Abstract
Background
Multimorbidity, the presence of more than one condition in a single individual, is a global health issue in primary care. Multimorbid patients tend to have a poor quality of life and suffer from a complicated care process. Clinical decision support systems (CDSSs) and telemedicine are the common information and communication technologies that have been used to reduce the complexity of patient management. However, each element of telemedicine and CDSSs is often examined separately and with great variability. Telemedicine has been used for simple patient education as well as more complex consultations and case management. For CDSSs, there is variability in data inputs, intended users, and outputs. Thus, there are several gaps in knowledge about how to integrate CDSSs into telemedicine and to what extent these integrated technological interventions can help improve patient outcomes for those with multimorbidity.
Objective
Our aims were to (1) broadly review system designs for CDSSs that have been integrated into each function of telemedicine for multimorbid patients in primary care, (2) summarize the effectiveness of the interventions, and (3) identify gaps in the literature.
Methods
An online search for literature was conducted up to November 2021 on PubMed, Embase, CINAHL, and Cochrane. Searching from the reference lists was done to find additional potential studies. The eligibility criterion was that the study focused on the use of CDSSs in telemedicine for patients with multimorbidity in primary care. The system design for the CDSS was extracted based on its software and hardware, source of input, input, tasks, output, and users. Each component was grouped by telemedicine functions: telemonitoring, teleconsultation, tele–case management, and tele-education.
Results
Seven experimental studies were included in this review: 3 randomized controlled trials (RCTs) and 4 non-RCTs. The interventions were designed to manage patients with diabetes mellitus, hypertension, polypharmacy, and gestational diabetes mellitus. CDSSs can be used for various telemedicine functions: telemonitoring (eg, feedback), teleconsultation (eg, guideline suggestions, advisory material provisions, and responses to simple queries), tele–case management (eg, sharing information across facilities and teams), and tele-education (eg, patient self-management). However, the structure of CDSSs, such as data input, tasks, output, and intended users or decision-makers, varied. With limited studies examining varying clinical outcomes, there was inconsistent evidence of the clinical effectiveness of the interventions.
Conclusions
Telemedicine and CDSSs have a role in supporting patients with multimorbidity. CDSSs can likely be integrated into telehealth services to improve the quality and accessibility of care. However, issues surrounding such interventions need to be further explored. These issues include expanding the spectrum of medical conditions examined; examining tasks of CDSSs, particularly for screening and diagnosis of multiple conditions; and exploring the role of the patient as the direct user of the CDSS.
Keywords: telemedicine, clinical decision support system, CDSS, primary care, multimorbidity, polypharmacy, chronic disease, pharmacy, pharmaceutic, telehealth, decision support, scoping, search strategy, review
Introduction
Multimorbidity, the presence of more than one condition in a single individual [1], is a global phenomenon that has impacted individual health and imposed national economic burdens [2-5]. People with multiple illnesses tend to have a lower quality of life, higher disability, and higher incidence of coexisting acute diseases compared to those without multiple illnesses [6,7]. It has been found that multimorbidity increases health and hospital expenditures through increased costs for medication, outpatient care, and hospitalization [2-5,8]. The raised workload on the medical staff, especially in a primary care setting, results in a lower quality of care. This results in patients with multimorbidity becoming less likely to receive patient-centered care and appropriate continuity of care [9,10].
Providing effective care for people with multimorbidity is complex, as patients and health providers face many challenges [11-15]. From the patient’s perspective, one of the barriers to reaching effective health outcomes is the complexity of the care process, which includes multiple appointments, refilling multiple prescriptions, and difficulty in understanding complex medical details. Patients are burdened by this process, particularly when all disease guidelines are followed [14]. Another barrier is the difficulty of access to care. As medical resources are scarce, the provision of health care facilities might be insufficient, leading to inadequate service centers, consultation restrictions, and limited geographic access [11-13]. From a provider’s perspective, multimorbidity contributes to more complex disease management and increased workload [15]. Patients with multiple diseases are also usually affected by polypharmacy, requiring more awareness of drug interactions and adverse events [16,17].
Information and communication technology (ICT) has been introduced to help deal with the complexities and challenges of caring for patients with multimorbidity [18]. Common ICT in health care services includes telemedicine and clinical decision support systems (CDSSs). Telemedicine, or telehealth, refers to the use of technology to improve patient outcomes by delivering health service at a distance. It comprises several basic medical care functions, including monitoring, consulting, mentoring, and providing medical knowledge [19]. Telemedicine has been claimed as an essential tool for improving health service access (increasing convenience of health care visits and removing geographic barriers) and reducing patient burdens from complex care processes (decreasing the frequency of appointments, providing necessary medical knowledge, and enhancing patient self-management) [19-23]. CDSSs are another common ICT used in health care. They have been developed in an attempt to assist health providers in making medical decisions by reducing the complexity of disease management. Computer-based programming has been used to analyze patient data from electronic health records (EHRs) and integrate the analyzed results with clinical knowledge and guidelines. The program then offers suitable choices for patients and providers through prompts, reminders, or treatment recommendations at the appropriate times [24]. These decision-support tools can be helpful for providers when managing patients with multiple conditions, as they reduce the burden and complexity of analyzing multiple problems and integrating different clinical guidelines. Assisting providers in complex decision-making allows patients to obtain better clinical outcomes and promotes both patient and provider satisfaction [25].
Both telemedicine and CDSSs could be potential tools for strengthening the capability and capacity of health care services in caring for multimorbidity in primary care settings where medical resources are limited [18]. However, each element of telemedicine and CDSSs is often examined separately as a tool for caring for multimorbidity, and there is great heterogeneity in implementation. Telemedicine has been used for simple patient education as well as more complex consultations and case management. CDSSs have variability in data inputs, intended users, and actions (ie, outputs). Thus, there are several gaps in knowledge about how to integrate CDSSs into telemedicine. Moreover, it is uncertain to what extent these integrated technological interventions can help improve outcomes for patients with multimorbidity. Therefore, the aims of this review are to (1) provide a broad review of how CDSSs have been integrated into each function of telemedicine for multimorbid patients in primary care, (2) summarize the effectiveness of telemedicine and CDSS interventions by looking at clinical and patient outcomes, and (3) identify gaps in the literature.
Methods
This scoping review used the PRISMA (Preferred Reporting Items for Scoping Reviews) checklist. The review protocol was registered on PROSPERO (CRD42021293444). Scoping reviews help identify how research is conducted in a given area, identify key characteristics related to particular concepts, and identify and analyze research gaps [26].
Search Strategy
An online literature search was conducted in September 2021 and updated in November 2021. The electronic databases included PubMed, Embase, CINAHL, and Cochrane. Only English articles published from 2015 to 2021 were included. We limited the publications to those published after 2015 due to the fact that CDSSs only started to be commonly used in health care, health science, and clinical informatics in 2015 [27]. Online databases showed a significant increase in the number of publications related to the use of CDSSs in telemedicine in 2015 and an exponential increase since the COVID-19 pandemic [28]. Additional checking of the reference lists of relevant papers was also done. The initial search terms were based on 2 concepts: clinical decision support system AND multimorbidity (eg, multiple conditions, multidrug therapy, concurrent illness, polypharmacy). The term telemedicine was considered in the articles that had both search terms. The full search terms are shown in Multimedia Appendix 1. An additional reference search was performed from articles included in the review. An extended search was then performed by adding more search terms for several specific conditions commonly examined in multimorbidity found in the initial searches. These conditions included diabetes, cardiovascular disease, hypertension, dyslipidemia, and heart failure. Additional search terms are shown in Multimedia Appendix 2. In this review, aside from having 2 or more conditions in a single individual, the word polypharmacy (ie, the act of taking ≥5 medicines) was inferred as implying multimorbidity, since prescribing 5 or more drugs for only 1 disease is rare [29].
Eligibility Criteria
The inclusion criteria were (1) the study focused on patients with multiple chronic conditions; (2) the intervention included any services using telemedicine and a CDSS; and (3) the setting was restricted to primary care.
We excluded (1) studies investigating telemedicine alone or a CDSS alone; (2) studies including patients with a single disease or multiple diseases that were the complication or consequence of each other; (3) studies without reported results, such as protocols, editorials, comments, perspectives, and correspondence; and (4) review articles.
Study Selection
Once duplicate results were removed, 4 authors (NW, SP, NB, and KP) were responsible for the screening and data extraction process. Abstract and full-paper screening was performed by at least 2 authors independently for each paper. Any disagreement on eligibility was identified and resolved by the senior author (C Angkurawaranon) if necessary.
Data Extraction
Similar to the screening process, 4 authors (NW, SP, NB, and KP) were responsible for data extraction. For each study selected, data were extracted by 2 authors working separately, and any disagreement was discussed with the senior author. The data included the author’s name, year of publication, year the study was conducted, country, study design, population characteristics, mean age of the population, sample size, details of the intervention, and outcomes of the studies in all methods of measurement (eg, mean difference, risk ratio, and odds ratio). Data from the same trial but with results published as multiple papers are summarized as 1 trial. The flow chart of the selection process is illustrated in Figure 1.
Figure 1.
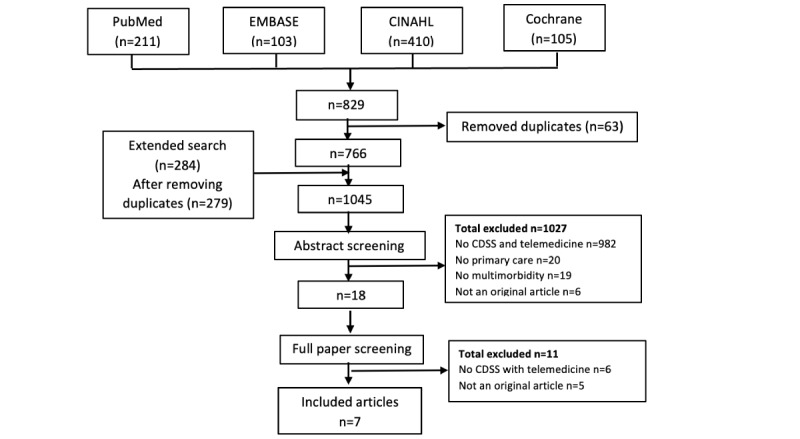
Flow chart of the selection process in different databases. CDSS: clinical decision support system.
Data Items
Telemedicine and CDSSs
We extracted data on telemedicine components and the structure of the CDSSs by adopting the definition of telemedicine components of Lee et al [19] and the structure of CDSSs from Fraccaro et al [30]. The structure of the CDSSs was extracted into 6 domains: software and hardware, source of input, input data, task, output (ie, content), and users and decision makers. The tasks of the CDSSs were categorized into 6 main purposes: disease prevention; disease diagnosis; patient management according to guidelines (or pathways); medication-related issues, such as drug interactions; patient education; and patient self-management.
For telemedicine, we used a classification modified from Lee et al [19]. We classified telemedicine into four categories: (1) tele-mentoring or teleconsultation, (2) tele–case management, (3) telemonitoring, and (4) tele-education. Tele-mentoring, which includes teleconsultation, is defined as the provision of medical guidance, disease-specific suggestions, and specific answers to patients by health providers. Tele–case management is defined as any communication between primary care providers and other specialists, case transferal, case referral, or medicine adjustment. The definitions of telemonitoring and tele-education followed the original taxonomy by Lee et al [19], defined as the process of distant transmission of patients’ health status and the use of technologies for the delivery of distance learning, respectively.
Effectiveness of Telemedicine and CDSSs
The effectiveness of the interventions was initially determined by looking at clinical outcomes or indicators of disease control, including biomarkers, such as hemoglobin A1C (HbA1c), fasting blood sugar level, blood pressure, body weight, and BMI.
Other outcomes of interest included medication management, patient self-management, and disease management. Medication management included documented adverse drug events (ADEs), medication errors, and medication discontinuation. Patient self-management included compliance with self-monitoring (such as home monitoring of blood pressure and capillary blood sugar level) and change in health behaviors. Finally, disease management was assessed by the ability of the system to provide diagnoses and further steps according to practice guidelines at the appropriate time. Other outcomes beyond this scope but related to the objectives were also included if reported.
Risk of Bias Assessment
As no observational studies were included, the Cochrane Risk of Bias Tool (version 2) was used to assess the risk of bias among experimental studies as low, high, or unclear [31]. The biases assessed had five aspects: (1) bias arising from the randomization process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in the measurement of the outcome, and (5) bias in the selection of the reported results. This step was performed by 2 authors independently, and any disagreement between them was resolved by discussion with the senior authors.
Synthesis of Results
For each study, the extracted data were qualitatively summarized. Key characteristics across studies were summarized using counts and percentages. The extracted data showed heterogeneity in study populations, designs, interventions, and outcomes. Therefore, meta-analysis of outcomes was not conducted.
Results
Search Selection
There were 829 potentially relevant publications from 4 online databases. Abstract screening for eligibility was done by 2 authors independently, and 25 full-text articles were assessed. Finally, 7 articles were included and extracted in this review. The flow chart of the selection process is illustrated in Figure 1.
Characteristics of Included Studies
All articles included in this review were experimental studies published between 2017 and 2020: 3 RCTs [32-34] and 4 non-RCTs [35-38]. They were mostly conducted in high-income countries such as the United States [32,33], England [38], Canada, Italy, and Spain [37]. Participants were patients with ≥2 chronic conditions aged from 33 years to older than 60 years. The existing chronic conditions included in this review were diabetes mellitus [32,34,35,38], hypertension [31,33,34,36,37], atrial fibrillation [37,38], and polypharmacy [33,36], with only 1 study focusing on diabetes mellitus in pregnancy [37]. The details of each study by year and chronic conditions are shown in Table 1.
Table 1.
Characteristics of included studies.
| Author (country), year of publication | Year conducted, design | Sample size (n), participant characteristics | Conditions | Age (years), mean | Intervention | Follow-up (months) | Control | Outcome |
| Fried [32] (US), 2017 | 2014-2016, RCTa | 128 community-dwelling veterans aged ≥65 years | HTb, DMc, polypharmacy | N/Ad | TRIMe app | 3 | TRIM telephone assessment plus usual care or usual care alone | Patient involvement; clinician-patient communication; correction of medication discrepancies; reduction in number of medications |
| Marcolino [35] (Brazil), 2021 | 2016-2018, quasi-experimental study | 4211 patients registered by primary care team, 25 physicians, 44 nurses, 27 other professionals | HT, DM | N/A for patients; median 33 for others | CDSSf | 6 | None | User satisfaction |
| McDonald [36] (Canada), 2019 | 2016-2017, experimental study using cohort as a control | 800 patients aged ≥65 years admitted to an IPDg and covered by health insurance and a prognosis of more than 3 months | Polypharmacy | Intervention: median 81; control 79 | MedSafer | 1 | Usual care: BPMHh with medication reconciliation at admission and discharge (no education or telemedicine) | Number of patients who had documented potentially inappropriate medications; adverse drug events within 30 days of hospital discharge |
| Peleg [37] (Italy, Spain), 2017 | 2010-2015, experimental study using cohort as a control | 266 pregnant woman, aged >35 years with AFi | Gestational diabetes mellitus with/without HT, AF | Intervention: 35.2; control: 34 | MobiGuide | 4 | Historical gestational diabetes mellitus cohort | Compliance to blood glucose, urine ketone, and blood pressure measurements; number of clinicians starting insulin therapy; compliance to electrocardiogram and blood pressure measurements; number of changes in diagnosis and treatment |
| Schiff [33] (US), 2019 | 2013-2015, RCT | 1152 patients aged >18 who were English or Spanish speakers | Polypharmacy | Intervention 57.2; control 59.7 | IVRj script and pharmacist protocol | 12 | Usual care | Adverse effect of medication; medication discontinuation with reasons |
| Willis [38] (England), 2020 | 2015-2016, RCT | 178 general practioners covering 2.2 million residents | DM, HT, AF, risky prescription | 38.4 | SystmOne | 11 | No intervention | DM control; risky prescribing; blood tests; pressure control; anticoagulation in AF |
| Prabhakaran [34] (India), 2018 | 2016-2017, RCT | 3324 patients aged ≥30 years intending to reside in the catchment area of the CHCk for ≥1 year | Uncontrolled HT or uncontrolled DM | Intervention 56.7; control 55.5 | mWellcare system | 12 | Usual care (clinical management guidelines for HT and DM) | Change in systolic blood pressure, hemoglobin A1C, free blood sugars, total cholesterol, cardiovascular disease risk, tobacco use, BMI, alcohol use, and depression score |
aRCT: randomized controlled trial.
bHT: hypertension.
cDM: diabetes mellitus.
dN/A: not available.
eTRIM: Tool to Reduce Inappropriate Medications.
fCDSS: clinical decision support system.
gIPD: inpatient department.
hBPMH: best possible medication history.
iAF: atrial fibrillation.
jIVR: interactive voice response.
kCHC: community health center.
Risk of Bias in Studies
In general, most studies were considered as having a low risk of bias for missing outcome data, measurement bias, and reporting bias. However, a high risk of bias due to a lack of randomization was found in several articles [35-37], and unclear evidence of performing an intention-to-treat analysis was found in 5 studies [33-38]. The results of the risk of bias assessment are illustrated in Table 2.
Table 2.
Risk of bias summary.
|
|
Bias arising from the randomization process | Bias due to deviations from intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported results |
| Fried et al [32] 2017 | No | No | No | No | No |
| McDonald et al [36] 2019 | Yes | Uncertain | No | No | No |
| Peleg et al [37] 2017 | Yes | Uncertain | No | No | No |
| Schiff et al [33] 2018 | No | Uncertain | No | Uncertain | No |
| Willis et al [38] 2020 | No | Uncertain | No | No | No |
| Prabhakaran et al [34] 2018 | No | No | No | No | No |
System Design of CDSSs in Telemedicine
Overview
Among the 7 included papers, telemedicine interventions were mainly used for monitoring, consulting, and case management (Multimedia Appendix 3 [32-38]). Only 1 study mentioned tele-education. This review found that the functions of telemedicine in treatment for multimorbidity often overlapped and that there often was more than 1 function in a single intervention, especially for tele–case management, which was not used alone without combination with other functions, such as telemonitoring. The tasks of CDSSs in telemedicine can be summarized into 6 categories. They were predominantly used for patient management according to guidelines, medication-related issues, disease diagnosis, patient education, and patient self-management, but not for disease prevention (Multimedia Appendix 4 [32-38]). This review found that CDSSs can complete several tasks, that is, they can manage multiple responsibilities at once, as demonstrated by their ability to provide notifications to patients and recommendations to providers at the same time. The following sections provide an overview of the system designs of CDSSs according to their main functions in telemedicine.
CDSS Designs for Use in Telemonitoring
Three papers reported interventions that used telemedicine for remote monitoring of patients with polypharmacy, atrial fibrillation, and gestational diabetes mellitus, as well as patients who were taking medicine to treat hypertension, diabetes, insomnia, and depression [32,33,37]. Sources of data input for the CDSSs varied, including manual assessment over the phone, wearable sensors, self-entering in a smartphone app, and EHRs. These sources gathered the necessary information from patients into the CDSS, which then detected ADEs, interpreted the data from sensors, and mapped patient data to clinical guidelines. The type of output depended on the task (Table 3). For example, CDSSs used for ADE detection and sensor interpretation may give feedback in the form of drug alerts and clinical issue notifications, respectively. In contrast, CDSSs used for guideline and health record interpretation may show outputs in the form of recommendations for patient management. Feedback was mostly sent to health providers, who were the main decision-makers in the patient care process. However, in one intervention, feedback was directly sent to patients [37], because they are the ones who decide their further management, such as visiting the outpatient department and modifying their behavior.
Table 3.
Overview of system designs used by clinical decision support systems for telemonitoring.
| Study (intervention), patient characteristics | Clinical decision support system | Outcomes of intervention | ||||||
|
|
Software/hardware | Source of input | Input | Task | Output (content) | Output users (ie, decision-makers) |
|
|
| Fried et al [32] (TRIMa), men aged ≥65 years with polypharmacy (diabetes mellitus, hypertension, and others) | Web application/ computer | EHRsb; patient telephone assessment | Patient cognition, social support, potential overtreatment, renal dosing, patient reports of adverse drug events | Evaluate medical appropriateness; generate algorithms for medication management | Patient-specific medication management (complete medication reconciliation, recommendations for discontinuation or dosage changes, recommendation of simplified regimen for patient with poor drug compliance) | Clinicians | Higher patient involvement rate; more active patient communication; more facilitative clinician communication; more medication-related communication; more correction of medication discrepancies; no reduction of medication number | |
| Peleg et al [37] (MobiGuide), atrial fibrillation patients | App/smart phone | Personal health records (EHRs, sensors, patient-reported symptoms, data) | Patient status, symptoms, ECGc measurement, blood pressure, international normalized ratio (taking warfarin), weight and exercise | Analyze data from ECG; map computer-interpretable guidelines to health record data | Patient recommendation, notification for patients (ECG, medications), notification for provider | Patients, clinicians | Higher compliance to ECG measurements and blood pressure measurements; more clinicians change their diagnosis and treatment | |
| Peleg et al [37] (MobiGuide), women aged 30-40 years with gestational diabetes mellitus (with or without hypertension) | App/smart phone | Personal health records (EHRs, sensors, patient-reported symptoms) | Patient status, symptoms, blood pressure, blood glucose level, ketonuria, exercise | Analyze data; map computer-interpretable guidelines to health record data | Patient recommendation, notification for patients, notification for provider | Patients, clinicians | Higher compliance to blood glucose levels, ketonuria, blood pressure; more clinicians start insulin therapy earlier; small number of patients received insulin therapy earlier | |
| Schiff et al [33], patients aged >18 years who newly received target medication with >7 doses | Interactive voice response/ telephone | EHRs, patient interviews | Patient status; number of primary care visits; target drug; history of diabetes mellitus, hypertension, depression, and insomnia; target medication adherence; drug-specific symptoms | Detect a positive symptom of adverse drug events | Adverse drug event alert | Pharmacists | More adverse drug event documentation in medical record; slightly more medication discontinuation with reasons | |
aTRIM: Tool to Reduce Inappropriate Medications.
bEHR: electronic health record.
cECG: electrocardiogram.
CDSS Designs for Use in Teleconsultation
Four studies investigated the use of telemedicine for consultation [34,35,37,38]. Health conditions examined included diabetes, hypertension, atrial fibrillation, and polypharmacy. CDSSs were used to confirm diagnoses based on patients’ information and diagnostic criteria, generate treatment plans based on the guidelines, and provide appropriate advisory materials for those with risky behaviors (Table 4). Some simple feedback created by the system was sent back to patients, including reminders for follow-up visits and medication adherence, general advice for patient self-care, and automated answers to patients’ common queries. However, for more complicated management and responses to more complex queries, health providers were offered treatment choices, and it was their task to answer patients’ questions using the information given by the CDSS.
Table 4.
Overview of system design of clinical decision support systems for teleconsultation.
| Study (intervention), patient characteristics | Clinical decision support system | Outcomes of intervention | ||||||
|
|
Software/hardware | Source of input | Input | Task | Output (content) | Output users (decision-makers shown in italics) |
|
|
| Peleg et al [37] (MobiGuide); atrial fibrillation patients | App/smart phone | Personal health records (EHRsa, sensors, patient-reported symptoms and data) | Patient status, symptoms, ECGb measurement, blood pressure, international normalized ratio (taking warfarin), weight and exercise | Analyze data from ECG; map computer-interpretable guidelines to health record data | Patient recommendations, notifications for patients (ECG, medications), notifications for providers | Patients, clinicians | Higher compliance to ECG and blood pressure measurements; more clinicians changed their diagnosis and treatment | |
| Peleg et al [37] (MobiGuide), women aged 30-40 years with gestational diabetes mellitus with or without hypertension | App/smart phone | Personal health records (EHRs, sensors, patient-reported symptoms) | Patient status, symptoms, blood pressure, blood glucose level, ketonuria, exercise | Analyze data; map computer-interpretable guidelines to health record data | Patient recommendations, notifications for patients, notifications for providers | Patients, clinicians | Higher compliance to blood glucose, urine ketone, and blood pressure; small number of patients received insulin therapy earlier | |
| Prabhakaran et al [34] (mWellcare), Patients aged ≥30 years with hypertension, diabetes mellitus, comorbid depression, and alcohol and tobacco use | Android app/ tablet computer | EHRs | Patient profile, diagnosed condition, comorbid conditions, previous and current medications | Generate treatment plan based on patient’s data and clinical guidelines | Recommended treatment plan, lifestyle modification advisory, date for the next follow-up visit, reminders for follow-up visit and medication adherence | Patients, clinicians | No improvement of biomedical parameters, no difference in tobacco or alcohol use | |
| Marcolino et al [35], patients with hypertension and diabetes mellitus | Application/computer | Data registry from health care professionals | Symptoms, medical history, physical examination, current medications, laboratory results and other complementary examinations | Screen for disease; confirm diagnosis; suggest clinical recommendations | Alerts and reminders of important clinical issues; recommendations for clinical management | Patients, health care professionals | All users were satisfied with the program | |
| Willis et al [37] (SystmOne), patients with hypertension, diabetes mellitus, atrial fibrillation, or risky prescription | Unknown/computer | EHRs, paper-based health records | Patient status, diagnosis, drug, duration of medication | Behavioral change technique delivery via audit and feedback and prompts and reminders | Audit and feedback (evidence-based clinical messages, answers to common queries, action-planning templates) and prompts and reminders (prompts for risky prescribing, prompts for adherence of quality-of-care indicators) | Patients, clinicians | The program has good performance for simple behaviors within the control of clinicians, but not for the behaviors that need patient engagement | |
aEHR: electronic health record.
bECG: electrocardiogram.
CDSS Designs for Use in Tele–Case Management
Two articles evaluated the use of CDSSs in managing patients with diabetes, hypertension, and polypharmacy [35,36]. Input data was mostly entered by health care providers from the patient registry and admission notes, including information regarding chief concerns, current illness, past medical history, current medications, laboratory results, and imaging. The outputs were notifications, clinical management recommendations, and medication management (Table 5). However, feedback was used among primary care providers and patients and could also be used between primary care providers and other specialists. For example, in the study by McDonald et al [36], patients’ prescriptions were circulated between community health providers and tertiary health facilities. Moreover, ICT systems were also designed for multiple-user registration, which increases the accessibility of systems to serve multidisciplinary teams, including physicians, nurses, nurse technicians, and support teams.
Table 5.
Overview of system design of clinical decision support systems for tele–case management and tele-education.
| Study (intervention), patient characteristics | Clinical decision support system | Outcomes of intervention | ||||||||||||||
|
|
Software/hardware | Source of input | Input | Task | Output (content) | Output users (decision-makers shown in italics) |
|
|||||||||
| Tele–case management | ||||||||||||||||
|
|
McDonald 2019 et al [36] (MedSafer), patients aged ≥65 years with polypharmacy |
|
Admission notes, EHRsa | Clinic visits, past hospitalization, past medical history, disease conditions | Identify deprescribing rules; screen for prescriptions; choose medication lists | Medication tapering instructions, patient education materials | Patients, caregivers, community pharmacists, community physicians | Increased documentation of patients with potentially inappropriate medications, slightly fewer ADEsb | ||||||||
|
|
Marcolino et al [35], patients with HT and DM | Application/computer | Data registry from health care professionals | Symptoms, medical history, physical examination, current medications, laboratory results and other complementary examinations | Screen for disease; confirm diagnosis; suggest clinical recommendation | Alerts and reminders of important clinical issues, recommendations for clinical management | Patients, health care professionals | All users were satisfied with the program | ||||||||
| Tele-education | ||||||||||||||||
|
|
McDonald et al [36] 2019 (MedSafer), patients aged ≥65 years with polypharmacy | N/Ac | Admission notes, EHRs | Clinic visits, past hospitalization, past medical history, disease conditions | Identify deprescribing rules; screen for prescriptions; choose medication lists | Medication tapering instructions, patient education materials | Patients, caregivers, community pharmacists, community physicians | Increased documentation of patients with potentially inappropriate medications, slightly fewer ADEs | ||||||||
aEHR: electronic health record.
bADE: adverse drug event.
cN/A: not available.
CDSS Designs for Use in Tele-Education
Only one study investigated the use of CDSSs for tele-education [36]. This system was used for patients with polypharmacy to interpret the patients’ medication data according to deprescribing rules and screening for high-risk prescriptions (Table 5). The system instructed patients with medication-related educational content and provided suggestions on tapering to providers. Patients or caregivers spontaneously received learning materials related to their current medications; for example, they learned details on the harms of polypharmacy and on deprescription.
Effectiveness of the Interventions and Other Outcomes
Six different types of outcomes were assessed in the 7 studies, including disease control, medication management, patient self-management, patient care, doctor-patient communication, and feasibility and satisfaction of intervention (Tables 2-5 and Multimedia Appendix 5 [32-38]). For disease control, 1 study conducted with patients with hypertension or diabetes showed that biomedical parameters were not significantly different from baseline to after completion of the intervention [34]. These parameters included blood pressure, BMI, fasting blood glucose level, HbA1c, and lipid profile. Three studies reported positive outcomes for medication management among the intervention groups, with more correction of medication discrepancies, fewer ADEs, and more appropriate medication discontinuation. However, the number of medications did not significantly decrease [32,33,36]. For patient self-management, 3 studies reported related outcomes [34,37,38]. Participants in the intervention group had higher compliance to biomarker monitoring (ie, electrocardiograms, blood glucose, blood pressure, and urine tests). Still, a significant change in risky behaviors was not seen (eg, tobacco and alcohol use). For disease management, the interventions improved the accuracy of diagnoses and shortened the time from diagnosis to initiation of treatment [37]. Clinicians who use CDSSs as smartphone apps were more likely to change their diagnoses and treatments to more appropriate ones; for example, they were more likely to start early insulin therapy for patients with diabetes.
Other associated outcomes assessed were communication between providers and patients and the program’s feasibility. For doctor-patient communication, the interventions enhanced patient participation, as can be seen by a higher patient involvement rate, more active patient communication, more facilitative clinician communication, and more medication-related communication [32]. Users from multidisciplinary teams that included physicians, nurses, and other health professionals demonstrated satisfaction with the intervention [35], but no satisfaction assessment was performed among patients.
Discussion
Principal Findings
This scoping review aims to explore how CDSSs can be integrated into telemedicine services for caring for people with multimorbidity. Our review found that CDSSs have been incorporated for telemonitoring, teleconsultation, tele–case management, and tele-education. However, the structure of CDSSs, such as data input, tasks, output, and intended users or decision-makers, varied. With limited studies, mostly from developed countries, and inconclusive evidence on the clinical effectiveness of the interventions, more research is needed to examine how CDSSs can be successfully integrated to improve patient outcomes in multimorbidity.
The evidence suggested that CDSSs can be integrated into telemedicine and used to improve the capacity of telehealth services. Telemonitoring is commonly used for multimorbidity, as it allows patients to update their health-related information (eg, blood tests and health behaviors) via online platforms and monitor patient health status (eg, blood pressure and electrocradiograms) in real-time by connecting with remote sensors or wearable devices. CDSSs play an important role in detecting abnormalities and giving alerts or feedback to patients and providers at an appropriate time. For multimorbidity, integration of CDSSs with telemonitoring could be helpful for patients who need close monitoring of their health status but do not have an indication for hospital admission, as they can be monitored at home and can keep a sense of contact with their providers [39]; this includes patients with cardiovascular disease [40], allergic diseases [41], and COVID-19 [42].
For consultation and case management, integrating CDSSs with strategies to shift tasks from more specialized to less specialized health providers could reduce the burden of management for multimorbidity and increase the impact of health care [43]. CDSSs can reduce the workload of medical staff by automating responses to common patient queries and increasing the potential of nonprofessional providers to manage patient problems. This is relevant for managing multimorbidity, as evidence-based and real-time data and algorithms can improve decision-making for community health workers and primary care providers, helping them to make more accurate decisions across the multitude of situations they encounter [44]. Online platforms allow them to consult with specialists when the situation needs expert opinion and management. However, when incorporating CDSSs into primary care and the community, it is important to develop a feasible and acceptable intervention for frontline health workers. Digital tool training and internet connectivity in the areas where the CDSS will be used must also be provided [45].
The review identified several gaps in evidence when examining the incorporation of CDSSs into telemedicine to manage multimorbidity. First, there was a small number of clinical trials and a lack of diversity in chronic conditions. This review summarized the interventions applied for people with hypertension, diabetes, polypharmacy, and atrial fibrillation, but these 4 conditions are likely inadequate to represent all major chronic diseases commonly found in patients with multimorbidity. A study conducted in Cambodia, Myanmar, Thailand, and Vietnam revealed that the 3 most common chronic conditions with comorbidities were hypertension (37.4%), depression (34.4%), and digestive diseases (32%) [46]. Other evidence shows that depression, diabetes, cancer, and cardiovascular disease are the most common starting conditions before the accumulation over time of comorbidities and conditions, such as osteoarthritis and dementia [47-49]. It should be kept in mind that mental illness and cancer, diseases often associated with multimorbidity that account for a large proportion of coexisting conditions [50-55], were not found in this review.
Second, there was limited evidence for using CDSSs for screening, whether for early prevention or early diagnosis. This might be due to the inclusion criteria of the studies, which allowed only patients who were already diagnosed with multiple diseases to enroll. However, the previous evidence shows that CDSSs could potentially be used to help identify health issues in people who are not currently experiencing symptoms (ie, early detection and screening), such as dementia [56], cardiovascular disease [57], breast cancer [58,59], and pancreatic cancer [60]. Studies investigating the role of CDSSs in detecting multiple chronic diseases are still in the area of active research and development.
Third, the role of CDSSs for patients with multimorbidity remained relatively underexamined. Most of the feedback (eg, prompts and alerts) was sent to providers rather than to patients. Patients are informed less, and providers are the main decision-makers who decide which further steps of treatment should be taken. Low patient engagement makes patient-centered services difficult and could lead to negative consequences, such as poorer health outcomes, decreased adherence to the treatment, and lack of trust in health care providers [61]. Therefore, the ICT system should provide more opportunities for patients to be involved, such as by regularly giving them feedback, informing them about their health status, and delegating some decision-making responsibility to them.
There is a lack of evidence on how telemedicine and CDSS interventions can be used in resource-limited areas. Articles in this review were mostly conducted in high-income countries. While it has been claimed that telemedicine can eliminate geographic barriers and improve access to care, several challenges have been identified as limiting patient access to interventions, including technology access, digital literacy, financial support, regulations on telemedicine use, socioeconomic status, and sociopolitical situations [62,63].
Moreover, the effectiveness of interventions in this review was examined mainly by measuring disease-specific biomarkers, patient behavioral change, and patient care processes in health care facilities. However, as previously mentioned, one major limitation in drawing inferences on effectiveness was the limited number of conditions being assessed. Furthermore, other measures of effectiveness, important for multimorbidity, have not been explored, such as patient-reported outcomes, care coordination, and cost-effectiveness. For multimorbidity, patient-reported outcome measures are an essential tool to assess patients’ perspectives on their health status and the impact of interventions. They provide information for achieving health system goals and evaluating patients’ quality of care [64]. Measuring care coordination, which includes establishing responsibility, communication, goal setting, care plan development, and monitoring, can potentially be useful [65]. And cost-effectiveness studies are likely needed to help analyze the price of inputs for one unit gained of health outcomes of the intervention. This analytic tool compares the cost of the intervention with the cost of alternatives to achieve the same goal [66,67]. This is highly relevant for ICT interventions where infrastructure and workforce to support delivery can still be limited in developing countries [68].
It should also be noted that the literature suggests that there are multiple barriers to adopting CDSSs and telemedicine [69]. Software development requires huge budgets and a large number of work hours; thus, the intervention’s cost and benefits need to be carefully estimated before investment, but as reflected in the review, data are limited for people with multimorbidity. Another barrier to implementation is acceptance by health care providers. On the one hand, CDSSs and telemedicine provide useful information to promote health, assist treatment, and improve patient care. On the other hand, it has been claimed by physicians that it causes significant delays in their daily routine. This finding was related to evidence that health care professionals will accept the intervention after considering how it affects patient disease management and workflows [70]. Some experts felt that this technology could not replace their skills and experience in disease diagnosis and management and that the ability of CDSSs to work across different care settings was still questionable [69]. Hence, making patients and health care workers satisfied with the technology calls for system designs that are both feasible and usable. A process evaluation of how to reduce time used for CDSSs and telemedicine in routine daily care workflows and how to make providers adopt the program is likely needed.
Strengths and Limitations
The novelty of this review is the attempt to summarize how CDSSs have been integrated into telemedicine services for patients with multimorbidity in primary care. However, there are some limitations. Telemedicine, CDSSs, and multimorbidity are broad concepts. Despite a comprehensive search strategy, some relevant literature may have been missed. A past review exploring the role of CDSSs and telemedicine in critical care [71] included only 2 studies, while a review examining the role of CDSSs and telemedicine in prehospital emergency care [72] included 7 studies. Our review was limited to 4 databases (PubMed, Embase, CINAHL, and Cochrane) and to literature published in English. Thus, non-English articles and articles that were published in other databases may have been missed. All the included studies were conducted in high-income countries with variations in design, sample size, study protocol, and outcomes. This makes it difficult to compare effectiveness across studies and make a clear summary or conclusion. Moreover, digital health is new in the medical research field, and the number of studies is still limited; ongoing research might have been missed.
Conclusion
There is a role for telemedicine and CDSSs in supporting patients with multimorbidity. CDSSs can likely be integrated into telehealth services to improve the quality and accessibility of care, resulting in a reduction in the burden of disease. However, various aspects of such interventions need to be explored, such as ways to expand the tasks included in CDSSs and the role of patient involvement, and interventions for a greater variety of conditions should be included. Additional assessments of the effectiveness of the intervention are required, including patient-reported outcomes, care coordination, and cost-effectiveness. Moreover, as the interventions have heterogeneous purposes and situational contexts, the feasibility and usability of the systems from the points of view of both health care providers and patients should be investigated in order to develop an ICT system that meets the needs of the local context.
Acknowledgments
The research work of C Angkurawaranon was partially supported by Chiang Mai University. This funder had no role in the design, execution, analyses, interpretation, or decision to publish.
Abbreviations
- ADE
adverse drug event
- CDSS
clinical decision support system
- EHR
electronic health record
- HbA1c
hemoglobin A1c
- ICT
information and communication technology
- PRISMA
Preferred Reporting Items for Scoping Reviews
- RCT
randomized control trial
Search terms in different databases.
Search terms for extended search.
The functions of telemedicine.
Types of clinical decision support system task in telemedicine.
Summary of types of intervention outcome.
Footnotes
Authors' Contributions: NW contributed to project administration; NW, PACM, SK, and C Angkurawaranon contributed to conceptualization; NW, PACM, SK, and C Angkurawaranon contributed to methodology; NW, C Aramrat, SP, NB, KP, and NN contributed to study selection and data extraction; NW contributed to formal analysis; NW contributed to visualization; NW and C Angkurawaranon contributed to writing of the original draft; C Aramrat, SP, NB, KP, NN, PACM, and SK contributed to review and editing of the manuscript; and PACM, SK, and C Angkurawaranon contributed to supervision. All authors have approved the manuscript.
Conflicts of Interest: None declared.
References
- 1.Johnston M, Crilly M, Black C, Prescott G, Mercer S. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health. 2019 Feb 01;29(1):182–189. doi: 10.1093/eurpub/cky098.5033670 [DOI] [PubMed] [Google Scholar]
- 2.Multimorbidity: a priority for global health research. The Academy of Medical Sciences. [2023-06-09]. https://acmedsci.ac.uk/file-download/82222577 .
- 3.Soley-Bori M, Ashworth M, Bisquera A, Dodhia H, Lynch R, Wang Y, Fox-Rushby J. Impact of multimorbidity on healthcare costs and utilisation: a systematic review of the UK literature. Br J Gen Pract. 2021 Jan;71(702):e39–e46. doi: 10.3399/bjgp20X713897. https://bjgp.org/lookup/pmidlookup?view=long&pmid=33257463 .bjgp20X713897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Zhang L, Zhao S, Zhang L. Impact of multimorbidity on health service use and catastrophic health expenditure in China: an analysis of data from a nationwide longitudinal survey. Lancet. 2019;394:S69. doi: 10.1016/S0140-6736(19)32405-5. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)32405-5/fulltext#articleInformation . [DOI] [Google Scholar]
- 5.Tran PB, Kazibwe J, Nikolaidis GF, Linnosmaa I, Rijken M, van Olmen J. Costs of multimorbidity: a systematic review and meta-analyses. BMC Med. 2022 Jul 19;20(1):234. doi: 10.1186/s12916-022-02427-9. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-022-02427-9 .10.1186/s12916-022-02427-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garin N, Olaya B, Moneta MV, Miret M, Lobo A, Ayuso-Mateos JL, Haro JM. Impact of multimorbidity on disability and quality of life in the Spanish older population. PLoS One. 2014 Nov 6;9(11):e111498. doi: 10.1371/journal.pone.0111498. https://dx.plos.org/10.1371/journal.pone.0111498 .PONE-D-14-18679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foguet-Boreu Q, Violan C, Roso-Llorach A, Rodriguez-Blanco T, Pons-Vigués Mariona, Muñoz-Pérez Miguel A, Pujol-Ribera E, Valderas JM. Impact of multimorbidity: acute morbidity, area of residency and use of health services across the life span in a region of south Europe. BMC Fam Pract. 2014 Mar 26;15(1):55. doi: 10.1186/1471-2296-15-55. https://bmcfampract.biomedcentral.com/articles/10.1186/1471-2296-15-55 .1471-2296-15-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Zhao S, Zhang L, Haregu TN, Wang H. Impacts of multimorbidity on medication treatment, primary healthcare and hospitalization among middle-aged and older adults in China: evidence from a nationwide longitudinal study. BMC Public Health. 2021 Jul 12;21(1):1380. doi: 10.1186/s12889-021-11456-7. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-021-11456-7 .10.1186/s12889-021-11456-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2011 Jan;61(582):e12–21. doi: 10.3399/bjgp11X548929. https://bjgp.org/lookup/pmidlookup?view=long&pmid=21401985 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricci-Cabello I, Violán Concepció, Foguet-Boreu Q, Mounce LTA, Valderas JM. Impact of multi-morbidity on quality of healthcare and its implications for health policy, research and clinical practice. A scoping review. Eur J Gen Pract. 2015;21(3):192–202. doi: 10.3109/13814788.2015.1046046.10.3109/13814788.2015.1046046 [DOI] [PubMed] [Google Scholar]
- 11.Huot S, Ho H, Ko A, Lam S, Tactay P, MacLachlan J, Raanaas RK. Identifying barriers to healthcare delivery and access in the Circumpolar North: important insights for health professionals. Int J Circumpolar Health. 2019 Dec;78(1):1571385. doi: 10.1080/22423982.2019.1571385. https://europepmc.org/abstract/MED/30696379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doetsch J, Pilot E, Santana P, Krafft T. Potential barriers in healthcare access of the elderly population influenced by the economic crisis and the troika agreement: a qualitative case study in Lisbon, Portugal. Int J Equity Health. 2017 Oct 25;16(1):184. doi: 10.1186/s12939-017-0679-7. https://equityhealthj.biomedcentral.com/articles/10.1186/s12939-017-0679-7 .10.1186/s12939-017-0679-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes Scott D, Song Eunyoung, Nam Sang, Choi Sarah J, Choi Seungyong. Identifying and intervening on barriers to healthcare access among members of a small Korean community in the southern USA. Patient Educ Couns. 2015 Apr;98(4):484–91. doi: 10.1016/j.pec.2015.01.001. https://europepmc.org/abstract/MED/25617908 .S0738-3991(15)00005-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann T, Jansen J, Glasziou P. The importance and challenges of shared decision making in older people with multimorbidity. PLoS Med. 2018 Mar;15(3):e1002530. doi: 10.1371/journal.pmed.1002530. https://dx.plos.org/10.1371/journal.pmed.1002530 .PMEDICINE-D-18-00524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bower P, Macdonald W, Harkness E, Gask L, Kendrick T, Valderas JM, Dickens C, Blakeman T, Sibbald B. Multimorbidity, service organization and clinical decision making in primary care: a qualitative study. Fam Pract. 2011 Oct;28(5):579–87. doi: 10.1093/fampra/cmr018.cmr018 [DOI] [PubMed] [Google Scholar]
- 16.Payne RA, Avery AJ. Polypharmacy: one of the greatest prescribing challenges in general practice. Br J Gen Pract. 2011 Feb;61(583):83–4. doi: 10.3399/bjgp11X556146. https://bjgp.org/lookup/pmidlookup?view=long&pmid=21276330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osanlou R, Walker L, Hughes DA, Burnside G, Pirmohamed M. Adverse drug reactions, multimorbidity and polypharmacy: a prospective analysis of 1 month of medical admissions. BMJ Open. 2022 Jul 04;12(7):e055551. doi: 10.1136/bmjopen-2021-055551. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=35788071 .bmjopen-2021-055551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aramrat C, Choksomngam Y, Jiraporncharoen W, Wiwatkunupakarn N, Pinyopornpanish K, Mallinson PAC, Kinra S, Angkurawaranon C. Advancing multimorbidity management in primary care: a narrative review. Prim Health Care Res Dev. 2022 Jul 01;23:e36. doi: 10.1017/S1463423622000238. https://europepmc.org/abstract/MED/35775363 .S1463423622000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SWH, Chan CKY, Chua SS, Chaiyakunapruk N. Comparative effectiveness of telemedicine strategies on type 2 diabetes management: A systematic review and network meta-analysis. Sci Rep. 2017 Oct 04;7(1):12680. doi: 10.1038/s41598-017-12987-z. doi: 10.1038/s41598-017-12987-z.10.1038/s41598-017-12987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Storch K, Graaf E, Wunderlich M, Rietz C, Polidori MC, Woopen C. Telemedicine-assisted self-management program for type 2 diabetes patients. Diabetes Technol Ther. 2019 Sep;21(9):514–521. doi: 10.1089/dia.2019.0056. [DOI] [PubMed] [Google Scholar]
- 21.Mileski M, Kruse CS, Catalani J, Haderer T. Adopting telemedicine for the self-management of hypertension: Systematic review. JMIR Med Inform. 2017 Oct 24;5(4):e41. doi: 10.2196/medinform.6603. https://medinform.jmir.org/2017/4/e41/ v5i4e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruse CS, Soma M, Pulluri D, Nemali NT, Brooks M. The effectiveness of telemedicine in the management of chronic heart disease - a systematic review. JRSM Open. 2017 Mar;8(3):2054270416681747. doi: 10.1177/2054270416681747. https://journals.sagepub.com/doi/abs/10.1177/2054270416681747?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_2054270416681747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Telemedicine: Opportunities and Developments in Member States: Report on the Second Global Survey on eHealth. World Health Organization. 2010. [2023-06-09]. https://apps.who.int/iris/handle/10665/44497 .
- 24.Njie GJ, Proia KK, Thota AB, Finnie RKC, Hopkins DP, Banks SM, Callahan DB, Pronk NP, Rask KJ, Lackland DT, Kottke TE, Community Preventive Services Task Force Clinical decision support systems and prevention: A community guide cardiovascular disease systematic review. Am J Prev Med. 2015 Nov;49(5):784–795. doi: 10.1016/j.amepre.2015.04.006. https://europepmc.org/abstract/MED/26477805 .S0749-3797(15)00190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bright TJ, Wong A, Dhurjati R, Bristow E, Bastian L, Coeytaux RR, Samsa G, Hasselblad V, Williams JW, Musty MD, Wing L, Kendrick AS, Sanders GD, Lobach D. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012 Jul 03;157(1):29–43. doi: 10.7326/0003-4819-157-1-201207030-00450. https://www.acpjournals.org/doi/abs/10.7326/0003-4819-157-1-201207030-00450?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .1206700 [DOI] [PubMed] [Google Scholar]
- 26.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018 Nov 19;18(1):143. doi: 10.1186/s12874-018-0611-x. https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-018-0611-x .10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenders RA. Advances in clinical decision support: Highlights of practice and the literature 2015-2016. Yearb Med Inform. 2017 Aug;26(1):125–132. doi: 10.15265/IY-2017-012. http://www.thieme-connect.com/DOI/DOI?10.15265/IY-2017-012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaghesh E, Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020 Aug 01;20(1):1193. doi: 10.1186/s12889-020-09301-4. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-020-09301-4 .10.1186/s12889-020-09301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Dahshan A, Al-Kubiasi N, Al-Zaidan M, Saeed W, Kehyayan V, Bougmiza I. Prevalence of polypharmacy and the association with non-communicable diseases in Qatari elderly patients attending primary healthcare centers: A cross-sectional study. PLoS One. 2020;15(6):e0234386. doi: 10.1371/journal.pone.0234386. https://dx.plos.org/10.1371/journal.pone.0234386 .PONE-D-19-22287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraccaro P, Arguello Casteleiro Mercedes, Ainsworth J, Buchan I. Adoption of clinical decision support in multimorbidity: a systematic review. JMIR Med Inform. 2015 Jan 07;3(1):e4. doi: 10.2196/medinform.3503. https://medinform.jmir.org/2015/1/e4/ v3i1e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne Jac, Savović J, Page Mj, Elbers Rg, Blencowe Ns, Boutron I, Cates Cj, Cheng H, Corbett Ms, Eldridge Sm, Emberson Jr, Hernán Miguel A, Hopewell S, Hróbjartsson Asbjørn, Junqueira Dr, Jüni Peter, Kirkham Jj, Lasserson T, Li T, McAleenan A, Reeves Bc, Shepperd S, Shrier I, Stewart La, Tilling K, White Ir, Whiting Pf, Higgins Jpt. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28;366:l4898. doi: 10.1136/bmj.l4898. https://eprints.whiterose.ac.uk/150579/ [DOI] [PubMed] [Google Scholar]
- 32.Fried TR, Niehoff KM, Street RL, Charpentier PA, Rajeevan N, Miller PL, Goldstein MK, O'Leary JR, Fenton BT. Effect of the tool to reduce inappropriate medications on medication communication and deprescribing. J Am Geriatr Soc. 2017 Oct;65(10):2265–2271. doi: 10.1111/jgs.15042. https://europepmc.org/abstract/MED/28804870 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiff GD, Klinger E, Salazar A, Medoff J, Amato MG, John Orav E, Shaykevich S, Seoane EV, Walsh L, Fuller TE, Dykes PC, Bates DW, Haas JS. Screening for adverse drug events: A randomized trial of automated calls coupled with phone-based pharmacist counseling. J Gen Intern Med. 2019 Feb;34(2):285–292. doi: 10.1007/s11606-018-4672-7. https://europepmc.org/abstract/MED/30291602 .10.1007/s11606-018-4672-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prabhakaran D, Jha D, Prieto-Merino D, Roy A, Singh K, Ajay VS, Jindal D, Gupta P, Kondal D, Goenka S, Jacob PD, Singh R, Kumar B G Prakash, Perel P, Tandon N, Patel V, Members of the Research Steering Committee‚Investigators‚Members of the Data SafetyMonitoring Board Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care:: The mWellcare cluster-randomized controlled trial. Circulation. 2019 Jan 15;139(3):380–391. doi: 10.1161/CIRCULATIONAHA.118.038192. https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.118.038192?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 35.Marcolino MS, Oliveira JAQ, Cimini CCR, Maia JX, Pinto VSOA, Sá Thábata Queiroz Vivas, Amancio K, Coelho L, Ribeiro LB, Cardoso CS, Ribeiro AL. Development and implementation of a decision support system to improve control of hypertension and diabetes in a resource-constrained area in Brazil: Mixed methods study. J Med Internet Res. 2021 Jan 11;23(1):e18872. doi: 10.2196/18872. https://www.jmir.org/2021/1/e18872/ v23i1e18872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald EG, Wu PE, Rashidi B, Forster AJ, Huang A, Pilote L, Papillon-Ferland L, Bonnici A, Tamblyn R, Whitty R, Porter S, Battu K, Downar J, Lee TC. The MedSafer study: a controlled trial of an electronic decision support tool for deprescribing in acute care. J Am Geriatr Soc. 2019 Sep;67(9):1843–1850. doi: 10.1111/jgs.16040. [DOI] [PubMed] [Google Scholar]
- 37.Peleg M, Shahar Y, Quaglini S, Broens T, Budasu R, Fung N, Fux A, García-Sáez Gema, Goldstein A, González-Ferrer Arturo, Hermens H, Hernando ME, Jones V, Klebanov G, Klimov D, Knoppel D, Larburu N, Marcos C, Martínez-Sarriegui Iñaki, Napolitano C, Pallàs Àngels, Palomares A, Parimbelli E, Pons B, Rigla M, Sacchi L, Shalom E, Soffer P, van Schooten Boris. Assessment of a personalized and distributed patient guidance system. Int J Med Inform. 2017 May;101:108–130. doi: 10.1016/j.ijmedinf.2017.02.010.S1386-5056(17)30049-7 [DOI] [PubMed] [Google Scholar]
- 38.Willis TA, Collinson M, Glidewell L, Farrin AJ, Holland M, Meads D, Hulme C, Petty D, Alderson S, Hartley S, Vargas-Palacios A, Carder P, Johnson S, Foy R, ASPIRE programme team An adaptable implementation package targeting evidence-based indicators in primary care: A pragmatic cluster-randomised evaluation. PLoS Med. 2020 Feb;17(2):e1003045. doi: 10.1371/journal.pmed.1003045. https://dx.plos.org/10.1371/journal.pmed.1003045 .PMEDICINE-D-19-02682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hussain M, Khan W, Afzal M, Lee S. Smart CDSS for smart homes. In: Donnelly M, Paggetti C, Nugent C, Mokhtari M, editors. Impact Analysis of Solutions for Chronic Disease Prevention and Management. Berlin, Germany: Springer; 2012. [Google Scholar]
- 40.Ventura Filipa, Sousa Pedro, Dixe Maria Anjos, Ferreira Paulo, Martinho Ricardo, Dias Sara Simões, Morais João, Gonçalves Lino M. A clinical decision support system for remote monitoring of cardiovascular disease patients: A clinical study protocol. Front Public Health. 2022;10:859890. doi: 10.3389/fpubh.2022.859890. https://europepmc.org/abstract/MED/35615041 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dramburg Stephanie, Marchante Fernández María, Potapova Ekaterina, Matricardi Paolo Maria. The potential of clinical decision support systems for prevention, diagnosis, and monitoring of allergic diseases. Front Immunol. 2020;11:2116. doi: 10.3389/fimmu.2020.02116. https://europepmc.org/abstract/MED/33013892 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moulaei K, Bahaadinbeigy K. Diagnosing, managing, and controlling COVID-19 using clinical decision support systems: a study to introduce CDSS applications. J Biomed Phys Eng. 2022 Apr;12(2):213–224. doi: 10.31661/jbpe.v0i0.2105-1336. https://europepmc.org/abstract/MED/35433521 .JBPE-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jindal D, Sharma H, Gupta Y, Ajay VS, Roy A, Sharma R, Ali M, Jarhyan P, Gupta P, Srinivasapura Venkateshmurthy N, Ali MK, Narayan KMV, Prabhakaran D, Weber MB, Mohan S, Patel SA, Tandon N. Improving care for hypertension and diabetes in India by addition of clinical decision support system and task shifting in the national NCD program: I-TREC model of care. BMC Health Serv Res. 2022 May 23;22(1):688. doi: 10.1186/s12913-022-08025-y. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-022-08025-y .10.1186/s12913-022-08025-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun R, Catalani C, Wimbush J, Israelski D. Community health workers and mobile technology: a systematic review of the literature. PLoS One. 2013;8(6):e65772. doi: 10.1371/journal.pone.0065772. https://dx.plos.org/10.1371/journal.pone.0065772 .PONE-D-13-00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feroz AS, Khoja A, Saleem S. Equipping community health workers with digital tools for pandemic response in LMICs. Arch Public Health. 2021 Jan 04;79(1):1. doi: 10.1186/s13690-020-00513-z. https://archpublichealth.biomedcentral.com/articles/10.1186/s13690-020-00513-z .10.1186/s13690-020-00513-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pengpid S, Peltzer K. Multimorbidity in chronic conditions: public primary care patients in four greater Mekong countries. Int J Environ Res Public Health. 2017 Sep 06;14(9):1019. doi: 10.3390/ijerph14091019. https://www.mdpi.com/resolver?pii=ijerph14091019 .ijerph14091019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jørgensen Isabella Friis, Aguayo-Orozco A, Lademann M, Brunak S. Age-stratified longitudinal study of Alzheimer's and vascular dementia patients. Alzheimers Dement. 2020 Jun;16(6):908–917. doi: 10.1002/alz.12091. https://europepmc.org/abstract/MED/32342671 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freisling H, Viallon V, Lennon H, Bagnardi V, Ricci C, Butterworth AS, Sweeting M, Muller D, Romieu I, Bazelle P, Kvaskoff M, Arveux P, Severi G, Bamia C, Kühn Tilman, Kaaks R, Bergmann M, Boeing H, Tjønneland Anne, Olsen A, Overvad K, Dahm CC, Menéndez Virginia, Agudo A, Sánchez Maria-Jose, Amiano P, Santiuste C, Gurrea AB, Tong TYN, Schmidt JA, Tzoulaki I, Tsilidis KK, Ward H, Palli D, Agnoli C, Tumino R, Ricceri F, Panico S, Picavet HSJ, Bakker M, Monninkhof E, Nilsson P, Manjer J, Rolandsson O, Thysell E, Weiderpass E, Jenab M, Riboli E, Vineis P, Danesh J, Wareham NJ, Gunter MJ, Ferrari P. Lifestyle factors and risk of multimorbidity of cancer and cardiometabolic diseases: a multinational cohort study. BMC Med. 2020 Jan 10;18(1):5. doi: 10.1186/s12916-019-1474-7. http://hdl.handle.net/2318/1766479 .10.1186/s12916-019-1474-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudesia P, Salimarouny B, Stanley M, Fortin M, Stewart M, Terry A, Ryan BL. The incidence of multimorbidity and patterns in accumulation of chronic conditions: A systematic review. J Multimorb Comorb. 2021;11:26335565211032880. doi: 10.1177/26335565211032880. https://journals.sagepub.com/doi/abs/10.1177/26335565211032880?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed .10.1177_26335565211032880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.You L, Yu Z, Zhang X, Wu M, Lin S, Zhu Y, Xu Z, Lu J, Wei F, Tang M, Wang J, Jin M, Chen K. Association Between multimorbidity and depressive symptom among community-dwelling elders in eastern China. Clin Interv Aging. 2019;14:2273–2280. doi: 10.2147/CIA.S221917. https://europepmc.org/abstract/MED/31908437 .221917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ansari S, Anand A, Hossain B. Multimorbidity and depression among older adults in India: Mediating role of functional and behavioural health. PLoS One. 2022;17(6):e0269646. doi: 10.1371/journal.pone.0269646. https://dx.plos.org/10.1371/journal.pone.0269646 .PONE-D-22-00909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walrave R, Beerten SG, Mamouris P, Coteur K, Van Nuland M, Van Pottelbergh G, Casas L, Vaes B. Trends in the epidemiology of depression and comorbidities from 2000 to 2019 in Belgium. BMC Prim Care. 2022 Jun 28;23(1):163. doi: 10.1186/s12875-022-01769-w. https://europepmc.org/abstract/MED/35764925 .10.1186/s12875-022-01769-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh S, Shri N, Dwivedi LK. An association between multi-morbidity and depressive symptoms among Indian adults based on propensity score matching. Sci Rep. 2022 Sep 15;12(1):15518. doi: 10.1038/s41598-022-18525-w. doi: 10.1038/s41598-022-18525-w.10.1038/s41598-022-18525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanners M, Barton C, Shakib S, Winefield H. The prevalence of depression amongst outpatients with multimorbidity. Health. 2013;05(04):805–810. doi: 10.4236/health.2013.54106. https://www.scirp.org/pdf/Health_2013042415045880.pdf . [DOI] [Google Scholar]
- 55.Boudoulas KD, Triposkiadis F, Gumina R, Addison D, Iliescu C, Boudoulas H. Cardiovascular disease, cancer, and multimorbidity interactions: Clinical implications. Cardiology. 2022;147(2):196–206. doi: 10.1159/000521680. https://doi.org10.1159/000521680 .000521680 [DOI] [PubMed] [Google Scholar]
- 56.Jayashree P, Janaka Sudha G, Srinivasan KS, Robert Wilson S. Clinical decision support system for early detection of Alzheimer's disease using an enhanced gradient boosted decision tree classifier. Health Informatics J. 2022;28(1):14604582221082868. doi: 10.1177/14604582221082868. https://journals.sagepub.com/doi/abs/10.1177/14604582221082868?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub0pubmed . [DOI] [PubMed] [Google Scholar]
- 57.Praveen D, Patel A, Raghu A, Clifford GD, Maulik PK, Mohammad Abdul A, Mogulluru K, Tarassenko L, MacMahon S, Peiris D. SMARTHealth India: development and field evaluation of a mobile clinical decision support system for cardiovascular diseases in rural India. JMIR Mhealth Uhealth. 2014 Dec 08;2(4):e54. doi: 10.2196/mhealth.3568. https://mhealth.jmir.org/2014/4/e54/ v2i4e54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarousi M, Bountris P, Daskalakis P, Koutsouris DD. Development of a cost-effective intelligent clinical decision support system for breast cancer early diagnosis and triage. Int J Onl Eng. 2022 Apr 12;18(05):43–64. doi: 10.3991/IJOE.V18I05.29067. https://online-journals.org/index.php/i-joe/article/view/29067/11145 . [DOI] [Google Scholar]
- 59.Alaa AM, Moon KH, Hsu W, van der Schaar M. ConfidentCare: A clinical decision support system for personalized breast cancer screening. IEEE Trans Multimedia. 2016 Oct;18(10):1942–1955. doi: 10.1109/tmm.2016.2589160. [DOI] [Google Scholar]
- 60.Adams E, Mital D, Mehta S. Clinical decision support system as a risk assessment tool to aid in earlier diagnosis of pancreatic cancer. Int J Med Eng Inform. 2017;9(2):87. doi: 10.1504/ijmei.2017.083087. [DOI] [Google Scholar]
- 61.Patient engagement: Technical series on safer primary care. World Health Organization. [2023-06-09]. https://apps.who.int/iris/bitstream/handle/10665/252269/9789241511629-eng.pdf .
- 62.Jefee-Bahloul H, Barkil-Oteo A, Augusterfer EF. Telemental Health in Resource-Limited Global Setting. Oxford, UK: Oxford University Press; 2017. [Google Scholar]
- 63.Scott R, Mars M. Telehealth in the developing world: current status and future prospects. Smart Homecare Technol Telehealth. 2015 Feb;:25. doi: 10.2147/SHTT.S75184. https://www.dovepress.com/getfile.php?fileID=23551 . [DOI] [Google Scholar]
- 64.Patient-reported outcome measures (PROMs) Canadian Institute for Health Information. [2022-12-01]. https://www.cihi.ca/en/patient-reported-outcome-measures-proms .
- 65.DiNoia C. The Benefits of Care Coordination. Lightbeam Health Solutions. [2023-06-09]. https://lightbeamhealth.com/the-benefits-of-care-coordination/
- 66.Mandelblatt JS, Fryback DG, Weinstein MC, Russell LB, Gold MR. Assessing the effectiveness of health interventions for cost-effectiveness analysis. Panel on cost-effectiveness in health and medicine. J Gen Intern Med. 1997 Sep;12(9):551–8. doi: 10.1046/j.1525-1497.1997.07107.x. https://europepmc.org/abstract/MED/9294789 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jamison DB, Breman JG, Measham AR. Priorities in Health. Washington, DC: World Bank; 2006. [PubMed] [Google Scholar]
- 68.Chib A, van Velthoven Michelle Helena, Car J. mHealth adoption in low-resource environments: a review of the use of mobile healthcare in developing countries. J Health Commun. 2015;20(1):4–34. doi: 10.1080/10810730.2013.864735. [DOI] [PubMed] [Google Scholar]
- 69.Chang J, Ronco C, Rosner MH. Computerized decision support systems: improving patient safety in nephrology. Nat Rev Nephrol. 2011 Jun;7(6):348–55. doi: 10.1038/nrneph.2011.50. https://europepmc.org/abstract/MED/21502973 .nrneph.2011.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamprinos I, Demski H, Mantwill S, Kabak Y, Hildebrand C, Ploessnig M. Modular ICT-based patient empowerment framework for self-management of diabetes: Design perspectives and validation results. Int J Med Inform. 2016 Jul;91:31–43. doi: 10.1016/j.ijmedinf.2016.04.006.S1386-5056(16)30055-7 [DOI] [PubMed] [Google Scholar]
- 71.Mackintosh N, Terblanche Marius, Maharaj Ritesh, Xyrichis Andreas, Franklin Karen, Keddie Jamie, Larkins Emily, Maslen Anna, Skinner James, Newman Samuel, De Sousa Magalhaes Joana Hiew, Sandall Jane. Telemedicine with clinical decision support for critical care: a systematic review. Syst Rev. 2016 Oct 18;5(1):176. doi: 10.1186/s13643-016-0357-7. https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-016-0357-7 .10.1186/s13643-016-0357-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim Y, Groombridge C, Romero L, Clare S, Fitzgerald MC. Decision support capabilities of telemedicine in emergency prehospital care: Systematic review. J Med Internet Res. 2020 Dec 08;22(12):e18959. doi: 10.2196/18959. https://www.jmir.org/2020/12/e18959/ v22i12e18959 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search terms in different databases.
Search terms for extended search.
The functions of telemedicine.
Types of clinical decision support system task in telemedicine.
Summary of types of intervention outcome.


