Abstract
Background:
Elucidating the neural basis of infant positive emotionality (PE) and negative emotionality (NE) can identify biomarkers of pathophysiological risk. Our goal was to determine how functional interactions among large-scale networks supporting emotional regulation influence WM microstructural-emotional behavior relationships in 3-month-old infants. We hypothesized that microstructural-emotional behavior relationships would be differentially mediated or suppressed by underlying resting-state functional connectivity (rsFC), particularly between Default Mode Network (DMN) and Central Executive Network (CEN) structures.
Methods:
The analytic sample comprised primary caregiver-infant dyads [52 infants (42% female, mean age at scan=15.10 weeks)], with infant neuroimaging and emotional behavior assessments at 3 months. Infant WM and rsFC were assessed by diffusion-weighted imaging/tractography and resting-state magnetic resonance imaging (MRI) during natural, non-sedated sleep. The Infant Behavior Questionnaire-R provided measures of infant PE and NE.
Results:
Following significant WM-emotional behavior relationships, multimodal analyses were performed using whole-brain voxelwise mediation. Results revealed that greater cingulum bundle volume was significantly associated with lower infant PE (ß = −0.263, p = 0.031); however, a pattern of lower rsFC between CEN and DMN structures suppressed this otherwise negative relationship. Greater uncinate fasciculus volume was significantly associated with lower infant NE (ß = −0.296, p = 0.022); however, lower orbitofrontal cortex (OFC)-amygdala rsFC, suppressed this otherwise negative relationship, while greater OFC-CEN rsFC mediated this relationship.
Conclusions:
Functional interactions among neural networks have an important influence on WM microstructural-emotional behavior relationships in infancy. These relationships can elucidate neural mechanisms contributing to future behavioral and emotional problems in childhood.
Keywords: Infant brain, Infant emotionality, Diffusion-weighted Imaging, Resting state, Multimodal, Mediation
Introduction
Temperamental features/emotional behaviors (i.e., low positive emotionality(PE), high negative emotionality(NE)), are early risk factors for subsequent behavioral and emotional problems(1–5). Elucidating the neural basis of infant emotional behaviors can identify objective biomarkers of risk that reflect underlying pathophysiological processes(6–8) and provide targets for interventions(9). Employing multimodal neuroimaging provides a deeper understanding of neural mechanisms underlying emotional behavior and has greater potential to reveal biomarkers of psychopathology than reliance on any single modality(10, 11).
In early infancy, the human brain undergoes complex and coordinated development of the neural architecture (12). Neuritic growth and myelination progress rapidly within the first postnatal year(12–16), and infant white matter (WM) development is associated with future cognitive processes and behavioral problems(17–19). The cingulum bundle(CB) and uncinate fasciculus(UF) connect key intrahemispheric regions implicated in emotional regulation, particularly anterior temporal structures, and anterior and posterior cingulate and orbitofrontal cortices, respectively(20–23). The forceps minor(FM) of the corpus callosum connects prefrontal and orbitofrontal regions and is important for interhemispheric communication(24). Although these structures reach full maturity in adulthood(25), they are identifiable in neonates(26); thus, a focus of infant multimodal neuroimaging research is to examine how WM development shapes clinically-relevant emotional behaviors. Diffusion-weighted imaging(DWI) provides proxy measures of WM structure, including structural integrity and fiber collinearity(e.g., fractional anisotropy, FA) and morphology(e.g., tract volume). We demonstrated that lower UF structural integrity and/or greater UF volume at 3 months predicted greater 9-month NE, which might reflect greater fiber arborization, potentially compromising emotional regulation capacity(27).
Neural network WM and function have a reciprocal relationship, and integration between neural network structure and functional connectivity allows global and integrative brain processes(28–30). Thus, another focus of infant multimodal neuroimaging is to examine relationships among large-scale network functional connectivity and measures of early emotionality. Resting-state functional connectivity(rsFC) reflects endogenous functioning and studies in neonates have shown that higher-order networks [e.g., default mode network(DMN), central executive network(CEN)] are topologically incomplete but become highly synchronized by age one, with increasing network-level integration(31, 32) and continued maturation beyond the first postnatal year(33–35). These higher-order, large-scale networks support emotional regulation processes(36, 37). The DMN(or medial frontoparietal network(38)) supports stimuli and perception value coding and self-monitoring; anchoring structures are medial prefrontal cortex, posterior cingulate cortex(PCC), and posterior inferior parietal lobule(36, 38, 39). The “limbic network,” comprising the orbitofrontal cortex(OFC) and anterior temporal lobes, is encompassed by the DMN(38). The CEN(or lateral frontoparietal network(38)) supports executive, goal-directed functions(38); anchoring structures are lateral prefrontal cortex(including dorsolateral prefrontal cortex, dlPFC) and the anterior inferior parietal lobule(36, 38, 40, 41). DMN-CEN connectivity is important for internally-directed attention(42), where DMN deactivation, greater CEN activation, and inverse CEN-DMN functional connectivity(or anticorrelation) during executive function(43, 44) reflects an adaptive reduction in self-monitoring processes(45–48). Aberrant positive(or reduced inverse) CEN-DMN connectivity is also linked to lower emotional regulation capacity and greater psychopathology risk(49–57).
We demonstrated significant positive relationships among infant smiling and amygdala rsFC, predominantly with CEN regions; and that caregiver affect-infant emotional behavior relationships were mediated by patterns of amygdala rsFC associated with lower infant smiling: greater amygdala-DMN rsFC and lower amygdala-CEN rsFC(58). The few other infant rsFC studies have also primarily focused on amygdala rsFC-emotional behavior relationships and reported that greater amygdala rsFC is associated with greater NE(i.e., fear and/or sadness)(59, 60) and less change in infant NE over time(61); however, none focused on rsFC between large-scale networks or on infant PE.
Critically, the CB, UF and FM connect key regions within these large-scale networks. The CB and UF form connecting pathways within the DMN; the CB connects temporal-parietal-frontal areas and the UF connects the temporal pole with the inferior frontal lobe(62) and posterior orbitofrontal areas(63). The CB also carries fibers connecting CEN and DMN anchors (dlPFC and PCC)(23). The FM provides lateral and medial PFC connectivity(64) across CEN and DMN, respectively. Given the integrative nature of WM structure and functional connectivity(28–30), it is possible that neural network function mediates the relationship between WM structure and behavior. Yet, few infant studies employed multimodal neuroimaging to examine intrinsic relationships among microstructural measures and endogenous network function(65–67). One study explored structure-function associations underlying noxious stimulus-evoked responses(65); others examined effects of prenatal conditions on multimodal neural outcomes(66, 67). Furthermore, most infant neuroimaging studies were performed at birth and 12 months(68). Yet, emerging emotional regulation(69) and neural functional specialization for processing negative emotion(70) occur around 3 months suggesting that this is an important developmental window for examining neural markers of early emotional behavior.
Neurodevelopment is influenced by the pre- and postnatal environment, including caregiver affect and socioeconomic status(SES)(17, 58, 71–74). Few studies of infant WM have examined emotion-related outcomes; one study showed that infant WM moderated the maternal psychopathology-infant NE relationship(75). Neonatal and infant rsFC is also sensitive to the pre- and postnatal environment(67, 72, 73, 76–79) and can predict later emotionality, including affective symptoms(e.g. internalizing)(59, 60, 80–82).
The goal of the present study was to employ multimodal neuroimaging to examine how functional interactions between large-scale neural networks supporting emotional regulation influence WM microstructural-emotional behavior relationships in 3-month-old infants. We first examined relationships between WM microstructure and infant PE and NE, focusing on three key WM tracts interconnecting regions in the DMN and CEN: CB, UF and FM. In order to integrate structural and functional measures, we used mediation models, where endogenous rsFC within these large-scale networks mediated relationships between WM and infant PE and NE. Measures of caregiver affect and socioeconomic status(SES) are included as additional predictors in our analyses.
Based on our prior work indicating a relationship between greater UF volume and higher later NE(27), and previous research showing relationships between greater CEN-DMN rsFC and lower emotional regulation capacity(49–57), we hypothesized that greater CEN-DMN connectivity would mediate(facilitate) relationships between greater WM volume and lower PE/higher NE, while lower CEN-DMN connectivity would suppress(reduce) those relationships.
Methods and Materials
Study Design and Participants
Overview
The study sample comprised caregiver-infant dyads, with both neuroimaging of the infant and caregiver report of infant emotional behavior at 3 months. Neuroimaging was completed at Children’s Hospital of Pittsburgh(CHP) between September 2018 and March 2020 (no caregiver or infant underwent study procedures during the COVID-19 shutdown period). All procedures were approved by the University of Pittsburgh Institutional Review Board.
Infants were healthy at birth with the following exclusion criteria: premature birth(prior to 37 weeks), low birthweight(<5.5lb; caregiver report, medical records), abnormal morphometry(i.e., small occipitofrontal circumference(<32cm)), abnormal APGAR scores(<7 at 5 mins) and extended hospitalization for physical health problems, including neurological illness. Infant MRI metal exclusion criteria were pacemakers, aneurysm clips and non-removable ferromagnetic material. Caregivers were excluded if they had prenatal/concurrent illicit substance use/substance use disorder(obstetric records/self-report), respectively, <2 hours/day care of their infant or were <18 years old(unable to give informed written consent).
Participants
Participants comprised 79 caregivers and their 3-month-old infants recruited from the community, including hospital postnatal wards, pediatric practices and a university research registry. Fifty-seven infants had usable DWI data; one was subsequently excluded for unreported low birthweight, and four others had incomplete behavioral data, leaving 52 caregiver-infant dyads(caregivers: 98% biological mothers, ages 20–42, mean=31.54 years, SD=4.09; infants: 42% female, ages 10–22 weeks, mean=15.10 weeks, SD=2.93; Supplement, Table S1.)
Magnetic Resonance Imaging (MRI) Procedures
At 3 months of age, infants underwent an MRI scan on a Siemens 3 Tesla Skyra system using a 32-channel head coil during natural, non-sedated sleep using the feed-and-wrap technique(83, 84).
DWI Acquisition and Preprocessing
DWI data were acquired using a multi-shell diffusion scheme with 2mm slice thickness; two sequences were acquired, one with posterior to anterior phase encoding with 10 b0 volumes(TR=2500 ms, TE=80 ms, voxel size=2.0× 2.0×2.0 mm3, FoV=200 mm), and another with posterior to anterior phase encoding with 159 volumes with b-values of 750 (50 directions) and 2000 (100 directions) s/mm2 (TR=3000 ms, TE=97 ms, voxel size = 2.0×2.0×2.0 mm3, FoV=200 mm).
DWI data were preprocessed using fMRI Software Library(FSL)’s TOPUP and EDDY tools. Using described methods(85), FSL’s TOPUP tool corrected for susceptibility-induced distortions. FSL’s BET removed non-brain tissue(86) and EDDY then corrected for eddy current distortions and subject motion(87). Images with artifact, signal loss or distortion were excluded from further analyses(n=22/79 infants; Supplement).
DWI Tractography
Using DSI Studio software, DWI data were reconstructed using a native-space generalized q-sampling imaging(GQI) approach(88). Tractography was performed for each targeted tract (e.g., left and right CB, left and right UF and FM) using the Automated Fiber Tracking(AutoTrack) function with consistent parameters across infants(Supplement, Table S2, Fig. S1). Only the frontoparietal/“standard” cingulum(22) was included in the tractography. Mean fractional anisotropy(FA)(89) and tract volume were extracted from each tract and averaged across hemispheres for CB and UF. Extracted axial and radial diffusivity, and intracranial volume (ICV, calculated from the brain mask of the b0 image) were used for post-hoc, exploratory analyses.
RsFC Acquisition and Preprocessing
To enhance the likelihood of acquiring usable data, two, five-minute resting-state imaging acquisitions were conducted. Resting-state data were acquired using echo-planar imaging (EPI)-blood-oxygenation-level dependent(BOLD), voxel size=3 mm3 isotropic, TR=800 ms, TE=32 ms, simultaneous multi-slice(SMS) factor=4,380 total frames acquired.
Previous methods(90) were used to minimize the risk of spurious resting-state correlations due to participant motion(Supplement). Five infants with usable DWI and behavioral data did not have usable rsFC data, leaving 47 infants for multimodal DWI and rsFC analyses.
Behavioral Assessments
Infant emotional behavior and caregiver (e.g., affect and sociodemographic) questionnaires were completed at an initial in-person, at home visit. The Edinburgh Postnatal Depression Scale(EPDS) was readministered if the scan visit fell beyond two weeks following the home visit.
Infant Emotional Behavior
Infant emotional behavior was measured via caregiver report at 3 months using the Infant Behavior Questionnaire Revised(IBQ-R)(91). PE and NE composites were calculated using the raw IBQ-R data with a mean of the items from each of the relevant subscales (i.e., PE - smiling, laughter and high pleasure; NE - sadness, distress, fear and falling reactivity subscales(58, 91)).
Caregiver Affect and Sociodemographic Covariates
As caregiver affect and SES are also important for neurodevelopment(17, 58, 71–74), we included these measures as additional predictors in analyses. Caregiver depressed mood and affective instability were assessed at 3 months using the EPDS(92) and the Personality Assessment Inventory-Borderline Features Scale(PAI-BOR(93)), respectively. A summary measure of public assistance was used as a SES proxy(Supplement, Table S1). (Supplement Table S3-correlations among behavioral and imaging variables.)
Statistical Analyses
WM-Emotional Behavior Relationships
Elastic net regressions using GLMNET in R (v.3.6.0) with cross validation(94) were performed for variable selection. We included 6 variables representing WM microstructure (FA and volume from each of the 3 tracts, UF, CB, FM), infant sex and age at scan(weeks), and caregiver age, SES, and EPDS and PAI-BOR scores at the time of the infant scan. Outcome variables included PE and NE evaluated in two separate models(Supplement, Figs. S2 & S3).
General Linear Models(GLM) were performed with elastic net-selected variables using a robust estimator(all variables and outcomes were Z-scored). Bootstrapped 95% confidence intervals(CIs) were generated using 1000 samples(bias corrected accelerated) and used to assess statistical significance. Post-hoc, exploratory GLMs substituting axial(AD) or radial diffusivity(RD) for significant WM volume variables were performed, as well as analyses including ICV as an additional predictor.
Mediation Analyses
For an overview of Mediation/Suppression analyses, see the Supplement. For rsFC, we included representative seed regions within the CEN(dlPFC) and DMN(PCC) with a whole-brain target. The medial OFC, a key UF region (21), was an additional seed(Supplement). The Shi et al. neonatal parcellation atlas provided seeds(95).
We then performed whole-brain voxelwise mediation analyses(n=47), with WM measures as the independent variables, rsFC as mediators, and NE and PE as separate outcome variables, focusing on the significant WM-emotional behavior relationships. The same covariates were used as in the elastic net regressions examining WM-emotional behavior relationships, adding a variable for resting-state image quality(square root of the number of retained functional MRI frames). Coefficients were extracted for a and b arms of the indirect effect, reflecting the WM-rsFC and rsFC-emotional behavior relationships, respectively; and the total effect, c, and direct effect, c’(Supplement). As there are not established directional relationships between WM structure, rsFC and behavior, we also performed secondary reverse mediations in which WM mediated the relationships between rsFC and infant emotionality(PE/NE) using the same seed regions for rsFC as in the main analyses.
Results
WM-Emotional Behavior Relationships
Positive Emotionality
Elastic net selected: FM FA (elastic net coefficients, 2.99), infant sex (0.33), 3-month infant age (0.08), caregiver postnatal depression (−0.04), CB volume (−1.85E-04) and FM volume (1.68E-04) as predicting PE at 3 months.
Post-hoc GLM showed that CB volume was the only significant predictor of PE (ß=−0.263, p=0.031, CI: −0.526,−0.049; Fig. 1, Table 1A). [EPDS approached significance (ß=−0.206, p=0.051), although not indicated by CIs.] Post-hoc, exploratory GLM substituting CB AD or RD for CB volume into the model did not reveal significant effects of CB AD or RD on PE (Supplement, Table S4&5). When ICV was included as an additional predictor in the PE model, averaged CB volume remains significant (ß=−0.252, p=0.035, Supplement, Table S6A).
Figure 1.
Lower CEN-DMN Resting-state Functional Connectivity Suppressed the Relationship between Cingulum Bundle Volume and Positive Emotionality. The plot displays the significant, negative relationship between cingulum bundle (CB) volume and positive emotionality (ß=−0.263, p=0.031), i.e., the direct effect (c’). Schematic depictions of the mediation analyses with resting-state functional connectivity (rsFC) of the dorsolateral prefrontal cortex (dlPFC/Anterior Central Executive Network, CEN) seed (Top) and the posterior cingulate cortex (PCC/Posterior Default Mode Network, DMN) seed (Bottom) depict the negative relationships (red arrows) of the a (CB volume-rsFC) and b (rsFC-PE) arms of the mediations. Images depict suppression effects (light blue) of dlPFC/Anterior CEN connectivity with predominantly DMN regions: middle temporal gyrus (MTG), angular gyrus (AG) and precuneus (PC), and suppression effects of PCC/Posterior DMN connectivity with a CEN region, the middle frontal gyrus (MFG). Thus, lower CEN-DMN/DMN-CEN connectivity suppressed the otherwise negative relationship between CB volume and infant PE (pFWE-corrected < 0.05).
Table 1.
General Linear Model Results: White Matter-Infant Emotionality Relationships
| A. Infant Positive Emotionality | |||
|---|---|---|---|
| Beta | Std. Error | p | |
| Infant Sex | .186 | .1070 | .083 |
| Infant Age (weeks, 3m) | .230 | .1272 | .070 |
| Postnatal Depression (EPDS) | −.206 | .1056 | .051 |
| Forceps Minor FA | .142 | .1204 | .237 |
| Cingulum Bundle Volume | −.263 | .1216 | .031 |
| Forceps Minor Volume | .189 | .1197 | .114 |
| B. Infant Negative Emotionality | |||
| Affective Instability (PAI-BOR) | .331 | .1488 | .026 |
| Forceps Minor FA | .175 | .1457 | .229 |
| Uncinate Fasciculus Volume | −.296 | .1295 | .022 |
Postnatal Depression (Edinburgh Postnatal Depression Scale, EPDS); Fractional Anisotropy (FA); Affective Instability (Personality Assessment Inventory– Borderline Features Scale, PAI-BOR)
Negative Emotionality
Elastic net selected: FM FA (elastic net coefficients, 0.47), caregiver affective instability (0.02) and UF volume (−4.12E−05) as predicting NE.
Post-hoc GLM showed that both caregiver affective instability (ß=0.331, p=0.026, CI: 0.017, 0.710) and UF volume (ß=−0.296, p=0.022, CI: −0.533,−0.087; Fig. 2) were significant predictors of NE (Table 1B). Post-hoc, exploratory GLM substituting UF AD or RD for UF volume into the model did not reveal significant effects of UF AD or RD on NE (Supplement, Table S4&5). When ICV was included as an additional predictor in the NE model, averaged UF volume was no longer significant (ß=−0.180, p=0.169, Supplement, Table 6B&C).
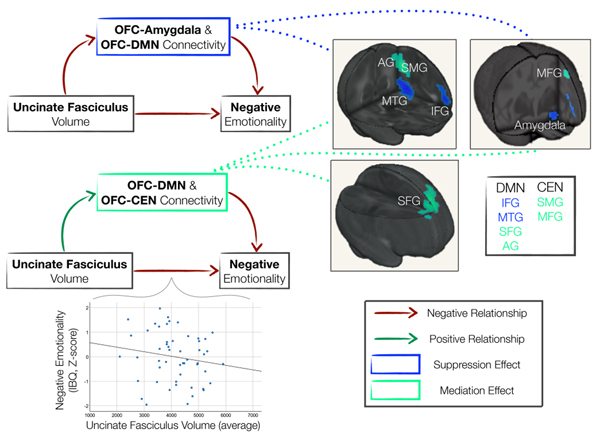
Figure 2.
Lower OFC-Amygdala and OFC-DMN Resting-state Functional Connectivity Suppressed the Relationship between Uncinate Fasciculus Volume and Negative Emotionality While Greater OFC-DMN and OFC-CEN Connectivity Mediated This Relationship. The plot displays the significant, negative relationship between uncinate fasciculus (UF) volume and negative emotionality (ß=−0.296, p=0.022), i.e., the direct effect (c’). Schematic depictions of the mediation analyses with resting-state functional connectivity (rsFC) of the orbitofrontal cortex (OFC) seed depict the negative (Top, red arrow) and positive relationships (Bottom, green arrow) of the a (UF volume-OFC rsFC) arm, and the negative relationships (red arrows) of the b (OFC rsFC-NE) arms of the mediations. Top: Images depict suppression effects (blue) of OFC connectivity with the amygdala and DMN regions, including inferior frontal gyrus (IFG) and middle temporal gyrus (MTG). Thus, lower OFC-amygdala and OFC-DMN connectivity suppressed the otherwise negative relationship between UF volume and infant NE. Bottom: Images depict mediation effects (light green) of OFC connectivity with DMN regions: superior frontal gyrus (SFG) and angular gyrus (AG), and CEN regions: supramarginal gyrus (SMG) and middle frontal gyrus (MFG). Thus, greater OFC-DMN and OFC-CEN connectivity mediated the negative relationship between UF volume and infant NE (pFWE-corrected < 0.05).
RsFC Mediates or Suppresses WM-Emotional Behavior Relationships
CB Volume, dlPFC rsFC & PE
The WM-emotional behavior relationship was a negative association between CB volume and infant PE. Mediation analyses (abbreviated results described in text, all pFWE-corrected < 0.05) revealed significant positive indirect relationships (suppression). Here, greater CB volume was associated with lower dlPFC (anterior CEN) connectivity with predominantly DMN regions: middle temporal gyrus, angular gyrus and precuneus; and lower dlPFC-DMN connectivity was associated with greater PE (Fig. 1, Fig. S4). Thus, lower CEN-DMN connectivity suppressed the otherwise negative relationship between CB volume and infant PE (Table 2A).
Table 2.
DlPFC & PCC Resting-state Connectivity Suppressed the Negative Relationship between Cingulum Bundle Volume and Infant Positive Emotionality
| A. Resting-state Connectivity Seed: Dorsolateral Prefrontal Cortex (dlPFC) – Anterior CEN | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Regions | Coordinates | k | a | b | Direct | Total | Indirect | Indirect CIs | M/S |
| Middle temporal gyrus left | −33, −36, 10 | 64 | −3.50E-04 | −7.72E-01 | −4.38E-04 | −1.68E-04 | 2.70E-04 | 0.000121, 0.001450 | S |
| Middle temporal gyrus right | 32, −40, 11 | 68 | −2.90E-04 | −7.00E-01 | −3.71E-04 | −1.68E-04 | 2.03E-04 | 0.000043, 0.000653 | S |
| Angular gyrus right | 29, −40, 22 | 78 | −2.92E-04 | −5.80E-01 | −3.38E-04 | −1.68E-04 | 1.69E-04 | 0.000036, 0.000500 | S |
| Angular gyrus left | −29, −39, 22 | 50 | −2.65E-04 | −4.52E-01 | −2.88E-04 | −1.68E-04 | 1.20E-04 | 0.000013, 0.000657 | S |
| Precuneus right | 8, −42, 26 | 68 | −3.30E-04 | −3.60E-01 | −2.87E-04 | −1.68E-04 | 1.19E-04 | 0.000017, 0.000640 | S |
| Postcentral gyrus left | −25, −20, 39 | 56 | 4.66E-04 | 2.37E-01 | −2.79E-04 | −1.68E-04 | 1.10E-04 | 0.000018, 0.000648 | S |
| Superior parietal gyrus left | −15, −39, 31 | 11 | −2.59E-04 | −4.17E-01 | −2.76E-04 | −1.68E-04 | 1.08E-04 | 0.000005, 0.000429 | S |
| Superior temporal gyrus right | 35, −30, 15 | 24 | −2.88E-04 | −3.44E-01 | −2.67E−04 | −1.68E-04 | 9.90E-05 | 0.000007, 0.000635 | S |
| Paracentral lobule left | −8, −22, 47 | 28 | 3.54E-04 | 2.67E-01 | −2.63E−04 | −1.68E-04 | 9.46E-05 | 0.000016, 0.000548 | S |
| Middle occipital gyrus left | −22, −43, 23 | 14 | −2.05E-04 | −4.40E-01 | −2.58E−04 | −1.68E-04 | 9.02E-05 | −0.000017, 0.000360 | S |
| Supramarginal gyrus right | 35, −31, 20 | 14 | −1.86E-04 | −2.87E-01 | −2.22E−04 | −1.68E-04 | 5.34E-05 | −0.000007, 0.000597 | S |
| Precuneus left | −9, −37, 29 | 41 | −1.80E-04 | −2.92E-01 | −2.21E−04 | −1.68E-04 | 5.26E-05 | −0.000012, 0.000456 | S |
| Superior occipital gyrus right | 14, −47, 25 | 20 | −2.31E-04 | −2.13E-01 | −2.17E−04 | −1.68E-04 | 4.92E-05 | −0.000007, 0.000361 | S |
| Cuneus right | 10, −48, 23 | 18 | −1.95E-04 | −1.63E-01 | −2.00E−04 | −1.68E-04 | 3.18E-05 | −0.000013, 0.000478 | S |
| Inferior parietal lobule left | −27, −29, 30 | 17 | 2.23E-04 | −1.04E-02 | −1.66E−04 | −1.68E-04 | −2.33E-06 | −0.000187, 0.000100 | M |
| B. Resting-state Connectivity Seed: Posterior Cingulate Cortex (PCC) – Posterior DMN | |||||||||
| Middle frontal gyrus left | −23, 16, 22 | 69 | −2.34E-04 | −6.02E-01 | −3.09E−04 | −1.68E-04 | 1.41E-04 | 0.000005, 0.000566 | S |
k – number of voxels within a cluster; a – independent variable-to-mediator relationship; b - mediator-to-dependent variable relationship; M/S – Mediation/Suppression.
CB Volume, PCC rsFC & PE
Mediation analyses revealed a significant positive indirect relationship (suppression). Here, greater CB volume was associated with lower PCC (posterior DMN) connectivity with a CEN region, the middle frontal gyrus; and lower PCC-middle frontal gyrus connectivity was associated with greater PE (Fig. 1, Fig. S5). Thus, lower DMN-CEN connectivity suppressed the otherwise negative relationship between CB volume and infant PE (Table 2B).
UF Volume, OFC rsFC & NE
The WM-emotional behavior relationship was a negative association between UF volume and infant NE. Mediation analyses revealed significant positive indirect relationships (suppression). Here, greater UF volume was associated with lower OFC connectivity with the amygdala (salience network) and DMN regions, including inferior frontal gyrus, middle temporal gyrus and intra-OFC (inferior) connectivity; and lower OFC-amygdala and OFC-DMN connectivity patterns were associated with greater NE (Fig. 2, Fig. S6). Thus, lower OFC-amygdala and OFC-DMN connectivity suppressed the otherwise negative relationship between UF volume and infant NE (Table 3).
Table 3.
Orbitofrontal Cortex Resting-state Connectivity Suppressed and Mediated the Negative Relationship between Uncinate Fasciculus Volume and Infant Negative Emotionality
| Resting-state Connectivity Seed: Orbitofrontal Cortex (OFC) – Limbic, DMN | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Regions | k | a | b | Direct | Total | Indirect | Indirect CIs | M/S |
| Amygdala left | −18, −5, −10 | 12 | −4.53E-04 | −3.29E-01 | −3.91E-04 | −2.42E-04 | 1.49E-04 | 0.000042, 0.000418 | S |
| Inferior frontal gyrus triangular left | −30, 18, 7 | 86 | −4.69E-04 | −2.64E-01 | −3.65E-04 | −2.42E-04 | 1.24E-04 | 0.000050, 0.000388 | S |
| Middle temporal gyrus left | −34, −28, 0 | 60 | −4.58E-04 | −2.03E-01 | −3.35E-04 | −2.42E-04 | 9.30E-05 | 0.000022, 0.000323 | S |
| Orbitofrontal cortex inferior left | −25, 21, −2 | 13 | −4.63E-04 | −8.02E-02 | −2.79E-04 | −2.42E-04 | 3.71E-05 | 0.000001, 0.000291 | S |
| Superior frontal gyrus dorsal left | −10, 20, 30 | 39 | 4.36E-04 | −1.43E-01 | −1.79E-04 | −2.42E-04 | −6.26E-05 | −0.000321, −0.000009 | M |
| Superior frontal gyrus medial right | 5, 25, 25 | 10 | 3.57E-04 | −1.55E-01 | −1.86E-04 | −2.42E-04 | −5.52E-05 | −0.000432, −0.000006 | M |
| Supramarginal gyrus left | −34, −30, 22 | 18 | 2.65E-04 | −1.80E-01 | −1.94E-04 | −2.42E-04 | −4.76E-05 | −0.000221, 0.000019 | M |
| Superior temporal gyrus left | −30, −27, 14 | 15 | 1.91E-04 | −2.01E-01 | −2.03E-04 | −2.42E-04 | −3.85E-05 | −0.000185, 0.000021 | M |
| Middle frontal gyrus left | −19, 12, 31 | 32 | 3.39E-04 | −1.00E-01 | −2.08E-04 | −2.42E-04 | −3.39E−05 | −0.000279, 0.000001 | M |
| Angular gyrus left | −33, −37, 23 | 31 | 2.40E-04 | −1.34E-01 | −2.09E-04 | −2.42E-04 | −3.22E-05 | −0.000320, 0.000001 | M |
| Inferior parietal lobule left | −35, −30, 29 | 18 | 1.95E-04 | −1.35E-01 | −2.15E-04 | −2.42E-04 | −2.64E-05 | −0.000248, 0.000006 | M |
| Superior frontal gyrus medial left | −2, 25, 26 | 26 | 8.80E-05 | −2.09E-02 | −2.40E-04 | −2.42E-04 | −1.84E-06 | −0.000405, 0.000013 | M |
k – number of voxels within a cluster; a – independent variable-to-mediator relationship; b - mediator-to-dependent variable relationship; M/S – Mediation/Suppression.
Mediation analyses also revealed significant negative indirect relationships (mediation). Here, greater UF volume was associated with greater OFC connectivity with DMN regions: superior frontal gyrus (dorsal, medial) and angular gyrus, and CEN regions: supramarginal gyrus, middle frontal gyrus, and inferior parietal lobule; and greater OFC-DMN and OFC-CEN connectivity was associated with lower NE (Fig. 2, Fig. S6). Thus, greater OFC-DMN and OFC-CEN connectivity mediated the negative relationship between UF volume and infant NE (Table 3).
Including ICV as a covariate in this model produced largely similar network connectivity patterns of suppression and mediation (Supplement, Table S8, Fig. S7).
Reverse Mediations: WM Mediates or Suppresses rsFC-Emotional Behavior Relationships
Reverse mediations focused on the significant mediations described above; these analyses used dlPFC, PCC or OFC seed to whole-brain target rsFC as the independent variable, either PE or NE as the dependent variable and either CB volume or UF volume as the mediator. These reverse-direction mediation analyses largely confirmed the nature of the tripartite relationships among WM, rsFC and PE and NE shown in our main findings(Supplement).
Discussion
Dynamic reconfiguration of structural and functional connectivity across large-scale brain networks occurs throughout development, with associations between developmental changes in rsFC and WM structural connectivity(11). The present study extends this work to infancy, demonstrating that relationships between the morphology of key WM tracts within large-scale networks important for emotional regulation and early emotional behavior are influenced by underlying rsFC. We examined the CB, UF and FM, which link structures within the CEN and DMN(62). Our primary findings were that greater CB volume was significantly associated with lower PE; however, an adaptive pattern of lower rsFC between CEN and DMN structures suppressed this relationship, and was associated with greater PE. Greater UF volume was significantly associated with lower NE; however, lower OFC-amygdala and OFC-DMN rsFC suppressed this relationship, while greater OFC-CEN rsFC mediated this relationship.
The CB is an association pathway connecting frontal, parietal and temporal areas(23). We focused on the frontoparietal segment, which connects specific CEN (dlPFC) and DMN (PCC) anchors(23). The CB is implicated in emotion, memory and attention-related processes and has the longest developmental trajectory of the major WM bundles(25). Thus, early differences in CB microstructure may have long-lasting consequences for emotional regulation. Our finding that greater CB volume was associated with lower PE in 3-month-old infants might reflect differences in WM or other microstructure, e.g., greater extracellular volume and/or greater dispersion of axons within the tract(96), that diminish emotional regulation capacity. Alternatively, greater volume may indicate inefficient synaptic pruning contributing to dysfunction(97).
The negative relationship between CB volume and PE was suppressed by lower CEN-DMN rsFC(i.e., dlPFC connectivity with DMN structures, and PCC connectivity with MFG). Thus, lower CEN-DMN connectivity was associated with greater PE in the context of greater CB volume. Deactivation in DMN regions(43, 44), alongside greater CEN activity and inverse CEN-DMN rsFC (or anticorrelation) during executive function and working memory tasks(43–45) is shown in adults(45, 46) and youth(47, 48), and is thought to reduce interference from self-monitoring processes during cognitive tasks(46, 98–100). Aberrant positive (or reduced inverse) CEN-DMN rsFC is associated with lower emotional regulation capacity and greater psychopathology risk(49–57), with emotional regulation deficits in preschool and school-age children(101), and worsening depressive symptom severity in school-aged youth at familial risk for affective disorders(102). Thus, our findings indicate that infants with greater CB volume but lower CEN-DMN rsFC might have more efficient switching capacity between these networks; these findings support a growing literature linking lower CEN-DMN rsFC with more adaptive emotional behavior, and suggest that these relationships emerge early in infancy.
The UF is also a long-range association pathway(25), connecting cortical nuclei of the amygdala and the temporal pole/anterior temporal lobe with posterior orbitofrontal areas(63). The UF connects DMN structures (temporal pole and inferior frontal lobe)(62) and the limbic network (including OFC and amygdala(41)) encompassed by the DMN(38), and supports formations of associations that motivate behavior and socio-emotional processing(21). Our findings indicate that in 3-month-old infants, larger UF volume is associated with lower NE, indicating that larger UF volume might yield greater opportunity for efficient pruning and ultimately greater connectivity, facilitating greater emotional regulation and lower NE. Our previous preliminary findings indicated that lower structural integrity (normalized quantitative anisotropy) and greater volume within the UF at 3 months were associated with greater NE prospectively at 9 months(27), however, potentially reflecting lower organization and/or greater dispersion of axons in this tract, yielding lower emotional regulation capacity. Together, these findings suggest that the association between early UF volume and infant NE changes across development.
The negative relationship between UF volume and NE was suppressed by patterns of OFC-amygdala and OFC-DMN(inferior frontal gyrus(IFG) and middle temporal gyrus) rsFC, such that lower OFC-amygdala and OFC-DMN rsFC were associated with greater NE. The OFC supports emotional processing and regulating and maintaining emotional responses(103); and the amygdala transmits salience and valence information to the anterior temporal lobe and OFC via the UF to guide cognitive processes, such as decision making(21, 104). Thus, lower OFC-amygdala rsFC might be associated with diminished coupling of valence information transmitted to the OFC, leading to emotional dysregulation and NE in the context of greater UF volume. The left IFG supports empathy, semantic processing and executive processes, such as working memory(105); and evaluation of actions and forming internal representations(105). Lower OFC-IFG rsFC in infancy might thus be associated with diminished capacity to integrate emotional and cognitive processing, contributing to greater NE. By contrast, greater OFC-DMN(superior frontal gyrus(SFG)) and OFC-CEN rsFC were associated with lower NE in the context of greater UF volume. The role of the CEN in executive function(36, 38), and that of the SFG in working memory(106) and cognitive control(43) suggest that greater connectivity between the OFC and these regions enhances cognitive processes important for the expression and regulation of NE.
We primarily focused on how WM-emotionality relationships were mediated/suppressed by rsFC. While it is well known that relationships between brain structure and function are reciprocal and important for global and integrative brain processes(28–30), the neurodevelopmental directionality of these relationships is less well understood and difficult to disentangle. Notably, our reverse-direction mediation analyses with WM volume as the mediator largely confirmed the nature of the tripartite relationships among WM, rsFC and PE and NE shown in our main findings.
Our initial GLMs indicated more robust effects of WM tract volume (i.e., CB volume-PE and UF volume-NE) than FA. Greater volume may indicate inefficient synaptic pruning(97), contributing to a greater density of collinear fibers and thus, more efficient – i.e., stronger - functional coupling (i.e., connectivity) among regions connected by the tract, or a greater degree of dendritic arborization, leading to greater fiber dispersion and more inefficient, dysfunctional – i.e., weaker - functional coupling among connected regions. By contrast, FA reflects a combination of fiber collinearity(AD) and dispersion(RD), which would mask the differential patterns of relationships between collinearity-based and dispersion-based volume and NE/PE; and neither AD- nor RD-based analyses alone would capture the complexity of these relationships(Supplement).
There were some limitations to this study; the sample size is modest, although post-hoc power analyses for the GLMs were robust, there was insufficient statistical power for testing moderating effects of sex or caregiver affect. While postnatal depression and affective instability were included as additional predictors, we did not account for effects of lifetime maternal psychiatric history on infant emotionality(107, 108). This sample was comprised of healthy, term-born infants; future studies should examine relationships among brain structure, function and infant emotionality in samples born pre-term or populations with greater levels of higher-risk prenatal exposures (e.g., poverty). Further, future studies can examine prospective relationships between neural measures and emotional behaviors, and how the development of the CB and UF predicts emotional behavior in infancy and later childhood. We also employed caregiver report of emotional behavior; future studies can examine more objective measures of infant behavior(e.g., independent observations). PE and NE were examined without accounting for other developmental processes(e.g., orienting, processing speed), thus, a potential limitation is the specificity of the observed brain-behavior associations(109, 110). Another limitation is the use of DWI-derived tract volume, which can be subject to distortions and rely on specific tractography parameters; these limitations are partially mitigated by corrections and consistent parameters, however, future replication is necessary. Further, neurite orientation dispersion and density imaging(NODDI)-derived measures are outside the scope of the current analysis, however, future work will include these to delve into differences in intracellular and extracellular compartments(111).
To our knowledge, these findings are the first to demonstrate that in 3-month-old infants, relationships between WM and emotional behavior are differentially suppressed or mediated by underlying rsFC within large-scale networks. Examining relationships among neural network WM microstructure and endogenous network function is thus important for identifying neural markers of emotional dysregulation, and can guide the development of interventions that modulate these structure-function relationships in order to reduce risk for future behavioral health problems later in childhood and adolescence.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | ||||
| Bacterial or Viral Strain | ||||
| Biological Sample | ||||
| Cell Line | ||||
| Chemical Compound or Drug | ||||
| Commercial Assay Or Kit | ||||
| Deposited Data; Public Database | ||||
| Genetic Reagent | ||||
| Organism/Strain | ||||
| Peptide, Recombinant Protein | ||||
| Recombinant DNA | ||||
| Sequence-Based Reagent | ||||
| Software; Algorithm | Mediation code available by request | |||
| Transfected Construct | ||||
| Other |
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (R01MH115466 to MLP and AEH) and The Pittsburgh Foundation (MLP). The project described was also supported by the National Institutes of Health through Grant Number UL1 TR001857.
Footnotes
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Park S-Y, Belsky J, Putnam S, Crnic K (1997): Infant emotionality, parenting, and 3-year inhibition: Exploring stability and lawful discontinuity in a male sample. Developmental psychology. 33:218. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty LR, Klein DN, Durbin CE, Hayden EP, Olino TM (2010): Temperamental positive and negative emotionality and children’s depressive symptoms: A longitudinal prospective study from age three to age ten. Journal of Social and Clinical Psychology. 29:462–488. [Google Scholar]
- 3.Oldehinkel AJ, Hartman CA, De Winter AF, Veenstra R, Ormel J (2004): Temperament profiles associated with internalizing and externalizing problems in preadolescence. Development and psychopathology. 16:421–440. [DOI] [PubMed] [Google Scholar]
- 4.De Pauw SSW, Mervielde I (2011): The role of temperament and personality in problem behaviors of children with ADHD. Journal of Abnormal Child Psychology. 39:277–291. [DOI] [PubMed] [Google Scholar]
- 5.Crockenberg SC, Leerkes EM, Jó PSB (2008): Predicting aggressive behavior in the third year from infant reactivity and regulation as moderated by maternal behavior. Development and Psychopathology. 20:37–54. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Cicchetti D (2010): Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of child psychology and psychiatry. 51:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochanska G, Tjebkes JL, Fortnan DR (1998): Children’s emerging regulation of conduct: Restraint, compliance, and internalization from infancy to the second year. Child development. 69:1378–1389. [PubMed] [Google Scholar]
- 8.Cicchetti D, Ackerman BP, Izard CE (1995): Emotions and emotion regulation in developmental psychopathology. Development and psychopathology. 7:1–10. [Google Scholar]
- 9.Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, et al. (2011): Harnessing neuroplasticity for clinical applications. Brain. 134:1591–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldermann JC, Schüller T, Kohl S, Voon V, Li N, Hollunder B, et al. (2021): Connectomic Deep Brain Stimulation for Obsessive-Compulsive Disorder. Biological Psychiatry. 90:678–688. [DOI] [PubMed] [Google Scholar]
- 11.Uddin LQ, Supekar KS, Ryali S, Menon V (2011): Dynamic Reconfiguration of Structural and Functional Connectivity Across Core Neurocognitive Brain Networks with Development. Journal of Neuroscience. 31:18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L (2014): The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience. 276:48–71. [DOI] [PubMed] [Google Scholar]
- 13.Uda S, Matsui M, Tanaka C, Uematsu A, Miura K, Kawana I, et al. (2015): Normal Development of Human Brain White Matter from Infancy to Early Adulthood: A Diffusion Tensor Imaging Study. Dev Neurosci-basel. 37:182–194. [DOI] [PubMed] [Google Scholar]
- 14.Dubois J, Dehaene-Lambertz G, Perrin M, Mangin J-F, Cointepas Y, Duchesnay E, et al. (2007): Asynchrony of the early maturation of white matter bundles in healthy infants: Quantitative landmarks revealed noninvasively by diffusion tensor imaging. Human Brain Mapping. 29:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deoni SCL, Dean DC, O’Muircheartaigh J, Dirks H, Jerskey BA (2012): Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 63:1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, et al. (2011): Mapping Infant Brain Myelination with Magnetic Resonance Imaging. Journal of Neuroscience. 31:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borchers LR, Dennis EL, King LS, Humphreys KL, Gotlib IH (2021): Prenatal and postnatal depressive symptoms, infant white matter, and toddler behavioral problems. Journal of Affective Disorders. 282:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sket GM, Overfeld J, Styner M, Gilmore JH, Entringer S, Wadhwa PD, et al. (2019): Neonatal White Matter Maturation Is Associated With Infant Language Development. Frontiers in Human Neuroscience. 13:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowe KN, Planalp EM, Dean DC, Alexander AL, Davidson RJ, Goldsmith HH (2020): Early microstructure of white matter associated with infant attention. Developmental Cognitive Neuroscience. 45:100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catani M, De Schotten MT (2008): A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 44:1105–1132. [DOI] [PubMed] [Google Scholar]
- 21.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013): Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 136:1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DK, Christiansen KF, Chapman RJ, Aggleton JP (2013): Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia. 51:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heilbronner SR, Haber SN (2014): Frontal Cortical and Subcortical Projections Provide a Basis for Segmenting the Cingulum Bundle: Implications for Neuroimaging and Psychiatric Disorders. The Journal of Neuroscience. 34:10041–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catani M, De Schotten MT (2012): Atlas of human brain connections. Oxford University Press. [Google Scholar]
- 25.Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C (2012): Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 60:340–352. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, An H, Zhu H, Jewells V, Armao D, Shen D, et al. (2011): Longitudinal regression analysis of spatial–temporal growth patterns of geometrical diffusion measures in early postnatal brain development with diffusion tensor imaging. Neuroimage. 58:993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banihashemi L, Bertocci MA, Alkhars HM, Versace A, Northrup JB, Lee VK, et al. (2020): Limbic white matter structural integrity at 3 months prospectively predicts negative emotionality in 9-month-old infants_ a preliminary study. Journal of Affective Disorders. 273:538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honey CJ, Kötter R, Breakspear M, Sporns O (2007): Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proceedings of the National Academy of Sciences. 104:10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sporns O, Chialvo DR, Kaiser M, Hilgetag CC (2004): Organization, development and function of complex brain networks. Trends in Cognitive Sciences. 8:418–425. [DOI] [PubMed] [Google Scholar]
- 30.Sporns O, Tononi G, Edelman GM (2000): Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Networks. 13:909–922. [DOI] [PubMed] [Google Scholar]
- 31.Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W (2012): The Synchronization within and Interaction between the Default and Dorsal Attention Networks in Early Infancy. Cerebral Cortex. 23:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W (2015): Development of human brain cortical network architecture during infancy. Brain Structure and Function. 220:1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao W, Lin W, Grewen K, Gilmore JH (2017): Functional Connectivity of the Infant Human Brain. The Neuroscientist. 23:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Liu J, Chen Y, Salzwedel A, Cornea E, Gilmore JH, et al. (2021): Developmental heatmaps of brain functional connectivity from newborns to 6-year-olds. Developmental Cognitive Neuroscience. 50:100976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pendl SL, Salzwedel AP, Goldman BD, Barrett LF, Lin W, Gilmore JH, et al. (2017): Emergence of a hierarchical brain during infancy reflected by stepwise functional connectivity. Human Brain Mapping. 38:2666–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menon V (2013): Developmental pathways to functional brain networks: emerging principles. Trends in Cognitive Sciences. 17:627–640. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs RH, Jenkins LM, Gabriel LB, Barba A, Ryan KA, Weisenbach SL, et al. (2014): Increased Coupling of Intrinsic Networks in Remitted Depressed Youth Predicts Rumination and Cognitive Control. PLoS ONE. 9:e104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin LQ, Yeo BTT, Spreng RN (2019): Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topogr. 32:926–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raichle ME (2015): The Brain’s Default Mode Network. Annual Review of Neuroscience. 38:433–447. [DOI] [PubMed] [Google Scholar]
- 40.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 15:483–506. [DOI] [PubMed] [Google Scholar]
- 41.Seitzman BA, Snyder AZ, Leuthardt EC, Shimony JS (2019): The State of Resting State Networks. Top Magn Reson Imag. 28:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kam JWY, Lin JJ, Solbakk A-K, Endestad T, Larsson PG, Knight RT (2019): Default network and frontoparietal control network theta connectivity supports internal attention. Nature Human Behaviour. 3:1263–1270. [DOI] [PubMed] [Google Scholar]
- 43.Raichle ME (2015): The brain’s default mode network. Annual review of neuroscience. 38:433–447. [DOI] [PubMed] [Google Scholar]
- 44.Taylor SF, Stern ER, Gehring WJ (2007): Neural systems for error monitoring: recent findings and theoretical perspectives. The Neuroscientist. 13:160–172. [DOI] [PubMed] [Google Scholar]
- 45.Piccoli T, Valente G, Linden DEJ, Re M, Esposito F, Sack AT, et al. (2015): The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PloS one. 10:e0123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu H, Hu Y, Chen X, He Y, Yang Y (2019): Regional excitation-inhibition balance predicts default-mode network deactivation via functional connectivity. Neuroimage. 185:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang AS, Klein DN, Leung H-C (2016): Load-related brain activation predicts spatial working memory performance in youth aged 9–12 and is associated with executive function at earlier ages. Developmental cognitive neuroscience. 17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, et al. (2013): Functional maturation of the executive system during adolescence. Journal of Neuroscience. 33:16249–16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alonso - Lana, Moro N, McKenna PJ, Sarró S, Romaguera A, Monté GC, et al. (2019): Longitudinal brain functional changes between mania and euthymia in bipolar disorder. Bipolar Disorders. 21:449–457. [DOI] [PubMed] [Google Scholar]
- 50.Breukelaar IA, Erlinger M, Harris A, Boyce P, Hazell P, Grieve SM, et al. (2020): Investigating the neural basis of cognitive control dysfunction in mood disorders. Bipolar disorders. 22:286–295. [DOI] [PubMed] [Google Scholar]
- 51.Gärtner M, Ghisu ME, Scheidegger M, Bönke L, Fan Y, Stippl A, et al. (2018): Aberrant working memory processing in major depression: evidence from multivoxel pattern classification. Neuropsychopharmacology. 43:1972–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodríguez-Cano E, Sarró S, Monté GC, Maristany T, Salvador R, McKenna PJ, et al. (2014): Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychological Medicine. 44:3263–3273. [DOI] [PubMed] [Google Scholar]
- 53.Rose EJ, Simonotto E, Ebmeier KP (2006): Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage. 29:203–215. [DOI] [PubMed] [Google Scholar]
- 54.Fernández-Corcuera P, Salvador R, Monté GC, Sarró SS, Goikolea JM, Amann B, et al. (2013): Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. Journal of Affective Disorders. 148:170–178. [DOI] [PubMed] [Google Scholar]
- 55.Rodríguez - Cano E, Alonso - Lana S, Sarró S, Fernández - Corcuera P, Goikolea JM, Vieta E, et al. (2017): Differential failure to deactivate the default mode network in unipolar and bipolar depression. Bipolar disorders. 19:386–395. [DOI] [PubMed] [Google Scholar]
- 56.Pomarol-Clotet E, Moro N, Sarró S, Goikolea JM, Vieta E, Amann B, et al. (2012): Failure of de-activation in the medial frontal cortex in mania: evidence for default mode network dysfunction in the disorder. The World Journal of Biological Psychiatry. 13:616–626. [DOI] [PubMed] [Google Scholar]
- 57.Bartova L, Meyer BM, Diers K, Rabl U, Scharinger C, Popovic A, et al. (2015): Reduced default mode network suppression during a working memory task in remitted major depression. Journal of Psychiatric Research. 64:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillips ML, Schmithorst VJ, Banihashemi L, Taylor M, Samolyk A, Northrup JB, et al. (2021): Patterns of Infant Amygdala Connectivity Mediate the Impact of High Caregiver Affect on Reducing Infant Smiling: Discovery and Replication. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, et al. (2016): Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Developmental Cognitive Neuroscience. 18:12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas E, Buss C, Rasmussen JM, Entringer S, Ramirez JSB, Marr M, et al. (2019): Newborn amygdala connectivity and early emerging fear. Developmental Cognitive Neuroscience. 37:100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP, et al. (2015): Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Translational Psychiatry. 5:e508–e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nozais V, Forkel SJ, Petit L, Schotten MTd, Joliot M (2022): Atlasing white matter and grey matter joint contributions to resting-state networks in the human brain. Biorxiv.2022.2001.2010.475690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liakos F, Komaitis S, Drosos E, Neromyliotis E, Skandalakis GP, Gerogiannis AI, et al. (2021): The Topography of the Frontal Terminations of the Uncinate Fasciculus Revisited Through Focused Fiber Dissections: Shedding Light on a Current Controversy and Introducing the Insular Apex as a Key Anatomoclinical Area. World Neurosurg. 152:e625–e634. [DOI] [PubMed] [Google Scholar]
- 64.Tsolaki E, Sheth SA, Pouratian N (2021): Variability of white matter anatomy in the subcallosal cingulate area. Human Brain Mapping. 42:2005–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baxter L, Moultrie F, Fitzgibbon S, Aspbury M, Mansfield R, Bastiani M, et al. (2021): Functional and diffusion MRI reveal the neurophysiological basis of neonates’ noxious-stimulus evoked brain activity. Nat Commun. 12:2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lugo-Candelas C, Cha J, Hong S, Bastidas V, Weissman M, Fifer WP, et al. (2018): Associations Between Brain Structure and Connectivity in Infants and Exposure to Selective Serotonin Reuptake Inhibitors During Pregnancy. JAMA Pediatrics. 172:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Humphreys KL, Camacho MC, Roth MC, Estes EC (2020): Prenatal stress exposure and multimodal assessment of amygdala–medial prefrontal cortex connectivity in infants. Developmental Cognitive Neuroscience. 46:100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azhari A, Truzzi A, Neoh MJ-Y, Balagtas JPM, Tan HH, Goh PP, et al. (2020): A decade of infant neuroimaging research: What have we learned and where are we going? Infant Behavior and Development. 58:101389. [DOI] [PubMed] [Google Scholar]
- 69.Feldman R, Greenbaum CW, Yirmiya N, Mayes LC (1996): Relations between cyclicity and regulation in mother-infant interaction at 3 and 9 months and cognition at 2 years. Journal of Applied Developmental Psychology. 17:347–365. [Google Scholar]
- 70.Blasi A, Mercure E, Lloyd-Fox S, Thomson A, Brammer M, Sauter D, et al. (2011): Early specialization for voice and emotion processing in the infant brain. Current biology. 21:1220–1224. [DOI] [PubMed] [Google Scholar]
- 71.Dean I, Douglas C. , Planalp EM, Wooten W Kecskemeti SR Adluru N, Schmidt CK, et al. (2018): Association of Prenatal Maternal Depression and Anxiety Symptoms With Infant White Matter Microstructure. JAMA Pediatrics. 172:973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BFP, et al. (2019): Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Nature Publishing Group.1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, et al. (2015): Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cerebral Cortex. 25:2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dégeilh F, Beauchamp MH, Leblanc É, Daneault V, Bernier A (2020): Socioeconomic Status in Infancy and the Developing Brain: Functional Connectivity of the Hippocampus and Amygdala. Dev Neurosci-basel. 41:327–340. [DOI] [PubMed] [Google Scholar]
- 75.Nolvi S, Tuulari JJ, Lavonius T, Scheinin NM, Lehtola SJ, Lavonius M, et al. (2020): Newborn White Matter Microstructure Moderates the Association between Maternal Postpartum Depressive Symptoms and Infant Negative Reactivity. Social Cognitive and Affective Neuroscience. 15:nsaa081-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turesky TK, Jensen SKG, Yu X, Kumar S, Wang Y, Sliva DD, et al. (2019): The relationship between biological and psychosocial risk factors and resting - state functional connectivity in 2 - month - old Bangladeshi infants: A feasibility and pilot study. Developmental Science. 22:e12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scheinost D, Spann MN, McDonough L, Peterson BS, Monk C (2020): Associations between different dimensions of prenatal distress, neonatal hippocampal connectivity, and infant memory. Neuropsychopharmacology.1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graham AM, Rasmussen JM, Entringer S, Ward EB, Rudolph MD, Gilmore JH, et al. (2019): Maternal Cortisol Concentrations During Pregnancy and Sex-Specific Associations With Neonatal Amygdala Connectivity and Emerging Internalizing Behaviors. BPS. 85:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dufford AJ, Salzwedel AP, Gilmore JH, Gao W, Kim P (2021): Maternal trait anxiety symptoms, frontolimbic resting - state functional connectivity, and cognitive development in infancy. Dev Psychobiol. 63:e22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, et al. (2014): Development of Thalamocortical Connectivity during Infancy and Its Cognitive Correlations. The Journal of neuroscience. 34:9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rogers CE, Sylvester CM, Mintz C, Kenley JK, Shimony JS, Barch DM, et al. (2017): Neonatal Amygdala Functional Connectivity at Rest in Healthy and Preterm Infants and Early Internalizing Symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 56:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salzwedel AP, Stephens RL, Goldman BD, Lin W, Gilmore JH, Gao W (2019): Development of Amygdala Functional Connectivity During Infancy and Its Relationship With 4-Year Behavioral Outcomes. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 4:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haney B, Reavey D, Atchison L, Poull J, Dryer L, Anderson B, et al. (2010): Magnetic resonance imaging studies without sedation in the neonatal intensive care unit: safe and efficient. The Journal of perinatal & neonatal nursing. 24:256–266. [DOI] [PubMed] [Google Scholar]
- 84.Windram J, Grosse-Wortmann L, Shariat M, Greer M-L, Crawford MW, Yoo S-J (2012): Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatric Radiology. 42:183–187. [DOI] [PubMed] [Google Scholar]
- 85.Andersson JLR, Skare S (2002): A Model-Based Method for Retrospective Correction of Geometric Distortions in Diffusion-Weighted EPI. NeuroImage. 16:177–199. [DOI] [PubMed] [Google Scholar]
- 86.Smith SM (2002): Fast robust automated brain extraction. Human Brain Mapping. 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN (2016): Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage. 141:556–572. [DOI] [PubMed] [Google Scholar]
- 88.Yeh FC, Wedeen VJ, Tseng W (2010): Generalized q-sampling imaging. IEEE transactions on medical imaging. 29:1626. [DOI] [PubMed] [Google Scholar]
- 89.Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI (2013): Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLOS ONE. 8:e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gartstein MA, Rothbart MK (2003): Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development. 26:64–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cox JL, Holden JM, Sagovsky R (1987): Edinburgh postnatal depression scale (EPDS). Br J psychiatry. 150:782–786. [DOI] [PubMed] [Google Scholar]
- 93.Morey LC, Lowmaster SE (2010): Personality assessment inventory. The Corsini Encyclopedia of Psychology.1–4. [Google Scholar]
- 94.Friedman J, Hastie T, Simon N, Tibshirani R (2016): Package glmnet: lasso and elastic-net regularized generalized linear models ver 2.0. [Google Scholar]
- 95.Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, et al. (2011): Infant Brain Atlases from Neonates to 1- and 2-Year-Olds. PLOS ONE. 6:e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johansen-Berg H, Behrens TEJ (2009): Diffusion MRI: From quantitative measurement to in-vivo neuroanatomy. First ed.: Elsevier Science. [Google Scholar]
- 97.Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY, Kim DH, et al. (2017): Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Molecular Psychiatry. 22:1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tomasi D, Ernst T, Caparelli EC, Chang L (2006): Common deactivation patterns during working memory and visual attention tasks: An intra - subject fMRI study at 4 Tesla. Human brain mapping. 27:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zuo N, Salami A, Yang Y, Yang Z, Sui J, Jiang T (2019): Activation - based association profiles differentiate network roles across cognitive loads. Human brain mapping. 40:2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fuentes-Claramonte P, Martín-Subero M, Salgado-Pineda P, Alonso-Lana S, Moreno-Alcázar A, Argila-Plaza I, et al. (2019): Shared and differential default-mode related patterns of activity in an autobiographical, a self-referential and an attentional task. Plos one. 14:e0209376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roy AK, Bennett R, Posner J, Hulvershorn L, Castellanos FX, Klein RG (2018): Altered intrinsic functional connectivity of the cingulate cortex in children with severe temper outbursts. Development and psychopathology. 30:571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fournier JC, Bertocci M, Ladouceur CD, Bonar L, Monk K, Abdul-Waalee H, et al. (2021): Neural function during emotion regulation and future depressive symptoms in youth at risk for affective disorders. Neuropsychopharmacology. 46:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Milad MR, Rauch SL (2007): The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences. 1121:546–561. [DOI] [PubMed] [Google Scholar]
- 104.Smith DM, Torregrossa MM (2021): Valence encoding in the amygdala influences motivated behavior. Behav Brain Res. 411:113370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liakakis G, Nickel J, Seitz RJ (2011): Diversity of the inferior frontal gyrus—A meta-analysis of neuroimaging studies. Behav Brain Res. 225:341–347. [DOI] [PubMed] [Google Scholar]
- 106.Courtney Susan M, Petit L, Maisog José M, Ungerleider Leslie G, Haxby James V (1998): An Area Specialized for Spatial Working Memory in Human Frontal Cortex. Science. 279:1347–1351. [DOI] [PubMed] [Google Scholar]
- 107.Durbin CE, Wilson S (2012): Convergent validity of and bias in maternal reports of child emotion. Psychological assessment. 24:647. [DOI] [PubMed] [Google Scholar]
- 108.Durbin CE, Klein DN, Hayden EP, Buckley ME, Moerk KC (2005): Temperamental emotionality in preschoolers and parental mood disorders. Journal of abnormal Psychology. 114:28. [DOI] [PubMed] [Google Scholar]
- 109.Ellis CT, Skalaban LJ, Yates TS, Turk-Browne NB (2021): Attention recruits frontal cortex in human infants. Proceedings of the National Academy of Sciences. 118:e2021474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grayson DS, Fair DA (2017): Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. NeuroImage. 160:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012): NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 61:1000–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.