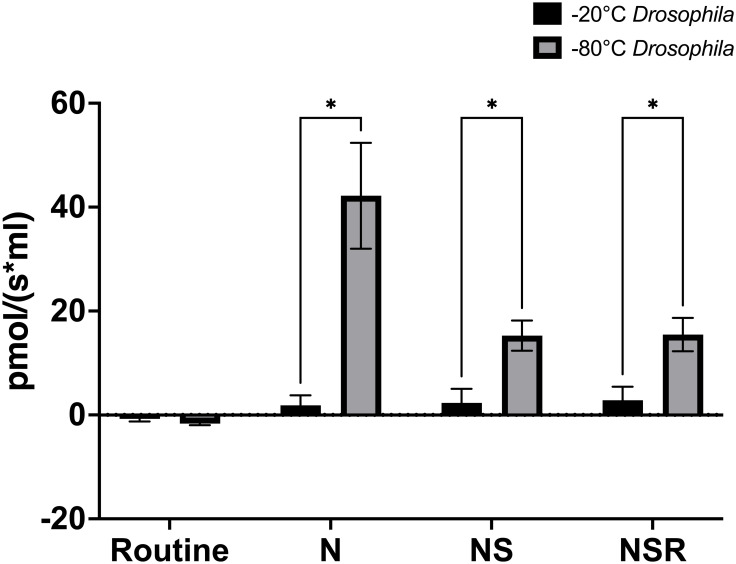
Using the same protocol, we assessed the activity of complex I and complex II as a function of oxygen consumption in D. melanogaster that had previously been frozen at either -20°C or -80°C (Figs 3 and 4). We report that oxygen consumption in D. melanogaster frozen at -20 °C is limited for titrations of both NADH and succinate (Table 2), but a strong signal is detected in D. melanogaster that had previously been frozen at -80°C.
Fig 3. Complex I and complex II-linked mitochondrial oxygen consumption of D. melanogaster frozen at -20°C and -80 °C.
The specific oxygen flux (pmol/(s*mL)) of D. melanogaster was assessed in samples that had been frozen at either -20°C (black) or -80°C (grey) in response to stepwise titrations of NADH (N), succinate (S), and rotenone (R), followed by antimycin A for background correction. D melanogaster -20°C (N = 3), D. melanogaster -80°C (N = 6). Error bars = SEM, * p < 0.05.
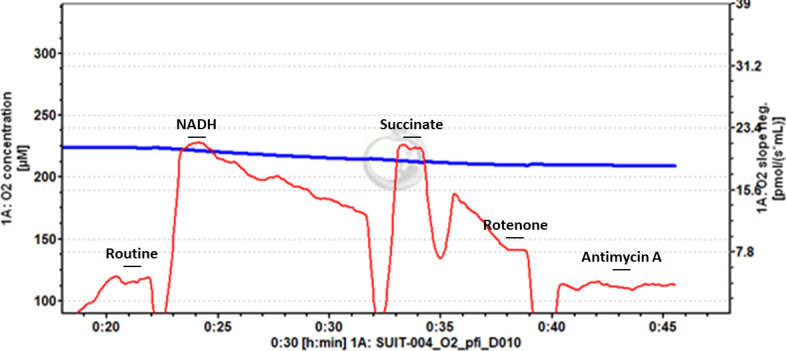
Fig 4. Example trace of -80°C frozen Drosophila.
Stepwise titration of NADH and succinate stimulate oxygen flux, that is then inhibited by titrations of rotenone and antimycin A. Titration of NADH leads to a peak of oxygen flux, before progressive decrease.
Table 2. Specific flux values of oxygen consumption from previously frozen D. melanogaster.
Mean specific flux values (pmol/(s*mL)) in homogenates of D. melanogaster previously frozen at -20°C and -80°C, in response to sequential titrations of substrates (NADH, succinate) and inhibitors (rotenone). D melanogaster -20°C (N = 3), D. melanogaster -80°C (N = 6), (SEM).
| Specific flux of substrate and inhibitor titrations (pmol/(s*mL)) | D. melanogaster | |
|---|---|---|
| -20 | -80 | |
| Routine | -0.745 (0.499) | -1.658 (0.310) |
| NADH | 1.847 (1.928) | 42.19 (10.176) |
| NADH, succinate | 2.334 (2.679) | 15.26 (2.915) |
| NADH, succinate, rotenone | 2.825 (2.608) | 15.48 (3.205) |
Discussion
We have demonstrated a simple method to assess the activity of mitochondrial electron transport complexes I and II as a function of mitochondrial oxygen consumption using the Oroboros Oxygraph-O2k. We demonstrated the feasibility of this protocol using lysates of different masses from mouse skeletal muscle, as well as comparing the values between D. melanogaster that had previously been frozen at either -20°C or -80°C.
We report that when the 2ml volume chambers are used the second smallest mass tested of mouse skeletal muscle homogenate, 5 mg, gives the strongest signals in response to the substrate and inhibitor titrations (Fig 1). The observation of a stronger signal being observed for this mass of tissue compared with 10 mg and 25 mg could be due to an excess of tissue obscuring the oxygen flux detection by the instrument and warrants additional investigation. Additionally, that 1 mg of tissue gave a value lower than the 5 mg of tissue would suggest that 5 mg is an optimum tissue mass for this method in mouse skeletal muscle, and that tissue mass optimisation is required in different tissue types and species that are investigated.
For the D. melanogaster, those that had been frozen at -80°C gave a significantly stronger signal than those frozen at -20°C (Fig 2), which is understandable due to the colder temperatures better maintaining the structural integrity of the relevant biomolecules.
While freezing of samples is known to compromise the integrity of the outer mitochondrial membrane [23, 24], thus preventing the study of coupled respiratory capacity and oxidative phosphorylation [18], this study shows that the electron transfer system is maintained in such a way that the activity of constituent enzymes can be assessed as a function of oxygen consumption by the system. This is in contrast with static assays which measure the specific activity of the single enzyme in isolation [20, 25].
We note that while in certain protocols that assess N-linked respiration followed by NS-linked respiration, our study reports a highly similar value for these respiratory states in mice and a lower NS-linked value in D. melanogaster. The titration of NADH lead to a rapid increase in oxygen flux, followed by a steady decrease, so we suggest that much of the oxygen flux reported when NS-linked respiration was measured was sustained by succinate oxidation. Titration of rotenone demonstrates the succinate-only sustained respiration.
Previous studies, including in the Oroboros Oxygraph-O2k [26], have presented methods to assess the oxygen consumption of the electron transfer system from cryopreserved samples [21, 22]. Through consideration of these studies, we have developed the methods reported in this study. We also believe that this protocol is highly useful for assessing the activities of respiratory complexes in samples that have previously been archived at -80°C, including valuable clinical samples. It may also be considered for use by research groups that are based in laboratories and institutions where access to high-quality fresh samples is a particular logistical challenge.
Further work using the principles outlined in this pilot study could be undertaken to explore the feasibility of assessing the activity of other electron transfer system enzymes, including proline dehydrogenase, the electron-transferring flavoprotein complex (cETF), and the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian systems.
Acknowledgments
We would like to thank Deniz Akbulut for his efforts in the laboratory during this time, and the interesting conversations that we had during this project.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/J014508/1), via an award to B.E. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shabihkhani M. et al. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin. Biochem. 47, 258–266 (2014). doi: 10.1016/j.clinbiochem.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming M. D. & Hamza I. Mitochondrial heme: An exit strategy at last. J. Clin. Invest. 122, 4328–4330 (2012). doi: 10.1172/JCI66607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giorgi C., Marchi S. & Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nature Reviews Molecular Cell Biology vol. 19 713–730 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Bock F. J. & Tait S. W. G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 21, 85–100 (2020). doi: 10.1038/s41580-019-0173-8 [DOI] [PubMed] [Google Scholar]
- 5.Hirst J. Mitochondrial Complex I. Annu. Rev. Biochem. 82, 551–575 (2013). doi: 10.1146/annurev-biochem-070511-103700 [DOI] [PubMed] [Google Scholar]
- 6.Watmough N. J. & Frerman F. E. The electron transfer flavoprotein: Ubiquinone oxidoreductases. Biochim. Biophys. Acta—Bioenerg. 1797, 1910–1916 (2010). doi: 10.1016/j.bbabio.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 7.Cecchini G. Function and structure of complex II of the respiratory chain. Annu. Rev. Biochem. 72, 77–109 (2003). doi: 10.1146/annurev.biochem.72.121801.161700 [DOI] [PubMed] [Google Scholar]
- 8.Pallag G. et al. Proline Oxidation Supports Mitochondrial ATP Production When Complex I Is Inhibited. Int. J. Mol. Sci. 23, 5111 (2022). doi: 10.3390/ijms23095111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mráček T., Drahota Z. & Houštěk J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim. Biophys. Acta—Bioenerg. 1827, 401–410 (2013). doi: 10.1016/j.bbabio.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 10.Gnaiger, E. Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. Bioenergetic Communications vol. 5th (2020).
- 11.Von Ballmoos C., Wiedenmann A. & Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annu. Rev. Biochem. 78, 649–672 (2009). doi: 10.1146/annurev.biochem.78.081307.104803 [DOI] [PubMed] [Google Scholar]
- 12.CHANCE B. & WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J. Biol. Chem. 217, 409–427 (1955). [PubMed] [Google Scholar]
- 13.Rogers G. W. et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One 6, (2011). doi: 10.1371/journal.pone.0021746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu X., Ma Y., Liu Y. & Wan Q. Measurement of mitochondrial respiration in adherent cells by Seahorse XF96 Cell Mito Stress Test. STAR Protoc. 2, 100245 (2021). doi: 10.1016/j.xpro.2020.100245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pesta D. & Gnaiger E. High-Resolution Respirometry: OXPHOS Protocols for Human Cells and Permeabilized Fibers from Small Biopsies of Human Muscle. in Mitochondrial Bioenergetics: Methods and Protocols (Methods in Molecular Biology, vol. 810) (eds. Palmeira C. M. & Moreno A. J.) vol. 810 25–58 (Humana Press, 2012). [DOI] [PubMed] [Google Scholar]
- 16.Doerrier C. et al. High-resolution fluorespirometry and oxphos protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. in Methods in Molecular Biology vol. 1782 31–70 (2018). doi: 10.1007/978-1-4939-7831-1_3 [DOI] [PubMed] [Google Scholar]
- 17.Araki T. Freezing injury in mitochondrial membranes. I. Susceptible components in the oxidation systems of frozen and thawed rabbit liver mitochondria. Cryobiology 14, 144–150 (1977). [DOI] [PubMed] [Google Scholar]
- 18.Thebud R. & Santarius K. A. Effects of freezing on isolated plant mitochondria. Planta 152, 242–247 (1981). doi: 10.1007/BF00385151 [DOI] [PubMed] [Google Scholar]
- 19.Štětina T., Des Marteaux L. E. & Koštál V. Insect mitochondria as targets of freezing-induced injury: Mitochondria and freeze tolerance. Proc. R. Soc. B Biol. Sci. 287, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollard A. K., Craig E. L. & Chakrabarti L. Mitochondrial complex 1 activity measured by spectrophotometry is reduced across all brain regions in ageing and more specifically in neurodegeneration. PLoS One 11, 1–13 (2016). doi: 10.1371/journal.pone.0157405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Roche M. et al. Respiratory analysis of coupled mitochondria in cryopreserved liver biopsies. Redox Biol. 17, 207–212 (2018). doi: 10.1016/j.redox.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acin-Perez R. et al. A novel approach to measure mitochondrial respiration in frozen biological samples. EMBO J. 39, 1–18 (2020). doi: 10.15252/embj.2019104073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nukala V. N., Singh I. N., Davis L. M. & Sullivan P. G. Cryopreservation of brain mitochondria: A novel methodology for functional studies. J. Neurosci. Methods 152, 48–54 (2006). doi: 10.1016/j.jneumeth.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi R. et al. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ. 14, 616–624 (2007). doi: 10.1038/sj.cdd.4402035 [DOI] [PubMed] [Google Scholar]
- 25.Rule C. S., Patrick M. & Sandkvist M. Measuring in vitro ATPase activity for enzymatic characterization. J. Vis. Exp. 2016, 3–7 (2016). doi: 10.3791/54305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuccolotto-dos-Reis F. H. et al. Acetyl-CoA-driven respiration in frozen muscle contributes to the diagnosis of mitochondrial disease. Eur. J. Clin. Invest. 51, 1–10 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.